Abstract
The innate immune pathways that contribute to the potent immunogenicity of recombinant adenovirus (rAd) vaccine vectors remain largely undefined. Previous studies assessing innate immunity triggered by vaccine vectors have largely focused on in vitro studies involving antigen-presenting cells and on early in vivo inflammatory responses. Here, we systematically explore the Toll-like receptor (TLR) signaling requirements for the generation of cellular immune responses by intramuscular immunization with common and alternative serotype rAd vectors in mice. Antigen-specific CD8+ T-lymphocyte responses elicited by these rAd vectors were significantly diminished in MyD88−/− mice but not in TRIF−/− or TLR3−/− mice, suggesting the importance of MyD88-dependent TLR signaling. However, the absence of each individual TLR resulted in minimal to no effect on vaccine-elicited cellular immune responses. Moreover, responses were not diminished in IL-1R−/− or IL-18R−/− mice. These data suggest that rAd vectors engage multiple MyD88-dependent signaling pathways, none of which are individually critical; rather, they are integrated to contribute to the potent immunogenicity of rAd vectors. Stimulation of multiple innate immune mechanisms may prove a generalizable property of potent vaccines, and this strategy could be harnessed in the development of next-generation vaccine vectors and adjuvants.
The discovery of pattern recognition receptors (PRRs) as front-line sensors for microbial pathogens has shed new light on the interface between innate and adaptive immunity. The ability of PRRs to recognize pathogens, facilitate maturation of antigen-presenting cells (APCs), and trigger cytokine cascades is critical for shaping subsequent adaptive immunity (3). Given the significance of innate immune sensors in initiating and determining long-lived immunity, there has been intense recent interest in defining the innate signaling requirements of both licensed and experimental vaccines. These insights may enable the design of optimized vaccine vectors and adjuvants against emerging infectious diseases.
Recent studies have demonstrated that the yellow fever vaccine and a modified poxvirus vector can activate multiple PRRs, including several Toll-like receptors (TLRs), suggesting that these vaccines initiate innate immunity by triggering multiple signaling pathways (9, 27, 28). These studies focused on in vitro stimulation of APCs to assess innate responses, and thus, the extent to which these innate responses influence adaptive immunity in vivo remains largely unknown. In this study, we systematically explore the impact of TLR signaling on the immunogenicity of common and alternative serotype replication-incompetent recombinant adenovirus (rAd) vaccine vectors (1, 32) in mice.
Engagement of TLRs initiates signaling cascades that proximally involve the intracellular adaptor protein MyD88 or TRIF (17). All of the known mammalian TLRs recruit MyD88, with the exception of TLR3, which recruits TRIF, and TLR4, which recruits both adaptor proteins. Initiation of downstream signaling cascades leads to activation of transcription factors and secretion of proinflammatory and antiviral cytokines (3, 33). TLR activation on APCs also provides a key signal for dendritic cell (DC) activation and maturation, enabling DCs to prime naïve T lymphocytes and providing a key link between innate and adaptive immunity (14).
Activation of innate immunity by adenoviruses has been reported to occur through both TLR-dependent and TLR-independent pathways (5, 8, 11-13, 25, 35, 38). The importance of TLR activation depends on the specific APC subsets studied, with a critical role reported for TLR9 as an adenovirus sensor in plasmacytoid DCs (pDCs) (8, 38). TLR-independent sensors also appear to contribute to the activation of other APCs by rAds, including DC subsets (11, 25), Kupffer cells, and macrophages (23, 38). In these studies, innate immune activation by rAds was elucidated through in vitro studies of APC subsets and by in vivo assessment of early cytokine expression after high-dose intravenous (i.v.) or intraperitoneal (i.p.) rAd administration. However, the impact of innate immune responses on adaptive cellular immune responses elicited by rAd vector vaccination has remained largely unexplored, and a comprehensive evaluation of TLR signaling requirements in vivo has not previously been conducted. This is true for the common serotype vector rAd type 5 (rAd5) (species C), as well as for the alternative serotype vectors rAd26 (species D) and rAd35 (species B), which are currently being evaluated as potential vaccine candidates in mice (1) and nonhuman primates (21) and in clinical trials. We also included a chimeric rAd5HVR48 vector in which the hexon hypervariable regions (HVRs) of Ad5 were exchanged with those from Ad48 (29).
Intramuscular (i.m.) administration of rAd5, rAd5HVR48, rAd26, and rAd35 vectors in mice resulted in antigen-specific CD8+ T-lymphocyte responses that were significantly dependent on MyD88 signaling but were not dependent on TRIF signaling. Furthermore, we conducted a systematic assessment of individual TLRs and interleukin 1 receptor (IL-1R) family members and demonstrated that no single MyD88-dependent TLR signaling pathway was individually critical, suggesting that rAd vectors interact with multiple TLRs and that the integrated innate immune signals induced by rAd vectors likely contribute to their potent immunogenicity.
MATERIALS AND METHODS
Vector production.
Replication-incompetent rAd5, rAd35, rAd26, and rAd5HVR48 vectors with E1/E3 deleted expressing SIVmac239 Gag were prepared as previously described (1, 7, 24, 29, 34). Briefly, adaptor plasmids containing the left end of the Ad genome in which the E1 region was replaced by the SIVmac239 Gag gene cassette under transcriptional control of the human full-length immediate-early cytomegalovirus (CMV) promoter, and cosmids containing the majority of the Ad genome with a deletion of the E3 region were linearized prior to cotransfection of PER.55K cells with Lipofectamine in T25 flasks. The cells were passaged into T75 flasks after 48 h and maintained until virus cytopathic effect was observed. The vectors were plaque purified, analyzed for transgene expression, amplified in 24 triple-layer T175 flasks, purified by double CsCl gradient ultracentrifugation, and dialyzed into phosphate-buffered saline (PBS) containing 5% sucrose. The purified rAd vectors were stored at −80°C. Virus particle (vp) titers were determined by spectrophotometry. Specific infectivity was assessed by PFU assays.
Mice and immunizations.
