Abstract
Left ventricular outpouchings are increasingly detected on cardiovascular imaging. Herein, we describe the case of a 45-year-old man who underwent noncardiac preoperative imaging and was found to have an asymptomatic left ventricular outpouching. The patient underwent successful surgical repair of the structure.
When left ventricular outpouchings are detected, the main differential diagnoses are pseudoaneurysm, aneurysm, and diverticulum. The outcomes for these conditions differ substantially, and accurate diagnosis can be crucial in making clinical decisions. We review the relevant medical literature, outline the natural history of these left ventricular abnormalities, and discuss options in regard to their management.
Key words: Aneurysm, false/diagnosis/mortality/surgery; cardiomyopathies/diagnosis/epidemiology/therapy; diagnosis, differential; diverticulum/congenital/diagnosis/surgery; heart aneurysm/diagnosis/etiology/mortality/physiopathology/radiography/surgery; heart defects, congenital/complications/diagnosis/surgery; heart rupture, post-infarction/etiology/therapy; heart ventricles/abnormalities/pathology/surgery; myocardial infarction/complications; treatment outcome; ventricular septal rupture/etiology
Unexpected abnormal morphologic findings on cardiac imaging are cause for concern. In asymptomatic individuals, these findings can pose significant dilemmas for clinicians. Herein, we describe an illustrative case and review the differential diagnoses of left ventricular (LV) outpouchings.
Case Report
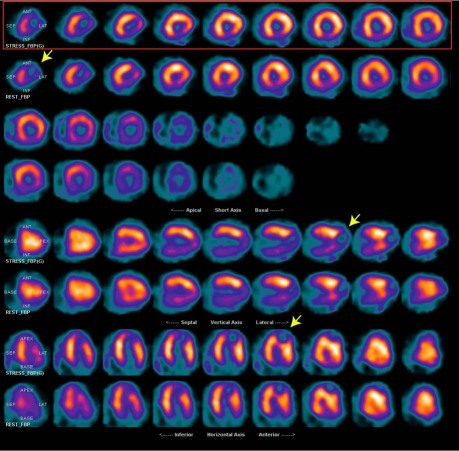
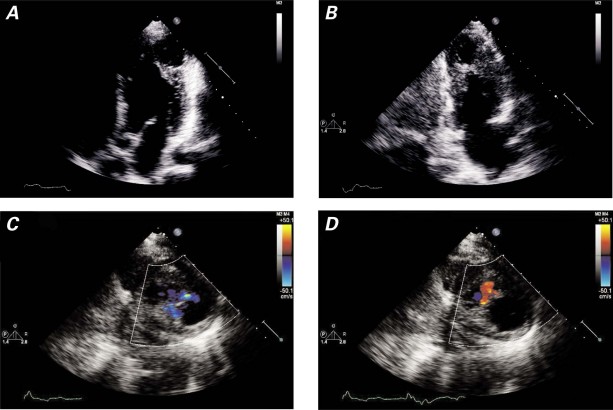
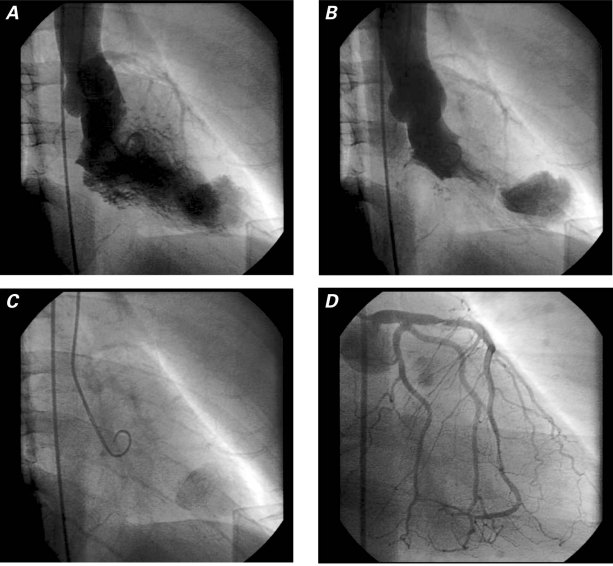
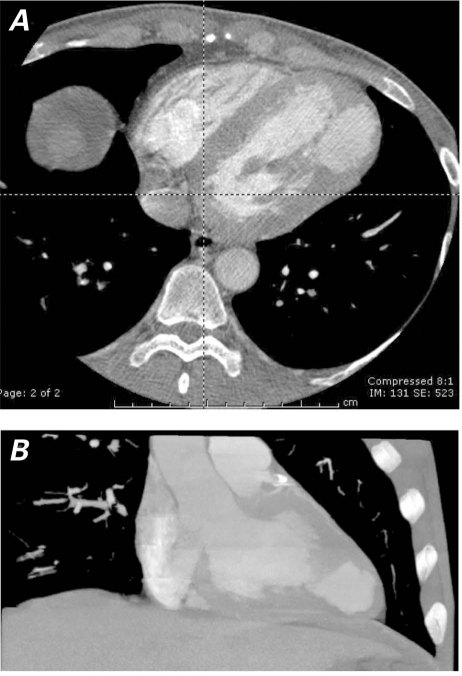
A 45-year-old man was referred to our institution for cardiac evaluation before a kidney transplant. His medical history included hypertension, diabetes mellitus type 1 for 20 years, and end-stage renal disease that was being treated with peritoneal dialysis. He had no history of myocardial infarction (MI), arrhythmias, or stroke. He had never experienced exertional dyspnea or chest pain. Results of a physical examination were within normal limits except for the presence of a peritoneal dialysis catheter. As part of the preoperative evaluation, the patient underwent a dipyridamole thallium-sestamibi dual-isotope stress test and 2-dimensional