Abstract
Hepatitis B virus (HBV) and hepatitis delta virus (HDV) interplay was investigated by examining liver and serum samples from 21 coinfected and 22 HBV-monoinfected patients with chronic liver disease. Different real-time PCR assays were applied to evaluate intrahepatic amounts of HBV DNA, covalently closed circular DNA (cccDNA), pregenomic RNA (pgRNA), pre-S/S RNAs, and HDV RNA. Besides HBV DNA and HDV RNA levels, HBsAg concentrations in the sera were also determined. HDV-coinfected cases showed significantly lower median levels of serum HBV DNA (−5 log), intrahepatic relaxed-circular DNA (−2 log), and cccDNA (−2 log) than those of HBV-monoinfected cases. Interestingly, pgRNA and pre-S/S RNA amounts were significantly lower (both −1 log) in HDV-positive patients, whereas serum HBsAg concentrations were comparable between the two patient groups. Pre-S/S RNA and HBsAg amounts per cccDNA molecule were higher in HDV-positive patients (3-fold and 1 log, respectively), showing that HBV replication was reduced, whereas synthesis of envelope proteins was not specifically decreased. The ratios of cccDNA to intracellular total HBV DNA showed a larger proportion of cccDNA molecules in HDV-positive cases. For these patients, both intrahepatic and serum HDV RNA amounts were associated with cccDNA but not with HBsAg or HBV DNA levels. Finally, HBV genomes with large deletions in the basal core promoter/precore region were detected in 5/21 HDV-positive patients but in no HDV-negative patients and were associated with lower viremia levels. These findings provide significant information about the interference exerted by HDV on HBV replication and transcription activities in the human liver.
Hepatitis delta virus (HDV) is a worldwide diffuse pathogen commonly associated with severe forms of liver disease (9, 21, 22, 35). HDV can establish infection only in individuals with continuing hepatitis B virus (HBV) infection, since it requires obligatory helper functions provided by HBV for in vivo infection. In particular, HDV needs to borrow the envelope proteins produced by HBV, and consequently, the two viruses share the same outer coats, consisting of the HBV surface antigen (HBsAg) (21, 35). In spite of this, HDV and HBV are completely different in terms of genome replication, with both showing several aspects that make their life cycles nearly unique among agents infecting animals. Very briefly, HDV is a small RNA virus with a single-stranded and circular genome of approximately 1,700 nucleotides (nt) that is replicated using a host RNA polymerase and contains a ribozyme able to self-cleave and self-ligate the circular HDV genome (30). In contrast, HBV is a closed, circular, partially double-stranded DNA virus of 3.2 kb containing four partially overlapping open reading frames that replicates via the formation of a circular covalently closed DNA (cccDNA) which serves as a template for the production of virus mRNAs, including an RNA pregenome that is reverse transcribed in the cytoplasm of hepatocytes for the synthesis of the DNA molecules (14). The HBV cccDNA can persist throughout life in the livers of infected individuals, and viral DNA may also be integrated directly into the host DNA (26). Apart from the above-mentioned envelope proteins supplied to HDV by HBV, the exact biological interactions between the two viruses are far from being understood completely. Both are parenterally transmitted agents with a striking hepatotropism, and their coexistence is traditionally believed to be characterized by suppression of HBV replication exerted by HDV (21). Although most HDV-positive patients have very low or even undetectable levels of serum HBV DNA, a number of them show serological patterns of active HBV replication, i.e., a positive e antigen (HBeAg) status and high viral DNA values, and these last cases are often associated with the most aggressive and rapidly evolving forms of chronic liver disease (21, 39). Moreover, recent studies suggested that HBV DNA and HDV RNA serum levels in coinfected patients may not be stable and may change over time (18, 24). Indeed, it is of utmost importance to clarify the interplay between the two viruses, not only from the biological point of view but also because it might open up a way to find adequate therapeutic approaches for the treatment of HDV-related liver diseases that are presently nearly incurable. HDV and HBV interactions in humans have been investigated so far essentially by evaluating the circulating viruses, whereas the molecular patterns of the two viruses have been investigated very little at the intrahepatic level.
The aim of this study was to explore the HBV and HDV replicative and transcriptional activities by analyzing liver and serum nucleic acid extracts from HDV-infected individuals with various HBV serological patterns.
MATERIALS AND METHODS
Patients and samples.
We studied 21 HBV- and HDV-coinfected patients (HDV-positive patients) who consecutively underwent needle liver biopsy at the liver centers in Bari, Naples, and Messina, Italy, in 2008 and 22 HBV-monoinfected patients (HDV-negative patients) consecutively undergoing liver biopsy at the Messina liver center in the same period. All of the HDV-positive patients (11 men and 10 women; median age, 43.5 years; age range, 30 to 58 years) were Italians; 3 of them were HBeAg positive, and 18 were anti-HBe positive. All HDV-negative patients (17 men and 5 women; median age, 43 years; age range, 14 to 62 years) were Italians, with the exception of one Chinese individual; 8 of them were HBeAg positive, and 14 were anti-HBe positive (Table 1).
TABLE 1.
Demographic, virologic, and histological characteristics of patients with and without HDV infection
| Parameter | Value |
P valuea | |
|---|---|---|---|
| HDV-positive patients | HDV-negative patients | ||
| No. of males/total no. of patients | 11/21 | 17/22 | NS |
| Median (range) age (yr) | 43.5 (30-58) | 43 (14-62) | NS |
| No. of HBeAg-positive patients/no. of HBeAg-negative patients | 3/18 | 8/14 | 0.007 |
| No. of patients with HBV genotype | |||
| D | 20 | 18 | NS |
| A | 1 | 3 | NS |
| C | 0 | 1 | NS |
| No. of patients with stage of fibrosisb | |||
| 0-1 | 5 | 6 | NS |
| 2 | 6 | 5 | NS |
| 3-4 | 10 | 11 | NS |
| No. of patients with grade of activityb | |||
| 1 | 5 | 6 | NS |
| 2 | 11 | 15 | NS |
| 3 | 5 | 1 | NS |
NS, not significant.
Histological staging and grading were performed according to the classification method of Scheuer (25).
For each patient, part of the liver biopsy specimen was processed for histologic examination, and the rest was immediately frozen in RNAlater (Applied Biosystems/Ambion, Austin, TX) and stored at −80°C until molecular analysis. Serum samples from each patient were collected and frozen the same day as the liver biopsy specimen. None of the patients were positive for hepatitis C virus or human immunodeficiency virus, were on treatment, or had received antiviral therapy in the past. The study protocol was performed according to the principles of the Declaration of Helsinki, and informed consent was obtained from all patients.
