Abstract
Murine fetal thymic organ culture was used to investigate the mechanism by which adenosine deaminase (ADA) deficiency causes T-cell immunodeficiency. C57BL/6 fetal thymuses treated with the specific ADA inhibitor 2′-deoxycoformycin exhibited features of the human disease, including accumulation of dATP and inhibition of S-adenosylhomocysteine hydrolase enzyme activity. Although T-cell receptor (TCR) Vβ gene rearrangements and pre–TCR-α expression were normal in ADA-deficient cultures, the production of αβ TCR+ thymocytes was inhibited by 95%, and differentiation was blocked beginning at the time of β selection. In contrast, the production of γδ TCR+ thymocytes was unaffected. Similar results were obtained using fetal thymuses from ADA gene-targeted mice. Differentiation and proliferation were preserved by the introduction of a bcl-2 transgene or disruption of the gene encoding apoptotic protease activating factor–1. The pan-caspase inhibitor carbobenzoxy-Val-Ala-Asp-fluoromethyl ketone also significantly lessened the effects of ADA deficiency and prevented the accumulation of dATP. Thus, ADA substrates accumulate and disrupt thymocyte development in ADA deficiency. These substrates derive from thymocytes that undergo apoptosis as a consequence of failing to pass developmental checkpoints, such as β selection.
Introduction
Adenosine deaminase (ADA) deficiency was recognized as a cause of severe combined immunodeficiency (SCID) by Giblett and colleagues in 1972 (1). This serendipitous observation revealed the importance of purine metabolism for the development of the human immune system. Concentrations of the ADA substrates adenosine (Ado) and 2′-deoxyadenosine (dAdo) were elevated in the plasma of ADA-deficient patients and dAdo was elevated in urine as well (2). A number of mechanisms by which these two compounds may compromise the development of a normal immune system have been postulated (2, 3). However, progress in evaluating the mechanism of T-cell toxicity due to ADA-deficient SCID has been hampered by lack of an appropriate experimental system. Even the stage in T-cell development that is affected has not been identified because of the difficulty in obtaining thymic tissue from ADA-deficient patients. Likewise, the source of lymphotoxic ADA substrates has not been identified, although early work by Chan (4) and Smith and Henderson (5) suggested that bone marrow macrophages that ingest nuclei extruded from normoblasts during erythropoiesis play an important role. In vitro culture models require the addition of exogenous ADA substrates, making it impossible to draw conclusions about the relative contribution of Ado and dAdo in vivo. Conventional ADA-deficient mice die of liver failure in the immediate perinatal period (6, 7), and normal mice treated with the specific and potent ADA inhibitor 2′-deoxycoformycin (dCF) (8) show signs of hepatic and adrenal toxicity (9). The problem of liver toxicity in ADA-deficient mice has been solved by the introduction of an Ada minigene expressed in the placenta (10). These mice display profound T- and B-cell lymphopenia and reduced concentrations of serum immunoglobulins by 2 weeks of age. They also exhibit several nonimmune phenotypes described in some ADA-deficient patients, including abnormal kidney pathology, enlargement of costochondral junctions, severe rib curvature, and pulmonary insufficiency. This last condition is believed to be responsible for their death at 3 weeks of age. However, stress-induced corticosteroids may also contribute to the immunodeficiency seen in these mice. Therefore, we established fetal thymic organ cultures (FTOCs) from ADA-deficient mice and dCF-treated FTOCs from normal mice as models for human ADA deficiency.
The developmental steps leading to αβ T-cell production in mice have been delineated on the basis of surface expression of CD4 and CD8. Immature cells, expressing neither CD4 nor CD8 (double negative, or DN cells) constitute 1–3% of the normal adult thymus. Cells of intermediate maturity, expressing both CD4 and CD8 (double positive, or DP cells) account for 80% of thymocytes. The remaining approximately 15% of thymocytes are mature single-positive CD4+ or CD8+ cells with high levels of CD3/αβ T-cell receptor (TCR) expression. DN cells can be divided into four subsets according to the expression of CD44 and CD25: CD44+CD25– (DNI), CD44+CD25+ (DNII), CD44–CD25+ (DN III), and CD44–CD25– (DNIV) (11, 12). Cells in the DNI and DNII stages have TCR genes largely in germline configuration; those in the DNIII stage show extensive TCR D→Jβ rearrangements and some V→DJβ rearrangements (13). When productive TCR β-chain rearrangement takes place and a functional pre-TCR is expressed, the cells transit to the DNIV stage (14), a process known as β selection. Approximately four of nine early thymocytes die because they fail to make a productively rearranged TCR-β chain (15). The consequences of β selection include rapid proliferation, phenotypic maturation, allelic exclusion at the TCR Vβ locus, and germline transcription of the TCR-α locus. These events are mediated by signaling through the pre-TCR (16), which consists, at least in part, of the pre–TCR-α chain, a productively rearranged TCR-β chain, and certain components of the CD3 complex (17). Signaling through the pre-TCR requires the lymphocyte-specific tyrosine kinase lck and results in both downregulation of CD25 and upregulation of CD4 and CD8 (18). Thus, mice with targeted disruptions of the RAG, pre–TCR-α, or TCR-β genes, as well as mice expressing a dominant negative form of lck, are arrested at the CD44–CD25+ DNIII stage (11, 18–22).
We show here that thymocyte development is also inhibited beginning at the DNIII stage in ADA-deficient FTOCs. Given that exogenous substrates are not needed to see the effects of a lack of ADA in this model, murine FTOC provides an excellent system to delineate the biochemical mechanism(s) that leads to a failure of T-cell development in ADA-deficient SCID (3). We also show that the accumulation of dATP, presumably derived from the ADA substrate dAdo, and the inhibition of thymocyte development, depend on the induction of apoptosis, as the effects of ADA deficiency in culture can be significantly lessened by the pan-caspase inhibitor carbobenzoxy-Val-Ala-Asp-fluoromethyl ketone (z-VADfmk). Even more protection is afforded by a bcl-2 transgene or targeted deletion of the gene encoding apoptotic protease–activating factor-1 (Apaf-1), implicating mitochondrial-dependent apoptosis as a key feature of the T-cell immunodeficiency in ADA-deficient SCID.
