Abstract
Chronic angina pectoris affects millions of patients every year. During the past 2 decades, advances in medical therapy have led to substantial reductions in the symptoms of angina. Nonetheless, many patients continue to experience persistent angina that causes debilitating symptoms and lifestyle changes. Moreover, many current therapeutic agents cause side effects that can induce substantial morbidity on their own. In major clinical trials, the drug ranolazine has been shown to bring symptomatic relief to large numbers of patients who have chronic angina. Herein, we review the physiology of the sodium channel; the pharmacology of ranolazine; clinical trials that support use of the drug; recent evidence about ranolazine's therapeutic effect on diastolic heart failure, glycemic control, and atrial fibrillation and other arrhythmias; officially approved clinical indications; and avenues of future study.
Key words: Angina pectoris/drug therapy/prevention & control; anti-arrhythmia agents/pharmacology/therapeutic use; cardiovascular agents/pharmacology/therapeutic use; clinical trials as topic; coronary disease/drug therapy; dose-response relationship, drug; heart conduction system/drug effects/metabolism/physiopathology; myocardial ischemia/drug therapy; piperazines/therapeutic use; ranolazine/administration & dosage; sodium channel blockers/pharmacology/therapeutic use; sodium channels/drug effects/metabolism/physiology
Cardiovascular disease is the leading cause of death in the western world, accounting for more than 1 million deaths every year. As part of the morbidity that is associated with coronary artery disease, stable angina pectoris affects more than 9 million individuals in the United States alone.1 In almost all cases, the condition is caused by a pathologic imbalance in myocardial oxygen demand and supply. Myocardial oxygen demand is determined by heart rate, systolic blood pressure, contractility, and left ventricular wall stress, whereas myocardial oxygen supply is primarily dependent upon coronary blood flow and diastolic perfusion pressure. Therefore, past efforts to reduce angina focused on controlling these determinants.
The primary goal of therapy with calcium-channel blockers and nitrates has been to improve oxygen supply. Nonetheless, despite the aggressive use of conventional therapies, many patients experience persistent angina. In fact, in the recently published COURAGE trial, at least 25% of patients who were on medically optimized regimens or who underwent percutaneous coronary intervention still had clinically relevant angina at 5-year follow-up.2 Other patients could not tolerate the medications due to substantial side effects such as hypotension, bradycardia, and sexual dysfunction, which lead to noncompliance with prescribed medical regimens.
Ranolazine, a piperazine derivative, is a new antianginal drug with a novel mechanism of action that appears to offer freedom from most adverse hemodynamic effects. Ranolazine was approved by the U.S. Food and Drug Administration (FDA) in 2006 for the treatment of stable angina pectoris. Herein, we review ranolazine's effects, current and potential clinical applications, and avenues of future study.
The Sodium Channel
Myocardial ischemia alters adenosine triphosphate flux, which causes a functional decrease in the energy available to the proteins that are responsible for myocardial contraction and relaxation. Some of these proteins are integral to the maintenance of myocyte ion homeostasis. Disruption of this electrolyte balance leads to abnormally elevated intracellular levels of sodium and calcium, which are thought to contribute to the discrepancy between myocardial oxygen demand and supply.3 It is postulated that the increase in intracellular sodium concentrations is mediated, in part, by late activation of the inward sodium channel current (INaL). During the last 10 years, investigators have sought to identify the physiologic properties of the inward INaL and the implications of its dysfunction in regard to the pathogenesis of angina and its cellular corollary, the ischemic myocyte.
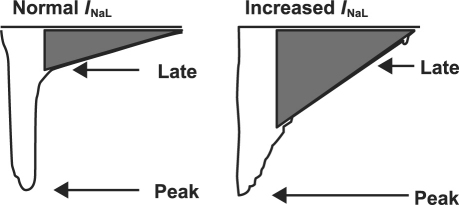
To appreciate the complexity of the pathologic INa, it is important to understand its normal electrophysiologic properties. In normal myocytes, depolarization of the myocardial membrane leads to rapid activation of the INa, followed by its rapid inactivation.4 The initial activation is the primary determinant of the rapid upstroke and the amplitude of the cardiac action potential that follows. After activation, the INa rapidly inactivates, which elevates intracellular sodium concentration (Fig. 1). Voltage-dependent calcium channels then open and initiate a large outflow of sarcoplasmic calcium into the cytoplasm. The cytosolic release of calcium ions initiates myocardial contraction through the coupling of actin and myosin filaments.5
Fig. 1 Late inhibition of inward sodium channel current (INaL) under normal and pathologic increased states. Adapted from: Hasenfuss G, Maier LS. Mechanism of action of the new anti-ischemia drug ranolazine. Clin Res Cardiol 2008;97(4):222–6. Used by permission.
