Abstract
Many aspects of the assembly of hepatitis C virus (HCV) remain incompletely understood. To characterize the role of NS2 in the production of infectious virus, we determined NS2 interaction partners among other HCV proteins during productive infection. Pulldown assays showed that NS2 forms complexes with both structural and nonstructural proteins, including E1, E2, p7, NS3, and NS5A. Confocal microscopy also demonstrated that NS2 colocalizes with E1, E2, and NS5A in dot-like structures near lipid droplets. However, NS5A did not coprecipitate with E2 and interacted only weakly with NS3 in pulldown assays. Also, there was no demonstrable interaction between p7 and E2 or NS3 in such assays. Therefore, NS2 is uniquely capable of interacting with both structural and nonstructural proteins. Among mutations in p7, NS2, and NS3 that prevent production of infectious virus, only p7 mutations significantly reduced NS2-mediated protein interactions. These p7 mutations altered the intracellular distribution of NS2 and E2 and appeared to modulate the membrane topology of the C-terminal domain of NS2. These results suggest that NS2 acts to coordinate virus assembly by mediating interactions between envelope proteins and NS3 and NS5A within replication complexes adjacent to lipid droplets, where virus particle assembly is thought to occur. p7 may play an accessory role by regulating NS2 membrane topology, which is important for NS2-mediated protein interactions and therefore NS2 function.
The majority of hepatitis C virus (HCV) infections result in chronic liver disease that often progresses to liver cirrhosis and hepatocellular carcinoma (2, 27). With more than 170 million people infected with this agent, HCV is a significant health threat worldwide. HCV is a small enveloped virus belonging to the Hepacivirus genus in the Flaviviridae family. It possesses a positive-sense, single-stranded RNA genome encoding a polyprotein that is processed by cellular and viral proteases into 10 different proteins, including structural proteins (core, E1, and E2) and nonstructural proteins (p7, NS2, NS3, NS4A, NS4B NS5A, and NS5B) (4, 31, 33). The development of in vitro infectious HCV culture systems derived from genotype 1a (H77S) and genotype 2a (JFH1) viruses has facilitated study of the entire life cycle of HCV, including viral particle assembly and release (16, 36, 41, 42). These studies suggest that a number of nonstructural proteins of HCV are engaged in this late step in the virus life cycle. p7, NS2, NS3, and NS5A have all been implicated in virus particle assembly and maturation (3, 7, 11, 12, 21, 22, 28, 34, 35, 39, 40). However, the precise roles of these proteins in the production of infectious virus remain unclear.
The HCV p7 protein is a small, hydrophobic protein that consists of two transmembrane domains connected by a short stretch of basic residues (10, 20). It forms either a hexameric or heptameric complex to function as a cationic ion channel (6, 20). Mutations in conserved residues in p7, including residues critical for ion channel activity, interfere with a late step in virus production (12, 34). NS3 is a multifunctional protein possessing protease, helicase, and nucleoside triphosphatase (NTPase) activities (4, 15, 30). The protease activity of NS3 and its cofactor NS4A is responsible for processing the viral polyprotein at NS3-NS4A, NS4A-NS4B, NS4B-NS5A, and NS5A-NS5B junction sites, while the helicase/NTPase plays an essential but unknown role in viral RNA replication. In previous studies, we found that a compensatory mutation (Q221L) within the helicase domain was essential for assembly of infectious particles produced by an intergenotypic chimeric genome (HJ3-5) containing RNA encoding the nonstructural proteins of H77 (core to NS2) in the background of JFH1 (21). This genetic evidence suggests that NS3 plays an essential role in particle assembly. NS5A is a phosphoprotein and is expressed in cells replicating HCV in both hypo- and hyperphosphorylated forms (14, 26). Several groups have reported recently that the C-terminal domain of NS5A, and in particular a cluster of serine residues near the C terminus, which are potential target sites of CK2-mediated phosphorylation, are important for infectious particle assembly (3, 22, 35).
NS2 is a transmembrane protein containing an autoprotease responsible for cis cleavage at the NS2-NS3 junction within its C-terminal domain (19, 32, 38). A role for NS2 in the late stages of the virus life cycle is suggested by several studies. First, the capacity of chimeric genomes containing the nonstructural proteins of the JFH1 virus and structural proteins of other genotypes to produce infectious virus is highly dependent upon the specific position of the chimeric junction site within NS2 (29, 39). Second, deletion or substitution mutations within NS2 significantly affect the production of infectious virus (7, 12, 28). Moreover, we have shown previously that the S168 residue of NS2 is involved in regulating a post-particle assembly step in infectious virus production (40). The defects in virus production caused by mutation of this residue can be trans complemented by ectopic expression of wild-type (wt) NS2.
Importantly, NS2 and NS3 have been shown to interact with each other regardless of whether they are expressed as individual proteins or as an NS2-NS3 precursor (8, 13). Dimitrova et al. (8) have presented data suggesting that NS2 also interacts with NS4A, NS4B, NS5A, and NS5B using in vitro translated proteins. The interactions with NS3, NS4A, and NS5B were confirmed in studies with recombinant adenoviruses (8). It is curious that NS2 interacts with most of the nonstructural proteins, since the NS3-5B segment of the polyprotein is sufficient for the autonomous replication of subgenomic HCV replicon RNAs and the addition of the NS2-coding region does not enhance HCV RNA replication (17). This suggests that the interaction of NS2 with other nonstructural proteins, if confirmed in the context of replicating virus, may be involved in a step in the viral cycle other than RNA replication. In this context, it is interesting to note that defects in virus production caused by mutations in NS2 can be reversed by second-site mutations in the nonstructural proteins, including p7, NS2, NS3, NS4A, and NS5A, as well as in the envelope proteins, E1 and E2 (28, 39). These results prompted us to investigate the interactions of NS2 with both structural proteins and nonstructural proteins in the context of the chimeric HJ3-5 HCV genome, which efficiently produces infectious virus when transfected as RNA into cultured Huh7 hepatoma cells (39, 40). Importantly, HJ3-5 virus produced from this RNA is infectious both in Huh7 cells and in the chimpanzee model of hepatitis C (M. Yi et al., unpublished data).
Our results show that NS2 forms complexes with the envelope proteins, E1 and E2, as well as with p7 and other nonstructural proteins in cells supporting the production of infectious virus. They suggest that NS2 may be responsible for mediating interactions between the envelope proteins and nonstructural proteins engaged in infectious particle assembly. In addition, we show that in the absence of functional p7, the ability of NS2 to interact with these other HCV proteins is effectively abrogated.
MATERIALS AND METHODS
Plasmids.
Manipulations of HCV cDNA in this study were carried out in the background of pHJ3-5, which has been described previously (39, 40). To construct pHJ3-5/NS2YFP, first overlapping PCR was carried out to insert a yellow fluorescent protein (YFP) sequence in frame between p7 and NS2 within a fragment corresponding to the H77 sequence from position 1881 to 3466. This fragment was digested with SacI and BglII and ligated to two other fragments derived from pHJ3-5 (EcoRI/SacI and EcoRI/BglII fragments) to make pHJ3-5/NS2YFP. The hemagglutinin (HA) epitope tag (YPYDVPDYA) was fused in frame to the first residue of E1 within pHJ3-5/NS2YFP by using the QuikChange II XL site-directed mutagenesis kit (Stratagene) to make pHJ3-5/E1HA/NS2YFP. We inserted an HA epitope tag within the E2 sequence by replacing the hypervariable region 1 (HVR-1) residues 393 to 400 of pHJ3-5 with HA epitope sequence to make pHJ3-5/E2HA/NS2YFP, pHJ3-5/E2HA/p7YFP, and pHJ3-5/E2HA/NS5AYFP. To construct pHJ5-3/NS5AYFP, we created ClaI and NruI recognition sites within pHJ3-5, replacing the JFH1 nucleotide sequence from position 7412 to 7420 (NS5A residues 382 to 384). Next, the YFP sequence was amplified by PCR by using a forward primer containing a ClaI site and reverse primer containing an NruI site. Following digestion with ClaI and NruI, the YFP fragment was ligated to three other fragments derived from pHJ3-5 digested with ClaI/NsiI, NsiI/EcoRI, and EcoRI/NruI, respectively, to make pHJ5-3/NS5AYFP.
pHJ3-5/p7/YFP and pHJ3-5/p7YFP were constructed in multiple steps. First, we introduced a unique PmeI restriction site between p7 and the NS2 region of HJ3-5 (pHJ3-5/PmeI). Second, overlapping PCR was carried out to generate an encephalomyocarditis virus (EMCV) internal ribosome entry site (IRES)-NS2 fragment with an N-terminal PmeI site. Third, this fragment was digested with PmeI and BglII (located within NS2) restriction enzymes and ligated to PmeI/BglII fragments derived from pHJ3-5/PmeI to make pHJ3-5/p7-EMCV-NS2. Fourth, we deleted the sequence between p7 and YFP in pHJ3-5/NS5AYFP to make a p7/YFP in-frame fusion. To prepare a p7YFP fusion construct, we inactivated the signal sequence cleavage site located at the C terminus of p7 by mutating the terminal residue of p7 from Ala to Arg. These plasmids were digested with BamHI/NruI and ligated to two fragments generated from pHJ3-5/p7-EMCV-NS2 following digestion of this plasmid with PmeI/EcoRI and EcoRI/BamHI, respectively. The p7(KRAA), NS2(SG), and NS3(Qwt) mutations were introduced into the indicated plasmids by PCR-based mutagenesis. The sequences of the regions manipulated within each plasmid were verified by DNA sequencing.