echocardiography. The stress test showed a fixed defect of the apical–lateral LV wall with thinning, bulging, and dyskinesis, suspicious for an aneurysm (Fig. 1). Uptake of nuclear isotope in the aneurysmal wall suggested the presence of myocytes. No ischemia or scarring was seen elsewhere. The echocardiogram showed normal ventricular size and function but confirmed an aneurysm in the apical–lateral walls, with systolic inflow and diastolic outflow (Fig. 2). Left ventriculography by use of cardiac catheterization revealed an apical, narrow-necked aneurysm that expanded slightly in systole, consistent with a pseudoaneurysm (Fig. 3A–C). There were 80% stenoses in the proximal and distal left anterior descending coronary artery, and no significant obstructive coronary disease elsewhere (Fig. 3D). Cardiac computed tomographic angiography revealed a 4.5 × 3.1 × 4.3-cm extra chamber arising from the inferoapical/apical–lateral wall of the LV (Fig. 4). Myocardium with prominent trabeculation was seen along the lateral wall of the chamber, but neither thinning along its upper lateral aspect nor infarction could be ruled out. Wall-motion analysis showed that the muscle of the chamber thickened during systole. No thrombus was seen. Because of the possibility of rupture, we decided to perform surgical excision.
Fig. 1 Myocardial single-photon-emission computed tomographic (SPECT) perfusion was performed with use of a dipyridamole thallium-sestamibi dual-isotope protocol. The left ventricle was dilated and hypertrophied with thinning and bulging in the region of the apical–lateral wall, suggesting aneurysm (arrows). There was homogenous uptake of thallium and sestamibi in the remainder of the myocardium, with no evidence of ischemia.
Fig. 2 Echocardiographic A) 4-chamber and B) 2-chamber apical views show the apical–lateral aneurysm-like abnormality. Off-axis images of the outpouching, with the use of color-flow Doppler echocardiography, show C) systolic inflow and D) diastolic outflow.
Fig. 3 Left ventriculography shows A) the filling of the apical aneurysm-like structure in diastole, B) the persistence of contrast medium in systole, and C) lingering contrast medium several beats after its clearance from the left ventricle. D) Coronary angiography shows lesions in the proximal and distal left anterior descending coronary artery.