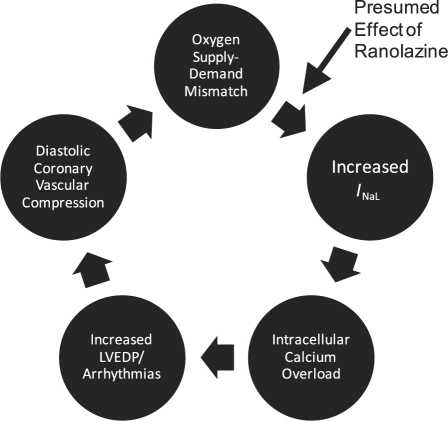
In pathologic states such as ischemia, oxidative stress, myocardial stretch, and left ventricular hypertrophy, the INa either fails to close or it reopens too late, leading to an abnormally high increase in intracellular sodium.6 This sodium elevation causes intracellular calcium to increase through the sodium–calcium exchanger. The increase can alter downstream pathways and lead to increased diastolic stiffness, diastolic coronary vascular compression, and poor oxygen demand–supply matching (Fig. 2).7-9
Fig. 2 Presumed effect of ranolazine on myocardial oxygen demand–supply mismatch. LVEDP = left ventricular end-diastolic pressure; INaL = late inward sodium channel current Adapted from: Stone PH. Ranolazine: new paradigm for management of myocardial ischemia, myocardial dysfunction, and arrhythmias. Cardiol Clin 2008;26(4):603–14.4 Used by permission.
Ranolazine's initial mechanism of action was thought to be the inhibition of cardiac metabolism of fatty acids. However, the concentration of the drug that is required to inhibit fatty-acid β-oxidation (>100 µM) is much higher than the therapeutic concentration (<10 µM). This suggested to investigators that the inhibition of fatty-acid metabolism could not validly explain ranolazine's mechanism of action.10 It was later proposed that ranolazine blocks the INaL, interrupting this pathway early in the ischemic cascade and eventually preventing most downstream effects.11 In animal models, the drug was shown to be a potent inhibitor of the INaL through a concentration-, voltage-, and frequency-dependent inhibition.12
The Pharmacology of Ranolazine
Ranolazine is a racemic mixture that contains enantiomeric forms (S-ranolazine and R-ranolazine) that inhibit the INaL. Ranolazine is rapidly metabolized in the liver, primarily through the cytochrome P-450 3A enzyme (CYP3A) pathway, and in the intestine. More than 70% of the drug is excreted in the urine. This pharmacokinetic profile necessitates careful dosage adjustments in patients who are elderly, who weigh less than 60 kg, and who have mild-to-moderate renal insufficiency or mild hepatic impairment, and in patients who are in New York Heart Association functional class III–IV. Ranolazine is contraindicated in patients with severe renal impairment (glomerular filtration rate, <30 mL/min/1.73 m2) or moderate-to-severe hepatic impairment (Child-Pugh classes B and C). The use of ranolazine by patients who are undergoing renal replacement therapy has not been studied.13
Drug Interactions
Due to the dependence of ranolazine on CYP3A metabolic pathways, the coadministration of a wide variety of drugs can affect its clearance. In particular, ketoconazole, a potent CYP3A inhibitor, can raise steady-state concentrations of ranolazine to more than 3 times the expected value; therefore, ketoconazole is contraindicated in patients who are taking ranolazine. Moderate inhibitors of CYP3A, such as diltiazem and verapamil, should be used with caution. Simvastatin, a weak inhibitor of CYP3A, does not seem to increase ranolazine levels. Macrolide antibiotics, human immunodeficiency virus protease inhibitors, and grapefruit juice—all of which inhibit CYP3A to varying degrees—should be used with caution. Ranolazine also inhibits P-glycoprotein, so it should be used with caution by patients who are taking verapamil, because plasma levels of that drug may increase. Ranolazine has been shown to increase serum digoxin levels by 1.5 times, leading to the recommendation that digoxin dosages be altered in patients who are taking both drugs.14
Clinical Evidence of Ranolazine's Efficacy
The therapeutic efficacy of ranolazine has been studied in patients with chronic stable angina pectoris and in patients who experienced unstable angina and non-ST-elevation myocardial infarction.