Cells.
FT3-7 and Huh-7.5 cells are clonal derivatives of Huh7 human hepatoma cells (5, 39). They were grown in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) and 1× penicillin-streptomycin at 37°C in a 5% CO2 environment.
HCV RNA transfection and virus production.
HCV RNAs were transcribed in vitro and electroporated into cells. In brief, for electroporation, 10 μg of in vitro-synthesized HCV RNA was mixed with 5 × 106 FT3-7 cells in a 4-mm cuvette and pulsed once at 270 V and 950 μF in a Gene Pulser Xcell Total System (Bio-Rad). Cells were subsequently seeded into 12-well plates for analysis of HCV RNA. For virus production, transfected cells were seeded into 25-cm2 flasks and fed with medium containing 10% FBS.
Quantitation of HCV RNA.
Viral RNA was detected by a quantitative TaqMan reverse transcription-PCR (RT-PCR) assay (40). Total RNA was isolated from cell lysates by using an RNeasy kit (Qiagen, Valencia, CA) in accordance with the manufacturer's instructions. Quantitative real-time TaqMan RT-PCR analysis was carried out in a Bio-Rad iQ5 real-time PCR detection system using primer pairs and a probe targeting a conserved 221-base sequence within the 5′ nontranslated RNA segment of the genome: HCV84FP, GCCATGGCGTTAGTATGAGTGT; HCV JFH_303RP, CGCCCTATCAGGCAGTACCACAA; and HCV146BHQ, FAM-TCTGCGGAACCGGTGAGTACACC-DBH1. Reaction mixtures were incubated at 50°C for 2 min, 60°C for 45 min, and 95°C for 2 min, followed by 40 cycles of 95°C for 20 s and 60°C for 1 min.
HCV infectivity assays.
For virus titration, 100-μl aliquots of serial 10-fold dilutions of supernatant cell culture fluids, clarified by low-speed centrifugation, were inoculated onto naïve Huh-7.5 cells seeded 24 h previously into 48-well plates at 1 × 105 cells/well. Cells were maintained at 37°C in a 5% CO2 environment and fed with 100 μl of medium 24 h later. Following 48 h of additional incubation, cells were fixed in 1:1 methanol-acetone at room temperature for 9 min and then stained with monoclonal antibody C7-50 to core protein (Affinity BioReagents; 1:600) for 2 h at 37°C, washed with phosphate-buffered saline (PBS) twice, and incubated with Alexa Fluor 488-conjugated goat anti-mouse IgG antibody diluted 1:200 (Invitrogen). Clusters of infected cells staining for core antigen were considered to constitute a single, infectious focus-forming unit (FFU). Infectivity titers (FFU/ml) were calculated from the results of sample dilutions yielding 5 to 100 FFU.
Coimmunoprecipitation experiments.
FT3-7 cells were electroporated with the indicated genomic RNA. Then, 48 h later, cells were washed twice with PBS and lysed with ice-cold lysis buffer (PBS, 1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate [CHAPS] [Sigma catalog no. 05-2605], 1× protease inhibitor cocktail [Roche]) for 10 min on ice. The lysates were centrifuged at 14,000 rpm at 4°C for 10 min to remove debris. Anti-green fluorescent protein (anti-GFP) or anti-HA pulldown experiments were carried out using a μMACS GFP or HA isolation kit (Miltenyi Biotech) according to the manufacturer's suggestions. Briefly, 50 μl of anti-GFP MicroBeads or anti-HA MicroBeads was added to clarified supernatants (500 μg protein in 500 μl of lysis buffer) and incubated for 30 min on ice. These solutions were applied to μ columns that had been set up in the magnetic field of the μMACS separator. Subsequently we washed the columns four times using 200 μl of wash buffer 1 (150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris-HCl [pH 8.0]) for each wash, followed by one wash with wash buffer 2 (20 mM Tris-HCl [pH 7.5]). The immune complexes were eluted by applying a preheated 95°C elution solution.
Immunoblotting of viral proteins.
Immunoblots of cell lysates were probed with antibody to core (C7-50; Affinity BioReagents; 1:30,000 dilution), anti-NS3 (9-G2; Virogen; 1:1,000), anti-NS5A (9E10 [16], kindly provided by Charles Rice, Rockefeller University; 1:7,000), anti-HA (Sigma; A2095; 1:1,000), and anti-GFP (Clontech; 1:2,000 dilution) followed by horseradish peroxidase-conjugated anti-mouse IgG (Southern Biotech; 1:30,000). Alternatively, the immunoblots were probed with polyclonal rabbit anti-NS2 antibody (1:10,000 dilution) followed by horseradish peroxidase-conjugated anti-rabbit IgG (Southern Biotech; 1:30,000). Polyclonal rabbit anti-NS2 antibody was produced by immunization with two synthetic peptides encoding NS2 residues 145 to 161 and 203 to 217 (TPLRDWAHNGLRDLA and LGPADGMVSKGWRLL, respectively), derived from the C-terminal domain of genotype 1a NS2. Proteins were visualized by chemiluminescence by using reagents provided with the ECL Advance kit (Amersham Biosciences). Alternatively, IRDye 800CW goat anti-mouse and IRDye 680 goat anti-rabbit secondary antibodies (LI-COR Biosciences, Lincoln, NE) were used to probe immunoblots, followed by scanning with an Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE).
Laser scanning confocal microscopy.
Transfected cells were seeded onto eight-well chamber slides and 2 days later washed three times with PBS, fixed with 4% paraformaldehyde (PFA), and permeabilized with 0.1% Triton X-100 for 10 min. For Fig. 6A, cells were labeled with monoclonal anti-NS5A (9E10; 1:7,500) and anti-HA (Sigma, A2095; 1:200 dilution) antibodies, followed by detection with goat anti-mouse IgG2a conjugated to Alexa-633 (Invitrogen; 1:200) and goat anti-mouse IgG1a conjugated to Alexa-594 (Invitrogen; 1:200), respectively. For Fig. 6B and C, rabbit anti-NS2 antibodies (1:3,000) and human monoclonal anti-E2 antibodies (CBH-7, kindly provided by Steven Foung, Stanford University; 1:100) were used to detect NS2 and E2 proteins. Nuclei were visualized by counterstaining with DAPI (4′,6′-diamidino-2-phenylindole) (1:1000). In Fig. 6D, Neutral lipids present in lipid droplets (LDs) were visualized by staining with BODIPY493/503 (Invitrogen).
For the staining shown in Fig. 7, FT3-7 cells were electroporated and seeded onto eight-well chamber slides. After 48 h, cells were washed twice with KHM (110 mM potassium acetate [KOAc], 20 mM HEPES, 2 mM MgCl2), incubated with 50 μg/ml digitonin or KHM for 1 to 2 min, and washed twice with KHM. Live cells were stained for 12 min at 37°C with anti-protein disulfide isomerase (anti-PDI) mouse IgG1a (Stressgen; SPA-891; 1:50), anti-NS5A mouse IgG2a (9E10; 1:1,500), and anti-NS2 rabbit polyclonal antibody (1:100). Following three washes with PBS, cells were fixed with 4% PFA for 20 min. For the slides fixed with KHM alone without digitonin, cells were permeabilized for 10 min with 0.5% Triton X-100 and washed twice with PBS, followed by staining with primary antibodies to PDI (1:500), NS5A (1:15,000), and NS2 (1:1,200). Both subsets of slides were incubated with appropriate secondary antibodies: Alexa Fluor 633 goat anti-mouse IgG1a (1:1,000), Alexa Fluor 594 goat anti-mouse IgG2a (1:2,000), and Alexa Fluor 488 goat anti-rabbit (1:1,000). Nuclei were visualized by counterstaining with DAPI (1:1,000). Slides were examined with an Olympus FluoView FV1000 laser scanning confocal microscope.
Proteinase K treatment.
