Abstract
In the past decade, the prevalence, significance, and regulatory mechanisms of vascular calcification have gained increasing recognition. Over a century ago, pathologists recognized atherosclerotic calcification as a form of extraskeletal ossification. Recent studies are identifying the mechanisms of this remarkable process as a recapitulation of embryonic endochondral ossification through phenotypic plasticity of vascular cells that function as adult mesenchymal stem cells. These embryonic developmental programs, involving bone morphogenetic proteins and potent osteochondrogenic transcription factors, are triggered and modulated by a variety of inflammatory, metabolic, and genetic disorders particularly hyperlipidemia, chronic kidney disease, diabetes, hyperparathyroidism, and osteoporosis. They are also triggered by loss of powerful inhibitors, such as fetuin-A, matrix GLA protein, and pyrophosphate, which ordinarily restrict biomineralization to skeletal bone. Teleologically, soft tissue calcification probably serves to create a wall of bone to sequester noxious foci such as chronic infections, parasites, and foreign bodies. This review focuses on atherosclerotic calcification, with reference to other forms of cardiovascular calcification, such as medial and valvular calcification, each of which warrants its own review. The capacity of the asculature to produce mineral in culture and to produce de novo, vascularized, trabecular bone and cartilage tissue, even in patients with osteoporosis, should intrigue investigators in tissue engineering and regenerative biology.
Introduction
More than a century ago, investigators recognized vascular calcification as a form of extraskeletal ossification.1, 2 This concept was forgotten in the past century, when cholesterol dominated the field. Vascular calcium deposits became regarded as passive, inevitable, unregulated, and degenerative consequences of aging. In the past decade, the prevalence, significance, and regulatory mechanisms of vascular calcification have gained recognition among clinicians.3
A recent meta-analysis of 30 prospective cohort studies demonstrated the consistent finding that presence of calcification poses an increased risk for cardiovascular and all-cause mortality.4 By far, the most extensive vascular calcification occurs in patients with renal disease (CKD),5 followed by those with type II diabetes.6 Nearly all cardiovascular disease (CVD) patients have some degree of calcification,7 and in asymptomatic adults, prevalence of coronary calcification corresponds roughly with age: among 60-year-olds, approximately 60% have calcific vasculopathy. 3, 4
Etiology
Vascular calcification is the culmination of several distinct pathological processes, many of which overlap, such as in chronic kidney disease (CKD), which Towler aptly named the “perfect storm” for vascular calcification.8 These processes largely follow developmental programs that recapitulate embryonic ossification, with modulation by inflammatory or metabolic phenomena (Figure 1). Both activators and inhibitors participate, and many of them interact. The developmental programs play out in vascular cells exhibiting lineage plasticity and inflammatory responses to chronic oxidative stress.
Figure 1.
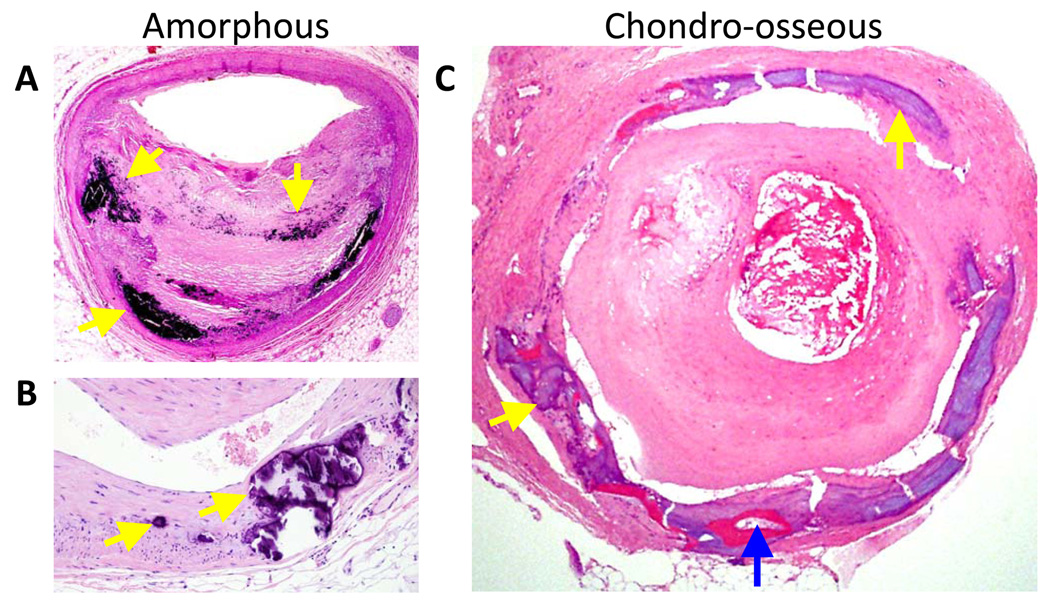
Histological sections of human vascular calcification. Medial arterial calcification, osseous metaplasia, and cartilaginous metaplasia (mineral is stained balck by the von Kossa method and eosin counterstain; original magnification X 40; provided by M. Fishbein, Department of Pathlogy and Laboratory Medicine, University of California, Los Angeles, CA, USA.) (B) Higher-power view of a sequential section from (A) showing chondrocytes in a basophilic matrix (arrows). (C) The tunica media of a human artery (hematoxylin and eosin stain; the lumen contains a thrombus). The yellow arrows indicate amorphous mineral, and the blue arrow identifies a region of osseous tissue that includes a marrow space. (Original magnification X 100; provided by J. H. Qiao, Department of Pathology, California Hospital Medical Center, Los Angeles, CA, USA.)
Given the potential adverse consequences of uncontrolled biomineralization, calcium-phosphate metabolism is tightly regulated and mineralization ordinarily limited to skeletal bone by circulating and local inhibitors. Early investigators believed that the process of vascular calcification was a passive process possible only when inhibitors were absent or deficient. The current view is that it is an active process that occurs despite the presence of inhibitors and, once underway, recapitulates regulated osteogenesis.