Six- to 12-week-old C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Transgenic mice deficient in TRIF, IL-1R1, or IL-18R were also obtained from the Jackson Laboratory (Bar Harbor, ME). MyD88- and TLR9-deficient mice were kindly provided by S. Akira to B. Pulendran. TLR2-, TLR3-, TLR4-, TLR5-, TLR6-, and TLR7-deficient mice were kindly provided by S. Akira to P. Greenberg. All knockout mice were bred on a C57BL/6 background. In all experiments, internal wild-type controls were included to account for variability among laboratories and shipping conditions. The mice were injected i.m. with various amounts of replication-incompetent rAd vectors expressing simian immunodeficiency virus (SIVmac239) Gag in 100 μl sterile PBS in both quadriceps muscles.
Tetramer binding assays.
Tetrameric H-2Db complexes folded around the immunodominant SIV Gag AL11 epitope (AAVKNWMTQTL) (7) were prepared and utilized to stain peptide-specific CD8+ T lymphocytes from mice as previously described (4, 7, 16). For the assessment of peripheral responses, mouse blood was collected in RPMI 1640 containing 40 U/ml heparin. Following lysis of red blood cells, 0.1 μg of phycoerythrin (PE)-labeled Db/AL11 tetramer in conjunction with anti-CD8α-allophycocyanin (Ly-2; Caltag, San Francisco, CA) was utilized to stain AL11-specific CD8+ T lymphocytes. The cells were washed in PBS containing 2% fetal bovine serum (FBS) and fixed in 0.15 ml PBS containing 1.5% paraformaldehyde. Samples were analyzed by two-color flow cytometry on a FACS Array (BD Biosciences, San Diego, CA). Gated CD8+ T lymphocytes were examined for staining with the Db/AL11 tetramer. For the assessment of tissue-specific tetramer-positive responses, lymphocytes were isolated from tissues as described below and stained for multiparametric flow analysis as previously described (16). CD8+ T lymphocytes from naïve mice were utilized as negative controls and exhibited <0.1% background tetramer staining.
ELISPOT assays.
Gag-specific cellular immune responses in vaccinated mice were assessed with gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assays as described previously (7, 24). Overlapping 15-amino-acid peptides spanning the SIVmac239 Gag protein were obtained from the NIH AIDS Research and Reference Reagent Program. Ninety-six-well multiscreen plates (Millipore, Bedford, MA) were coated overnight with 100 μl/well of 10 μg/ml anti-mouse or anti-human IFN-γ (BD Biosciences, San Diego, CA) in endotoxin-free Dulbecco's PBS (D-PBS). The plates were then washed three times with D-PBS containing 0.25% Tween 20 (D-PBS-Tween), blocked for 2 h with RPMI 1640 containing 10% FBS at 37°C, washed three times with D-PBS-Tween, and incubated with 2 μg/ml of pooled Gag peptides or AL11, KV9, or DD13 epitope peptides and 5 × 105 murine splenocytes in triplicate in 100-μl reaction mixture volumes. Following 18 h of incubation at 37°C, the plates were washed nine times with PBS-Tween and once with distilled water. The plates were then incubated with 2 μg/ml biotinylated anti-mouse IFN-γ (BD Biosciences, San Diego, CA) for 2 h at room temperature, washed six times with PBS-Tween, and incubated for 2 h with a 1:500 dilution of streptavidin-alkaline phosphatase (Southern Biotechnology Associates, Birmingham, AL). Following five washes with PBS-Tween and one with PBS, the plates were developed with nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate chromogen (Pierce, Rockford, IL), stopped by being washed with tap water, air dried, and read using an ELISPOT reader (Cellular Technology Ltd., Cleveland, OH). The numbers of spot-forming cells (SFC) per 106 cells were calculated. The medium background levels were typically <10 SFC per 106 cells.
Intracellular cytokine staining (ICS) assays.
The magnitudes and phenotypes of Gag-specific cellular immune responses in vaccinated mice were assessed by multiparameter ICS assays (20). Splenocytes (106) were incubated for 6 h at 37°C with RPMI 1640 containing 10% FBS and 2% penicillin-streptomycin, the SIVmac239 Gag peptide pool consisting of 2 μg/ml of each peptide or 10 pg/ml phorbol myristate acetate and 1 μg/ml ionomycin (Sigma-Aldrich, St. Louis, MO). Monensin (GolgiStop; BD Biosciences, San Diego, CA) and brefeldin A (GolgiPlug; BD Biosciences, San Diego, CA) were added to the cultures after 2 h. The cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD3 (145-2C11) and PerCPCy5.5-conjugated anti-CD8 (53-6.7) (BD Biosciences), fixed, and permeabilized with Cytofix/Cytoperm (BD Biosciences). The cells were then stained intracellularly with allophycocyanin-conjugated anti-IFN-γ (XMG1.2; BD Biosciences) and PE-conjugated anti-IL-2 (JES6-5H4; BD Biosciences). For some experiments, further functionality was assessed with FITC-conjugated antibodies to anti-CD107a and -b (1D4B and ABL-93; BD Biosciences) and PE-Cy7-conjugated anti-tumor necrosis factor alpha (TNF-α) (MP6-X22; BD Biosciences) as described previously (15). The cells were washed and fixed with 1.5% paraformaldehyde. Samples were analyzed with an LSRII Flow Cytometer (BD Biosciences) and analyzed using FlowJo software (TreeStar, Ashland, OR). Approximately 500,000 events were collected per sample. Medium control background staining was typically <0.04% of gated CD8+ T lymphocytes.
Mucosal-lymphocyte isolation.
Mucosal-lymphocyte populations were isolated as previously described (16). Briefly, the small and large bowels, vaginal tract, and respiratory tract were dissected free of associated connective tissue and cut into small pieces. Bowel specimens were incubated with Hanks balanced salt solution supplemented with 0.2 mM EDTA and 10% FBS at 37°C for 30 min with vigorous shaking to release the intraepithelial lymphocyte (IEL) population. Cells from the supernatant were resuspended in 40% Percoll (Sigma) and layered over 67% Percoll; samples were centrifuged at 1,000 × g for 20 min, and lymphocytes were isolated from the interface. After IEL removal, the bowel specimens were washed three times with RPMI 1640 containing 5% FBS supplemented with type II collagenase (Sigma) at 300 U/ml with vigorous shaking. The supernatants from two serial incubations were then pooled and purified on a Percoll gradient as described above. Lymphoid tissues were isolated as previously described (16).