At 2 days postelectroporation, microsomal membranes were prepared from cells replicating HJ3-5 RNA with or without a p7(KRAA) mutation according to the protocols of Allan and Balch (1), with only slight modifications. Briefly, cells were scraped and homogenized by passing them 25 times through a 26-gauge needle in homogenization buffer (20 mM HEPES [pH 7.4], 250 mM sucrose). Following centrifugation at 720 × g for 5 min at 4°C, the postnuclear supernatant was mixed with 0.5 volume of buffer A (20 mM HEPES [pH 7.4], 210 mM potassium acetate, 3 mM magnesium acetate) and pelleted by centrifugation at 12,000 × g for 2 min at 4°C. The pellets were washed once in MSB buffer (150 mM potassium acetate, 5 mM magnesium acetate, 50 mM HEPES [pH 7.4], 1 mM dithiothreitol [DTT]) and resuspended in 5 volumes of MSB buffer before being stored in a −80°C freezer for future use. For proteinase K digestion, 20 μl of microsomal fraction was mixed with 20 μl each of following solutions: PK buffer (20 mM HEPES, 50 mM NaCl), PK buffer plus 50 μg/ml proteinase K, PK buffer plus 100 μg/ml proteinase K, and PK buffer plus 50 μg/ml proteinase K and 2% Triton X-100. The reaction was carried out on ice for 1 h and stopped by adding phenylmethylsulfonyl fluoride (PMSF) to final concentration of 20 mM. NS2 Western blot analysis was performed according to the protocols described above.
RESULTS
HJ3-5 RNA encoding NS2YFP is capable of producing infectious virus.
As reported previously, HJ3-5 is an intergenotypic chimeric HCV genome in which RNA encoding the core-to-NS2 segment of the H77 polyprotein is fused to JFH1 at the junction site between NS2 and NS3 and in which two compensatory mutations located in E1 and NS3 are responsible for robust virus production (Fig. 1A) (40). To facilitate the detection of interactions between NS2 and other HCV proteins, we inserted the YFP-coding sequence at the N terminus of NS2 in HJ3-5 to make virus expressing a YFP-fused NS2 protein (HJ3-5/NS2YFP) (Fig. 1A). Inserting YFP in frame within the NS2 sequence had minimal impact on RNA replication, as shown by quantitative RT-PCR following electroporation of the RNA into FT3-7 cells (Fig. 1B). Also, we detected significant levels of infectious HJ3-5/NS2YFP virus production, with titers reaching ∼2 × 104 to 3 × 104 FFU/ml by 2 days following the electroporation of HJ3-5/NS2YFP RNA into the FT3-7 cells (Fig. 1C and 2A). Although this level of virus yield is about 1.5 log units lower than that from the unmodified HJ3-5 RNA (Fig. 1C and 2A), it is significantly higher (10- to 20-fold) than that from genotype 2a JFH1 RNA (data not shown) (21, 40).
FIG. 1.
RNA replication and infectious virus release from chimeric HJ3-5 based genomes. (A) Organization of chimeric HJ3-5 RNAs encoding the genotype 1a H77-derived structural region in the background of JFH1 modified to express HA-fused E1 and E2 and YFP-fused p7, NS2, and NS5A. The genotype 1a H77-derived sequence is shaded. (B) Semiquantitative RT-PCR assays for HCV RNA in lysates of electroporated FT3-7 cells. (C) At day 2 postelectroporation, titers of infectious virus released from FT3-7 cells were determined by using naïve Huh7.5 cells. Error bars indicate standard deviations.
FIG. 2.
Differential effects of particle assembly-related mutations on NS2YFP protein-mediated pulldown of NS3. The interaction of NS2YFP with the nonstructural proteins NS3 and NS5A was determined by NS2YFP pulldown from cell lysates prepared at day 2 postelectroporation of HJ3-5/NS2YFP RNA. (A) The top panel shows the location of each mutation in the HJ3-5/NS2YFP genome. The bottom panel shows the virus titer determined at day 2 postelectroporation of the indicated RNAs into FT3-7 cells. Error bars indicate standard deviations. (B) Western blot results following anti-GFP (α-GFP) antibody immunoprecipitation (IP). In addition to NS3- and NS5A-specific antibodies, anti-GFP was used to detect NS2YFP.
NS2YFP coimmunoprecipitates with NS3 but not in the presence of the p7(KRAA) mutation.
To assess interactions between NS2 and other nonstructural proteins, we electroporated HJ3-5/NS2YFP RNA into FT3-7 cells. Two days later, cell lysates were obtained and NS2YFP-interacting proteins were determined by a GFP pulldown experiment. As shown in Fig. 2B, NS2YFP coimmunoprecipitated strongly with NS3. Interestingly, we were not able to demonstrate an interaction between NS2YFP and NS5A by the same method, although we were able to detect an interaction between the two proteins by a reciprocal approach (see below). Next, we introduced three different mutations, each of which has been shown to affect different steps in infectious particle production, into the HJ3-5/NS2YFP genomic background to determine whether these mutations affect the interaction between NS2 and NS3. Dual mutations in p7 changing conserved residues Lys-33 and Arg-35 to Ala have been shown to severely reduce (in the case of genotype 2a chimeras containing J6 structural proteins) or completely block (in the case of JFH1 or a chimera derived from genotype 1b Con1 and JFH1 sequences) infectious virus production without affecting HCV RNA replication (12, 34). These mutations ablate the putative ion channel activity of p7, but the exact mechanism by which these mutations block virus assembly is not clear. When we introduced these p7 mutations, K33A and R35A [or p7(KRAA)], into HJ3-5 or HJ3-5/NS2YFP RNA, they completely prevented infectious particle release from cells electroporated with these RNAs without significantly reducing RNA replication (Fig. 1B and 2A). Interestingly, the p7(KRAA) mutations also completely abrogated the interaction between NS2YFP and NS3 observed in the GFP pulldown assay (Fig. 2B). This result indicates that p7, and possibly the ion channel activity of p7, influences the interaction of NS2 with NS3 and could otherwise affect NS2 function (see below).
We demonstrated previously that a Ser-to-Gly substitution at residue 168 of NS2 [NS2(SG) mutant] blocks infectious virus production from HJ3-5 RNA at a post-particle assembly step (40). Interestingly, the YFP fusion with NS2 rescued virus production by the NS2(SG) mutant, albeit minimally (Fig. 2A). This compensatory effect of the YFP fusion on virus production was specific for the NS2(SG) mutant and was not due to a general enhancement of virus production, since the titer of HJ3-5/NS2YFP was lower than that of HJ3-5 (Fig. 2A). The NS2(SG) mutation did not impair the interaction between NS2 and NS3 (Fig. 2B). We also analyzed the impact of a Leu-to-Gln substitution at NS3 residue 221 [NS3(Qwt)] in HJ3-5/NS2YFP on the NS2-NS3 interaction. Residue 221 is Gln in the wild-type JFH1, and its replacement with Leu is the key compensatory mutation in the intergenotypic HJ3-5 chimera that allows it to produce infectious virus (21). The Q221L compensatory mutation is required for virus particle assembly, and its reversion to the wt Gln in the NS3(Qwt) mutant completely blocks virus production not only from HJ3-5 but also from HJ3-5/NS2YFP (Fig. 2A) (21). However, the NS3(Qwt) mutation does not affect the interaction of NS2YFP with NS3. This result indicates that the interaction between NS2 and NS3 occurs independently of particle formation and likely occurs prior to it during the process of new virus assembly and release. Thus, of the three mutations investigated here that block infectious virus production by HJ3-5 RNA, only the p7 mutation ablates the interaction of NS2 with NS3, while this phenomenon was not observed with mutations in NS2 or NS3 itself.
The interaction of E1 and E2 with NS2 is impaired by the p7 mutation.
Next, we investigated the interaction between NS2 and the structural proteins. To facilitate the detection of the E1 envelope protein, we inserted an HA epitope tag within the N-terminal region of E1 in the HJ3-5/NS2YFP genome. The resulting HJ3-5/E1HA/NS2YFP RNA replicated efficiently in a manner similar to that of HJ3-5 and HJ3-5/NS2YFP. However, this modification of E1 resulted in a low level of virus production, corresponding to about a 2-log10-unit reduction in virus yield compared to HJ3-5/NS2YFP and about a 3.5-log10-unit reduction in yield compared to HJ3-5. The insertion of the HA tag within the N-terminal region of E1 caused a reduction in the relative infectivity of virus released into culture supernatant fluids (infectious particle per RNA copy number), which suggested to us that viral entry was affected (see Fig. S1D in the supplemental material). It is less likely that the HA tag affected the virus assembly and release step, since the E1-tagged construct showed no differences in viral RNA secretion into the medium or in the ratio of intra- to extracellular infectivity compared to the RNA secretion and infectivity of its counterpart lacking an HA tag (see Fig. S1B and C in the supplemental material). To determine whether E1 can be shown to interact with NS2 during the production of virus particles, HJ3-5/E1HA/NS2YFP RNA was electroporated into FT3-7 cells, and 2 days later lysates were prepared and analyzed by anti-GFP immunoprecipitation followed by immunoblot detection of NS2YFP with anti-GFP antibody and of E1HA with anti-HA antibody. The results demonstrated strong complex formation between NS2YFP and E1HA (Fig. 3A). The p7(KRAA) mutation severely reduced this interaction between NS2 and E1, while neither the NS2(SG) nor the NS3(Qwt) mutation affected the interaction (Fig. 3A).