Recapitulation of osteogenesis
One of the first, and most compelling, clues that vascular calcification recapitulates osteogenesis was the presence of bone-like tissue in atherosclerotic arteries and valves.2, 9 In 10–20% of atherosclerotic human vessels and valves, 9 architecturally-complete, trabecular bone emerges from amorphous mineralized matrix. All stages of endochondral ossification are found in a careful survey of such lesions,10 even fully-formed marrow cavities with hematopoietic cells, vascular sinusoids, marrow adipocytes, and marrow stromal cells.1, 10, 11 Spontaneous bone formation inside the wall of adult arteries is difficult to explain,
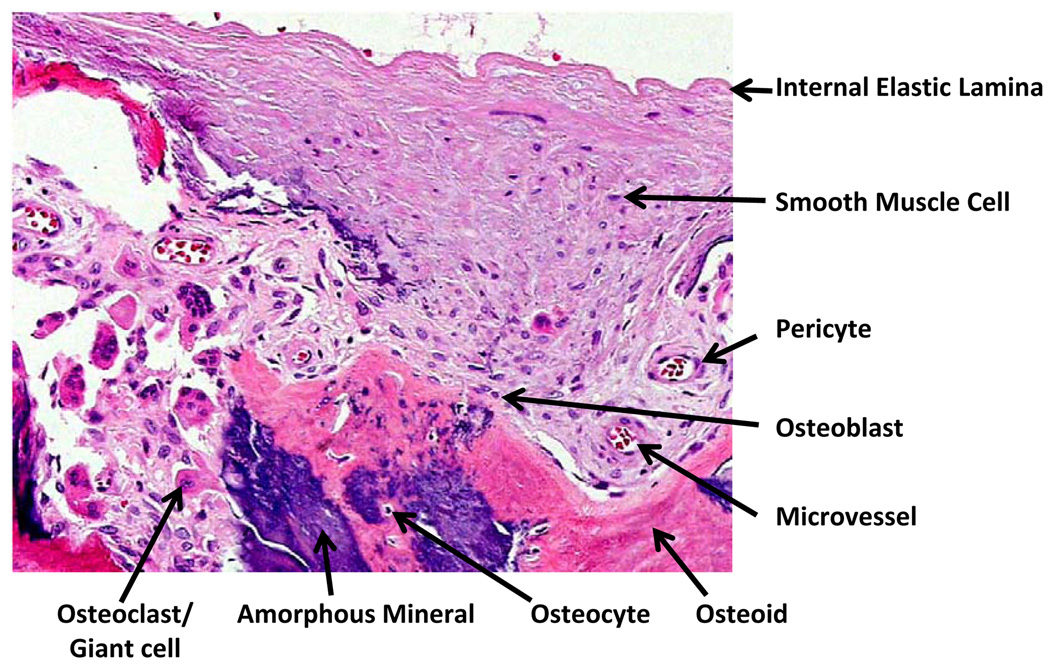
Vascular calcium deposits resemble bone from the macroscale to the nanoscale levels. Macroscopically, cortical bone osteons resemble a parallel array of calcified vessels, each containing concentric layers of mesenchymal cells surrounding the subendothelial basement membrane of a central vessel. The mineral deposits contain “osteoid” (bone matrix), which consists primarily of collagen I, but also has non-collagenous bone matrix proteins, including osteopontin, bone sialoprotein, osteocalcin, fetuin, and matrix GLA protein. Cellular components include osteoblasts, osteoclasts, chondroblasts, chondroclasts, osteocytes, lymphocytes, and vascular cells. Figure 2
Figure 2.
Chondro-osseous calcific vasculopathy of the medial layer of an atherosclerotic human artery. Histological section (hematoxylin and eosin stain; original magnification X100; provided by Qiao, J.H., Mertens, R. B., Fishbein, M.C. & Geller S.A. Cartilagenous metaplasia in calcified diabetic peripheral vascular disease: morphologic evidence of endochondral ossification. Hum. Pathol. 34(4), 402–407© 2003, with permission from Elsevier).
Analysis of the mineral in vascular lesions and cell cultures by energy dispersive x-ray analysis consistently yields a calcium-phosphate molar ratio of approximately 1.6, matching that of hydroxyapatite (bone) mineral.12, 13 The non-mineral components of the deposits include cells, proteins, proteoglycans,14 and lipids.15 Solid-state nuclear magnetic resonance spectroscopy reveals molecular surface features matching those of bone.14.
Cellular plasticity and vascular stem cells
Ectopic bone in the artery wall is dramatic evidence of mesenchymal cell plasticity. The notion that smooth muscle cells undergo “de-differentiation into a synthetic phenotype” and “phenotypic modulation” already has gained acceptance,16 and these terms may be viewed as euphemisms for “transdifferentiation.” Indeed, conventional distinctions among many cell lineages are becoming less clear as investigators report that: endothelial cells differentiate to smooth muscle cells;17 adult mesoangioblasts to myocytes and cardiomyocytes;18 adipocytes to vascular cells;19 osteoclasts to dendritic cells;20 dendritic cells to osteoclasts;21 smooth muscle cells to osteoblasts;22, 23 and microvascular pericytes to osteoblasts, myocytes and adipocytes.24, 25 The doctrines of lineage commitment and terminal differentiation are giving way to the view of mesenchymal cell plasticity.
Mature vascular cells, such as SMCs and valvular interstitial cells, may transdifferentiate22 or dedifferentiate-redifferentiate into osteochondrogenic cells. As with other cells, vascular cell differentiation responds to microenvironmental26 and mechanical cues. As evidence of the mechanical influence, mineral deposits co-localize with sites of mechanical stress on valves.27 Furthermore, substrates of greater stiffness, such as fibronectin, induce osteochondrogenic differentiation, whereas distensible substrates, such as laminin, promote smooth muscle and/or adipogenic differentiation. 28 Since cross-linking increases matrix stiffness, this may account for the vascular calcification induced by the matrix cross-linker, transglutaminase-2.29 Positive feedback may occur when calcium deposition increases plaque stiffness, which would induces osteochondrogenic differentiation of adjoining cells.
Adult mesenchymal stem cells residing in the artery wall have a lineage repertoire similar to that of marrow stromal cells.18, 23, 25 Located primarily in the subendothelial (perivascular) basement membrane,19 they appear to form an anatomic continuum30 ranging from the microvascular pericytes to the macrovascular intimal cells, once known as “atherophils” and “intimacytes.” Even the adventitial layer has subendothelial pericytes in its microvessels, the vasa vasorum.31, 32
Paradox of vascular calcification and osteoporosis
Interestingly, aortic calcification is often greater in patients with osteoporosis, independently of age.33, 34 Public health programs currently promote calcium supplements to prevent osteoporosis, giving the impression that dietary calcium is the limiting factor for bone growth. However, the fact that the vascular tissues of osteoporotic patients can produce mature, well-mineralized bone tissue raises questions about the need for dietary supplements and emphasizes the role of local, tissue-specific factors, such as inflammation, in osteoporosis.
If the relationship is independent of age, possible explanations for the correlation include: 1) vascular calcification causes osteoporosis, 2) osteoporosis causes vascular calcification, or 3) the two processes share a common etiology. A potential shared etiology is oxidant stress from hyperlipidemia. Though most widely recognized in the vessel wall, hyperlipidemia leads to deposition and nonenzymatic oxidation of lipoprotein particles in tissue. The pro-inflammatory lipid oxidation products initiate atherogenesis. They also accumulate in other tissues, including bone.35, 36
This concept has to the “lipid hypothesis of osteoporosis.”37 High fat-fed mice develop skeletal bone loss,38 which is accelerated in hyperlipidemia.39 Lipid oxidation products have direct effects on both bone-forming and bone-resorbing cells. They directly inhibit differentiation of osteoblasts37 while directly inducing differentiation of osteoclasts.40 They also regulate osteoclastogenic cytokines produced by osteoblasts41 and T lymphocytes.42 Recent studies have shown that patients with hyperlipidemia may not achieve efficacy with intermittent PTH treatment for osteoporosis because the oxidized lipids blunt the bone anabolic effects of PTH.43, 44
Mechanisms of initiation
While intracellular osteogenic phenomena are becoming clear, the extracellular crystal initiation mechanism(s) remains less clear. In general, biomineralization is restricted to tissues that express both collagen I and alkaline phosphatase; engineered co-expression of these proteins suffices to induce ectopic calcification.45 Type I collagen, but not ALP, is present in normal artery wall. Both type I collagen and ALP colocalize with mineral deposits in calcific atherosclerosis46 and are produced in vascular cells in vitro.47, 48
In mature osteoid, mineral is associated with fibrillar collagen I. In earlier stages, crystallization may originate within extracellular membrane vesicles, such as matrix vesicles or apoptotic bodies, which offer a microenvironment high in calcium and phosphate and carry membrane-bound ALP.49 By electron microscopy, such crystalline matrix vesicles, are often found in close proximity to apoptotic cells,50 within atherosclerotic plaques and, when isolated, retain the ability to concentrate calcium and phosphate and initiate new crystal formation.51, 52 Matrix vesicles may be specialized forms of general-purpose, membrane vesicles used for intercellular communication53 while apoptotic bodies may represent a pathophysiological surrogate.54
Elastic lamellae are degraded in atherosclerotic plaque, and their degradation products may contribute to crystal initiation.55, 56
Pyrophosphate, a water softener and a cause of pseudogout, potently inhibits calcium phosphate crystal formation. Although ALP produces Pi, its main role in calcification is the breakdown of PPi. Extracellular PPi is produced in the extracellular space from nucleotide triphosphates, such as ATP, by enzymes such as nucleotide pyrophosphatase (NPP)1. PPi produced inside cells is exported via the transmembrane protein, Ank. Deficiency of either NPP1 or Ank in mice results in vascular and joint calcification with spontaneous chondrogenic metaplasia.57
Human genetic disorders
A lethal congenital disorder that causes myocardial infarction in infants, previously known as idiopathic infantile arterial calcinosis, is now recognized as homozygous NPP1 deficiency and has been renamed infantile arterial calcification.58 Patients with another hereditary human disorder, fibrodysplasia ossificans progressiva (FOP), develop ectopic bone at sites of muscle and soft tissue injury, gradually causing petrification of its victims.59 The ectopic bone formation is mediated by cells of vascular origin,60 that derive from a Tie2-positive, but not SM-MHC positive lineage, suggesting an endothelial or more primitive progenitor stem cell origin. Patients with homozygous familial hypercholesterolemia have premature severe calcific aortopathy and valvulopathy, suggesting a role for hyperlipidemia in both.61
Categories of vascular calcification
Vascular calcification can be distinguished by location as intimal (atherosclerotic), medial, or valvular, each having somewhat distinct mechanisms.