Fig. 4 Cardiac computed tomographic angiography shows a 4.1 × 3.1 × 4.3-cm chamber-like structure arising from the left ventricle in the A) apical–lateral and B) inferoapical region. The wall of the chamber appears to have myocardium of variable thickness, with substantial thinning at the upper lateral aspect.
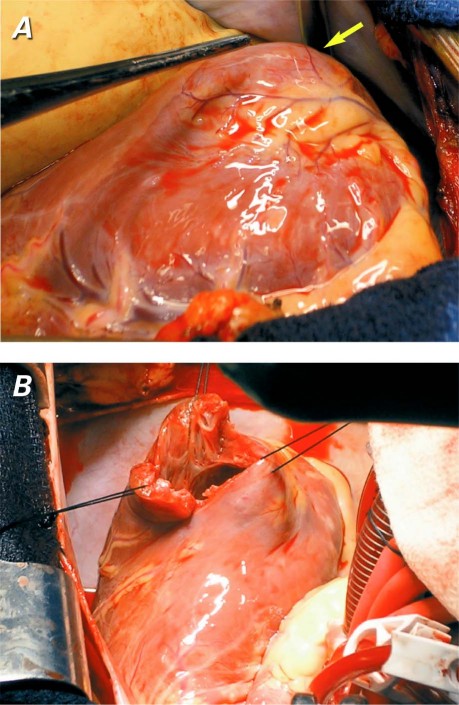
Intraoperative inspection of the LV confirmed a dyskinetic outpouching that had muscular and scar tissue (Fig. 5A). When the aneurysmal region was explored, a septum with a small hole was seen to separate the accessory chamber from the main ventricular cavity (Fig. 5B). The aneurysmal area was resected, a Dacron patch was applied, and the ventricular muscle was closed. The left internal mammary artery was anastomosed to the left anterior descending coronary artery. The patient recovered uneventfully and was discharged from the hospital on the 6th postoperative day. At his 3-month follow-up visit, he was doing well, had maintained his baseline functional status, and was awaiting kidney transplantation.
Fig. 5 Intraoperative photographs show A) a left ventricular outpouching (arrow), and B) a septum, with a small hole, that separates the accessory chamber from the main ventricular cavity.
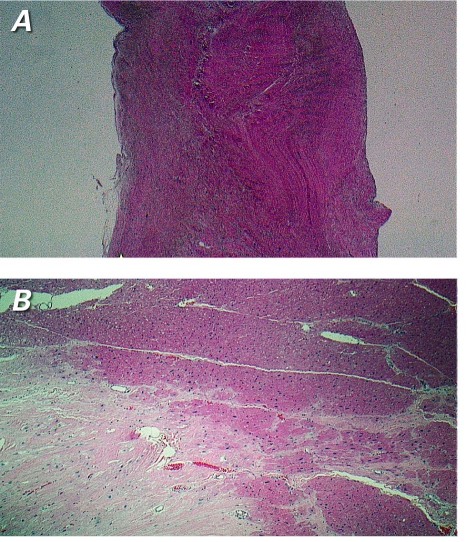
Pathologic examination revealed that the wall of the outpouching consisted of intact myocardium with myocyte hypertrophy and patchy interstitial infiltrate (Fig. 6). An area of fibrous scar tissue or scarring indicated a healed MI, which contributed to the aneurysmal appearance of the structure. The fenestrated septum that separated the outpouching from the main ventricular cavity consisted of focally hyalinized fibrous tissue with a few interspersed cardiac muscle bundles. The final diagnosis was a congenital septate diverticulum of the LV, with a region of scarring that was secondary to a healed MI.
Fig. 6 Photomicrographs of the congenital fenestrated septum show dense fibrous tissue. A) No myocardial elements are seen in this section (H & E, orig. ×2). B) The lateral wall of the outpouching shows myocardial tissue with an area of fibrous scarring that indicates a healed myocardial infarction (H & E, orig. ×4).