Effect on Chronic Stable Angina
The initial study of ranolazine versus placebo was performed by Pepine and Wolff.15 Their investigation showed that exercise duration, exercise time until the onset of angina, and exercise time until the development of 1-mm ST-segment depression increased with the use of immediate-release ranolazine. However, the effect was not continuous and in fact was not seen at trough levels (plasma concentrations immediately before the next scheduled dose).15 This discovery led to 3 multicenter trials that sought to evaluate the efficacy of an extended-release formulation of the drug.
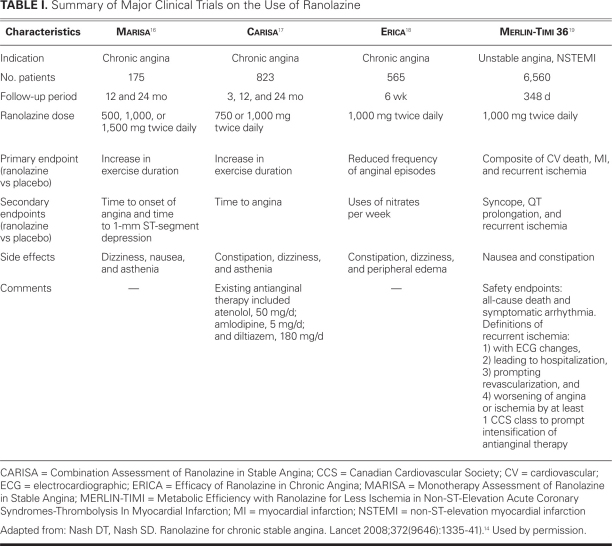
The 1st trial was the Monotherapy Assessment of Ranolazine in Stable Angina (MARISA),16 in which ranolazine, as monotherapy for stable angina, was compared with placebo in a double-blinded crossover study of 175 patients. All patients were required to undergo angina-limited exercise stress tests and to discontinue previous antianginal therapy 1 week before randomization to placebo or to twice-daily regimens of 500, 1,000, or 1,500 mg of ranolazine. At peak and trough levels, all 3 ranolazine regimens led to statistically significant increases versus placebo in exercise duration (500 mg, 23.7 s, P <0.003; 1,000 mg, 33.7 s, P <0.001; and 1,500 mg, 45.9 s, P <0.001), in time until the onset of angina (500 mg, 27 s, P <0.005; 1,000 mg, 45.9 s, P <0.001; and 1,500 mg, 59.6 s, P <0.001), and in time until the development of 1-mm ST-segment depression (27.6, 44.5, and 64.6 s, respectively; all P <0.001). Although the 1,500-mg regimen had the greatest effect, the side-effect profile was also highest at that dose.16
The 2nd study, the Combination Assessment of Ranolazine in Stable Angina (CARISA) trial,17 investigated the benefit of ranolazine as part of combined therapy. Ranolazine response at 750 mg and 1,000 mg twice daily was compared with response to placebo in 823 patients who were already receiving antianginal therapy. Patients in both ranolazine groups showed statistically significant improvement in exercise duration at trough dosing (750 mg, 23.7 s and 1,000 mg, 24 s; both P <0.03). Secondary endpoints (exercise duration at 4 hr after dosing, and times to angina, electrocardiographic [ECG] evidence of myocardial ischemia, and frequency of anginal episodes) were also significantly longer in both ranolazine groups than in the placebo groups.17
In the 3rd trial, Efficacy of Ranolazine in Chronic Angina (ERICA),18 ranolazine was evaluated versus placebo in 565 patients in whom angina persisted despite maximal doses of amlodipine (10 mg/d). Patients with a 60% stenosis in at least 1 major coronary artery, a stress-induced defect on perfusion imaging, chronic stable angina for at least 3 months, and at least 3 anginal episodes per week during a 2-week period were randomized to receive either 1,000 mg of ranolazine twice daily or placebo. The primary endpoint of self-reported anginal episodes per week was lower in the ranolazine group than in the placebo group (mean, 2.9 vs 3.3 episodes; P <0.028). A similar effect was seen in all subgroups, including women, elderly patients (age, >65 yr), and patients on ongoing nitrate therapy. Ranolazine was more beneficial in patients who had more than 4.5 anginal episodes per week than in patients who experienced fewer episodes.18
Effect on Unstable Angina and Non-ST-Elevation Myocardial Infarction
Ranolazine use was also studied in patients with unstable angina and non-ST-elevation myocardial infarction, in the Metabolic Efficiency with Ranolazine for Less Ischemia in Non-ST-Elevation Acute Coronary Syndromes-Thrombolysis in Myocardial Infarction (MERLIN-TIMI) 36 trial.19 This randomized, double-blinded, placebo-controlled, multinational clinical trial involved 6,560 patients who presented within 48 hours of ischemic symptoms and who were treated with either intravenous ranolazine followed by sustained-release oral ranolazine (1,000 mg twice daily) or placebo. The study's authors agreed that although the goal of this trial was to evaluate the efficacy of ranolazine in reducing major outcomes in patients with acute coronary syndrome (ACS), there was concomitant interest in evaluating ranolazine's effect on chronic ischemia and in establishing the safety and tolerability of the drug in a large cohort of patients.