Calcific atherosclerosis occurs in the same distribution as atherosclerosis. Small (5–10 µm) hydroxyapatite mineral crystals arise in early lesions in the 3rd decade of life.62 It was previously believed that calcification occurred only in the most advanced, end-stage plaque in the elderly. Yet, it is now used as a marker for early atherosclerosis.63
Calcific valvular stenosis, a lethal disorder formerly attributed to wear-and-tear injury, is now seen as an atherosclerotic process that shares risk factors and etiological factors with calcific atherosclerosis. Chondroosseous metaplasia is a common feature.64 Oxidant stress, 65 lipids66, 67 and Wnt/beta-catenin/LRP5 pathway68, 69 have been implicated.
Medial calcification occurs primarily in association with chronic kidney disease and diabetes, independently of atherosclerosis, and it resembles embryonic membranous (vs. endochondral) ossification. In microvessels, medial calcification is known as calcific uremic arteriolopathy, previously calciphylaxis.
Both medial calcification and calcific valvular stenosis have been reviewed extensively elsewhere. 5, 6, 64, 70, 71 The present review focuses primarily on atherosclerotic calcification and its mechanisms.
Clinical significance
Controversy remains with respect to whether vascular calcification is a cause or consequence of cardiovascular disease. Most likely, it is both: a cause, in that atherosclerosis induces cellular osteogenic differentiation; and a consequence, in that vascular calcification stiffens the aorta and affects plaque stability. In the coronary arteries, calcified plaque independently predicts a 1.7-fold increase in mortality. In peripheral arteries, it independently predicts amputation and mortality.72 When coronary calcification is extensive, the risk is 60-fold higher.3
Aortic stiffness is an important consequence of medial calcification, and calcification has the greatest effect on aortic rigidity.73, 74 Aortic rigidity results in hypertension, left ventricular hypertrophy, ischemia, heart failure, amputation, and death.6
Plaque stability may be affected by calcium deposits because they introduce compliance mismatch at the interface of the rigid mineral with the more distensible artery wall tissue. Under mechanical stress, this interface has a greater risk for mechanical failure (plaque rupture). Such plaque rupture is believed to cause most myocardial infarction and stroke.75 Based on finite element analysis, calcium deposits dramatically redistribute stress in plaque, reducing it in some regions at the cost of increasing it in others,76 with the net effect on risk of rupture depending on the anatomic orientation of the calcium deposits relative to the plaque and any necrotic core.77, 78
Regulatory factors
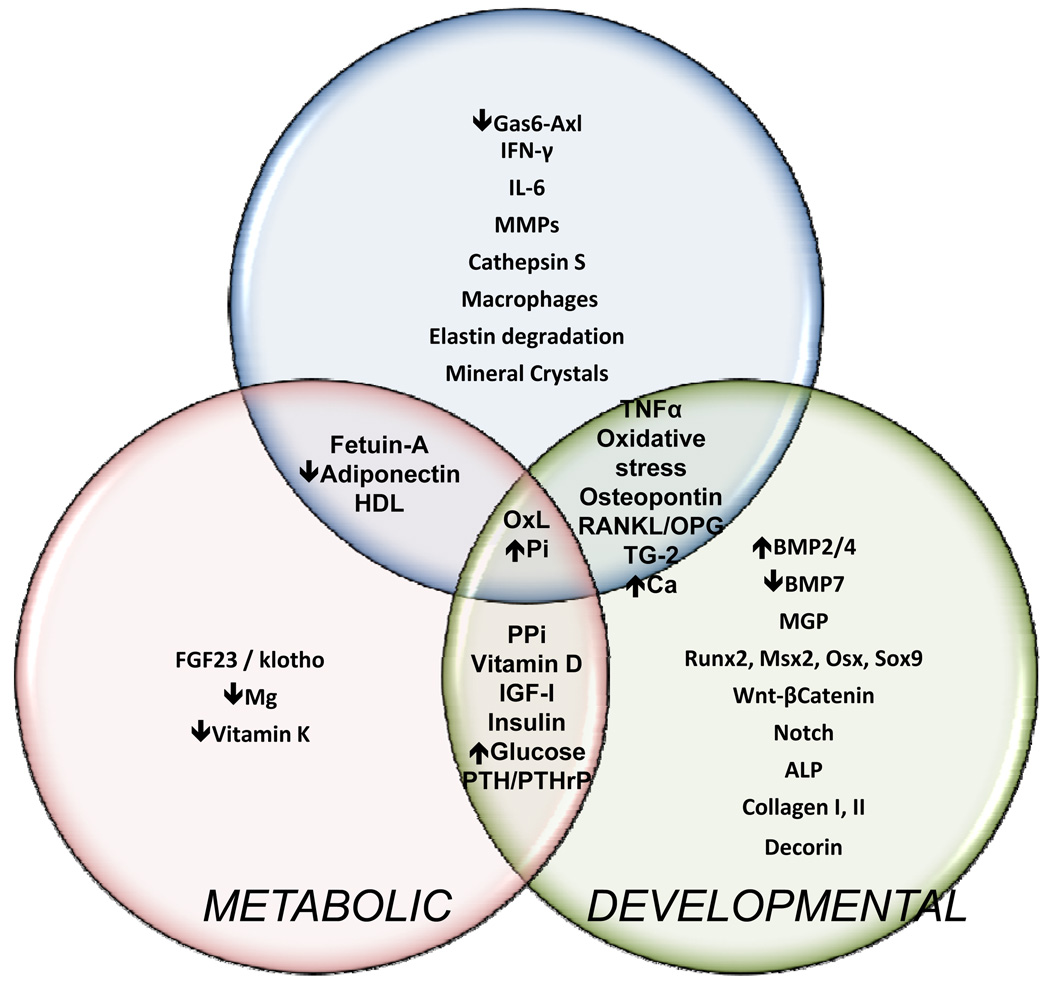
Factors that regulate vascular calcification are shown in Figure 3. Developmental, inflammatory and metabolic factors all impact on this process.
