Abstract
Gag orchestrates the assembly and release of human immunodeficiency virus type 1 (HIV-1) particles. We explored here the potential of anti-Gag RNA aptamers to inhibit HIV-1 replication. In vitro, RNA aptamers raised against an HIV-1 Gag protein, lacking the N-terminal myristate and the C-terminal p6 (DP6-Gag), could bind to matrix protein (MA), nucleocapsid protein (NC), or entire DP6-Gag protein. Upon cotransfection with pNL4-3.Luc molecular clone into 293T cells, six of the aptamers caused mild inhibition (2- to 3-fold) in the extracellular capsid levels, and one aptamer displayed 20-fold inhibition. The reduction was not due to a release defect but reflected Gag mRNA levels. We hypothesized that the aptamers influence genomic RNA levels via perturbation of specific Gag-genomic RNA interactions. Binding studies revealed that the “NC-binders” specifically compete with the packaging signal (ψ) of HIV-1 for binding to DP6-Gag. Therefore, we tested the ability of two NC-binders to inhibit viruses containing ψ-region deletions (ΔSL1 or ΔSL3) and found that the NC-binders were no longer able to inhibit Gag synthesis. The inability of these aptamers to inhibit ψ-deleted viruses correlated with the absence of competition with the corresponding ψ transcripts lacking SL1 or SL3 for binding DP6-Gag in vitro. These results indicate that the NC-binding aptamers disrupt Gag-genomic RNA interaction and negatively affect genomic RNA transcription, processing, or stability. Our results reveal an essential interaction between HIV-1 Gag and the ψ-region that may be distinct from that which occurs during the encapsidation of genomic RNA. Thus, anti-Gag aptamers can be an effective tool to perturb Gag-genomic RNA interactions.
Gag polyprotein, a major structural protein of human immunodeficiency virus type 1 (HIV-1), is synthesized in the cytoplasm of infected cells and is the only protein that is necessary and sufficient for the formation of noninfectious virus-like particles (VLPs) (20, 39, 45). Gag is modified cotranslationally by an N-terminal myristate that aids in its trafficking to the plasma membrane of the infected cell, where it assembles by multimerization. During or soon after budding, the viral protease (PR) is activated and cleaves Gag into its component proteins: matrix protein (MA), capsid protein (CA), nucleocapsid protein (NC), the late domain (p6), and two small, spacer peptides, SP1 and SP2 (see Fig. 1A). This process, termed maturation, leads to virion core condensation and also makes the particles infectious (22, 32). The various domains of Gag play specific roles in the life cycle of HIV-1 and mutations in any of them lead to defects in particle assembly and/or loss of infectivity. The MA domain includes an N-terminal myristate and a patch of basic amino acid residues, both of which facilitate binding of Gag to the plasma membrane (46). The CA domain plays critical roles in Gag multimerization and participates in the formation of the mature viral core. CA consists of two distinct domains, an N-terminal domain (NTD) and a C-terminal domain (CTD) separated by a flexible linker (3, 18, 19, 21). The CTD contains the major homology domain (MHR), a highly conserved stretch of 20 amino acids present in all retroviruses (45), that promotes Gag-Gag interactions. NC is a small, basic protein that contains two conserved zinc-finger motifs, which play a critical role in the specific recognition and subsequent encapsidation of the unspliced viral genomic RNA into the virion particles (2). During early events, the NC protein functions as a nucleic acid chaperone and enhances the efficiency of reverse transcription (15, 26, 36, 42). The late domain, p6, contains the PTAP and LYPXnL motifs, which interact with components of the cellular ESCRT machinery and facilitate virus release (10).
FIG. 1.
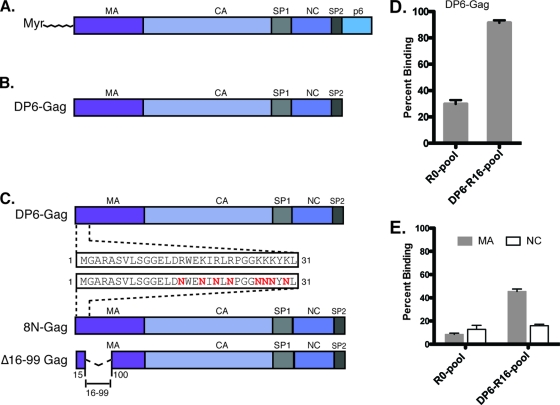
Diagrammatic representation of the various proteins used for RNA selections and aptamer analysis. (A) Wild-type HIV-1 Gag polyprotein. (B) The DP6-Gag protein used for selections lack the N-terminal myristate and the late domain p6. (C) 8N-Gag with eight basic amino acid residues of MA mutated to asparagines and Δ16-99-Gag, with a deletion in between amino acid residues 16 and 99. (D) The binding of RNAs from the R0 and R16 pools to DP6-Gag. (E) The binding of RNAs from the R0 and R16 pools to nonmyristoylated matrix (gray bars) and nucleocapsid (open bars). All binding reactions were performed with 10 nM aptamer RNAs and 1 μM protein, in the presence of a 5-fold molar excess of yeast tRNA to eliminate nonspecific binding.
While a preventive vaccine for AIDS does not appear to be on the horizon, conventional drug-based therapies such as highly active antiretroviral therapy (HAART) have been successful in suppressing HIV replication. Despite its success, HAART is also associated with a multitude of side effects due to toxicity. Nonadherence to therapy and the consequent emergence of drug-resistant viruses frequently hamper effective treatment. Furthermore, since the latently infected, long lived memory CD4+ T cells cannot be eliminated, therapy must continue for dozens of years, making it difficult to sustain high costs. Therefore, newer, more effective forms of antiretroviral therapy are required. Gene therapy-based approaches are attractive due to the promise of one-time treatment making them cost-effective and have immense potential as adjuvant therapy, along with HAART. RNA-based anti-HIV molecules that have been demonstrated to be effective in inhibiting HIV-1 replication include ribozymes, antisense RNAs, RNA decoys, and aptamers (27, 33, 37, 40).
Aptamers are single-stranded nucleic acid molecules that bind with high affinity and specificity to their target. They can be raised against any target of interest by SELEX (i.e., selective enrichment of ligands by exponential enrichment), an in vitro molecular evolution technique (12, 43). Aptamers have numerous advantages over conventional drugs because of their high specificity to their target, and lack of toxicity or immunogenicity. Aptamers have been raised against numerous HIV-1 proteins, including reverse transcriptase (RT), Rev, Tat, integrase, gp120, Gag, and NC (for a review, see reference 25). Aptamers have shown immense promise in controlling HIV-1 replication as we and others, have shown that anti-RT RNA aptamers inhibit HIV replication (6, 27). Such aptamers, when stably expressed in Jurkat T cells, strongly inhibited the replication of multiple subtypes of HIV-1 and drug-resistant isolates. We subsequently demonstrated that while both aptamers against HIV-1 RT and shRNAs directed to HIV-1 genome produced a comparable level of inhibition at a lower multiplicity of infection, only the aptamers retained potency in inhibiting HIV-1 replication when challenged at higher multiplicities (28). While aptamers have been raised against Gag and several of its component proteins, none of them have been tested for efficacy in inhibiting HIV-1 replication (30, 34, 35).