Serum HBV DNA and HBsAg quantification.
Serum HBV DNA was quantified using the Cobas AmpliPrep/Cobas TaqMan HBV test (Roche Molecular Systems, Branchburg, NJ), with a lower limit of detection of 70 copies/ml (12 IU/ml), and HBsAg quantification was performed by the Architect HBsAg assay (Abbott Laboratories, Chicago, IL) according to the manufacturer's instructions.
DNA and RNA extraction from liver specimens.
Total DNA and RNA extractions from liver biopsy specimens were performed through the approach described by Volz et al. (32), with minor modifications. Briefly, cryopreserved liver tissue specimens from individual patients were homogenized by use of a TissueRupter instrument (Qiagen, Milano, Italy) in 500 μl homogenization buffer (50 mM Tris-HCl, pH 8.0, 1 mM EDTA, 150 mM NaCl) at 4°C and then divided into two equal parts, with one used for DNA extraction and the other used for RNA extraction.
Total liver DNA was extracted from one part of the homogenate by digestion in 150 mM NaCl, 50 mM Tris-HCl (pH 7.4), 10 mM EDTA, 1% sodium dodecyl sulfate, and proteinase K (800 μg/ml) overnight at 37°C. After extraction with phenol-chloroform, the nucleic acids were precipitated in 2 volumes of pure cold ethanol. Nucleic acids were then resuspended and digested with pancreatic RNase (100 μg/ml), followed by extraction with phenol-chloroform and reprecipitation in pure cold ethanol. The DNA was resuspended in 10 mM Tris-HCl (pH 7.4) and 1 mM EDTA. Total liver RNA was extracted from the other half of the liver tissue homogenate by use of TRIzol reagent (Invitrogen) as recommended by the manufacturer. RNA quality and quantity were monitored on agarose gels by ethidium bromide staining and UV absorption.
RNA and DNA concentrations were measured using an ND-1000 spectrophotometer (NanoDrop Technologies) at 260 nm.
Quantification of HBV DNA in liver specimens.
Quantification of total intracellular HBV DNA and HBV cccDNA was performed by adapting the methods described by Werle-Lapostolle et al. (36) for the “utility channel” of a Cobas TaqMan 48 (Roche, Basel, Switzerland) instrument. Briefly, real-time PCR to evaluate total HBV DNA was performed using a 75-μl reaction volume containing 10 μl of DNA extract, 3 mM MgCl2, 0.5 μM (each) forward and reverse primers, 0.2 μM 3′-fluorescein (FL)-labeled probe, and 0.4 μM 5′-Red640 (R640)-labeled probe. Amplification was performed under the following conditions: 95°C for 10 min and then 60 cycles of 95°C for 30 s, 57°C for 20 s, and 72°C for 20 s. Serial dilutions of a plasmid containing a monomeric HBV insert (Alfa Wasserman, Milan, Italy) were used as quantification standards.
To perform quantification of cccDNA, aliquots of liver DNA extracts were treated for 1 h at 37°C with 10 U of plasmid-safe DNase (Epicentre, Madison, WI). Real-time PCR experiments were then performed using a 75-μl reaction volume containing 20 ng of DNA, 3 mM MgCl2, 0.5 μM (each) forward and reverse primers, 0.2 μM 3′-FL-labeled probe, and 0.4 μM 5′-R640-labeled probe. Amplification was performed under the following conditions: 95°C for 10 min and then 60 cycles of 95°C for 30 s, 58°C for 10 s, 63°C for 30 s, and 72°C for 20 s. The efficacy of DNase treatment in the elimination of open circular and single-stranded forms of HBV DNA prior to PCR was confirmed by abrogation of the PCR amplification of HBV DNA extracted from serum viral particles by use of nonselective HBV-specific oligonucleotide primers targeting the HBs open reading frame. To normalize the number of viral genomes present in each liver sample, the number of haploid genomes was evaluated by using a β-globin gene kit (Roche DNA control kit; Roche Diagnostics). To evaluate intrahepatic concentrations of relaxed circular replicative DNA (rcDNA), cccDNA amounts were subtracted from total intracellular HBV DNA values.
Molecular analyses of HDV RNA from serum samples.
HDV RNA was extracted from 200 μl of serum by use of TRIzol reagent (Invitrogen, Paisley, Scotland) as recommended by the manufacturer and then was dissolved in 50 μl diethyl pyrocarbonate (DEPC)-treated water. Five microliters of extracted RNA was used as a template for first-strand cDNA synthesis by use of a Super Script reverse transcriptase kit (Invitrogen) and oligo(dT) primers. HDV genotype determination was performed through reverse transcription-PCR (RT-PCR) amplification and sequencing analysis of the R0 region of the HDV genome, which covers the 3′ end of the HD gene (nucleotides 885 to 1285 [numbering according to reference 33]), as previously described by Radjef et al. (17).
Primers and probes for HDV real-time PCR assays were designed by taking into account the genetic variability of HDV. The sequences and nucleotide positions of the primers and probes were as follows: DELTAF, 5′-AAGGGGGACTCCGGGACTC-3′ (nt 1063 to 1081); DELTAR, 5′-CCTCAGCAAGGAGGAAGAAGA-3′ (nt 1236 to 1216); DELTA/FL (FRET hybridization probe), 5′-CAGACTGGGGACGAAGCCGCCCC-FL-3′ (nt 1086 to 1108); and DELTA/LC (FRET hybridization probe), 5′-LC-R640-CGCTCCCCTCGATCCACCTTCGAGG-PH-3′ (nt 1113 to 1137). Real-time PCR by use of the “utility channel” of a Cobas TaqMan 48 instrument was performed under the following conditions: 95°C for 10 min and then 60 cycles of 95°C for 30 s, 57°C for 20 s, and 72°C for 20 s.
The plasmid pCRII-delta-R0, containing one copy of the R0 region of the HDV genome (nt 853 to 1308), was used as a standard for HDV cDNA quantification. The plasmid was digested with EcoRI (New England Biolabs GmbH, Frankfurt, Germany), and the R0 sequence was gel purified using the Wizard SV gel and PCR clean-up system (Promega, Madison, WI). The concentration of purified R0 DNA was determined with an ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE), and the corresponding copy number was calculated. A series of 10-fold dilutions of the plasmid pCRII-delta-R0 was used as a standard for HDV cDNA quantification. Serum samples from HDV-negative patients were analyzed as negative controls.
