Abstract
Vesicular stomatitis virus (VSV) is a rhabdovirus that alters host nuclear and cytoplasmic function upon infection. We have investigated the effect of VSV infection on cellular signaling through the phosphatidylinositol-3 kinase (PI3k)/Akt signaling pathway. Akt phosphorylation at both threonine 308 (Thr308) and serine 473 (Ser473) was inhibited in cells infected with VSV. This inhibition was rapid (beginning within the first 2 to 3 h postinfection) and correlated with the dephosphorylation of downstream effectors of Akt, such as glycogen synthase kinase 3β (GSK3β) and mammalian target of rapamycin (mTOR). The dephosphorylation of Akt occurred in the presence of growth factor stimulation and was not overcome through constitutive membrane targeting of Akt or high levels of phosphatidylinositol-3,4,5-triphosphate (PIP3) accumulation in the membrane. Akt dephosphorylation was not a result of alterations in PDK1 phosphorylation or activity, changes in phosphatase and tensin homologue deleted on chromosome 10 (PTEN) levels, or the downregulation of PI3k signaling. Inactivation of Akt was caused by the expression of the viral M protein in the absence of other viral components, and an M protein mutant that does not inhibit RNA polymerase II (Pol II) transcription and nuclear/cytoplasmic transport was also defective in inhibiting Akt phosphorylation. These data illustrate that VSV utilizes a novel mechanism to alter this central player in cell signaling and oncogenesis. It also suggests an inside-out model of signal transduction where VSV interruption of nuclear events has a rapid and significant effect on membrane signaling events.
Viral infection results in the activation of a wide variety of host intracellular signaling pathways that are, in part, required to mount a host antiviral response to infection (23, 43, 52). These responses include the suppression of signals for growth, the induction of proapoptotic signals, and the release of specific inflammatory cytokines. Many of the host responses are part of the innate immune response designed to aid clearance of the viral pathogen. Thus, if a successful viral replication is to occur, the virus must counter these stress signals or evolve to be insensitive to them.
Many viruses are known to alter signal transduction to benefit viral replication in various ways (23, 25, 52). One such signaling pathway known to be affected is the phosphatidylinositol-3-kinase (PI3k)/Akt kinase-signaling cascade. Normally, signaling through this pathway is initiated by the stimulation of a receptor tyrosine kinase (RTK) with a hormone or a growth factor, such as insulin or epidermal growth factor (EGF), at the cell surface. Activation of the RTK recruits and activates the PI3k, which converts phosphatidylinositol-4,5-biphosphate (PIP2) to the phosphatidylinositol-3,4,5-triphosphate (PIP3) form. PIP3 recruits Akt from the cytosol to the plasma membrane, where it binds to PIP3 via its pleckstrin homology domain.
PIP3 also serves as a nucleation site for the colocalization of Akt with its activating kinase, phosphoinositide-dependent protein kinase 1 (PDK1), which phosphorylates Akt at threonine 308 (Thr308). This activating phosphorylation leads to a second phosphorylation event by the mammalian target of rapamycin C2 (mTORC2) on Akt at serine 473 (Ser473), which potentiates kinase activity (4, 17, 29, 61). Once activated, Akt can phosphorylate and inhibit proapoptotic factors such as Bad (51) and promote cellular translation through glycogen synthase kinase 3 (GSK3) phosphorylation and activation of mTORC1, which inactivates the translation suppressor 4E-BP1 (64). In addition to having these functions, Akt can also act to stimulate the immune response (33).
The PI3k/Akt pathway has long been recognized as an important signaling pathway activated by virus infection. There are many examples of both DNA (Epstein-Barr virus [EBV], herpes simplex virus type 1 [HSV-1], and simian virus 40 [SV40]) and RNA (influenza A virus, hepatitis C virus [HCV], Ebola virus, and respiratory syncytial virus [RSV]) viruses that induce or activate PI3k/Akt signaling during infection (reviewed in references 16, 23, and 25). These viruses appear to benefit from the antiapoptotic properties of this pathway (23).
For other viruses, the role of the PI3k/Akt pathway in virus replication is less clear. Vesicular stomatitis virus (VSV), the prototype negative-strand RNA virus, is an excellent example of this. It has been described previously that mammalian target of rapamycin (mTOR) (2), 4E-BP1 (21), and rpS6 (42), which are all downstream substrates and effectors of the PI3k/Akt pathway, are dephosphorylated during VSV replication. These data suggest that VSV can block some aspect of this signaling pathway. In contrast, it has been suggested that the kinase activity of PI3k is important for viral entry (38) and that Akt activity is essential for VSV replication (59). Studies with two primary cell types that are resistant to VSV infection have reached opposite conclusions. It was reported that macrophages stimulate Akt phosphorylation following exposure to VSV (55) but that Drosophila cells infected with VSV appear to downregulate Akt phosphorylation (57).
We were interested in determining the interaction of VSV with the Akt signaling pathway to determine where the virus might interact with the pathway. We found that in classically permissive cells, infection with VSV actively inhibits Akt activation in a manner dependent on virus replication but that the accumulation of PIP3 is unhindered. It is particularly relevant that VSV, currently being developed as an oncolytic virus, appears to have a unique mechanism of blocking Akt signaling. Akt is a transforming kinase (18), which is frequently activated in cancer cells (53).
MATERIALS AND METHODS
Tissue culture and virus infections.
BHK, HeLa, and Vero cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 7% fetal bovine serum (FBS) (Invitrogen) and 2 mM glutamine (Invitrogen). HEK-TERST and HEK-TERV (a kind gift from William C. Hahn) cell lines (30) were cultured in MEM Alpha (Invitrogen) supplemented with 10% FBS and 2 mM glutamine. BSR-T7/5 cells were cultured in Glasgow MEM (GMEM) (Invitrogen) supplemented with 1 mg/ml G418 (Invitrogen), 10% FBS, 2 mM glutamine, and 1× nonessential amino acids (Invitrogen). Cells were grown to 85 to 95% confluence and then infected with VSV (Indiana serotype, Orsay strain) in growth medium at a multiplicity of infection (MOI) of 10 PFU/cell.
Cytosol and membrane fractionation.
Cytosolic and membrane fractionation were essentially performed as described previously (40). Cells were harvested on ice, and all procedures were performed at 4°C. Cells were gently washed once with ice-cold phosphate-buffered saline (PBS) and then scraped into homogenization buffer containing 25 mM Tris-HCl (pH 7.4), 2 mM EDTA, 10 mM NaCl, and 0.25 M sucrose and supplemented with a phosphatase inhibitor cocktail (PhosSTOP) and a protease inhibitor cocktail (COmplete), as directed by the manufacturer (Roche Applied Science). The cells were allowed to swell on ice for 10 min and then homogenized with 25 strokes of a glass homogenizer. Cell lysates were collected and centrifuged at 2,000 × g for 5 min at 4°C; supernatants were then centrifuged at 100,000 × g for 30 min, and the resulting supernatant was used as the cytosolic fraction. The pellet was gently rinsed with PBS three times and extracted with homogenization buffer containing 1% Triton X-100 for 30 min. The Triton X-100-soluble component was centrifuged at 14,000 × g for 20 min at 4°C, and the resulting supernatant was used as the membrane fraction. Protein concentrations were determined by the Bio-Rad (CA) protein assay using bovine serum albumin (BSA) as a standard.
Immunoblotting and detection.