We describe here RNA aptamers against the HIV-1 Gag protein and their in vitro characterization. Although some of the aptamers, in addition to binding a Gag protein lacking p6 (DP6-Gag), specifically recognized either the purified MA or NC proteins in vitro, the binding of the remainder could not be mapped to specific subdomains of Gag. We also tested the potency of anti-Gag aptamers to inhibit HIV-1 replication and found that 7 of our 11 aptamers caused a reduction in extracellular p24 levels. We demonstrate that the anti-Gag aptamers mediate their antiviral effects by lowering the levels of viral genomic RNA in producer cells. Furthermore, we show that the anti-NC aptamers compete with the Ψ-packaging signal for binding to DP6-Gag in vitro. Our data indicate that the perturbation of Gag-genomic RNA interaction that occurs at Ψ or other sites along the genome leads to the inhibition of HIV-1 genomic RNA levels, thus affecting the levels of intracellular Gag protein.
MATERIALS AND METHODS
Purified proteins.
The DP6-, 8N-, and Δ16-99 Gag proteins and the CA-NC protein were purified as described previously (4, 5, 8, 9). The nonmyristoylated HIV-1 matrix (MA) and capsid (CA) proteins were provided by Michael Summers and Wesley Sundquist, respectively. The HIV-1 nucleocapsid (HIV-1MN p7) protein was provided by Robert Gorelick through the NIH AIDS Research and Reference Reagent Program.
Preparation of the RNA library.
The single-stranded DNA library was synthesized in the Ellington laboratory using standard phosphoramidite chemistry and a phosphoramidite molar ratio of 1.5:1.5:1.0:1.2 (dA/dC/dG/dT). The library comprised of random sequences 100 nucleotides long, with an overall complexity of ∼1014. Each DNA had the sequence 5′-GGACAGCAAGCGTACATCTA-N100-TGCTAGCCTGAAGTCATACG-3′, where N is any nucleotide, and the flanking sequences are specific constant regions required for in vitro transcription and cloning. The DNA was transcribed into RNA by using the Ampliscribe T7 high-yield transcription kit (Epicenter Biotechnologies). To perform RNA selections, ∼660 pmol of the DP6-Gag protein was incubated with equimolar quantities of the RNA library in binding buffer (20 mM Tris-HCl [pH 7.4], 250 mM NaCl, 5 mM MgCl2, and 100 μM Tris(2-carboxyethyl)phosphine [TCEP]) for 15 min at 37°C, after which the reaction was filtered through HAWP nitrocellulose filters (Millipore, Bedford, MA). RNA bound to the nitrocellulose was eluted into 7 M urea, after which it was ethanol precipitated, and the pellet was resuspended in sterile water. The recovered RNA was reverse transcribed by using a Superscript III cDNA synthesis kit (Invitrogen), and the cDNA was used as a template for the synthesis of the second round of the RNA pool. A second round of selection was performed using this new pool, and the procedure was repeated several times until a significant enhancement of binding to DP6 was observed. With increasing rounds of selections, the ratio of RNA to protein was increased to improve the stringency of selections. A negative selection was performed after every third round in order to eliminate filter-binding RNA species. Binding assays were performed after every four rounds of selection to monitor the progress of selection.
Binding assays and determination of dissociation constants.
Radiolabeled aptamer RNAs, obtained via in vitro transcription of 100 ng of DNA template, were internally labeled using [α-32P]UTP (Perkin-Elmer) by using the Ampliscribe T7 high-yield transcription kit (Epicenter Biotechnologies). Transcribed RNAs (140 nucleotides in length) were gel purified from 7.5% polyacrylamide-7 M urea gel into 0.3 M sodium acetate, 0.1% sodium dodecyl sulfate (SDS), and 1 mM EDTA by incubation at 37°C overnight. The radiolabeled RNAs were precipitated and resuspended in sterile water. Gag binding assays were performed in the binding buffer described above. Selections were performed in “high-salt” conditions (250 mM NaCl) in order to maintain the proteins in solution, as well as to allow for a stringent selection. Binding assays with purified nucleocapsid protein were also performed under high-salt (250 mM NaCl, 5 mM MgCl2) conditions as described previously (14, 16). Prior to their use in binding assays, aptamer RNAs were unfolded at 70°C for 5 min in binding buffer and refolded by incubation on ice for 10 to 15 min. When necessary, yeast tRNA was used in the reactions to eliminate nonspecific binding. Radiolabeled aptamers (10 nM) were added to the protein (0 to 1,000 nM), followed by incubation at 37°C for 15 min, after which the reaction was filtered through two filters: a nitrocellulose filter to trap the protein (and any RNA bound to it) and a nylon filter to capture any unbound RNA. The percentage of aptamers bound to the protein was quantitated by using a PhosphorImager (Molecular Dynamics) and was used to determine the dissociation constants (Kd) of the aptamer-protein interaction using the sigmoidal dose-response equation in Prism.
Plasmid construction.
The various anti-Gag aptamers were expressed under the control of the cytomegalovirus (CMV) promoter by using the RNA expression plasmid, pSilencer 4.1-CMV-Puro (Ambion). In order to minimize misfolding, the aptamers needed to be expressed in cells without extraneous flanking sequences. This was achieved by placing hammerhead ribozymes flanking the aptamer sequences as described previously (1, 27). The pSilencer-HIV-1-Ψ plasmid that expresses the HIV-1-Ψ (SL1 to SL4) flanked by the ribozymes was constructed in a similar manner.
Cells, transfections, and infections.
The pNL4-3.Luc.R-E− molecular clone is envelope deficient, and its nef gene was replaced with the gene encoding firefly luciferase (7, 23; NIH AIDS Research and Reference Reagent program). The HIV-Luc reporter viruses were pseudotyped with the vesicular stomatitis virus envelope (VSV-G) from the plasmid pVSV-G (Clontech). Cotransfections of 293T cells were carried out with Lipofectamine 2000 (Invitrogen) using 1.5 μg each of pNL4-3.Luc.R-E− and pVSV-G and 7.5 μg of the various pSilencer-aptamer plasmids. Transfection efficiency was monitored in two ways. First, all transfections included the plasmid peGFP, resulting in green fluorescence as an indicator of transfection efficiency. Second, the producer cells were lysed, and their luciferase activity (from pNL4-3.Luc.R-E− transfection) was measured. The transfection efficiencies measured by both of these methods were found to be ∼90%. Viruses released into the supernatant were harvested 48 h posttransfection, centrifuged at 3,000 rpm for 15 min at 4°C, and filtered through 0.45-μm-pore-size filters, after which the virion-associated CA (p24) levels were measured by using an HIV-1 p24 antigen capture assay kit (Advanced BioScience Laboratories, Inc.) according to the manufacturer's instructions. Virus release efficiency was calculated as 100 × (virus-associated p24/total p24), where total p24 is the sum of the virus-associated and the cell-associated p24 values. For infection experiments, virus supernatants, normalized for p24 levels, were used to infect 293T cells. The cells were harvested 48 h postinfection and lysed in reporter lysis buffer (Promega), and their luciferase activity was measured.
Generation of pNL4-3.Luc.ΔSL mutants.
The pNL4-3.Luc.ΔSL1 and pNL4-3.Luc.ΔSL3 plasmids, which lack the SL1 (nucleotides 697 to 731 in pNL4-3) or SL3 (nucleotides 766 to 779 in pNL4-3) stem-loops in their Ψ-packaging signals, respectively, were generated as follows. The AatII-SpeI fragment of pNL4-3.Luc, which spans the entire Ψ-region of HIV-1 (SL1 to SL4), was subcloned into pUC19 to generate pUC19-Ψ. The SL1 or SL3 stem-loops in pUC19-Ψ were then removed by using a QuikChange site-directed mutagenesis kit (Stratagene). Upon confirming the deletions by sequencing, the AatII-SpeI fragment was recloned into pNL4-3.Luc to generate pNL4-3.Luc.ΔSL1 or pNL4-3.Luc.ΔSL3.