Methods
Mice.
C57BL/6 mice (Taconic Farms, Germantown, New York, USA; or The Jackson Laboratory, Bar Harbor, Maine, USA), RAG-2–/– mice (Taconic Farms), and transgenic mice expressing bcl-2 under the control of the lck proximal promoter (The Jackson Laboratory) (23) were bred in our animal facility under specific pathogen-free conditions. ADA-deficient mice have been described previously (7); the targeted allele has been designated “m1.” Apaf-1–/– mice (24) were obtained from T. Mak and H. Yoshida (Amgen Research Institute, Toronto, Canada). NotchIC (25) transgenic mice were provided by E. Robey (University of California, Berkeley, California, USA).
FTOC.
ADA-deficient fetuses were obtained from matings of heterozygous Adam1/+ parents. Fetal thymuses were removed from timed pregnant mice on day 14 or 15 of gestation (plug day = day 0) and separated into individual lobes. Both lobes from each thymus were cultured at 37°C in an humidified atmosphere of 5% CO2 in air in individual wells of 24-well culture plates on cellulose ester filters (Millipore Corp., Bedford, Massachusetts, USA) resting upon Gelfoam sponges (Pharmacia and Upjohn Co., Kalamazoo, Michigan, USA) in 2 ml of serum-free medium (HY-CCM-1; Hyclone Laboratories, Logan, Utah, USA) supplemented with 2 mM glutamine, nonessential amino acids, 5 × 10–5 M 2-mercaptoethanol, penicillin (100 U/ml), streptomycin (100 μg/ml), and amphotericin B (2.5 μg/ml). FCS was not used as it contains ADA. ADA enzyme assays (26) were performed on each liver to determine which fetuses were ADA deficient. The medium was changed after 3 days. When the lobes were harvested on day 5, two pools were made, one consisting of Adam1/m1 lobes and the other, Adam1/+ plus Ada+/+ lobes. Single-cell suspensions were made by pushing the lobes through 70-μm nylon screens, and the thymocytes were counted and characterized for cell-surface phenotype. In experiments using C57BL/6 fetuses, thymuses were separated into individual lobes that were randomized and cultured on Gelfoam rafts in RPMI 1640 supplemented with 10% FCS, 2 mM glutamine, nonessential amino acids, 5 × 10–5 M 2-mercaptoethanol, penicillin (100 U/ml), streptomycin (100 μg/ml), and amphotericin B (2.5 μg/ml) in the presence and absence of 5 μM dCF (Parke Davis, Ann Arbor, Michigan, USA; and SuperGen Inc., San Ramon, California, USA). S-adenosylhomocysteine (SAH) hydrolase enzyme assays were performed as described previously (6).
The pan-caspase inhibitor z-VADfmk and control peptide z-YVADfmk were obtained from Enzyme Systems Products (Livermore, California, USA) and stored frozen in DMSO. In FTOCs using this compound, all cultures contained equivalent concentrations of DMSO (0.25%).
Antibodies and immunofluorescent staining.
The following antibodies were used: FITC-rat anti-CD4, PE-rat anti-CD8, biotinylated hamster anti-αβ TCR or biotinylated hamster anti-γδ TCR + streptavidin Cy-Chrome or FITC-avidin, PE-hamster anti-αβ TCR, PE-hamster anti-γδ TCR, FITC-rat anti-CD25, PE-rat anti-CD44, Cy-Chrome-rat anti-CD4, and Cy-Chrome-rat anti-CD8. All antibodies were from PharMingen (San Diego, California, USA). Staining was performed as described previously (27) using isotype-matched rat myeloma proteins or irrelevant hamster mAb’s as negative controls. Propidium iodide (PI) was added to all single- or two-color stains so that dead cells could be excluded from analysis. Data were collected on 10,000 cells for single-color stains and on 20,000–50,000 cells for multicolor stains using a Becton-Dickinson FACScan or FACSCalibur (Mountain View, California, USA). The data were analyzed using Lysis II or CellQuest software (Becton-Dickinson).
Cell-cycle analysis.
Thymic lobes were harvested at various times after the initiation of FTOCs and single cell suspensions were made. Cell cycle analysis was performed by staining with PI as described previously (28). PI staining was evaluated with a FACScan utilizing doublet discrimination software.
Pre–TCR-α and T early α expression.
Pre–TCR-α and T early α (TEA) expression were evaluated by semiquantitative RT-PCR. RNA was isolated from FTOCs initiated on day 15 of gestation and cultured for 3 (TEA) or 5 (pre–TCR-α) days ± 5 μM dCF. After RT with oligo-dT and AMV RT, the resulting cDNA was subjected to PCR. For the pre–TCR-α, the primers were: 5′, 5′-CTGCAACTGGGTCATGCTTC-3′ and 3′, 5′-TCAGACGGGTGGGTAAGATC-3′ (29). Each PCR cycle consisted of 95°C for 1 minute, 58°C for 1 minute, and 72°C for 1 minute. Aliquots were removed after 26, 29, 32, and 35 cycles, and the PCR products were separated on 2% agarose gels and visualized with ethidium bromide (EtBr) staining. The identity of the pre–TCR-α bands was confirmed by Southern blotting and probing with the following end-labeled oligo: 5′-TTCAAACTGCTTCTGCTC-3′. The amount of pre–TCR-α signal was compared with that obtained with a 1:5 dilution of the cDNA and β actin primers under the same PCR conditions. For TEA transcripts, the PCR primers were: 5′, 5′-GGACAACCTGGCTTAATGGATACG-3′ and 3′, 5′-TTCTCGGTCAACGTGGCATCACAG-3′. PCR was carried out for 38 cycles consisting of 95°C for 30 seconds, 68°C for 45 seconds, and 72°C for 45 seconds. The identity of the PCR product was confirmed by hybridization to an internal probe (5′-CAGCACATCTGCAGGCAGAG-3′).
Anti-CD3 stimulation.
Fetal thymic lobes were removed from RAG-2–/– fetuses at day 15 of gestation and cultured in the presence of hamster anti-CD3 mAb 145.2C-11 (gift of J. Bluestone, University of Chicago, Chicago, Illinois, USA) as described previously (30). The cultures were harvested after 3 days, and RNA was isolated for evaluation of TEA transcripts by RT-PCR. RNA from C57BL/6 day 17 fetal thymocytes was used as a positive control.
dATP measurements.
