Abstract
Cytomegalovirus (CMV) utilizes multiple strategies to modulate immunity and promote lifelong, persistent/latent infection, including suppressing T cell activation pathways. Here we examined the role of B7 costimulatory ligands in establishing immune détente from both the host and virus perspectives. Mice lacking both B7.1 and B7.2 showed reduced early expansion of CMV-specific CD4 T cells, consequently allowing for enhanced levels of persistent virus replication. In turn, a CMV mutant lacking expression of the m138 and m147.5 gene products, which restrict B7.1 and B7.2 expression in infected antigen-presenting cells, induced a more robust CD4 T cell response and showed decreased persistence. Together, these data reveal a requirement for B7-mediated signaling in regulating the CMV-specific CD4 T cell response and establishing host-virus equilibrium.
Herpesviruses have coevolved with their vertebrate hosts for over one hundred million years (29), resulting in a finely tuned equilibrium with the immune system. Human cytomegalovirus (HCMV/HHV5 [a betaherpesvirus]) infects the majority of the world's population, establishing a lifelong, largely asymptomatic infection in immunocompetent persons but causing severe disease in immunocompromised neonates and adults (35). Excessive accumulation of CMV-specific T cells occurs in persistently infected hosts (18, 43, 46), a phenomenon termed memory inflation (25), and has been associated with an immune risk profile and immune senescence in elderly patients (34, 47).
Both innate and adaptive immune responses control CMV infection. Innate defenses mounted by type I interferons in the initial hours (40) and by NK and NKT cells during the first days largely limit acute replication (6, 45). Following this initial phase, adaptive immune responses develop. The generation of CMV-specific CD4 T cells correlates strongly with disease protection in patients (10, 11). Experimental models of CMV infection have shown that CD4 T cells can control primary systemic CMV infection, restrict persistent replication in select tissues, and promote antibody responses (22-24). In turn, CD8 T cells can protect immunocompromised humans and mice from CMV disease and restrict viral reactivation from latency (38, 39).
In order for antigen-presenting cells (APCs) such as dendritic cells (DCs) to effectively activate T cells, costimulatory signals must be induced in combination with T cell receptor (TCR) ligation. Positive cosignals enhance initial T cell activation, promote cell division, augment cell survival, and induce effector functions. The B7-CD28 costimulatory pathway is critical for T cell responses against numerous pathogens (5, 14, 41). The ligands B7.1 (CD80) and B7.2 (CD86) are rapidly upregulated upon activation of APCs, while their positive costimulatory receptor CD28 is constitutively expressed on both naïve and activated T cells (41). B7.1/2-induced T cell activation is abrogated in later phases of the response by upregulation of CTLA-4 (CD152), a negative cosignaling receptor for both ligands. Additional negative (e.g., PD-1) and positive (e.g., CD27, OX40, and 4-1BB) cosignals work in concert with B7 ligands to precisely tune the numbers and function of T cells that expand during an acute response, as well as to establish their eventual set point during the memory phase.
A large percentage of the CMV genome is recognized to encode immunomodulatory genes (8), several of which target the cellular machinery involved in T cell activation (36). Mouse CMV (MCMV) encodes two gene products that inhibit expression of B7.1 and B7.2 (m138 and m147.5, respectively) (26, 31), and HCMV similarly downregulates these two costimulatory ligands (17, 32). Targeting of B7 signaling by both human and mouse CMVs implies it imposes a strong selective pressure on the interplay between CMV and its host. Here we show that B7-CD28 signaling, as well as MCMV modulation of this system, regulates the MCMV-specific CD4 T cell response and impacts persistent replication.
MATERIALS AND METHODS
Mice.
C57BL/6 (B6) wild-type (WT), CD28−/−, B7.1−/−, B7.2−/−, and CD28−/− mice (all on a B6 background) and BALB/c mice were purchased from the Jackson Laboratory (Bar Harbor, ME). B7.1−/− and B7.2−/− double-deficient mice (B7.1/2−/−) on a B6 background were kindly provided by A. Sharpe. CD90.1 (Thy1.1) OT-II and CD90.1 CD28−/− OT-II TCR-transgenic mice were bred in-house. Mice were maintained under specific-pathogen-free conditions in the Department of Laboratory Animal Care at the La Jolla Institute for Allergy and Immunology. All experiments were approved by the La Jolla Institute IACUC in accordance with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care.
Generation of MCMV-Δm138/m147.5 mutant virus.
A mutant MCMV deficient for expression of m138 and m147.5 was generated from the previously constructed RV-AL22 BAC that contains four stop codons in the positions of amino acids 48, 49, 50, and 52 of the m147.5 open reading frame (ORF) abrogating the production of a functional m147.5 protein (26). MCMV-Δm138/m147.5 was generated in both the C3X (pSM3FR) and green fluorescent protein (GFP) (pSM3FR-GFP) bacterial artificial chromosome (BAC)-cloned MCMV genome containing the aforementioned mutation (28). Utilizing the ET-BAC mutagenesis method (26), a 130-bp region (between nucleotides 193070 and 193201) was replaced with the sequences encoding a kanamycin resistance gene (26). The following primers were utilized to generate the deletion in the m138 gene: forward, 5′-cactggaggtgaaagcgtagttccatgtgtcgacgtcgtacagcatcaagCTACAAGGACGACGACGACAAGTAA-3′, and reverse, 5′gactgaagtcgccaacagagtctgctgtaagctcgatgattaccataactGTGACA CAGGAACACTTAA CGGCTGA-3′. Sequences that anneal to the BAC MCMV sequence are lowercase, and those that anneal to the Kanr gene are uppercase. The MCMV-Δm138/m147.5 mutant virus was verified by restriction digest and sequencing and was confirmed to be unable to downregulate B7.1 and B7.2 in infected cells.
