Abstract
Influenza A viruses are human and animal pathogens that cause morbidity and mortality, which range from mild to severe. The 2009 H1N1 pandemic was caused by the emergence of a reassortant H1N1 subtype (H1N1pdm) influenza A virus containing gene segments that originally circulated in human, avian, and swine virus reservoirs. The molecular determinants of replication and pathogenesis of H1N1pdm viruses in humans and other mammals are poorly understood. Therefore, we set out to elucidate viral determinants critical to the pathogenesis of this novel reassortant using a mouse model. We found that a glutamate-to-glycine substitution at residue 158 of the PB2 gene (PB2-E158G) increased the morbidity and mortality of the parental H1N1pdm virus. Results from mini-genome replication assays in human cells and virus titration in mouse tissues demonstrated that PB2-E158G is a pathogenic determinant, because it significantly increases viral replication rates. The virus load in PB2-E158G-infected mouse lungs was 1,300-fold higher than that of the wild-type virus. Our data also show that PB2-E158G had a much stronger influence on the RNA replication and pathogenesis of H1N1pdm viruses than PB2-E627K, which is a known pathogenic determinant. Remarkably, PB2-E158G substitutions also altered the pathotypes of two avian H5 viruses in mice, indicating that this residue impacts genetically divergent influenza A viruses and suggesting that this region of PB2 could be a new antiviral target. Collectively, the data presented in this study demonstrate that PB2-E158G is a novel pathogenic determinant of influenza A viruses in the mouse model. We speculate that PB2-E158G may be important in the adaptation of avian PB2 genes to other mammals, and BLAST sequence analysis identified a naturally occurring human H1N1pdm isolate that has this substitution. Therefore, future surveillance efforts should include scrutiny of this region of PB2 because of its potential impact on pathogenesis.
Influenza A viruses are important human and animal pathogens that cause disease that ranges from relatively benign subclinical infections to severe infections that result in very high mortality. Influenza A viruses have a segmented genome composed of eight negative-sense single-stranded RNA molecules (vRNAs), and they infect multiple species, including avian, swine, equine, and canine species; marine mammals; and humans (33, 43). The segmented RNA genome, interspecies transmission, and coinfections enable the generation of completely new reassortant viruses that have pandemic potential.
The pathogenesis of influenza A viruses is a polygenic trait, and understanding the molecular mechanisms important to host range and pathogenesis are critical for prevention and treatment of influenza A virus infections. The viral RNA-dependent RNA polymerase (RDRP), which is a heterotrimer formed by the PB1, PB2, and PA subunits, is a major determinant of species specificity, transmission, and pathogenesis. Factors that increase RDRP activity and virus replication efficiency in mammals, such as a glutamate-to-lysine substitution at residue 627 (E627K) or an aspartic acid-to-asparagine substitution at residue 701 (D701N) in the PB2 subunit, facilitate the adaptation of H5N1 and other avian viruses to mammals and increase transmission and/or pathogenesis in humans, mice, ferrets, and guinea pigs (3, 5-8, 10, 13, 14, 22-24, 29, 32, 35-38, 45).
The 2009 influenza A virus pandemic was caused by a novel quadruple-reassortant H1N1 virus (H1N1pdm) containing gene segments that originally circulated in human, avian, and swine virus reservoirs. For example, the RDRP gene segments are derived from human (PB1) and avian (PB2 and PA) lineage viruses. This novel virus was transmitted efficiently from person to person and was more pathogenic than the seasonal influenza viruses in the naïve pediatric population and in experimental animals (2, 11, 18, 25, 27, 31); however, the molecular mechanisms involved in the transmission and pathogenesis of the H1N1pdm viruses in humans and other mammals are poorly understood.
Mice are an excellent small-animal model for the study of influenza A viruses, and they are particularly useful for identifying pathogenic determinants of avian, human, and other mammalian lineage viruses (13, 26-28). To gain a better understanding of viral determinants critical to the pathogenesis of this new human pathogen in a mammalian model, we serially passaged a low-pathogenic, plasmid-derived H1N1pdm virus in mice. We hypothesized that serial passage would result in the purifying selection of a variant(s) that was highly pathogenic for this host and identify molecular determinants that are important in the pathogenesis of H1N1pdm viruses in mice and potentially in humans. We obtained a virus that was highly pathogenic for mice, and genomic analysis identified a novel amino acid substitution in the PB2 gene that increased the pathogenicity of the H1N1pdm virus. Furthermore, when introduced into two unrelated avian H5 viruses with high and low pathogenicity in chickens, this mutation also substantially increased the mortality resulting from infection by the two viruses in mice. In vitro and in vivo analyses demonstrated that this PB2 mutation significantly enhanced RNA polymerase activity and viral replication.
MATERIALS AND METHODS
Biosafety and animal care.
All experiments with infectious virus were performed using procedures and facilities that met or exceeded the requirements set forth by the U.S. Department of Health and Human Services for propagation of influenza A viruses or their use in animals. All experiments involving avian H5 influenza A viruses were conducted using enhanced biosafety level 3 practices. Experiments involving animals were performed in biosafety level 3 or enhanced biosafety level 3 containment laboratories approved for such use by the Centers for Disease Control and Prevention and the U.S. Department of Agriculture and were conducted under approved animal care and use protocols at the Wadsworth Center, New York State Department of Health (NYSDOH). All experiments were conducted in compliance with the requirements of federal and state regulatory agencies, and animal experiments used husbandry and procedures to limit discomfort, distress, pain, or injury.
Cell culture.
Human embryonic kidney 293T (HEK-293T) cells and mouse rectum epithelial carcinoma (CMT-93) cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Madin-Darby canine kidney (MDCK) cells were maintained in Eagle's minimum essential medium (EMEM) supplemented with 10% FBS. Human lung epithelial (Calu-3) cells were maintained in EMEM supplemented with 5% FBS, 1% nonessential amino acids, and 1 mM sodium pyruvate.
MDCK, CMT-93, and Calu-3 cells were obtained from the American Type Culture Collection, Rockville, MD. HEK-293T cells were kindly provided by Yoshihiro Kawaoka (University of Wisconsin, Madison, WI).
Serial passage in mice.
A recombinant wild-type (WT) pandemic H1N1 influenza A virus, A/New York/1682/2009 (rNY1682-WT), was created by reverse genetics using plasmids that corresponded to the consensuses sequence obtained from a human swab specimen collected in New York State in April 2009 (46). Five-week-old female BALB/cJ mice (Jackson Laboratory, Bar Harbor, ME) were separated into microisolator cages to acclimate them to the ABSL3 laboratory for 6 to 14 days prior to the studies. Two 6-week-old female BALB/cJ mice were anesthetized with isoflurane and inoculated intranasally with 104 50% tissue culture infective doses (TCID50) of rNY1682-WT in 50 μl of phosphate-buffered saline (PBS) diluent. Four days postinoculation (p.i.), the lungs were collected and homogenized, and aliquots were pooled, diluted 1:100, and used to inoculate two 6- to 8-week-old naïve BALB/cJ mice. This procedure was repeated for 6 passages to obtain NY1682-MAP7. NY1682-MAP7 was propagated in MDCK cells to obtain working stocks for sequencing and animal studies.
Sequencing of the NY1682-MAP7 virus.