FIG. 3.

Interactions between NS2YFP and structural proteins, including core, E1, and E2. FT3-7 cells were electroporated with HJ3-5/E1HA/NS2YFP (A), HJ3-5/E2HA/NS2YFP (B) and HJ3-5/NS2YFP (C) with the indicated substitution or deletion mutations. At day 2 postelectroporation, cell lysates were subjected to anti-GFP IP followed by Western blot analysis to detect NS2YFP (anti-GFP antibody), E1HA or E2HA (anti-HA antibody), and NS3 or core (specific antibodies).
A previous study showed that replacement of the HVR-1 region of E2 with a Flag tag sequence did not adversely affect RNA replication and allowed production of infectious virus from JFH1 (24). We followed a similar strategy to insert the HA epitope into the H77 E2-coding sequence, replacing the HVR-1 region in HJ3-5/NS2YFP with the HA epitope sequence (see Materials and Methods). This modification did not substantially affect RNA replication (Fig. 1B) but did result in a moderate reduction in the yield of the resulting HJ3-5/E2HA/NS2YFP virus, by ∼5-fold, compared to that of HJ3-5/NS2YFP (Fig. 1C). Using this virus, however, we were able to demonstrate the presence of a complex containing NS2YFP and E2HA in lysates of cells transfected with HJ3-5/E2HA/NS2YFP RNA and its cognate NS2(SG) and NS3(Qwt) mutants (Fig. 3B). The minor differences apparent in the amounts of the tagged E2 protein precipitating with NS2 in lysates of cells transfected with the NS2(SG) and NS3(Qwt) mutants in Fig. 3B were not reproducible in subsequent experiments. In contrast, we observed no E2-NS2 complex formation in cells transfected with the p7(KRAA) mutant (Fig. 3B, middle panel). However, we also observed that processing between E2 and p7 appeared to be severely impaired with the p7(KRAA) mutant, resulting in an E2 HA protein of greater-than-expected size (Fig. 3B, input lanes, middle panel). To determine whether the lack of processing between E2 and p7 was responsible for the lack of interaction between NS2 and E2, we deleted the entire p7-coding sequence in HJ3-5/E2HA/NS2YFP and examined the interaction between the two proteins. Even though E2 processing appeared to be normalized in this construct, as suggested by the apparent molecular mass of E2 in SDS-PAGE, E2HA did not coimmunoprecipitate with NS2YFP in lysates from cells transfected with the Δp7 mutant (Fig. 3B, middle panel). Likewise NS2YFP failed to pull down NS3 from cell lysates derived from p7(KRAA) and Δp7 mutants (Fig. 3B, bottom panel). In addition, the p7 mutation G32A, which caused a ca. 30-fold reduction in the HJ3-5/E2HA/NS2YFP infectious virus yield without affecting E2-p7 processing, also reduced the interaction between NS2 and E2 (data not shown). Therefore, it is unlikely that the p7(KRAA) mutation globally affected NS2-mediated protein interactions by inhibiting E2-p7 processing.
The interaction between NS2 and NS3 is dependent on the presence of wt p7 but not on expression of the E1 and E2 proteins, since unlike the impact of the p7 deletion, which resulted in a complete loss of NS2-NS3 complex formation, the deletion of the E1E2-coding region from HJ3-5/NS2YFP did not affect the NS2-NS3 interaction (Fig. 3C). However, the introduction of the p7(KRAA) mutation into the ΔE1E2 mutant significantly reduced the interaction between NS2 and NS3 (Fig. 3C). In contrast to its negative impact on E2-p7 processing, the p7(KRAA) mutation did not impair processing between core and p7 in the ΔE1E2 mutant (Fig. 3C, bottom panel, and data not shown). These results strongly suggest that p7 is responsible for regulation of NS2-mediated interactions with other HCV proteins. Importantly, under the same experimental conditions as those employed in the experiments described above, the core protein failed to coprecipitate with NS2YFP (Fig. 3C, bottom panel). We conclude from these experiments that NS2 forms complexes involving a specific set of both nonstructural (NS3) and structural (E1 and E2) proteins in cells producing infectious HCV.
NS5AYFP coimmunoprecipitates efficiently with NS2, weakly with NS3, and not with E2.
Although we were not able to demonstrate the formation of a complex involving NS2 and NS5A in the NS2YFP pulldown assay (see above), we were able to detect such interaction when we attempted NS5A-mediated immunoprecipitation of NS2. Several groups have demonstrated that the insertion of GFP-coding sequence into the C-terminal region of NS5A allows continued replication of HCV RNA as well as infectious virus production (25). Likewise, we found that the insertion of the YFP-coding sequence into the C-terminal region of HJ3-5 NS5A resulted in a replication-competent RNA that produced only 3-fold less infectious virus than did HJ3-5 (Fig. 1B and C). Analysis of cell lysates electroporated with HJ3-5/NS5AYFP RNA by a GFP pulldown assay showed that NS5AYFP coimmunoprecipitated NS2 (Fig. 4A and C), suggesting that there is indeed formation of a complex involving both proteins. The p7(KRAA) mutation impaired but did not absolutely prevent formation of the complex between NS5AYFP and NS2 (Fig. 4A and C). A reduction in complex formation was also observed with the NS2(SG) mutation, albeit to a lesser extent (Fig. 4A) (see Discussion). In contrast, the NS3(Qwt) mutation did not perturb the interaction between NS5A and NS2.
FIG. 4.
NS5AYFP and p7YFP interact with NS2 but not with E2. (A) The interaction between NS5A and NS2 in the presence of the indicated mutations was determined by Western analysis followed by anti-GFP IP. NS5AYFP was detected with an anti-GFP antibody, and NS2 was detected by an NS2-specific antibody. Typically four washes were carried out following IP. The result of eight washes is also shown for comparison. (B) Comparison of the relative strengths of interaction of NS3 with either NS2YFP or NS5AYFP. As a control of background interaction, HJ3-5 RNA was electroporated into EGFP-expressing FT3-7 cells before we further analyzed the lysates (HJ3-5 + eGFP). (C) Interaction of NS5AYFP and p7YFP with E2, NS2, and NS3 in the presence and absence of the p7(KRAA) mutation. The indicated RNA was electroporated into FT3-7 cells. At 2 days postelectroporation, immunoblot detection of the indicated proteins was carried out following anti-GFP-IP. The upper panel was probed with an anti-GFP antibody and the second panel with anti-HA antibody. The third and fourth panels were probed with anti-NS3 and -NS2 antibodies, respectively. HJ3-5+eGFP, control for background binding (see above).
Although we were able to detect a small amount of NS3 in NS5AYFP precipitates, it was only slightly above background levels and very different from the efficient pulldown of NS3 that we observed in NS2YFP precipitates (Fig. 4B, compare lanes 2 and 3). Immunoprecipitates of enhanced GFP (EGFP)-associated HCV proteins from lysates of FT3-7 cells expressing EGFP and electroporated with HJ3-5 RNA (HJ3-5 + eGFP in Fig. 4B and C) served as the background binding for these pulldown experiments. Interestingly, the p7(KRAA) mutation minimally affected the interaction between NS5A and NS3, in contrast to its much stronger inhibitory effect on the interaction between NS5A and NS2 (Fig. 4C [compare lanes 1 and 2 in the third and fourth panels] and A). These results confirm a prior report of a weak association between NS3 and NS5A demonstrated in pulldown experiments with lysates of cells containing subgenomic replicons expressing NS5A with a PSTCD tag (23). These subgenomic RNAs replicate autonomously but do not produce infectious virus, making it likely that the weak interaction between NS3 and NS5A that we observed here reflects the common presence of the two proteins in the replication complex.
To facilitate the detection of the interaction between NS5A and E2, we inserted an HA tag in the E2 HVR-1 region in HJ3-5/NS5AYFP by the methods described above. Although the yield of the resulting HJ3-5/E2HA/NS5AYFP virus was moderately decreased, as was the case with HJ3-5/E2HA/NS2YFP, it reached almost 104 FFU/ml. When lysates of cells electroporated with the wt or p7(KRAA) mutant HJ3-5/E2HA/NS5AYFP RNA were analyzed, we were readily able to detect E2HA in wt cell lysates and unprocessed E2HA-p7 from p7(KRAA) mutant cell lysates, as expected (Fig. 4C, input lanes 1 and 2 in second panel). However, NS5AYFP was not able to pull down either form of the E2 protein (Fig. 4C, lanes 1 and 2 in second panel). These results suggest that NS5A, unlike NS2, does not form a complex involving E2.