Figure 3.
Regulatory factors in vascular calcification. The Venn diagram illustrates overlap between the three categories of factors regulating vascular calcification. The importance of oxidized lipids (OxL) and hyperphosphatemia is reflected by their inclusion in each category.
Developmental factors
Several developmental regulatory factors, and their respective inhibitors, govern osteogenesis in skeletal bone and in vascular calcification. The master transcription factors, Msx2, Runx2 (Cbfa1), Osterix, and Sox9, designate cells for osteoblast vs. chondrocyte lineages through induction of downstream matrix components such as collagen I, alkaline phosphatase, osteopontin, tissue factor, and osteocalcin. Osteopontin, a multifunctional protein involved in diverse processes including inhibition mineralization, is reviewed in detail by Giachelli and colleagues.79
Bone morphogenetic proteins
BMP2 and 4, potent osteogenic differentiation factors originally isolated from bovine bone, induce ectopic ossification in muscle tissue in vivo and mineralization in SMC in vitro. BMP-2 acts through Runx2,80 which induces type I collagen and alkaline phosphatase. It is antagonized by noggin, chordin and matrix carboxyglutamic acid protein (MGP). 81 BMP4 is induced by RANKL in rat smooth muscle cells,82 and BMP7 inhibits calcification.83
MGP
MGP, the BMP inhibitor, associates with conventional cardiovascular risk factors (Framingham), but, surprisingly, not with coronary calcification.84 It is highly expressed in calcified vs. normal human arteries.46 Its inhibitory role in vascular calcification was revealed with the unexpected phenotype of the MGP deficient mouse – complete ossification of the aortic wall and major branches by calcified cartilage.85 Later studies showed two mechanisms of MGP inhibition of calcification: direct binding of nascent crystals, and direct binding and inhibition of BMP-2.86 MGP is blocked, in turn, by heat shock protein-70,87 and lack of vitamin K. Its function depends on vitamin K-dependent gamma-carboxylation of glutamate residues,88 a process inhibited by warfarin. Accordingly, long-term warfarin therapy is associated with increased femoral artery calcification,4 and its possible contribution to other vascular calcification is under investigation.
RANKL
RANKL, expressed by osteoblasts, is an essential regulator of osteoclast differentiation. Deficiency of osteoprotegerin (OPG), a decoy receptor for RANK, leads to medial calcifcation.89, 90 Unexpectedly, RANKL levels increase with age and reliably predict cardiovascular events.91 The OPG/RANKL system may govern differentiation of osteoclast-like cells at sites of vascular calcification.9, 92
Inflammatory factors
Inflammation is closely associated with calcification. Macrophages, lymphocytes, and dendritic cells infiltrate plaque and release cytokines that regulate calcification.20, 40 Perivascular adipose inflammation and systemic inflammation 93, 94 as well as systemic inflammation.95 In elegant work by Towler and colleagues, the link between inflammatory and developmental mechanisms was identified as the pro-osteogenic Msx2-Wnt-β-catenin signaling mechanism.
TNF-a
TNFα induces the Msx2-Wnt-beta-catenin signaling pathway96 as do oxidant stress6 and hyperphosphatemia.97 TNFα also promotes calcification in vitro by reducing anti-apoptotic Gas698 and by inducing ALP via PKA signaling.48 TNFα may also act on ALP via NADPH-mediated ROS and induction of Msx2.6, 99 In vivo, targeted TNFα overexpression in SMCs enhances Msx2-Wnt induced calcification in Ldlr−/− mice, and the clinically-used monoclonal antibody to TNFα, infliximab, inhibits Wnt activation and calcification in Ldlr−/− mice.96
Gas6/Axl
A prominent anti-apoptotic pathway in VSMCs is regulated by Axl tyrosine kinase and its ligand, Gas6, another GLA protein. Reduced expression of Axl and Gas6 correlates with progression of calcification in vitro,100 whereas restoration prevents calcification.101, 102 This pathway is a downstream effector for a number of key regulators of vascular calcification, including inorganic phosphate103 and TNFa98 as well as testosterone 104 and adiponectin.98 The role of Gas6 and Axl in cardiovascular diseases may be complex, since they also mediate atherogenesis, platelet function and immune cell activation.
Fetuin-A
Fetuin-A, an abundant serum protein produced in the liver, binds and complexes calcium phosphate nanocrystals, forming calcioprotein particles (CPPs), preventing aggregation into insoluble mineral crystals, and preventing further growth. Fetuin-A also promotes cellular uptake and removal of the complexes.105 Fetuin accumulates at sites of vascular calcification106 as well as in bone. Other bone-associated proteins also bind crystals particularly osteopontin.107 In vitro, fetuin taken up by VSMCs reduces the ability of their matrix vesicles to calcify.106, 108 Low fetuin-A levels are associated with increased vascular calcification and mortality in CKD patients.109
Crystals
Nanoscale hydroxyapatite crystals13, 14 may have direct biological effects on cells through physicochemical interactions that trigger inflammation and apoptosis. 13 These effects are known pyrophosphate and urate crystals, but less so for hydroxyapatite.110 A positive feedback loop may ensue when inflammation triggers mineralization and mineralization triggers inflammation. In osteoarthritis crystals induce MMPs and cell proliferation through Erk and calcium signaling.111
Metabolic factors
Excess lipids, phosphate and/or glucose, in chronic diseases, have both direct and indirect effects on vascular calcification. A central downstream mediator of these changes and their link with inflammatory and developmental processes described above may be oxidant stress.
Oxidant stress
Oxidant stress, alone, promotes vascular cell calcification,112 It may account for the procalcific effects of inflammatory cytokines, oxidized lipids and certain oxysterols. The classical oxidant stressor, H202, promotes osteochondrocytic differentiation of VSMC by upregulating Runx2.47 Reactive oxygen species are increased at sites of calcification in human valves.65, 113 Products of lipid oxidation, such as minimally-modified LDL and oxidized phospholipids, induce osteogenic37, 114 and apoptosis-mediated calcification of vascular cells.115 The osteogenic differentiation induced by TNF-alpha and H202 is inhibited by insulin-like growth factor-1.116
Hyperphosphatemia
The important role of hyperphosphatemia in the medial calcification associated with chronic kidney disease has been reviewed in detail.70, 97 Serum phosphate levels are regulated by FGF23, which is released from bone osteocytes and activates its coreceptor Klotho in the kidney, controlling phosphate elimination.
Vitamin D
High dose, dietary vitamin D reliably induces medial calcification; it is often used to generate animal models of vascular calcification.117 As a fat-soluble vitamin, dietary vitamin D may be carried by chylomicrons and lipoprotein particles, which are deposited into the artery wall, where it may be converted to active form by 1-alpha-hydroxylase in VSMC and in monocyte-macrophages. This raises interesting questions about the potential for vitamin D to promote atherosclerotic calcification and cardiovascular risk. The role of vitamin D in vascular calcification has been reviewed recently.118 Other metabolic factors that affect calcific vasculopathy include insulin and glucose as well as the adipose-derived factors, leptin and adiponectin, which promote and inhibit vascular calcification, respectively.119–121
Treatments
Currently, no therapy is available to reverse vascular calcification; several are available for underlying disorders such as atherosclerosis, CKD, diabetes mellitus, and osteoporosis. Interestingly, this disorder is now afforded such importance in CKD that new therapies are largely judged by their impact on vascular calcification.122 Under investigation are HMG-CoA reductase inhibitors, sevelamer, bisphosphonates, the phosphorus binder, lanthanum carbonate, bisphosphonates, alkaline phosphatase inhibitors, calcimimetics, , and the RANK blocker, denosumab. Importantly, procalcific treatments used for skeletal osteoporosis may have adverse effects on vascular calcification.
