Abstract
Atavism is the rare reappearance, in a modern organism, of a trait from a distant evolutionary ancestor. We describe an apparent case of atavism involving a 59-year-old man with chest pain whose coronary circulation and myocardial architecture resembled those of the reptilian heart. The chest pain was attributed to a coronary steal phenomenon. The patient was discharged from the hospital on a heightened regimen of β-blockers, and his symptoms improved significantly. To our knowledge, this is only the 2nd reported clinical case of a human coronary circulation similar to that of reptiles.
Key words: Anatomy, comparative; angina pectoris/etiology; coronary circulation/physiology; coronary steal; coronary vessels/anatomy & histology; embryology, cardiac; evolution; fistula/diagnosis; heart/anatomy & histology; heart defects, congenital/pathology/radiography; laser therapy; microcirculation; morphology, cardiac; reptiles; snakes; veins/anatomy & histology
The saying “ontogeny recapitulates phylogeny”1 means that an animal's embryologic development repeats the evolutionary development of the species. Over several million years, mammals have evolved from other classes of vertebrates, including reptiles. As a result of this long evolutionary process, the human heart might be susceptible to atavism. We describe a patient who presented with chest pain and was found to have a coronary circulation and myocardial architecture resembling that of reptiles. To our knowledge, this is the 2nd reported clinical case to involve a coronary circulation similar to that of reptiles.
Case Report
A 59-year-old man was referred to our institution for coronary artery bypass grafting and repair of a left ventricular (LV) fistula. He had originally been admitted to another hospital because of chest pain, progressively worsening shortness of breath (New York Heart Association functional class III–IV), and fatigue. His sharp, exertional chest pains were not relieved by nitrates. The patient's medical history included asthma and percutaneous coronary intervention in the right coronary artery (RCA). His family history was notable for coronary artery disease and diabetes mellitus. The patient had no relevant history of febrile illness or chest trauma. He was a cigarette smoker who was trying to quit, but he denied any use of alcohol or illicit drugs. Vital signs were normal except for mild tachycardia. Physical findings and laboratory results (including those for cardiac enzyme levels) were unremarkable. Cardiac auscultation revealed no murmur, gallop, or click.
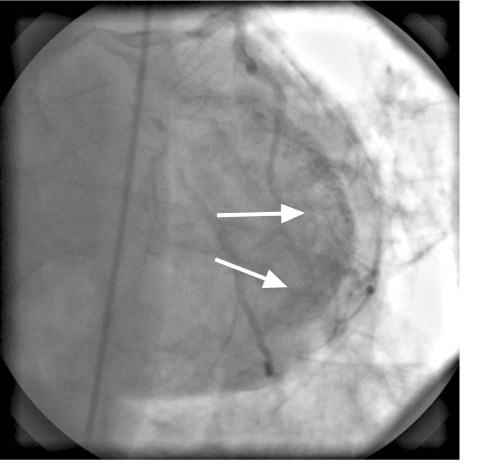
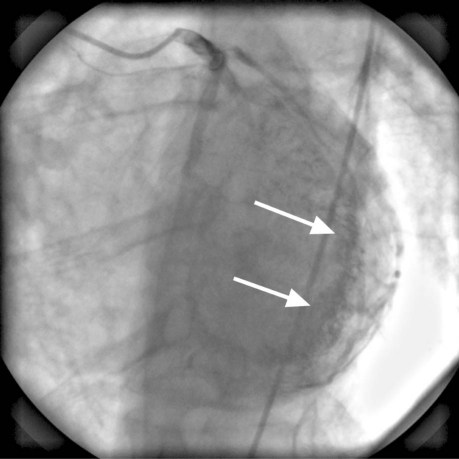
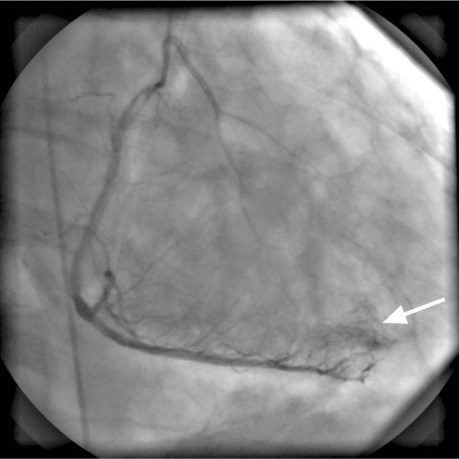
Electrocardiography and chest radiography showed no abnormality. Cardiac catheterization confirmed that the previously placed RCA stents were widely patent and that the RCA had no significant obstruction. The left anterior descending and left circumflex coronary arteries were free of significant obstruction but had multiple abnormal communications with the LV cavity (Figs. 1 and 2). The RCA branches, posterior descending coronary artery, and posterolateral LV artery drained into the LV through multiple coronary cameral fistulae. Angiography also showed a significant degree of noncompaction of the myocardium (Fig. 3). The venous drainage system of the heart was barely discernible. Two-dimensional Doppler echocardiography at rest showed mildly impaired LV systolic function, with an ejection fraction of 0.40 to 0.44, and impaired LV relaxation that suggested diastolic dysfunction. Trivial mitral and tricuspid regurgitation was present. We concluded that the patient's chest pains were caused by a coronary steal phenomenon that resulted from the loss of myocardial perfusion via the multiple coronary artery-to-LV fistulae. He was discharged on an elevated regimen of β-blockers, and his symptoms greatly improved.