Quantification of HDV RNA and HBV RNA from liver specimens.
Five micrograms of extracted RNA was treated with RQ1 RNase-free DNase (Promega, Madison, WI) for 1 h at 37°C and then used as a template for first-strand cDNA synthesis with Super Script reverse transcriptase (Invitrogen) and oligo(dT) primers.
The same primers, hybridization probes, and reaction conditions utilized for serum HDV RNA quantification were used to quantify intrahepatic HDV RNA. Serial dilutions of the plasmid pCRII-delta-R0 served as quantification standards. Liver biopsy specimens from HDV-negative patients were analyzed as negative controls. The sequences and nucleotide positions of the primers and probes specific for pregenomic HBV RNA (pgRNA) were as follows: HBV7, 5′-CCTCACCATACTGCACTCA-3′ (nt 2048 to 2066); HBV8, 5′-GAGGGAGTTCTTCTTCTAGG-3′ (nt 2385 to 2366); CORE/FL (FRET hybridization probe), 5′-AGTGTGGATTCGCACTCCTCCAGC-FL-3′ (nt 1086 to 1108); and CORE/LC (FRET hybridization probe), 5′-LC-R640-ATAGACCACCAAATGCCCCTATCTTATCAAC-PH-3′ (nt 2295 to 2325). The same primers and probes designed for total HBV DNA quantification were used to evaluate total HBV RNA levels (corresponding to S [2.1 kb], pre-S [2.4 kb], and C-E or pregenome [3.5 kb] mRNA). For each liver biopsy specimen, the amount of pre-S/S RNA was estimated by subtracting the pgRNA quantity from the total HBV RNA amount. Serial dilutions of plasmid containing a monomeric HBV insert (Alfa Wasserman) were used as quantification standards for HBV reverse-transcribed pgRNA and total RNA. For RNA normalization, evaluation of the number of haploid genomes by use of a β-globin gene kit (Roche DNA control kit; Roche Diagnostics) allowed us to determine the cell number in the liver biopsy homogenate and, consequently, also in the aliquot used for RNA analysis.
Precision and reproducibility of real-time PCR assays.
A linear relationship from 1 × 101 to 1 × 107 copies/ml was obtained between cycle threshold values and numbers of HBV DNA or HDV cDNA copies used as standards. The correlation coefficient was repeatedly >0.99, and the slope was 3.5 for both HBV DNA and HDV cDNA standard curves. In order to assess intra-assay reproducibility, different approaches were applied. First, four replicates of 10-fold standard dilutions ranging from 1 to 1 × 108 copies per reaction were tested in the same experiment. This was performed both with HBV DNA and with HDV RNA standards, which yielded coefficients of variation (CVs) ranging from 0.5% to 1.7% and from 0.6% to 2.3%, respectively. Second, liver tissue specimens from 2 HDV-positive and 2 HDV-negative individuals were processed independently for DNA and RNA extraction, cDNA synthesis, and real-time PCR in the same experiment in triplicate. Quantification of total HBV DNA, HBV RNA, and HDV RNA in the liver yielded CVs ranging from 2.4 to 6.7, from 3.5 to 7.8, and from 4.5 to 7, respectively. Third, all patient samples were tested independently in duplicate for either HBV DNA, HBV RNA, or HDV RNA in all real-time PCR experiments. To assess interassay reproducibility, DNAs and RNAs extracted from serum and liver samples of 3 HDV-positive patients (1 with high and 2 with low HBV DNA levels in the serum) were tested in 5 independent experiments on 5 different days. Interassay coefficients of variation were always less than 10%.
HBV sequence analysis.
HBV genotypes and basal core promoter (BCP)/precore (PC) HBV genomic region analyses were performed by PCR amplification and subsequent direct sequencing as previously described (16).
Statistical analysis.
Statistical analysis was performed with the SPSS, version 13.0, software package (SPSS Inc., Chicago, IL). Medians and minimum and maximum values were calculated for numerical data, and percentages were computed for categorical data. A nonparametric approach was used to examine variables showing an absence of a normal distribution, as verified by the Kolmogorov-Smirnov test. In particular, the interdependence between numerical variables was determined by use of the Spearman rank correlation test, whereas the Mann-Whitney test was applied to perform comparisons of continuously distributed variables between 2 independent groups. To evaluate the association between categorical variables, the log-likelihood ratio test was applied. P values of <0.05 were considered statistically significant.
RESULTS
Patient characteristics.
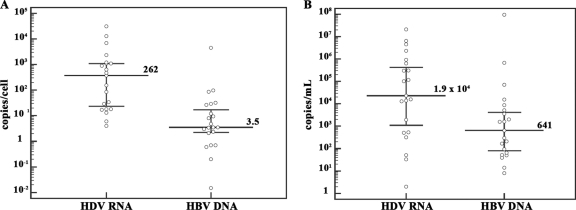
A total of 43 HBsAg-positive chronic hepatitis patients—21 of whom were coinfected with HDV—were studied. The characteristics of the patients are summarized in Table 1. Age and sex distributions within the groups of patients with and without HDV infection were similar. HBV genotype analysis showed that 20 of the 21 HDV-positive patients were infected with HBV genotype D and 1 was infected with genotype A, whereas among the 22 HDV-negative patients, 18 were infected with HBV genotype D, 3 were infected with genotype A, and 1 was infected with genotype C. No association was observed between HBV DNA levels and HBV genotypes. All 21 HDV-positive patients were infected with HDV genotype 1. Amounts of HDV RNA and HBV DNA in both sera and liver specimens from HDV-positive patients are reported in Fig. 1A and B. Finally, there was no significant difference between HDV-positive and HDV-negative patients in the degree of necroinflammation and fibrosis, according to Scheuer classification (25) (Table 1).
FIG. 1.
Amounts of HDV RNA and HBV DNA in liver and serum samples from HDV-positive patients. (A) Intrahepatic levels of HDV RNA and HBV DNA. (B) Serum levels of HDV RNA and HBV DNA. Median levels of HDV RNA and HBV DNA in the liver were 262 copies/cell (range, 4 to 3.1 × 105 copies/cell) and 3.5 copies/cell (range, 0.015 to 4.5 × 103 copies/cell), respectively, whereas median levels of HDV RNA and HBV DNA in serum were 1.9 × 104 copies/ml (range, 2 to 2.1 × 107 copies/ml) and 641 copies/ml (range, 70 to 9.4 × 107 copies/ml), respectively. Dots represent single patient measurements. The median is indicated with a long line, and the 25th and 75th percentiles are indicated with error bars.
