Abstract
Aortic pseudoaneurysm is a rare, life-threatening complication after cardiac or aortic root surgery. When a pseudoaneurysm has eroded bony structures in the chest, the surgeon's challenge is to choose the safest approach for sternotomy. Herein, we report the case of a 74-year-old woman who presented with a giant pseudoaneurysm of the ascending aorta, 8 years after undergoing aortic valve replacement. The 8.9 × 5.8-cm formation arose in the anterior aortic sinus, extended to the retrosternal region, exerted mass effect on the main pulmonary artery, and eroded the bony structures of the sternum and medial upper chest. A new aortic valved tissue conduit was placed, and the coronary arteries were reimplanted. The patient recovered without neurologic sequelae. We discuss the characteristics of this case and explain our surgical decisions.
Key words: Aneurysm, false/diagnosis/etiology/radiology/surgery; aortic aneurysm, thoracic/surgery; blood vessel prosthesis implantation; cardiac surgical procedures/adverse effects; cardiopulmonary bypass; circulatory arrest, deep hypothermia induced; reoperation; risk factors; tomography, X-ray computed; treatment outcome
WEB SITE FEATURE
Aortic pseudoaneurysms are rare, life-threatening sequelae of cardiac surgery.1 They can also occur consequent to infection, genetic disorders, or trauma.2–5 False aortic aneurysms result from disruption of the intima and media of a vessel. They are contained by the adventitia and the surrounding structures of the mediastinum.6
The incidence, risk factors, and natural history of aortic pseudoaneurysm are unknown, because so few cases have been reported. Symptoms include a pulsatile suprasternal mass, chest pain, dysphagia, and stridor. These aneurysmal formations usually rupture, so prompt surgical intervention is necessary.
When a false aneurysm has eroded the bony structures in the chest, the surgeon's challenge is to choose an approach for sternotomy that enables safe entry. Herein, we discuss our surgical management of an aortic pseudoaneurysm in a patient with a failed stentless porcine valve.
Case Report
In December 2008, a 74-year-old woman was transferred from a community hospital to our institution for tertiary care. She was a smoker who had diabetes mellitus, high blood pressure, and coronary artery disease. She presented with a week's history of progressive dyspnea, orthopnea, cough, and chest pain. At the referring institution, a 2-dimensional echocardiogram had revealed substantial aortic insufficiency with an apical akinetic area and a left ventricular ejection fraction of 0.40 to 0.45. The patient's medical history included aortic stenosis, which had been treated 8 years before by the placement of a stentless porcine aortic bioprosthesis.
Upon admission to the hospital, the patient (height, 165 cm; weight, 40 kg) had a temperature of 37.1 °C, blood pressure of 120/55 mmHg, a heart rate of 71 beats/min, a respiratory rate of 14 breaths/min, and oxygen saturation of 89% on room air. She was alert and in moderate respiratory distress. She reported no chest trauma, fever, chest pain, or headache. Cardiac auscultation revealed a regular heart rhythm and a complex murmur with systolic and diastolic components. Chest auscultation revealed diffuse crackles. An abdominal examination revealed nothing unusual. The peripheral pulses were present but weak. In the lower extremities, moderate edema was noted up to mid-calf level. Chest radiographs showed diffuse interstitial markings that were consistent with pulmonary edema. Laboratory results included hemoglobin, 10.5 g/dL; hematocrit, 31%; white blood cell count, 8,400/mm3; platelets, 255,000/mm3; creatine kinase–MB fraction, 1.1 ng/mL; and cardiac troponin I, negative. Analysis of arterial blood gases revealed respiratory acidosis with pH of 7.26, PCO2 of 54.2 mmHg, bicarbonate of 24 mEq/L, and PO2 of 51 mmHg. Urinalysis and liver function test results were normal.