Viral RNA was extracted from supernatant containing NY1682-MAP7 and used as a template for genomic amplification using multisegment reverse transcription (RT)-PCR (46). The genomic amplicons were sequenced, and adaptive mutations resulting from serial passage were identified by comparing the consensus NY1682-MAP7 sequence to the sequences of the various plasmids used to create the rNY1682 virus.
Generation of recombinant viruses.
Mutations were introduced into the NY1682 PB2 plasmid to generate the two mutant PB2 segments PB2-E158G and PB2-E627K. The recombinant viruses rNY1682-WT, rNY1682-E158G, and rNY1682-E627K were generated by cotransfection of eight reverse-genetics plasmids containing the double-stranded DNA (dsDNA) representing each gene segment into HEK-293T/MDCK cocultured monolayers adapted from the method of Hoffmann et al. (16). Briefly, 0.6 μg of plasmid for each gene segment was mixed and incubated with 15 μl of Lipofectamine 2000 (Invitrogen, Carlsbad, CA) at 20°C for 20 min. The Lipofectamine-DNA mixture was transferred to 90% confluent 293T/MDCK cell monolayers in a 35-mm tissue culture dish and incubated at 33°C with 5% CO2 for 8 h. The transfection supernatant was replaced with 3 ml of Opti-Mem I medium (Invitrogen) supplemented with 0.3% bovine serum albumin (BSA) fraction V (Invitrogen), 3 μg/ml tosylsulfonyl phenylalanyl chloromethyl ketone (TPCK)-trypsin (Worthington, Lakewood, NJ), and 1% antibiotic-antimycotic (Invitrogen). Three days posttransfection, the supernatant was collected and viruses were propagated in MDCK cells at 33°C. Titers of the viruses used in this study were determined by TCID50 in MDCK cells. The recombinant A/Turkey/Ontario/7732/1966 viruses (rTy/Ont-WT and rTy/Ont-E158G) and A/Mallard/Wisconsin/944/1982 viruses (rMal/WI-WT and rMal/WI-E158G) were generated similarly, except that the transfection supernatants were directly injected into 10-day-old embryonated eggs for virus isolation, and they were subsequently propagated in 10-day-old embryonated eggs to obtain the P1 stock used in this study.
Replication kinetics in vitro.
MDCK cell monolayers in 12-well plates were washed twice with PBS, and then 2 ml of virus growth medium (VGM) was added to each well. The cells were inoculated at a multiplicity of infection (MOI) of 0.01 TCID50/cell with recombinant NY1682 WT, E158G, and E627K viruses. Supernatants were collected at 2, 12, 24, 48, and 72 h p.i. Infections of Calu-3 and CMT-93 cells were performed similarly, except that an MOI of 0.02 TCID50/cell was used for Calu-3 cells. The VGM used for MDCK and CMT-93 cells was EMEM supplemented with 0.15% BSA fraction V, 2 μg/ml TPCK-trypsin, and 1% antibiotic-antimycotic, and the VGM used for Calu-3 cells was EMEM supplemented with 0.3% BSA fraction V, 1 μg/ml TPCK-trypsin, and 1% antibiotic-antimycotic.
Mini-genome replication assay.
To construct the pPolI-NS-Luc plasmid (pBZ81A36), primers tailed with the NY1682 NS noncoding terminal sequences were used to amplify the luciferase gene from the pGL4.33 vector (Promega, Madison, WI), which contains a destabilized firefly luciferase for improved temporal response. The NS-Luc cDNA was cloned into the recombination-based influenza virus reverse-genetics plasmid pG26A12 (46) between the RNA polymerase I promoter and terminator for expression of vRNA-like negative-sense luciferase RNA, which can be transcribed into mRNA and replicated (producing cRNA and vRNA) by viral RDRP. HEK-293T cells in 24-well plates were cotransfected with 0.2 μg each of pBZ81A36 and plasmids to express the NY1682 PB2 (PB2-WT, PB2-E158G, or PB2-E627K), PB1, PA, and NP proteins, and to control for transfection efficiency, 0.02 μg of the Renilla luciferase plasmid pRL-TK (Promega) was also cotransfected. Eighteen hours posttransfection, luciferase production was assayed using the dual-luciferase reporter assay system (Promega) according to the manufacturer's instructions. Firefly luciferase expression was normalized to Renilla luciferase expression (relative luciferase activity). The PB2-WT luciferase activity was set at 100%, and the activities of E158G and D627K were determined relative to that of the WT. All results shown are the averages from triplicate experiments, and the standard deviation was calculated and is shown.
Mouse studies.
To determine morbidity and mortality, groups of 6-week-old female BALB/cJ mice were anesthetized with isoflurane and inoculated intranasally with the recombinant viruses at the indicated doses in 50 μl of EMEM diluent or mock inoculated with EMEM to serve as controls. Body weight was measured daily for 14 days, and clinical observations were recorded. Mice that lost more than 25% of their original weight were euthanized for humane reasons.
To determine virus replication in the lungs and nasal airways of infected mice, 6-week-old mice were intranasally inoculated with 103 TCID50 of the indicated viruses, and three mice in each group were euthanized at 12, 24, 48, and 96 h postinoculation. Lungs were removed and homogenized using a 5-mm stainless steel BB in a Tissue Lyser II (Qiagen, Valencia, CA) in 1 ml of virus collection medium (VCM) (EMEM supplemented with 0.15% BSA fraction V and 1% antibiotic-antimycotic). Nasal washes were collected by insertion of a 26-gauge needle into the trachea and washing of the nasal turbinates with 1 ml of VCM, which was collected from the nostrils. Virus titers in the lungs and nasal washes were determined by TCID50 assay.
Statistical analysis.
Weight loss/morbidity and virus titers in supernatants and tissues were analyzed using Fisher (F) tests to compare trajectories estimated by classical least squares from all of the data points (graphs of data and the estimated curves used for analysis are not shown). For simple comparisons, Student's t test was used to examine the significance of differences observed, and the specific parameters used are indicated in the text.
Nucleotide sequence accession numbers.
The consensus sequence of each of the gene segments was deposited in GenBank as A/New York/WC3-MA7/2009(H1N1), which we called NY1682-MAP7 to simplify the nomenclature in this study. The accession numbers of the gene segments are as follows: PB2, CY054706; PB1, CY054705; PA, CY054704; hemagglutinin (HA), CY054699; NP, CY054702; NA, CY054701; M, CY054700; and NS, CY054703.
RESULTS
Serial passage generated a pandemic H1N1 variant that is highly pathogenic for mice.
A plasmid-derived recombinant 2009 pandemic H1N1 virus A/New York/1682/2009 (rNY1682-WT) was serially passaged in BALB/cJ mice to create NY1682-MAP7. The morbidity and mortality resulting from infection with either rNY1682-WT or NY1682-MAP7 were compared in vivo. Although rNY1682-WT caused little disease, mice infected with NY1682-MAP7 showed clinical symptoms of disease, including ruffled fur and hunched posture, and eventually became reluctant to move by 4 to 5 days p.i. Mice inoculated with 104 or 105 TCID50 of rNY1682-WT showed a maximum of 7% or 12% weight loss, respectively (Fig. 1A). In contrast, inoculation with 104 or 105 TCID50 of NY1682-MAP7 caused weight loss as early as 2 days p.i., and the animals had >25% weight loss by 5 days p.i. (Fig. 1A). Statistical analysis using F tests to compare the estimated trajectories of the data for each group (data not shown) indicated that both rNY1682-WT and NY1682-MAP7 caused significant weight loss (P < 10−13) and that NY1682-MAP7 caused significantly greater weight loss than rNY1682-WT (P < 10−13) when inoculated at the same dose. Survival curves also clearly showed that NY1682-MAP7 was highly pathogenic for mice, with all of the animals succumbing to infection by 5 days p.i. (Fig. 1B).