Conclusions
Mineral deposits in atherosclerotic plaque result from several different pathways involving metabolic and/or inflammatory processes that trigger reversion to embryonic developmental programs for osteochondrogenesis. What evolutionary advantage might this confer? One possibility is that soft tissue calcification in general is an immune response of last resort. Certain chronic infections, such as mycobacteria, parasitic worms, abscesses, or foreign body infections, may be unabated by cellular and humoral immunity. By surrounding such infections with a wall of bone, the body may contain the noxious focus. Radiologists have described such calcified structures as “ostrich eggs.” The capacity of the artery wall to generate complete, vascularized, trabecular bone tissue, as well as cartilage and fat, should capture the attention investigators in tissue regenerative and tissue engineering medicine.
Key Points
Calcific vasculopathy and valvulopathy encompass amorphous calcification and chondro-osseous metaplasia in atherosclerotic plaque, the medial layer of large arteries, and cardiac valves, with different, but overlapping mechanisms.
The process is associated with, and possibly driven by, developmental, inflammatory, and/or metabolic abnormalities. A disturbance of one or any combination of activating and inhibiting factors may be responsible. The list of regulatory factors continues to grow, and the complex feedback regulatory mechanisms require nonlinear and systems engineering analysis.
Vascular calcification associates with most conventional cardiovascular risk factors, and it is a significant, independent risk factor in itself.
Clinical consequences of calcific disease include heart failure, valvular sclerosis and stenosis, ventricular hypertrophy, diastolic dysfunction, and hypertension. Plaque calcification introduces mechanical discontinuities and compliance mismatch.
Targeting hyperlipidemia in CVD patients and hyperphosphatemia in CKD patients remain the current major approaches to preventing vascular calcification, but definitive studies remain in progress.
Acknowledgments
Funding:
This work was supported in part by the National Institutes of Health [DK081346 to YT, HL081202 to LLD] and American Heart Association [0825033F to APS].
References
- 1.Bunting CH. The Formation of True Bone with Cellular (Red) Marrow in a Sclerotic Aorta. J Exp Med. 1906;8:365–376. doi: 10.1084/jem.8.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virchow R. CEllular Pathology: As Based upon Physiological and Pathological Hisology. New York: Dover; 1863. [DOI] [PubMed] [Google Scholar]
- 3.Budoff MJ, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 4.Rennenberg RJ, et al. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag. 2009;5:185–197. doi: 10.2147/vhrm.s4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res. 2004;95:560–567. doi: 10.1161/01.RES.0000141775.67189.98. [DOI] [PubMed] [Google Scholar]
- 6.Shao JS, Cheng SL, Sadhu J, Towler DA. Inflammation and the osteogenic regulation of vascular calcification: a review and perspective. Hypertension. 2010;55:579–592. doi: 10.1161/HYPERTENSIONAHA.109.134205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Rourke RA, et al. American College of Cardiology/American Heart Association Expert Consensus document on electron-beam computed tomography for the diagnosis and prognosis of coronary artery disease. Circulation. 2000;102:126–140. doi: 10.1161/01.cir.102.1.126. [DOI] [PubMed] [Google Scholar]
- 8.Towler DA. Vascular calcification in ESRD: Another cloud appears in the perfect storm--but highlights a silver lining? Kidney Int. 2004;66:2467–2468. doi: 10.1111/j.1523-1755.2004.66095.x. [DOI] [PubMed] [Google Scholar]
- 9.Hunt JL, et al. Bone formation in carotid plaques: a clinicopathological study. Stroke. 2002;33:1214–1219. doi: 10.1161/01.str.0000013741.41309.67. [DOI] [PubMed] [Google Scholar]
- 10.Qiao JH, Mertens RB, Fishbein MC, Geller SA. Cartilaginous metaplasia in calcified diabetic peripheral vascular disease: morphologic evidence of enchondral ossification. Hum Pathol. 2003;34:402–407. doi: 10.1053/hupa.2003.72. [DOI] [PubMed] [Google Scholar]
- 11.Soor GS, Vukin I, Leong SW, Oreopoulos G, Butany J. Peripheral vascular disease: who gets it and why? A histomorphological analysis of 261 arterial segments from 58 cases. Pathology. 2008;40:385–391. doi: 10.1080/00313020802036764. [DOI] [PubMed] [Google Scholar]
- 12.Bostrom K, et al. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–1809. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ewence AE, et al. Calcium phosphate crystals induce cell death in human vascular smooth muscle cells: a potential mechanism in atherosclerotic plaque destabilization. Circ Res. 2008;103:e28–e34. doi: 10.1161/CIRCRESAHA.108.181305. [DOI] [PubMed] [Google Scholar]
- 14.Duer MJ, Friscic T, Murray RC, Reid DG, Wise ER. The mineral phase of calcified cartilage: its molecular structure and interface with the organic matrix. Biophys J. 2009;96:3372–3378. doi: 10.1016/j.bpj.2008.12.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarig S, et al. Detection of cholesterol associated with calcium mineral using confocal fluorescence microscopy. Lab Invest. 1994;71:782–787. [PubMed] [Google Scholar]
- 16.Iyemere VP, Proudfoot D, Weissberg PL, Shanahan CM. Vascular smooth muscle cell phenotypic plasticity and the regulation of vascular calcification. J Intern Med. 2006;260:192–210. doi: 10.1111/j.1365-2796.2006.01692.x. [DOI] [PubMed] [Google Scholar]
- 17.Frid MG, Kale VA, Stenmark KR. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation: in vitro analysis. Circ Res. 2002;90:1189–1196. doi: 10.1161/01.res.0000021432.70309.28. [DOI] [PubMed] [Google Scholar]
- 18.Cossu G, Bianco P. Mesoangioblasts--vascular progenitors for extravascular mesodermal tissues. Curr Opin Genet Dev. 2003;13:537–542. doi: 10.1016/j.gde.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Traktuev DO, et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 20.Li H, et al. Crosstalk between the bone and immune systems: osteoclasts function as antigen-presenting cells and activate CD4+ and CD8+ T cells. Blood. doi: 10.1182/blood-2009-11-255026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallois A, et al. Genome-Wide Expression Analyses Establish Dendritic Cells as a New Osteoclast Precursor Able to Generate Bone-Resorbing Cells More Efficiently Than Monocytes. J Bone Miner Res. 2009 doi: 10.1359/jbmr.090829. [DOI] [PubMed] [Google Scholar]
- 22.Speer MY, et al. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104:733–741. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tintut Y, et al. Multilineage potential of cells from the artery wall. Circulation. 2003;108:2505–2510. doi: 10.1161/01.CIR.0000096485.64373.C5. [DOI] [PubMed] [Google Scholar]