Comparison between HDV-positive and HDV-negative patient groups.
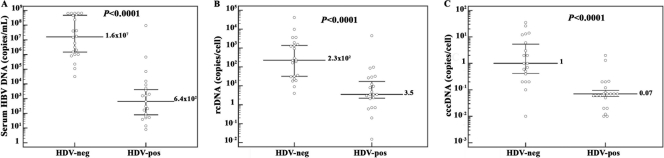
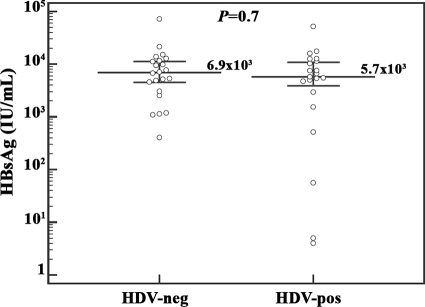
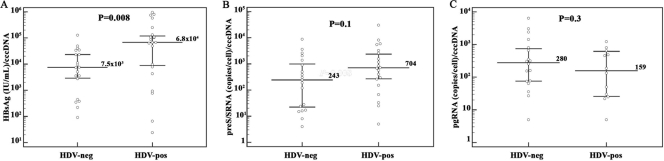
Real-time PCR analyses of paired serum and liver biopsy samples from each patient revealed that HDV-positive cases had significantly lower median levels of both serum HBV DNA (641 copies/ml; range, 70 to 9.4 × 107 copies/ml; P < 0.0001) and intrahepatic rcDNA (3.5 copies/cell; range, 0.015 to 4.5 × 103 copies/cell; P < 0.0001) than did HDV-negative cases (1.6 × 107 copies/ml of serum HBV DNA [range, 3.1 × 104 to 6.5 × 108 copies/ml] and 227 copies/cell of intrahepatic rcDNA [range, 4 to 4.1 × 104 copies/cell]) (Fig. 2A and B). Intracellular cccDNA amounts were also significantly lower in HDV-positive patients, with a median level of 0.07 copy/cell (range, 0.01 to 2 copies/cell; P < 0.0001), than in HDV-negative patients (median, 1 copy/cell; range, 0.01 to 35 copies/cell) (Fig. 2C). However, the evaluation of the ratio of cccDNA to intracellular total HBV DNA showed a higher, although not significant, proportion of cccDNA molecules in HDV-positive patients (2% versus 0.4%; P = 0.2), and median amounts of rcDNA produced per cccDNA molecule were 3-fold lower in HDV-positive subjects (median, 75 versus 245 rcDNA molecules/cccDNA molecule; P = 0.1). In addition, analysis of HBV transcription by transcript-specific real-time PCR revealed that HDV-positive patients had significantly lower median levels of both pgRNA (14.5 copies/cell; range, 0.5 to 2.4 × 103 copies/cell; P < 0.0001) and pre-S/S transcripts (62 copies/cell; range, 1 to 2.1 × 103 copies/cell; P = 0.03) than did HDV-negative patients (572 copies/cell of pgRNA [range, 11 to 3.4 × 104 copies/cell] and 400 copies/cell of pre-S/S transcripts). However, the evaluation of serum HBsAg by the Architect assay showed that HBsAg concentrations were comparable between HDV-positive and HDV-negative groups of patients (median, 5.7 × 103 versus 6.9 × 103 IU/ml; P = 0.7) (Fig. 3). In fact, HBsAg amounts per cccDNA molecule were significantly higher for HDV-positive patients (median, 6.8 × 104 IU/ml; range, 24 to 9.5 × 105 IU/ml; P = 0.008) than for HDV-negative patients (median, 7.5 × 103 IU/ml; range, 91 to 1.2 × 105 IU/ml) (Fig. 4A). To investigate whether the smaller amounts of serum HBV DNA, intrahepatic HBV replicative intermediates, and transcripts detected in HDV-positive patients were due to a reduced transcriptional activity of cccDNA molecules, ratios of pre-S/S RNA to cccDNA and pgRNA to cccDNA were evaluated for each patient. Interestingly, no statistically significant differences were found between concentrations of pre-S/S RNA and pgRNA produced per cccDNA molecule (704 versus 243 pre-S/S RNA molecules/cccDNA molecule [P = 0.1] and 159 versus 280 pgRNA molecules/cccDNA molecule [P = 0.3]) in HDV-positive and HDV-negative patients (Fig. 4B and C). In addition, although statistical significance was not achieved, it has to be pointed out that the ratio of pre-S/S RNA to cccDNA molecules was almost 3-fold higher in HDV-positive than in HDV-negative patients (Fig. 4B), justifying the significantly larger amounts of HBsAg per cccDNA molecule observed in HDV-positive patients. Finally, the evaluation of the ratio of pgRNA to total HBV RNA revealed a significantly smaller proportion of pregenome molecules (0.1% [range, 0.01% to 4%] versus 0.8% [range, 0.08% to 14%]; P = 0.02) in HDV-positive patients.
FIG. 2.
Serum and intrahepatic amounts of HBV DNA differ significantly between patients with and without HDV infection. Median levels of serum HBV DNA (A), intrahepatic HBV rcDNA (B), and HBV cccDNA (C) are shown for HDV-negative and HDV-positive patients. Dots represent single patient measurements. The median is indicated with a long line, and the 25th and 75th percentiles are indicated with error bars.
FIG. 3.
Median levels of HBsAg production in HDV-negative and HDV-positive patients. Dots represent single patient measurements. The median is indicated with a long line, and the 25th and 75th percentiles are indicated with error bars.
FIG. 4.
HBV cccDNA transcription and replicative activities appear to be disconnected in HDV-infected patients. Serum HBsAg (A), intrahepatic pre-S/S RNA (B), and pgRNA (C) concentrations were normalized to cccDNA amounts in the livers of corresponding patients in the HDV-negative or HDV-positive group. Dots represent single patient measurements. The median is indicated with a long line, and the 25th and 75th percentiles are indicated with error bars.
Correlations between different variables in HDV-positive and HDV-negative patients.