Statistical analysis.
Statistical analysis was performed with GraphPad Prism version 4.01 (GraphPad Software, Inc.; 2004). In all studies, immune responses were determined in individual mice, and the results are presented as means with standard errors. Comparisons among groups were performed by Wilcoxon two-sample tests. For the composite analysis of responses to rAd vectors, immune responses to individual rAd vectors were pooled and comparisons were performed by Wilcoxon two-sample tests. In all cases, P values of <0.05 were considered significant.
RESULTS
Vaccine-elicited CD8+ T-lymphocyte responses are diminished in MyD88-deficient mice.
To determine the significance of TLR signaling for vaccine-elicited CD8+ T-lymphocyte responses, we assessed the immunogenicity of rAd5, rAd5HVR48, rAd26, and rAd35 vectors expressing SIV Gag in wild-type C57BL/6 mice and in MyD88−/− mice. Groups of mice (n = 12 to 16/group) were immunized once i.m. with 3 × 108 vp of rAd5-Gag, rAd5HVR48-Gag, rAd26-Gag, or rAd35-Gag (1, 29, 34). SIV Gag-specific CD8+ T-lymphocyte responses to the immunodominant AL11 epitope (AAVKNWMTQTL) were measured by Db/AL11 tetramer binding assays (7).
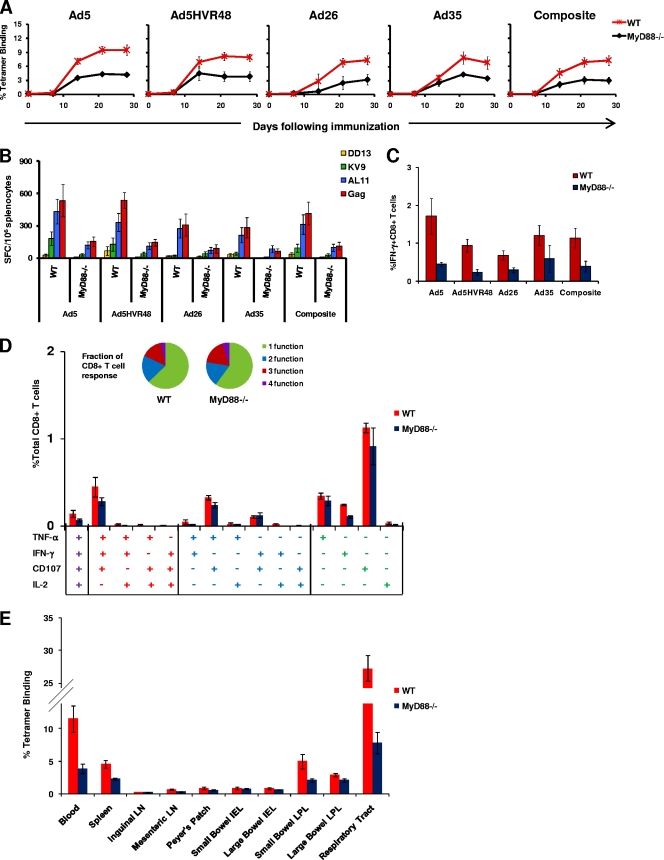
As shown in Fig. 1A, all four vaccine vectors elicited diminished tetramer-positive CD8+ T-lymphocyte responses in MyD88−/− mice compared with wild-type mice. By day 28 postimmunization, rAd5-Gag generated a mean tetramer-positive response of 4.2% in MyD88−/− mice compared with 9.5% in wild-type mice (P < 0.001). Similarly, rAd5HVR48-Gag generated a mean tetramer-positive response of 4.2% in MyD88−/− mice compared with 7.9% in wild-type mice (P < 0.001), rAd26-Gag elicited a mean tetramer response of 3.2% in MyD88−/− mice compared with 7.4% in wild-type mice (P = 0.002), and rAd35-Gag induced a mean tetramer response of 3.4% in MyD88−/− mice compared with 6.9% in wild-type mice (P = 0.027). As the responses appeared similar for all rAd serotypes, we also performed a composite analysis, which showed a mean tetramer response of 3.7% in MyD88−/− mice compared with 7.9% in wild-type mice on day 28 (P < 0.001). These data indicate that MyD88 signaling contributed substantially to the immunogenicity of various rAd vectors in vivo. These differences diminished when this experiment was repeated with a higher dose of 1 × 109 vp of these vectors, suggesting that this partial MyD88 dependence could be overcome by increasing the vector dose (data not shown).
FIG. 1.
Gag-specific CD8+ T-lymphocyte responses in MyD88−/− mice. C57BL/6 (wild type [WT]) and MyD88−/− mice (n = 12 to 16 mice/group) were immunized with a single i.m. injection of 3 × 108 vp of rAd5, rAd5HVR48, rAd26, or rAd35 expressing SIV Gag. (A to C) Gag-specific cellular immune responses were assayed by Db/AL11 tetramer binding assays at multiple time points following injection (A); IFN-γ ELISPOT assays in response to a Gag peptide pool, the Db-restricted CD8+ T-lymphocyte epitopes AL11 and KV9, and the CD4+ T-lymphocyte epitope DD13 (B); and IFN-γ ICS assays by CD8+ T lymphocytes in response to a Gag peptide pool (C). The composite responses represent pooled data from all mice that received rAd vectors representing 4 independent experiments for each of the 4 vectors. (D) Further assessment of the quality of functional Gag-specific responses was performed by multiparameter ICS assays for IFN-γ, IL-2, TNF-α, and CD107 in WT and MyD88−/− mice (n = 4 mice/group) immunized with 3 × 108 vp of rAd26-Gag. Collated data are shown for each individual combination of functions, and the fraction of the total response that is multifunctional (inset). ELISPOT and ICS assays were performed 5 weeks after immunization. (E) Db/AL11 tetramer binding responses in wild-type and MyD88−/− mice (n = 4 mice/group) were determined 2 weeks after immunization i.m. with 1 × 108 vp of rAd5-Gag in multiple anatomic compartments. LN, lymph nodes; IEL, intraepithelial lymphocytes; LPL, lamina propria lymphocytes. Mean responses with standard errors are shown.