P7YFP efficiently coimmunoprecipitates with NS2 but not with NS3 and E2.
Since p7 mutations greatly affected NS2-mediated protein interactions, we considered it possible that p7, like NS2, might be directly involved in the formation of multiprotein complexes involving both structural and nonstructural viral proteins. To determine whether p7 interacts with HCV proteins in a manner similar to that of NS2, we inserted the YFP-coding sequence at the C terminus of p7 in the HJ3-5 genome (see Materials and Methods). Since this leads to concomitant inactivation of the signal peptidase cleavage site at the end of the p7-coding region, it was necessary to overcome the processing defect between p7 and NS2. We accomplished this by introducing a picornaviral IRES between p7YFP and NS2. We also introduced the HA epitope tag into E2 protein, as described above. The resulting genome (HJ3-5/E2HA/p7YFP/IRES) replicated similarly to HJ3-5 but failed to produce infectious virus. We detected a small amount of unprocessed E2HA-p7YFP in addition to p7YFP in lysates of cells electroporated with this RNA (Fig. 4C, first panel, lane 4). However, the inclusion of the p7(KRAA) mutation resulted in the detection of E2HA-p7YFP as the major product detected with anti-GFP antibody (Fig. 4C, first panel, lane 3). Thus, the p7(KRAA)-mediated processing defect between E2 and p7 was not influenced by the YFP fusion to p7. Pulldown experiments demonstrated that p7YFP, with or without the KRAA mutations, efficiently coimmunoprecipitated NS2 (Fig. 4C, fourth panel, lanes 3 and 4). In the case of the p7(KRAA) mutant, it is likely that E2HA-p7YFP, rather than p7YFP, coprecipitated the NS2, since most p7 appeared to be expressed in the form of unprocessed E2HA-p7YFP in cells transfected with this RNA (Fig. 4C, lane 3). We were not able to detect an interaction between wt p7 and E2 or NS3, or between the p7(KRAA) mutant and NS3, in the same experiments (Fig. 4C, second and third panels, lanes 3 and 4).
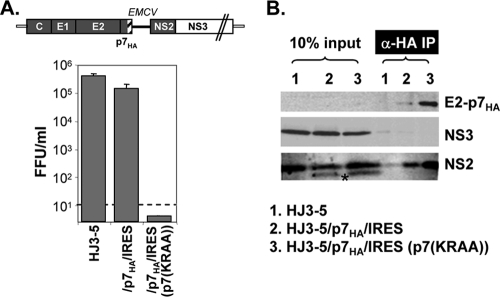
Since the HJ3-5/E2HA/p7YFP/IRES RNA failed to produce infectious virus, most likely at an early step in morphogenesis (see Fig. S1 in the supplemental material), it was not clear whether the p7YFP-mediated interactions observed here reflect actual interactions involved in infectious particle formation. However, as reported previously with the genotype 2a chimera J6/JFH1, the introduction of an IRES sequence between p7 and NS2 within HJ3-5 only minimally decreased infectious virus production (data not shown) (12). Thus, the dicistronic expression of HJ3-5 polyprotein per se is not responsible for the failure of the HJ3-5/E2HA/p7YFP/IRES RNA to produce virus. Removing the HA tag from the E2 protein in this construct did not restore virus production to any significant level (Fig. 1C, HJ3-5/p7YFP/IRES). However, when the signal sequence at the C terminus of p7 was restored, allowing processing of the junction between p7 and YFP, we were able to detect efficient virus production (HJ3-5/p7/YFP/IRES) (Fig. 1C). Therefore, the YFP fusion to p7 seems to have inhibited some critical function of p7 required for infectious virus production. To overcome this problem, we introduced an HA epitope tag at the C terminus of p7, replacing YFP to make HJ3-5/p7HA/IRES, with the rationale that the smaller HA tag would be less likely to interfere with p7 function than the much larger YFP tag. As expected, HJ3-5/p7HA/IRES RNA robustly produced infectious virus (Fig. 5A). However, despite several attempts, we were not able to detect a distinctive p7HA protein band in lysates of FT3-7 cells electroporated with HJ3-5/p7HA/IRES RNA (data not shown), probably due to its small size and technical difficulties with the immunoblot procedure. On the other hand, we were able to detect a distinct band representing unprocessed E2-p7HA following anti-HA immunoprecipitation of lysates from cells transfected with a HJ3-5/p7HA/IRES mutant containing the p7(KRAA) mutations (Fig. 5B, lane 3). We also detected a low abundance of E2-p7HA in immunoprecipitates of HJ3-5/p7HA/IRES-transfected cells (Fig. 5B, lane 2), probably due to inefficient processing between E2 and p7HA Importantly, NS2, but not NS3, was coimmunoprecipitated with p7HA in lysates from cells transfected with either HJ3-5/p7HA/IRES or its p7(KRAA) mutant at above background level (Fig. 5B). These results are similar to those we obtained with anti-GFP precipitation of HJ3-5/p7YFP/IRES lysates (Fig. 4C). In aggregate, these results indicate that p7 forms a complex with NS2 but may not be involved in multiprotein complexes involving NS2 and NS3 or E2 (see Discussion).
FIG. 5.
Infectious virus release from HJ3-5/p7HA-IRES-transfected cells and p7HA-mediated pulldown of NS2 and NS3 proteins. (A) The upper panel shows the organization of HJ3-5/p7HA-IRES. The lower panel shows infectious virus release into cell culture fluid at day 2 postelectroporation of the indicated RNA into FT3-7 cells. Error bars indicate standard deviations. (B) Western analysis of proteins following anti-HA IP. The proteins were run on 10% PAGE and probed with anti-HA, NS3, or NS2 antibodies, as noted. The asterisk indicates an additional band detectable by NS2 antibody. We speculate that this band may represent an aberrant initiation product by EMCV IRES, since this band appeared only from HJ3-5/p7HA/IRES-related constructs.
Mutations in p7 alter the distribution of NS2 and E2 within cells and disrupt NS2 and E2 colocalization with NS5A.
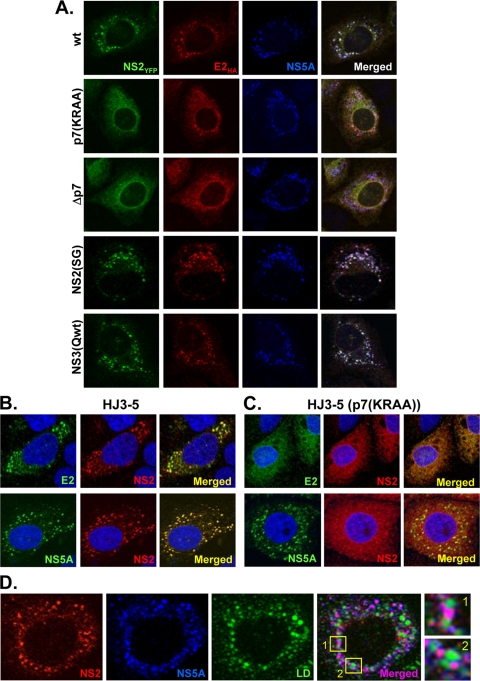
The distribution of NS2, E2, and NS5A within cells was determined by laser scanning confocal microscopy at 2 days after electroporation of HJ3-5/NS2YFP/E2HA RNA into FT3-7 cells. NS2YFP showed a predominantly punctate cytoplasmic distribution as detected by YFP autofluorescence and colocalized strongly with E2HA and NS5A (Fig. 6A, top row). NS5A is generally distributed within the cytoplasm of cells in a distinct punctuate pattern corresponding to replication complexes (25, 37) and is also recruited to the surfaces of lipid droplets (LDs) decorated with core protein (3, 24), which are believed to be sites of actual particle assembly (see below) (3, 24, 25, 37). Thus, it is no surprise that we found NS5A to be distributed in this distinct punctate fashion. However, both NS2 and E2 have been reported previously to be localized to the endoplasmic reticulum (ER) and to show an ER-like reticular distribution when expressed individually (9, 40). Therefore, it was surprising to find NS2 and E2, expressed in the context of replicating HCV RNA producing infectious virus, colocalized with the focal, punctate distribution of NS5A (in more than 70% of cells replicating this RNA). Interestingly, the introduction of the p7(KRAA) mutation or deletion of p7 resulted in an ER-like distribution of both NS2YFP and E2HA in more than 90% of the cells supporting replication of these RNAs (Fig. 6A, second and third rows). We observed similar results when we used HJ3-5/E1HA/NS2YFP to determine the localization of E1 in the presence and absence of p7 mutations (data not shown). Thus, E1HA was localized to distinct foci and colocalized with NS2 and NS5A, while mutations of p7 in the form of either p7(KRAA) or deletion of p7 led to a diffuse distribution of E1 and NS2 within the cytoplasm (data not shown). Since these p7 mutations block infectious virus production (Fig. 2A), it is reasonable to assume that the colocalization of the envelope proteins, NS2, and NS5A demonstrated in Fig. 6 may be essential for HCV assembly and release. Importantly, the distribution pattern of NS5A was unchanged by the p7 mutations (Fig. 6A, second and third rows), despite the fact that the p7 mutation disrupted the interaction we observed between NS2 and NS5A (Fig. 4A). This indicates that NS5A localization is independent of its interaction with NS2 but is consistent with NS2 localization being dependent on its interaction with NS5A. The NS2(SG) and NS3(Qwt) mutations did not alter the localization pattern of NS2YFP and E2HA, despite their lethal effect on infectious virus production (Fig. 6A, fourth and fifth rows). This is consistent with the result of NS2-dependent pulldown assays described above and suggests that they block an alternative step in infectious virus production.