In HDV-positive patients, intrahepatic HDV RNA levels showed significant correlations with both serum HDV RNA (r = 0.569; P = 0.01) and HBV cccDNA (r = 0.595; P = 0.009) levels. However, no significant association was found between serum or intrahepatic HDV RNA concentrations and HBsAg levels, as well as serum and intrahepatic HBV DNA amounts. In addition, HBV DNA measured in the sera of these patients showed no correlation with concentrations of HBsAg (r = 0.329; P = 0.1), intrahepatic HBV DNA (r = 0.173; P = 0.4), or cccDNA (r = 0.205; P = 0.3), whereas a significant correlation was found between intrahepatic HBV DNA and cccDNA amounts (r = 0.438; P < 0.04). When the HDV-positive/HBeAg-negative subgroup of patients was analyzed separately, a significant correlation was found only between intrahepatic HBV DNA and cccDNA amounts (r = 0.577; P < 0.01). For the HDV-positive/HBeAg-positive subgroup, the correlation between variables could not be evaluated because of the small number of patients included in it.
For HDV-negative patients, highly significant correlations were found between amounts of cccDNA and both intrahepatic rcDNA levels (r = 0.76; P < 0.0001) and serum HBV DNA levels (r = 0.818; P < 0.0001) and between intrahepatic rcDNA and serum HBV DNA levels (r = 0.83; P < 0.0001). However, different results were obtained when the HBeAg-positive and HBeAg-negative patients of the HDV-negative group were evaluated separately. For both the HBeAg-positive and HBeAg-negative subgroups, a significant correlation was found only between intrahepatic rcDNA amounts and serum viral titers (r = 0.874 and P = 0.005 for the HBeAg-positive group and r = 0.732 and P = 0.003 for the HBeAg-negative group), and no correlation was found between cccDNA and either intrahepatic rcDNA levels (r = 0.595 and P = 0.1 for the HBeAg-positive group and r = 0.336 and P = 0.2 for the HBeAg-negative group) or serum viral titers (r = 0.659 and P = 0.07 for the HBeAg-positive group and r = 0.530 and P = 0.07 for the HBeAg-negative group). In addition, for all groups and subgroups of patients, we evaluated whether serum HBsAg concentrations correlated directly with intrahepatic amounts of HBV DNA. For both HDV-positive and HDV-negative groups of patients and their HBeAg-positive and HBeAg-negative subgroups, we found no correlation between HBsAg concentrations and either intrahepatic HBV DNA or cccDNA amounts. Comparisions between HBeAg-positive and HBeAg-negative subgroups of HDV-positive and HDV-negative patients are provided as supplimental material.
Analysis of HBV BCP and PC region variability.
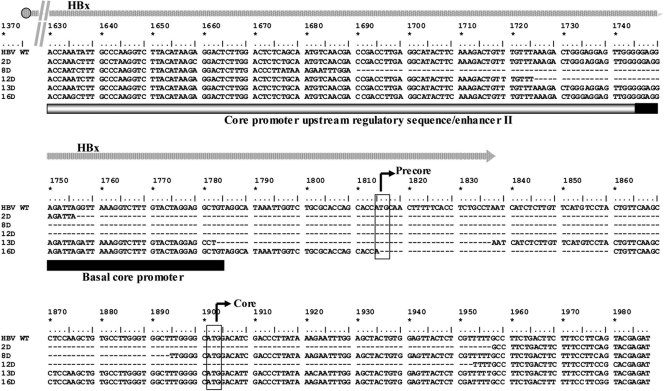
To evaluate whether mutations in the PC and BCP regions of HBV might have any influence on viral replication and transcription, cccDNA molecules from liver biopsy specimens were analyzed by direct sequencing. Of interest, 5 of the 21 (23.8%) HDV-positive and none of the HDV-negative patients (P = 0.01) carried major HBV populations with large deletions (ranging from 45 to 228 bp) in the BCP and PC regions (Fig. 5). The presence of such deletions was associated with lower viremia levels (r = −0.458; P = 0.04). However, the previously reported statistical significances obtained by comparing HDV-positive and HDV-negative patients were maintained when these 5 patients were excluded from the analysis. Among the remaining 16 HDV-positive patients, 5 (31%) were infected with HBV strains carrying the G1986A nucleotide substitution, introducing a stop codon in the PC region, and 4 of them also had a double BCP mutation at nucleotide positions 1762 and 1764. The presence of PC and/or BCP mutations showed no association with serum or intrahepatic HBV DNA levels. Major HBV populations from 12 (54%) of the 22 HDV-negative patients (2 HBeAg-positive and 10 HBeAg-negative patients) carried mutations in the BCP/PC regions. Notably, the presence of such mutations in the HBeAg-negative subgroup was associated with higher HBV DNA amounts in both serum (r = 0.808; P = 0.003) and the liver (r = 0.866; P = 0.001).
FIG. 5.
Alignment of HBV DNA nucleotide sequences corresponding to the basal core promoter region and precore region for wild-type (WT) HBV genotype D and the dominant viral populations of 5 HDV-infected patients (2D, 8D, 12D, 13D, and 16D). The transcription initiation sites of the precore and core genes are indicated by black arrows. The structures of the core promoter and enhancer II are indicated by open and filled boxes. Deletions are indicated by hyphens. The overlapping region of the HBx open reading frame is indicated by a gray arrow. Numbering is according to the work of Galibert et al. (7).
DISCUSSION
It is estimated that about 15 to 20 million of the 400 million HBsAg carriers worldwide are coinfected with HDV, and most of these patients suffer from severe and evolving forms of liver diseases (21, 35). At present, only interferon-based therapies are licensed for chronic HDV hepatitis treatment, but these approaches achieve the objective of curing the infection (namely, HDV clearance) in only a very few cases (5). Chronic hepatitis D is thus considered a nearly incurable liver disease. Moreover, since HDV does not produce its own enzymatic proteins (21, 30), there is no room for typical antiviral therapeutic approaches based on the use of specific inhibitors of viral enzymes (20). Natural recovery from HDV infection is a rare event, usually occurring only in the event of HBsAg seroclearance (21, 35), thus confirming that studying the mechanisms of HBV and HDV interplay may have great importance not only from the virological point of view but also in identifying new methods for the cure of HDV-related diseases.