Infected or mock-infected cells were lysed in 35-mm 6-well dishes for 5 min at 4°C using 250 μl of NP-40 lysis buffer (Boston BioProducts Inc.) supplemented with a phosphatase inhibitor cocktail (PhosSTOP) and a protease inhibitor cocktail (COmplete) as directed by the manufacturer (Roche Applied Science). Lysates were collected and spun at 10,000 × g for 5 min at 4°C, and then 100 μl of the supernatant was added to 20 μl of 6× sample buffer (Boston BioProducts Inc.) for SDS-PAGE. Equal volumes of lysate were electrophoresed on either 12% or 15% SDS-PAGE gels. After electrophoresis, gels were electroblotted onto a polyvinylidene difluoride (PVDF) membrane (0.2 μm; Bio-Rad) and blocked with 5% (wt/vol) nonfat dry milk in TBS-T (Tris-buffered saline [pH 7.6], 0.1% Tween 20). Primary antibodies were diluted in 5% (wt/vol) BSA (fraction V)-TBS-T as recommended by the manufacturer (Cell Signaling Technologies, Danvers, MA). Anti-mouse IgG and anti-rabbit IgG horseradish peroxidase (HRP)-linked antibodies (Cell Signaling Technologies, Danvers, MA) were diluted to 1:2,000 in 5% (wt/vol) nonfat dry milk in TBS-T.
Detection and quantification of cellular PIP3 levels.
Total cellular PIP3 levels were determined by using a PIP3 mass strip kit (K-2400; Echelon Biosciences Inc.). The extraction and quantification of total cellular PI (3,4,5)P3 levels from cells was carried out by following the supplier's protocol. Briefly, cells were scraped off and collected at 4°C in 4 ml of 0.5 M trichloroacetic acid (TCA), pelleted at 1,500 rpm, and washed with 5% TCA, 1 mM EDTA. After extraction of neutral lipids with MeOH-CHCl3 (2:1), acidic lipids were extracted with CHCl3, MeOH, 12 N HCl (40:80:1) and vacuum dried (SpeedVac; Savant). Dried samples were redissolved in CHCl3-MeOH-H2O (1:2:0.8) and spotted onto nitrocellulose membranes containing prespotted PIP3 standards, and the membranes were processed by serial incubation in blocking solution (3% fatty acid-free BSA in PBS), PIP3 detector, secondary detector solution, and tertiary detector solution and then detected by chemiluminescent developing solution.
Transfections.
Plasmid transfections into BSR-T7/5 cells were performed with Lipofectamine 2000 reagent as described in the manufacturer's protocol (Invitrogen). Briefly, monolayers of subconfluent BSR-T7/5 cells (a kind gift from Kurt Conzelmann) grown in 35-mm dishes were transfected with a transfection mixture containing 4 μg of plasmid DNA (unless otherwise stated) and 10 μl Lipofectamine 2000 (Invitrogen) in 500 μl Opti-MEM (Invitrogen). After 5 h at 37°C, the transfection mixture was removed and replaced with 2 ml of growth medium (without selection) and incubation continued for a further 16 h at 37°C, after which cells lysates were harvested for analysis. All mock transfections included 4 μg of the pTZ18 vector. Plasmid transfections into COS-7 cells were performed with FuGENE 6 transfection reagent as described in the manufacturer's protocol (Roche).
Plasmids.
The VSV protein expression plasmids pBS-N, pBS-P, pBS-M, pBS-G, pBS-L, and pBS-M-NCP12.1 (31) were a kind gift from Mike A. Whitt. The plasmids pLNCX-myr-HA-Akt1 (which encodes Akt1 with an N-terminal src myristoylation sequence followed by the hemagglutinin [HA] epitope), pLNCX-myr-HA-Akt1 (K179M) (which carries a mutation in the kinase domain), and the empty vector pLNCX were a kind gift from William Sellers (51).
Chemicals, reagents, and antibodies.
All chemicals unless otherwise stated were purchased from Sigma-Aldrich. Insulin (catalog no. I0516) was purchased from Sigma-Aldrich, and epidermal growth factor (EGF) (8916SF) was from purchased from Cell Signaling Technologies (Danvers, MA). Antibodies specific to Akt, phosphorylated Akt (Thr308) [p-Akt (Thr308)], p-Akt (Ser473), mTOR, p-mTOR (Ser2481), GSK3β, p-GSK3β (Ser9), PDK1, p-PDK1 (Ser241), p-PTEN (Ser380), and p-RSK2 (Ser227) were purchased from Cell Signaling Technologies (Danvers, MA) and used at the manufacturer's recommended dilution. The phospho-specific antibody p-PKCγ (Thr514) (1:5,000; PKCγ is protein kinase C γ) was purchased from Epitomics. The antibody against β-actin (1:5,000) was purchased for Santa Cruz Inc. Anti-VSV M, anti-VSV G, and anti-VSV N were a kind gift from Doug Lyles (Wake Forest University) (39).
RESULTS
VSV inactivates the Akt/mTORC1 signaling pathway.
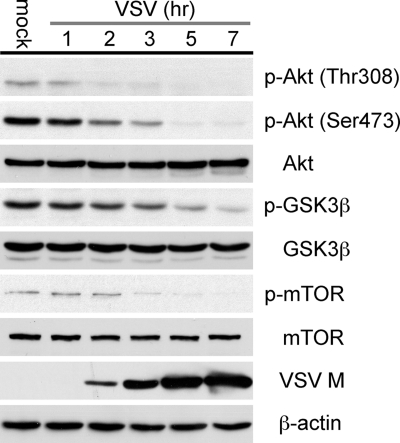
To determine how VSV interacts with the PI3k/Akt signaling pathway, we determined the level of Akt phosphorylation during a VSV infection. BHK cells were infected with VSV at an MOI of 10, and cell lysates were collected at various times between 1 and 7 h postinfection. The lysates were analyzed by immunoblotting to determine the cellular levels of the VSV matrix protein and the levels of Akt phosphorylation at positions 308 (Thr) and 473 (Ser).
As shown in Fig. 1, we could detect Akt phosphorylation in mock-infected cells at both the Thr308 and the Ser473 position. Concurrent with the detection of the VSV matrix protein at 2 h postinfection, we observed a decrease in the level of Akt phosphorylation at both the Thr308 and the Ser473 position. By 7 h postinfection, Akt phosphorylation at both positions was barely detectable. The level of total Akt remained constant at all time points, indicating that the drop in the level of Akt phosphorylation at Thr308 and Ser473 was not due to changes in the levels of cellular Akt but rather to dephosphorylation. In addition, the phosphorylation levels of a direct substrate of Akt, GSK3β (Ser9), and a downstream effector of Akt, mTOR (Ser2481), also showed decreases in their levels of phosphorylation by 2 to 3 h postinfection. This is consistent with the dephosphorylation of Akt and subsequent inactivation of its kinase activity.
FIG. 1.
Akt/mTOR signaling pathway changes following infection with VSV. BHK cells were either mock infected or infected with VSV at an MOI of 10. Cell lysates were collected at various time points and assayed by immunoblotting with antibodies specific to Akt, p-Akt (Thr308), p-Akt (Ser473), mTOR, p-mTOR (Ser2481), GSK3β, p-GSK3β (Ser9), VSV matrix protein (M), and β-actin.
Inactivation of Akt occurs at a step postentry and requires virus replication.
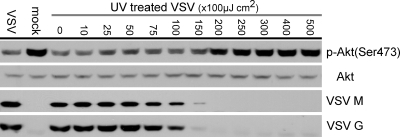
As we observed that Akt dephosphorylation/inactivation occurred between approximately 2 and 3 h postinfection, we postulated that inactivation of the Akt pathway by VSV was replication dependent and not mediated by viral entry. To test this hypothesis, we utilized VSV that had been exposed to increasing amounts of UV-C irradiation. Inactivation of VSV by UV-C irradiation blocks viral RNA genome replication, viral mRNA synthesis, and, consequently, viral protein synthesis but is thought to have little effect on virus receptor binding and the subsequent entry of the virus into the cell (10, 63). HeLa cells were infected with untreated virus or virus that had been treated with increasing amounts of UV-C irradiation at a preirradiation MOI of 10. Cell lysates were collected at 3 h postinfection and analyzed by Western blotting to determine the level of viral protein synthesis and the level of Akt phosphorylation at Ser473.