- 24.Dellavalle A, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 25.Farrington-Rock C, et al. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226–2232. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- 26.Nelson CM, Vanduijn MM, Inman JL, Fletcher DA, Bissell MJ. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314:298–300. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoen FJ, Levy RJ. SnapShot: calcification of bioprosthetic heart valves. Biomaterials. 2009;30:4445–4446. doi: 10.1016/j.biomaterials.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 28.Yip CY, Chen JH, Zhao R, Simmons CA. Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arterioscler Thromb Vasc Biol. 2009;29:936–942. doi: 10.1161/ATVBAHA.108.182394. [DOI] [PubMed] [Google Scholar]
- 29.Johnson KA, Polewski M, Terkeltaub RA. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circ Res. 2008;102:529–537. doi: 10.1161/CIRCRESAHA.107.154260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andreeva ER, Pugach IM, Gordon D, Orekhov AN. Continuous subendothelial network formed by pericyte-like cells in human vascular bed. Tissue Cell. 1998;30:127–135. doi: 10.1016/s0040-8166(98)80014-1. [DOI] [PubMed] [Google Scholar]
- 31.Crisan M, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Doherty MJ, et al. Vascular pericytes express osteogenic potential in vitro and in vivo. J Bone Miner Res. 1998;13:828–838. doi: 10.1359/jbmr.1998.13.5.828. [DOI] [PubMed] [Google Scholar]
- 33.Persy V, D'Haese P. Vascular calcification and bone disease: the calcification paradox. Trends Mol Med. 2009;15:405–416. doi: 10.1016/j.molmed.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Szulc P, Kiel DP, Delmas PD. Calcifications in the abdominal aorta predict fractures in men: MINOS study. J Bone Miner Res. 2008;23:95–102. doi: 10.1359/jbmr.070903. [DOI] [PubMed] [Google Scholar]
- 35.Niemeier A, et al. Uptake of postprandial lipoproteins into bone in vivo: impact on osteoblast function. Bone. 2008;43:230–237. doi: 10.1016/j.bone.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 36.Tintut Y, Morony S, Demer LL. Hyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivo. Arterioscler Thromb Vasc Biol. 2004;24:e6–e10. doi: 10.1161/01.ATV.0000112023.62695.7f. [DOI] [PubMed] [Google Scholar]
- 37.Parhami F, et al. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–687. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 38.Parhami F, et al. Atherogenic high-fat diet reduces bone mineralization in mice. J Bone Miner Res. 2001;16:182–188. doi: 10.1359/jbmr.2001.16.1.182. [DOI] [PubMed] [Google Scholar]
- 39.Hirasawa H, et al. ApoE gene deficiency enhances the reduction of bone formation induced by a high-fat diet through the stimulation of p53-mediated apoptosis in osteoblastic cells. J Bone Miner Res. 2007;22:1020–1030. doi: 10.1359/jbmr.070330. [DOI] [PubMed] [Google Scholar]
- 40.Tintut Y, et al. 8-Isoprostaglandin E2 enhances receptor-activated NFkappa B ligand (RANKL)-dependent osteoclastic potential of marrow hematopoietic precursors via the cAMP pathway. J Biol Chem. 2002;277:14221–14226. doi: 10.1074/jbc.M111551200. [DOI] [PubMed] [Google Scholar]
- 41.Tseng W, et al. Regulation of IL-6 expression in osteoblasts by oxidized phospholipids. J Lipid Res. 2009 doi: 10.1194/jlr.M001099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graham LS, et al. Oxidized lipids enhance RANKL production by T lymphocytes: implications for lipid-induced bone loss. Clin Immunol. 2009;133:265–275. doi: 10.1016/j.clim.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang MS, et al. Hyperlipidemia impairs osteoanabolic effects of PTH. J Bone Miner Res. 2008;23:1672–1679. doi: 10.1359/JBMR.080513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang MS, et al. Atherogenic phospholipids attenuate osteogenic signaling by BMP-2 and parathyroid hormone in osteoblasts. J Biol Chem. 2007;282:21237–21243. doi: 10.1074/jbc.M701341200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murshed M, Harmey D, Millan JL, McKee MD, Karsenty G. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev. 2005;19:1093–1104. doi: 10.1101/gad.1276205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tyson KL, et al. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol. 2003;23:489–494. doi: 10.1161/01.ATV.0000059406.92165.31. [DOI] [PubMed] [Google Scholar]
- 47.Byon CH, et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283:15319–15327. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tintut Y, Parhami F, Bostrom K, Jackson SM, Demer LL. cAMP stimulates osteoblast-like differentiation of calcifying vascular cells. Potential signaling pathway for vascular calcification. J Biol Chem. 1998;273:7547–7553. doi: 10.1074/jbc.273.13.7547. [DOI] [PubMed] [Google Scholar]
- 49.Harmey D, et al. Concerted regulation of inorganic pyrophosphate and osteopontin by akp2, enpp1, and ank: an integrated model of the pathogenesis of mineralization disorders. Am J Pathol. 2004;164:1199–1209. doi: 10.1016/S0002-9440(10)63208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kockx MM, et al. Apoptosis and related proteins in different stages of human atherosclerotic plaques. Circulation. 1998;97:2307–2315. doi: 10.1161/01.cir.97.23.2307. [DOI] [PubMed] [Google Scholar]
- 51.Hsu HH, Camacho NP, Sun F, Tawfik O, Aono H. Isolation of calcifiable vesicles from aortas of rabbits fed with high cholesterol diets. Atherosclerosis. 2000;153:337–348. doi: 10.1016/s0021-9150(00)00425-1. [DOI] [PubMed] [Google Scholar]
- 52.Tanimura A, McGregor DH, Anderson HC. Matrix vesicles in atherosclerotic calcification. Proc Soc Exp Biol Med. 1983;172:173–177. doi: 10.3181/00379727-172-41542. [DOI] [PubMed] [Google Scholar]
- 53.Clarke MC, et al. Chronic apoptosis of vascular smooth muscle cells accelerates atherosclerosis and promotes calcification and medial degeneration. Circ Res. 2008;102:1529–1538. doi: 10.1161/CIRCRESAHA.108.175976. [DOI] [PubMed] [Google Scholar]
- 54.Proudfoot D, et al. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ Res. 2000;87:1055–1062. doi: 10.1161/01.res.87.11.1055. [DOI] [PubMed] [Google Scholar]
- 55.Aikawa E, et al. Arterial and aortic valve calcification abolished by elastolytic cathepsin S deficiency in chronic renal disease. Circulation. 2009;119:1785–1794. doi: 10.1161/CIRCULATIONAHA.108.827972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hosaka N, et al. Elastin degradation accelerates phosphate-induced mineralization of vascular smooth muscle cells. Calcif Tissue Int. 2009;85:523–529. doi: 10.1007/s00223-009-9297-8. [DOI] [PubMed] [Google Scholar]
- 57.Johnson K, Polewski M, van Etten D, Terkeltaub R. Chondrogenesis mediated by PPi depletion promotes spontaneous aortic calcification in NPP1−/− mice. Arterioscler Thromb Vasc Biol. 2005;25:686–691. doi: 10.1161/01.ATV.0000154774.71187.f0. [DOI] [PubMed] [Google Scholar]