Very few data are currently available on intrahepatic HBV and HDV molecular status for coinfected patients (2, 12), and most of the existing evidence derives from experiments with animal models (1, 8, 15, 29). In this study, we applied sensitive molecular assays to evaluate serologic and intrahepatic quantitative profiles of HBV and HDV in chronic infections. In accordance with previous studies (9, 10, 23, 40), our results seem to confirm that HDV exerts a dominant role over HBV, as indicated by its higher levels of replication and by the lower levels of serum and intrahepatic HBV DNA, cccDNA, and HBV transcripts detected in the majority of coinfected patients than those in HBV-monoinfected individuals. However, in agreement with previously reported data, we found that serum HBsAg levels were comparable between HDV-positive and HDV-negative patients (8) and that amounts of pre-S/S RNAs and HBsAg produced per cccDNA molecule were higher in HDV-positive patients. Considering that HDV depends on HBV only to acquire HBsAg and that HDV nucleoproteins may be considered competitors of replicating HBV genomes (21, 35), our findings support the hypothesis that HDV might be able to induce an opposing effect on HBV replication and on HBV transcription (3, 13). In fact, our results showing a significantly smaller proportion of pgRNA among HBV transcripts imply that the reduction of virion production might be due mainly to a selective suppression of pgRNA transcription, thus suggesting a kind of dissociation between pgRNA and pre-S/S RNA production in cases of HDV coinfection. This hypothesis is biologically plausible, since the transcription of pgRNA and that of pre-S/S RNAs are under the control of two different HBV genomic regulatory regions (14, 26). However, the molecular mechanisms possibly implicated in the discrepancy between pgRNA and pre-S/S RNA steady-state levels are unknown, and one cannot exclude that the discrepancy might be dependent on differences in transcript stabilities. During the course of chronic HBV infection, the emergence and selection of viral variants mutated at the PC and/or BCP region which are able to affect HBV gene expression and replication are quite frequent occurrences (11, 31, 32). To evaluate whether these HBV variants might be involved in the impairment of HBV virion production in HDV patients, we investigated BCP/PC genomic region variability at the cccDNA level. We found that one-fourth of our HDV-positive patients were infected with HBVs carrying large deletions in the BCP/PC region, and the presence of these deletions was associated with lower levels of HBV DNA in both serum and the liver. These deletions might deeply impair pgRNA transcription and HBV replication, and future studies on larger numbers of patients, together with in vitro functional analyses of the deleted HBV strains, will verify the importance of the selection of these HBV mutants in HDV coinfection. Notably, HBV isolates carrying the frequently occurring point mutations at nucleotide positions 1762, 1764, and 1896 in the BCP/PC region were significantly associated with higher viremia levels in HDV-negative/HBeAg-negative patients than in HDV-positive/HBeAg-negative ones, further confirming that HDV may be able to overcome some of the molecular mechanisms regulating HBV activity.
Concerning the large amounts of HBsAg production in HDV-positive patients, previous reports suggested that a casual integration into the host genome of the HBV region coding for the envelope proteins may occur, establishing an independent source of HBsAg production (6, 34). However, studies on the frequency and molecular characteristics of HBV integration in HDV patients have never been performed so far, and in any case, this hypothetical integration might account for the high levels of HBsAg only in the case of a clonal expansion of the hepatocytes containing such integrants, a condition usually occurring in tumoral lesions, not in chronic hepatitis.
A few in vitro studies on HDV/HBV interference have been performed so far, and they showed that the small delta antigen dramatically reduces the expression of HBV 3.5- and 2.1-kb RNAs and suppresses HBV virion production (38). Very recently, it was demonstrated that both small and large HDV proteins are able to inhibit HBV activities, through a strong repression of HBV enhancers, and that the large protein transactivates the alpha interferon-inducible MxA gene, which encodes a protein known to inhibit HBV replication (37). However, none of the available evidence sheds any light on the mechanisms allowing the small number of HBV cccDNA molecules detected in HDV coinfection to synthesize amounts of envelope proteins comparable to those produced in HBV monoinfection. Whatever the molecular events implicated, the discrepancy between HBV production and HBsAg synthesis turns to HDV's advantage, since it can secure sufficient amounts of envelope proteins for virion assembly and release and for propagation of the infection.
As expected, we found a significant association between serum and intrahepatic HDV RNA quantities. Of interest, we also observed a direct significant correlation between both serum and intrahepatic HDV RNA and HBV cccDNA amounts but no correlation between HDV RNA and HBV DNA concentrations, at both the serum and intrahepatic levels. Considering that HDV does not depend on HBV to replicate in host cells (30), these results tempt us to speculate that the direct correlation between HDV RNA and HBV cccDNA might be a consequence of the sequestration of envelope proteins by HDV, which by hindering envelopment of HBV DNA-containing nucleocapsids might favor their recycling into the nuclei of hepatocytes.
In contrast with previous reports (13, 27, 28, 40), we did not find any correlation between HBsAg and HDV RNA serum amounts. However, Shih et al. (28) clearly reported that this correlation exists mainly for patients with undetectable serum HBV DNA, not for patients with actively replicating HBV. In fact, all of our HDV-positive patients had circulating viral DNA, and half of them showed viremia levels of >2,000 copies/ml. Interestingly, recent longitudinal studies have demonstrated that the two viruses frequently show complex, dynamic replicative profiles (18, 24) and that circulating amounts of HBsAg and HDV RNA may fluctuate over time, independent of each other (24). The fluctuation patterns, together with the known large differences in stability and half-life for HBsAg and HDV RNA (3, 4, 15, 18, 24), may also explain the lack of correlation between them.
All of the results we obtained by comparing HDV-positive and HDV-negative patients were substantially confirmed when the same evaluations were performed by the comparison of HBeAg-positive and HBeAg-negative subgroups of both HDV-positive and HDV-negative patients. Incidentally, in contrast with previous reports (11, 32), we found that steady-state levels of pgRNA and pre-S/S RNAs, as well as synthesis of envelope proteins, were not reduced in HBeAg-negative individuals of the HDV-negative group. In particular, we detected significantly larger amounts of HBsAg per cccDNA molecule in these patients than in HBeAg-positive patients. Such discrepancies with previous reports could be imputable to possible differences in the virologic characteristics of the study populations, considering that all of our HBeAg-negative patients had highly active HBV infections, as also demonstrated by their median HBV DNA levels in the serum and in the liver, which were both higher than those reported in the above-mentioned previous studies (11, 32).
In conclusion, our study provides new information on the “smart” mode of inhibition of HBV activities exerted by HDV, which is apparently able to provoke a selective suppression of HBV functions, with maintenance of the capacity to synthesize large amounts of envelope proteins necessary for the formation of HDV virions and for the propagation of the infection.
However, regardless of HDV's ability to strongly suppress HBV, in no case does it allow HBV infection to enter into its “occult” phase, since this condition—characterized by a deep suppression of both HBV replication and S gene expression (19)—is disadvantageous for HDV itself.