Thymuses were harvested after 2 days of culture in FTOC and extracted overnight in ten volumes of 60% methanol at –20°C. Cellular dATP levels were determined by HPLC as described previously (7).
Results
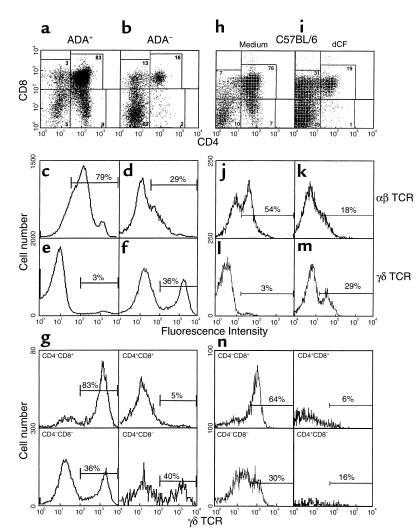
αβ T-cell production is inhibited in ADA-deficient FTOCs, but γδ T cells are spared.
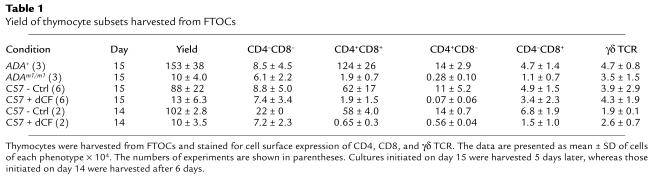
FTOCs were performed on gestational day 15 with fetuses derived from the mating of heterozygous Ada gene-targeted (Adam1/+) mice. After 5 days, thymic lobes from ADA-expressing (i.e., Ada+/+ and Adam1/+) fetuses were pooled and analyzed separately from those from ADA-deficient (i.e., Adam1/m1) fetuses. The numbers of thymocytes recovered from ADA-deficient lobes were only about 5% of those recovered from ADA-positive lobes (Table 1), and differentiation was inhibited past the double negative stage (Figure 1, a and b), resulting in a dramatic decrease in the production of αβ T cells (Figure 1, c and d). The absolute numbers of CD4+CD8+ and CD4+CD8– cells were decreased by 98%. In contrast, the numbers of CD4–CD8+ cells were only 75% reduced, and most of these were γδ T cells (Figure 1g). The relative sparing of γδ T cells resulted in a 12-fold increase in the percentages of γδ T cells in ADA-deficient cultures compared with controls (Figure 1, e and f). The absolute numbers of γδ T cells recovered from ADA-deficient lobes were 75% of those from ADA+ lobes (Table 1). Three-color immunofluorescence showed that most of the γδ T cells were from the CD4–CD8+ and CD4–CD8– populations (Figure 1, b and g). These data show a dramatic difference in the sensitivity of αβ versus γδ T-cell differentiation in ADA-deficient FTOC. It is interesting to note that the numbers of γδ T cells were elevated about twofold in conventional Ada gene-targeted mice compared with control littermates (6). It is important to determine whether a selective sparing of γδ T cells also occurs in ADA-deficient humans.
Table 1.
Yield of thymocyte subsets harvested from FTOCs
Figure 1.
Production of αβ T cells is inhibited in ADA-deficient FTOCs, but γδ T cells are spared. FTOCs were initiated on gestational day 15 using ADA-deficient fetuses (b, d, f, g) and control littermates (a, c, e) or C57BL/6 fetuses in the presence (i, k, m, n) and absence (h, j, l) of 5 μM dCF. After 5 days of culture, thymocytes were harvested and counted. CD4 and CD8 (a, b, h, i), αβ (c, d, j, k), and γδ TCR expression (e, f, l, m) were evaluated with FITC-rat anti-CD4, PE-rat anti-CD8, hamster anti-αβ TCR, and hamster anti-γδ mAb’s. Data from one representative experiment of four to six are presented. γδ TCR expression was evaluated in the four major thymocyte subsets in FTOCs from ADA-deficient (g) or dCF-treated (n) fetal thymuses using three-color flow cytometry. Representative results from one of two experiments are shown. Note: the fluorescence intensities of cells in c–g are different from those in j–n because antibodies conjugated to different fluorochromes were used.
Because the use of Adam1/m1 fetuses in FTOC was cumbersome, we evaluated the ability of the specific and potent ADA inhibitor dCF (8) to mimic the genetically ADA-deficient state. 2′-deoxycoformycin at 5 μM inhibited the production of thymocytes by 85% in FTOCs initiated on day 15 of gestation with C57BL/6 fetuses and harvested 5 days later (Table 1). This concentration inhibited thymic ADA activity by greater than 99% (15 vs. 9,423 nmol/h/mg protein). SAH hydrolase enzyme activity was also inhibited by 90% (12 vs. 132 nmol/h/mg protein), similar to what is seen in erythrocytes from ADA-deficient patients (2). As with genetically ADA-deficient FTOC, thymocyte differentiation past the double negative stage was inhibited (Figure 1, h and i), and the production of αβ TCR+ thymocytes was markedly reduced (Figure 1, j and k). The numbers of CD4+CD8+ and CD4+CD8– cells were decreased by 95% and 99%, respectively (Table 1). As in ADA– cultures, the numbers of CD4–CD8+ cells were more modestly reduced, and most of these were γδ T cells (Figure 1n). The numbers of γδ T cells recovered were equal to or greater than those recovered from control cultures and represented a 10- to 40-fold increase over the numbers of γδ T cells present at the time the cultures were initiated (data not shown). Thus, the outcome of FTOCs performed with the ADA inhibitor dCF closely mimicked that of genetically deficient fetal thymuses. Therefore, most subsequent experiments were performed with normal C57BL/6 fetal thymuses ± dCF. A more limited series of experiments were done with Adam1/m1 fetal thymuses to confirm the results.
Thymocyte differentiation is inhibited within the CD4–CD8– stage under ADA-deficient conditions.