As shown in Fig. 2, preirradiation of VSV with UV-C light between 0 and 100 × 100 μJ cm2 had little or no effect on the level of viral protein synthesis and the virus-mediated dephosphorylation of Akt at Ser473 (the level of Akt phosphorylation at Ser473 was comparable to that of mock-irradiated virus). Preirradiation of VSV with 150 × 100 μJ cm2 of UV light reduced the level of viral protein synthesis, but this level of viral gene expression was still able to induce the dephosphorylation of Akt. VSV irradiated with UV-C at 200 × 100 μJ cm2 or greater could not drive viral protein synthesis and did not induce the dephosphorylation of p-Akt (Ser473) (the level of Akt phosphorylation at Ser473 was comparable to that of mock-infected cells). This result demonstrated that viral replication is required for the dephosphorylation of Akt.
FIG. 2.
Effect of virus inactivation on Akt dephosphorylation. VSV was inactivated by UV irradiation to determine if viral attachment and entry were sufficient to cause Akt dephosphorylation. HeLa cells were infected with VSV or VSV preirradiated with UV-C light at an MOI of 10. At 3 h postinfection, total cell lysates were collected and subjected to Western blotting to determine the levels of p-Akt (Ser473) and total Akt. The total intracellular levels of the VSV matrix (M) and glycoprotein (G) proteins were also determined to identify the level of UV irradiation necessary to completely inactivate VSV.
VSV-induced dephosphorylation of Akt is dominant over extracellular activation signals.
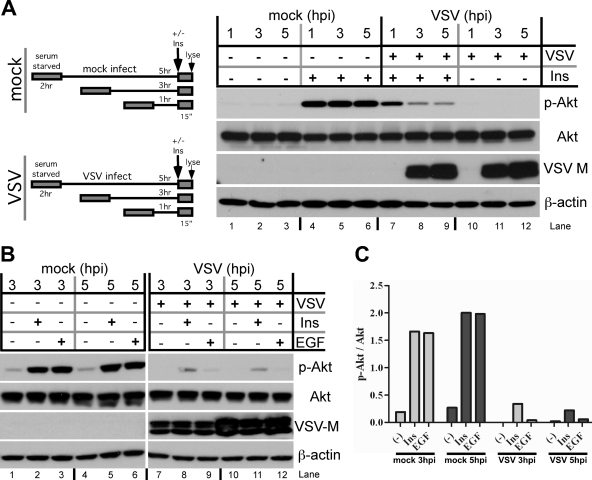
We next wanted to determine if VSV replication rendered Akt insensitive to signaling from extracellular stimuli. To do this, we determined whether a VSV infection could block the receptor tyrosine kinase-driven activation of Akt by insulin stimulation. We examined the phosphorylation status of Akt at Ser473 in VSV- or mock-infected cells at 1, 3, and 5 h postinfection in the absence or presence of insulin stimulation. These experiments were done in serum-starved cells so that growth factors found in serum that can stimulate Akt phosphorylation (the cause of basal phosphorylation levels seen in Fig. 1 and 2) would not complicate interpretation.
In cells that were stimulated with insulin but not infected, Akt phosphorylation at Ser473 was robustly induced after insulin treatment at all three time points (Fig. 3A, lanes 4, 5, and 6). In contrast, Akt phosphorylation after insulin stimulation in VSV-infected cells was noticeably reduced at the 1-h time point (Fig. 3A, lane 7) compared to that of mock-infected cells and markedly inhibited at both the 3- and 5-h time points (Fig. 3A, lanes 8 and 9) compared to that of mock-infected cells stimulated with insulin. Quantification of the data shows that a VSV infection can reduce insulin-induced Akt phosphorylation by 40% at 1 h postinfection and by 80 to 83% at 3 and 5 h postinfection (data not shown). This result demonstrates that VSV can block the phosphorylation of Akt by insulin stimulation during infection.
FIG. 3.
Effect of virus infection on RTK-induced Akt phosphorylation. To measure the stimulation of Akt phosphorylation by insulin (Ins) and EGF in VSV-infected cells, cells were serum deprived for 2 h (45) before being either mock infected or infected with VSV at an MOI 10. (A) Cartoon depicting the experimental procedure. Vero cells were mock infected or infected with VSV at an MOI of 10 in serum-free medium. At 1, 3, and 5 h postinfection (hpi), cells were either mock exposed or exposed to insulin (100 nM) for 10 min. Total cell lysates were collected and subjected to SDS-PAGE and then immunoblotted with antibodies directed against p-Akt (Ser473), total Akt, and the VSV matrix protein (M). Total Akt also served as a loading control. (B) The procedure was as described for panel A, except that cells were either treated with EGF (100 nM) or insulin (100 nM) for 10 to 15 min or left untreated. Total cell lysates were collected and immunoblotted with antibodies directed against p-Akt (Ser473), total Akt, the VSV matrix protein (M), and β-actin. Representative results from two independent experiments are shown. (C) The levels of p-Akt (Ser473) in insulin- and EGF-stimulated cells (Fig. 3B) were quantified by densitometry. The levels of p-Akt (Ser473) were normalized with respect to the level of total Akt found in each lane. Representative results from two independent experiments are shown.
To determine whether VSV can block the activation of Akt by a different class of tyrosine kinase receptors, we stimulated infected cells with insulin or epidermal growth factor (EGF) and again determined Akt Ser473 phosphorylation levels. Both the insulin and EGF tyrosine kinase receptors recruit PI3k to the membrane, but they do so through different mechanisms. The insulin receptor signals through the adaptor protein IRS1 to activate PI3k (34, 44), and the EGF tyrosine kinase receptor (EGFR) signals through direct recruitment of PI3k (47). Thus, we were interested in whether VSV infection blocked one or both signaling strategies. As described for Fig. 3A, we examined the phosphorylation status of Akt (Ser473) in VSV- or mock-infected cells at 3 and 5 h postinfection, in the absence or presence of insulin and EGF. In mock-infected cells, Akt phosphorylation at Ser473 was robustly induced after both insulin and EGF treatment (Fig. 3B, lanes 2, 3, 5, and 6). In contrast, the stimulation of Akt phosphorylation by either insulin or EGF was markedly inhibited at both the 3- and 5-h postinfection time points in VSV-infected cells (Fig. 3B, lanes 8, 9, 11, and 12). Quantification of the data shows that a VSV infection can block both insulin- and EGF-induced Akt phosphorylation by greater than 80% at both the 3- and 5-h postinfection time points (Fig. 3C).
VSV is dominant over a membrane-targeted, constitutively active form of Akt.
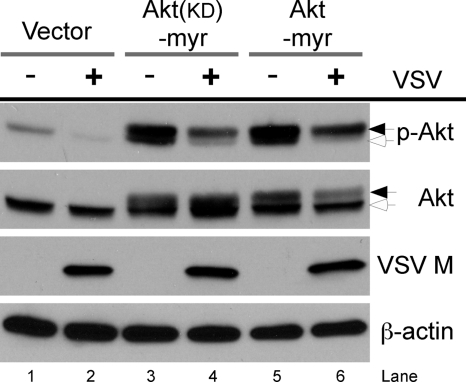
We next tested whether expression of a membrane-targeted, constitutively active form of Akt would be dephosphorylated by VSV replication. For this purpose, we utilized a recombinant clone of Akt that carried a myristoylation signal (myr-AKT). It has previously been established that myr-Akt is activated independently of all upstream signaling events (51). Transfection of cells with either the constitutively active form of Akt (pLNCX-myr-HA-Akt) or a kinase-defective form [pLNCX-myr-HA-Akt (K179M)] resulted in expression of the myr-Akt forms, as confirmed by Western blot analysis (Fig. 4). The slower-migrating band represents the myr-HA-tagged forms of Akt (Fig. 4, lanes 3 to 6, black arrows), and a faster-migrating band represents the endogenous form of Akt seen in all lanes (Fig. 4, white arrows). In mock-infected cells, endogenous Akt and the myr-tagged-Akt forms were found to be strongly phosphorylated at Ser473 (Fig. 4, lanes 1, 3, and 5). In contrast, the levels of Akt phosphorylation at Ser473 in both the endogenous form and the myr-Akt forms were found to be reduced in VSV-infected cells (Fig. 4, lanes 2, 4, and 6), demonstrating that VSV can alter the phosphorylation of both normally and constitutively active forms of Akt.