MCMV stock preparation, infection, and quantification.
The MCMV-Smith strain (referred to as “MCMV” throughout) was obtained from the American Type Culture Collection (VR-194, Manassas, VA), and salivary gland stocks were prepared in BALB/c mice as described previously (40). MCMV-OVA has been described (42). MCMVC3X-Δm138/m147.5, MCMVGFP-Δm138/m147.5, and the respective wild-type viruses were derived after BAC DNA electroporation into NIH 3T3 cells (30), and viral stocks were subsequently generated in the same cells. Age (8- to 12-week-old)- and gender-matched mice were infected intraperitoneally (i.p.) with 5 × 104 PFU of salivary gland-derived MCMV-Smith or with 5 × 105 PFU of BAC-derived viruses. Bone marrow-derived dendritic cells cultured in granulocyte-macrophage colony-stimulating factor (GM-CSF) were infected as previously described (multiplicity of infection [MOI], 10) (4), and 48 h postinfection these cells were GFP+ sorted and adoptively transferred into recipient mice (5 × 104 GFP+ cells per mouse). MCMV PFU in infected organs were determined as described previously (40).
In vivo antibody treatment.
Hybridomas were cultured in Life Technologies protein-free hybridoma medium-II (Invitrogen, San Diego, CA), and monoclonal antibodies (MAbs) were purified by dialysis of supernatants. To deplete CD4 T cells, 200 μg of anti-mouse CD4 MAb (clone GK1.5) was injected i.p. on days −3, −1, and +3 after infection. To deplete CD8 T cells, 200 μg anti-mouse CD8 MAb (clone 2.43) was injected i.p. on days +3 and +5 after infection. For in vivo blockade of cosignaling molecules, 200 μg of anti-mouse B7.1 (clone 16-10A1) and/or anti-mouse B7.2 (clone GL1) or anti-CTLA-4 MAb (clone UC10) was injected i.p. on days −1, 0 and +2 of infection. All MAbs were administered in 200 μl phosphate-buffered saline (PBS).
Flow cytometry and intracellular staining.
Spleens were harvested and single-cell suspensions were prepared by mincing the tissue through a 70-μm cell strainer (BD Biosciences). Lymphocytes from perfused livers and lungs were obtained by mincing the tissue, followed by collagenase treatment for 0.5 h and lympholyte gradient. Erythrocytes were lysed in a hypotonic (0.82%) ammonium chloride buffer. For cell surface staining, cells were resuspended in staining buffer (PBS + 2% fetal calf serum [FCS] + 0.05% sodium azide) and incubated with fluorescent conjugated antibodies for 30 min at 4C°. For intracellular cytokine staining, cells were in vitro restimulated in 96-well flat-bottom plates (1.5 × 106 splenocytes per well). CD8 T cells were stimulated in medium containing 2 μg/ml peptide and 1 μg/ml brefeldin A for 5 h, and CD4 T cells were stimulated in medium with 5 μg/ml peptide for 8 h, of which the last 6 h were in the presence of 1 μg/ml brefeldin A. After stimulation, cells were transferred to U-bottom 96-well plates and cell surface stained with fluorescent conjugated antibodies at 4°C for 0.5 h in staining buffer. After being washed, cells were fixed with 1% paraformaldehyde at 4C° for >0.5 h followed by intracellular staining for cytokines at 4C° for 0.5 to 1 h in Perm/Wash buffer (BD Biosciences). After washing and resuspending in staining buffer, cells were acquired using a BD LSRII flow cytometer and data were analyzed using FlowJo software (Tree Star). Fluorochrome-conjugated monoclonal antibodies specific for CD4, CD8, CD62L, CD90.1, gamma interferon [IFN-γ], tumor necrosis factor [TNF], and Vα2 were purchased from BD Biosciences (San Diego, CA) or eBioscience (San Diego, CA).
Peptides.
The following MCMV peptide epitopes were used: major histocompatibility complex (MHC) class I-restricted M45985-993, M38316-323, M57816-824, and m139419-426 and MHC class II-restricted m09133-147, m18872-886, M25409-423, m139560-574, m141181-195, and m14224-38. Peptides were high-performance liquid chromatography (HPLC) purified and synthesized by A&A Systems (San Diego, CA).
Adoptive transfer.
For adoptive transfer experiments, 5 × 104 WT OT-II or CD28−/− OT-II T cells were transferred into naïve recipient WT mice, which were subsequently infected i.p. with 5 × 105 PFU MCMV-OVA. OT-II T cell expansion in the spleens of recipient mice was determined 8 days posttransfer by flow cytometric analysis of the transgenic TCRa chain (Va2) and the congenic marker (CD90.1/Thy1.1) on CD4 T cells.
Statistical analysis.
The statistical significance of viral titers was determined with the Mann-Whitney test, and statistical significance of T cell responses was determined with the two-tailed Student t test. Differences between groups were considered significant at P values of <0.05 (*, P < 0.05; **, P < 0.005).
RESULTS
B7-mediated CD4 T cell responses control MCMV replication.