Although the investigators found no statistically significant difference between groups in the primary efficacy endpoint (the composite of cardiovascular death, myocardial infarction, and recurrent ischemia), they reported a significant reduction in the endpoint of recurrent ischemia in the ranolazine group. In addition, the study revealed a similar reduction in recurrent ischemic complications in the ranolazine group, specifically in 30-day cardiovascular death, myocardial infarction, severe recurrent ischemia, and positive Holter monitoring for ischemia (P=0.55). Because the investigations revealed no difference in mortality rates or symptomatic arrhythmia between the 2 groups, the results established the safety of ranolazine as antianginal therapy in a large population.19
Table I shows summary information for the MARISA, CARISA, ERICA, and MERLIN-TIMI trials.14
TABLE I. Summary of Major Clinical Trials on the Use of Ranolazine
Post Hoc Analyses
Some post hoc analyses have revealed important information about the use of ranolazine in certain subgroups of patients. Ischemic events in patients with diabetes mellitus were significantly reduced (hazard ratio, 0.75; 95% confidence interval, 0.61–0.93; P=0.008).20 In subgroups of patients with prior chronic angina who presented with ACS, the reduction in ischemia was more substantial and led to a statistically significant composite outcome.21
In patients with known coronary artery disease who underwent exercise-treadmill nuclear stress testing, ranolazine therapy concomitant with conventional antianginal medication led to improvements on quantitative myocardial perfusion imaging and to decreased ischemic burdens.22 In a subgroup analysis of the MERLIN-TIMI 36 trial, the frequency of anginal episodes was greater and the level of brain natriuretic peptide (BNP) was higher in women than in men, which suggests a particularly useful role for ranolazine therapy in women.23
The Antiarrhythmic Effects of Ranolazine
The complex nature of ranolazine's antiarrhythmic effects is largely a result of the drug's effect on multiple ion channels: it inhibits the INaL, the late rectifying potassium channel, and the late L-type calcium channel. Whereas inhibition of the potassium channel increases the action-potential duration, inhibition of the other 2 channels shortens the action potential.24 This physiologic effect seems to explain the modest increase in QTC interval that was observed in some clinical trials. In the CARISA trial, the mean increase in QTC was 6.1 ms in the 750-mg ranolazine group and 9.2 ms in the 1,000-mg group. Similar increases over the baseline QTC interval were seen in the MARISA trial in all 3 trough-dosing regimens (increase at 500 mg, 6 ms; at 1,000 mg, 7 ms; and at 1,500 mg, 11 ms).25
Ranolazine's effect on the QT interval raised concern about drug-induced torsades de pointes. However, none of the 4 major clinical trials produced evidence of that phenomenon. The absence of this expected effect is partially explained by the absence of early afterdepolarization and increased dispersion of ventricular repolarization. Both of these electrophysiologic events are often needed to initiate malignant ventricular tachyarrhythmias in the presence of a prolonged QT interval. In a canine model, ranolazine did not increase the dispersion of repolarization, and it decreased the incidence of early afterdepolarization.11
In a subgroup analysis of the MERLIN-TIMI 36 trial, the continuous ECGs of 6,351 patients were analyzed. The results showed that, in comparison with placebo, treatment with ranolazine resulted in fewer episodes of ventricular tachycardia that lasted 8 beats or longer (5.3% vs 8.3%; P <0.001), and in fewer episodes of supraventricular tachycardia (44.7% vs 55%; P <0.001) and new-onset atrial fibrillation (1.7% vs 2.4%; P=0.08). In addition, there were no differences in the incidence of polymorphic ventricular tachycardia or sudden cardiac death, a concern that had arisen after previous observations of prolonged QT intervals.26