FIG. 1.
Pathogenicity of the parental H1N1pdm virus (rNY1682-WT) and the mouse-adapted variant (NY1682-MAP7) in mice. Six-week-old female BALB/cJ mice (n = 5/group) were inoculated intranasally with 50 μl containing 104 or 105 TCID50 of rNY1682-WT (WT) or NY1682-MAP7 (MAP7) viruses or mock inoculated (Mock; n = 4). (A) Morbidity was examined by recording the body weights of inoculated mice daily, and it is represented as a percentage of the weight on the day of inoculation (day 0). The average of each group is shown, and the error bars represent the standard deviations from the mean (SD). *, F tests indicated that MAP7 caused greater weight loss than the WT (P < 10−13). (B) Mouse mortality after inoculation with 50 μl containing 104 or 105 TCID50 of rNY1682-WT (WT) or NY1682-MAP7 (MAP7) or diluent (Mock).
PB2-E158G substitution enhanced the pathogenicity of the H1N1pdm virus.
To determine the potential substitutions responsible for the increased pathogenicity of NY1682-MAP7, the consensus sequence of its complete genome was determined. Sequence analysis identified an adenosine-to-guanosine substitution at nucleotide position 500 (cDNA sense) in the avian-derived PB2 gene segment, which results in a glutamate-to-glycine substitution at residue 158 of the PB2 protein (PB2-E158G). Since the NY1682-MAP7 virus stock actually exists as a large population of closely related variants derived from rNY1682, multiple mutations present in virus subpopulations could be responsible for the high pathogenicity observed. Therefore, to determine if the PB2-E158G substitution was solely responsible for the high pathogenicity of NY1682-MAP7, we used reverse genetics to create a recombinant virus that differed from the parental rNY1682-WT virus only at residue 158 (rNY1682-E158G). We also created a virus containing the E627K substitution in PB2 (rNY1682-E627K) in order to compare the PB2-E158G substitution with a known pathogenic determinant important in the adaptation of avian viruses to humans and other mammals.
To determine the pathotypes of these single-amino-acid variants, mice were inoculated intranasally with 105 TCID50 of rNY1682-WT, rNY1682-E158G, or rNY1682-E627K. The rNY1682-E158G virus caused 25 to 30% weight loss (Fig. 2A), and all the mice succumbed to infection within 6 days p.i. (Fig. 2B). In contrast, both the rNY1682-WT and rNY1682-E627K viruses caused moderate weight loss (10 to 12%) (Fig. 2A), and all mice survived infection (Fig. 2B). F tests of the estimated trajectories of the raw data (data not shown) indicated that the weight loss caused by rNY1682-E158G was significantly greater than that caused by rNY1682-WT (P < 10−13). To further evaluate the magnitude of the increased pathogenicity rendered by the PB2-E158G substitution, we intranasally inoculated mice with 102, 103, 104, or 105 TCID50 of the rNY1682-E158G virus. All of the mice infected with rNY1682-E158G (102 to 105) lost 15 to 25% of their starting weights (Fig. 3A), and all showed greater morbidity than is typically observed in mice inoculated with 105 TCID50 of the parental rNY1682-WT virus (compare Fig. 3A and 1A). Inoculation with 105 or 104 TCID50 of rNY1682-E158G resulted in 100% mortality, and a 60% fatality rate was observed in the mice receiving 103 TCID50 of the virus (Fig. 3B). The 50% mouse lethal dose (MLD50) of rNY1682-E158G was 6 × 102 TCID50, which is at least 10,000-fold lower than the MLD50 of the parental virus rNY1682-WT (>6 × 106 TCID50).
FIG. 2.
Pathogenicity of rNY1682-WT, rNY1682-E158G, and rNY1682-E627K viruses in mice. Six-week-old female BALB/cJ mice (n = 5/group) were inoculated intranasally with 50 μl containing 105 TCID50 of different recombinant viruses (rNY1682-WT [WT], rNY1682-E158G [E158G], or rNY1682-E627K [E627K]) or mock inoculated (Mock). (A) Morbidity was assessed by weight changes over a 14-day period and is graphed as a percentage of the animals' weights on the day of inoculation (day 0). The average body weight for each group is shown, with error bars representing the SD. *, an F test indicated that the weight loss caused by rNY1682-E158G was significantly greater than that caused by rNY1682-WT (P < 10−13). (B) Mortality associated with infection by the recombinant virus (rNY1682-WT [WT], rNY1682-E158G [E158G], or rNY1682-E627K [E627K]) was also examined.
FIG. 3.
Determination of the mouse lethal dose of rNY1682-E158G. Six-week-old female BALB/cJ mice (n = 10/group) were inoculated intranasally with 50 μl containing 1 × 102, 1 × 103, 1 × 104, or 1 × 105 TCID50 of rNY1682-E158G or mock inoculated. (A) Morbidity was assessed by weight changes over a 14-day period, and it is graphed as a percentage of the weight on the day of inoculation (day 0). The average body weight of each group is shown, with error bars representing SD. (B) Mortality of the mice was also monitored for 14 days postinoculation.
The PB2-E158G virus replicates efficiently in cells derived from multiple species.
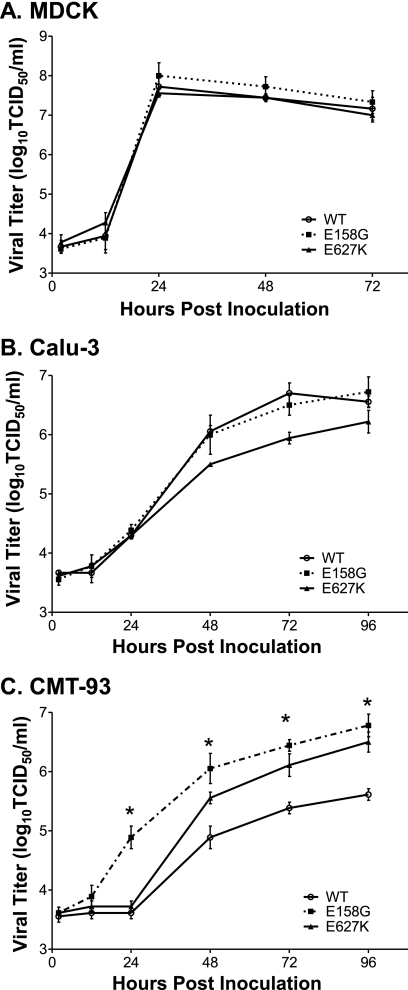
To begin to investigate the mechanism for the increased pathogenicity rendered by the PB2-E158G change, we examined the multistep replication kinetics of rNY1682-WT, rNY1682-E158G, and rNY1682-E627K in canine, human, and murine epithelial cell lines. MDCK cells, which are optimal substrates for propagation of influenza A virus, were inoculated at an MOI of 0.01 TCID50/cell, and all the viruses grew to nearly identical titers (107.5 to 108 TCID50) at 24 h p.i. (Fig. 4A). Calu-3 cells were used to determine if PB2-E158G affected replication in a human lung epithelial cell line. We found that rNY1682-WT and rNY1682-E158G grew to similar titers, but rNY1682-E627K was moderately attenuated in the Calu-3 cell line (Fig. 4B). A difference between rNY1682-WT and rNY1682-E158G growth kinetics was seen when the two viruses were analyzed using CMT-93 cells, which are mouse rectum epithelial carcinoma cells (Fig. 4C). The rNY1682-E158G viral titer was more than 10-fold higher than that of the rNY1682-WT virus at 24 h p.i., and that trend was maintained throughout the time course (Fig. 4C). Statistical analysis indicated that the titers of rNY1682-E158G were significantly different from those of rNY1682-WT at 24 to 96 h p.i. (P = 2 × 10−10; F test). The replication kinetics of rNY1682-E627K was also significantly faster than that of rNY1682-WT (P = 1 × 10−7; F test) and slower than that of rNY1682-E158G (Fig. 4C). Overall, the virus containing the PB2-E158G substitution has the greatest replication advantage over the virus containing the PB2-WT, and this is most evident at early times postinfection.