- 58.Rutsch F, et al. Hypophosphatemia, hyperphosphaturia, and bisphosphonate treatment are associated with survival beyond infancy in generalized arterial calcification of infancy. Circ Cardiovasc Genet. 2008;1:133–140. doi: 10.1161/CIRCGENETICS.108.797704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaplan FS, Pignolo RJ, Shore EM. The FOP metamorphogene encodes a novel type I receptor that dysregulates BMP signaling. Cytokine Growth Factor Rev. 2009;20:399–407. doi: 10.1016/j.cytogfr.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hegyi L, et al. Stromal cells of fibrodysplasia ossificans progressiva lesions express smooth muscle lineage markers and the osteogenic transcription factor Runx2/Cbfa-1: clues to a vascular origin of heterotopic ossification? J Pathol. 2003;201:141–148. doi: 10.1002/path.1413. [DOI] [PubMed] [Google Scholar]
- 61.Awan Z, et al. Vascular calcifications in homozygote familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2008;28:777–785. doi: 10.1161/ATVBAHA.107.160408. [DOI] [PubMed] [Google Scholar]
- 62.Stary HC. Natural history of calcium deposits in atherosclerosis progression and regression. Z Kardiol. 2000;89 Suppl 2:28–35. doi: 10.1007/s003920070097. [DOI] [PubMed] [Google Scholar]
- 63.Lee CD, Jacobs DR, Jr, Schreiner PJ, Iribarren C, Hankinson A. Abdominal obesity and coronary artery calcification in young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2007;86:48–54. doi: 10.1093/ajcn/86.1.48. [DOI] [PubMed] [Google Scholar]
- 64.Mohler ER., 3rd Mechanisms of aortic valve calcification. Am J Cardiol. 2004;94:1396–1402. doi: 10.1016/j.amjcard.2004.08.013. A6. [DOI] [PubMed] [Google Scholar]
- 65.Miller JD, et al. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol. 2008;52:843–850. doi: 10.1016/j.jacc.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Olsson M, Thyberg J, Nilsson J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler Thromb Vasc Biol. 1999;19:1218–1222. doi: 10.1161/01.atv.19.5.1218. [DOI] [PubMed] [Google Scholar]
- 67.Shao JS, Cheng SL, Charlton-Kachigian N, Loewy AP, Towler DA. Teriparatide (human parathyroid hormone (1–34)) inhibits osteogenic vascular calcification in diabetic low density lipoprotein receptor-deficient mice. J Biol Chem. 2003;278:50195–50202. doi: 10.1074/jbc.M308825200. [DOI] [PubMed] [Google Scholar]
- 68.Rajamannan NM, Subramaniam M, Caira F, Stock SR, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced calcification in the aortic valves via the Lrp5 receptor pathway. Circulation. 2005;112:I229–I234. doi: 10.1161/01.CIRCULATIONAHA.104.524306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shao JS, et al. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115:1210–1220. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giachelli CM. Vascular calcification mechanisms. J Am Soc Nephrol. 2004;15:2959–2964. doi: 10.1097/01.ASN.0000145894.57533.C4. [DOI] [PubMed] [Google Scholar]
- 71.Shanahan CM. Mechanisms of vascular calcification in renal disease. Clin Nephrol. 2005;63:146–157. doi: 10.5414/cnp63146. [DOI] [PubMed] [Google Scholar]
- 72.Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–942. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 73.Demer LL. Effect of calcification on in vivo mechanical response of rabbit arteries to balloon dilation. Circulation. 1991;83:2083–2093. doi: 10.1161/01.cir.83.6.2083. [DOI] [PubMed] [Google Scholar]
- 74.London GM, Marchais SJ, Guerin AP, Metivier F. Impairment of arterial function in chronic renal disease: prognostic impact and therapeutic approach. Nephrol Dial Transplant. 2002;17 Suppl 11:13–15. doi: 10.1093/ndt/17.suppl_11.13. [DOI] [PubMed] [Google Scholar]
- 75.Virmani R, Ladich ER, Burke AP, Kolodgie FD. Histopathology of carotid atherosclerotic disease. Neurosurgery. 2006;59:S219–S227. doi: 10.1227/01.NEU.0000239895.00373.E4. discussion S3–13. [DOI] [PubMed] [Google Scholar]
- 76.Hoshino T, et al. Mechanical stress analysis of a rigid inclusion in distensible material: a model of atherosclerotic calcification and plaque vulnerability. Am J Physiol Heart Circ Physiol. 2009;297:H802–H810. doi: 10.1152/ajpheart.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–1170. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 78.Lutgens E, et al. Atherosclerotic plaque rupture: local or systemic process? Arterioscler Thromb Vasc Biol. 2003;23:2123–2130. doi: 10.1161/01.ATV.0000097783.01596.E2. [DOI] [PubMed] [Google Scholar]
- 79.Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2302–2309. doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- 80.Li X, Yang HY, Giachelli CM. BMP-2 promotes phosphate uptake, phenotypic modulation, and calcification of human vascular smooth muscle cells. Atherosclerosis. 2008;199:271–277. doi: 10.1016/j.atherosclerosis.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zebboudj AF, Imura M, Bostrom K. Matrix GLA protein, a regulatory protein for bone morphogenetic protein-2. J Biol Chem. 2002;277:4388–4394. doi: 10.1074/jbc.M109683200. [DOI] [PubMed] [Google Scholar]
- 82.Panizo S, et al. RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circ Res. 2009;104:1041–1048. doi: 10.1161/CIRCRESAHA.108.189001. [DOI] [PubMed] [Google Scholar]
- 83.Mathew S, Davies M, Lund R, Saab G, Hruska KA. Function and effect of bone morphogenetic protein-7 in kidney bone and the bone-vascular links in chronic kidney disease. Eur J Clin Invest. 2006;36 Suppl 2:43–50. doi: 10.1111/j.1365-2362.2006.01663.x. [DOI] [PubMed] [Google Scholar]
- 84.O'Donnell CJ, et al. Matrix Gla protein is associated with risk factors for atherosclerosis but not with coronary artery calcification. Arterioscler Thromb Vasc Biol. 2006;26:2769–2774. doi: 10.1161/01.ATV.0000245793.83158.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luo G, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 86.Bostrom K, Tsao D, Shen S, Wang Y, Demer LL. Matrix GLA protein modulates differentiation induced by bone morphogenetic protein-2 in C3H10T1/2 cells. J Biol Chem. 2001;276:14044–14052. doi: 10.1074/jbc.M008103200. [DOI] [PubMed] [Google Scholar]
- 87.Yao Y, Watson AD, Ji S, Bostrom KI. Heat shock protein 70 enhances vascular bone morphogenetic protein-4 signaling by binding matrix Gla protein. Circ Res. 2009;105:575–584. doi: 10.1161/CIRCRESAHA.109.202333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schurgers LJ, et al. Post-translational modifications regulate matrix Gla protein function: importance for inhibition of vascular smooth muscle cell calcification. J Thromb Haemost. 2007;5:2503–2511. doi: 10.1111/j.1538-7836.2007.02758.x. [DOI] [PubMed] [Google Scholar]
- 89.Bucay N, et al. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rattazzi M, et al. Calcification of advanced atherosclerotic lesions in the innominate arteries of ApoE-deficient mice: potential role of chondrocyte-like cells. Arterioscler Thromb Vasc Biol. 2005;25:1420–1425. doi: 10.1161/01.ATV.0000166600.58468.1b. [DOI] [PubMed] [Google Scholar]
- 91.Kiechl S, et al. Soluble receptor activator of nuclear factor-kappa B ligand and risk for cardiovascular disease. Circulation. 2007;116:385–391. doi: 10.1161/CIRCULATIONAHA.106.686774. [DOI] [PubMed] [Google Scholar]
- 92.Jeziorska M, McCollum C, Wooley DE. Observations on bone formation and remodelling in advanced atherosclerotic lesions of human carotid arteries. Virchows Arch. 1998;433:559–565. doi: 10.1007/s004280050289. [DOI] [PubMed] [Google Scholar]