FIG. 4.
VSV is dominant over the activation of a membrane-targeted form of Akt. Cell lines transiently expressing recombinant clones of Akt were generated by transfecting COS-7 cells with 1 μg of the expression plasmids pLNCX-myr-HA-Akt, pLNCX-myr-HA-Akt (K179M) [lanes Akt(kd)-myr], and pLNCX as an empty vector control. Cell lines were mock infected or infected with VSV at an MOI of 10. At 3 h postinfection (hpi), total cell lysates were collected and subjected to SDS-PAGE and then immunoblotted with antibodies specific for Akt, p-Akt (Ser473), VSV matrix protein (M), and β-actin. Total β-actin served as a loading control. Black arrows, myr-HA-tagged forms of Akt; white arrows, endogenous form of Akt.
VSV is able to bypass the inhibition of Akt dephosphorylation by SV40 ST.
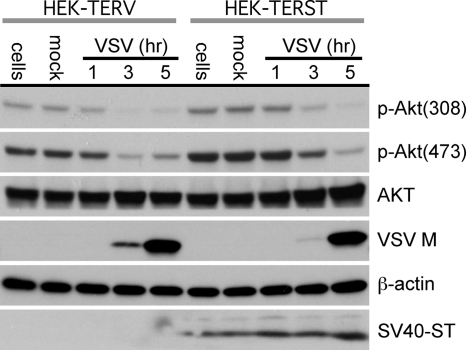
As the phosphate at position 308 of Akt is removed by the serine-threonine protein phosphatase 2A (PP2A) (5, 37), we wanted to test whether VSV induces the dephosphorylation of Akt through PP2A activation. To test this hypothesis, we determined whether VSV was (still) able to induce the dephosphorylation of Akt in cells constitutively expressing the SV40 small t antigen (ST). Previous studies have shown that the SV40 ST can bind to PP2A (54) and inhibit PP2A phosphatase activity (20, 65). The inhibitory effect of ST on PP2A activity leads to an increased and sustained activation of Akt (19).
Subconfluent monolayers of HEK-TERST cells (expressing the ST) and HEK-TERV cells (the parental cell line) (30) were infected with VSV at an MOI of 10 and assayed for viral protein expression and levels of Akt phosphorylation at various time points. As shown in Fig. 5, the detection of VSV M protein demonstrates that VSV was able to infect and replicate in both cell lines and induce the dephosphorylation of Akt at both position 308 and position 473 in each cell line in a time frame similar to that shown in Fig. 1. These data suggest that VSV is able to induce the dephosphorylation of Akt in a manner that can bypass the inhibitory effects of ST on PP2A.
FIG. 5.
VSV is able to overcome SV40 ST-induced Akt phosphorylation. The cell line HEK-TERST, which constitutively expresses the SV40 ST, and its parental cell line, HEK-TERV, were infected with VSV at an MOI of 10. Cell lysates were harvested for analysis at 1, 3, and 5 h postinfection. The phosphorylation levels of Akt and the total protein levels of Akt, VSV M, β-actin, and SV40 ST were determined.
Lipid but not protein regulators of Akt is altered by virus infection.
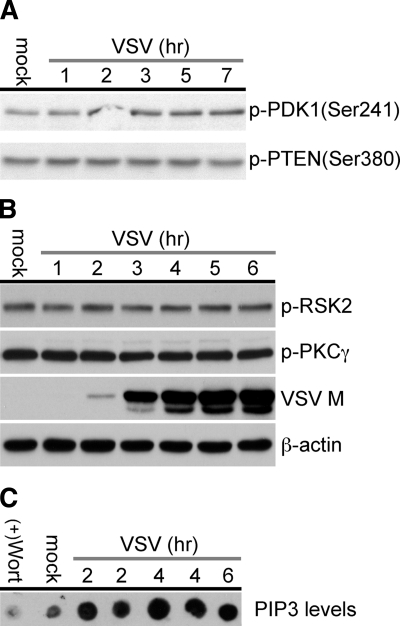
VSV was able to block a positive signal (Fig. 3) that normally drives Akt activation and the phosphorylation of a myr-Akt clone, which suggested that the virus may block upstream signaling proteins in this pathway. To determine which upstream effectors might be inhibited by virus infection, we analyzed cell lysates with phospho-specific antibodies to detect changes in the phosphorylation of PDK1, the activating kinase of Akt, and in phosphatase and tensin homologue deleted on chromosome 10 (PTEN), the PIP3 phosphatase. As shown in Fig. 6A, there was no significant decrease in the level of p-PDK1 (Ser241) or p-PTEN (Ser380) during the VSV time course of infection from 1 to 7 h, suggesting that neither the activation nor the stability of these proteins was altered by VSV.
FIG. 6.
Levels of p-PDK1, p-PTEN, and PIP3 in infected and uninfected cells. (A) Total cell lysates harvested from BHK cells were either mock infected or infected with VSV at an MOI of 10. Cell lysates were collected at various time points and assayed by immunoblotting with antibodies specific to p-PDK1 (Ser241) and p-PTEN (Ser380). (B) As described for panel A, cells were mock infected or infected with VSV at an MOI of 10. Cell lysates were collected at various time points and assayed by immunoblotting with antibodies specific to p-PKCγ (Thr514) and p-RSK2 (Ser227), VSV matrix protein (M), and β-actin. (C) HeLa cells were either mock treated or treated with 10 μM wortmannin for 30 min before being mock infected or infected with VSV at an MOI of 10. Cell lysates were harvested at various time points and the levels of total PIP3 determined as described in Materials and Methods.
We next tested the hypothesis that PDK1's catalytic activity was inhibited and that all substrates of this kinase were no longer being phosphorylated. Both PKCγ and RSK2 are serine/threonine kinases that are phosphorylated by PDK1 within their activation segment at Thr514 and Ser227, respectively (32, 49). Analysis of the level of phosphorylation on the PDK1 substrates PKCγ and RSK2 during VSV infection between 1 and 6 h postinfection (Fig. 6B) demonstrated that VSV replication did not significantly affect the level of either PKCγ (Thr514) or RSK2 (Ser227) phosphorylation. These data demonstrate that VSV replication does not block the phosphorylation of PKCγ or RSK2 by PDK1 and that the kinase activity of PDK1 is still functional.
These results led us to investigate whether levels of lipid cofactors important for Akt activation were changed during virus infection. The presence of PIP3 at the membrane is essential for the activation of Akt through colocalization of Akt and PDK1. Cells were mock infected or infected with VSV at an MOI of 10, and then at increasing times postinfection, PIP3 levels were determined from the lipid extracts of infected cells (Echelon PIP3 mass strip assay kit). Surprisingly, compared to the levels of PIP3 in mock-infected cells, the levels of PIP3 in VSV-infected cells increased significantly above the basal level (mock infected) with time (Fig. 6C). PIP3 levels rose from ∼1 pmol in mock-infected cells to ∼2 pmol by 2 h postinfection and ∼4 pmol by 4 to 6 h postinfection. The data suggest that the PI(4,5)P2 kinase, PI3k, is still active (functional) during a VSV infection and that VSV upregulates PI3k enzyme activity in the cell (Fig. 6C).
VSV replication causes Akt to accumulate at the membrane.