The importance of MyD88 for rAd immunogenicity was confirmed by functional IFN-γ ELISPOT and ICS assays performed 5 weeks postimmunization. As shown in Fig. 1B, IFN-γ ELISPOT responses to a Gag peptide pool, the CD8+ T-lymphocyte epitopes AL11 and KV9 (7), and the CD4+ T-lymphocyte epitope DD13 (19) were substantially diminished in MyD88−/− mice compared with wild-type mice for all the rAd vectors studied (P < 0.001), consistent with the tetramer data. ICS assays using a Gag peptide pool further confirmed that antigen-specific IFN-γ+ CD8+ T-lymphocyte responses were reduced in all MyD88 groups (0.39%) compared with wild-type mice (1.13%) (P = 0.028), as shown in Fig. 1C. The functionality of vaccine-elicited CD8+ T-lymphocyte responses was further assessed by multiparameter ICS assays measuring IFN-γ, IL-2, TNF-α, and the cytotoxic degranulation marker CD107 at 5 weeks following immunization of wild-type and MyD88−/− mice with 3 × 108 vp of rAd5-Gag (n = 4 mice/group). As shown in Fig. 1D, the magnitudes of multiple different subsets of CD8+ T-lymphocyte responses were reduced in MyD88−/− mice, although the overall functional profiles were similar.
To determine whether the anatomic distribution of rAd-elicited cellular immune responses was altered by the absence of MyD88, we measured tetramer-positive responses in multiple anatomic compartments following rAd5-Gag immunization. As shown in Fig. 1E, the magnitude of tetramer-positive responses in MyD88−/− mice was not only diminished in the spleen and blood, but trended lower across all other anatomic compartments studied, including the small bowel, large bowel, and respiratory tract. These data show that MyD88−/− mice developed decreased vaccine-elicited CD8+ T-lymphocyte responses in multiple peripheral and mucosal tissue compartments.
Vaccine-elicited CD8+ T-lymphocyte responses are not diminished in TRIF- or TLR3-deficient mice.
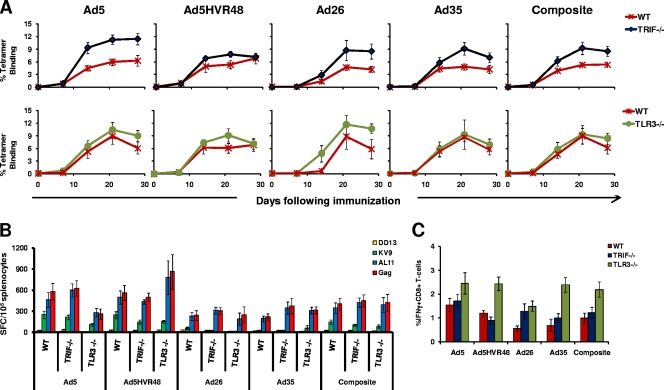
Although most TLRs recruit MyD88 upon activation, TLR3 exclusively recruits the adaptor protein TRIF, and TLR4 may signal through both MyD88 and TRIF (33). To determine whether TRIF or TLR3 contributed to the immunogenicity of rAd vectors, we assessed T-lymphocyte responses elicited by the rAd vectors described above in wild-type, TRIF−/−, and TLR3−/− C57BL/6 mice. In each experiment, internal wild-type C57BL/6 mouse controls were included. Mice (n = 4 to 8/group) were immunized once i.m. with 3 × 108 vp of rAd5-Gag, rAd5HVR48-Gag, rAd26-Gag, or rAd35-Gag. As shown in Fig. 2A, a trend toward higher tetramer-positive responses was observed in TRIF−/− mice compared with the wild-type mice, but these differences were not statistically significant and were not reflected in the functional T-lymphocyte assays (Fig. 2B and C). Similarly, tetramer-positive responses in TLR3−/− mice were comparable to those in wild-type controls. IFN-γ ELISPOT and ICS assays confirmed that responses in the TRIF−/− mice and TLR3−/− mice were comparable to those in wild-type mice (Fig. 2B and C). These data show that antigen-specific CD8+ T-lymphocyte responses were not diminished in the absence of TRIF or TLR3, suggesting that these signaling molecules were not required for rAd vector cellular immunogenicity in this system.
FIG. 2.
Gag-specific CD8+ T-lymphocyte responses in TRIF−/− and TLR3−/− mice. C57BL/6 (WT), TRIF−/−, and TLR3−/− mice (n = 4 to 8 mice/group) were immunized with a single i.m. injection of 3 × 108 vp of rAd5, rAd5HVR48, rAd26, or rAd35 expressing SIV Gag. Gag-specific cellular immune responses were assayed by Db/AL11 tetramer binding assays at multiple time points following injection (A); IFN-γ ELISPOT assays in response to a Gag peptide pool, the CD8+ T-lymphocyte epitopes AL11 and KV9, and the CD4+ T-lymphocyte epitope DD13 (B); and IFN-γ ICS assays by CD8+ T lymphocytes in response to a Gag peptide pool performed 5 weeks postimmunization (C). The composite responses represent pooled data from all mice that received rAd vectors. Mean responses with standard errors are shown.
Vaccine-elicited CD8+ T-lymphocyte responses in TLR2-, TLR4-, TLR5-, TLR6-, TLR7-, and TLR9-deficient mice.