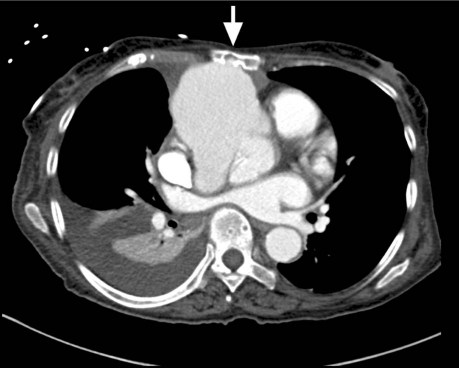
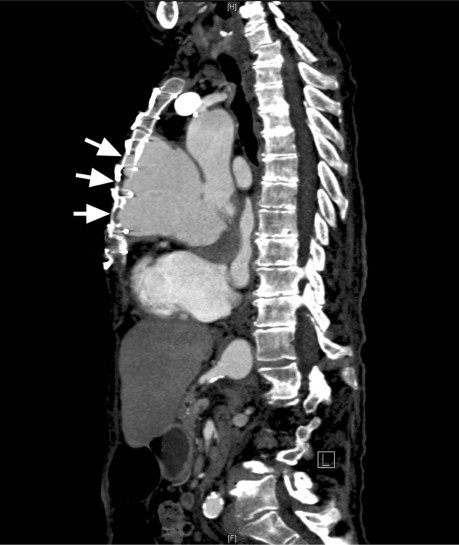
The patient was initially treated with diuretics. Cardiac catheterization revealed severe aortic insufficiency and contrast extravasation into a large pseudoaneurysm. No significant coronary artery disease was found. Thrombus was seen in the proximal ascending aorta. Computed tomography with contrast agent showed cardiomegaly and a giant pseudoaneurysm, which arose from the anterior aortic sinus and extended to the retrosternal region (Fig. 1). The aneurysm, which was 8.9 × 5.8 cm at its greatest transverse and anteroposterior diameters, exerted mass effect on the main pulmonary artery and had eroded the bony structures of the sternum and medial upper chest (Fig. 2). The patient consented to surgical treatment.
Fig. 1 Preoperative computed tomogram with contrast agent shows a pseudoaneurysm (greatest transverse and anteroposterior diameter, 8.9 × 5.8 cm) arising from the anterior aortic sinus. The mass extends to the posterior aspect of the sternum and the mid-posterior chest wall (arrow), and remodels the bony anatomy.
Fig. 2 Computed tomogram of the chest (with contrast agent) shows a large pseudoaneurysm in the craniocaudal extent. Abnormalities are caused by the mass and by the aortic pseudoaneurysm's erosion (arrows) of the retrosternal bony structures.
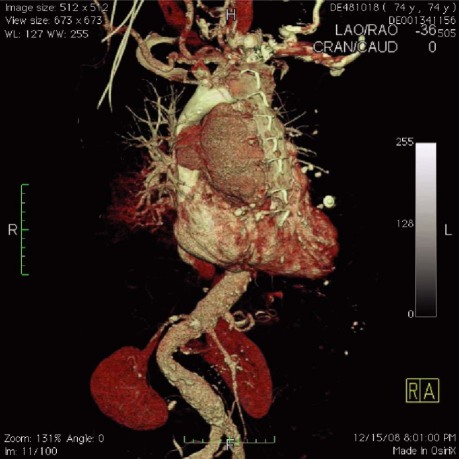
The risk of massive hemorrhage upon surgical entry was high due to the pseudoaneurysm's size, anterior location, and erosion into the outer sternum (Fig. 3). Perioperative transesophageal echocardiography revealed severe aortic insufficiency and blood that collected into a periaortic cavity. There was minimal mitral regurgitation and no tricuspid regurgitation.
Fig. 3 Three-dimensional computed tomographic chest reconstruction. The size and anterior location of the pseudoaneurysm increased the risk of hemorrhage during surgical entry. Real-time motion image is available at www.texasheart.org/journal.
Before sternotomy, cardiopulmonary bypass (CPB) was instituted by means of right femoral artery perfusion and right femoral venous drainage. Hypothermic circulatory arrest was established. However, the targeted core temperature of 19.1 °C was not attained, because the patient developed ventricular fibrillation at 26 °C. To avoid left ventricular distention, the sternum was entered expeditiously. Active bleeding from the cavity of the pseudoaneurysm was seen, and a defect in the proximal ascending aorta was exposed. To avoid exsanguination and to enable a better surgical view, perfusion pressure and flow were stopped almost completely for 2 minutes. Afterwards, CPB was resumed by placing a cross-clamp on the distal ascending aorta just below the brachiocephalic artery. Lysis of previous adhesions was performed by means of sharp dissection. Cardioplegic solution was supplied retrograde through the coronary sinus. There was no apparent purulent material or chronic granulation tissue to suggest active endocarditis. The aortic root had been disrupted from the mid ascending aorta down to the right coronary ostia. Part of the stentless valve had been torn away, and there was no anterior ascending aortic wall—the aortic suture line had given way. No felt or prosthetic material had been used to reinforce the prior closure.