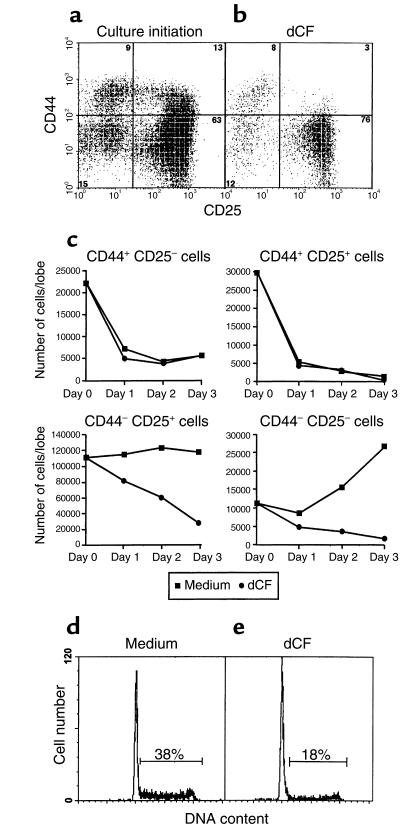
To more precisely delineate the block in thymocyte differentiation caused by a lack of ADA, the CD4–CD8– cells from FTOCs performed with C57BL/6 mice and dCF were characterized for the expression of CD25 and CD44. At the initiation of cultures on day 15 of gestation, about 75% of the thymocytes expressed CD25, and of these, 15–20% were CD44+ (Figure 2a). After 5 days in FTOC with dCF, the percentages of CD25+ cells (in the CD4–CD8– compartment) were essentially unchanged, and almost all of these were CD44– (Figure 2b). Kinetic experiments revealed that the absolute numbers of DNIII and DNIV thymocytes were both markedly reduced in dCF-treated FTOCs harvested on days 1 through 3 of culture (Figure 2c). Similar results were obtained with FTOCs performed with day 15 fetal thymocytes from ADA-deficient mice (data not shown). Therefore, ADA deficiency prevents the entry of thymocytes into, and/or the survival of thymocytes within, the DNIII and DNIV compartments. These data are reminiscent of those obtained when thymocyte development is arrested at the time of β selection (11, 18–22).
Figure 2.
Characterization of thymocytes from ADA-inhibited FTOCs. CD25 and CD44 expression were compared in freshly isolated thymocytes from C57BL/6 fetuses at day 15 of gestation (a) and in those harvested from day 15 FTOCs after 5 days of culture with 5 μM dCF (b). Single-cell suspensions were stained with Cy-Chrome-rat anti-CD4, Cy-Chrome-rat anti-CD8, and FITC-rat anti-CD25 followed by PE-rat anti-CD44. Finally, the stained cells were incubated with PI to allow the exclusion of dead cells. CD25 versus CD44 (a and b) staining of cells negative for PI and Cy-chrome staining is shown for one of three representative experiments. In other experiments (c), cells were harvested on days 1, 2, and 3 of culture, counted, and then stained with PE-anti-CD4, PE-anti-CD8, PE-anti-γδ TCR, FITC-anti-CD25, and biotinylated anti-CD44 plus RED 613-streptavidin. Cells expressing CD4, CD8, and/or γδ TCR (i.e., PE-labeled cells) were excluded from the analysis, and CD44 and CD25 were assessed on the remaining double negative population. In other experiments, thymocytes were harvested from day 15 FTOCs after 2 days of culture in the presence (e) or absence (d) of 5 μM dCF. Cell-cycle analysis was performed by PI staining, and DNA content was evaluated with a FACScan. Data from one representative experiment of four are shown.
ADA deficiency reduces the percentages of dividing cells in FTOCs.
To dissect the mechanism by which a lack of ADA blocks thymocyte production, cell-cycle analyses were performed at various time intervals after the initiation of dCF-treated cultures at day 15 of gestation. After 2 days of culture under control conditions, 38% of thymocytes were in [S + G2 + M] (Figure 2d). In contrast, ADA-inhibited thymic lobes contained only 18% of such cells (Figure 2e). Similar reductions were seen on days 3 and 5 of culture, although the percentages of cells in [S + G2 + M] in both control and treated lobes were smaller at later times (P = 0.031 by a paired t test with four independent experiments). These data are consistent with a failure of thymocytes from ADA-deficient cultures to reach the CD44–D25– DNIV stage, which usually contains a high percentage of cycling cells (13).
ADA deficiency affects thymocyte development between the time of TCR-β and TCR-α gene rearrangements.
Given that ADA deficiency inhibited thymic development at about the time a productively rearranged TCR-β gene is needed for further differentiation, TCR-β gene rearrangements were analyzed in control and dCF-treated thymic lobes using PCR with genomic DNA as described by Lalli et al. (31). The cultures were initiated on day 14 of gestation, before the onset of TCR-Vβ gene rearrangements as detected by this method. The extent of TCR-Vβ3, -Vβ6, -Vβ8, and -Vβ17 gene rearrangements were similar in DNA isolated from fetal thymic lobes cultured for 6 days in medium alone or with 5 μM dCF (data not shown). In contrast, evaluation of TCR-Vα5H, -VαF3, and -Vα2C rearrangements by the same method revealed that little TCR-Vα gene rearrangement had occurred in either dCF-treated or genetically ADA-deficient cultures (data not shown). This is consistent with the observed greater than 95% reduction in the number of CD4+CD8+ thymocytes in these cultures (Table 1). The low numbers of CD4+CD8+ thymocytes cannot be explained by a lack of α-chain gene rearrangement, however, as normal numbers of CD4+CD8+ thymocytes have been reported in TCR-α chain gene–targeted mice (32). Thus, a lack of ADA enzyme activity in FTOC does not appear to inhibit TCR-Vβ gene rearrangements, but blocks differentiation before the stage at which TCR-α rearrangements normally occur.
Pre–TCR-α expression is not inhibited in ADA-inhibited FTOCs.
Because a lack of pre–TCR-α expression could also potentially cause a block in thymocyte differentiation at the CD44–CD25+ DN stage, we evaluated pre–TCR-α expression in control and ADA-inhibited cultures. Semiquantitative RT-PCR showed that dCF treatment did not inhibit expression of the pre–TCR-α in FTOCs (data not shown). Given that both the TCR-β chain and the pre–TCR-α appeared to be expressed normally in dCF-treated FTOCs, the observed block in differentiation is unlikely to be caused by a failure of pre-TCR expression.