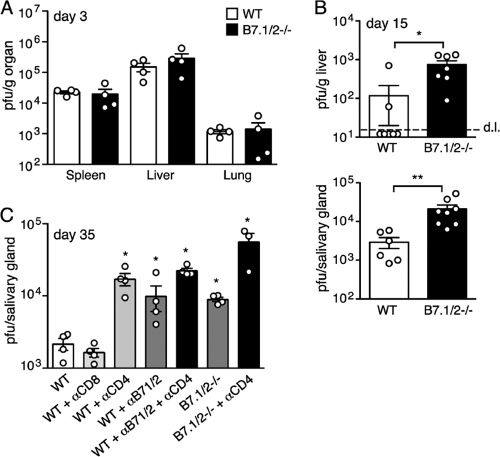
Mice genetically deficient for both B7.1 and B7.2 (B7.1/2−/−) were utilized to determine the role of B7-CD28 signaling during MCMV infection. At day 3 postinfection, no differences in viral replication were observed in the spleen, liver, or lung when comparing wild-type (WT) and B7.1/2−/− mice (Fig. 1A). Since NK cells are critical for controlling MCMV at early times, this result suggests that B7.1/2−/− mice mount a normal NK response to this virus. Indeed, no defects in the expression of activation markers (CD25 and CD69) by splenic NK cells (NK1.1+DX5+) were observed at day 3 in MCMV-infected B7.1/2−/− mice (data not shown), consistent with recently published results examining NK cell numbers and proliferation in these mice after MCMV infection (9). In contrast, at day 15, when T cells contribute to immune control, MCMV replication was markedly higher in the livers of B7.1/2−/− mice (Fig. 1B). Virus production was also significantly increased in the salivary glands of B7.1/2−/− mice at day 15 and day 35 postinfection (Fig. 1B and C).
FIG. 1.
B7-mediated costimulation is required to control MCMV replication. (A) WT and B7.1/2−/− mice were infected with MCMV, and 3 days later PFU were determined in the spleen, lung, and liver. (B) At day 15 postinfection PFU in the liver and salivary glands of MCMV-infected WT and B7.1/2−/− mice were determined. Dashed line represents detection limit (d.l.). (C) MCMV-infected WT and B7.1/2−/− mice were antibody depleted of CD8 or CD4 T cells (αCD8/CD4) or treated with blocking B7.1 and B7.2 MAbs (αB7.1/2). Graphs depict MCMV PFU present in salivary glands 35 days postinfection. All bar graphs display means with standard errors, and each symbol (○) represents an individual mouse. Data shown are representative of two independent experiments, and statistical significance was analyzed using Mann-Whitney tests (*, P < 0.05; **, P < 0.005).
CD4 T cells play the principal role in controlling persistent MCMV replication in the salivary gland (22), so the contribution of B7-CD28 signaling in the presence and absence of this cellular subset was examined. As expected, depletion of CD4 T cells in WT mice enhanced MCMV production in the salivary glands of WT mice by ∼8-fold at day 35 following infection (Fig. 1C). This increase was slightly higher than that seen in B7.1/2−/− mice or WT mice injected with blocking B7.1/2 antibodies in the absence of depletion (∼4- to 5-fold), and depletion of CD4 T cells in B7 signaling-deficient mice further enhanced replication. No increase in salivary gland replication was detected when CD8 T cells were depleted in WT mice. Taken together, these data strongly imply that the impaired MCMV-specific CD4 T cell responses in mice lacking B7 signaling are largely responsible for the observed increase in persistent replication in this organ.
Optimal MCMV-specific CD4 T cell responses are dependent on B7-mediated costimulation.
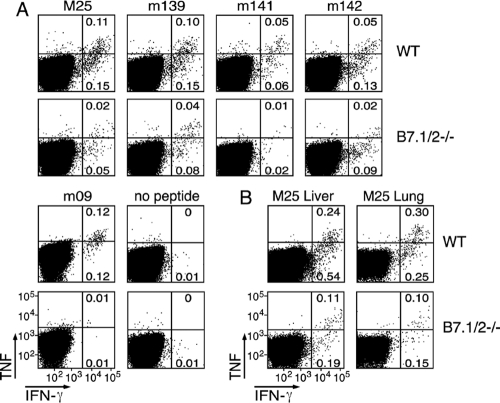
The increased levels of MCMV salivary gland replication in B7.1/2−/− mice suggested that B7-CD28 signaling contributes to the development of the virus-specific CD4 T cell response. To assess this directly, the levels of several MCMV epitope-specific CD4 T cell responses (3) were determined in WT mice and B7.1/2−/− mice (Fig. 2A). CD4 T cell responses recognizing MHC class II-restricted epitopes that peak during acute infection (e.g., m18, M25, m139, m141, m142) were significantly decreased at day 8 postinfection in the spleens of B7.1/2−/− mice compared to those in WT mice (Fig. 2A), and similar reductions were also observed in the lungs and liver (shown for M25; Fig. 2B). In addition, the response to a peptide derived from m09, which peaks at ∼day 40, was significantly reduced in B7.1/2−/− mice (Fig. 2A).
FIG. 2.
Optimal MCMV-specific CD4 T cell responses are dependent on B7-mediated costimulation. WT and B7.1/2−/− mice were infected with MCMV. (A) Flow cytometric plots represent the percentage of gated splenic CD4 T cells of WT or B7.1/2−/− mice producing IFN-γ and TNF after restimulation with the MHC class II restricted peptide epitopes derived from the M25, m139, m141, m142, and m18 proteins at day 8 postinfection. Restimulation with the m09 peptide epitope was performed at day 40 postinfection and with vehicle-stimulated splenocytes at day 8 after infection (no peptide) (B) Flow plots show gated CD4 T cells that were isolated from livers and lungs at day 8 postinfection and restimulated with the M25 peptide epitope. Data shown are representative of two independent experiments.
MCMV-specific CD4 T cell responses are dependent on CD28 interaction with B7.1 and B7.2.