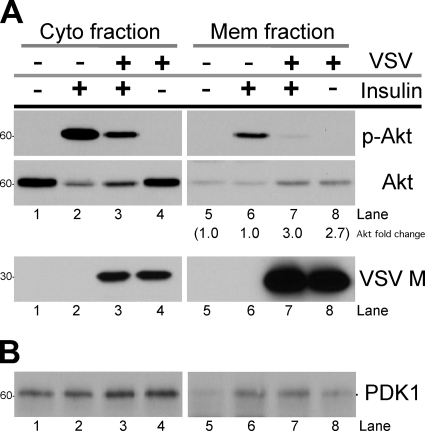
An increase in the level of PIP3 at the plasma membrane is normally associated with the recruitment and colocalization of Akt and PDK1 to the membrane. This promotes protein-protein interaction between the two kinases and leads to the activation of Akt. We asked whether VSV replication blocks the membrane translocation of Akt and/or PDK1 through analysis of the cytosolic and membrane fractions.
In mock-infected cells, total Akt was present primarily in the cytosolic fraction (Fig. 7A, compare Akt lane 1 to lane 5). Upon stimulation with insulin, a portion moved out of the cytosol fraction (compare Akt lane 1 to lane 2), resulting in a marked increase in the levels of Akt phosphorylation in the cytosol and membrane fraction (Fig. 7A, p-Akt lanes 2 and 6). This relocalization of Akt is consistent with that demonstrated in previous reports on the activation of Akt by insulin and growth factors (4, 17, 29). In VSV-infected cells, we observed the same redistribution of Akt from the cytosol upon insulin stimulation (Fig. 7A, Akt lanes 4 and 3), but Akt did not become phosphorylated to the same extent in the cytosolic or membrane fraction (Fig. 7A, p-Akt lanes 3 and 7). We found that there was approximately 2.7- to 3-fold more total Akt in the membrane fractions from VSV-infected cells than the amount seen in the mock-infected membrane fractions (Fig. 7A, compare Akt lanes 5 and 6 to 7 and 8). This was initially unexpected but, when taken together with the increase in PIP3 levels found during a VSV infection (Fig. 6C), demonstrates that Akt is able to translocate to the plasma membrane during a VSV infection, where it accumulates, but that it is not capable of being phosphorylated by PDK1 once it reaches this site.
FIG. 7.
Membrane-associated levels of Akt and PDK1 during VSV infection. (A and B) Vero cells were serum starved for 2 h (45) before being mock infected or infected with VSV at an MOI of 10 in serum-free medium. At 5 h postinfection, cells were stimulated with insulin for 10 to 15 min before being harvested to collect the cytosol (Cyto) and membrane (Mem) fractions as described in Materials and Methods. Equivalents of each fraction were separated on an SDS-PAGE gel and analyzed by immunoblotting with antibodies specific to p-Akt (Ser473), total Akt, and PDK1. The level of VSV M protein was also determined. The level of total Akt in the membrane fractions was determined by densitometry. The level of Akt in mock-infected/mock-treated cells (lane 5) was considered 1.0 and then used to determine the levels in lanes 6 to 8 (shown in parentheses). Data are representative of two experiments.
Unlike the altered behavior of Akt in virus-infected cells, the distributions of PDK1 in the membrane and cytosolic fractions were found to be similar for both mock-infected and VSV-infected cells, with or without insulin stimulation (Fig. 7B). The levels of PDK1 detected in the cytosolic fractions did not significantly change after insulin stimulation (Fig. 7B, compare lane 1 to lane 2 and lane 4 to lane 3), while in the membrane fractions there was found to be a slight increase (Fig. 7B, compare lane 5 to lane 6 and lane 8 to lane 7). The increase in membrane-associated PDK1 is consistent with a portion of cytosolic PDK1 translocating to the membrane after insulin stimulation (3, 56).
Matrix protein induces Akt dephosphorylation in the absence of other viral components.
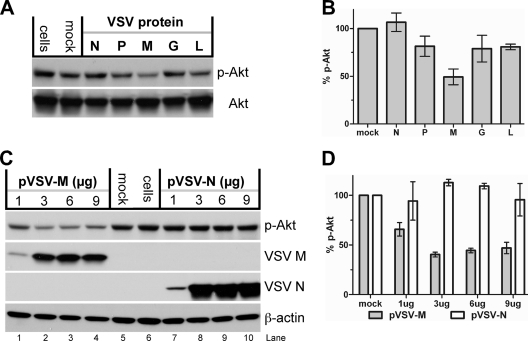
To investigate if expression of a single viral protein was sufficient to induce Akt dephosphorylation, each VSV protein was transiently expressed in cells, and the phosphorylation of Akt was determined. Since transient expression of the VSV matrix protein inhibits polymerase II (Pol II) transcription (1), we expressed the viral proteins using the BSR-T7/5 cell cytoplasmic expression system (15). T7 promoter-driven plasmids encoding each of the five VSV structural proteins (the kind gift of Mike Whitt, UTHS) were transfected into BSR-T7/5 cells, and their effect on Akt phosphorylation was determined. As shown in Fig. 8A, transient expression of the VSV matrix (M) protein appeared to induce the most significant level of Akt dephosphorylation. Quantification of the data shows that expression of the VSV M protein can reduce Akt phosphorylation by approximately 55% (Fig. 8B), leading us to investigate the effect of increasing concentrations of M on Akt phosphorylation.
FIG. 8.
Transient expression of recombinant VSV proteins. (A) BSR-T7/5 cells were transfected with plasmids encoding the VSV nucleocapsid (pBS-N), phosphoprotein (pBS-P), matrix (pBS-M), glycoprotein (pBS-G), and viral polymerase (pBS-L) individually. After an overnight incubation at 37°C, cell lysates were harvested and the levels of Akt (Thr308) phosphorylation and total Akt determined. (B) The levels of p-Akt in panel A were determined by densitometry. The levels of p-Akt were normalized with respect to the level of total Akt found in each lane and compared to the level in mock-transfected cells, which was considered 100%. The graph represents the data from three independent experiments normalized against each mock-transfected cell lane, which was considered 100%. (C) BSR-T7/5 cells were transfected with various amounts of pBS-M and pBS-N plasmid DNA (μg). Cell lysates were harvested, and the levels of Akt phosphorylation, VSV M, VSV N, and β-actin were determined. (D) The level of Akt phosphorylation in panel C was determined by densitometry. The level of Akt phosphorylation in the liposome-treated lane (mock) was considered 100%.
As shown in Fig. 8C, the expression of low levels of M protein in the cells (transfection of 1 μg DNA [lane 1]) resulted in a reduction of Akt phosphorylation that was further reduced as the level of M protein expression increased (3 to 6 μg DNA [lanes 2 and 3]). No significant decrease in Akt phosphorylation was detected when cells were transfected with 1 to 9 μg of the N protein plasmid (lanes 7 to 10), which served as a control for high levels of cellular expression of another viral protein (Fig. 8D).
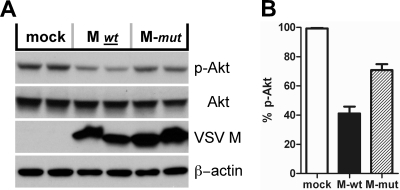
To test whether M protein's known roles in blocking host cell transcription and nuclear/cytoplasmic transport (13, 14, 50) are associated with the dephosphorylation of Akt, we determined whether a mutant M protein with the mutations M33A and M51A [M-(M33,51A)], which is deficient (attenuated) in these functions, would still cause a decrease in Akt phosphorylation. As show in Fig. 9A, both the M wild type and M-(M33,51A) mutant were expressed to similar levels in the cells, but the mutant M-(M33,51A) did not force Akt dephosphorylation to the same extent as wild-type M. When these results were quantified, the level of Akt phosphorylation in M-(M33,51A)-transfected cells was found to be 70% of that of mock-transfected cells versus 40% of that in wild-type-M-transfected cells (Fig. 9B).
FIG. 9.