Ranolazine also has a presumed antiarrhythmic effect on atrial myocytes. Recent evidence suggests that atrial myocytes have fundamentally different electrophysiologic properties than do ventricular myocytes.27,28 An increased INaL can cause early afterdepolarization and cellular calcium overload in the atria, which can trigger sustained atrial electrical activity—a possible cause of atrial fibrillation.29,30 In canine models, ranolazine more effectively inhibited the INaL in the atrium than in the ventricle.31 Also in dogs, ranolazine effectively suppressed the triggers of atrial fibrillation that originated from the sleeves of pulmonary veins.32 However, these results were seen in animals with “healthy” atria, and it is unclear what effect, if any, there is on atria that have undergone chronic remodeling in the presence of long-standing atrial arrhythmias.32 Finally, in a small study of 7 patients, ranolazine was initiated soon after atrial fibrillation ablation and was found to be useful in maintaining sinus rhythm.33 When considered together, these limited analyses suggest the value of exploring ranolazine's role in treating atrial arrhythmias, particularly atrial fibrillation.
Ranolazine in Heart Failure
Due to its effect on the INaL, ranolazine is thought to affect myocardial contractility and diastolic tension.34 As intracellular sodium increases, diastolic tension increases, causing diastolic dysfunction. In muscle-strip preparations from dogs in end-stage heart failure that were stimulated at high frequencies, ranolazine reduced the increase in diastolic tension that typically accompanied the fast stimulation rates.35 In healthy and failing dog hearts, ranolazine reduced end-diastolic pressure and increased left ventricular ejection fraction and stroke volume without significant changes in heart rate or blood pressure.35 In patients with ischemic heart disease, ranolazine increased regional peak filling rate and regional wall thickening during the isovolumetric relaxation period, indicating improved diastolic function. Of note, this improvement was seen without a concomitant decrease in fractional shortening, indicating no negative inotropic effect of the drug.36 In a subgroup analysis of the MERLIN-TIMI 36 trial that categorized patients into 2 groups on the basis of BNP levels, ranolazine reduced the composite endpoint in patients who had elevated BNP levels.37
The Metabolic Effects of Ranolazine
In the CARISA trial, ranolazine improved glycemic control in patients who had diabetes mellitus. In particular, the drug reduced hemoglobin A1C (HbA1C) concentrations by approximately 0.5% in the diabetes subgroup, an effect that persisted during long-term therapy. Comparing HbA1C levels was not an initial endpoint of the study, so the results may not have reflected true randomization. The reduction in HbA1C was seen in the same patients who experienced an increase in exercise tolerance, and the improved glycemic control logically could have been attributed to increased exercise. However, the level of exercise that would have been needed to sustain such a reduction in HbA1C was far greater than the moderate improvement in exercise tolerance that was noted in the trial. Accordingly, the lowered HbA1C was thought to be due in part to increased insulin sensitivity.38 The same effect was seen in the recently published subgroup analysis of the MERLIN-TIMI 36 trial.20 The drug lowered HbA1C concentrations in patients with known diabetes and decreased the incidence of impaired fasting glucose-tolerance levels during the follow-up period. However, further clarification is needed regarding these mechanisms, because ion channels on pancreatic >β-cells may have a role in insulin secretion.
Ranolazine has also been shown to improve endothelial function. In a study of 27 patients who had stable coronary artery disease, ranolazine therapy significantly increased endothelial vasodilation in comparison with placebo.39 Other inflammatory markers, such as C-reactive protein and symmetric dimethylarginine levels, also decreased in the treatment group. The precise mechanisms of these effects need to be clarified.