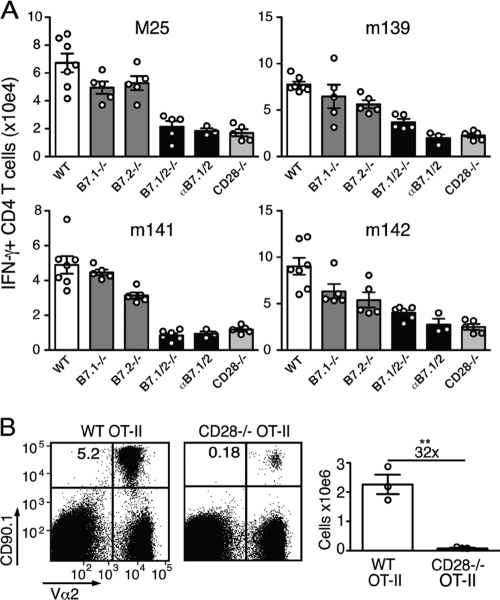
To examine the respective roles of B7.1 and B7.2 in regulating the MCMV-specific CD4 T cell response, single B7 knockout (B7.1−/− and B7.2−/− mice) and blocking monoclonal αB7.1 and αB7.2 antibodies were utilized (Fig. 3A). MCMV-specific CD4 T cell numbers were only slightly decreased in the singly deficient mice, demonstrating that B7.1 and B7.2 function redundantly to a large degree during MCMV infection (Fig. 3A). CD28−/− mice essentially mirrored what was observed in B7.1/2−/− mice. Mice treated with antibodies blocking B7.1 and/or B7.2 recapitulated the results observed in genetically deficient mice, with both B7 molecules needing to be blocked to observe marked reductions in MCMV-specific CD4 T cell responses (Fig. 3A and data not shown). To verify that CD28 signaling in the CD4 T cell itself is critical for B7-mediated expansion of MCMV-specific CD4 T cell responses, naïve ovalbumin (OVA)-specific CD4 T cells (OT-II) from WT or CD28−/− mice were transferred into WT mice, which were subsequently infected with MCMV expressing the ovalbumin protein (MCMV-OVA). At day 8 postinfection, the expansion of CD28−/− OT-II cells was markedly reduced compared to that of WT OT-II cells (∼30-fold; Fig. 3B). Taken together, these data indicate that CD28 ligation of CD4 T cells by both B7.1 and B7.2 regulates their expansion during MCMV infection.
FIG. 3.
Both B7.1 and B7.2 promote the expansion of MCMV-specific CD4 T cells. (A) Graphs depict the total number of IFN-γ+ CD4 T cells in the spleen of MCMV-infected WT, B7.1−/−, B7.2−/−, and B7.1/2−/− and CD28−/− mice or WT mice treated with blocking B7.1 and B7.2 antibodies (αB7.1/2). (B) Congenically marked (CD90.1) WT and CD28−/− OVA-specific TCR transgenic CD4 T cells (OT-II) were adoptively transferred into mice that were subsequently infected with MCMV-OVA. The number of transgenic T cells was determined in the spleen 8 days following transfer. Statistical significance was determined by Student's t test (**, P < 0.005). Fold difference is indicated. Bar graphs are shown as means with standard errors, and each symbol (○) represents an individual mouse. Data shown are representative of two independent experiments.
Enhancing B7-CD28 interactions by blocking CTLA-4 increases MCMV-specific CD4 T cell responses.
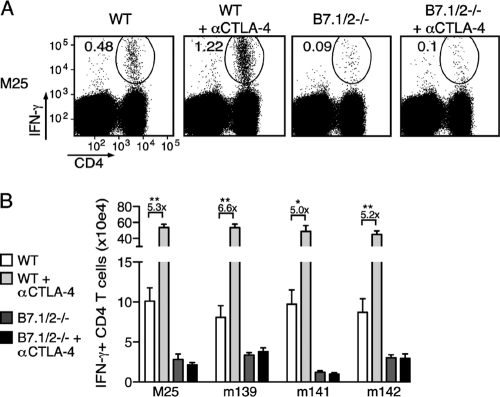
To determine whether inhibitory counterbalancing mechanisms for B7-CD28-mediated costimulation are in place during MCMV infection, the binding of B7.1 and B7.2 to their inhibitory receptor CTLA-4 was neutralized by injecting blocking antibodies during initial infection (αCTLA-4). αCTLA-4 increased the frequencies of MCMV-specific CD4 T cells (shown for M25) in the spleen ∼3-fold (Fig. 4A). As αCTLA-4 also induces splenomegaly, the effect was even more pronounced when absolute numbers were determined, revealing an ∼5-fold increase for each epitope-specific response (Fig. 4B). As anticipated, αCTLA-4 did not alter antigen-specific CD4 T cell responses in B7.1/2−/− mice.
FIG. 4.
Impact of CTLA-4 blockade on the expansion of MCMV-specific CD4 T cells in WT and B7.1/2−/− mice. (A) MCMV-infected WT or B7.1/2−/− mice were treated with blocking αCTLA-4 or left untreated, and 8 days later MCMV-specific CD4 T cells present in the spleen were analyzed after restimulation with class II peptide epitopes. Dot plots depict the frequency of IFN-γ-producing M25-specific CD4 T cells from spleens, and numbers represent the percentage of IFN-γ+ CD4+ cells within the total CD4 T cell population. (B) Same conditions as in panel A, and the total numbers of M25-, m139-, m141-, or m142-specific CD4 T cells producing IFN-γ+ are depicted. The data are representative of two independent experiments each with four or five mice per group. Numbers are shown as means with standard errors. Statistical significance was determined by Student's t test.