Effect of transient expression of a VSV M mutant on Akt dephosphorylation. (A) BSR-T7/5 cells were transfected with liposomes only (mock) or with the plasmids pBS-M (M wt) and pBS-M-NCP12.1(M33,51A) (M-mut). Cell lysates were harvested and the levels of Akt phosphorylation and VSV M protein determined. (B) The level of Akt phosphorylation in panel A was determined by densitometry. The level of Akt phosphorylation in the liposome-treated lane (mock) was considered 100%.
DISCUSSION
Here we demonstrate that VSV causes the dephosphorylation and subsequent inactivation of Akt and its signaling pathway at an early stage of infection and that dephosphorylation is found to be dependent on virus replication. This finding is in agreement with previous observations that VSV replication induces the dephosphorylation of 4EB-P1 (21) and downstream effectors of Akt (42) and that VSV replication is not dependent on an active PI3k/Akt signaling pathway (24). This runs counter to what has been seen for other viruses and even other negative-strand RNA viruses, such as influenza A virus and RSV, which are known to activate Akt (26, 41, 60). VSV's inactivation of Akt is reminiscent of the Akt inhibition seen during measles infection (6). Measles virus is thought to inactivate Akt in a replication-independent manner through the induction of a cellular lipid phosphatase that alters the concentration of PIP3 at the membrane (7), while we find that VSV blocks in a replication-dependent manner that is independent of PIP3 and involves the viral matrix protein.
VSV was able to interrupt normal receptor tyrosine kinase-driven Akt activation. Insulin and EGF stimulation was markedly blunted in infected cells, and this dominance of signaling was present throughout the course of the infection. This appears to be due to the effect of virus infection on Akt specifically and not due to the inactivation of tyrosine kinase signaling, as signaling to PI3k to synthesize PIP3 and activate the mitogen-activated protein (MAP) kinase extracellular signal-regulated kinases 1/2 (ERK1/2) (data not shown) was still intact. Thus, virus infection effectively decouples Akt activation from growth factor-mediated stimulation.
This decoupling/inactivation of Akt highlights a novel mechanism of interacting with this signaling pathway. Infection of cells with virus did decrease phosphorylation of Akt but did not alter total cellular levels or the activity of PDK1 (Fig. 6A), PDK1's subcellular localization (Fig. 7), or the levels of phosphorylation of other PDK1 substrates (Fig. 6B). Analysis of subcellular fractions determined that VSV did not keep Akt from translocating to the membrane. Akt levels at the membrane were in fact found to be approximately 3-fold higher than found in mock-infected cells. This observation is consistent with the significant increase in PIP3 levels detected during VSV replication. Thus, VSV must block the activation of Akt after membrane localization by either disrupting the interaction of PDK1 and Akt at the membrane during infection or blocking access to the phosphorylation site(s) on Akt.
Our data are consistent with the model in which VSV replication blocks the phosphorylation of Akt, and this block is dominant over the external stimuli of growth factors to phosphorylate and activate Akt. The rapid decrease in the level of phosphorylated Akt detected during VSV replication is likely due to constitutive cellular phosphatase activity leading to “run-down.”
This block/disruption of Akt phosphorylation appears to be mediated at least in part by the viral matrix protein (M). M, a peripheral membrane protein, was sufficient to induce the dephosphorylation of Akt (Fig. 9A) in transfection experiments. This control of Akt is due at least in part to the protein's ability to block transcription and nuclear/cytoplasmic transport, as a mutant of M that is defective in blocking nuclear/cytoplasmic transport and host transcription was defective in forcing Akt inactivation (Fig. 9A and B). The generation and characterization of new M protein mutants may help further identify which amino acids are important for M-induced Akt dephosphorylation and whether a specific cellular localization of M is necessary for this phenotype.
A moderate reduction in Akt phosphorylation (∼20% with significant variation) was also found in cells transiently expressing either the VSV P, G, or L protein (Fig. 8B). This effect was not as dramatic as with the M protein, but it is possible that during a virus infection there could be an additive effect of the combination of these single factors that leads to the greatly reduced levels of Akt phosphorylation that we observe. During the course of our studies, we also noticed that increasing the incubation time of VSV G transient expression (>20 h) resulted in a significant drop in the level of Akt phosphorylation (data not shown). We did not pursue this finding further, as this time point also correlated with complete syncytia of the cell monolayer, a phenotype not observed during a VSV infection and therefore one that we presumed to be an artifact of transfecting cells in tissue culture.
What gain does the virus derive from Akt inactivation? Earlier publications have suggested that active Akt signaling can decrease VSV replication (42). In addition, Akt signaling has recently been shown to be essential for generating the interferon (IFN)-dependent antiviral response and to complement the function of IFN-activated JAK-STAT pathways in cells (33). Thus, the inactivation of Akt by VSV may serve to blunt the IFN response in productively infected cells.
One aspect of interest from these findings pertains to VSV's potential as an oncolytic agent. VSV has previously been shown to be an effective oncolytic agent in a variety of tumor models (8, 9, 22, 58), both on its own and in combination with other therapies (2, 46, 62). While there have been several studies analyzing why cancer cells are susceptible to infection (11), the primary signaling pathway by which the virus induces apoptosis in these cells has not been elucidated, though both the Bcl-2 pathway and ASK1/DAXX pathways have been implicated (27, 48). Inactivation of Akt/PKB can stimulate both of these pathways (12, 35), suggesting that this action is a key regulator of VSV-mediated cell killing and may explain how cells can be directed into different apoptotic pathways (28, 36).
Our findings could help guide the future development of new oncolytic VSV strains. The natural ability of VSV to block oncogenic signaling through Akt can be useful in identifying potential synergistic effects of combination therapies. As an example, Alain et al. (2) recently reported that pretreatment of a malignant glioma with the mTORC1 inhibitor rapamycin potentiated the oncolytic effect of VSV in vivo and ex vivo. Based on our findings, the combination of VSV and the mTOR inhibitor is predicted to have delivered a “double hit” to the Akt signaling axis (2) making it a highly potent antiproliferative combination.
Acknowledgments
We thank Rachel Fearns, Geoffrey M. Cooper, and members of the Connor lab for critical reading of the manuscript, Kurt K. Conzelmann for kindly sharing BSR-T7/5 cells, William C. Hahn for the HEKTERV and HEKTER-ST cell lines, and Peter Sellers for sharing the constitutively active Akt constructs. Both Mike A. Whitt and Douglas S. Lyles generously shared reagents helpful for this study.
This work was supported by Public Health Service grant AI064606 from the NIH and NERCE grant NIAID U54 AI057159. J.H.C. was supported in part through a Peter T. Paul Career Development Professor Award.
Footnotes
Published ahead of print on 27 October 2010.