Discussion
The differential diagnosis of LV outpouchings includes aneurysm, pseudoaneurysm, and diverticulum. These must be distinguished from LV noncompaction, which is characterized by a prominent trabecular meshwork with a distinctly spongy appearance and deep intertrabecular recesses that are believed to be caused by an arrest in normal embryogenesis.1
Left Ventricular Pseudoaneurysm
The diagnosis with the worst prognosis is LV pseudoaneurysm, which is a contained rupture of the LV free wall.2 Causes of LV pseudoaneurysm include MI, cardiac surgery, infectious endocarditis, and chest trauma. The pericardium overlying the site of rupture adheres to the LV and contains extravasation from the ventricle, which prevents frank tamponade. Data from the United States National Registry of Myocardial Infarction (1996) suggested that, although the incidence of cardiac rupture among patients with MI was less than 1%, cardiac rupture was responsible for 7.3% of in-hospital deaths, and patients who were given thrombolysis were at higher risk.3 However, the true incidence of LV pseudoaneurysm is not known. The available data are from case reports, case series, or analyses of case reports in the medical literature.
Frances and colleagues4 reviewed abstracted data from 290 case reports in 201 articles that were published from 1966 through 1997. Approximately 12% of the patients with LV pseudoaneurysm were asymptomatic upon diagnosis. Most of the LV pseudoaneurysms were posterior. The mortality rate for patients who underwent surgery was 23%, versus 48% for patients who were managed medically. These numbers reflect aggregated data from published case reports and cannot be generalized. Considering the improvements in surgical techniques since 1997, operative mortality rates are now likely to be lower.
In contrast to the conclusions drawn by Frances and colleagues, some studies indicate that medically managed patients can survive long-term. Patients have been diagnosed with LV pseudoaneurysm many years after their index infarction.5 Yeo and colleagues6 performed a retrospective analysis of 52 LV pseudoaneurysm patients in a single institution, 25 of whom were asymptomatic. Ten were medically treated because they refused surgery, had small aneurysms, or had comorbid conditions. Six of the 10 died after a median survival of 2.1 years; however, none died of cardiac rupture. Of the surgically treated patients, the surgical mortality rate was 7%, and of 13 subsequent deaths, none was due to cardiac rupture. This study suggests that the LV pseudoaneurysm itself is less predictive of death than are the underlying conditions, and that conservative management may be appropriate in asymptomatic patients who have substantial comorbidities and a LV pseudoaneurysm that was found incidentally upon imaging. This reasoning is further supported by Moreno and colleagues.7 They monitored 9 patients with such pseudoaneurysms who underwent conservative management. The mortality rate was 26% after 4 years, and no deaths were due to cardiac rupture. Of note was a high incidence of stroke (32.5% at 4 yr). Sakai and co-authors8 reported that 7 of 8 patients who were treated conservatively for LV pseudoaneurysm after mitral valve repair experienced no adverse consequences during a mean follow-up period of 57 months (range, 5–136 mo).
The prognosis for patients with LV pseudoaneurysm is influenced by the nature, chronicity, underlying cause, and location of the pseudoaneurysm. A prudent approach to postinfarction LV pseudoaneurysms is to consider performing surgery in patients with good comorbid status and in whom the precipitating event was recent, and to favor conservative management in asymptomatic patients with substantial comorbidities and a stable LV pseudoaneurysm that was found incidentally, late after the causative event.9 Cases of successful percutaneous closure have been reported, and this treatment may be considered in patients who are poor candidates for surgery.10,11 The compilation of an international registry may be a way to better understand the natural history of this condition.