FIG. 4.
Replication kinetics of rNY1682-WT, rNY1682-E158G, and rNY1682-E627K in various cell lines. Confluent monolayers of the various cell lines were inoculated with the rNY1682-WT (WT), rNY1682-E158G (E158G), and rNY1682-E627K (E627K) viruses. Culture supernatants were harvested from MDCK cells at 2, 12, 24, 48, and 72 h p.i. (A) and from human lung epithelial cells (Calu-3) (B) or mouse rectal epithelial carcinoma cells (CMT-93) (C) at 2, 12, 24, 48, 72, and 96 h p.i. Viral titers were determined by TCID50 assay using MDCK cells. Averages of triplicate experiments are shown, with error bars representing SD. *, statistical analysis indicated that the titers of rNY1682-E158G were significantly greater than those of rNY1682-WT at 24 to 96 h p.i. (P = 2 × 10−10; F test).
PB2-E158G increases viral RNA polymerase activity to a greater extent than PB2-E627K in human cells.
The different replication kinetics of the three NY1682-PB2 variants in the various cell lines led us to use a mini-genome replication assay to focus our analysis on the influence that PB2-E158G has on the transcription/replication of vRNAs. HEK-293T cells were cotransfected with plasmids expressing PB1, PA, NP, and PB2 (WT, E158G, or E627K) and an NS-Luc reporter plasmid that generates a vRNA-like molecule, which expresses destabilized firefly luciferase upon transcription by the viral RNA polymerase complex. PB2-E627K is known to enhance RNA polymerase activity in other viruses, and as anticipated, when this substitution was incorporated into NY1682 (H1N1pdm) PB2, it had 400% greater activity than PB2-WT (Fig. 5). PB2-E158G had a very striking increase of 1,500% in the context of the H1N1pdm RDRP (Fig. 5). Statistical analysis using a two-tailed t test with unequal variance and the Bonferroni correction showed that PB2-E158G had significantly greater activity than either PB2-WT (P = 1.6 × 10−5) or PB2-E627K (P = 4.2 × 10−5). These luciferase-based replication assay results were also confirmed with an enhanced green fluorescent protein (EGFP)-mediated mini-genome assay system, in which NS-Luc was replaced by the NS-EGFP plasmid (data not shown). The mini-genome replication assays show that the PB2-E158G substitution has a stronger impact on the H1N1pdm viral RNA polymerase activity than the PB2-E627K change does in human cells.
FIG. 5.
Viral RNA polymerase activity of PB2-WT, PB2-E158G, and PB2-E627K in human cells. HEK-293T cells were transfected with a pPolI-NS-Luc plasmid (pBZ81A36) that expresses negative-sense virus-like RNA encoding a destabilized firefly luciferase enzyme that can be transcribed by the viral RDRP. The HEK-293T cells were also cotransfected with plasmids expressing NY1682 PB1, PA, and NP and one of the PB2 clones (WT, E158G, or E627K) to generate different viral RDRPs. Cells were also cotransfected with a Renilla luciferase expression plasmid to control for transfection efficiency. Eighteen hours posttransfection, both firefly and Renilla luciferase production levels were measured, and Renilla expression was used to normalize the data. The averages of triplicate experiments are shown, with error bars that represent SD. *, PB2-E158G had significantly greater activity than either PB2-WT (P = 1.6 × 10−5) or PB2-E627K (P = 4.2 × 10−5) when analyzed using a t test with unequal variance and Bonferroni's correction.
PB2-E158G significantly increases virus replication in mouse respiratory tissues.
Our multistep replication experiments and the mini-genome replication assays strongly suggested that the high pathogenicity of rNY1682-E158G resulted from enhanced replication of the virus, which is conferred by the PB2-E158G substitution. To determine if rNY1682-E158G replicates more efficiently than rNY1682-WT and rNY1682-E627K viruses in vivo, we inoculated mice with 103 TCID50 of each virus and analyzed virus titers in lungs and nasal washes at various times postinoculation (Fig. 6). The titer of rNY1682-E158G virus was 1,300-fold higher than that of the rNY1682-WT virus at 12 and 24 h p.i. and reached very high peak titers (108 TCID50/ml) by 48 h p.i., whereas the peak titers of rNY1682-WT and rNY1682-E627K were less than 107 TCID50/ml (Fig. 6A). Statistical analysis by F tests showed that rNY1682-E158G had significantly higher lung titers than rNY1682-WT (P = 6 × 10−10), whereas the differences between rNY1682-WT and rNY1682-E627K were insignificant. The average virus titer in the nasal wash was approximately 100-fold higher in mice infected with the PB2-E158G variant than in mice infected with the PB2-WT virus at 24 h p.i. (Fig. 6B), which was statistically significant (P = 0.005; F test). The high level of replication achieved in the lungs early after infection that was sustained throughout the course of infection by rNY1682-E158G explains why the PB2-E158G substitution causes severe morbidity and mortality in mice. We also examined the potential for virus replication in other tissues, including brain, kidney, spleen, serum, and intestine. Small amounts of both rNY1682-WT and rNY1682-E158G were sporadically detected in the kidney, spleen, serum, and intestine; however, there was not an obvious difference between the extrarespiratory tropisms of the WT and PB2-E158G viruses (data not shown).
FIG. 6.
Analysis of viral replication efficiency in the respiratory tracts of mice. Six-week-old female BALB/cJ mice (n = 3/group/time point) were inoculated intranasally with 50 μl containing 103 TCID50 of rNY1682-WT (WT), rNY1682-E158G (E158G), or rNY1682-E627K (E627K). Animals were euthanized at 12, 24, 48, and 96 h p.i. An entire lung of each animal was homogenized in 1 ml of medium and clarified by centrifugation, and nasal washes were collected from each mouse in 1 ml of medium. Viral titers of the clarified lung homogenates (A) and nasal washes (B) were determined by TCID50 assay using MDCK cells. The average of each group is shown, with error bars representing the SD. *, rNY1682-E158G had significantly higher lung titers than rNY1682-WT at all of the time points (P = 6 × 10−10; F test). **, rNY1682-E158G had significantly higher titers than rNY1682-WT in the nasal wash at 24 h p.i. (P = 0.005; F test). The dotted line (1.5 log10 units) indicates the lower limit of detection of infectious virus.