B7-CD28 regulation of CD4 T cell expansion is operable primarily during the early phase of MCMV infection.
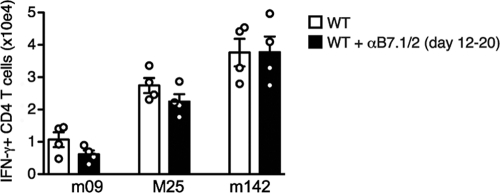
The experiments in genetically deficient mice indicated that B7-CD28 signals are required for expansion of the MCMV-specific CD4 T cell response during the primary phase of acute infection (i.e., the first 8 days). To determine whether B7-mediated cosignals might also regulate the magnitude of the CD4 T cell response at later times, B7.1/2 was neutralized by injection of blocking antibodies from day 12 to 20 postinfection. Quantifying CD4 T cell levels at this later time also allowed for the analysis of the m09-specific CD4 T cells, as this response is undetectable until later times of infection (3). MCMV-specific CD4 T cells (m09, M25, and m142) tended to be only very slightly reduced after this treatment, with no reductions reaching significance (Fig. 5), indicating that the critical phase for B7-CD28 regulation of this response is early during infection.
FIG. 5.
Dispensable role of B7-CD28 for CD4 T cells at later times of MCMV infection. MCMV-infected WT mice were treated with blocking B7.1 and B7.2 antibodies (αB7.1/2) from day 12 to day 20 postinfection. Graph shows the total number of IFN-γ+ CD4 T cells in the spleen at day 20 after restimulation with m09-, M25-, and m142-derived peptide epitopes. Bar graphs are shown as means with standard errors, and each symbol (○) represents an individual mouse. Experiment was performed twice with similar results.
Inhibition of B7.1 and B7.2 expression by MCMV restricts the CD4 T cell response and promotes persistent replication.
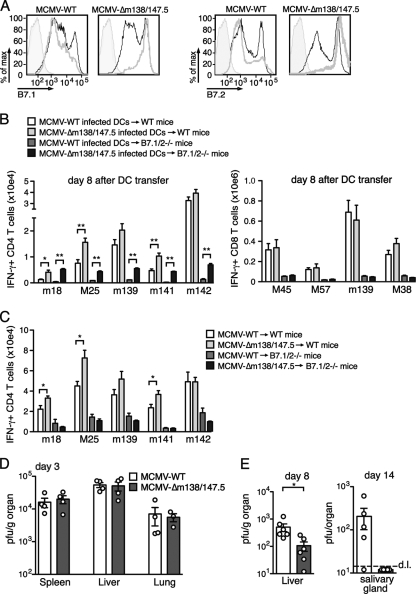
Given that both B7 molecules are crucial for the host to develop MCMV-specific CD4 T cell responses and control persistent replication, we wished to examine the role of these molecules from the virus perspective. Consequently, an MCMV mutant disrupted for expression of both m138 and m147.5 was generated, the proteins responsible for down-modulating B7.1 and B7.2, respectively. Infection of bone marrow-derived DCs with MCMV-Δm138/m147.5 revealed this mutant to be unable to restrict B7.1/2 expression compared to DCs infected with WT (Fig. 6A). MCMV-WT- or MCMV-Δm138/m147.5-infected DCs were then adoptively transferred into WT recipient mice, and the magnitudes of the m18-, M25-, and m141-specific CD4 T cell responses were significantly increased in mice receiving MCMV-Δm138/m147.5-infected DCs (Fig. 6B). Moreover, when these two infected DC populations were transferred into B7.1/2−/− recipients, MCMV-specific CD4 T cell responses were dramatically enhanced in mice receiving mutant virus infected cells. In contrast, MCMV epitope-specific CD8 T cell responses were equivalent whether transferred DCs were infected with WT or mutant virus. In total, these results prove unequivocally that B7 downmodulation in DCs themselves preferentially impacts the expansion of MCMV-specific CD4 T cells (Fig. 6B).
FIG. 6.
Restriction of B7.1 and B7.2 expression by MCMV suppresses CD4 T cell responses and enhances viral replication. (A) Bone marrow-derived dendritic cells (DCs) were treated with lipopolysaccharide (LPS) for 24 h and then infected with MCMVGFP-WT or MCMVGFP-Δm138/m147.5 at an MOI of ∼0.3. Cell surface expression of B7.1 and B7.2 was determined 24 h later. Thick gray histograms represent GFP+ DCs, thin black histograms are GFP− DCs in the same culture, and shaded histograms are isotype control antibody staining. (B) Bone marrow-derived DCs were infected with MCMVGFP-WT or MCMVGFP-Δm138/m147.5, and 48 h later GFP+ DCs were adoptively transferred into WT or B7.1/2−/− mice. Eight days later, the numbers of MCMV epitope-specific CD4 T cells (left panel) or CD8 T cells (right panel) were determined after peptide restimulation of splenocytes and intracellular cytokine staining. (C) WT and B7.1/2−/− mice were infected with MCMVC3X-WT or MCMVC3X-Δm138/m147.5, and 8 days later the numbers of IFN-γ+ MCMV-specific CD4 T cells in spleens were determined. (D) MCMV PFU were determined in the spleen, lung, and liver at day 3 postinfection. (E) PFU were determined from livers of WT B6 mice infected with MCMVC3X-WT or MCMVC3X-Δm138/m147.5 at day 8 and at day 14 from salivary glands of infected BALB/c mice. Dotted line represents detection limit (d.l.). The data are representative of two independent experiments each with three to six mice per group. Bar graphs are shown as means with standard errors, and each symbol (○) represents an individual mouse. Statistical significance of viral titers was determined with the Mann-Whitney test, and statistical significance of T cell responses was determined with the two-tailed Student t test. Differences between groups were considered significant at P values of <0.05 (*, P < 0.05; **, P < 0.005).