Fig. 1 Coronary angiogram (anteroposterior cranial view) shows multiple fistulous communications (arrows) between the left anterior descending and left circumflex coronary arteries and the left ventricular cavity.
Fig. 2 Coronary angiogram (left anterior oblique cranial view) shows multiple fistulous communications (arrows) between the left anterior descending and left circumflex coronary arteries and the left ventricular cavity.
Fig. 3 Coronary angiogram (left anterior oblique cranial view) shows right coronary artery branches, the posterior descending artery, and the posterolateral left ventricular artery draining into the left ventricle through multiple coronary cameral fistulae (arrow).
Discussion
Coronary angiography showed that our patient had a noncompacted myocardium, along with multiple coronary cameral fistulae that drained blood from the right and left coronary artery systems directly into the LV cavity. Remarkably, the morphology resembled that of the reptilian heart—that is, it featured direct communications to the ventricular cavity and had the sinusoidal characteristics of noncompacted myocardium. To our knowledge, this is only the 2nd reported case involving such morphology, the 1st case having been reported by Osman and associates.2
The basic anatomy and physiology of the mammalian heart is very different from that of the reptilian heart.3 Unlike mammals, reptiles have a single ventricle responsible for distributing mixed blood to both the systemic and pulmonary circulations, which are not completely separate from each other. Hence, the reptilian heart has been called the “transitional heart.” In poikilothermic (“cold-blooded”) reptiles, the heart has a unique adaptive value that enables the conservation of energy during prolonged hibernation.3-6
The mammalian coronary circulation is also distinctly different from that of nonmammalian vertebrates. Not until the appearance of birds and mammals did the coronary arteries develop. In the human heart, the arterial supply of the myocardium is provided by the left and right coronary arteries, and venous drainage occurs via the anterior cardiac veins,7-9 which drain most of the right and left coronary arteries and the coronary sinus.10,11 In reptiles, however, a thin periphery, consisting of about one twelfth of the myocardial mass, is supplied by small, external coronary vessels. The bulk of the myocardium is supplied directly by luminal blood from the ventricular cavity.12 The reptilian heart is made up of noncompacted myocardium, with sinusoids that channel the blood to the myocardium directly from the lumen of the ventricle.13 This type of circulation is sufficient for poikilothermic reptiles, because of their lower metabolic demands, but it is not sufficient for mammals. In mammals, coronary arteries form communications with a sponge-like sinusoidal network to form capillaries, which then anastomose with the coronary veins to create a more efficient circulation.14 In human beings, the sinusoids deliver oxygenated blood to the myocardium. Therefore, in a normal human heart, the myocardium is compacted and is supplied by 2 large coronary artery systems and their tributaries, instead of by sinusoids. Although prominent trabeculae are seen in a normal right ventricle, the persistence of prominent LV trabeculae is not normally apparent after birth.15
The evolution of a species is reiterated during embryonic development. In early stages of embryonic development, the human heart resembles that of a fish, in that each chamber is undivided and blood exits through a single aorta.16 In later stages of development, the human heart—now with fully separated atria and a partially separated ventricle—resembles a reptilian heart.6 In its final stage, the human heart has 4 separate chambers composed of compacted myocardium, and blood is supplied by 3 large epicardial arteries.