The old valve was removed. Tissue samples from the aorta and the valve were sent to the microbiology laboratory, which detected no evidence of active endocarditis. A new 19-mm Carpentier-Edwards® porcine bioprothesis (Edwards Lifesciences LLC; Irvine, Calif) was sutured to a 22-mm aortic Hemashield® graft (Boston Scientific Corporation; Natick, Mass). A 6-mm woven graft (Boston Scientific) was anastomosed to the right coronary ostium, and an 8-mm woven graft (BostonScientific) was sutured to the left coronary ostium. Theprepared valved conduit was sutured into place, trimmed to proper length, and anastomosed to the transected aorta. The left coronary ostial graft was anastomosed to the aortic conduit, and the right coronary ostial graft was piggybacked onto the left graft. The heart was de-aired, and the cross-clamp was released. The total cross-clamp time was 118 min. Temporary atrial and ventricular epicardial wires were placed, and atrioventricular sequential pacing was begun at a rate of 90 beats/min. The patient was weaned from CPB with satisfactory hemodynamic results and was transferred to the intensive care unit in stable condition.
The patient's early postoperative course was uneventful. She experienced no neurologic sequelae and was extubated 3 days after surgery. Anticoagulation was started due to atrial fibrillation. On the 9th postoperative day, she became oliguric secondary to a hemopericardium, which was drained. She recovered well thereafter. Shortly before her discharge from the hospital, computed tomography of the chest showed no leakage from the aortic anastomotic site, and she underwent the elective placement of a pacemaker because of sick sinus syndrome.
After 3 months, the patient reported an uneventful recovery and her enrollment in the hospital's cardiac rehabilitation program. As of 6- and 9-month follow-up visits, she was participating in her usual daily activities.
Discussion
Ascending aortic pseudoaneurysms are associated with high morbidity and mortality rates.1,7–9 In some patients, the progressive growth of an aortic pseudoaneurysm can erode the bony structures of the sternum. Rupture is an imminent sequela of large ascending aortic pseudoaneurysms. Because of sternal erosion and the anterior location of the aneurysm in our patient, the risk of rupture was quite high. In addition, the large size of the mass crippled her cardiopulmonary system, producing signs and symptoms of heart failure. For these reasons, urgent surgical correction was necessary.
A major surgical challenge is to choose an approach that enables safe entry into the chest when a pseudoaneurysm has eroded the bony structures. The use of appropriate technique will avoid catastrophic hemorrhage during sternotomy. The chief objectives are to control the aortic defect during mediastinal dissection and to preserve adequate cerebral perfusion.
Other authors have described their methods for instituting CPB before sternotomy in these instances.7,10,11 The best approach to CBP remains undefined and depends upon the site and size of the ascending aortic pseudoaneurysm. Femoral or axillary arterial cannulation for CPB has been suggested. Mohammadi and colleagues11 used femorofemoral and bilateral carotid artery cannulation in 9 patients. In 2007, Bachet and associates10 described direct bilateral carotid cannulation in 5 patients, stating that the technique preserves cerebral circulation and maintains systemic CPB flow. However, patients in both series experienced neurologic sequelae. Because of the risk of stroke, carotid artery cannulation has not attained wide use.
Hypothermic circulatory arrest, which is used to control the depletion of intravascular volume and to enable the preservation of cerebral function, provides time to perform the mediastinal dissection and to control the aortic defect. Sudden and extreme blood loss can be avoided by using this approach. When the aorta is cross-clamped, CPB is again established.
Pettersson and associates12 used an intra-aortic occlusion balloon catheter to minimize the risk of rupture of an aortic wall defect. Although this novel technique could be used in the treatment of small-necked ascending aortic pseudoaneurysms, it does not minimize ventricular distention in patients with aortic insufficiency. Other investigators have suggested the use of a left atrial vent via a left thoracotomy.12 However, placing a vent does not guarantee the avoidance of ventricular distention in the presence of severe aortic insufficiency. In addition, vent placement compounds the risk of aneurysmal rupture and prolongs the surgical procedure.