When WT mice were infected directly with MCMV-Δm138/m147.5, the CD4 T cell responses specific for the peptides m18, M25, and m141 were significantly enhanced after infection (Fig. 6C). Importantly, no differences in CD4 T cell responses were observed in B7.1/2−/− mice directly infected with MCMV-Δm138/m147.5 or WT, proving that the inability of MCMV-Δm138/m147.5 to regulate B7.1/2 expression is responsible for the enhanced T cell responses observed (Fig. 6C).
To test whether the increased MCMV-specific CD4 T cell response induced by MCMV-Δm138/m147.5 impacts viral replication, viral titers were determined at several time points after infection. At day 3, when innate immunity is dominant, MCMV-WT and MCMV-Δm138/m147.5 replicated to similar titers in the liver, spleen, and lung (Fig. 6D). At day 8 postinfection, however, the titers of MCMV-Δm138/m147.5 were reduced in the liver compared to those of the WT (Fig. 6E). Additionally, replication of MCMV-WT was detectable in the salivary glands of BALB/c mice at day 14 postinfection, whereas MCMV-Δm138/m147.5 was not (Fig. 6E). Taken together, these data indicate that m138 and m147.5 function to restrict the MCMV-specific CD4 T cell response, translating to an increased duration of persistent phase replication.
DISCUSSION
Like all the herpesviruses, CMV has coevolved with its vertebrate hosts for many millions of years (29), and the diverse populations of CMV-specific T cells that develop over a lifetime of infection reflect the complexity of this immunological relationship (2, 13, 25, 37). The contribution of B7-CD28 signaling in promoting T cell responses is pathogen dependent (5), and the modulation of B7 ligand expression by CMV suggested potential importance. Consistent with this hypothesis, we show in this study that B7-CD28 signaling is essential for developing CD4 T cell-mediated immunity against MCMV and that MCMV inhibition of this cosignaling system dampens the virus-specific CD4 T cell response and enhances persistent replication.
In a recently published study, Cook et al. reported decreased pan-T cell expansion in the liver but not the spleen of mice lacking B7-CD28 signaling at day 6 after MCMV infection (CD3+NK1.1−, i.e., not MCMV specific) (9). Our results significantly extend these findings by examining the contribution of B7-CD28 signaling in promoting the MCMV-specific CD4 T cell response and assessing how this translates to control of persistent phase infection. It is clear that the expansion of the MCMV-specific T cell response is dramatically decreased both in the spleen and in the nonlymphoid tissues of B7.1/2−/− mice when using MCMV-specific peptide epitopes to measure these responses. Additionally, antibody blockade of B7.1 and B7.2 revealed that B7-CD28 signaling is primarily operable during the first week of infection. This places the importance of B7-CD28 signaling earlier than that of OX40-OX40L and 4-1BB/4-1BBL in the regulation of the MCMV-specific T cell response. Interaction of these TNF receptor superfamily members does not promote MCMV-specific CD4 T cell expansion but instead regulates the eventual memory set point during infection with this persistent virus (19, 20).
It is often speculated that the multitude of CMV strategies targeting adaptive immunity likely evolved to enable the establishment of persistence. This study identifies specific CMV gene products (i.e., m138 and m147.5) that impact the magnitude of CD4 T cell responses and viral load in vivo. In contrast, others have shown that an MCMV mutant unable to restrict MHC class I-dependent antigen presentation does not alter the CD8 T cell pool after primary infection (12, 33, 36), although NK control and CD8 T cell restriction of replication in the salivary gland are compromised (27). It is likely that the majority of CD8 T cells are cross-primed by uninfected APCs (4, 7, 33), and therefore CMV immune-modulating proteins whose expression is confined to infected cells are likely unable to impact CD8 T cell responses generated by this mechanism. Consistent with this hypothesis, MCMV-Δm138/m147.5-infected DCs did not alter MCMV-specific CD8 T cell responses when adoptively transferred into mice. Intriguingly, it was recently shown that a rhesus CMV mutant lacking MHC class I-modulating capacity induced a normal rhesus CMV-specific T cell response upon primary infection of monkeys, which was identical to the result with MCMV. However, this mutant was unable to reinfect preimmune monkeys, a feat which can be accomplished by WT CMV and has led to an effort to develop this virus as a vaccine vector (15, 16). This result adds another dimension to how immune evasion genes must be considered to function in the course of CMV pathogenesis, and it would be interesting to know whether viral genes such as m138 and m147.5 might also impact CMV reinfection.
Because MCMV-Δm138/m147.5 induced a more robust MCMV-specific CD4 T cell response when mice were directly infected with this mutant virus and when infected DCs were transferred, this is strong evidence that these viral immune-modulating genes can function in APCs and shape the MHC class II-restricted response. This idea is also consistent with recent data from Andrews et al., where the MCMV-specific CD4 T cell response was shown to vary in magnitude depending upon the number of APCs that are productively infected (1). Although m138 is responsible for restricting B7.1 expression, it is also an Fc receptor orthologue (44) and restricts expression of several NK cell-activating ligands (21). As this same study showed the robustness of NK cell activation during MCMV infection can also impact the CD4 T cell response (1), it is possible that m138 targeting of both innate and adaptive responses makes it an especially effective immune-modulatory protein.
Acknowledgments
We thank Michael Croft for excellent discussions and Antje Rhode for technical assistance.