PB2-E158G substantially alters the pathotypes of unrelated avian H5 viruses.
To determine if PB2-E158G is a pathogenic determinant specifically in the genetic constellation of H1N1pdm viruses or if this substitution represents an adaptive strategy that enhances the pathogenesis of unrelated avian viruses in mice, we created two sets of avian viruses that differ only at PB2-158. We used plasmid-based reverse genetics to create wild-type and PB2-E158G variants of a highly pathogenic avian H5N9 virus, A/Turkey/Ontario/7732/1966 (rTy/Ont) (21), and a low-pathogenic avian H5N2 virus, A/Mallard/Wisconsin/944/1982 (rMal/WI). Remarkably, substitution of E158G in the PB2s of these avian H5 viruses increased the morbidity and mortality observed in mice (Fig. 7). Although rTy/Ont has a highly cleavable HA and is highly pathogenic for chickens, it is normally not lethal to mice. The rTy/Ont-WT virus caused a maximum of ∼10% weight loss, and it was not lethal to the mice (Fig. 7A and B). In contrast, infection by rTy/Ont-E158G caused >25% weight loss and 100% mortality by 5 days p.i. (Fig. 7A and B). The increase in pathogenicity caused by the PB2-E158G substitution was more remarkable in the context of the normally low-pathogenic rMal/WI viruses. Mice infected by rMal/WI-WT did not lose weight, and 100% survived infection (Fig. 7C and D). On the other hand, infection with rMal/WI-E158G caused >25% weight loss and 100% mortality by 6 days p.i. (Fig. 7C and D).
FIG. 7.
PB2-E158G radically alters the pathotypes of avian H5 viruses in mice. Six-week-old female BALB/cJ mice (n = 5/group) were intranasally inoculated with 50 μl of the recombinant avian H5 viruses created by reverse genetics. (A and B) Mice were inoculated with 5 × 105 TCID50 of either rA/Ty/Ont/7732/66-wild type (rTy/Ont-WT) or rA/Ty/Ont/7732/66-E158G (rTy/Ont-E158G) virus or mock inoculated (Mock). (A) Morbidity was assessed by weight changes over a 10-day period and is graphed as a percentage of the animals' weights on the day of inoculation (day 0). (B) Mortality associated with infection by the recombinant A/Ty/Ont/7732/66 (H5N9) viruses was also examined. (C and D) Mice were inoculated with 1 × 105 TCID50 of rA/Mal/WI/944/82-WT (rMal/WI-WT) or rA/Mal/WI/944/82-E158G (rMal/WI-E158G) or mock inoculated (Mock). The morbidity (C) and mortality (D) of mice infected with the recombinant Mal/WI (H5N2) viruses were recorded daily for 10 days. The average weight loss of each group (A and C) is shown, with error bars representing the SD.
DISCUSSION
Zoonosis and subsequent human-to-human transmission of influenza A viruses requires the emerging virus to overcome a variety of species-specific barriers to infection, and amino acid substitutions in the viral RDRP are one of the major adaptive mechanisms that avian viruses exploit for efficient replication, transmission, and pathogenesis in mammals. Although the avian (PB2 and PA)/human (PB1) RDRP of H1N1pdm viruses previously acquired amino acid substitutions that enhance its replication in mammals (e.g., PB2-G590S/Q591R) (30, 44) during its evolution as part of the swine triple-reassortant virus polymerase complex, additional evolutionary changes will undoubtedly be acquired as the virus adapts to humans.
Using mice as a model for the mammalian adaptation of the H1N1pdm virus, we showed that a highly pathogenic variant evolved from a plasmid-derived parental stock after limited serial passage. Additionally, we proved that a single amino acid substitution (PB2-E158G) identified in the consensus sequence of NY1682-MAP7 was the virulence determinant responsible for the increased pathogenicity observed. The PB2 gene binds host capped mRNAs, targeting them for cleavage by the PA subunit, and it is an important pathogenic determinant; however, amino acid substitutions critical to the pathogenesis of avian PB2s in mammals often map to the carboxy-terminal region of the protein (e.g., E627K and D701N). Therefore, PB2-E158G represents a unique amino acid change in the amino-terminal region of the protein that had not been described previously. Comparison of the pathotypes of rNY1682 viruses containing PB2-WT, PB2-E627K, and PB2-E158G demonstrated that the PB2-E158G substitution has a much greater impact on morbidity and mortality than the PB2-E627K change in the context of H1N1pdm viruses. Although we were initially surprised that rNY1682-E627K did not have enhanced pathogenicity, our data are consistent with three other PB2-E627K studies using different strains of the H1N1pdm virus (15, 19, 47). Intriguingly, mouse adaptation of two H1N1pdm clinical isolates (A/CA/04/09 and A/TN/1-560/09) identified PB2-E158G/A, along with eight other amino acid changes in PA (L295P), NP (D101G and H289Y), and HA (K119N, G155E, S183P, R221K, and D222G), as putative pathogenic determinants that were identified in lethal plaque-picked variants selected after nine serial passages in BALB/c mice (17). Although Ilyushina et al. did not use reverse genetics to define the roles that the individual amino acid changes played in vivo (17), the data that they presented and the data from our study independently demonstrate that PB2 residue 158 is an important determinant in the pathogenesis of the pandemic H1N1 virus in the mouse model.
To investigate the mechanism(s) responsible for the increased pathogenicity rendered by PB2-E158G, we compared this change with PB2-WT and PB2-E627K using assays for viral RDRP activity and virus replication in vitro and in vivo. We were not surprised that all of the viruses grew to similar titers in MDCK cells, which is one of the best cell lines for the propagation of influenza A viruses. We also observed similar replication kinetics between rNY1682-WT and rNY1682-E158G in human lung epithelial (Calu-3) cells, which indicates that the PB2-E158G substitution does not have a negative impact on replication in human lung cells (Fig. 4B). In contrast, the rNY1682-E627K virus showed subtle attenuation in these cells, which is consistent with a recent study using the A549 human lung epithelial cell line (19). The attenuation of rNY1682-E627K in Calu-3 cells is likely due to the fact that the H1N1pdm viruses have an arginine substitution at position 591, which compensates for the glutamate typically in position 627, and these two residues interact in the three-dimensional structure of this domain of the PB2 protein (30, 44). We used a mouse rectum epithelial cell line (CMT-93) to further investigate the effects that PB2-E158G and PB2-E627K have on virus replication, and these data clearly demonstrated that PB2-E158G enhances virus replication (Fig. 4C). Notably, rNY1682-E627K also grew better than the wild-type virus in CMT-93 cells, although its replication kinetics were delayed compared to those of rNY1682-E158G. PB2-E627K is not regarded as a mouse-specific adaptive mutation because it also enhances replication of avian influenza A viruses in ferrets, pigs, and humans, as well as in a wide variety of mammalian cell lines (14, 38). Thus, the high level of virus replication observed for rNY1682-E158G and rNY1682-E627K in CMT-93 cells may not be related to the fact that CMT-93 cells are derived from mice. We speculate that PB2-E158G may enhance replication in cells of many mammalian species and that its influence is evident under stringent conditions, such as in CMT-93 cells or in respiratory epithelial cells in vivo.