Left Ventricular Aneurysm
Left ventricular aneurysm describes a discrete region of ventricular wall that is thinner than the adjacent myocardial segments, balloons outward, and exhibits either akinesis or dyskinesis. During surgery, this region appears flaccid and puckered after the LV is evacuated. Histopathologic examination of resected LV aneurysms reveals dense collagenous or hyaline connective tissue, occasionally with a few muscle fibers surrounded by scarring, and foci of mononuclear cells.12 The pericardial cavity is obliterated by dense adhesions, and intramural clots are often found. In the vast majority of cases, the underlying cause is transmural MI. Trauma, iatrogenic injury, Chagas disease, hypertrophic cardiomyopathy, mucopolysaccharidosis, and sarcoidosis are other reported causes.13-16
The incidence of LV aneurysm has decreased significantly due to improvements in the treatment of acute MI. The pathology of MI has changed substantially in the post-interventional era.17 According to 1 report, the use of thrombolytic agents has decreased the incidence of LV aneurysm from 18.8% to 7.2%.18
The major complications of LV aneurysm include thromboembolism, ventricular arrhythmia, congestive heart failure, refractory angina (possibly from altered hemodynamic levels), and, rarely, cardiac rupture. Early investigators who performed autopsy studies concluded that the natural history of LV aneurysm was poor, with mortality rates as high as 80%. More recently, however, investigators have found 5-year mortality rates of 10% to 50%, depending upon aneurysmal size.19 Data from the Coronary Artery Surgery Study (CASS) registry showed that survival in patients with LV aneurysm was related to age, LV function, and the clinical severity of heart failure, rather than to the presence of the aneurysm itself.20
Left Ventricular Diverticulum
Left ventricular diverticulum is defined as an outpouching structure that contains endocardium, myocardium, and pericardium and displays normal contraction. Left ventricular diverticula are distinguished from LV aneurysms, which have fibrous walls and exhibit paradoxical motion. No recent studies have been conducted to examine the epidemiology of LV diverticulum, although earlier studies have been cited as reporting a prevalence of 0.4%, or 3 of 750 cardiac necropsy cases.21,22
Left ventricular diverticula are considered to be congenital if there is no history of conditions that have injured the myocardium. An outpouching can occur in a weak area of the ventricular wall in the first 2 or 3 weeks of embryonic life. In utero viral infection, muscle and connective-tissue defects, and excessive primordial-cell stimulation have been proposed as causes.23 Midline thoracoabdominal defects and other congenital cardiac malformations are found in a substantial proportion of patients who have LV diverticula. Most LV diverticula are found in the apex.24 When LV diverticulum is associated with midline thoracoabdominal congenital abnormalities, diaphragmatic and sternal defects, and partial absence of the inferoapical pericardium, the condition is called Cantrell syndrome.25
The natural history of LV diverticulum has not been systematically studied. Major concerns are thrombosis, embolism, rupture, congestive heart failure, ventricular arrhythmias, and valvular abnormalities. The true incidence of these complications is not known because of the rarity of LV diverticulum. A review of 22 patients with congenital ventricular outpouchings found that apical diverticula were associated with midline thoracoabdominal defects and other heart malformations, whereas nonapical diverticula were isolated.26 Among the 10 patients with LV diverticulum who were not surgically treated, 2 experienced spontaneous regression, and 8 were alive without symptoms after a mean follow-up period of 8.4 years. In 1 report, an LV diverticulum did not increase in size over 13 years, suggesting that the clinical course may be benign.27 However, cases of associated sustained monomorphic ventricular tachycardia have required treatment with an implantable cardioverter-defibrillator or with cryoablative surgery.28-30 Some authors have recommended immediate surgical resection, in order to prevent complications.31 In view of the inadequate data for universal guidance, decisions on the management of LV diverticulum should be tailored to the clinical characteristics of each patient, taking into consideration associated abnormalities and potential complications.
All noninvasive and minimally invasive techniques are useful in the diagnosis of LV outpouchings. In our patient, computed tomography best defined the anatomy of the LV and showed the myocardial contraction in the wall of the outpouching.
Conclusion
The prognosis of LV outpouchings can vary from benign to catastrophic, depending upon the underlying cause. Accurate diagnosis is required to guide management decisions. High-quality imaging will characterize LV outpouchings well, helping clinicians to better understand the natural history of these conditions and to manage them appropriately.