FIG. 6.
Mutations in p7 perturb the colocalization of NS2 and E2 with NS5A. (A) FT3-7 cells were electroporated with HJ3-5/E2HA/NS2YFP RNA with the mutations indicated on the left side and 2 days later were immunostained with anti-HA antibody to detect E2HA (red) and anti-NS5A (blue) antibody. NS2YFP was detected by GFP autofluorescence signal (green). The fluorescence signals were examined by using an Olympus FluoView FV1000 laser scanning confocal microscope. Merged images are shown at the right. (B and C) NS2 (red), E2 (green), and NS5A (green) were detected at 2 days postelectroporation of HJ3-5 RNA without (B) or with (C) the p7(KRAA) mutation. Merged images are shown at the right. (D) HJ3-5 RNA was electroporated into FT3-7 cells, and at day 2 postelectroporation, NS2 (red) and NS5A (blue) were immunostained and LDs (green) were stained with BODIPY493/503 and analyzed with a laser scanning confocal microscope. The left three panels show individual staining signals, and the forth panel is a merged image. At the right is the enlarged area from the merged image showing NS2 and NS5A (pink) localized adjacent to LD (green).
We detected similar patterns of HCV protein localization in cells electroporated with HJ3-5 RNA with or without the p7(KRAA) mutation, as shown in Fig. 6B and C. We detected the colocalization of NS2 with E2 or NS5A in dot-like foci from cells electroporated with HJ3-5 RNA (Fig. 6B). Importantly, these complexes containing NS2 and NS5A were localized immediately adjacent to LDs (Fig. 6D). Introduction of the p7(KRAA) mutation into HJ3-5 resulted in an ER-like distribution of NS2 and E2, without affecting the punctate distribution of NS5A and its association with LDs (Fig. 6C and data not shown). Combined with protein interaction data, these results suggest that NS2 interacts with NS3 and NS5A in replication complexes adjacent to LDs and also interacts with envelope proteins to support virus assembly.
p7(KRAA) mutation and NS2 topology.
The results described above indicate that p7 modulates the intracellular localization of NS2 and its interaction with both structural and nonstructural proteins. Since p7 appears to interact with NS2 but not NS3 (Fig. 4C and 5B) or E2 (Fig. 4C), we considered the possibility that p7 might influence the function of NS2 by altering its topology with respect to membranes. Previous reports suggest that NS2 is a transmembrane protein with the potential to adopt different topologies (32, 38). To be active as the NS2/NS3 protease, the C-terminal domain of NS2 must be localized to the cytoplasm (19). On the other hand, experimental data indicate that the C-terminal domain of NS2 may also reside within the ER lumen (32, 38). We asked whether mutations ablating p7 function could influence these topologies adopted by NS2, thereby altering the ability of NS2 to interact with other HCV proteins and, secondarily, its distribution within the cell. To address this question, we modified the approach described by Lorenz et al. (18) to determine the membrane topology of proteins in living cells, using differential membrane permeabilization protocols followed by immunofluorescence analysis with an antibody directed against the C-terminal domain of NS2 (see Fig. S2 in the supplemental material). FT 3-7 cells transfected with HJ3-5 or the related p7(KRAA) mutant were treated with digitonin to permeabilize the plasma membrane or with Triton X-100 to permeabilize both the plasma and intracellular membranes. Control antigens studied in parallel in this experiment included the ER luminal protein PDI and the cytoplasmic HCV protein NS5A. As shown in Fig. 7A, digitonin treatment did not allow the detection of PDI, as anticipated. However, NS2 was readily detected using an antibody directed against its carboxy-terminal domain, as was the cytoplasmic antigen NS5A, which also identifies cells in which HJ3-5 RNA was replicating. In contrast, Triton X-100 permeabilization led to the detection of all three proteins, including PDI. These results suggest that the C-terminal domain of NS2 expressed during productive replication of HJ3-5 RNA is localized to the cytoplasm. However, when cells transfected with the HJ3-5[p7(KRAA)] mutant were examined, NS2 was no longer detectable following digitonin treatment, but only after treatment with Triton X-100 (Fig. 7B). These results suggest that the C-terminal domain of NS2 likely resides in the ER lumen when expressed in cells transfected with HJ3-5[p7(KRAA)] RNA, which is unable to produce infectious virus. In general, we observed a correlation between NS2 detection following digitonin treatment and a punctate localization of NS2, which was seen in about 70% of the cells with HJ3-5 (see above). However, we were not able to detect NS2 in digitonin-treated cells when it was localized in a diffuse pattern, as was the case in the majority of cells transfected with the p7(KRAA) mutant.
FIG. 7.
Perturbation of NS2 topology in the presence of the p7(KRAA) mutation. (A and B) FT3-7 cells were electroporated with HJ3-5 RNA without (A) or with (B) the p7(KRAA) mutation and placed on eight-well chamber slides. Two days later, one group of cells was permeabilized with digitonin to detect cytoplasmic antigen and the other group of cells with Triton X-100 to detect antigens located at intracellular organelles. Cells were probed with antibodies directed against the C-terminal domains of NS2, NS5A, and PDI. Detection of the cytoplasmic protein NS5A in digitonin-treated cells, without concurrent staining of the ER luminal protein PDI, confirmed that the plasma membrane was selectively permeabilized, while the ER membrane remained intact. NS5A also served as a control for HCV-positive cells. (C) Proteinase K digestion pattern of NS2 present in microsomes isolated from cells replicating HJ3-5 with or without p7(KRAA) mutations. PrK 25, PrK 50, and PrK 25/T indicate treatment of microsomes with 25 μg/ml proteinase K, 50 μg/ml proteinase K, and 25 μg/ml proteinase K plus 1% Triton X-100, respectively.
A similar conclusion was suggested by an analysis of the susceptibility of NS2 in microsome preparations to proteinase K digestion in the presence or absence of 1% Triton X-100. As shown in Fig. 7C, in the absence of Triton X-100, immunoblots of microsomes containing the p7(KRAA) mutant revealed a major proteinase K-resistant fragment that was smaller than intact NS2. This disappeared with proteinase K treatment in the presence of Triton X-100. In contrast, no NS2 band was detected following proteinase K digestion of microsomes derived from HJ3-5-transfected cells, in either the presence or absence of Triton X-100. Since the antibody used in these blots recognizes the C terminus of NS2, this result suggests that the C-terminal domain of NS2 is localized to the cytoplasm in cells transfected with the wild-type HJ3-5 RNA but is protected from proteinase K digestion in the p7 (KRAA) mutant because it is localized to the ER lumen.
Collectively, these results suggest that p7 function may be essential for proper localization of the NS2 C-terminal domain within the cytoplasm and that this localization is critical for NS2 interactions with other viral proteins.
DISCUSSION
The recent development of fully infectious molecular clones of HCV has allowed studies of the mechanism of action of individual viral proteins involved in virus assembly and release. Several nonstructural proteins, especially p7, NS2, NS3, and NS5A have been shown to be critical for these late steps in virus replication (3, 7, 11, 12, 21, 22, 28, 34, 35, 39, 40). However, the specific contributions of these proteins to this process remain unclear. In this report, we describe studies aimed at understanding the mechanism by which NS2 promotes infectious virus production by characterizing its interactions with other HCV proteins in cells supporting the replication of an HCV RNA that produces robust yields of infectious virus.