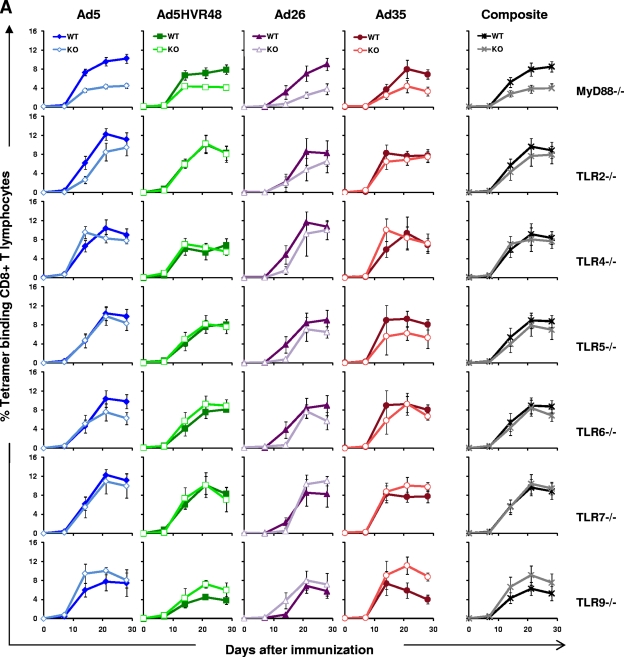
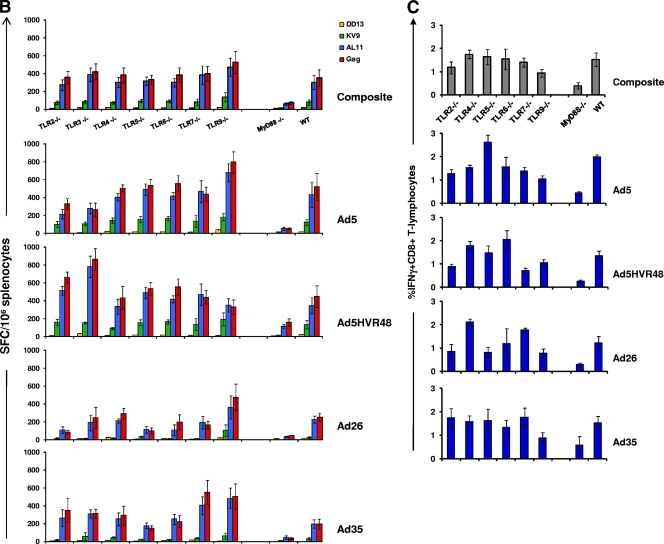
Since multiple TLRs recruit MyD88 upon activation, we performed a systematic evaluation to determine which TLRs were responsible for the MyD88 dependence of rAd immunogenicity. We obtained all the well-characterized individual TLR knockout mice that signal through MyD88, with the exception of TLR11, whose only known ligand is a protozoan antigen from Toxoplasma gondii (36). Note that TLR1 functions as a heterodimer with TLR2, and TLR8 and TLR10 are nonfunctional in mice. We immunized MyD88−/−, TLR2−/−, TLR4−/−, TLR5−/−, TLR6−/−, TLR7−/−, and TLR9−/− mice, as well as wild-type mice (n = 4 mice/group), once i.m. with 3 × 108 vp of rAd5-Gag, rAd5HVR48-Gag, rAd26-Gag, or rAd35-Gag and assessed Gag-specific CD8+ T-lymphocyte responses as described previously.
As shown in Fig. 3A, tetramer-positive responses to all four vectors were diminished in the absence of MyD88, as we observed in the previous experiment (Fig. 1A), but they were not significantly diminished when any individual TLR was absent. Thus, no individual TLR recapitulated the MyD88 dependence of rAd vectors. In TLR4−/− and TLR7−/− mice, tetramer-positive responses were comparable to those in wild-type mice for all vectors studied. There were slight trends toward lower responses to rAd5 and rAd26 in TLR2−/− mice, to rAd26 and rAd35 in TLR5−/− mice, and to rAd5 and rAd26 in TLR6−/− mice, but none of these differences were statistically significant. The tetramer-positive responses to all four vectors in TLR9−/− mice trended higher than responses in wild-type mice, but these differences also were not statistically significant (P = 0.34). The composite analysis of all rAd-immunized mice (n = 16 mice/combined group) confirmed the clear suppression of CD8+ T-lymphocyte responses in MyD88−/− mice, but not in any individual TLR−/− mice. These results were confirmed by functional IFN-γ ELISPOT and ICS assays performed 5 weeks postimmunization, as shown in Fig. 3B and C. Consistent with the tetramer data, functional antigen-specific T-lymphocyte responses were significantly diminished in MyD88−/− mice, but not in the absence of TLR2, TLR4, TLR5, TLR6, TLR7, or TLR9. These data show that the rAd dependence on MyD88 signaling did not rely exclusively upon activation of any single TLR, suggesting that this effect was mediated instead by signaling by multiple TLRs or, alternatively, by a MyD88-dependent non-TLR signaling pathway (2, 23).
FIG. 3.
Gag-specific CD8+ T-lymphocyte responses in MyD88−/−, TLR2−/−, TLR4−/−, TLR5−/−, TLR6−/−, TLR7−/−, and TLR9−/− mice. C57BL/6 (WT) and the respective TLR−/− mice (n = 4 mice/group) were immunized with a single i.m. injection of 3 × 108 vp of rAd5, rAd5HVR48, rAd26, or rAd35 expressing SIV Gag. Gag-specific cellular immune responses were assayed by Db/AL11 tetramer binding assays at multiple time points following injection (A); IFN-γ ELISPOT assays in response to a Gag peptide pool, the CD8+ T-lymphocyte epitopes AL11and KV9, and the CD4+ T-lymphocyte epitope DD13 (B); and IFN-γ ICS assays by CD8+ T lymphocytes in response to a Gag peptide pool (C). ELISPOT and ICS assays were performed 5 weeks after immunization. The composite responses represent pooled data from all mice that received rAd vectors. Mean responses with standard errors are shown.
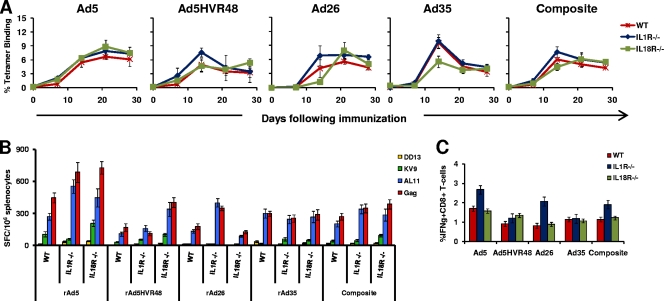
Vaccine-elicited CD8+ T-lymphocyte responses in IL-1R- and IL-18R-deficient mice.
