Nearly 20 years ago, 2 independent teams led by Parodi1 and Volodos2 performed the first endovascular aortic repair procedures. However, it was not until 1999—when the U.S. Food and Drug Administration approved the use of endografts for the repair of abdominal aortic aneurysms (EVAR)—that EVAR was rapidly adopted. In 2005, the Food and Drug Administration also approved the use of endografts for descending thoracic endovascular aortic repair (TEVAR). Early reports of low procedural mortality and morbidity rates associated with endografts contributed to their widespread use for the repair of both abdominal and descending thoracic aortic aneurysms. Consequently, the use of open surgical approaches for the repair of abdominal and descending thoracic aortic aneurysms has decreased.3
However, the increased use of endovascular approaches has now increased the number of reported complications. The complications of EVAR affect both short-term and long-term outcomes. Recently, a study reporting the long-term outcomes of patients who underwent either EVAR or open surgical repair showed that the early survival advantage conferred by EVAR does not progress to better long-term survival: survival in the long term was similar for both treatments.4 Moreover, the results of the long-awaited investigation of stent-grafts in patients with type B aortic dissection (INSTEAD) trial revealed no advantage (over medical therapy alone) in using TEVAR in addition to optimal medical therapy to treat uncomplicated distal aortic dissection.5 As a consequence, questions remain in regard to the overall effectiveness of endovascular repair.
Complications of endovascular repair can be classified as problems associated with the endograft (for example, device fractures), the endograft delivery system (for example, inflexible or too-large delivery sheaths), or the particular circumstances of an individual patient (for example, the patient's unsuitability for endovascular repair). Potential complications include device or delivery failure, access-vessel injury, retrograde proximal dissection, stroke, distal embolization, paraplegia, mesenteric ischemia, late mechanical failure, migration, endoleak, infection, and continued aneurysmal expansion. Although endograft manufacturers have been quick to respond to reports of design- and delivery-related complications, the risk of other types of complications—which can occur at any time—remains. Numerous strategies have evolved not only to mitigate or avoid these problems, but also to better identify patients who are most likely to benefit from endovascular repair.
Many early complications of endovascular repair, including rupture and stroke, are directly related to the manipulation of the endograft delivery system within the access vessels and the aorta. Patients with narrow, calcified, or tortuous vessels are at particularly high risk of complications related to endograft deployment. Detailed preprocedural imaging can assist in identifying patients with suboptimal vessels, so that an alternative plan for device deployment can be developed. Often, a conduit can be attached to an access vessel to ease deployment of the endograft.
A relatively common and potentially deadly complication of TEVAR is retrograde aortic dissection, which converts an easily treatable, localized descending thoracic aortic aneurysm into an acute, life-threatening problem that involves the entire thoracic aorta. When retrograde dissection occurs, the usual culprits are an oversized endograft or over-aggressive balloon expansion during that phase of the repair. Other problems associated with endovascular repair include stent-graft misdeployment, device migration, endograft kinking, endograft infection, and the development of endoleaks. An endoleak is a persistent flow of blood into the aneurysmal sac, readily seen on radiologic imaging. This flow of blood leads to continuous pressurization of the sac, which can cause expansion or rupture over time. Endoleaks are categorized by the cause or origin of the leak. The most common variety is a type II endoleak—caused by backflow into the sac from collateral arteries. Type II endoleaks that do not resolve during a period of observation are often treated through interventional approaches, such as coil embolization. Any increase in aneurysm size during the observation period indicates the need for treatment.
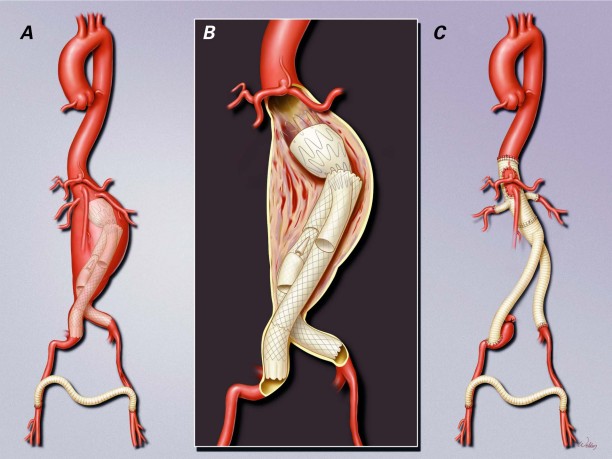
Ultimately, when endograft failure is not amenable to further endovascular treatment, open explantation of the endograft and graft replacement of the involved aortic segments is warranted (Fig. 1). When removing infected aortic endografts during in situ repair, the surgeon can take steps to prevent recurrent graft infection. In the absence of infection, the surgeon can occasionally reduce the overall extent of repair by salvaging portions of the stent-graft.