Pre-TCR signaling is intact in ADA-deficient FTOC.
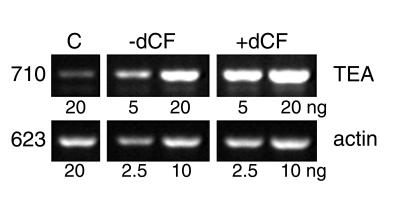
A hallmark of pre-TCR signaling, germline transcription of the TEA genetic element, was examined in ADA-deficient FTOCs. It is difficult to evaluate TEA transcripts in normal thymocyte development because the region of genomic DNA encoding TEA mRNA is deleted as the TCR-α locus rearranges (33). Thus, to look at this aspect of pre-TCR signaling, FTOCs were performed with RAG-2–/– fetal thymuses stimulated with anti-CD3, as the TCR-α locus cannot rearrange in these mice. As shown in Figure 3, the levels of TEA transcripts were similar in control and dCF-treated cultures, demonstrating that a lack of ADA does not inhibit pre-TCR signaling.
Figure 3.
TEA transcription is intact in ADA-inhibited FTOCs. FTOCs were performed with RAG-2–/– fetal thymuses at day 15 of gestation treated with anti-CD3 mAb 145.2C-11 ± 5 μM dCF. After 3 days, thymocytes were harvested and RNA was isolated. TEA transcripts were evaluated by RT-PCR with the indicated amounts of RNA; β-actin mRNA was evaluated as a loading control. The sizes of the two transcripts are indicated in the left margin. The lane labeled “C” contains RNA from a day 17 C57BL/6 fetal mouse thymus as a positive control.
Evaluation of apoptosis in ADA-deficient FTOC.
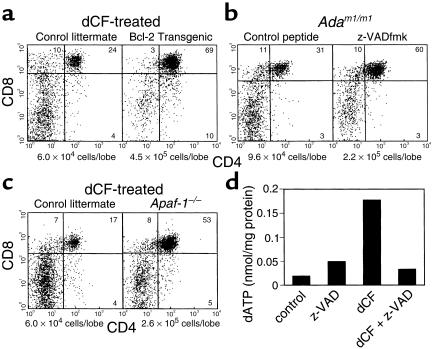
Because pre-TCR signaling appeared to be functional in ADA-deficient FTOCs, we examined apoptosis as an alternative explanation for the observed block in thymocyte differentiation. Cell-cycle analysis by PI staining failed to reveal a significant sub-G0 peak (Figure 2, e and g), and TUNEL staining on tissue sections showed similar percentages of apoptotic cells in control and ADA-deficient cultures (data not shown). However, thymic macrophages are remarkably efficient at removing apoptotic thymocytes (34), so a moderate increase in apoptosis may not be detectable by either of these methods. Indeed, introduction of a bcl-2 transgene under the control of the proximal lck promoter abrogated the consequences of ADA inhibition in FTOCs (Figure 4a). Compared with ADA-inhibited FTOCs performed with control littermates, those with bcl-2 transgenic mice yielded an average 7.6-fold increase in total cell yield and a 35-fold increase in the absolute number of double positive thymocytes. Similarly, the pan-caspase inhibitor z-VADfmk at 100 μM significantly lessened the effects of ADA deficiency in FTOC with Adam1/m1 fetuses (Figure 4b). Compared with cultures with the control peptide z-YVADfmk, those treated with z-VADfmk had a 2.3-fold increase in cell yield and a 4.4-fold increase in the absolute number of double positive thymocytes. Finally, the effects of dCF treatment were also largely overcome in FTOCs performed with Apaf-1–deficient mice (Figure 4c). These mice have a targeted deletion in the gene encoding Apaf-1, a key component of the so-called apoptosome that initiates the apoptotic cascade by coordinating procaspase-9 activation when cytochrome c is released from mitochondria. Compared with ADA-inhibited FTOCs performed with control littermates, those with Apaf-1–/– mice yielded a fourfold increase in total cell yield and a 13-fold increase in the absolute number of double positive thymocytes. Taken together, these data suggest that mitochondrial-dependent apoptosis is an important component of the mechanism that interferes with thymocyte development under ADA-deficient conditions.
Figure 4.
Inhibitors of apoptosis abrogate the effects of ADA deficiency in FTOC. FTOCs were performed with bcl-2 transgenic mice (a), Adam1/m1 mice (b), or Apaf-1–/– mice and control littermates at day 15 of gestation. The ADA inhibitor dCF (5 μM) was added to cultures performed with bcl-2 transgenic mice and Apaf-1–/– mice and control littermates. The pan-caspase inhibitor z-VADfmk or a control peptide, z-YVADfmk, which was demonstrated to be ineffective in abrogating the effects of ADA deficiency in C57BL/6 FTOCs (data not shown), was added at 100 μM to FTOCs performed with Adam1/m1 mice. After 2 days, the cultures were harvested and thymocytes were counted and stained with FITC-anti-CD4 plus PE-anti-CD8. Dead cells were excluded by PI staining. The viable cell recoveries per lobe are shown beneath the dot plots. Representative results are shown from two to four independent experiments. In a separate experiment (d), dATP was measured by HPLC in extracts of 30–35 fetal thymic lobes from normal C57BL/6 mice cultured for 2 days under control conditions or in the presence of z-VADfmk (100 μM), dCF (5 μM), or a combination of both. All cultures contained equivalent concentrations of DMSO (0.25%). The data are representative of three independent experiments.
The accumulation of ADA substrates depends on cells undergoing apoptosis in the thymus.