Side Effects of Ranolazine
The most common side effects of ranolazine are dizziness, nausea, constipation, and headache. Less than 2% of patients experience these side effects.17-19 In most cases, the symptoms are mild, and they occur within the 1st few weeks of therapy. Although some patients must discontinue taking the drug, most can tolerate reduced dosages.
Official Recommendations for Clinical Use
On the basis of the data from the clinical trials, the FDA approved ranolazine as a 1st-line agent in the treatment of chronic stable angina, either as a primary agent or as an adjunct to ongoing β-blocker and nitrate therapy. Patients who take ranolazine as a 1st-line agent should begin with 500 mg twice daily, gradually increasing to 1,000 mg twice daily within a 4-to 6-week period. When ranolazine is prescribed as adjunctive therapy, the CARISA trial results suggest that it is not necessary to maximize the dosing regimens of other antianginal medications. It should be noted that ranolazine is not approved for the treatment of ACS: the results of the MERLIN-TIMI 36 trial established the safety and tolerability of the drug in a large cohort but did not provide evidence of its benefit in ACS. On the basis of the available clinical data and ranolazine's pharmacokinetics, the drug should be used with caution in treating patients who have substantial renal impairment (glomerular filtration rate, <50 mL/min/1.73 m2). Finally, there are insufficient data to support ranolazine treatment in patients with reduced left ventricular systolic function, and it has not been approved for use in that group.
Conclusion
Chronic angina pectoris remains a widespread health problem despite multiple, evolving medical therapies. The side effects of traditional antianginal drugs can cause morbidity and contribute to noncompliance with therapeutic regimens. Ranolazine seems not to cause many of these side effects. It is a potential supplement and alternative to conventional medications. The clinical efficacy, safety, and tolerability of the drug have been established in large clinical trials. Subgroup analyses have shown that patients with chronic angina appear to derive significant clinical and symptomatic benefit from ranolazine, and possibly reductions in ischemia.
Ranolazine appears to have other promising nonanginal effects, including glycemic control, improvements in endothelial function, and decreases in the incidence of atrial fibrillation and other arrhythmias. These initial findings warrant further investigation as independent outcomes. Finally, further clinical studies are needed to analyze the role of ranolazine therapy in patients with systolic heart failure and in those who are dependent on dialysis—cohorts of patients who have, up to this point, been excluded from efficacy and safety trials of the drug. Nonetheless, ranolazine may be an effective antianginal treatment for millions of individuals who have chronic angina.
Footnotes
Address for reprints: Bharath M. Reddy, MD, Fellow, Cardiovascular Disease, Leon H. Charney Division of Cardiology, New York University School of Medicine, 550 First Ave., NBV 17-S-5, New York, NY 10016
E-mail: Bharath.Reddy@nyumc.org
References
- 1.Fraker TD Jr, Fihn SD, 2002 Chronic Stable Angina Writing Committee, American College of Cardiology, American Heart Association, Gibbons RJ, et al. 2007 chronic angina focused update of the ACC/AHA 2002 guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to develop the focused update of the 2002 guidelines for the management of patients with chronic stable angina [published erratum appears in J Am Coll Cardiol 2007;50(23):e1]. J Am Coll Cardiol 2007;50(23):2264–74. [DOI] [PubMed]
- 2.Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356 (15):1503–16. [DOI] [PubMed]
- 3.Zaza A, Belardinelli L, Shryock JC. Pathophysiology and pharmacology of the cardiac “late sodium current.” Pharmacol Ther 2008;119(3):326–39. [DOI] [PubMed]