Northern blot analysis of cellular RNA.
Total RNA was extracted from 293T cells 48 h posttransfection using TRIzol reagent (Invitrogen). A portion (10 μg) of the extracted RNA was electrophoresed on a 7.5% polyacrylamide-7 M urea gel and transferred to Hybond nylon membranes. The transferred RNA was UV-cross-linked and probed for the presence of aptamers using antisense DNA oligonucleotides end labeled with [γ-32P]ATP that recognizes a region common to all aptamers. Hybridization was performed using Ultrahyb hybridization buffer (Applied Biosystems) according to the manufacturer's instructions.
Western blot analysis.
After 48 h of transfection, 293T cells were harvested, and total protein was extracted by using radioimmunoprecipitation assay (RIPA) lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1% NP-40, 1% Triton X-100, 0.5% sodium deoxycholate, 1 mM phenylmethylsulfonyl fluoride). These lysates were also used to determine intracellular Gag levels (as measured by the intracellular p24 levels) using a p24-capture enzyme-linked immunosorbent assay (ELISA) as described above. For the analysis of viral proteins, purified viruses (normalized by their p24 values) were pelleted in 30% polyethylene glycol at 4°C and resuspended in RIPA lysis buffer. The extracted proteins were run on a 12% SDS-polyacrylamide gel, transferred to nitrocellulose membranes, and probed for the presence of viral proteins using anti-HIV-1 immunoglobulin G (NIH AIDS Research and Reference Reagent Program).
Real-time quantitative PCR analysis.
Total RNA was extracted from producer cells at 48 h posttransfection by using RNeasy (Qiagen) and converted to cDNA using the SuperScript III first-strand synthesis system for real-time PCR (RT-PCR; Invitrogen). The cDNA was subjected to RT-PCR by using an Applied Biosystems 7300 Fast Real-Time PCR system. Primer pairs were designed against the antiviral interferon-stimulated genes (ISGs)—OAS2 (2′,5′-oligoadenylate synthetase 2), PKR (protein kinase R), and MxA (myxovirus resistance A) (31, 38, 41, 44), as well as HIV-1 Gag—and their relative changes were compared to that of the housekeeping gene (GAPDH) by using a Maxima SYBR green qPCR Master Mix (2×; Fermentas).
In vitro competition assays.
To perform competition assays, DP6-Gag protein was incubated with a mixture of radiolabeled aptamer (10 nM) and unlabeled competitor RNAs (250 nM). The reaction was incubated at 37°C for 30 min, after which they were filtered, and the total binding of the aptamer to the protein was quantitated as described above. DNA templates (with a T7 promoter) encoding the competitor RNAs were amplified by PCR from either pNL4-3, pNL4-3.Luc.ΔSL1, or pNL4-3.Luc.ΔSL3, using primers specific for these regions. The amplified PCR product was gel purified, after which they were used as templates for in vitro transcription. The transcribed RNAs were purified, refolded, and used as competitors in the binding reaction.
RESULTS
Selection of aptamers against HIV-1 Gag protein.
In order to generate aptamers that bind specifically to the Gag protein via SELEX, the protein must exist in its native folded conformation. Wild-type HIV-1 Gag protein has poor solubility and is prone to proteolysis in bacterial systems. These problems were previously overcome by the expression of a truncated form of Gag lacking p6 and the N-terminal myristate (DP6-Gag) (4). After 16 rounds of selections performed against DP6-Gag, there was a significant increase in the total binding of the round 16 (R16) RNA pools (∼90%) compared to that of the round zero (R0) pool (∼25%) (Fig. 1D; SELEX progression [see Fig. S1 in the supplemental material]). A total of 44 clones from the DP6-R16 RNA pools were sequenced, and sequences with <5% variation were grouped into sequence families; the topmost sequence was used as a representative member for binding and efficacy studies. The aptamers were named “DP6-x,” where x refers to the clone from which the aptamer was sequenced. Eleven unique aptamers were isolated with one sequence (DP6-1) making up nearly half of the pool (24/44) and, hence, was the largest family of aptamers. Several smaller families of aptamers (DP6-5, DP6-7, DP6-32, and DP6-19) were also enriched, along with six individual aptamers (DP6-12, DP6-10, DP6-21, DP6-22, DP6-42, and DP6-44) (see Fig. S2 in the supplemental material). The dissociation constants (Kd) of binding of the aptamers to DP6-Gag ranged from approximately 80 to 200 nM (Table. 1).
TABLE 1.
Binding affinities for the binding of selected anti-Gag aptamers to DP6-Gag and two mutant Gag proteins, 8N-Gag and Δ16-99-Gaga
| Aptamer | Binding specificitya | Mean Kd (nM)b ± SD |
||
|---|---|---|---|---|
| DP6-Gag | 8N-Gag | Δ16-99-Gag | ||
| DP6-1 | MA | 178 ± 31 | ND | >956.4 |
| DP6-5 | Gag* | 161 ± 10.4 | 104 ± 15.5 | 121 ± 7.2 |
| DP6-7 | Gag* | 179 ± 12.2 | 337 ± 10.6 | 143 ± 10 |
| DP6-32 | Gag* | 203 ± 22 | 515 ± 50.7 | 238 ± 18 |
| DP6-19 | NC | 113 ± 9.9 | 209 ± 17.1 | 122 ± 22.2 |
| DP6-12 | MA, NC | 130 ± 9.3 | 342 ± 76.3 | 156 ± 33.2 |
| DP6-10 | Gag* | 144 ± 11.2 | 208 ± 28.8 | 72.4 ± 6.7 |
| DP6-21 | MA | 233 ± 32.2 | ND | >593.4 |
| DP6-22 | Gag* | 100 ± 3.4 | 177 ± 11.6 | 159 ± 9.5 |
| DP6-42 | MA | 91.3 ± 4.4 | ND | >494 |
| DP6-44 | Gag* | 82.5 ± 2.1 | 77.9 ± 22.8 | 41.9 ± 5.3 |
Aptamers that did not bind to any of the Gag component proteins tested—MA, CA, or NC—are indicated by an asterisk (*).
Binding affinities (Kd) were determined by filter binding. The specificities were determined by testing the percent binding of each selected aptamer to recombinant purified MA, CA, and NC proteins. ND, not detected.
Anti-DP6-Gag aptamers bind preferentially to MA, NC, or both.
In binding assays with recombinant, purified MA, CA, or NC proteins, the R16 pool bound preferentially to MA (∼45%) compared to the R0 pool (∼10%) (Fig. 1E, gray bars). The R16 pool also bound, to some extent, to NC (Fig. 1E, open bars); however, the specific binding to the R16 pool over the R0 pool was masked by the inherent ability of the NC protein to bind RNA, giving rise to a high level of binding to the R0 pool even in the presence of a 5-fold molar excess of yeast tRNA in the reaction. Aptamers that recognized the CA domain were not observed (data not shown).