Considering that direct anti-HDV therapeutic approaches appear far from being identified, anti-HBV drugs are generally considered an indirect solution for the cure of HDV-related diseases. Unfortunately, however, the currently available anti-HBV treatments [nucleos(t)ide analogs and alpha interferon] have no or very little effect on HBV S gene expression and intracellular cccDNA contents, and consequently, they are largely ineffective against HDV hepatitis (5, 20, 34). To reach the goal of HBV eradication, new drugs able to prevent reinfection of hepatocytes or inducing depletion of cccDNA must be produced, and these drugs would also be a true solution for HDV infection.
Supplementary Material
Acknowledgments
This work was supported by grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC), the Ministero Italiano dell'Istruzione Università e Ricerca (MIUR), and the Italian Progetti di Ricerca di Interesse Nazionale del Ministero dell'Istruzione, dell'Universita e della Ricerca.
We are grateful to John Taylor for helpful scientific discussions and precious technical advice.
Footnotes
Published ahead of print on 20 October 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Bordier, B. B., J. Ohkanda, P. Liu, S. Y. Lee, F. H. Salazar, P. L. Marion, K. Ohashi, L. Meuse, M. A. Kay, J. L. Casey, S. M. Sebti, A. D. Hamilton, and J. S. Glenn. 2003. In vivo antiviral efficacy of prenylation inhibitors against hepatitis delta virus. J. Clin. Invest. 112:407-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, P. J., P. M. Yang, C. R. Chen, and D. S. Chen. 1989. Characterization of the transcripts of hepatitis D and B viruses in infected human livers. J. Infect. Dis. 160:944-947. [DOI] [PubMed] [Google Scholar]
- 3.Chulanov, V. P., G. A. Shipulin, S. Schaefer, and W. H. Gerlich. 2003. Kinetics of HBV DNA and HBsAg in acute hepatitis B patients with and without coinfection by other hepatitis viruses. J. Med. Virol. 69:313-323. [DOI] [PubMed] [Google Scholar]
- 4.Dandri, M., J. M. Murray, M. Lutgehetmann, T. Volz, A. W. Lohse, and J. Petersen. 2008. Virion half-life in chronic hepatitis B infection is strongly correlated with levels of viremia. Hepatology 48:1079-1086. [DOI] [PubMed] [Google Scholar]
- 5.Farci, P., L. Chessa, C. Balestrieri, G. Serra, and M. E. Lai. 2007. Treatment of chronic hepatitis D. J. Viral Hepat. 14(Suppl. 1):58-63. [DOI] [PubMed] [Google Scholar]
- 6.Feitelson, M. A., and J. Lee. 2007. Hepatitis B virus integration, fragile sites, and hepatocarcinogenesis. Cancer Lett. 252:157-170. [DOI] [PubMed] [Google Scholar]
- 7.Galibert, F., E. Mandart, F. Fitoussi, P. Tiollais, and P. Charnay. 1979. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature 281:646-650. [DOI] [PubMed] [Google Scholar]
- 8.Gerin, J. L. 2001. Animal models of hepatitis delta virus infection and disease. ILAR J. 42:103-106. [DOI] [PubMed] [Google Scholar]
- 9.Heidrich, B., K. Deterding, H. L. Tillmann, R. Raupach, M. P. Manns, and H. Wedemeyer. 2009. Virological and clinical characteristics of delta hepatitis in Central Europe. J. Viral Hepat. 16:883-894. [DOI] [PubMed] [Google Scholar]
- 10.Jardi, R., F. Rodriguez, M. Buti, X. Costa, M. Cotrina, R. Galimany, R. Esteban, and J. Guardia. 2001. Role of hepatitis B, C, and D viruses in dual and triple infection: influence of viral genotypes and hepatitis B precore and basal core promoter mutations on viral replicative interference. Hepatology 34:404-410. [DOI] [PubMed] [Google Scholar]
- 11.Laras, A., J. Koskinas, E. Dimou, A. Kostamena, and S. J. Hadziyannis. 2006. Intrahepatic levels and replicative activity of covalently closed circular hepatitis B virus DNA in chronically infected patients. Hepatology 44:694-702. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Talavera, J. C., M. Buti, J. Casacuberta, H. Allende, R. Jardi, R. Esteban, and J. Guardia. 1993. Detection of hepatitis delta virus RNA in human liver tissue by non-radioactive in situ hybridization. J. Hepatol. 17:199-203. [DOI] [PubMed] [Google Scholar]
- 13.Manesis, E. K., M. Schina, F. Le Gal, O. Agelopoulou, C. Papaioannou, C. Kalligeros, V. Arseniou, S. Manolakopoulos, E. S. Hadziyannis, E. Gault, J. Koskinas, G. Papatheodoridis, and A. J. Archimandritis. 2007. Quantitative analysis of hepatitis D virus RNA and hepatitis B surface antigen serum levels in chronic delta hepatitis improves treatment monitoring. Antivir. Ther. 12:381-388. [PubMed] [Google Scholar]
- 14.Nassal, M. 2008. Hepatitis B viruses: reverse transcription a different way. Virus Res. 134:235-249. [DOI] [PubMed] [Google Scholar]
- 15.Netter, H. J., K. Kajino, and J. M. Taylor. 1993. Experimental transmission of human hepatitis delta virus to the laboratory mouse. J. Virol. 67:3357-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollicino, T., G. Raffa, L. Costantino, A. Lisa, C. Campello, G. Squadrito, M. Levrero, and G. Raimondo. 2007. Molecular and functional analysis of occult hepatitis B virus isolates from patients with hepatocellular carcinoma. Hepatology 45:277-285. [DOI] [PubMed] [Google Scholar]
- 17.Radjef, N., E. Gordien, V. Ivaniushina, E. Gault, P. Anais, T. Drugan, J. C. Trinchet, D. Roulot, M. Tamby, M. C. Milinkovitch, and P. Deny. 2004. Molecular phylogenetic analyses indicate a wide and ancient radiation of African hepatitis delta virus, suggesting a deltavirus genus of at least seven major clades. J. Virol. 78:2537-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raimondo, G., M. R. Brunetto, P. Pontisso, A. Smedile, A. M. Maina, C. Saitta, G. Squadrito, and N. Tono. 2006. Longitudinal evaluation reveals a complex spectrum of virological profiles in hepatitis B virus/hepatitis C virus-coinfected patients. Hepatology 43:100-107. [DOI] [PubMed] [Google Scholar]