We demonstrated that NS2 forms complexes involving the two HCV envelope proteins (E1 and E2), p7, and the nonstructural proteins NS3 and NS5A. While our data do not exclude indirect interactions between NS2 and these proteins, perhaps through bridges provided by other viral or cellular proteins, they do suggest the presence of several different NS2-containing complexes. For example, p7 pulldown of NS2 did not result in the coprecipitation of NS3 (Fig. 4C and 5B) or E2 (data not shown), despite strong evidence for interactions between NS2 and both NS3 and E2 (Fig. 2 and 3). Also, while NS5A interacted with NS2, it could not pull down E2 (Fig. 4A). In addition, although the p7(KRAA) mutation disrupted the NS2-mediated pulldowns of E1, E2, and NS3 (Fig. 2 and 3), the same mutation did not significantly affect the interaction of p7 with NS2 (Fig. 4C and 5B) or that of NS5A with NS3 (Fig. 4C). Importantly, the interaction between NS2 and NS5A was detected only when we used cell lysates from HJ3-5/NS5AYFP to pull down NS2 with NS5AYFP and not when we used those from HJ3-5/NS2YFP RNA-transfected cells to pull down NS5A with NS2YFP (Fig. 2 and 5). This is curious, since NS2YFP efficiently pulled down other HCV proteins, including E1, E2, and NS3. It is possible that the GFP antibody preferentially precipitated NS2YFP-containing protein complexes devoid of interaction with NS5A due to steric hindrance for antibody binding resulting from the interaction of NS2 with NS5A, rather than a lack of interaction between the two proteins in HJ3-5/NS2YFP replicating cells. Consistent with this hypothesis, we detected strong colocalization of NS2YFP and NS5A (Fig. 6A). On the other hand, our data show that the NS2(SG) mutation, which inhibits a post-particle assembly step in virus production, did not affect the interaction of NS2 with E1, E2, and NS3. However, this mutation significantly weakened the interaction between NS2 and NS5A. It is possible that the NS2 and NS5A interaction may be involved in a process, such as postassembly maturation, different from that requiring the interaction of NS2 with E1, E2, and NS3. Our previous data show that the NS2(SG)-mediated defect in virus production can be compensated for by a second-site mutation in NS5A, strongly supporting this hypothesis (40). In this context, it is tempting to speculate that NS2 may be involved in at least two different complexes, one containing E1, E2, and NS3 and the other containing NS5A. The former complex may be functioning at the initiation stage of viral particle formation, and the latter complex may be functioning at a post-particle assembly step. However, further study will be necessary to prove this theory.
The p7 mutations, p7(KRAA) and Δp7, interrupt virus production and inhibit the interaction between NS2 and each of the other HCV proteins that we tested. Since the p7(KRAA) mutant protein coimmunoprecipitated with NS2 (Fig. 4A), we considered the possibility that the mutant p7 might have an altered cellular distribution and, through its interaction with NS2, lead to the aberrant ER-like location for NS2 shown in Fig. 6A. However, according to Griffin et al. (10), the p7(KRAA) mutations do not change p7 localization, when p7 is expressed either alone or as part of a C-E1-E2-p7 polyprotein. We also detected no difference in p7YFP localization in the presence or absence of the p7(KRAA) mutations in cells electroporated with HJ3-5/p7YFP/IRES (data not shown). Our attempt to detect the localization of HA-p7 from cells replicating HJ3-5/p7HA/IRES was unsuccessful, probably due to low-level expression of p7HA protein (Fig. 5B and data not shown). Finally, the p7 deletion mutant also induced an ER-like distribution of NS2 in a manner similar to that of the p7(KRAA) mutant, indicating that it is the absence of functional p7 that induces the aberrant distribution of NS2.
On the other hand, our results suggest that the dramatic effect of the ablation of p7 function, by either introduction of the p7(KRAA) mutations or deletion of p7 entirely, on NS2-mediated protein interactions may result from an alteration in the membrane topology of the C-terminal domain of NS2. As shown in Fig. 7, we used two different technical approaches to investigate the cytoplasmic localization of the C-terminal domain of NS2 in cells replicating HJ3-5 RNA. Both immunofluorescence microscopy with limited membrane permeabilization and ex vivo proteinase K sensitivity studies suggested that the p7(KRAA) mutation induces an alteration in the membrane topology of the C-terminal domain of NS2, redirecting it from the cytoplasm to the lumen of the ER, where it appears to be unable to interact with envelope proteins and nonstructural proteins. Since the p7(KRAA) mutation has been shown to inhibit infectious particle assembly without affecting RNA replication, these results are consistent with the notion that the NS2-mediated protein interactions we have demonstrated here are critical for infectious particle assembly. Although it has been shown previously that NS2 may adopt different membrane topologies when expressed ectopically, it has not been clear whether NS2 expressed from replicating viral RNA is similarly capable of adopting different topologies. The technical approach that we used cannot distinguish between complete localization (single topology) versus partial localization (mixed topologies) of the C-terminal domain of NS2 in the cytoplasm. Further studies will be necessary to determine if NS2 topology plays a significant role in virus production, as we suspect it might. However, this subject is beyond the scope of the current study.
LDs play a critical role in infectious HCV production (24). The HCV core protein coats LDs, and the replication complex is directed to a site adjacent to the LD by an interaction between core and NS5A (3, 24). However, it has not been clear how envelope proteins could be directed to this site to initiate virus particle formation. Our data suggest that NS2 plays a key role in this process. Our data show that the HCV envelope proteins, NS2, and NS5A colocalize in dot-like structures in cells actively producing infectious virus. NS2 and the envelope proteins have been shown to assume an ER-like distribution when expressed alone (9, 40). On the other hand, NS5A is known to be distributed into punctate foci due to its association with replication complex (37). It is likely that the complexes formed by NS2 and E1 and E2, as well as the nonstructural NS3 and NS5A proteins, result in the colocalization of these proteins at the nexus of the replication complex and the LD. Consistent with this hypothesis, the introduction of p7 mutations [p7(KRAA) or Δp7] that block interactions between NS2 and other HCV proteins led to a disruption of the colocalization of envelope proteins and NS2 with NS5A, effectively redistributing the envelope proteins and NS2 to an ER-like distribution. On the other hand, the p7 mutations did not affect NS5A localization, probably because NS5A is associated with relatively stable replication complexes independent of its interaction with NS2. We suggest that NS2 acts to recruit the envelope proteins to replication complexes adjacent to LDs through its interactions with both the envelope proteins and nonstructural proteins associated with replication complex, effectively acting as a scaffold promoting the assembly of the infectious virus particles.
Supplementary Material
Acknowledgments
We are grateful to Charles Rice for providing Huh-7.5 cells and NS5A antibodies and to Takaji Wakita for providing the JFH1 cDNA clone.