In order to determine if there was a non-TLR signaling pathway responsible for the observed MyD88 dependence, we studied immune responses in mice deficient for the IL-1 receptor family molecules IL-1R and IL-18R, which recruit MyD88 when activated (2, 6). IL-1R has also been reported to be required for the activation of hepatic macrophages by rAd5 (10). We immunized wild-type C57BL/6, IL-1R−/−, and IL-18R−/− mice (n = 4 mice/group) once i.m. with 3 × 108 vp of rAd5-Gag, rAd5HVR48-Gag, rAd26-Gag, or rAd35-Gag. As shown in Fig. 4A, Gag-specific CD8+ T-cell responses to each of the four rAd vectors were comparable in the IL-1R−/− and IL-18R−/− mice compared with the wild-type mice. Functional IFN-γ ELISPOT and ICS assays confirmed comparable responses in wild-type, IL-1R−/−, and IL-18R−/− mice (Fig. 4B and C). These data indicate that neither IL-1R nor IL-18R was required for CD8+ T-lymphocyte responses elicited by rAd vectors.
FIG. 4.
Gag-specific CD8+ T-lymphocyte responses in IL-1R−/− and IL-18R−/− mice. C57BL/6, IL-1R−/−, and IL-18R−/− mice (n = 4 mice/group) were immunized with a single i.m. injection of 3 × 108 vp of rAd5, rAd5HVR48, rAd26, or rAd35 expressing SIV Gag. Gag-specific cellular immune responses were assayed by Db/AL11 tetramer binding assays at multiple time points following injection (A); IFN-γ ELISPOT assays in response to a Gag peptide pool, the CD8+ T-lymphocyte epitopes AL11and KV9, and the CD4+ T-lymphocyte epitope DD13 (B); and IFN-γ ICS assays by CD8+ T lymphocytes in response to a Gag peptide pool (C). ELISPOT and ICS assays were performed 5 weeks after immunization. The composite responses represent pooled data from all mice that received rAd vectors. Mean responses with standard errors are shown.
DISCUSSION
Recombinant Ad vectors represent promising vaccine candidates for a variety of pathogens due to their ability to elicit robust and functional cellular immunity. Elucidating the contribution of innate immunity to the immunogenicity of rAd vectors could potentially assist in the rational design of improved vectors and adjuvants. In this study, we present the first systematic in vivo evaluation of the innate immune signaling requirements for a series of rAd vectors in a vaccination model in mice. Our data show a clear and consistent MyD88 dependence of responses elicited by rAd5, rAd5HVR48, rAd26, and rAd35. However, antigen-specific CD8+ T-lymphocyte responses elicited by these rAd vectors were not significantly diminished in mice lacking TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, or TLR9 or in mice lacking IL-1R or IL-18R. These results indicate that no single TLR or IL-1R family member was individually responsible for the observed MyD88 dependence.
Previous studies exploring innate immune responses to rAd vectors have focused primarily on defining the early inflammatory cytokine responses following i.v. administration of high doses of rAd5 vectors (22, 30). Intravenous delivery of rAd vectors has been shown to activate tissue-specific macrophages in the liver and spleen (10) that likely act as key mediators for secretion of proinflammatory cytokines. In particular, rapid induction of inflammatory responses in the liver due to activation of macrophages (18, 37), as well as direct activation of other hepatocytes (5), has been demonstrated to occur within minutes of i.v. rAd administration (10). In these studies, MyD88 recruitment was shown to contribute significantly to the induction of inflammatory and antiviral cytokines (13, 35, 38). Several APC subsets have also been demonstrated to recognize rAds by non-TLR sensors (25, 38). Additional studies have shown that splenic DCs recognize rAds through an unknown cytosolic sensor (11) and that NALP3 inflammasomes in hepatic macrophages are activated by rAds (23).
The present study confirms and extends these previous results by showing that a series of rAd vaccine vectors exhibit partial MyD88 dependence for the induction of antigen-specific CD8+ T-lymphocyte responses in mice. In particular, MyD88−/− mice vaccinated i.m. with rAd-Gag vectors generated lower-magnitude antigen-specific CD8+ T-lymphocyte responses than wild-type mice, suggesting that one or more TLRs are activated by rAds. However, responses were not completely abolished in MyD88−/− mice, and the partial MyD88 dependence could be overcome by increasing the vector dose (data not shown), indicating that rAds may also activate non-MyD88-dependent innate immune pathways.
To explore the TLR requirements of the observed MyD88 dependence, we systematically assessed responses elicited by rAd-Gag vaccine vectors in a series of mice lacking individual TLRs. Previous studies have reported that TLR9 is critical for pDC activation by rAds in vitro and for cytokine secretion by rAds in vivo, and TLR9 deficiency has been correlated with mildly diminished adaptive immune responses (5, 38). TLR2 has also been reported to contribute to in vivo cytokine secretion and immune responses elicited by rAds (38). In contrast with these previous reports, we observed that antigen-specific CD8+ T-lymphocyte responses elicited by rAd vaccine vectors in vivo were not diminished when any individual TLR was absent. Surprisingly, responses were not blunted and may have even been slightly increased in the absence of TLR9, indicating that TLR9 activation was not critical for the induction of adaptive immune responses by rAd vectors in this model. The differences between our data and prior studies likely reflect different experimental specifics, such as i.m. administration of substantially lower doses of rAd vectors in the present studies. Our data suggest that multiple individual TLRs likely contribute in an additive or synergistic fashion to MyD88 signaling to shape the subsequent adaptive immune responses elicited by rAd vectors in vivo.