This work was supported by a Veni grant from the Netherlands Organization for Research to R.A. (916.155), a Deutsche Forschungsgemeinschaft fellowship to A.L. (DFG number 1421/1-1), and NIH grants AI076864 and AI069298 to C.A.B., CA80261 and AI76972 to S.P.S., and AI048073 and AI057840 to C.F.W.
Footnotes
Published ahead of print on 27 October 2010.
REFERENCES
- 1.Andrews, D. M., M. J. Estcourt, C. E. Andoniou, M. E. Wikstrom, A. Khong, V. Voigt, P. Fleming, H. Tabarias, G. R. Hill, R. G. van der Most, A. A. Scalzo, M. J. Smyth, and M. A. Degli-Esposti. 2010. Innate immunity defines the capacity of antiviral T cells to limit persistent infection. J. Exp. Med. 207:1333-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J. McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379-385. [DOI] [PubMed] [Google Scholar]
- 3.Arens, R., P. Wang, J. Sidney, A. Loewendorf, A. Sette, S. P. Schoenberger, B. Peters, and C. A. Benedict. 2008. Cutting edge: murine cytomegalovirus induces a polyfunctional CD4 T cell response. J. Immunol. 180:6472-6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benedict, C. A., A. Loewendorf, Z. Garcia, B. R. Blazar, and E. M. Janssen. 2008. Dendritic cell programming by cytomegalovirus stunts naive T cell responses via the PD-L1/PD-1 pathway. J. Immunol. 180:4836-4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertram, E. M., W. Dawicki, and T. H. Watts. 2004. Role of T cell costimulation in anti-viral immunity. Semin. Immunol. 16:185-196. [DOI] [PubMed] [Google Scholar]
- 6.Biron, C. A., K. B. Nguyen, G. C. Pien, L. P. Cousens, and T. P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189-220. [DOI] [PubMed] [Google Scholar]
- 7.Bohm, V., C. O. Simon, J. Podlech, C. K. Seckert, D. Gendig, P. Deegen, D. Gillert-Marien, N. A. Lemmermann, R. Holtappels, and M. J. Reddehase. 2008. The immune evasion paradox: immunoevasins of murine cytomegalovirus enhance priming of CD8 T cells by preventing negative feedback regulation. J. Virol. 82:11637-11650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brocchieri, L., T. N. Kledal, S. Karlin, and E. S. Mocarski. 2005. Predicting coding potential from genome sequence: application to betaherpesviruses infecting rats and mice. J. Virol. 79:7570-7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook, C. H., L. Chen, J. Wen, P. Zimmerman, Y. Zhang, J. Trgovcich, Y. Liu, and J. X. Gao. 2009. CD28/B7-mediated co-stimulation is critical for early control of murine cytomegalovirus infection. Viral Immunol. 22:91-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gamadia, L. E., E. B. Remmerswaal, J. F. Weel, F. Bemelman, R. A. Van Lier, and I. J. Ten Berge. 2002. Primary immune responses to human CMV a critical role for IFN-gamma-producing CD4+ T cells in protection against CMV-disease. Blood 101:2686-2692. [DOI] [PubMed] [Google Scholar]
- 11.Gamadia, L. E., R. J. Rentenaar, R. A. van Lier, and I. J. ten Berge. 2004. Properties of CD4(+) T cells in human cytomegalovirus infection. Hum. Immunol. 65:486-492. [DOI] [PubMed] [Google Scholar]
- 12.Gold, M. C., M. W. Munks, M. Wagner, C. W. McMahon, A. Kelly, D. G. Kavanagh, M. K. Slifka, U. H. Koszinowski, D. H. Raulet, and A. B. Hill. 2004. Murine cytomegalovirus interference with antigen presentation has little effect on the size or the effector memory phenotype of the CD8 T cell response. J. Immunol. 172:6944-6953. [DOI] [PubMed] [Google Scholar]
- 13.Gratama, J. W., R. A. Langelaar, M. A. Oosterveer, J. A. van der Linden, A. den Ouden-Noordermeer, A. M. Naipal, J. W. Visser, G. C. de Gast, and H. J. Tanke. 1989. Phenotypic study of CD4+ and CD8+ lymphocyte subsets in relation to cytomegalovirus carrier status and its correlate with pokeweed mitogen-induced B lymphocyte differentiation. Clin. Exp. Immunol. 77:245-251. [PMC free article] [PubMed] [Google Scholar]
- 14.Greenwald, R. J., G. J. Freeman, and A. H. Sharpe. 2005. The B7 family revisited. Annu. Rev. Immunol. 23:515-548. [DOI] [PubMed] [Google Scholar]
- 15.Hansen, S. G., C. J. Powers, R. Richards, A. B. Ventura, J. C. Ford, D. Siess, M. K. Axthelm, J. A. Nelson, M. A. Jarvis, L. J. Picker, and K. Fruh. 2010. Evasion of CD8+ T cells is critical for superinfection by cytomegalovirus. Science 328:102-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen, S. G., C. Vieville, N. Whizin, L. Coyne-Johnson, D. C. Siess, D. D. Drummond, A. W. Legasse, M. K. Axthelm, K. Oswald, C. M. Trubey, M. Piatak, Jr., J. D. Lifson, J. A. Nelson, M. A. Jarvis, and L. J. Picker. 2009. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med. 15:293-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hertel, L., V. G. Lacaille, H. Strobl, E. D. Mellins, and E. S. Mocarski. 2003. Susceptibility of immature and mature Langerhans cell-type dendritic cells to infection and immunomodulation by human cytomegalovirus. J. Virol. 77:7563-7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holtappels, R., M. F. Pahl-Seibert, D. Thomas, and M. J. Reddehase. 