We subsequently focused specifically on the function of the viral RDRP using a luciferase-based mini-genome assay to better understand why the PB2-E158G change led to enhanced production of infectious virus particles. Although the RDRPs containing PB2-E627K and PB2-E158G both showed higher luciferase expression, PB2-E158G had an impressive 15-fold increase, which indicates that this change substantially enhances RDRP activity in human cells (Fig. 5). The increased RNA polymerase activity of PB2-E158G mutants in the mini-genome replication assay correlates strongly with the fact that mice infected with rNY1682-E158G had 1,300-fold-higher lung titers than mice infected with the wild-type virus. The titers of rNY1682-E158G in nasal washes from infected mice also showed the same trend, which suggests that PB2-E158G may also enhance transmission between hosts, because high-level replication in the upper respiratory tract is likely to increase transmission. In contrast, the titers of rNY1682-E627K and the wild type were nearly identical in lungs and nasal washes from infected mice. These results directly correlate with the pathotype of each of the PB2 mutants and demonstrate that PB2-E158G is a virulence determinant in mice because it drastically enhances early viral replication in this mammalian host. The viral titers and associated mortality we observed for the rNY1682-E158G virus are reminiscent of studies conducted with the highly pathogenic 1918 pandemic H1N1 virus (1, 9, 41), and the RDRP of the 1918 virus is central to its high pathogenicity (42). Remarkably, PB2-E158G substitutions in the natural gene constellations of two avian H5 viruses also increased their morbidity and mortality in mice. The results of the avian H5 virus studies powerfully demonstrate that PB2-E158G has far-reaching impacts on the pathogenicity of divergent influenza A viruses isolated from different hosts over the last 43 years (i.e., turkeys in 1966, ducks in 1984, and humans in 2009) in the mouse model. Collectively, our data show that this region of the PB2 protein is an important virulence factor in the adaptation of avian PB2 genes in mice and potentially in other mammals.
The majority of studies on mutations in PB2 that enhance replication of avian viruses in mammalian systems are focused on residues close to the carboxy terminus (e.g., E627K, D701N, and G590S/Q591R), which are believed to either facilitate interaction with a host stimulatory factor or prevent interaction with a host inhibitory factor. However, enhanced mini-genome replication in human cells was recently observed when threonine 271, commonly found in avian viruses, was changed to the alanine conserved in mammalian PB2 genes (4), which indicates that residues in other regions of the protein also play critical roles in replication. The H1N1pdm viruses already have alanine 271, so E158G represents an additional adaptive mutation that enhances H1N1pdm RDRP activity in human cells. Although high-resolution crystal structures are available for the central and carboxy-terminal domains of PB2 (12, 20, 39, 40), there is no structural information available around residue 158. Studies using PB2 deletion mutants implicate this region of the molecule in interaction with the viral NP, and interactions with specific host factors, such as those involved in recognition of host capped mRNAs, have not been identified (34). Amino acid sequence analysis of this region predicts that residue 158 is in a hydrophilic loop that is likely to be exposed on the surface of the PB2 protein. The residues near position 158 are highly conserved in PB2; however, sequence database searches identified a human H1N1pdm virus isolated in New Zealand (A/Auckland/1/2009) and four avian influenza viruses that have the PB2-E158G mutation. Detailed information about the human H1N1pdm PB2-158G mutant and the case history are not available, so we do not know the disease severity associated with this infection. Nonetheless, our data indicate that future molecular epidemiological surveillance of this region of PB2 is warranted. Since the PB2-E158G virus replicates efficiently in human lung epithelial cells and the RDRP activity of this PB2 substitution is enhanced in human kidney cells, we speculate that PB2-E158G may enhance replication in many mammalian species in addition to mice. However, additional studies on the influence of the PB2-E158G substitution in other animal models, such as ferrets or primates, are needed to determine if this mutation enhances pathogenicity in these models and if it is likely to impact virulence in humans.
Acknowledgments
We thank Anju Subba, Chrystal Chadwick, and Amanda Mercer for their expert assistance with the mouse studies; Matthew Shudt in the Wadsworth Center Applied Genomic Technologies Core for his assistance in the sequencing of plasmids and RT-PCR amplicons; and Noel Espina, Jianzhong Tang, and Richard Cadagan for tissue culture support. We also thank Andrew Reilly in the Wadsworth Center bioinformatics core for his help with statistical analysis. We are grateful to Peter Palese and Adolfo García-Sastre for providing the pDZ reverse-genetics plasmid and Yoshihiro Kawaoka for providing the pHH21 reverse-genetics plasmid and HEK-293T cells. We thank Joachim Jaeger for his help with various predictive structural algorithms.
These studies were supported in part by Health Research Inc. and the Wadsworth Center, NYSDOH, Director's office, and genomic sequencing of NY1682-MA7 was supported by NIH Genomic Sequencing Center contract HHSN272200900007C. Y.L., Anju Subba, D.E.W., and the biosafety level-3 (BSL-3) vivarium at the Wadsworth Center that was used are funded in part as a core facility by NIH/NIAID U54-AI057158 (Northeast Biodefense Center-Lipkin). D.E.W. was also supported by NIH/NIAID P01AI059576-05.