- 19.Raimondo, G., T. Pollicino, I. Cacciola, and G. Squadrito. 2007. Occult hepatitis B virus infection. J. Hepatol. 46:160-170. [DOI] [PubMed] [Google Scholar]
- 20.Rizzetto, M. 2010. Hepatitis D: clinical features and therapy. Dig. Dis. 28:139-143. [DOI] [PubMed] [Google Scholar]
- 21.Rizzetto, M. 2009. Hepatitis D: thirty years after. J. Hepatol. 50:1043-1050. [DOI] [PubMed] [Google Scholar]
- 22.Romeo, R., E. Del Ninno, M. Rumi, A. Russo, A. Sangiovanni, R. de Franchis, G. Ronchi, and M. Colombo. 2009. A 28-year study of the course of hepatitis delta infection: a risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology 136:1629-1638. [DOI] [PubMed] [Google Scholar]
- 23.Sakugawa, H., H. Nakasone, T. Nakayoshi, Y. Kawakami, T. Yamashiro, T. Maeshiro, F. Kinjo, A. Saito, and H. Zukeran. 2001. Hepatitis B virus concentrations in serum determined by sensitive quantitative assays in patients with established chronic hepatitis delta virus infection. J. Med. Virol. 65:478-484. [PubMed] [Google Scholar]
- 24.Schaper, M., F. Rodriguez-Frias, R. Jardi, D. Tabernero, M. Homs, G. Ruiz, J. Quer, R. Esteban, and M. Buti. 2010. Quantitative longitudinal evaluations of hepatitis delta virus RNA and hepatitis B virus DNA shows a dynamic, complex replicative profile in chronic hepatitis B and D. J. Hepatol. 52:658-664. [DOI] [PubMed] [Google Scholar]
- 25.Scheuer, P. J. 1991. Classification of chronic viral hepatitis: a need for reassessment. J. Hepatol. 13:372-374. [DOI] [PubMed] [Google Scholar]
- 26.Seeger, C., and W. S. Mason. 2000. Hepatitis B virus biology. Microbiol. Mol. Biol. Rev. 64:51-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheldon, J., B. Ramos, C. Toro, P. Rios, J. Martinez-Alarcon, M. Bottecchia, M. Romero, J. Garcia-Samaniego, and V. Soriano. 2008. Does treatment of hepatitis B virus (HBV) infection reduce hepatitis delta virus (HDV) replication in HIV-HBV-HDV-coinfected patients? Antivir. Ther. 13:97-102. [PubMed] [Google Scholar]
- 28.Shih, H. H., K. S. Jeng, W. J. Syu, Y. H. Huang, C. W. Su, W. L. Peng, I. J. Sheen, and J. C. Wu. 2008. Hepatitis B surface antigen levels and sequences of natural hepatitis B virus variants influence the assembly and secretion of hepatitis D virus. J. Virol. 82:2250-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sureau, C., J. Taylor, M. Chao, J. W. Eichberg, and R. E. Lanford. 1989. Cloned hepatitis delta virus cDNA is infectious in the chimpanzee. J. Virol. 63:4292-4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor, J. M. 2006. Hepatitis delta virus. Virology 344:71-76. [DOI] [PubMed] [Google Scholar]
- 31.Tong, S., K. H. Kim, C. Chante, J. Wands, and J. Li. 2005. Hepatitis B virus e antigen variants. Int. J. Med. Sci. 2:2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volz, T., M. Lutgehetmann, P. Wachtler, A. Jacob, A. Quaas, J. M. Murray, M. Dandri, and J. Petersen. 2007. Impaired intrahepatic hepatitis B virus productivity contributes to low viremia in most HBeAg-negative patients. Gastroenterology 133:843-852. [DOI] [PubMed] [Google Scholar]
- 33.Wang, K. S., Q. L. Choo, A. J. Weiner, J. H. Ou, R. C. Najarian, R. M. Thayer, G. T. Mullenbach, K. J. Denniston, J. L. Gerin, and M. Houghton. 1986. Structure, sequence and expression of the hepatitis delta viral genome. Nature 323:508-514. [DOI] [PubMed] [Google Scholar]
- 34.Wedemeyer, H. 2010. Re-emerging interest in hepatitis delta: new insights into the dynamic interplay between HBV and HDV. J. Hepatol. 52:627-629. [DOI] [PubMed] [Google Scholar]
- 35.Wedemeyer, H., and M. P. Manns. 2010. Epidemiology, pathogenesis and management of hepatitis D: update and challenges ahead. Nat. Rev. Gastroenterol. Hepatol. 7:31-40. [DOI] [PubMed] [Google Scholar]
- 36.Werle-Lapostolle, B., S. Bowden, S. Locarnini, K. Wursthorn, J. Petersen, G. Lau, C. Trepo, P. Marcellin, Z. Goodman, W. E. T. Delaney, S. Xiong, C. L. Brosgart, S. S. Chen, C. S. Gibbs, and F. Zoulim. 2004. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology 126:1750-1758. [DOI] [PubMed] [Google Scholar]
- 37.Williams, V., S. Brichler, N. Radjef, P. Lebon, A. Goffard, D. Hober, R. Fagard, D. Kremsdorf, P. Deny, and E. Gordien. 2009. Hepatitis delta virus proteins repress hepatitis B virus enhancers and activate the alpha/beta interferon-inducible MxA gene. J. Gen. Virol. 90:2759-2767. [DOI] [PubMed] [Google Scholar]
- 38.Wu, J. C., P. J. Chen, M. Y. Kuo, S. D. Lee, D. S. Chen, and L. P. Ting. 1991. Production of hepatitis delta virus and suppression of helper hepatitis B virus in a human hepatoma cell line. J. Virol. 65:1099-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu, J. C., T. Z. Chen, Y. S. Huang, F. S. Yen, L. T. Ting, W. Y. Sheng, S. H. Tsay, and S. D. Lee. 1995. Natural history of hepatitis D viral superinfection: significance of viremia detected by polymerase chain reaction. Gastroenterology 108:796-802. [DOI] [PubMed] [Google Scholar]
- 40.Zachou, K., C. Yurdaydin, U. Drebber, G. N. Dalekos, A. Erhardt, Y. Cakaloglu, H. Degertekin, S. Gurel, S. Zeuzem, H. Bozkaya, V. Schlaphoff, H. P. Dienes, T. C. Bock, M. P. Manns, and H. Wedemeyer. 2010. Quantitative HBsAg and HDV-RNA levels in chronic delta hepatitis. Liver Int. 30:430-437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.