Conclusion
In patients with giant pseudoaneurysms of the ascending aorta, sternal entry is a formidable challenge. Careful preoperative planning can enable safe entry. A combination of techniques can mitigate and minimize blood loss. Methods for preservation of cerebral perfusion should be considered. The surgeon's decision to institute axillary, femoral, or carotid CPB should depend on the nature of each case. The appropriate use of hypothermic circulatory arrest enables mediastinal dissection and aortic control. Every case of ascending aortic pseudoaneurysm is individual and should be managed accordingly.
Acknowledgments
The authors thank Mario Caballero, PA, Aarmal Hunter, PA, Alex Jonusas, PA, and the surgical cardiothoracic team for their expertise in the treatment of this patient.
Supplementary Material
Footnotes
Address for reprints: Juan D. Garisto, MD, Department of Cardiothoracic Surgery, University of Miami Hospital, 1295 NW 14th St., Suite H, Miami, FL 33125
E-mail: jgaristomd@yahoo.com
References
- 1.Dhadwal AK, Abrol S, Zisbrod Z, Cunningham JN Jr. Pseudoaneurysms of the ascending aorta following coronary artery bypass surgery. J Card Surg 2006;21(3):221–4. [DOI] [PubMed]
- 2.Brunner S, Engelmann MG, Nabauer M. Thoracic mycotic pseudoaneurysm from Candida albicans infection. Eur Heart J 2008;29(12):1515. [DOI] [PubMed]
- 3.Coselli JS, Crawford ES, Williams TW Jr, Bradshaw MW, Wiemer DR, Harris RL, Safi HJ. Treatment of postoperative infection of ascending aorta and transverse aortic arch, including use of viable omentum and muscle flaps. Ann Thorac Surg 1990;50(6):868–81. [DOI] [PubMed]
- 4.Mulder BJ. The distal aorta in the Marfan syndrome. Neth Heart J 2008;16(11):382–6. [DOI] [PMC free article] [PubMed]
- 5.Bizzarri F, Mattia C, Ricci M, Chirichilli I, Santo C, Rose D, et al. Traumatic aortic arch false aneurysm after blunt chest trauma in a motocross rider. J Cardiothorac Surg 2008;3:23. [DOI] [PMC free article] [PubMed]
- 6.Dumont E, Carrier M, Cartier R, Pellerin M, Poirier N, Bouchard D, Perrault LP. Repair of aortic false aneurysm using deep hypothermia and circulatory arrest. Ann Thorac Surg 2004;78(1):117–21. [DOI] [PubMed]
- 7.Katsumata T, Moorjani N, Vaccari G, Westaby S. Mediastinal false aneurysm after thoracic aortic surgery. Ann Thorac Surg 2000;70(2):547–52. [DOI] [PubMed]
- 8.Razzouk A, Gundry S, Wang N, Heyner R, Sciolaro C, Van Arsdell G, et al. Pseudoaneurysms of the aorta after cardiac surgery or chest trauma. Am Surg 1993;59(12):818–23. [PubMed]
- 9.Sullivan KL, Steiner RM, Smullens SN, Griska L, Meister SG. Pseudoaneurysm of the ascending aorta following cardiac surgery. Chest 1988;93(1):138–43. [DOI] [PubMed]
- 10.Bachet J, Pirotte M, Laborde F, Guilmet D. Reoperation for giant false aneurysm of the thoracic aorta: how to reenter the chest? Ann Thorac Surg 2007;83(5):1610–4. [DOI] [PubMed]
- 11.Mohammadi S, Bonnet N, Leprince P, Kolsi M, Rama A, Pavie A, Gandjbakhch I. Reoperation for false aneurysm of the ascending aorta after its prosthetic replacement: surgical strategy. Ann Thorac Surg 2005;79(1):147–52. [DOI] [PubMed]
- 12.Pettersson G, Nores M, Gillinov AM. Transfemoral control of ruptured aortic pseudoaneurysm at aortic root reoperation. Ann Thorac Surg 2004;77(1):311–2. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.