- 4.Stone PH. Ranolazine: new paradigm for management of myocardial ischemia, myocardial dysfunction, and arrhythmias. Cardiol Clin 2008;26(4):603–14. [DOI] [PubMed]
- 5.Noble D, Noble PJ. Late sodium current in the pathophysiology of cardiovascular disease: consequences of sodium-calcium overload. Heart 2006;92 Suppl 4:iv1-iv5. [DOI] [PMC free article] [PubMed]
- 6.Le Grand B, Pignier C, Letienne R, Colpaert F, Cuisiat F, Rolland F, et al. Na+ currents in cardioprotection: better to be late. J Med Chem 2009;52(14):4149–60. [DOI] [PubMed]
- 7.Hale SL, Shryock JC, Belardinelli L, Sweeney M, Kloner RA. Late sodium current inhibition as a new cardioprotective approach. J Mol Cell Cardiol 2008;44(6):954–67. [DOI] [PubMed]
- 8.Saint DA. The cardiac persistent sodium current: an appealing therapeutic target? Br J Pharmacol 2008;153(6):1133–42. [DOI] [PMC free article] [PubMed]
- 9.Hayashida W, van Eyll C, Rousseau MF, Pouleur H. Effects of ranolazine on left ventricular regional diastolic function in patients with ischemic heart disease. Cardiovasc Drugs Ther 1994;8(5):741–7. [DOI] [PubMed]
- 10.MacInnes A, Fairman DA, Binding P, Rhodes J, Wyatt MJ, Phelan A, et al. The antianginal agent trimetazidine does not exert its functional benefit via inhibition of mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res 2003;93 (3):e26–32. [DOI] [PubMed]
- 11.Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, et al. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation 2004;110(8):904–10. [DOI] [PMC free article] [PubMed]
- 12.Fraser H, Belardinelli L, Wang L, Light PE, McVeigh JJ, Clanachan AS. Ranolazine decreases diastolic calcium accumulation caused by ATX-II or ischemia in rat hearts. J Mol Cell Cardiol 2006;41(6):1031–8. [DOI] [PubMed]
- 13.Ranexa® dosing information [cited 2010 Nov 1]. Available at: http://www.ranexa.com/HCP/Dosing/Default.aspx.
- 14.Nash DT, Nash SD. Ranolazine for chronic stable angina. Lancet 2008;372(9646):1335–41. [DOI] [PubMed]
- 15.Pepine CJ, Wolff AA. A controlled trial with a novel anti-ischemic agent, ranolazine, in chronic stable angina pectoris that is responsive to conventional antianginal agents. Ranolazine Study Group. Am J Cardiol 1999;84(1):46–50. [DOI] [PubMed]
- 16.Chaitman BR, Skettino SL, Parker JO, Hanley P, Meluzin J, Kuch J, et al. Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J Am Coll Cardiol 2004;43(8):1375–82. [DOI] [PubMed]
- 17.Chaitman BR, Pepine CJ, Parker JO, Sokpal J, Chumakova G, Kuch J, et al. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA 2004;291(3):309–16. [DOI] [PubMed]
- 18.Stone PH, Gratsiansky NA, Blokhin A, Huang IZ, Meng L; ERICA Investigators. Antianginal efficacy of ranolazine when added to treatment with amlodipine: the ERICA (Efficacy of Ranolazine in Chronic Angina) trial. J Am Coll Cardiol 2006;48(3):566–75. [DOI] [PubMed]
- 19.Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, Murphy SA, Budaj A, Varshavsky S, et al. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA 2007;297(16):1775–83. [DOI] [PubMed]
- 20.Morrow DA, Scirica BM, Chaitman BR, McGuire DK, Murphy SA, Karwatowska-Prokopczuk E, et al. Evaluation of the glycometabolic effects of ranolazine in patients with and without diabetes mellitus in the MERLIN-TIMI 36 randomized controlled trial. Circulation 2009;119(15):2032–9. [DOI] [PubMed]
- 21.Wilson SR, Scirica BM, Braunwald E, Murphy SA, Karwatowska-Prokopczuk E, Buros JL, et al. Efficacy of ranolazine in patients with chronic angina observations from the randomized, double-blind, placebo-controlled MERLIN-TIMI (Metabolic Efficiency with Ranolazine for Less Ischemia in Non-ST-Segment Elevation Acute Coronary Syndromes) 36 Trial. J Am Coll Cardiol 2009;53(17):1510–6. [DOI] [PubMed]
- 22.Venkataraman R, Belardinelli L, Blackburn B, Heo J, Iskandrian AE. A study of the effects of ranolazine using automated quantitative analysis of serial myocardial perfusion images. JACC Cardiovasc Imaging 2009;2(11):1301–9. [DOI] [PubMed]
