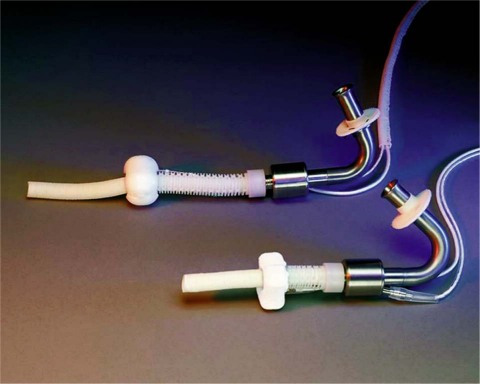
The development of the DeBakey axial-flow ventricular assist device (VAD) began in 1988, after a visit with NASA engineers. The initial design was a collaborative effort among Drs. Michael E. DeBakey and George P. Noon, the NASA engineers, and Baylor College of Medicine. In 1996, MicroMed Technology, a commercial company, was formed to continue pump development and in vitro and in vivo animal testing, and then to proceed to clinical trials. The final pump was made of titanium; the revolutions per minute (rpm) ranged from 7,500 to 12,500, with flow rates up to 10 L/min. A flow meter was incorporated for continuous real-time flow measurements. Pumps for both adults and children were developed (Fig. 1).
Fig. 1 The latest version of the MicroMed DeBakey Noon ventricular assist devices for adults and children. (Courtesy of MicroMed Cardiovascular, Inc.; Houston, Tex)
The first human implant of the MicroMed DeBakey Noon VAD was in Berlin, Germany, in November 1998. This was the first continuous-flow VAD implanted in man. It was used as a bridge to transplant. The ensuing clinical trials showed that continuous (non-or less-pulsatile) flow could successfully provide circulatory support for heart-failure patients—resulting in resuscitation, rehabilitation, and maintenance of these patients similar to that provided by larger, more cumbersome, pulsatile VADs. The first United States implant of the MicroMed DeBakey Noon VAD was performed in 2002 as a bridge to transplant. Thus far, there have been 451 implants (155 of those in the U.S.), including 19 pediatric. In light of the clinical trial findings, some changes have been incorporated into the design over the years, chiefly in regard to the prevention of pump thrombus and power fluctuation.
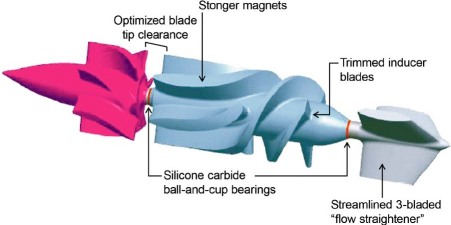
The tools used in this ongoing design evaluation included computational fluid dynamics, particle image velocimetry, and in vitro and in vivo animal testing. We also performed studies of hemolysis and platelets. The improvements resulting from multiple iterations of the pump have been incorporated into the present design, the Heart Assist 5™. These revisions have included, for example, a change from the original shaft-and-ring bearing to a silicone carbide cup-and-ball bearing, and changes to the inflow cannula, to the front and rear gaps, and to the inducer and flow-straightener configurations. Stronger magnets were implanted in the impeller (Fig. 2). After incorporating these pump modifications, animal testing in calves was performed without anticoagulation. There were 10 non-GLP (good laboratory practices) 30-day studies and 10 GLP 60-day studies performed in calves. In these animal implants, we have not encountered any thrombus formation or power fluctuations. Adult and pediatric versions of the HeartAssist 5 are ready for human implantation.
Fig. 2 Summary of improvements in the HeartAssist 5™, the modern DeBakey Noon ventricular assist device. (Courtesy of MicroMed Cardiovascular, Inc.; Houston, Tex)
In addition to the changes in the pump that resulted in the HeartAssist 5, MicroMed has improved the controller, the Clinical Data Acquisition System, and the Patient Home Support System—and has combined the last 2 into the HeartAttendant, which is used in the hospital and at home by the patient and VAD personnel (Fig. 3). The Attendant has a touch-screen display. When first connected to the controller, the Attendant is used to initialize the pump and controller and to set pump parameters. When it is connected to the controller, the Attendant displays and stores wave forms and other data. It has Internet connectivity for transmitting information and alarm alerts to a data support center and the patient's transplant center. Four batteries can be charged and reconditioned simultaneously. The Attendant also provides electrical power from the wall to the controller and pump.

Fig. 3 The HeartAssist 5™ System: the HeartAttendant, the controller, and the HA5 pump. (Courtesy of MicroMed Cardiovascular, Inc.; Houston, Tex)
In summary, the HeartAttendant programs the controller and sets pump rpm and alarm thresholds. It collects and stores pump parameters when connected to the controller and enables remote monitoring via the Internet. It charges and reconditions the batteries and can provide electrical power from the wall. It eliminates the need for the Clinical Data Acquisition System and the Patient Home Support System. The new controller, in all modes, displays pump flow (L/min), current/amps, power/watts, rpm, and diagnostic and emergency alarms.
The HeartAssist 5 is CE-approved in Europe. A new U.S. Food and Drug Administration study of the HeartAssist 5 as a bridge to transplant is being finalized.
Footnotes
Address for reprints: George P. Noon, MD, Division of Transplant & Assist Devices, Department of Surgery, Baylor College of Medicine, 6565 Fannin St., A979, Houston, TX 77030
E-mail: gnoon@bcm.edu
Presented at the Joint Session of the Denton A. Cooley Cardiovascular Surgical Society and the Michael E. DeBakey International Surgical Society; Austin, Texas, 10–13 June 2010