Footnotes
Published ahead of print on 20 October 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Basler, C. F., and P. V. Aguilar. 2008. Progress in identifying virulence determinants of the 1918 H1N1 and the Southeast Asian H5N1 influenza A viruses. Antiviral Res. 79:166-178. doi: 10.1016/j.antiviral.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bautista, E., T. Chotpitayasunondh, Z. Gao, S. A. Harper, M. Shaw, T. M. Uyeki, S. R. Zaki, F. G. Hayden, D. S. Hui, J. D. Kettner, A. Kumar, M. Lim, N. Shindo, C. Penn, and K. G. Nicholson. 2010. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N. Engl. J. Med. 362:1708-1719. [DOI] [PubMed] [Google Scholar]
- 3.Brown, E. G., H. Liu, L. C. Kit, S. Baird, and M. Nesrallah. 2001. Pattern of mutation in the genome of influenza A virus on adaptation to increased virulence in the mouse lung: identification of functional themes. Proc. Natl. Acad. Sci. U. S. A. 98:6883-6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bussey, K. A., T. L. Bousse, E. A. Desmet, B. Kim, and T. Takimoto. 2010. PB2 residue 271 plays a key role in enhanced polymerase activity of influenza A viruses in mammalian host cells. J. Virol. 84:4395-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fornek, J. L., L. Gillim-Ross, C. Santos, V. Carter, J. M. Ward, L. I. Cheng, S. Proll, M. G. Katze, and K. Subbarao. 2009. A single-amino-acid substitution in a polymerase protein of an H5N1 influenza virus is associated with systemic infection and impaired T-cell activation in mice. J. Virol. 83:11102-11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabriel, G., B. Dauber, T. Wolff, O. Planz, H. D. Klenk, and J. Stech. 2005. The viral polymerase mediates adaptation of an avian influenza virus to a mammalian host. Proc. Natl. Acad. Sci. U. S. A. 102:18590-18595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabriel, G., A. Herwig, and H. D. Klenk. 2008. Interaction of polymerase subunit PB2 and NP with importin alpha1 is a determinant of host range of influenza A virus. PLoS. Pathog. 4:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao, Y., Y. Zhang, K. Shinya, G. Deng, Y. Jiang, Z. Li, Y. Guan, G. Tian, Y. Li, J. Shi, L. Liu, X. Zeng, Z. Bu, X. Xia, Y. Kawaoka, and H. Chen. 2009. Identification of amino acids in HA and PB2 critical for the transmission of H5N1 avian influenza viruses in a mammalian host. PLoS Pathog. 5:e1000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Sastre, A., and R. J. Whitley. 2006. Lessons learned from reconstructing the 1918 influenza pandemic. J. Infect. Dis. 194(Suppl. 2):S127-S132. [DOI] [PubMed] [Google Scholar]
- 10.Govorkova, E. A., J. E. Rehg, S. Krauss, H. L. Yen, Y. Guan, M. Peiris, T. D. Nguyen, T. H. Hanh, P. Puthavathana, H. T. Long, C. Buranathai, W. Lim, R. G. Webster, and E. Hoffmann. 2005. Lethality to ferrets of H5N1 influenza viruses isolated from humans and poultry in 2004. J. Virol. 79:2191-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guarner, J., and R. Falcón-Escobedo. 2009. Comparison of the pathology caused by H1N1, H5N1, and H3N2 influenza viruses. Arch. Med. Res. 40:655-661. doi: 10.1016/j.arcmed.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Guilligay, D., F. Tarendeau, P. Resa-Infante, R. Coloma, T. Crepin, P. Sehr, J. Lewis, R. W. H. Ruigrok, J. Ortin, D. J. Hart, and S. Cusack. 2008. The structural basis for cap binding by influenza virus polymerase subunit PB2. Nat. Struct. Mol. Biol. 15:500-506. doi: 10.1038/nsmb.1421. [DOI] [PubMed] [Google Scholar]
- 13.Hatta, M., P. Gao, P. Halfmann, and Y. Kawaoka. 2001. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 293:1840-1842. [DOI] [PubMed] [Google Scholar]
- 14.Hatta, M., Y. Hatta, J. H. Kim, S. Watanabe, K. Shinya, T. Nguyen, P. S. Lien, Q. M. Le, and Y. Kawaoka. 2007. Growth of H5N1 influenza A viruses in the upper respiratory tracts of mice. PLoS. Pathog. 3:1374-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herfst, S., S. Chutinimitkul, J. Ye, E. de Wit, V. J. Munster, E. J. Schrauwen, T. M. Bestebroer, M. Jonges, A. Meijer, M. Koopmans, G. F. Rimmelzwaan, A. D. Osterhaus, D. R. Perez, and R. A. Fouchier. 2010. Introduction of virulence markers in PB2 of pandemic swine-origin influenza virus does not result in enhanced virulence or transmission. J. Virol. 84:3752-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann, E., G. Neumann, Y. Kawaoka, G. Hobom, and R. G. Webster. 2000. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc. Natl. Acad. Sci. U. S. A. 97:6108-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ilyushina, N. A., A. M. Khalenkov, J. P. Seiler, H. L. Forrest, N. V. Bovin, H. Marjuki, S. Barman, R. G. Webster, and R. J. Webby. 2010. Adaptation of pandemic H1N1 influenza viruses in mice. J. Virol. doi: 10.1128/JVI.00159-10. [DOI] [PMC free article] [PubMed]
- 18.Itoh, Y., K. Shinya, M. Kiso, T. Watanabe, Y. Sakoda, M. Hatta, Y. Muramoto, D. Tamura, Y. Sakai-Tagawa, T. Noda, S. Sakabe, M. Imai, Y. Hatta, S. Watanabe, C. Li, S. Yamada, K. Fujii, S. Murakami, H. Imai, S. Kakugawa, M. Ito, R. Takano, K. Iwatsuki-Horimoto, M. Shimojima, T. Horimoto, H. Goto, K. Takahashi, A. Makino, H. Ishigaki, M. Nakayama, M. Okamatsu, K. Takahashi, D. Warshauer, P. A. Shult, R. Saito, H. Suzuki, Y. Furuta, M. Yamashita, K. Mitamura, K. Nakano, M. Nakamura, R. Brockman-Schneider, H. Mitamura, M. Yamazaki, N. Sugaya, M. Suresh, M. Ozawa, G. Neumann, J. Gern, H. Kida, K. Ogasawara, and Y. Kawaoka. 2009. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature 460:1021-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jagger, B. W., M. J. Memoli, Z.-M. Shi, L. Qi, R. J. Hrabal, G. L. Allen, V. G. Dugan, R. Wang, P. Digard, J. C. Kash, and J. K. Taubenberger. 2010. The PB2-E627K mutation attenuates viruses containing the 2009 H1N1 influenza pandemic polymerase. mBio 1:e00067-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuzuhara, T., D. Kise, H. Yoshida, T. Horita, Y. Murazaki, A. Nishimura, N. Echigo, H. Utsunomiya, and H. Tsuge. 2009. Structural basis of the influenza A virus RNA polymerase PB2 RNA-binding domain containing the pathogenicity-determinant lysine 627 residue. J. Biol. Chem. 284:6855-6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang, G., O. Narayan, B. T. Rouse, A. E. Ferguson, and M. C. Connell. 1968. A new influenza A virus infection in turkeys. II. A highly pathogenic variant, A/turkey/ontario/7732/66. Can. Vet. J. 9:151-160. [PMC free article] [PubMed] [Google Scholar]