The presence of a noncompacted myocardium in human beings has been well described.17-20 In a large number of cases, it can lead to nonischemic cardiomyopathy, as well as thromboembolic complications.15 Following the example of the sinusoids, investigators have devised new techniques for revascularizing the ischemic myocardium.13,21–24 Several have attempted to divert cavitary blood into the sinusoids by inserting conduits or performing direct acupuncture. These procedures aim to de-evolve the human heart to that of a primitive reptile.14 In 1982, Mirhoseini and co-authors25,26 proposed drilling transmyocardial channels with a carbon dioxide laser. Later, Cooley and colleagues21 used this approach in patients with chronic refractory coronary artery disease. In a randomized study, transmyocardial laser revascularization—the only such technique still used for treating refractory angina—was shown to improve perfusion and symptoms.27
Coronary cameral fistulae are usually observed in right-sided structures.28 Coronary artery-to-LV fistulae are usually silent.29 Such fistulae can result in a coronary steal phenomenon that can lead to decreased myocardial perfusion and account for exertional chest pain.30,31 The literature contains multiple descriptions of coronary cameral fistulae; most of these fistulae have been identified incidentally by means of angiography.32,33 In symptomatic patients in whom the coronary steal from the fistula is considered to be the cause of chest pain, the fistula can be closed either surgically or percutaneously.28,34 Our patient had no important obstruction in his epicardial vessels, so a coronary steal from the fistula was the most likely reason for his chest pain. Because the fistulous connections were small and multiple, they were not amenable to any closure technique. A higher dosage of β-blockers relieved the patient's angina, perhaps by reducing the myocardial oxygen demand.
Conclusion
To our knowledge, this is only the 2nd reported case of an unusual morphologic variant involving a nonmammalian, reptile-like cardiac anatomy that exemplifies atavism in the human heart. Medical management successfully relieved the patient's symptoms, and his myocardial function was preserved.
Acknowledgment
The authors thank Ms Virginia Fairchild, of the Texas Heart Institute's Department of Scientific Publications, for her editorial support.
Footnotes
Address for reprints: Reynolds M. Delgado III, MD, 6624 Fannin, Suite 2420, Houston, TX 77030
E-mail: ReynoldsDelgadoMD@gmail.com
References
- 1.Haeckel E. The riddle of the universe. Amherst (NY): Prometheus Books; 1992.
- 2.Osman F, Qaisar S, Murray RG. Images in cardiology. Human coronary circulation mimicking reptilian cardiac physiology. Heart 2006;92(4):550. [DOI] [PMC free article] [PubMed]
- 3.Solc D. The heart and heart conducting system in the kingdom of animals: a comparative approach to its evolution. Exp Clin Cardiol 2007;12(3):113–8. [PMC free article] [PubMed]
- 4.Victor S, Nayak VM. Evolutionary anticipation of the human heart. Ann R Coll Surg Engl 2000;82(5):297–302. [PMC free article] [PubMed]
- 5.Webster DB, Webster M. Comparative vertebrate morphology. New York: Academic Press; 1974.
- 6.Kardong KV. Vertebrates. Comparative anatomy, function, evolution. 2nd ed. Boston: William C. Brown; 1995.
- 7.Gregg DE, Shipley RE, Bidder TG. The anterior cardiac veins. Their functional importance in the venous drainage of the right heart. Am J Physiol 1943;139(5):732–41.
- 8.Gregg DE. The coronary circulation. Physiol Rev 1946;26(1): 28–46. [DOI] [PubMed]
- 9.von Ludinghausen M. The venous drainage of the human myocardium. Adv Anat Embryol Cell Biol 2003;168:I-VIII, 1–104. [DOI] [PubMed]
- 10.Gilard M, Mansourati J, Etienne Y, Larlet JM, Truong B, Boschat J, Blanc JJ. Angiographic anatomy of the coronary sinus and its tributaries. Pacing Clin Electrophysiol 1998;21(11 Pt 2):2280–4. [DOI] [PubMed]
- 11.Uemura H, Ho SY, Anderson RH, Devine WA, Smith A, Shinohara T, et al. The surgical anatomy of coronary venous return in hearts with isomeric atrial appendages. J Thorac Cardiovasc Surg 1995;110(2):436–44. [DOI] [PubMed]