We also assessed responses in IL-1R- and IL-18R-deficient mice, as these receptors also recruit MyD88 upon activation. Furthermore, IL-1R is important for the initiation of acute inflammatory responses after i.v. rAd administration (10, 31), and cytokine secretion in response to rAds has been shown to be significantly diminished in IL-1R−/− mice (31). However, the impact of IL-1R on adaptive immune responses elicited by rAds has not previously been reported. We observed that IL-1R and IL-18R signaling did not significantly contribute to rAd vector cellular immunity and thus did not account for the decreased adaptive immune responses observed in MyD88−/− mice. However, we cannot exclude the possibility that IL-1R and IL-18R may still play contributory roles in certain settings.
Defining the innate immune signaling requirements of vaccines will likely be critical for a deeper understanding of the safety and immunogenicity of licensed and experimental vaccines and in the development of next-generation vaccine vectors and adjuvants (26). In this study, we focused on the partial MyD88 dependence of rAds, but other innate immune pathways are likely also triggered by rAds (11, 23), and future studies should examine additional potential innate pathways, such as inflammasomes, RNA helicases, and cytosolic DNA receptors. The successful yellow fever vaccine 17D likely signals through at least four TLRs and a RIG-I-like receptor to generate a highly effective T-cell response, demonstrating the importance of polyvalent innate immune responses (28). Our data suggesting that rAd vaccine vectors also signal through multiple innate immune pathways thus may help explain their potent immunogenicity. These findings also suggest the possibility that highly immunogenic vaccines may in general utilize multiple overlapping innate immune pathways.
Acknowledgments
We thank S. Akira, J. Goudsmit, A. Riggs, M. Lifton, K. Furr, A. Hovav, and M. Cayabyab for generous advice, assistance, and reagents.
This work was supported by Bill and Melinda Gates Foundation grant 38614 (D.H.B.) and NIH grants AI078526 (D.H.B.), AI066924 (D.H.B.), AI066305 (D.H.B.), AI048638 (B.P.), DK057665 (B.P.), and AI057266 (B.P.).
Footnotes
Published ahead of print on 20 October 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Abbink, P., A. A. Lemckert, B. A. Ewald, D. M. Lynch, M. Denholtz, S. Smits, L. Holterman, I. Damen, R. Vogels, A. R. Thorner, K. L. O'Brien, A. Carville, K. G. Mansfield, J. Goudsmit, M. J. Havenga, and D. H. Barouch. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 81:4654-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. [DOI] [PubMed] [Google Scholar]
- 3.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. [DOI] [PubMed] [Google Scholar]
- 4.Altman, J. D., P. A. Moss, P. J. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. [DOI] [PubMed] [Google Scholar]
- 5.Appledorn, D. M., S. Patial, A. McBride, S. Godbehere, N. Van Rooijen, N. Parameswaran, and A. Amalfitano. 2008. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J. Immunol. 181:2134-2144. [DOI] [PubMed] [Google Scholar]
- 6.Arend, W. P., G. Palmer, and C. Gabay. 2008. IL-1, IL-18, and IL-33 families of cytokines. Immunol. Rev. 223:20-38. [DOI] [PubMed] [Google Scholar]
- 7.Barouch, D. H., M. G. Pau, J. H. Custers, W. Koudstaal, S. Kostense, M. J. Havenga, D. M. Truitt, S. M. Sumida, M. G. Kishko, J. C. Arthur, B. Korioth-Schmitz, M. H. Newberg, D. A. Gorgone, M. A. Lifton, D. L. Panicali, G. J. Nabel, N. L. Letvin, and J. Goudsmit. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 172:6290-6297. [DOI] [PubMed] [Google Scholar]
- 8.Basner-Tschakarjan, E., E. Gaffal, M. O'Keeffe, D. Tormo, A. Limmer, H. Wagner, H. Hochrein, and T. Tuting. 2006. Adenovirus efficiently transduces plasmacytoid dendritic cells resulting in TLR9-dependent maturation and IFN-alpha production. J. Gene Med. 8:1300-1306. [DOI] [PubMed] [Google Scholar]
- 9.Delaloye, J., T. Roger, Q. G. Steiner-Tardivel, D. Le Roy, M. Knaup Reymond, S. Akira, V. Petrilli, C. E. Gomez, B. Perdiguero, J. Tschopp, G. Pantaleo, M. Esteban, and T. Calandra. 2009. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 5:e1000480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Di Paolo, N. C., E. A. Miao, Y. Iwakura, K. Murali-Krishna, A. Aderem, R. A. Flavell, T. Papayannopoulou, and D. M. Shayakhmetov. 2009. Virus binding to a plasma membrane receptor triggers interleukin-1 alpha-mediated proinflammatory macrophage response in vivo. Immunity 31:110-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fejer, G., L. Drechsel, J. Liese, U. Schleicher, Z. Ruzsics, N. Imelli, U. F. Greber, S. Keck, B. Hildenbrand, A. Krug, C. Bogdan, and M. A. Freudenberg. 2008. Key role of splenic myeloid DCs in the IFN-alphabeta response to adenoviruses in vivo. PLoS Pathog. 4:e1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartman, Z. C., D. M. Appledorn, and A. Amalfitano. 2008. Adenovirus vector induced innate immune responses: impact upon efficacy and toxicity in gene therapy and vaccine applications. Virus Res. 132:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartman, Z. C., A. Kiang, R. S. Everett, D. Serra, X. Y. Yang, T. M. Clay, and A. Amalfitano. 2007. Adenovirus infection triggers a rapid, MyD88-regulated transcriptome response critical to acute-phase and adaptive immune responses in vivo. J. Virol. 81:1796-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987-995. [DOI] [PubMed] [Google Scholar]