The ability of a bcl-2 transgene, z-VADfmk, and targeted deletion of the Apaf-1 gene to protect thymocytes from the consequences of ADA deficiency has two possible interpretations. First, accumulated ADA substrates might disrupt thymocyte development by inducing apoptosis. Alternatively, ADA substrates might be derived from the large numbers of cells that normally undergo apoptosis during thymic development. To attempt to distinguish between these two possibilities, dATP was measured in fetal thymic lobes cultured with dCF for 2 days in the presence and absence of z-VADfmk. Elevated dATP is a hallmark of ADA deficiency and correlates with the severity of the disease. As expected, dATP was elevated in dCF-treated thymic lobes compared with controls (Figure 4d). The addition of z-VADfmk completely prevented the intrathymic accumulation of dATP, indicating that ADA substrates were derived from apoptotic cells. These results do not rule out the possibility that accumulated ADA substrates or their metabolites may also induce further apoptosis.
Discussion
Production of αβ T cells is profoundly inhibited in ADA-deficient FTOCs beginning at the CD44–CD25+ DNIII stage. The percentages of cycling cells are reduced compared with control cultures, consistent with their reduced capacity to reach the CD44–CD25– DNIV stage that normally contains the highest percentages of rapidly cycling thymocytes in DN populations (13). Even the small numbers of cells that do reach the DNIV compartment show only low mitotic activity. Mice with targeted deletions of the RAG-1 (11, 22), RAG-2 (21), TCR-β (11), pre–TCR-α (20), or CD3ε (35) genes, as well as mice expressing a dominant negative lck transgene (18), show similar blocks in differentiation, as lck-mediated signaling through the pre-TCR is required for the CD44–CD25+ DNIII → CD44–CD25– DNIV transition. However, a lack of pre-TCR signaling does not appear to be responsible for the failure of thymocyte development in our cultures for the following reasons. First, all the components of the pre-TCR appear to be available. ADA inhibition does not compromise the ability of developing thymocytes to rearrange their TCR-β chain genes. Pre-TCR-α expression is also normal as evaluated by semiquantitative RT-PCR, and CD3ε is expressed as shown by the ability of an anti-CD3ε antibody to induce TEA transcription. Second, and most important, a hallmark of pre-TCR signaling, TEA transcription, is intact in ADA-inhibited cultures.
A lack of ADA does not affect the ability of γδ T cells to differentiate and proliferate in FTOC. While αβ T-cell production is severely inhibited, γδ T-cell numbers increase 10- to 40-fold and approach control levels. One explanation could be increased IL-7 signaling, as this cytokine has been shown (36) to promote the growth of γδ T cells while blocking αβ-cell production in FTOCs. It is unlikely that ADA inhibition acts by dysregulating IL-7 signaling, however, as our results do not mimic what is seen when IL-7 is added to FTOCs and both IL-7 and IL-7 receptor mRNA levels were similar in control and dCF-treated cultures (data not shown). Another scenario is that an abnormal purine metabolite skews the αβ versus γδ lineage decision. Although it appears that TCR gene rearrangements influence this decision (37), other factors such as the transmembrane receptor Notch are also important. Robey and coworkers found that an activated form of Notch favored the αβ over the γδ T-cell fate (25). However, αβ T-cell production was still inhibited by dCF in FTOCs using mice expressing a constitutively active Notch transgene (data not shown). The simplest explanation for our observed difference in the sensitivity of γδ T cells and pre-αβ T cells to the accumulation of ADA substrates is that the two cell types express distinct patterns of purine metabolizing enzymes. This could be because developing αβ T cells undergo extensive proliferation compared with γδ T cells and, therefore, may have adapted to express high levels of deoxynucleoside kinases to facilitate the salvage of deoxynucleosides as DNA precursors from thymocytes undergoing apoptosis. It is difficult to address this issue experimentally, however, as there are no markers to distinguish γδ versus αβ T-cell precursors.
FTOC has several advantages which make it an ideal system to delineate the biochemical mechanism(s) by which a loss of ADA enzyme activity leads to a failure of T-cell differentiation. First, thymocyte differentiation in FTOCs is remarkably similar to that which occurs in vivo (38), making FTOC a more appropriate system than immortalized T-cell lines or mature peripheral blood T cells that have been heavily used in the past. Second, a lack of ADA inhibits thymocyte maturation in FTOCs without the addition of exogenous Ado or dAdo. ADA substrates must accumulate in situ to sufficient concentrations to inhibit thymocyte maturation. This is in striking contrast to other in vitro systems in which dCF alone has no deleterious consequences on cells cultured in suspension (reviewed in ref. 2). Third, ADA-deficient FTOCs exhibit biochemical hallmarks of human ADA deficiency. dATP accumulates and SAH hydrolase enzyme activity is inhibited by about 90%. These features of the human disease were also observed in thymuses of Ada gene-targeted mice (6, 7). Fourth, the use of dCF to mimic ADA deficiency makes it possible to perform experiments with transgenic and gene-targeted mice, which may give insight into the pathogenesis of the disease. Finally, ADA-deficient mice suffer from multiorgan system toxicity. This problem has now been solved by treatment of placentally rescued ADA-deficient mice with low concentrations of ADA conjugated to polyethylene glycol (PEG-ADA) such that their pulmonary insufficiency is ameliorated but their immune deficiency remains intact. In fact, thymocyte differentiation is inhibited past the DN stage in these mice, similar to what was seen in our FTOC experiments (39).
With FTOC, thymocytes are allowed to differentiate in a totally ADA-deficient environment (created either by the use of Adam1/m1 thymuses or normal thymuses plus dCF) in isolation from other organ systems that might influence thymopoiesis. Our data clearly demonstrate that ADA deficiency in the mouse inhibits αβ T-cell differentiation and that thymocytes undergoing apoptosis are the source of ADA substrates that cause lymphotoxicity. We believe that late DN thymocytes are the primary targets because this is the first stage of differentiation when substantial numbers of cells die and provide a source of toxic metabolites. Indeed, it is estimated that four of nine DN thymocytes die because they do not receive a survival signal through the pre-TCR as a consequence of failing to productively rearrange their TCR Vβ genes (15). Based on the early work of Chan (4) and Smith and Henderson (5), macrophages probably ingest apoptotic nuclei and secrete purines derived from degraded DNA. Although these investigators speculated that bone marrow macrophages were largely responsible for the deoxyadenosine observed in ADA-deficient patients, our data show that thymic macrophages can produce enough ADA substrates locally to inhibit thymocyte development. Later stages of thymocyte development may also be sensitive to ADA deficiency, as additional ADA substrates are probably generated by double positive thymocytes that are negatively selected or fail positive selection.