To determine binding specificity of individual aptamers to MA, CA, NC, or DP6-Gag, binding assays were performed with each of the aptamers and the various purified proteins. Aptamers DP6-1, DP6-12, DP6-21, and DP6-42 bound to MA, and this interaction was retained even in the presence of excess yeast tRNA in the binding reaction, indicating their specificity (Fig. 2A). However, aptamer DP6-1 had a unique specificity to MA, as seen by its inability to bind to either NC (Fig. 2B) or a fusion protein, CA-NC (see Fig. S3 in the supplemental material). All 11 anti-Gag aptamers could bind to the NC protein nonspecifically, presumably via the two zinc-finger domains and/or the basic residues, in the absence of yeast tRNA (a nonspecific RNA) in the reaction (data not shown). However, only aptamers DP6-19 and DP6-12 specifically bound to the NC protein in the presence of 5-fold (Fig. 2B) or 30-fold (see Fig. S4 in the supplemental material) molar excess of yeast tRNA in the reaction, indicating that their binding to NC was specific. It is worth noting that DP6-12 was unique in that it had bivalent specificity: MA and NC. The binding of anti-Gag aptamers to these two domains is consistent with the fact that these two represent the most basic regions on the Gag polyprotein.
FIG. 2.
Specific binding of individual aptamers to MA and NC. Binding of the anti-Gag aptamers (individual RNA molecules from the R16 pool) to purified Gag component proteins in the presence of 5-fold molar excess yeast-tRNA. (A) Binding to nonmyristoylated matrix. (B) Binding to nucleocapsid.
The N-terminal region of MA is important for the aptamer-binding ability of HIV-1 Gag.
To further characterize the binding of the aptamers, we used two Gag proteins that had mutations at their N termini (Fig. 1C). In 8N-Gag, eight of the basic amino acid residues at the N terminus of MA have been replaced with asparagines (46), whereas Δ16-99 Gag has a large deletion in the globular domain of MA (13). The aptamers bound with various affinities to the two mutant Gag proteins (Table 1). Binding of the three MA-specific aptamers—DP6-1, DP6-21, and DP6-42—to the two mutant Gag proteins was completely abrogated, indicating that these aptamers require the N terminus of MA for binding. However, binding of DP6-12 (MA and NC-binder) and DP6-19 (NC-binder) to the two mutant Gag proteins was minimally affected, with only about 2- to 2.5-fold reduction in binding affinity for 8N-Gag. In some cases (e.g., DP6-10 or DP6-44), deletions near the N terminus of MA appeared to minimally increase binding (<2-fold) to Δ16-99 Gag compared to DP6-Gag. In general, both 8N-Gag and Δ16-99-Gag proteins bound poorly to the MA-specific aptamers compared to the DP6-Gag protein, and this result is in agreement with studies done by others on MA mutants with mutations at their N termini that have impaired RNA binding (35).
Anti-HIV-1 Gag aptamers affect extracellular virion levels, but not their infectivity.
We then tested whether intracellular expression of these anti-Gag aptamers would interfere with the assembly and release of virus particles. 293T cells were cotransfected with pNL4-3.Luc (pVSV-G), along with the various pSilencer plasmids, and virus-associated p24-levels were measured at 48 h posttransfection. We observed a wide range of effects on the levels of extracellular virus with our 11 anti-Gag aptamers, reflected in p24-levels that ranged from 5 to 120% that of the empty vector control (MCS). Cotransfections of pNL4-3.Luc/VSV-G with pSilencer plasmids that expressed aptamers DP6-1, -5, -7, -32, -19, and -22 resulted in an inhibition in the range of 2- to 5-fold compared to MCS (Fig. 3A). The most significant inhibition was observed with DP6-12, which resulted in a 20-fold inhibition of virus production, an effect that is comparable to that seen with an shRNA against the Tat/Rev region of HIV-1. It is interesting that DP6-12 is also bivalent in terms of its in vitro binding specificity. Among the aptamers that did not result in any inhibition upon cotransfection (DP6-10, -21, -42, and -44), DP6-42 consistently resulted in a 2.5-fold enhancement of virus production.
FIG. 3.
Efficacy of the anti-Gag aptamers to inhibit HIV-1 replication. 293T cells were cotransfected with pNL4-3.Luc/VSV-G and various pSilencer plasmids. Virus-containing supernatants were harvested at 48 h posttransfection, and the effect of the aptamer on both early and late events was analyzed. (A) Effect of aptamer expression on virus production. The panel shows nanograms of p24/ml of culture supernatant. (B) Effect of aptamer expression in producer cells on virion infectivity. The virus input was normalized by the use of equal amounts of p24 (2.5 ng), and the luciferase activity was determined at 48 h postinfection. MCS refers to the empty-vector control that does not express any aptamers, and shRNA refers to the plasmid expressing an shRNA against the Tat/Rev region of the HIV-1 genome. (C) Release efficiencies of the viruses in the presence of the aptamers were calculated as the percentage of extracellular p24 (data from Fig. 3A) over the total (sum of extracellular and intracellular p24), i.e., the virus release efficiency = 100 × (virus-associated p24/total p24).
Northern blot analysis of total RNA from producer cells, showed that all 11 aptamers were being processed properly by the ribozymes (see Fig. S5A in the supplemental material). Upon normalizing aptamer expression levels to the levels of cellular 5S-rRNA, we observed that the aptamer expression levels varied only by ca. 10% (the fully processed aptamers varied by 5.3%, partially processed aptamers by 2.2%, unprocessed aptamers by 10.6%, and total aptamers by 9.7%) and, therefore, the variation in virus production could not be attributed to differential expression of aptamers. A Western blot analysis of the viruses released and those of the producer cell lysates indicated minor processing defects with aptamers DP6-5, -32, -19, -12, and -22. However, there was no major effect of the aptamers on the processing of the viral proteins by the protease (see Fig. S5B and S5C in the supplemental material; see below). Infecting 293T cells with viruses produced in the presence of aptamers, after normalizing for the input p24, showed no significant effect (Fig. 3B).
Anti-Gag aptamers do not affect virus release but downregulate intracellular Gag protein and Gag mRNA levels.
We wanted to understand whether the reduction in extracellular virion levels brought about by aptamers is due to a release defect or simply a reflection of intracellular Gag levels. Therefore, we also determined the levels of intracellular Gag and calculated the virus release efficiency. We observed a reduction in the levels of intracellular Gag protein similar to that seen for extracellular p24 levels, both by ELISA and in the Western blots, of producer cell lysates (see Fig. S5C in the supplemental material). The virus release efficiencies for all of the aptamers were comparable to that of the control plasmid pMCS (Fig. 3C), indicating that the efficacious aptamers do not directly interfere with steps affecting virus release.
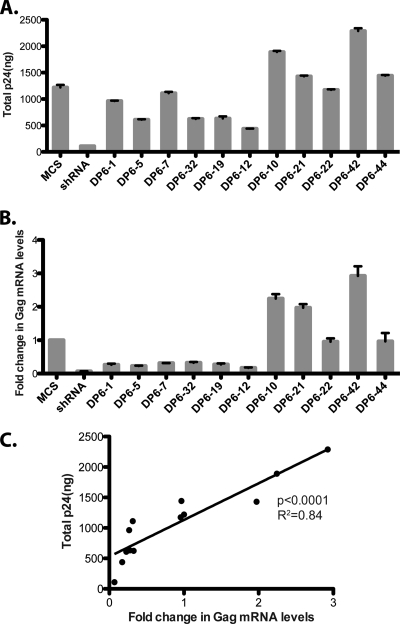
We then compared the levels of total Gag protein (the sum of intracellular Gag and virus-associated p24) to the intracellular Gag mRNA levels (Fig. 4A and B). When the fold change in the Gag mRNA levels was plotted as a function of the total Gag protein levels, we observed that the levels of viral RNA were directly proportional to the total Gag protein levels (Fig. 4C; P < 0.0001, R2 = 0.84). However, this was not a global effect of the aptamers on all cellular RNAs, since the levels of GAPDH mRNA remained unaffected (data not shown).
