The CardioWest™ TAH-t (temporary total artificial heart), now known as the SynCardia temporary Total Artificial Heart (SynCardia Systems, Inc.; Tucson, Ariz), was approved by the U.S. Food and Drug Administration in 2004 for temporary use in patients with irreversible biventricular heart failure who are potential candidates for cardiac transplantation. This approval was granted on the basis of a multi-institutional study of 80 patients.1 Surgical teams at 23 centers in the U.S. and 20 in Europe and Australia have been trained to implant this device, which is the only TAH to be approved in either the United States or Europe. More than 888 of these devices have been implanted since 1993. The leading centers have been in Paris; Berlin and Bad Oeynhausen, Germany; Tucson, Arizona; and Richmond, Virginia (Medical College of Virginia).
The SynCardia TAH-t is a pneumatic biventricular pulsatile pump that weighs 160 g, displaces 400 mL of space, and routinely pumps 7 to 9 L/min of blood at a central venous pressure of 10 to 15 mmHg when implanted. Once the console has been set up, it rarely needs any adjustment. Thanks to the pneumatic mechanism, it automatically increases output as the patient exercises or experiences any other increase in blood volume; there is no blood-balance discrepancy between the left and right ventricles. The ventricles are attached to the left and right atria, which are left entirely intact and have normal compliance and filling pressures. Therefore, this pump does not create negative pressure within the thorax at any time.
Indications for implantation have included severe heart failure that requires treatment with multiple high-dose inotropic agents and vasopressors and often with mechanical ventilation, intra-aortic balloon counterpulsation, and temporary extracorporeal cardiac and pulmonary support. The survival rate has been 79% to the time of transplantation; 86% of those survivors have lived for one year after transplantation. Sixty-nine percent of the TAH recipients, compared with 37% of a matched control group, have reached the endpoint of successful post-transplantation survival (P <0.01). Complications with this pump have been no worse than those reported with left ventricular assist devices. Stroke, the most dreaded complication of mechanical circulatory support devices, was seen in 10% of patients, but nearly all strokes occurred at the time of implantation or explantation. The stroke rate during device support was lower than 2%.
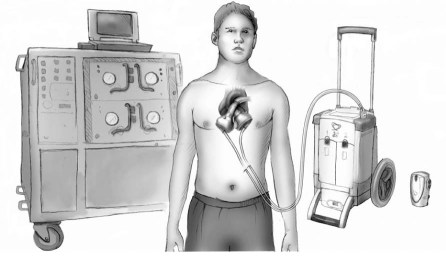
Since 2003, out-of-hospital care of TAH recipients has been a reality in Germany, where the Bad Oeynhausen group started discharging patients from the hospital on a portable driver. Now, more than 68 patients have been discharged home in Germany, France, and Italy for periods of up to 903 days. In the U.S., 2 new drivers, the Companion II (45 lb) and the Freedom (13.5 lb) drivers (SynCardia), have been tried in the laboratory. They are now ready for human use in Europe and the U.S. (Fig. 1). There are approximately 20 Companion II and 8 Freedom drivers attached to patients in Europe and Australia. The first patient discharged home on the 13.5-lb driver was in Moscow, the second in Phoenix. Before the production of the Companion II and Freedom drivers, only 36 large (Big Blue) consoles for driving the TAH were available in the world. This placed limitations on use, which will disappear over the next 18 months as the portable drivers become more available.
Fig. 1 The relative sizes of 3 drivers used for the SynCardia temporary total artificial heart. Illustration courtesy of SynCardia Systems, Inc.
Multivariate analyses of risks associated with the use of various types of mechanical circulatory support device have been used in an effort to identify favorable populations for each device type.2 The result has been that ventricular assist devices are associated with higher mortality rates in patients with right ventricular failure, either by itself or in combination with failure of the kidneys, the liver, or the lungs—and the SynCardia TAH-t does not increase the risk for patients with these conditions.
As the new, smaller drivers are approved and released, out-of-hospital care of TAH patients will be possible, first in Europe and later in the U.S. This should enable the cost of a TAH to be cut drastically. The quality of life of patients will be dramatically improved, and surgeons and hospitals will have a device-support option to offer the very sickest patients.
Footnotes
Address for reprints: Jack Copeland, MD, Cardiothoracic Surgery, MC 8892, University of California, San Diego, 200 W. Arbor Dr., San Diego, CA 92103
E-mail: jackcope3@gmail.com
Dr. Copeland holds an equity interest in SynCardia Systems, Inc.
Presented at the Joint Session of the Denton A. Cooley Cardiovascular Surgical Society and the Michael E. DeBakey International Surgical Society; Austin, Texas, 10–13 June 2010
References
- 1.Copeland JG, Smith RG, Arabia FA, Nolan PE, Sethi GK, Tsau PH. Cardiac replacement with a total artificial heart as a bridge to transplantation. N Engl J Med 2004;351(9):859–67. [DOI] [PubMed]
- 2.Copeland JG, Smith RG, Bose RK, Tsau PH, Nolan PE, Slepian MJ. Risk factor analysis for bridge to transplantation with the CardioWest total artificial heart. Ann Thorac Surg 2008;85(5):1639–44. [DOI] [PubMed]