REFERENCES
- 1.Ahmed, M., and D. S. Lyles. 1998. Effect of vesicular stomatitis virus matrix protein on transcription directed by host RNA polymerases I, II, and III. J. Virol. 72:8413-8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alain, T., X. Lun, Y. Martineau, P. Sean, B. Pulendran, E. Petroulakis, F. J. Zemp, C. G. Lemay, D. Roy, J. C. Bell, G. Thomas, S. C. Kozma, P. A. Forsyth, M. Costa-Mattioli, and N. Sonenberg. 2010. Vesicular stomatitis virus oncolysis is potentiated by impairing mTORC1-dependent type I IFN production. Proc. Natl. Acad. Sci. U. S. A. 107:1576-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, K. E., J. Coadwell, L. R. Stephens, and P. T. Hawkins. 1998. Translocation of PDK-1 to the plasma membrane is important in allowing PDK-1 to activate protein kinase B. Curr. Biol. 8:684-691. [DOI] [PubMed] [Google Scholar]
- 4.Andjelkovic, M., D. R. Alessi, R. Meier, A. Fernandez, N. J. Lamb, M. Frech, P. Cron, P. Cohen, J. M. Lucocq, and B. A. Hemmings. 1997. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 272:31515-31524. [DOI] [PubMed] [Google Scholar]
- 5.Andjelkovic, M., T. Jakubowicz, P. Cron, X. F. Ming, J. W. Han, and B. A. Hemmings. 1996. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc. Natl. Acad. Sci. U. S. A. 93:5699-5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avota, E., A. Avots, S. Niewiesk, L. P. Kane, U. Bommhardt, V. ter Meulen, and S. Schneider-Schaulies. 2001. Disruption of Akt kinase activation is important for immunosuppression induced by measles virus. Nat. Med. 7:725-731. [DOI] [PubMed] [Google Scholar]
- 7.Avota, E., H. Harms, and S. Schneider-Schaulies. 2006. Measles virus induces expression of SIP110, a constitutively membrane clustered lipid phosphatase, which inhibits T cell proliferation. Cell. Microbiol. 8:1826-1839. [DOI] [PubMed] [Google Scholar]
- 8.Balachandran, S., and G. N. Barber. 2000. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life 50:135-138. [DOI] [PubMed] [Google Scholar]
- 9.Balachandran, S., M. Porosnicu, and G. N. Barber. 2001. Oncolytic activity of vesicular stomatitis virus is effective against tumors exhibiting aberrant p53, Ras, or myc function and involves the induction of apoptosis. J. Virol. 75:3474-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ball, L. A., and C. N. White. 1976. Order of transcription of genes of vesicular stomatitis virus. Proc. Natl. Acad. Sci. U. S. A. 73:442-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barber, G. N. 2005. VSV-tumor selective replication and protein translation. Oncogene 24:7710-7719. [DOI] [PubMed] [Google Scholar]
- 12.Bergmann, A. 2002. Survival signaling goes BAD. Dev. Cell 3:607-608. [DOI] [PubMed] [Google Scholar]
- 13.Black, B. L., and D. S. Lyles. 1992. Vesicular stomatitis virus matrix protein inhibits host cell-directed transcription of target genes in vivo. J. Virol. 66:4058-4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blondel, D., G. G. Harmison, and M. Schubert. 1990. Role of matrix protein in cytopathogenesis of vesicular stomatitis virus. J. Virol. 64:1716-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchholz, U. J., S. Finke, and K. K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchkovich, N. J., Y. Yu, C. A. Zampieri, and J. C. Alwine. 2008. The TORrid affairs of viruses: effects of mammalian DNA viruses on the PI3K-Akt-mTOR signalling pathway. Nat. Rev. Microbiol. 6:266-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calleja, V., D. Alcor, M. Laguerre, J. Park, B. Vojnovic, B. A. Hemmings, J. Downward, P. J. Parker, and B. Larijani. 2007. Intramolecular and intermolecular interactions of protein kinase B define its activation in vivo. PLoS Biol. 5:e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carpten, J. D., A. L. Faber, C. Horn, G. P. Donoho, S. L. Briggs, C. M. Robbins, G. Hostetter, S. Boguslawski, T. Y. Moses, S. Savage, M. Uhlik, A. Lin, J. Du, Y. W. Qian, D. J. Zeckner, G. Tucker-Kellogg, J. Touchman, K. Patel, S. Mousses, M. Bittner, R. Schevitz, M. H. Lai, K. L. Blanchard, and J. E. Thomas. 2007. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature 448:439-444. [DOI] [PubMed] [Google Scholar]
- 19.Chen, W., R. Possemato, K. T. Campbell, C. A. Plattner, D. C. Pallas, and W. C. Hahn. 2004. Identification of specific PP2A complexes involved in human cell transformation. Cancer Cell 5:127-136. [DOI] [PubMed] [Google Scholar]
- 20.Chen, Y., Y. Xu, Q. Bao, Y. Xing, Z. Li, Z. Lin, J. B. Stock, P. D. Jeffrey, and Y. Shi. 2007. Structural and biochemical insights into the regulation of protein phosphatase 2A by small t antigen of SV40. Nat. Struct. Mol. Biol. 14:527-534. [DOI] [PubMed] [Google Scholar]
- 21.Connor, J. H., and D. S. Lyles. 2002. Vesicular stomatitis virus infection alters the eIF4F translation initiation complex and causes dephosphorylation of the eIF4E binding protein 4E-BP1. J. Virol. 76:10177-10187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connor, J. H., C. Naczki, C. Koumenis, and D. S. Lyles. 2004. Replication and cytopathic effect of oncolytic vesicular stomatitis virus in hypoxic tumor cells in vitro and in vivo. J. Virol. 78:8960-8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooray, S. 2004. The pivotal role of phosphatidylinositol 3-kinase-Akt signal transduction in virus survival. J. Gen. Virol. 85:1065-1076. [DOI] [PubMed] [Google Scholar]
- 24.Dunn, E. F., R. Fearns, and J. H. Connor. 2009. Akt inhibitor Akt-IV blocks virus replication through an Akt-independent mechanism. J. Virol. 83:11665-11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehrhardt, C., and S. Ludwig. 2009. A new player in a deadly game: influenza viruses and the PI3K/Akt signalling pathway. Cell. Microbiol. 11:863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehrhardt, C., T. Wolff, S. Pleschka, O. Planz, W. Beermann, J. G. Bode, M. Schmolke, and S. Ludwig. 2007. Influenza A virus NS1 protein activates the PI3K/Akt pathway to mediate antiapoptotic signaling responses. J. Virol. 81:3058-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaddy, D. F., and D. S. Lyles. 2007. Oncolytic vesicular stomatitis virus induces apoptosis via signaling through PKR, Fas, and Daxx. J. Virol. 81:2792-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaddy, D. F., and D. S. Lyles. 2005. Vesicular stomatitis viruses expressing wild-type or mutant M proteins activate apoptosis through distinct pathways. J. Virol. 79:4170-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galetic, I., M. Andjelkovic, R. Meier, D. Brodbeck, J. Park, and B. A. Hemmings. 1999. Mechanism of protein kinase B activation by insulin/insulin-like growth factor-1 revealed by specific inhibitors of phosphoinositide 3-kinase—significance for diabetes and cancer. Pharmacol. Ther. 82:409-425. [DOI] [PubMed] [Google Scholar]
- 30.Hahn, W. C., S. K. Dessain, M. W. Brooks, J. E. King, B. Elenbaas, D. M. Sabatini, J. A. DeCaprio, and R. A. Weinberg. 2002. Enumeration of the simian virus 40 early region elements necessary for human cell transformation. Mol. Cell. Biol. 22:2111-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jayakar, H. R., and M. A. Whitt. 2002. Identification of two additional translation products from the matrix (M) gene that contribute to vesicular stomatitis virus cytopathology. J. Virol. 76:8011-8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen, C. J., M. B. Buch, T. O. Krag, B. A. Hemmings, S. Gammeltoft, and M. Frodin. 1999. 90-kDa ribosomal S6 kinase is phosphorylated and activated by 3-phosphoinositide-dependent protein kinase-1. J. Biol. Chem. 274:27168-27176. [DOI] [PubMed] [Google Scholar]
- 33.Kaur, S., A. Sassano, B. Dolniak, S. Joshi, B. Majchrzak-Kita, D. P. Baker, N. Hay, E. N. Fish, and L. C. Platanias. 2008. Role of the Akt pathway in mRNA translation of interferon-stimulated genes. Proc. Natl. Acad. Sci. U. S. A. 105:4808-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keller, S. R., L. Lamphere, B. E. Lavan, M. R. Kuhne, and G. E. Lienhard. 1993. Insulin and IGF-I signaling through the insulin receptor substrate 1. Mol. Reprod. Dev. 35:346-352. [DOI] [PubMed] [Google Scholar]