Experiments are in progress to determine which ADA substrate, Ado or dAdo, is responsible for the disruption of thymocyte differentiation that occurs in ADA deficiency. It is tempting to speculate that dATP, derived from dAdo, both induces the release of cytochrome c from mitochondria as shown by Yang and Cortopassi in vitro (40), and also participates in apoptosome formation with Apaf-1, cytochrome c, and procaspase-9 as described by Zou et al. (41). This would then initiate processing of procaspase-9, leading to activation of caspase-3 as demonstrated in cytosolic extracts of HeLa cells by Li et al. (42). Our findings that a bcl-2 transgene and disruption of the Apaf-1 gene both protected FTOCs from the consequences of ADA deficiency are consistent with this hypothesis. Bcl-2 would be expected to inhibit dATP-induced cytochrome c release from mitochondria and Apaf-1 deficiency would prevent activation of caspase-9 and initiation of the apoptotic cascade. However, it is also possible that bcl-2 and Apaf-1 deletion are protective because they prevent the accumulation of ADA substrates as was shown for z-VADfmk. Further experimentation will be necessary to distinguish between these two scenarios. It is also important to note that although z-VADfmk normalized intracellular dATP concentrations, it did not completely abrogate the effects of ADA deficiency in FTOC, so mechanisms besides apoptosis may also be important. These might include aberrant adenosine receptor signaling or inhibition of methylation as a consequence of suicide inactivation of SAH hydrolase. Further exploitation of both FTOC and PEG-ADA–treated ADA-deficient mice will resolve these issues and yield important insight not only into biochemical abnormalities that cause immunodeficiency, but also into novel mechanisms regulating normal and malignant thymocyte development.
Acknowledgments
We gratefully acknowledge Paul Kincade, Carol Webb, Xiao-Hong Sun, Mark Coggeshall, Howard Petrie, and Albert Zlotnik for helpful discussions and Ellen Robey, Xiao-Hong Sun, Hiroki Yoshida, and Tak Mak for the gifts of transgenic mice. We also thank Viji Dandapani for flow cytometric analyses and the OASIS Word Processing Center for figure preparation. This work was supported by grants from the National Institutes of Health (AI 18220 and HD36044 to L.F. Thompson; CA08906 to Regina Resta; HD07843 to M.R. Blackburn; DK46207 to R.E. Kellems; and DK20902 to M.S. Hershfield) and from Enzon Inc. (M.S. Hershfield) and the Oklahoma Center for the Advancement of Science and Technology, OCAST (H97-068, L.F. Thompson).
Footnotes
Hong Jiang’s present address is: Curatek Pharmaceuticals, Elk Grove Village, Illinois, USA.
Regina Resta’s present address is: Albany Regional Cancer Center, Albany, New York, USA.
References
- 1.Giblett ER, Anderson JE, Cohen F, Pollara B, Meuwissen HJ. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972;2:1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- 2.Hershfield, M.S., and Mitchell, B.S. 1995. Immunodeficiency diseases caused by adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency. In The metabolic and molecular bases of inherited disease. C.R. Scriver, A.L. Beaudet, W.S. Sly, and D. Valle, editors. McGraw-Hill Inc., New York, New York, USA. 1725–1768.
- 3.Resta R, Thompson LF. SCID: the role of adenosine deaminase deficiency. Immunol Today. 1997;18:371–374. doi: 10.1016/s0167-5699(97)01047-5. [DOI] [PubMed] [Google Scholar]
- 4.Chan T. Purine excretion by mouse peritoneal macrophages lacking adenosine deaminase activity. Proc Natl Acad Sci USA. 1979;76:925–929. doi: 10.1073/pnas.76.2.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith CM, Henderson JF. Deoxyadenosine triphosphate accumulation in erythrocytes of deoxycoformycin-treated mice. Biochem Pharmacol. 1982;31:1545–1551. doi: 10.1016/0006-2952(82)90379-3. [DOI] [PubMed] [Google Scholar]
- 6.Migchielsen AAJ, et al. Adenosine-deaminase-deficient mice die perinatally and exhibit liver-cell degeneration, atelectasis and small intestinal cell death. Nat Genet. 1995;10:279–287. doi: 10.1038/ng0795-279. [DOI] [PubMed] [Google Scholar]
- 7.Wakamiya M, et al. Disruption of the adenosine deaminase gene causes hepatocellular impairment and perinatal lethality in mice. Proc Natl Acad Sci USA. 1995;92:3673–3677. doi: 10.1073/pnas.92.9.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal RP, Spector T, Parks RE., Jr Tight-binding inhibitors-IV. Inhibition of adenosine deaminases by various inhibitors. Biochem Pharmacol. 1977;26:359–367. doi: 10.1016/0006-2952(77)90192-7. [DOI] [PubMed] [Google Scholar]
- 9.Ratech H, Hirschhorn R, Thorbecke GJ. Effects of deoxycoformycin in mice. III. A murine model reproducing multi-system pathology of human adenosine deaminase deficiency. Am J Pathol. 1985;119:65–72. [PMC free article] [PubMed] [Google Scholar]
- 10.Blackburn MR, Datta SK, Kellems RE. Adenosine deaminase-deficient mice generated using a two-stage genetic engineering strategy exhibit a combined immunodeficiency. J Biol Chem. 1998;273:5093–5100. doi: 10.1074/jbc.273.9.5093. [DOI] [PubMed] [Google Scholar]
- 11.Godfrey DI, Kennedy J, Mombaerts P, Tonegawa S, Zlotnik A. Onset of TCR-beta gene rearrangement and role of TCR-beta expression during CD3-CD4-CD8- thymocyte differentiation. J Immunol. 1994;152:4783–4792. [PubMed] [Google Scholar]
- 12.Pearse M, et al. A murine early thymocyte developmental sequence is marked by transient expression of the interleukin-2 receptor. Proc Natl Acad Sci USA. 1989;86:1614–1618. doi: 10.1073/pnas.86.5.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tourigny MR, Mazel S, Burtrum DB, Petrie HT. T cell receptor (TCR)-beta gene recombination: dissociation from cell cycle regulation and developmental progression during T cell ontogeny. J Exp Med. 1997;185:1549–1556. doi: 10.1084/jem.185.9.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson SJ, Perlmutter RM. A signaling pathway governing early thymocyte maturation. Immunol Today. 1995;16:99–105. doi: 10.1016/0167-5699(95)80096-4. [DOI] [PubMed] [Google Scholar]