This work was supported by grants R01-AI075090, U19-AI40035, and N01-AI25488 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Published ahead of print on 20 October 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Allan, B. B., and W. E. Balch. 2001. In vitro analysis of endoplasmic-reticulum-to-Golgi transport in mammalian cells. Curr. Protoc. Cell Biol. 11:11.3. [DOI] [PubMed] [Google Scholar]
- 2.Alter, H. J. 2005. HCV natural history: the retrospective and prospective in perspective. J. Hepatol. 43:550-552. [DOI] [PubMed] [Google Scholar]
- 3.Appel, N., M. Zayas, S. Miller, J. Krijnse-Locker, T. Schaller, P. Friebe, S. Kallis, U. Engel, and R. Bartenschlager. 2008. Essential role of domain III of nonstructural protein 5A for hepatitis C virus infectious particle assembly. PLoS Pathog. 4:e1000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartenschlager, R. 1999. The NS3/4A proteinase of the hepatitis C virus: unravelling structure and function of an unusual enzyme and a prime target for antiviral therapy. J. Viral Hepat. 6:165-181. [DOI] [PubMed] [Google Scholar]
- 5.Blight, K. J., J. A. McKeating, and C. M. Rice. 2002. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J. Virol. 76:13001-13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke, D., S. Griffin, L. Beales, C. S. Gelais, S. Burgess, M. Harris, and D. Rowlands. 2006. Evidence for the formation of a heptameric ion channel complex by the hepatitis C virus p7 protein in vitro. J. Biol. Chem. 281:37057-37068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dentzer, T. G., I. C. Lorenz, M. J. Evans, and C. M. Rice. 2009. Determinants of the hepatitis C virus nonstructural protein 2 protease domain required for production of infectious virus. J. Virol. 83:12702-12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimitrova, M., I. Imbert, M. P. Kieny, and C. Schuster. 2003. Protein-protein interactions between hepatitis C virus nonstructural proteins. J. Virol. 77:5401-5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duvet, S., L. Cocquerel, A. Pillez, R. Cacan, A. Verbert, D. Moradpour, C. Wychowski, and J. Dubuisson. 1998. Hepatitis C virus glycoprotein complex localization in the endoplasmic reticulum involves a determinant for retention and not retrieval. J. Biol. Chem. 273:32088-32095. [DOI] [PubMed] [Google Scholar]
- 10.Griffin, S. D., R. Harvey, D. S. Clarke, W. S. Barclay, M. Harris, and D. J. Rowlands. 2004. A conserved basic loop in hepatitis C virus p7 protein is required for amantadine-sensitive ion channel activity in mammalian cells but is dispensable for localization to mitochondria. J. Gen. Virol. 85:451-461. [DOI] [PubMed] [Google Scholar]
- 11.Jirasko, V., R. Montserret, N. Appel, A. Janvier, L. Eustachi, C. Brohm, E. Steinmann, T. Pietschmann, F. Penin, and R. Bartenschlager. 2008. Structural and functional characterization of nonstructural protein 2 for its role in hepatitis C virus assembly. J. Biol. Chem. 283:28546-28562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones, C. T., C. L. Murray, D. K. Eastman, J. Tassello, and C. M. Rice. 2007. Hepatitis C virus p7 and NS2 proteins are essential for production of infectious virus. J. Virol. 81:8374-8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiiver, K., A. Merits, M. Ustav, and E. Zusinaite. 2006. Complex formation between hepatitis C virus NS2 and NS3 proteins. Virus Res. 117:264-272. [DOI] [PubMed] [Google Scholar]
- 14.Koch, J. O., and R. Bartenschlager. 1999. Modulation of hepatitis C virus NS5A hyperphosphorylation by nonstructural proteins NS3, NS4A, and NS4B. J. Virol. 73:7138-7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin, C., and J. L. Kim. 1999. Structure-based mutagenesis study of hepatitis C virus NS3 helicase. J. Virol. 73:8798-8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindenbach, B. D., M. J. Evans, A. J. Syder, B. Wolk, T. L. Tellinghuisen, C. C. Liu, T. Maruyama, R. O. Hynes, D. R. Burton, J. A. McKeating, and C. M. Rice. 2005. Complete replication of hepatitis C virus in cell culture. Science 309:623-626. [DOI] [PubMed] [Google Scholar]
- 17.Lohmann, V., F. Korner, J. Koch, U. Herian, L. Theilmann, and R. Bartenschlager. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110-113. [DOI] [PubMed] [Google Scholar]
- 18.Lorenz, H., D. W. Hailey, and J. Lippincott-Schwartz. 2006. Fluorescence protease protection of GFP chimeras to reveal protein topology and subcellular localization. Nat. Methods 3:205-210. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz, I. C., J. Marcotrigiano, T. G. Dentzer, and C. M. Rice. 2006. Structure of the catalytic domain of the hepatitis C virus NS2-3 protease. Nature 442:831-835. [DOI] [PubMed] [Google Scholar]
- 20.Luik, P., C. Chew, J. Aittoniemi, J. Chang, P. Wentworth, Jr., R. A. Dwek, P. C. Biggin, C. Venien-Bryan, and N. Zitzmann. 2009. The 3-dimensional structure of a hepatitis C virus p7 ion channel by electron microscopy. Proc. Natl. Acad. Sci. U. S. A. 106:12712-12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma, Y., J. Yates, Y. Liang, S. M. Lemon, and M. Yi. 2008. NS3 helicase domains involved in infectious intracellular hepatitis C virus particle assembly. J. Virol. 82:7624-7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masaki, T., R. Suzuki, K. Murakami, H. Aizaki, K. Ishii, A. Murayama, T. Date, Y. Matsuura, T. Miyamura, T. Wakita, and T. Suzuki. 2008. Interaction of hepatitis C virus nonstructural protein 5A with core protein is critical for the production of infectious virus particles. J. Virol. 82:7964-7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCormick, C. J., S. Maucourant, S. Griffin, D. J. Rowlands, and M. Harris. 2006. Tagging of NS5A expressed from a functional hepatitis C virus replicon. J. Gen. Virol. 87:635-640. [DOI] [PubMed] [Google Scholar]
- 24.Miyanari, Y., K. Atsuzawa, N. Usuda, K. Watashi, T. Hishiki, M. Zayas, R. Bartenschlager, T. Wakita, M. Hijikata, and K. Shimotohno. 2007. The lipid droplet is an important organelle for hepatitis C virus production. Nat. Cell Biol. 9:1089-1097. [DOI] [PubMed] [Google Scholar]
- 25.Moradpour, D., M. J. Evans, R. Gosert, Z. Yuan, H. E. Blum, S. P. Goff, B. D. Lindenbach, and C. M. Rice. 2004. Insertion of green fluorescent protein into nonstructural protein 5A allows direct visualization of functional hepatitis C virus replication complexes. J. Virol. 78:7400-7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neddermann, P., A. Clementi, and R. De Francesco. 1999. Hyperphosphorylation of the hepatitis C virus NS5A protein requires an active NS3 protease, NS4A, NS4B, and NS5A encoded on the same polyprotein. J. Virol. 73:9984-9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pawlotsky, J. M. 2004. Pathophysiology of hepatitis C virus infection and related liver disease. Trends Microbiol. 12:96-102. [DOI] [PubMed] [Google Scholar]
- 28.Phan, T., R. K. Beran, C. Peters, I. C. Lorenz, and B. D. Lindenbach. 2009. Hepatitis C virus NS2 protein contributes to virus particle assembly via opposing epistatic interactions with the E1-E2 glycoprotein and NS3-NS4A enzyme complexes. J. Virol. 83:8379-8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pietschmann, T., A. Kaul, G. Koutsoudakis, A. Shavinskaya, S. Kallis, E. Steinmann, K. Abid, F. Negro, M. Dreux, F. L. Cosset, and R. Bartenschlager. 2006. Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proc. Natl. Acad. Sci. U. S. A. 103:7408-7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Preugschat, F., D. R. Averett, B. E. Clarke, and D. J. Porter. 1996. A steady-state and pre-steady-state kinetic analysis of the NTPase activity associated with the hepatitis C virus NS3 helicase domain. J. Biol. Chem. 271:24449-24457. [DOI] [PubMed] [Google Scholar]
- 31.Reed, K. E., A. Grakoui, and C. M. Rice. 1995. Hepatitis C virus-encoded NS2-3 protease: cleavage-site mutagenesis and requirements for bimolecular cleavage. J. Virol. 69:4127-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santolini, E., L. Pacini, C. Fipaldini, G. Migliaccio, and N. Monica. 1995. The NS2 protein of hepatitis C virus is a transmembrane polypeptide. J. Virol. 69:7461-7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimotohno, K., Y. Tanji, Y. Hirowatari, Y. Komoda, N. Kato, and M. Hijikata. 1995. Processing of the hepatitis C virus precursor protein. J. Hepatol. 22:87-92. [PubMed] [Google Scholar]
- 34.Steinmann, E., F. Penin, S. Kallis, A. H. Patel, R. Bartenschlager, and T. Pietschmann. 2007. Hepatitis C virus p7 protein is crucial for assembly and release of infectious virions. PLoS Pathog. 3:e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tellinghuisen, T. L., K. L. Foss, and J. Treadaway. 2008. Regulation of hepatitis C virion production via phosphorylation of the NS5A protein. PLoS Pathog. 4:e1000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wakita, T., T. Pietschmann, T. Kato, T. Date, M. Miyamoto, Z. Zhao, K. Murthy, A. Habermann, H. G. Krausslich, M. Mizokami, R. Bartenschlager, and T. J. Liang. 2005. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 11:791-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolk, B., B. Buchele, D. Moradpour, and C. M. Rice. 2008. A dynamic view of hepatitis C virus replication complexes. J. Virol. 82:10519-10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamaga, A. K., and J. H. Ou. 2002. Membrane topology of the hepatitis C virus NS2 protein. J. Biol. Chem. 277:33228-33234. [DOI] [PubMed] [Google Scholar]
- 39.Yi, M., Y. Ma, J. Yates, and S. M. Lemon. 2007. Compensatory mutations in E1, p7, NS2, and NS3 enhance yields of cell culture-infectious intergenotypic chimeric hepatitis C virus. J. Virol. 81:629-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yi, M., Y. Ma, J. Yates, and S. M. Lemon. 2009. Trans-complementation of an NS2 defect in a late step in hepatitis C virus (HCV) particle assembly and maturation. PLoS. Pathog. 5:e1000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yi, M., R. A. Villanueva, D. L. Thomas, T. Wakita, and S. M. Lemon. 2006. Production of infectious genotype 1a hepatitis C virus (Hutchinson strain) in cultured human hepatoma cells. Proc. Natl. Acad. Sci. U. S. A. 103:2310-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhong, J., P. Gastaminza, G. Cheng, S. Kapadia, T. Kato, D. R. Burton, S. F. Wieland, S. L. Uprichard, T. Wakita, and F. V. Chisari. 2005. Robust hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. U. S. A. 102:9294-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.