FIG. 4.
Aptamers affect steady-state levels of Gag mRNA in producer cells. (A) 293T cells were cotransfected with pNL4-3.Luc/VSV-G and pSilencer plasmids as indicated, and their total p24 (sum of intracellular and virus-associated p24) levels were measured by using ELISA. (B) Total RNA was extracted from producer cells 48 h posttransfection, and the fold changes in the levels of HIV-1 Gag mRNA were analyzed. (C) The fold change in the Gag mRNA is plotted as a function of the total Gag protein levels in producer cells (calculated in panels A and B).
The presence of structured RNAs in the cytoplasm of infected cells may cause a nonspecific induction of interferons, which is likely to lead to an antiviral effect. To exclude this possibility, we analyzed changes in the levels of ISGs—OAS2, PKR, and MxA—in producer cells but did not observe any significant induction in their levels (see Fig. S6 in the supplemental material). Similar results were also obtained when we used the replication-competent clone pNL4-3, indicating that the aptamers could exert their inhibitory effect even in the context of wild-type virus (data not shown).
NC-specific aptamers compete with the Ψ-packaging signal of HIV-1 for binding to DP6-Gag in vitro.
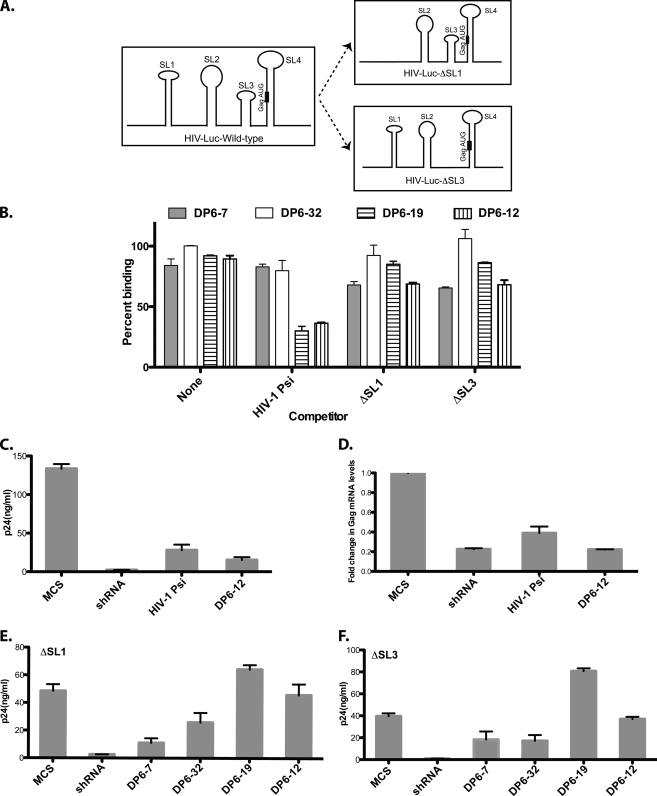
The binding of the aptamers to the Gag protein in vivo could perturb the interactions of the latter with the viral genomic RNA. The roles of Ψ-packaging signal in HIV-1 genomic RNA are well characterized. The Ψ-packaging signal consists of four major stem-loops: SL1 (major dimer initiation site), SL2 (splice donor), SL3 (minimal packaging signal), and SL4 (has the Gag AUG) (11) (Fig. 5A). To test whether NC-specific aptamers compete with the Ψ site for binding to the Gag polyprotein, we performed an in vitro competition assay where a mixture of radiolabeled-aptamer RNA and unlabeled competitor RNA (RNA transcripts encoding the Ψ-region of HIV-1) was added to DP6-Gag protein. The ability of the aptamer to compete for binding with the Ψ-packaging signal would be reflected by the amount of radiolabeled aptamer bound to the protein. For the in vitro competition assays, we selected four aptamers that had different binding specificities in vitro and had moderate to severe effects on virus production (see Fig. 3A and Table 1): the NC-binder DP6-19, the MA and NC-binder DP6-12, and the Gag binders- DP6-7 and DP6-32. We observed that the two NC-binders, DP6-19 and DP6-12, competed with HIV-1 Ψ for binding to DP6-Gag, as indicated by the strong reduction in total binding (∼60% of no-competitor control), whereas DP6-7 and -32 could not (Fig. 5B). To probe the importance of the various stem-loops of HIV-1 Ψ, we performed competition assays using HIV-1 Ψ-RNA transcripts lacking either SL1 or SL3. Upon deletion of either stem-loop, we observed that these RNAs could no longer compete with the NC-specific aptamers for binding to DP6-Gag (Fig. 5B). The binding of aptamers DP6-7 and DP6-32 to DP6-Gag remained refractory to the presence of wild-type HIV-1 Ψ-RNA, or those lacking SL1 or SL3 stem-loops, indicating that these aptamers may perturb Gag interaction with another part of the HIV genome.
FIG. 5.
The Ψ-packaging signal plays a critical role in the aptamer-mediated inhibition. (A) Diagrammatic representation of the Ψ-packaging signal of HIV-1 and the deletions that were performed. (B) In vitro competition assay performed with selected aptamers and using wild-type-Ψ or those lacking the SL1 or SL3 stem-loops as competitors. (C and D) 293T cells were cotransfected with pNL4-3.Luc (pVSV-G) and a plasmid that expressed the HIV-1 Ψ-RNA transcript as an exogenous transcript, and the p24 levels in the culture supernatant (C) and the fold change in the Gag mRNA levels (D) were measured. (E and F) 293T cells were cotransfected with pNL4-3.Luc.ΔSL1 or pNL4-3.Luc.ΔSL3 (pVSV-G), along with selected aptamers, and the virus-associated p24-levels were measured.
An exogenous RNA-transcript that encodes the Ψ-packaging signal of HIV-1 inhibits virus production.
Since the two NC-specific aptamers, DP6-19 and DP6-12, could compete for binding with the Ψ-packaging signal of HIV-1 in vitro, it is likely that when expressed intracellularly, these aptamers compete with the viral genomic RNA for binding to Gag, eventually resulting in an inhibition of virus production. Hence, their presence in the cytoplasm along with the viral genomic RNA in transfected cells might exert a “dominant-negative-like” effect for interaction with the Gag protein. This would imply that the overexpression of Ψ-sequences in the producer cells could also parallel this effect. To test this hypothesis, we cloned the Ψ-packaging signal of HIV-1 (SL1 to SL4) into pSilencer4.1-CMV-Puro to create pSilencer-HIV-1-Ψ. We cotransfected the latter plasmid along with pNL4-3.Luc (pVSV-G) into 293T cells and examined its effect on virus production. Interestingly, expression of HIV-1 Ψ as an exogenous transcript resulted in a significant inhibition to virus production. The reduction in the levels of virus-associated p24 levels was comparable to that observed with the most efficacious aptamer, DP6-12 (Fig. 5C). There was also a concomitant reduction in the levels of the Gag mRNA (similar to that observed with the efficacious aptamers) in cells transfected with pSilencer-HIV-1-Ψ (Fig. 5D). This result suggested the possibility that HIV-1 Ψ, as well as the NC-specific aptamers, could perturb specific interactions between the Gag protein and the viral RNA, which eventually results in an inhibition of virus production.