- 12.Sen PK, Udwadia TE, Kinare SG, Parulkar GB. Transmyocardial acupuncture: a new approach to myocardial revascularization. J Thorac Cardiovasc Surg 1965;50:181–9. [PubMed]
- 13.Goldman A, Greenstone SM, Preuss FS, Strauss SH, Chang ES. Experimental methods for producing a collateral circulation to the heart directly from the left ventricular. J Thorac Surg 1956;31(3):364–74. [PubMed]
- 14.Tsang JC, Chiu RC. The phantom of “myocardial sinusoids”: a historical reappraisal. Ann Thorac Surg 1995;60(6):1831–5. [DOI] [PubMed]
- 15.Varnava AM. Isolated left ventricular non-compaction: a distinct cardiomyopathy? Heart 2001;86(6):599–600. [DOI] [PMC free article] [PubMed]
- 16.Morris EW. Embryology of some congenital cardiac anomalies. Thorax 1965;20:158–69. [DOI] [PMC free article] [PubMed]
- 17.Burke A, Mont E, Kutys R, Virmani R. Left ventricular noncompaction: a pathological study of 14 cases. Hum Pathol 2005;36(4):403–11. [DOI] [PubMed]
- 18.Junqueira FP, Fernandes FD, Coutinho AC, De Pontes PV, Domingues RC. Case report. Isolated left ventricular myocardium non-compaction: MR imaging findings from three cases. Br J Radiol 2009;82(974):e37–41. [DOI] [PubMed]
- 19.Moreira FC, Miglioransa MH, Mautone MP, Muller KR, Lucchese F. Noncompaction of the left ventricle: a new cardiomyopathy is presented to the clinician. Sao Paulo Med J 2006;124(1):31–5. [DOI] [PMC free article] [PubMed]
- 20.Ivan D, Flamm SD, Abrams J, Kindo M, Heck K, Frazier OH. Isolated ventricular non-compaction in adults with idiopathic cardiomyopathy: cardiac magnetic resonance and pathologic characterization of the anomaly. J Heart Lung Transplant 2005;24(6):781–6. [DOI] [PubMed]
- 21.Cooley DA, Frazier OH, Kadipasaoglu KA, Pehlivanoglu S, Shannon RL, Angelini P. Transmyocardial laser revascularization. Anatomic evidence of long-term channel patency. Tex Heart Inst J 1994;21(3):220–4. [PMC free article] [PubMed]
- 22.Effler DB, Groves LK, Sones FM Jr, Shirey EK. Increased myocardial perfusion by internal mammary artery implant: Vineberg's operation. Ann Surg 1963;158:526–36. [DOI] [PMC free article] [PubMed]
- 23.Vineberg A. Coronary vascular anastomoses by internal mammary artery implantation. Can Med Assoc J 1958;78(11):871–9. [PMC free article] [PubMed]
- 24.Vineberg A, Deliyannis T, Pablo G. The Ivalon sponge procedure for myocardial revascularization. Surgery 1960;47:268–89. [PubMed]
- 25.Mirhoseini M, Muckerheide M, Cayton MM. Transventricular revascularization by laser. Lasers Surg Med 1982;2(2):187–98. [DOI] [PubMed]
- 26.Mirhoseini M, Shelgikar S, Cayton MM. Transmyocardial laser revascularization: a review. J Clin Laser Med Surg 1993; 11(1):15–9. [DOI] [PubMed]
- 27.Frazier OH, March RJ, Horvath KA. Transmyocardial revascularization with a carbon dioxide laser in patients with end-stage coronary artery disease. N Engl J Med 1999;341(14): 1021–8. [DOI] [PubMed]
- 28.Lopez-Candales A, Kumar V. Coronary artery to left ventricle fistula. Cardiovasc Ultrasound 2005;3:35. [DOI] [PMC free article] [PubMed]
- 29.Liberthson RR, Sagar K, Berkoben JP, Weintraub RM, Levine FH. Congenital coronary arteriovenous fistula. Report of 13 patients, review of the literature and delineation of management. Circulation 1979;59(5):849–54. [DOI] [PubMed]
- 30.Cheng TO. Left coronary artery-to-left ventricular fistula: demonstration of coronary steal phenomenon. Am Heart J 1982;104(4 Pt 1):870–2. [DOI] [PubMed]
- 31.Kiso I, Itoh T, Morishita M, Kato K, Ishikura Y. Blood flow and pressure measurements of right coronary artery to left ventricle fistula. Thorax 1978;33(2):253–6. [DOI] [PMC free article] [PubMed]
- 32.Ahmed SS, Haider B, Regan TJ. Silent left coronary artery-cameral fistula: probable cause of myocardial ischemia. Am Heart J 1982;104(4 Pt 1):869–70. [DOI] [PubMed]
- 33.Cha SD, Singer E, Maranhao V, Goldberg H. Silent coronary artery-left ventricular fistula: a disorder of the thebesian system? Angiology 1978;29(2):169–73. [DOI] [PubMed]
- 34.Strecker T, Hakami L, Singer H, Weyand M, Cesnjevar R. Congenital coronary artery fistula draining into the right ventricle: successful surgical closure after failed transcatheter coil embolization. Thorac Cardiovasc Surg 2006;54(1):61–3. [DOI] [PubMed]