- 22.Le, Q. M., M. Ito, Y. Muramoto, P. V. Hoang, C. D. Vuong, Y. Sakai-Tagawa, M. Kiso, M. Ozawa, R. Takano, and Y. Kawaoka. 2010. Pathogenicity of highly pathogenic avian H5N1 influenza A viruses isolated from humans from 2003 to 2008 in Northern Vietnam. J. Gen. Virol. 91:2485-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le, Q. M., Y. Sakai-Tagawa, M. Ozawa, M. Ito, and Y. Kawaoka. 2009. Selection of H5N1 influenza virus PB2 during replication in humans. J. Virol. 83:5278-5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, Z., H. Chen, P. Jiao, G. Deng, G. Tian, Y. Li, E. Hoffmann, R. G. Webster, Y. Matsuoka, and K. Yu. 2005. Molecular basis of replication of duck H5N1 influenza viruses in a mammalian mouse model. J. Virol. 79:12058-12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Libster, R., J. Bugna, S. Coviello, D. R. Hijano, M. Dunaiewsky, N. Reynoso, M. L. Cavalieri, M. C. Guglielmo, M. S. Areso, T. Gilligan, F. Santucho, G. Cabral, G. L. Gregorio, R. Moreno, M. I. Lutz, A. L. Panigasi, L. Saligari, M. T. Caballero, R. M. Egües Almeida, M. E. Gutierrez Meyer, M. D. Neder, M. C. Davenport, M. P. Del Valle, V. S. Santidrian, G. Mosca, M. Garcia Domínguez, L. Alvarez, P. Landa, A. Pota, N. Bolonati, R. Dalamon, V. I. Sanchez Mercol, M. Espinoza, J. C. Peuchot, A. Karolinski, M. Bruno, A. Borsa, F. Ferrero, A. Bonina, M. Ramonet, L. C. Albano, N. Luedicke, E. Alterman, V. Savy, E. Baumeister, J. D. Chappell, K. M. Edwards, G. A. Melendi, and F. P. Polack. 2010. Pediatric hospitalizations associated with 2009 pandemic influenza A (H1N1) in Argentina. N. Engl. J. Med. 362:45-55. doi: 10.1056/NEJMoa0907673. [DOI] [PubMed] [Google Scholar]
- 26.Lu, X., T. M. Tumpey, T. Morken, S. R. Zaki, N. J. Cox, and J. M. Katz. 1999. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J. Virol. 73:5903-5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maines, T. R., A. Jayaraman, J. A. Belser, D. A. Wadford, C. Pappas, H. Zeng, K. M. Gustin, M. B. Pearce, K. Viswanathan, Z. H. Shriver, R. Raman, N. J. Cox, R. Sasisekharan, J. M. Katz, and T. M. Tumpey. 2009. Transmission and pathogenesis of swine-origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science 325:484-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maines, T. R., X. H. Lu, S. M. Erb, L. Edwards, J. Guarner, P. W. Greer, D. C. Nguyen, K. J. Szretter, L. M. Chen, P. Thawatsupha, M. Chittaganpitch, S. Waicharoen, D. T. Nguyen, T. Nguyen, H. H. Nguyen, J. H. Kim, L. T. Hoang, C. Kang, L. S. Phuong, W. Lim, S. Zaki, R. O. Donis, N. J. Cox, J. M. Katz, and T. M. Tumpey. 2005. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J. Virol. 79:11788-11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mase, M., N. Tanimura, T. Imada, M. Okamatsu, K. Tsukamoto, and S. Yamaguchi. 2006. Recent H5N1 avian influenza A virus increases rapidly in virulence to mice after a single passage in mice. J. Gen. Virol. 87:3655-3659. [DOI] [PubMed] [Google Scholar]
- 30.Mehle, A., and J. A. Doudna. 2009. Adaptive strategies of the influenza virus polymerase for replication in humans. Proc. Natl. Acad. Sci. U. S. A. 106:21312-21316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munster, V. J., E. de Wit, J. M. van den Brand, S. Herfst, E. J. Schrauwen, T. M. Bestebroer, D. van de Vijver, C. A. Boucher, M. Koopmans, G. F. Rimmelzwaan, T. Kuiken, A. D. Osterhaus, and R. A. Fouchier. 2009. Pathogenesis and transmission of swine-origin 2009 A(H1N1) influenza virus in ferrets. Science 325:481-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munster, V. J., E. de Wit, D. van Riel, W. E. Beyer, G. F. Rimmelzwaan, A. D. Osterhaus, T. Kuiken, and R. A. Fouchier. 2007. The molecular basis of the pathogenicity of the Dutch highly pathogenic human influenza A H7N7 viruses. J. Infect. Dis. 196:258-265. [DOI] [PubMed] [Google Scholar]
- 33.Palese, P., and M. L. Shaw. 2007. Orthomyxoviridae: The viruses and their replication, p. 1647-1690. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology. Lippincott Williams and Wilkins, Philadelphia, PA.
- 34.Poole, E., D. Elton, L. Medcalf, and P. Digard. 2004. Functional domains of the influenza A virus PB2 protein: identification of NP- and PB1-binding sites. Virology 321:120-133. doi: 10.1016/j.virol.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 35.Shinya, K., S. Hamm, M. Hatta, H. Ito, T. Ito, and Y. Kawaoka. 2004. PB2 amino acid at position 627 affects replicative efficiency, but not cell tropism, of Hong Kong H5N1 influenza A viruses in mice. Virology 320:258-266. [DOI] [PubMed] [Google Scholar]
- 36.Shinya, K., S. Watanabe, T. Ito, N. Kasai, and Y. Kawaoka. 2007. Adaptation of an H7N7 equine influenza A virus in mice. J. Gen. Virol. 88:547-553. [DOI] [PubMed] [Google Scholar]
- 37.Steel, J., A. C. Lowen, S. Mubareka, and P. Palese. 2009. Transmission of influenza virus in a mammalian host is increased by PB2 amino acids 627K or 627E/701N. PLoS Pathog. 5:e1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subbarao, E. K., W. London, and B. R. Murphy. 1993. A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. J. Virol. 67:1761-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tarendeau, F., J. Boudet, D. Guilligay, P. J. Mas, C. M. Bougault, S. Boulo, F. Baudin, R. W. H. Ruigrok, N. Daigle, J. Ellenberg, S. Cusack, J. P. Simorre, and D. J. Hart. 2007. Structure and nuclear import function of the C-terminal domain of influenza virus polymerase PB2 subunit. Nat. Struct. Mol. Biol. 14:229-233. doi: 10.1038/nsmb1212. [DOI] [PubMed] [Google Scholar]
- 40.Tarendeau, F., T. Crepin, D. Guilligay, R. W. H. Ruigrok, S. Cusack, and D. J. Hart. 2008. Host determinant residue lysine 627 lies on the surface of a discrete, folded domain of influenza virus polymerase PB2 subunit. PLoS Pathog. 4:e1000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tumpey, T. M., C. F. Basler, P. V. Aguilar, H. Zeng, A. Solorzano, D. E. Swayne, N. J. Cox, J. M. Katz, J. K. Taubenberger, P. Palese, and A. Garcia-Sastre. 2005. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science 310:77-80. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe, T., S. Watanabe, K. Shinya, J. H. Kim, M. Hatta, and Y. Kawaoka. 2009. Viral RNA polymerase complex promotes optimal growth of 1918 virus in the lower respiratory tract of ferrets. Proc. Natl. Acad. Sci. U. S. A. 106:588-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wright, P. F., G. Neumann, and Y. Kawaoka. 2007. Orthomyxoviruses, p. 1691-1740. In: D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, S. E. Straus, M. A. Martin, and B. Roizman (ed.), Fields virology, 5th ed. Lippincott Williams and Wilkins, Philadelpha, PA.
- 44.Yamada, S., M. Hatta, B. L. Staker, S. Watanabe, M. Imai, K. Shinya, Y. Sakai-Tagawa, M. Ito, M. Ozawa, T. Watanabe, S. Sakabe, C. Li, J. H. Kim, P. J. Myler, I. Phan, A. Raymond, E. Smith, R. Stacy, C. A. Nidom, S. M. Lank, R. W. Wiseman, B. N. Bimber, D. H. O'Connor, G. Neumann, L. J. Stewart, and Y. Kawaoka. 2010. Biological and structural characterization of a host-adapting amino acid in influenza virus. PLoS. Pathog. 6:e1001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao, Y., L. J. Mingay, J. W. McCauley, and W. S. Barclay. 2001. Sequences in influenza A virus PB2 protein that determine productive infection for an avian influenza virus in mouse and human cell lines. J. Virol. 75:5410-5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou, B., M. E. Donnelly, D. T. Scholes, K. St George, M. Hatta, Y. Kawaoka, and D. E. Wentworth. 2009. Single-reaction genomic amplification accelerates sequencing and vaccine production for classical and swine origin human influenza A viruses. J. Virol. 83:10309-10313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu, H., J. Wang, P. Wang, W. Song, Z. Zheng, R. Chen, K. Guo, T. Zhang, J. S. Peiris, H. Chen, and Y. Guan. 2010. Substitution of lysine at 627 position in PB2 protein does not change virulence of the 2009 pandemic H1N1 virus in mice. Virology 401:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]