- 15.Kaufman, D. R., M. Bivas-Benita, N. L. Simmons, D. Miller, and D. H. Barouch. 2010. Route of adenovirus-based HIV-1 vaccine delivery impacts the phenotype and trafficking of vaccine-elicited CD8+ T-lymphocytes. J. Virol. 84:5986-5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufman, D. R., J. Liu, A. Carville, K. G. Mansfield, M. J. Havenga, J. Goudsmit, and D. H. Barouch. 2008. Trafficking of antigen-specific CD8+ T lymphocytes to mucosal surfaces following intramuscular vaccination. J. Immunol. 181:4188-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawai, T., and S. Akira. 2007. TLR signaling. Semin. Immunol. 19:24-32. [DOI] [PubMed] [Google Scholar]
- 18.Lieber, A., C. Y. He, L. Meuse, D. Schowalter, I. Kirillova, B. Winther, and M. A. Kay. 1997. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J. Virol. 71:8798-8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, J., B. A. Ewald, D. M. Lynch, M. Denholtz, P. Abbink, A. A. Lemckert, A. Carville, K. G. Mansfield, M. J. Havenga, J. Goudsmit, and D. H. Barouch. 2008. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J. Virol. 82:4844-4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, J., B. A. Ewald, D. M. Lynch, A. Nanda, S. M. Sumida, and D. H. Barouch. 2006. Modulation of DNA vaccine-elicited CD8+ T-lymphocyte epitope immunodominance hierarchies. J. Virol. 80:11991-11997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu, J., K. L. O'Brien, D. M. Lynch, N. L. Simmons, A. La Porte, A. M. Riggs, P. Abbink, R. T. Coffey, L. E. Grandpre, M. S. Seaman, G. Landucci, D. N. Forthal, D. C. Montefiori, A. Carville, K. G. Mansfield, M. J. Havenga, M. G. Pau, J. Goudsmit, and D. H. Barouch. 2009. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457:87-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muruve, D. A., M. J. Barnes, I. E. Stillman, and T. A. Libermann. 1999. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum. Gene Ther. 10:965-976. [DOI] [PubMed] [Google Scholar]
- 23.Muruve, D. A., V. Petrilli, A. K. Zaiss, L. R. White, S. A. Clark, P. J. Ross, R. J. Parks, and J. Tschopp. 2008. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452:103-107. [DOI] [PubMed] [Google Scholar]
- 24.Nanda, A., D. M. Lynch, J. Goudsmit, A. A. Lemckert, B. A. Ewald, S. M. Sumida, D. M. Truitt, P. Abbink, M. G. Kishko, D. A. Gorgone, M. A. Lifton, L. Shen, A. Carville, K. G. Mansfield, M. J. Havenga, and D. H. Barouch. 2005. Immunogenicity of recombinant fiber-chimeric adenovirus serotype 35 vector-based vaccines in mice and rhesus monkeys. J. Virol. 79:14161-14168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nociari, M., O. Ocheretina, J. W. Schoggins, and E. Falck-Pedersen. 2007. Sensing infection by adenovirus: Toll-like receptor-independent viral DNA recognition signals activation of the interferon regulatory factor 3 master regulator. J. Virol. 81:4145-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pulendran, B., and R. Ahmed. 2006. Translating innate immunity into immunological memory: implications for vaccine development. Cell 124:849-863. [DOI] [PubMed] [Google Scholar]
- 27.Querec, T., S. Bennouna, S. Alkan, Y. Laouar, K. Gorden, R. Flavell, S. Akira, R. Ahmed, and B. Pulendran. 2006. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J. Exp. Med. 203:413-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Querec, T. D., R. S. Akondy, E. K. Lee, W. Cao, H. I. Nakaya, D. Teuwen, A. Pirani, K. Gernert, J. Deng, B. Marzolf, K. Kennedy, H. Wu, S. Bennouna, H. Oluoch, J. Miller, R. Z. Vencio, M. Mulligan, A. Aderem, R. Ahmed, and B. Pulendran. 2009. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat. Immunol. 10:116-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts, D. M., A. Nanda, M. J. Havenga, P. Abbink, D. M. Lynch, B. A. Ewald, J. Liu, A. R. Thorner, P. E. Swanson, D. A. Gorgone, M. A. Lifton, A. A. Lemckert, L. Holterman, B. Chen, A. Dilraj, A. Carville, K. G. Mansfield, J. Goudsmit, and D. H. Barouch. 2006. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 441:239-243. [DOI] [PubMed] [Google Scholar]
- 30.Schnell, M. A., Y. Zhang, J. Tazelaar, G. P. Gao, Q. C. Yu, R. Qian, S. J. Chen, A. N. Varnavski, C. LeClair, S. E. Raper, and J. M. Wilson. 2001. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol. Ther. 3:708-722. [DOI] [PubMed] [Google Scholar]
- 31.Shayakhmetov, D. M., Z. Y. Li, S. Ni, and A. Lieber. 2005. Interference with the IL-1-signaling pathway improves the toxicity profile of systemically applied adenovirus vectors. J. Immunol. 174:7310-7319. [DOI] [PubMed] [Google Scholar]
- 32.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 33.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 34.Vogels, R., D. Zuijdgeest, R. van Rijnsoever, E. Hartkoorn, I. Damen, M. P. de Bethune, S. Kostense, G. Penders, N. Helmus, W. Koudstaal, M. Cecchini, A. Wetterwald, M. Sprangers, A. Lemckert, O. Ophorst, B. Koel, M. van Meerendonk, P. Quax, L. Panitti, J. Grimbergen, A. Bout, J. Goudsmit, and M. Havenga. 2003. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J. Virol. 77:8263-8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamaguchi, T., K. Kawabata, N. Koizumi, F. Sakurai, K. Nakashima, H. Sakurai, T. Sasaki, N. Okada, K. Yamanishi, and H. Mizuguchi. 2007. Role of MyD88 and TLR9 in the innate immune response elicited by serotype 5 adenoviral vectors. Hum. Gene Ther. 18:753-762. [DOI] [PubMed] [Google Scholar]
- 36.Yarovinsky, F., S. Hieny, and A. Sher. 2008. Recognition of Toxoplasma gondii by TLR11 prevents parasite-induced immunopathology. J. Immunol. 181:8478-8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaiss, A. K., Q. Liu, G. P. Bowen, N. C. Wong, J. S. Bartlett, and D. A. Muruve. 2002. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J. Virol. 76:4580-4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu, J., X. Huang, and Y. Yang. 2007. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J. Virol. 81:3170-3180. [DOI] [PMC free article] [PubMed] [Google Scholar]