The SL1 and SL3 stem-loops are essential for the antiviral effects mediated by the NC-specific aptamers.
To further understand the role played by the Ψ-packaging signal in aptamer-mediated inhibition of virus production, we deleted the SL1 or SL3 stem-loops in pNL4-3.Luc to generate pNL4-3.Luc.ΔSL1 and pNL4-3.Luc.ΔSL3, respectively (Fig. 5A). We cotransfected pNL4-3.Luc.ΔSL1 or pNL4-3.Luc.ΔSL3 (pVSV-G) along with one of the four pSilencer plasmids encoding the chosen aptamers (DP6-7, -32, -19, and -12) and studied the effect of the deletions on virus production. The most striking effect of the deletions was observed in the case of the two NC-specific aptamers, DP6-19 and DP6-12. In the absence of either stem-loop, neither aptamer could inhibit virus production, as reflected by the rescue of virus-associated p24 levels in the culture supernatant (Fig. 5E and F). The virus-associated p24 levels in these cases were comparable (or increased in the case of ΔSL3 and DP6-19) to that of the MCS. Consistent with the in vitro competition data (Fig. 5B), the deletion of the stem-loops had no effect on the efficacies of aptamers DP6-7 and -32, indicating that these aptamers mediated their antiviral effects via interaction with other parts of Gag mRNA.
The strong correlation (P < 0.0001) between the total p24 levels and Gag mRNA in the producer cells observed with pNL4-3.Luc (Fig. 4C) was also lost upon deletion of SL1 or SL3 (data not shown). As with pNL4-3.Luc, the aptamers did not interfere with the release efficiencies of the viruses produced in the absence of SL1 or SL3 (data not shown). These results, taken together, suggest that the two NC-specific aptamers inhibit virus production by disrupting interactions of the Gag protein with the viral mRNA and the Ψ-packaging signal is critical to mediate this inhibition.
DISCUSSION
In this study, we have raised RNA aptamers against near full-length Gag protein of HIV-1, characterized their interaction with Gag in vitro, and tested their efficacy in blocking HIV replication in cultured cells. Previous reports on aptamers against the Gag protein have used glutathione S-transferase fusion proteins or purified component proteins of Gag (29, 34, 35). Thus, the final folded structure of the protein during selections in vitro are likely to be different and consequently affect the types of aptamers obtained. We performed RNA selections against an untagged form of the Gag protein, DP6-Gag (Fig. 1B). The dissociation constants (Kd) for binding of the aptamers to DP6-Gag ranged from approximately 80 to 200 nM (Table 1). We acknowledge the fact that aptamers raised by previous groups displayed much lower Kds than the ones we have isolated (30, 34). However, there are significant differences between our selection conditions and the ones reported earlier. First, we raised the aptamers against a form of protein (DP6-Gag) whose assembly properties closely resembles that of the wild-type Gag protein (4). Second, our target protein had no affinity tags that might otherwise interfere with protein folding or RNA selections. Third, our RNA library is composed of longer sequences (100N versus 50N or 70N), which allows for a greater complexity in both structures and motifs in the RNA pools used for selections and provide greater surface area for interaction with Gag. However, despite the differences between our results and those obtained by Lochrie et al. (34), both sets of aptamers recognized the Gag or its MA and NC domains. The affinity of the aptamer RNAs to either the MA or NC domains is probably due to the presence of basic patches of amino acid residues in these two proteins, which share a feature of binding to nucleic acids.
It has also been reported that aptamers that bind to HIV-1 MA contain an RNA sequence that is highly homologous to the pol open reading frame in the HIV-1 genome (35). However, the MA-specific aptamers we identified lacked this or any other consensus sequence. It is known that HIV-1 NC binds with high affinity to DNA (or RNA) sequences which have a composition of d(TG)n (16, 17). Although NC binds many different sequence motifs, the examination of the NC-specific aptamers did not reveal them. It is likely that the aptamers contain a unique, novel sequence or fold, which promotes NC binding. The only aptamers that included these sequence motifs (TGTGT or GTGTG) were DP6-21 and DP6-44, neither of which was NC specific or efficacious.
To our knowledge, this is the first report of anti-HIV-1 Gag aptamers mediating a significant inhibition of HIV-1 replication. Although Kim and Jeong have demonstrated inhibition of HIV-1 genomic RNA encapsidation upon the expression of anti-NC aptamers (29), the effect of the intracellularly expressed aptamer on HIV-1 production has not been studied. We believe that the enhanced inhibition to HIV-1 production (20-fold) that we observed with at least one of the anti-DP6-Gag aptamers is a combination of two major factors. First, to minimize misfolding of the aptamers due to the flanking sequences, we placed hammerhead ribozymes on each side of the aptamer. It is important to note that the ribozymes per se do not have any antiviral effect, as shown previously. With such constructs, we have previously demonstrated the ability of anti-RT aptamers to inhibit HIV-1 replication (27). Second, the longer aptamers (100N) could potentially access a larger surface of the protein. For instance, DP6-12 could recognize both MA and NC domains in vitro. This could be important because the ability of DP6-12 to interact with both the ψ element and a different region of viral mRNA simultaneously could enhance the overall affinity of the aptamer to Gag.
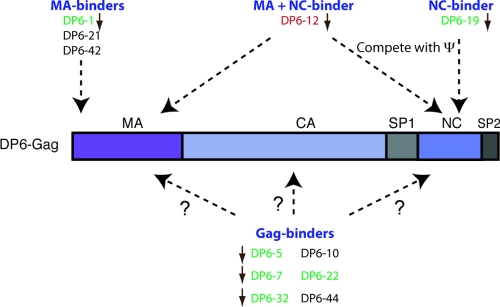
Upon cotransfection of the anti-DP6-Gag aptamers along with pNL4-3.Luc, while the aptamers led up to 20-fold inhibition to virus production, there was no significant effect on the infectivity of the released viruses. One possible explanation for this result is that the infectivity readout represents viruses released by cells that did not receive the pSilencer plasmid and, hence, were fully infectious. It is also possible that these aptamers only interfered with late events of the viral life cycle. The roles played by certain Gag component proteins, such as MA, in early events are believed to be redundant. For instance, it has been shown that the nuclear localization signal (NLS) of Vpr could facilitate the nuclear entry of preintegration complexes (PICs) in the absence of a functional NLS in MA (24). So, other viral proteins could override the functions of Gag and mask the effect of the aptamer during entry. A schematic summary of the various interactions made by the aptamers with the Gag protein is in Fig. 6.
FIG. 6.
Summary of the various interactions of the aptamers with Gag. The figure depicts the domains on Gag that are recognized by the various aptamers. The aptamers are color-coded based on their efficacy in vivo: red, strong; green, moderate; and black, no inhibition to virus production. The brown arrows next to the aptamers indicate that the aptamers also lowered the viral mRNA levels. Aptamers DP6-19 and DP6-12 also compete with HIV-1 ψ for binding to DP6-Gag.