- 93.Takaoka M, et al. Periadventitial adipose tissue plays a critical role in vascular remodeling. Circ Res. 2009;105:906–911. doi: 10.1161/CIRCRESAHA.109.199653. [DOI] [PubMed] [Google Scholar]
- 94.Tieu BC, et al. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119:3637–3651. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shroff RC, et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008;118:1748–1757. doi: 10.1161/CIRCULATIONAHA.108.783738. [DOI] [PubMed] [Google Scholar]
- 96.Al-Aly Z, et al. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr−/− mice. Arterioscler Thromb Vasc Biol. 2007;27:2589–2596. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- 97.Hruska KA, Saab G, Mathew S, Lund R. Renal osteodystrophy, phosphate homeostasis, and vascular calcification. Semin Dial. 2007;20:309–315. doi: 10.1111/j.1525-139X.2007.00300.x. [DOI] [PubMed] [Google Scholar]
- 98.Son BK, et al. Adiponectin antagonizes stimulatory effect of tumor necrosis factor-alpha on vascular smooth muscle cell calcification: regulation of growth arrest-specific gene 6-mediated survival pathway by adenosine 5'-monophosphate-activated protein kinase. Endocrinology. 2008;149:1646–1653. doi: 10.1210/en.2007-1021. [DOI] [PubMed] [Google Scholar]
- 99.Lee HL, Woo KM, Ryoo HM, Baek JH. Tumor necrosis factor-alpha increases alkaline phosphatase expression in vascular smooth muscle cells via MSX2 induction. Biochem Biophys Res Commun. 391:1087–1092. doi: 10.1016/j.bbrc.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 100.Collett G, et al. Receptor tyrosine kinase Axl modulates the osteogenic differentiation of pericytes. Circ Res. 2003;92:1123–1129. doi: 10.1161/01.RES.0000074881.56564.46. [DOI] [PubMed] [Google Scholar]
- 101.Collett GD, et al. Axl/phosphatidylinositol 3-kinase signaling inhibits mineral deposition by vascular smooth muscle cells. Circ Res. 2007;100:502–509. doi: 10.1161/01.RES.0000258854.03388.02. [DOI] [PubMed] [Google Scholar]
- 102.Son BK, et al. Gas6/Axl-PI3K/Akt pathway plays a central role in the effect of statins on inorganic phosphate-induced calcification of vascular smooth muscle cells. Eur J Pharmacol. 2007;556:1–8. doi: 10.1016/j.ejphar.2006.09.070. [DOI] [PubMed] [Google Scholar]
- 103.Son BK, et al. Statins protect human aortic smooth muscle cells from inorganic phosphate-induced calcification by restoring Gas6-Axl survival pathway. Circ Res. 2006;98:1024–1031. doi: 10.1161/01.RES.0000218859.90970.8d. [DOI] [PubMed] [Google Scholar]
- 104.Son BK, et al. Androgen receptor-dependent transactivation of growth arrest-specific gene 6 mediates inhibitory effects of testosterone on vascular calcification. J Biol Chem. 285:7537–7544. doi: 10.1074/jbc.M109.055087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Heiss A, et al. Hierarchical role of fetuin-A and acidic serum proteins in the formation and stabilization of calcium phosphate particles. J Biol Chem. 2008;283:14815–14825. doi: 10.1074/jbc.M709938200. [DOI] [PubMed] [Google Scholar]
- 106.Reynolds JL, et al. Multifunctional roles for serum protein fetuin-a in inhibition of human vascular smooth muscle cell calcification. J Am Soc Nephrol. 2005;16:2920–2930. doi: 10.1681/ASN.2004100895. [DOI] [PubMed] [Google Scholar]
- 107.Holt C, Sorensen ES, Clegg RA. Role of calcium phosphate nanoclusters in the control of calcification. FEBS J. 2009;276:2308–2323. doi: 10.1111/j.1742-4658.2009.06958.x. [DOI] [PubMed] [Google Scholar]
- 108.Chen NX, et al. Fetuin-A uptake in bovine vascular smooth muscle cells is calcium dependent and mediated by annexins. Am J Physiol Renal Physiol. 2007;292:F599–F606. doi: 10.1152/ajprenal.00303.2006. [DOI] [PubMed] [Google Scholar]
- 109.Ketteler M, et al. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet. 2003;361:827–833. doi: 10.1016/S0140-6736(03)12710-9. [DOI] [PubMed] [Google Scholar]
- 110.Nadra I, et al. Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase C and MAP kinase pathways: a vicious cycle of inflammation and arterial calcification? Circ Res. 2005;96:1248–1256. doi: 10.1161/01.RES.0000171451.88616.c2. [DOI] [PubMed] [Google Scholar]
- 111.Major ML, Cheung HS, Misra RP. Basic calcium phosphate crystals activate c-fos expression through a Ras/ERK dependent signaling mechanism. Biochem Biophys Res Commun. 2007;355:654–660. doi: 10.1016/j.bbrc.2007.01.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31:509–519. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 113.Liberman M, et al. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler Thromb Vasc Biol. 2008;28:463–470. doi: 10.1161/ATVBAHA.107.156745. [DOI] [PubMed] [Google Scholar]
- 114.Bear M, Butcher M, Shaughnessy SG. Oxidized low-density lipoprotein acts synergistically with beta-glycerophosphate to induce osteoblast differentiation in primary cultures of vascular smooth muscle cells. J Cell Biochem. 2008;105:185–193. doi: 10.1002/jcb.21812. [DOI] [PubMed] [Google Scholar]
- 115.Proudfoot D, Davies JD, Skepper JN, Weissberg PL, Shanahan CM. Acetylated low-density lipoprotein stimulates human vascular smooth muscle cell calcification by promoting osteoblastic differentiation and inhibiting phagocytosis. Circulation. 2002;106:3044–3050. doi: 10.1161/01.cir.0000041429.83465.41. [DOI] [PubMed] [Google Scholar]
- 116.Radcliff K, et al. Insulin-like growth factor-I regulates proliferation and osteoblastic differentiation of calcifying vascular cells via extracellular signal-regulated protein kinase and phosphatidylinositol 3-kinase pathways. Circ Res. 2005;96:398–400. doi: 10.1161/01.RES.0000157671.47477.71. [DOI] [PubMed] [Google Scholar]
- 117.Price PA, June HH, Buckley JR, Williamson MK. Osteoprotegerin inhibits artery calcification induced by warfarin and by vitamin D. Arterioscler Thromb Vasc Biol. 2001;21:1610–1616. doi: 10.1161/hq1001.097102. [DOI] [PubMed] [Google Scholar]
- 118.Hsu JJ, Tintut Y, Demer LL. Vitamin D and osteogenic differentiation in the artery wall. Clin J Am Soc Nephrol. 2008;3:1542–1547. doi: 10.2215/CJN.01220308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Luo XH, et al. Development of arterial calcification in adiponectin-deficient mice: adiponectin regulates arterial calcification. J Bone Miner Res. 2009;24:1461–1468. doi: 10.1359/jbmr.090227. [DOI] [PubMed] [Google Scholar]
- 120.Parhami F, Tintut Y, Ballard A, Fogelman AM, Demer LL. Leptin enhances the calcification of vascular cells: artery wall as a target of leptin. Circ Res. 2001;88:954–960. doi: 10.1161/hh0901.090975. [DOI] [PubMed] [Google Scholar]
- 121.Zeadin M, et al. Effect of leptin on vascular calcification in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2009;29:2069–2075. doi: 10.1161/ATVBAHA.109.195255. [DOI] [PubMed] [Google Scholar]
- 122.London GM, Marchais SJ, Guerin AP, Metivier F. Arteriosclerosis, vascular calcifications and cardiovascular disease in uremia. Curr Opin Nephrol Hypertens. 2005;14:525–531. doi: 10.1097/01.mnh.0000168336.67499.c0. [DOI] [PubMed] [Google Scholar]