- 35.Kim, A. H., G. Khursigara, X. Sun, T. F. Franke, and M. V. Chao. 2001. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol. Cell. Biol. 21:893-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kopecky, S. A., and D. S. Lyles. 2003. Contrasting effects of matrix protein on apoptosis in HeLa and BHK cells infected with vesicular stomatitis virus are due to inhibition of host gene expression. J. Virol. 77:4658-4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuo, Y. C., K. Y. Huang, C. H. Yang, Y. S. Yang, W. Y. Lee, and C. W. Chiang. 2008. Regulation of phosphorylation of Thr-308 of Akt, cell proliferation, and survival by the B55alpha regulatory subunit targeting of the protein phosphatase 2A holoenzyme to Akt. J. Biol. Chem. 283:1882-1892. [DOI] [PubMed] [Google Scholar]
- 38.Le Blanc, I., P. P. Luyet, V. Pons, C. Ferguson, N. Emans, A. Petiot, N. Mayran, N. Demaurex, J. Faure, R. Sadoul, R. G. Parton, and J. Gruenberg. 2005. Endosome-to-cytosol transport of viral nucleocapsids. Nat. Cell Biol. 7:653-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lefrancios, L., and D. S. Lyles. 1982. The interaction of antibody with the major surface glycoprotein of vesicular stomatitis virus. I. Analysis of neutralizing epitopes with monoclonal antibodies. Virology 121:157-167. [PubMed] [Google Scholar]
- 40.Li, J., M. R. Hellmich, G. H. Greeley, Jr., C. M. Townsend, Jr., and B. M. Evers. 2002. Phorbol ester-mediated neurotensin secretion is dependent on the PKC-alpha and -delta isoforms. Am. J. Physiol. Gastrointest. Liver Physiol. 283:G1197-G1206. [DOI] [PubMed] [Google Scholar]
- 41.Luthra, P., D. Sun, M. Wolfgang, and B. He. 2008. AKT1-dependent activation of NF-κB by the L protein of parainfluenza virus 5. J. Virol. 82:10887-10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minami, K., Y. Tambe, R. Watanabe, T. Isono, M. Haneda, K. Isobe, T. Kobayashi, O. Hino, H. Okabe, T. Chano, and H. Inoue. 2007. Suppression of viral replication by stress-inducible GADD34 protein via the mammalian serine/threonine protein kinase mTOR pathway. J. Virol. 81:11106-11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mogensen, T. H., and S. R. Paludan. 2001. Molecular pathways in virus-induced cytokine production. Microbiol. Mol. Biol. Rev. 65:131-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Myers, M. G., Jr., J. M. Backer, X. J. Sun, S. Shoelson, P. Hu, J. Schlessinger, M. Yoakim, B. Schaffhausen, and M. F. White. 1992. IRS-1 activates phosphatidylinositol 3′-kinase by associating with src homology 2 domains of p85. Proc. Natl. Acad. Sci. U. S. A. 89:10350-10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ng, Y., G. Ramm, J. A. Lopez, and D. E. James. 2008. Rapid activation of Akt2 is sufficient to stimulate GLUT4 translocation in 3T3-L1 adipocytes. Cell Metab. 7:348-356. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen, T. L., H. Abdelbary, M. Arguello, C. Breitbach, S. Leveille, J. S. Diallo, A. Yasmeen, T. A. Bismar, D. Kirn, T. Falls, V. E. Snoulten, B. C. Vanderhyden, J. Werier, H. Atkins, M. J. Vaha-Koskela, D. F. Stojdl, J. C. Bell, and J. Hiscott. 2008. Chemical targeting of the innate antiviral response by histone deacetylase inhibitors renders refractory cancers sensitive to viral oncolysis. Proc. Natl. Acad. Sci. U. S. A. 105:14981-14986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olayioye, M. A., R. M. Neve, H. A. Lane, and N. E. Hynes. 2000. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 19:3159-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pearce, A. F., and D. S. Lyles. 2009. Vesicular stomatitis virus induces apoptosis primarily through Bak rather than Bax by inactivating Mcl-1 and Bcl-XL. J. Virol. 83:9102-9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pearce, L. R., D. Komander, and D. R. Alessi. 2010. The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 11:9-22. [DOI] [PubMed] [Google Scholar]
- 50.Petersen, J. M., L. S. Her, and J. E. Dahlberg. 2001. Multiple vesiculoviral matrix proteins inhibit both nuclear export and import. Proc. Natl. Acad. Sci. U. S. A. 98:8590-8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramaswamy, S., N. Nakamura, F. Vazquez, D. B. Batt, S. Perera, T. M. Roberts, and W. R. Sellers. 1999. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc. Natl. Acad. Sci. U. S. A. 96:2110-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Randall, R. E., and S. Goodbourn. 2008. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 89:1-47. [DOI] [PubMed] [Google Scholar]
- 53.Robey, R. B., and N. Hay. 2009. Is Akt the “Warburg kinase”? Akt-energy metabolism interactions and oncogenesis. Semin. Cancer Biol. 19:25-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rundell, K. 1987. Complete interaction of cellular 56,000- and 32,000-Mr proteins with simian virus 40 small-t antigen in productively infected cells. J. Virol. 61:1240-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schabbauer, G., J. Luyendyk, K. Crozat, Z. Jiang, N. Mackman, S. Bahram, and P. Georgel. 2008. TLR4/CD14-mediated PI3K activation is an essential component of interferon-dependent VSV resistance in macrophages. Mol. Immunol. 45:2790-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scheid, M. P., M. Parsons, and J. R. Woodgett. 2005. Phosphoinositide-dependent phosphorylation of PDK1 regulates nuclear translocation. Mol. Cell. Biol. 25:2347-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shelly, S., N. Lukinova, S. Bambina, A. Berman, and S. Cherry. 2009. Autophagy is an essential component of Drosophila immunity against vesicular stomatitis virus. Immunity 30:588-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stojdl, D. F., B. D. Lichty, B. R. tenOever, J. M. Paterson, A. T. Power, S. Knowles, R. Marius, J. Reynard, L. Poliquin, H. Atkins, E. G. Brown, R. K. Durbin, J. E. Durbin, J. Hiscott, and J. C. Bell. 2003. VSV strains with defects in their ability to shut down innate immunity are potent systemic anti-cancer agents. Cancer Cell 4:263-275. [DOI] [PubMed] [Google Scholar]
- 59.Sun, M., S. M. Fuentes, K. Timani, D. Sun, C. Murphy, Y. Lin, A. August, M. N. Teng, and B. He. 2008. Akt plays a critical role in replication of nonsegmented negative-stranded RNA viruses. J. Virol. 82:105-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomas, K. W., M. M. Monick, J. M. Staber, T. Yarovinsky, A. B. Carter, and G. W. Hunninghake. 2002. Respiratory syncytial virus inhibits apoptosis and induces NF-kappa B activity through a phosphatidylinositol 3-kinase-dependent pathway. J. Biol. Chem. 277:492-501. [DOI] [PubMed] [Google Scholar]
- 61.Toker, A. 2000. Protein kinases as mediators of phosphoinositide 3-kinase signaling. Mol. Pharmacol. 57:652-658. [PubMed] [Google Scholar]
- 62.Tumilasci, V. F., S. Oliere, T. L. Nguyen, A. Shamy, J. Bell, and J. Hiscott. 2008. Targeting the apoptotic pathway with BCL-2 inhibitors sensitizes primary chronic lymphocytic leukemia cells to vesicular stomatitis virus-induced oncolysis. J. Virol. 82:8487-8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weck, P. K., and R. R. Wagner. 1979. Transcription of vesicular stomatitis virus is required to shut off cellular RNA synthesis. J. Virol. 30:410-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wullschleger, S., R. Loewith, and M. N. Hall. 2006. TOR signaling in growth and metabolism. Cell 124:471-484. [DOI] [PubMed] [Google Scholar]
- 65.Yang, S. I., R. L. Lickteig, R. Estes, K. Rundell, G. Walter, and M. C. Mumby. 1991. Control of protein phosphatase 2A by simian virus 40 small-t antigen. Mol. Cell. Biol. 11:1988-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]