Our results suggest the intriguing possibility that the aptamer interaction with Gag disrupts interactions between Gag protein and its mRNA, specifically those with the ψ, which leads to negative regulation of Gag mRNA metabolism (transcription, processing, or stability). This interaction could be either the same interaction that must occur in the process of encapsidation of viral genomic RNA by Gag protein or a distinct interaction that occurs well before the assembly begins. Although our results do not distinguish between these two possibilities, we speculate that these two are one and the same interaction. Although our data suggest that the NC-binding aptamers likely compete with Ψ for binding to Gag, it is interesting that the virus particles produced retain infectivity. The presence of cells that received HIV-Luc DNA but did not receive the aptamer may only provide partial explanation for this. Another explanation could be that encapsidation of the RNA genome requires Gag interaction with other determinants on the RNA for which the aptamers cannot compete. These questions remain currently unanswered.
Although the role of Ψ element in this interaction appears to be important, it may not be the only region on Gag involved in enhancing mRNA stability by interaction. For example, the inhibition by certain aptamers (e.g., DP6-7 and DP6-32) could not be explained by the competition with the Ψ element of viral genomic RNA. In fact, we do not know what part of Gag polyprotein interacts with these aptamers. Similarly, the most frequently appearing sequence among the aptamers obtained from DP6-Gag selections, DP6-1, also seemed to be independent of Ψ interaction for its mild efficacy (data not shown). Nevertheless, all efficacious aptamers appeared to reduce viral RNA levels concomitantly with the reduction in Gag protein. It appears that these aptamers (DP6-7, DP6-32, etc.) interfere with the Gag-mRNA interactions at regions other than ψ. It is also likely that some of the aptamers affect other aspects of late events. For example, aptamers DP6-32 and -12 had significant levels of reduction in virus release efficiency (ca. 25%) and appeared to cause minor processing defects (DP6-5, -32, -19, -12, and -22). It remains to be seen whether these defects can be enhanced by ensuring high-level aptamer expression using stable cell-lines. The basis for the absence of effect by some of the aptamers is not understood and did not correlate with their Kds. In fact, some of them even increase the amount of Gag protein and Gag mRNA consistently. It is possible that these aptamers either become unfolded in vivo or have other issues that compromise their inhibitory ability.
In summary, we demonstrate that aptamers raised against the Gag polyprotein of HIV-1 can potently inhibit virus production and, hence, have the potential as anti-HIV gene-therapeutic agents. Hematopoietic stem cell (HSC)-based gene therapy, where HSCs are engineered to express anti-HIV molecules, is one of the rapidly developing fields in the treatment of AIDS. The long-term stable expression of RNA aptamers in HSCs and the development of methods to optimize the repopulation of human bone marrow with such genetically modified HSC is an area of active investigation that will pave the way for future clinical testing of such anti-HIV aptamers for in vivo efficacy.
Supplementary Material
Acknowledgments
We thank Na Li and Carlos Garcia for their assistance with the initial rounds of selections; Michael Summers and Wesley Sundquist for purified MA and CA proteins, respectively; Nathaniel Landau for the pNL4-3.Luc molecular clone; Eric Barklis for many helpful suggestions, Matthew Levy and Scott Garforth for critically reading the manuscript; and the NIH AIDS Research and Reference Reagent Program.
S.D. was supported by an institutional NIH AIDS training grant (T32 AI007501) and an NIH MARC fellowship (F31-GM78730). The research described in this report was supported by NIH grants R37AI030861 and P01 AI 061797 to V.R.P. and in part by the Intramural Research Program at the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
Published ahead of print on 27 October 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Benedict, C. M., W. Pan, S. E. Loy, and G. A. Clawson. 1998. Triple ribozyme-mediated down-regulation of the retinoblastoma gene. Carcinogenesis 19:1223-1230. [DOI] [PubMed] [Google Scholar]
- 2.Berkowitz, R., J. Fisher, and S. P. Goff. 1996. RNA packaging. Curr. Top. Microbiol. Immunol. 214:177-218. [DOI] [PubMed] [Google Scholar]
- 3.Berthet-Colominas, C., S. Monaco, A. Novelli, G. Sibai, F. Mallet, and S. Cusack. 1999. Head-to-tail dimers and interdomain flexibility revealed by the crystal structure of HIV-1 capsid protein (p24) complexed with a monoclonal antibody Fab. EMBO J. 18:1124-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell, S., and A. Rein. 1999. In vitro assembly properties of human immunodeficiency virus type 1 Gag protein lacking the p6 domain. J. Virol. 73:2270-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell, S., and V. M. Vogt. 1995. Self-assembly in vitro of purified CA-NC proteins from Rous sarcoma virus and human immunodeficiency virus type 1. J. Virol. 69:6487-6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaloin, L., M. J. Lehmann, G. Sczakiel, and T. Restle. 2002. Endogenous expression of a high-affinity pseudoknot RNA aptamer suppresses replication of HIV-1. Nucleic Acids Res. 30:4001-4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 8.Datta, S. A., and A. Rein. 2009. Preparation of recombinant HIV-1 gag protein and assembly of virus-like particles in vitro. Methods Mol. Biol. 485:197-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Datta, S. A., Z. Zhao, P. K. Clark, S. Tarasov, J. N. Alexandratos, S. J. Campbell, M. Kvaratskhelia, J. Lebowitz, and A. Rein. 2007. Interactions between HIV-1 Gag molecules in solution: an inositol phosphate-mediated switch. J. Mol. Biol. 365:799-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demirov, D. G., and E. O. Freed. 2004. Retrovirus budding. Virus Res. 106:87-102. [DOI] [PubMed] [Google Scholar]
- 11.D'Souza, V., and M. F. Summers. 2005. How retroviruses select their genomes. Nat. Rev. Microbiol. 3:643-655. [DOI] [PubMed] [Google Scholar]
- 12.Ellington, A. D., and J. W. Szostak. 1990. In vitro selection of RNA molecules that bind specific ligands. Nature 346:818-822. [DOI] [PubMed] [Google Scholar]