- 23.Mega JL, Hochman JS, Scirica BM, Murphy SA, Sloan S, McCabe CH, et al. Clinical features and outcomes of women with unstable ischemic heart disease: observations from metabolic efficiency with ranolazine for less ischemia in non-ST-elevation acute coronary syndromes-thrombolysis in myocardial infarction 36 (MERLIN-TIMI 36). Circulation 2010;121(16):1809–17. [DOI] [PubMed]
- 24.Schram G, Zhang L, Derakhchan K, Ehrlich JR, Belardinelli L, Nattel S. Ranolazine: ion-channel-blocking actions and in vivo electrophysiological effects. Br J Pharmacol 2004;142(8): 1300–8. [DOI] [PMC free article] [PubMed]
- 25.Chaitman BR. Efficacy and safety of a metabolic modulator drug in chronic stable angina: review of evidence from clinical trials. J Cardiovasc Pharmacol Ther 2004;9 Suppl 1:S47–64. [DOI] [PubMed]
- 26.Scirica BM, Morrow DA, Hod H, Murphy SA, Belardinelli L, Hedgepeth CM, et al. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: results from the Metabolic Efficiency with Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation 2007;116(15):1647–52. [DOI] [PubMed]
- 27.Burashnikov A, Antzelevitch C. Atrial-selective sodium channel block for the treatment of atrial fibrillation. Expert Opin Emerg Drugs 2009;14(2):233–49. [DOI] [PMC free article] [PubMed]
- 28.Burashnikov A, Antzelevitch C. Atrial-selective sodium channel blockers: do they exist? J Cardiovasc Pharmacol 2008;52 (2):121–8. [DOI] [PMC free article] [PubMed]
- 29.Dobrev D. Cardiomyocyte Ca2+ overload in atrial tachycardia: is blockade of L-type Ca2+ channels a promising approach to prevent electrical remodeling and arrhythmogenesis? Naunyn Schmiedebergs Arch Pharmacol 2007;376(4):227–30. [DOI] [PubMed]
- 30.Song Y, Shryock JC, Belardinelli L. An increase of late sodium current induces delayed afterdepolarizations and sustained triggered activity in atrial myocytes. Am J Physiol Heart Circ Physiol 2008;294(5):H2031–9. [DOI] [PubMed]
- 31.Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation 2007;116(13):1449–57. [DOI] [PMC free article] [PubMed]
- 32.Sicouri S, Glass A, Belardinelli L, Antzelevitch C. Antiarrhythmic effects of ranolazine in canine pulmonary vein sleeve preparations. Heart Rhythm 2008;5(7):1019–26. [DOI] [PMC free article] [PubMed]
- 33.Murdock DK, Overton N, Kersten M, Kaliebe J, Devecchi F. The effect of ranolazine on maintaining sinus rhythm in patients with resistant atrial fibrillation. Indian Pacing Electrophysiol J 2008;8(3):175–81. [PMC free article] [PubMed]
- 34.Ver Donck L, Borgers M, Verdonck F. Inhibition of sodium and calcium overload pathology in the myocardium: a new cytoprotective principle. Cardiovasc Res 1993;27(3):349–57. [DOI] [PubMed]
- 35.Undrovinas AI, Belardinelli L, Undrovinas NA, Sabbah HN. Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol 2006;17 Suppl 1:S169-S77. [DOI] [PMC free article] [PubMed]
- 36.Sossalla S, Wagner S, Rasenack EC, Ruff H, Weber SL, Schondube FA, et al. Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts–role of late sodium current and intracellular ion accumulation. J Mol Cell Cardiol 2008;45(1):32–43. [DOI] [PubMed]
- 37.Morrow DA, Scirica BM, Sabatine MS, de Lemos JA, Murphy SA, Jarolim P, et al. B-type natriuretic peptide and the effect of ranolazine in patients with non-ST-segment elevation acute coronary syndromes: observations from the MERLIN-TIMI 36 (Metabolic Efficiency with Ranolazine for Less Ischemia in Non-ST Elevation Acute Coronary-Thrombolysis In Myocardial Infarction 36) trial. J Am Coll Cardiol 2010;55(12): 1189–96. [DOI] [PubMed]
- 38.Timmis AD, Chaitman BR, Crager M. Effects of ranolazine on exercise tolerance and HbA1c in patients with chronic angina and diabetes. Eur Heart J 2006;27(1):42–8. [DOI] [PubMed]
- 39.Deshmukh SH, Patel SR, Pinassi E, Mindrescu C, Hermance EV, Infantino MN, et al. Ranolazine improves endothelial function in patients with stable coronary artery disease. Coron Artery Dis 2009;20(5):343–7. [DOI] [PubMed]