Acknowledgments
We are grateful to Linda Bogar, MD, Ramalingaier Parameswaran, MD, Anoop Parameswaran-Chandrika, MD, Huyen Tran, MD, and Shahriar Yazdanfar, MD, for their role in the care of the patient and in image acquisition. We also thank Tuisha Makkuni for her help with graphic design.
Footnotes
Address for reprints: Vincent M. Figueredo, MD, Director, Cardiovascular Diseases Fellowship Programs, Levy 3232, Albert Einstein Medical Center, 5501 Old York Rd., Philadelphia, PA 19141
E-mail: FigueredoV@einstein.edu
References
- 1.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006; 113(14):1807–16. [DOI] [PubMed]
- 2.Roberts WC, Morrow AG. Pseudoaneurysm of the left ventricle. An unusual sequel of myocardial infarction and rupture of the heart. Am J Med 1967;43(4):639–44. [DOI] [PubMed]
- 3.Becker RC, Gore JM, Lambrew C, Weaver WD, Rubison RM, French WJ, et al. A composite view of cardiac rupture in the United States National Registry of Myocardial Infarction. J Am Coll Cardiol 1996;27(6):1321–6. [DOI] [PubMed]
- 4.Frances C, Romero A, Grady D. Left ventricular pseudoaneurysm. J Am Coll Cardiol 1998;32(3):557–61. [DOI] [PubMed]
- 5.Natarajan MK, Salerno TA, Burke B, Chiu B, Armstrong PW. Chronic false aneurysms of the left ventricle: management revisited. Can J Cardiol 1994;10(9):927–31. [PubMed]
- 6.Yeo TC, Malouf JF, Oh JK, Seward JB. Clinical profile and outcome in 52 patients with cardiac pseudoaneurysm. Ann Intern Med 1998;128(4):299–305. [DOI] [PubMed]
- 7.Moreno R, Gordillo E, Zamorano J, Almeria C, Garcia-Rubira JC, Fernandez-Ortiz A, Macaya C. Long term outcome of patients with postinfarction left ventricular pseudoaneurysm. Heart 2003;89(10):1144–6. [DOI] [PMC free article] [PubMed]
- 8.Sakai K, Nakamura K, Ishizuka N, Nakagawa M, Hosoda S. Echocardiographic findings and clinical features of left ventricular pseudoaneurysm after mitral valve replacement. Am Heart J 1992;124(4):975–82. [DOI] [PubMed]
- 9.Eren E, Bozbuga N, Toker ME, Keles C, Rabus MB, Yildirim O, et al. Surgical treatment of post-infarction left ventricular pseudoaneurysm: a two-decade experience. Tex Heart Inst J 2007;34(1):47–51. [PMC free article] [PubMed]
- 10.Gladding PA, Ruygrok PN, Greaves SC, Gerber IL, Hamer AW. Images in cardiovascular medicine. Percutaneous closure of a left ventricular free-wall rupture site. Circulation 2006; 113(18):e748–9. [DOI] [PubMed]
- 11.Elshershari H, Gossett JG, Hijazi ZM. Percutaneous closure of left ventricular pseudoaneurysms after Ross procedure. Ann Thorac Surg 2008;85(2):634–6. [DOI] [PubMed]
- 12.Lillehei CW, Levy MJ, Dewall RA, Warden HE. Resection of myocardial aneurysms after infarction during temporary cardiopulmonary bypass. Circulation 1962;26:206–17. [DOI] [PubMed]
- 13.Glower DD, Lowe JE. Left ventricular aneurysm. In: Cohn LH, Edmunds LH, editors. Cardiac surgery in the adult. 2nd ed. New York: McGraw-Hill; 2003. p. 771–8.