- 15.Gärtner F, et al. Immature thymocytes employ distinct signaling pathways for allelic exclusion versus differentiation and expansion. Immunity. 1999;10:537–546. doi: 10.1016/s1074-7613(00)80053-9. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman ES, et al. Productive T-cell receptor beta-chain gene rearrangement: coincident regulation of cell cycle and clonality during development in vivo. Genes Dev. 1996;10:948–962. doi: 10.1101/gad.10.8.948. [DOI] [PubMed] [Google Scholar]
- 17.Groettrup M, et al. A novel disulfide-linked heterodimer on pre-T cells consists of the T cell receptor beta chain and a 33 kd glycoprotein. Cell. 1993;75:283–294. doi: 10.1016/0092-8674(93)80070-u. [DOI] [PubMed] [Google Scholar]
- 18.Levin SD, Anderson SJ, Forbush KA, Perlmutter RM. A dominant-negative transgene defines a role for p56lck in thymopoiesis. EMBO J. 1993;12:1671–1680. doi: 10.1002/j.1460-2075.1993.tb05812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fehling HJ, Krotkova A, Saint-Ruf C, von Boehmer H. Crucial role of the pre-T-cell receptor α gene in development of αβ but not γδ T cells. Nature. 1995;375:795–798. doi: 10.1038/375795a0. [DOI] [PubMed] [Google Scholar]
- 20.Fehling HJ, et al. Restoration of thymopoiesis in pTα–/– mice by anti-CD3ε antibody treatment or with transgenes encoding activated Lck or tailless pTα. Immunity. 1997;6:703–714. doi: 10.1016/s1074-7613(00)80446-x. [DOI] [PubMed] [Google Scholar]
- 21.Shinkai Y, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 22.Mombaerts P, et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 23.Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, Korsmeyer SJ. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991;67:879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida H, et al. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell. 1998;94:739–750. doi: 10.1016/s0092-8674(00)81733-x. [DOI] [PubMed] [Google Scholar]
- 25.Washburn T, et al. Notch activity influences the alpha/beta versus gamma/delta T cell lineage decision. Cell. 1997;88:833–843. doi: 10.1016/s0092-8674(00)81929-7. [DOI] [PubMed] [Google Scholar]
- 26.Boss GR, Thompson LF, O’Connor RD, Ziering RW, Seegmiller JE. Ecto-5′-nucleotidase deficiency: association with adenosine deaminase deficiency and non-association with deoxyadenosine toxicity. Clin Immunol Immunopathol. 1981;19:1–7. doi: 10.1016/0090-1229(81)90042-8. [DOI] [PubMed] [Google Scholar]
- 27.Fox RI, Thompson LF, Huddlestone JR. T gamma cells express T-lymphocyte associated antigens. J Immunol. 1981;126:2062–2063. [PubMed] [Google Scholar]
- 28.Resta R, et al. Insights into thymic purine metabolism and adenosine deaminase deficiency revealed by transgenic mice over expressing ecto-5′-nucleotidase (CD73) J Clin Invest. 1997;99:676–683. doi: 10.1172/JCI119211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saint-Ruf C, et al. Analysis and expression of a cloned pre-T cell receptor gene. Science. 1994;266:1208–1212. [PubMed] [Google Scholar]
- 30.Levelt CN, Ehrfeld A, Eichmann K. Regulation of thymocyte development through CD3. I. Timepoint of ligation of CD3 epsilon determines clonal deletion or induction of developmental program. J Exp Med. 1993;177:707–716. doi: 10.1084/jem.177.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lalli E, Sassone-Corsi P, Ceredig R. Block of T lymphocyte differentiation by activation of the cAMP-dependent signal transduction pathway. EMBO J. 1996;15:528–537. [PMC free article] [PubMed] [Google Scholar]
- 32.Mombaerts P, et al. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu T, Takeshita S, Muto M, Kubo E, Sado T, Yamagishi H. Mouse germline transcript of TCR α joining region and temporal expression in ontogeny. Int Immunol. 1993;5:155–160. doi: 10.1093/intimm/5.2.155. [DOI] [PubMed] [Google Scholar]
- 34.Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100–102. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 35.Malissen M, et al. Altered T cell development in mice with a targeted mutation of the CD3-ε gene. EMBO J. 1995;14:4641–4653. doi: 10.1002/j.1460-2075.1995.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plum J, De Smedt M, Leclercq G. Exogenous IL-7 promotes the growth of CD3-CD4-CD8-CD44+CD25+/– precursor cells and blocks the differentiation pathway of TCR-alpha/beta cells in fetal thymus organ culture. J Immunol. 1993;150:2706–2716. [PubMed] [Google Scholar]
- 37.Aifantis I, Azogui O, Feinberg J, Saint-Ruf C, von Boehmer H. On the role of the pre-T cell receptor in alphabeta versus gamma-delta T lineage commitment. Immunity. 1998;9:649–655. doi: 10.1016/s1074-7613(00)80662-7. [DOI] [PubMed] [Google Scholar]
- 38.Jenkinson EJ, Owen JJT. T cell differentiation in thymus organ cultures. Semin Immunol. 1990;2:51–58. [PubMed] [Google Scholar]
- 39.Blackburn, M.R., et al. 2000. The use of enzyme therapy to regulate the metabolic and phenotypic consequences of adenosine deaminase deficiency in mice: differential impact on pulmonary and immunologic abnormalities. J. Biol. Chem. In press. [DOI] [PubMed]
- 40.Yang JC, Cortopassi GA. dATP causes specific release of cytochrome C from mitochondria. Biochem Biophys Res Comm. 1998;250:454–457. doi: 10.1006/bbrc.1998.9333. [DOI] [PubMed] [Google Scholar]
- 41.Zou H, Li Y, Liu X, Wang X. An APAF-1 cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- 42.Li P, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]