- 13.Facke, M., A. Janetzko, R. L. Shoeman, and H. G. Krausslich. 1993. A large deletion in the matrix domain of the human immunodeficiency virus gag gene redirects virus particle assembly from the plasma membrane to the endoplasmic reticulum. J. Virol. 67:4972-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng, Y. X., S. Campbell, D. Harvin, B. Ehresmann, C. Ehresmann, and A. Rein. 1999. The human immunodeficiency virus type 1 Gag polyprotein has nucleic acid chaperone activity: possible role in dimerization of genomic RNA and placement of tRNA on the primer binding site. J. Virol. 73:4251-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng, Y. X., T. D. Copeland, L. E. Henderson, R. J. Gorelick, W. J. Bosche, J. G. Levin, and A. Rein. 1996. HIV-1 nucleocapsid protein induces “maturation” of dimeric retroviral RNA in vitro. Proc. Natl. Acad. Sci. U. S. A. 93:7577-7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher, R. J., M. J. Fivash, A. G. Stephen, N. A. Hagan, S. R. Shenoy, M. V. Medaglia, L. R. Smith, K. M. Worthy, J. T. Simpson, R. Shoemaker, K. L. McNitt, D. G. Johnson, C. V. Hixson, R. J. Gorelick, D. Fabris, L. E. Henderson, and A. Rein. 2006. Complex interactions of HIV-1 nucleocapsid protein with oligonucleotides. Nucleic Acids Res. 34:472-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher, R. J., A. Rein, M. Fivash, M. A. Urbaneja, J. R. Casas-Finet, M. Medaglia, and L. E. Henderson. 1998. Sequence-specific binding of human immunodeficiency virus type 1 nucleocapsid protein to short oligonucleotides. J. Virol. 72:1902-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gamble, T. R., F. F. Vajdos, S. Yoo, D. K. Worthylake, M. Houseweart, W. I. Sundquist, and C. P. Hill. 1996. Crystal structure of human cyclophilin A bound to the amino-terminal domain of HIV-1 capsid. Cell 87:1285-1294. [DOI] [PubMed] [Google Scholar]
- 19.Gamble, T. R., S. Yoo, F. F. Vajdos, U. K. von Schwedler, D. K. Worthylake, H. Wang, J. P. McCutcheon, W. I. Sundquist, and C. P. Hill. 1997. Structure of the carboxyl-terminal dimerization domain of the HIV-1 capsid protein. Science 278:849-853. [DOI] [PubMed] [Google Scholar]
- 20.Gheysen, D., E. Jacobs, F. de Foresta, C. Thiriart, M. Francotte, D. Thines, and M. De Wilde. 1989. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell 59:103-112. [DOI] [PubMed] [Google Scholar]
- 21.Gitti, R. K., B. M. Lee, J. Walker, M. F. Summers, S. Yoo, and W. I. Sundquist. 1996. Structure of the amino-terminal core domain of the HIV-1 capsid protein. Science 273:231-235. [DOI] [PubMed] [Google Scholar]
- 22.Gross, I., H. Hohenberg, T. Wilk, K. Wiegers, M. Grattinger, B. Muller, S. Fuller, and H. G. Krausslich. 2000. A conformational switch controlling HIV-1 morphogenesis. EMBO J. 19:103-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 69:6705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinzinger, N. K., M. I. Bukinsky, S. A. Haggerty, A. M. Ragland, V. Kewalramani, M. A. Lee, H. E. Gendelman, L. Ratner, M. Stevenson, and M. Emerman. 1994. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc. Natl. Acad. Sci. U. S. A. 91:7311-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Held, D. M., J. D. Kissel, J. T. Patterson, D. G. Nickens, and D. H. Burke. 2006. HIV-1 inactivation by nucleic acid aptamers. Front. Biosci. 11:89-112. [DOI] [PubMed] [Google Scholar]
- 26.Ji, X., G. J. Klarmann, and B. D. Preston. 1996. Effect of human immunodeficiency virus type 1 (HIV-1) nucleocapsid protein on HIV-1 reverse transcriptase activity in vitro. Biochemistry 35:132-143. [DOI] [PubMed] [Google Scholar]
- 27.Joshi, P., and V. R. Prasad. 2002. Potent inhibition of human immunodeficiency virus type 1 replication by template analog reverse transcriptase inhibitors derived by SELEX (systematic evolution of ligands by exponential enrichment). J. Virol. 76:6545-6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joshi, P. J., T. W. North, and V. R. Prasad. 2005. Aptamers directed to HIV-1 reverse transcriptase display greater efficacy over small hairpin RNAs targeted to viral RNA in blocking HIV-1 replication. Mol. Ther. 11:677-686. [DOI] [PubMed] [Google Scholar]
- 29.Kim, M. Y., and S. Jeong. 2004. Inhibition of the functions of the nucleocapsid protein of human immunodeficiency virus-1 by an RNA aptamer. Biochem. Biophys. Res. Commun. 320:1181-1186. [DOI] [PubMed] [Google Scholar]
- 30.Kim, S. J., M. Y. Kim, J. H. Lee, J. C. You, and S. Jeong. 2002. Selection and stabilization of the RNA aptamers against the human immunodeficiency virus type-1 nucleocapsid protein. Biochem. Biophys. Res. Commun. 291:925-931. [DOI] [PubMed] [Google Scholar]
- 31.Kochs, G., M. Trost, C. Janzen, and O. Haller. 1998. MxA GTPase: oligomerization and GTP-dependent interaction with viral RNP target structures. Methods 15:255-263. [DOI] [PubMed] [Google Scholar]
- 32.Krausslich, H. G. 1991. Human immunodeficiency virus proteinase dimer as component of the viral polyprotein prevents particle assembly and viral infectivity. Proc. Natl. Acad. Sci. U. S. A. 88:3213-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee, T. C., B. A. Sullenger, H. F. Gallardo, G. E. Ungers, and E. Gilboa. 1992. Overexpression of RRE-derived sequences inhibits HIV-1 replication in CEM cells. New Biol. 4:66-74. [PubMed] [Google Scholar]
- 34.Lochrie, M. A., S. Waugh, D. G. Pratt, Jr., J. Clever, T. G. Parslow, and B. Polisky. 1997. In vitro selection of RNAs that bind to the human immunodeficiency virus type-1 gag polyprotein. Nucleic Acids Res. 25:2902-2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purohit, P., S. Dupont, M. Stevenson, and M. R. Green. 2001. Sequence-specific interaction between HIV-1 matrix protein and viral genomic RNA revealed by in vitro genetic selection. RNA 7:576-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rein, A., L. E. Henderson, and J. G. Levin. 1998. Nucleic-acid-chaperone activity of retroviral nucleocapsid proteins: significance for viral replication. Trends Biochem. Sci. 23:297-301. [DOI] [PubMed] [Google Scholar]
- 37.Sarver, N., E. M. Cantin, P. S. Chang, J. A. Zaia, P. A. Ladne, D. A. Stephens, and J. J. Rossi. 1990. Ribozymes as potential anti-HIV-1 therapeutic agents. Science 247:1222-1225. [DOI] [PubMed] [Google Scholar]
- 38.Silverman, R. H. 2007. Viral encounters with 2′,5′-oligoadenylate synthetase and RNase L during the interferon antiviral response. J. Virol. 81:12720-12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith, A. J., N. Srinivasakumar, M. L. Hammarskjold, and D. Rekosh. 1993. Requirements for incorporation of Pr160gag-pol from human immunodeficiency virus type 1 into virus-like particles. J. Virol. 67:2266-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sullenger, B. A., H. F. Gallardo, G. E. Ungers, and E. Gilboa. 1990. Overexpression of TAR sequences renders cells resistant to human immunodeficiency virus replication. Cell 63:601-608. [DOI] [PubMed] [Google Scholar]
- 41.Talloczy, Z., H. W. t. Virgin, and B. Levine. 2006. PKR-dependent autophagic degradation of herpes simplex virus type 1. Autophagy 2:24-29. [DOI] [PubMed] [Google Scholar]
- 42.Tanchou, V., C. Gabus, V. Rogemond, and J. L. Darlix. 1995. Formation of stable and functional HIV-1 nucleoprotein complexes in vitro. J. Mol. Biol. 252:563-571. [DOI] [PubMed] [Google Scholar]
- 43.Tuerk, C., and L. Gold. 1990. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249:505-510. [DOI] [PubMed] [Google Scholar]
- 44.Weber, F., O. Haller, and G. Kochs. 2000. MxA GTPase blocks reporter gene expression of reconstituted Thogoto virus ribonucleoprotein complexes. J. Virol. 74:560-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wills, J. W., and R. C. Craven. 1991. Form, function, and use of retroviral gag proteins. AIDS 5:639-654. [DOI] [PubMed] [Google Scholar]
- 46.Zhou, W., L. J. Parent, J. W. Wills, and M. D. Resh. 1994. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J. Virol. 68:2556-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.