- 14.Studer MA, Jefferies JL, McKenzie ED, Ing FF. Traumatic cardiac rupture and left ventricular aneurismal formation in childhood. Am J Cardiol 2008;101(3):413–4. [DOI] [PubMed]
- 15.Oudit GY, Butany J, Williams WG, Clarke JT, Iwanochko RM. Images in cardiovascular medicine. Left ventricular aneurysm associated with mucopolysaccharidosis type VI syndrome (Maroteaux-Lamy syndrome). Circulation 2007;115 (5):e60–2. [DOI] [PubMed]
- 16.De La Calzada CS, Verdugo AL, Perez JT, Alcaine CC, Fernandez AS. Hypertrophic cardiomyopathy associated with left ventricular aneurysm and normal coronary arteries: case study indicating genetic tendencies of cardiomyopathy. Cardiovasc Dis 1981;8(1):73–83. [PMC free article] [PubMed]
- 17.Pasotti M, Prati F, Arbustini E. The pathology of myocardial infarction in the pre- and post-interventional era. Heart 2006;92(11):1552–6. [DOI] [PMC free article] [PubMed]
- 18.Tikiz H, Balbay Y, Atak R, Terzi T, Genc Y, Kutuk E. The effect of thrombolytic therapy on left ventricular aneurysm formation in acute myocardial infarction: relationship to successful reperfusion and vessel patency. Clin Cardiol 2001;24 (10):656–62. [DOI] [PMC free article] [PubMed]
- 19.Cohen M, Packer M, Gorlin R. Indications for left ventricular aneurysmectomy. Circulation 1983;67(4):717–22. [DOI] [PubMed]
- 20.Faxon DP, Ryan TJ, Davis KB, McCabe CH, Myers W, Lesperance J, et al. Prognostic significance of angiographically documented left ventricular aneurysm from the Coronary Artery Surgery Study (CASS). Am J Cardiol 1982;50(1):157–64. [DOI] [PubMed]
- 21.Pressoir R, Downing JW. Congenital diverticula of the right ventricle of the heart: a case report. J Natl Med Assoc 1980;72 (3):262–4. [PMC free article] [PubMed]
- 22.Skapinker S. Diverticulum of the left ventricle of the heart; review of the literature and report of a successful removal of the diverticulum. AMA Arch Surg 1951;63(5):629–34. [DOI] [PubMed]
- 23.Mardini MK. Congenital diverticulum of the left ventricle. Report of two unusual cases. Br Heart J 1984;51(3):321–6. [DOI] [PMC free article] [PubMed]
- 24.Walton-Shirley M, Smith SM, Talley JD. Left ventricular diverticulum: case report and review of the literature. Cathet Cardiovasc Diagn 1992;26(1):31–3. [DOI] [PubMed]
- 25.Cantrell JR, Haller JA, Ravitch MM. A syndrome of congenital defects involving the abdominal wall, sternum, diaphragm, pericardium, and heart. Surg Gynecol Obstet 1958;107(5): 602–14. [PubMed]
- 26.Marijon E, Ou P, Fermont L, Concordet S, Le Bidois J, Sidi D, Bonnet D. Diagnosis and outcome in congenital ventricular diverticulum and aneurysm. J Thorac Cardiovasc Surg 2006;131(2):433–7. [DOI] [PubMed]
- 27.Archbold RA, Robinson NM, Mills PG. Long-term follow-up of a true contractile left ventricular diverticulum. Am J Cardiol 1999;83(5):810–3, A11. [DOI] [PubMed]
- 28.Sierra M, Huynh H, Machado C. Congenital ventricular diverticulum presenting as sustained monomorphic ventricular tachycardia. Int J Cardiol 2009;133(2):e70–2. [DOI] [PubMed]
- 29.Deepak BV, Alsous F, Mathur R, Zarich S. Congenital left ventricular diverticulum presenting as ventricular tachycardia in an elderly woman. Am J Geriatr Cardiol 2007;16(4):262–5. [DOI] [PubMed]
- 30.Shen EN, Fukuyama O, Herre JM, Yee E, Scheinman MM. Ventricular tachycardia with congenital ventricular diverticulum. Chest 1991;100(1):283–5. [DOI] [PubMed]
- 31.Tanaka M, Kawahito K, Kaneda H, Saito S. Congenital left ventricular diverticulum in an adult. Eur Heart J 2007;28 (12):1537. [DOI] [PubMed]