Fig. 1 Late conversion to open repair in a 65-year-old man who A) underwent multiple endovascular reinterventions and B) developed an infection. C) Open extent IV (Safi's classification) intravenous thoracoabdominal aortic repair was performed to remove the infected aortic endografts and to replace them with an antibiotic-soaked polyester graft. The graft was also covered with omentum to reduce the risk of recurrent infection. The patient recovered without further complication and was discharged from the hospital on postoperative day 6, to complete 8 weeks of intravenous vancomycin therapy. Illustration © 2010 Baylor College of Medicine; used by permission.
A potential procedural complication of TEVAR is paraplegia. Cerebrospinal fluid drainage—traditionally used as a surgical adjunct in extensive open thoracoabdominal aortic repair—has been adopted by many endovascular centers to mitigate the risk of paraplegia during TEVAR. The risk of paraplegia is greatest when a large section of the descending thoracic aorta is covered by one or more endografts; cerebrospinal fluid drainage can be initiated after repair, should paraplegia result from spinal cord ischemia. Although not all complications related to stent-grafts pose a risk of death or significant disability, endovascular repair should be performed by qualified teams that are capable of managing a variety of problems. Because late complications are relatively common, a stringent surveillance protocol should be implemented.
Acknowledgments
The author expresses gratitude to Stephen N. Palmer, PhD, ELS, and Nicole Stancel, PhD, of the Texas Heart Institute, and Susan Y. Green, MPH, for editorial assistance; and Scott A. Weldon, MA, CMI, for creating the illustrations and assisting with image selection.
Footnotes
Address for reprints: Joseph S. Coselli, MD, Division of Cardiothoracic Surgery, Michael E. DeBakey Department of Surgery, Baylor College of Medicine, BCM 390, One Baylor Plaza, Houston, TX 77030
E-mail: jcoselli@bcm.edu
Presented at the Joint Session of the Denton A. Cooley Cardiovascular Surgical Society and the Michael E. DeBakey International Surgical Society; Austin, Texas, 10–13 June 2010
Dr. Coselli has participated as principal investigator in the TX2 Thoracic Stent-Graft Trial (Cook, Inc.); as principal investigator in the Valor II and THRIVE Stent-Graft Trials (Medtronic, Inc.); as a consultant and speaker for Medtronic; as a principal investigator in the Gore Conformable Descending/Dissection Thoracic Stent-Graft Trial (W.L. Gore & Assoc.); and as a speaker and consultant for W.L. Gore. He also has received an educational grant from Vascutek Terumo and royalties for the Coselli Branched Graft for thoracoabdominal aortic aneurysm repairs, and he serves as a consultant to Vascutek Terumo.
References
- 1.Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg 1991;5(6):491–9. [DOI] [PubMed]
- 2.Volodos NL, Karpovich IP, Troyan VI, Kalashnikova YuV, Shekhanin VE, Ternyuk NE, et al. Clinical experience of the use of self-fixing synthetic prostheses for remote endoprosthetics of the thoracic and the abdominal aorta and iliac arteries through the femoral artery and as intraoperative endoprosthesis for aorta reconstruction. Vasa Suppl 1991;33:93–5. [PubMed]
- 3.Giles KA, Pomposelli F, Hamdan A, Wyers M, Jhaveri A, Schermerhorn ML. Decrease in total aneurysm-related deaths in the era of endovascular aneurysm repair. J Vasc Surg 2009; 49(3):543–51. [DOI] [PMC free article] [PubMed]
- 4.Goodney PP, Tavris D, Lucas FL, Gross T, Fisher ES, Finlayson SR. Causes of late mortality after endovascular and open surgical repair of infrarenal abdominal aortic aneurysms. J Vasc Surg 2010;51(6):1340–7.e1. [DOI] [PMC free article] [PubMed]
- 5.Nienaber CA, Rousseau H, Eggebrecht H, Kische S, Fattori R, Rehders TC, et al. Randomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial. Circulation 2009;120(25):2519–28. [DOI] [PubMed]