2000. Enrichment of immediate-early 1 (m123/pp89) peptide-specific CD8 T cells in a pulmonary CD62Llo memory-effector cell pool during latent murine cytomegalovirus infection of the lungs. J. Virol. 74:11495-11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humphreys, I. R., S. W. Lee, M. Jones, A. Loewendorf, E. Gostick, D. A. Price, C. A. Benedict, C. F. Ware, and M. Croft. 2010. Biphasic role of 4-1BB in the regulation of mouse cytomegalovirus-specific CD8(+) T cells. Eur. J. Immunol. 40:2762-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humphreys, I. R., A. Loewendorf, C. de Trez, K. Schneider, C. A. Benedict, M. W. Munks, C. F. Ware, and M. Croft. 2007. OX40 costimulation promotes persistence of cytomegalovirus-specific CD8 T cells: a CD4-dependent mechanism. J. Immunol. 179:2195-2202. [DOI] [PubMed] [Google Scholar]
- 21.Jonjic, S., M. Babic, B. Polic, and A. Krmpotic. 2008. Immune evasion of natural killer cells by viruses. Curr. Opin. Immunol. 20:30-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonjic, S., W. Mutter, F. Weiland, M. J. Reddehase, and U. H. Koszinowski. 1989. Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J. Exp. Med. 169:1199-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jonjic, S., I. Pavic, P. Lucin, D. Rukavina, and U. H. Koszinowski. 1990. Efficacious control of cytomegalovirus infection after long-term depletion of CD8+ T lymphocytes. J. Virol. 64:5457-5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jonjic, S., I. Pavic, B. Polic, I. Crnkovic, P. Lucin, and U. H. Koszinowski. 1994. Antibodies are not essential for the resolution of primary cytomegalovirus infection but limit dissemination of recurrent virus. J. Exp. Med. 179:1713-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karrer, U., S. Sierro, M. Wagner, A. Oxenius, H. Hengel, U. H. Koszinowski, R. E. Phillips, and P. Klenerman. 2003. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J. Immunol. 170:2022-2029. [DOI] [PubMed] [Google Scholar]
- 26.Loewendorf, A., C. Kruger, E. M. Borst, M. Wagner, U. Just, and M. Messerle. 2004. Identification of a mouse cytomegalovirus gene selectively targeting CD86 expression on antigen-presenting cells. J. Virol. 78:13062-13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu, X., A. K. Pinto, A. M. Kelly, K. S. Cho, and A. B. Hill. 2006. Murine cytomegalovirus interference with antigen presentation contributes to the inability of CD8 T cells to control virus in the salivary gland. J. Virol. 80:4200-4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathys, S., T. Schroeder, J. Ellwart, U. H. Koszinowski, M. Messerle, and U. Just. 2003. Dendritic cells under influence of mouse cytomegalovirus have a physiologic dual role: to initiate and to restrict T cell activation. J. Infect. Dis. 187:988-999. [DOI] [PubMed] [Google Scholar]
- 29.McGeoch, D. J., A. Dolan, and A. C. Ralph. 2000. Toward a comprehensive phylogeny for mammalian and avian herpesviruses. J. Virol. 74:10401-10406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Messerle, M., I. Crnkovic, W. Hammerschmidt, H. Ziegler, and U. H. Koszinowski. 1997. Cloning and mutagenesis of a herpesvirus genome as an infectious bacterial artificial chromosome. Proc. Natl. Acad. Sci. U. S. A. 94:14759-14763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mintern, J. D., E. J. Klemm, M. Wagner, M. E. Paquet, M. D. Napier, Y. M. Kim, U. H. Koszinowski, and H. L. Ploegh. 2006. Viral interference with B7-1 costimulation: a new role for murine cytomegalovirus fc receptor-1. J. Immunol. 177:8422-8431. [DOI] [PubMed] [Google Scholar]
- 32.Moutaftsi, M., A. M. Mehl, L. K. Borysiewicz, and Z. Tabi. 2002. Human cytomegalovirus inhibits maturation and impairs function of monocyte-derived dendritic cells. Blood 99:2913-2921. [DOI] [PubMed] [Google Scholar]
- 33.Munks, M. W., A. K. Pinto, C. M. Doom, and A. B. Hill. 2007. Viral interference with antigen presentation does not alter acute or chronic CD8 T cell immunodominance in murine cytomegalovirus infection. J. Immunol. 178:7235-7241. [DOI] [PubMed] [Google Scholar]
- 34.Nikolich-Zugich, J. 2008. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat. Rev. Immunol. 8:512-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pereira, L., E. Maidji, S. McDonagh, and T. Tabata. 2005. Insights into viral transmission at the uterine-placental interface. Trends Microbiol. 13:164-174. [DOI] [PubMed] [Google Scholar]
- 36.Reddehase, M. J. 2002. Antigens and immunoevasins: opponents in cytomegalovirus immune surveillance. Nat. Rev. Immunol. 2:831-844. [DOI] [PubMed] [Google Scholar]
- 37.Reddehase, M. J., W. Mutter, K. Munch, H. J. Buhring, and U. H. Koszinowski. 1987. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J. Virol. 61:3102-3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddehase, M. J., C. O. Simon, C. K. Seckert, N. Lemmermann, and N. K. Grzimek. 2008. Murine model of cytomegalovirus latency and reactivation. Curr. Top. Microbiol. Immunol. 325:315-331. [DOI] [PubMed] [Google Scholar]
- 39.Riddell, S. R., and P. D. Greenberg. 1997. T cell therapy of human CMV and EBV infection in immunocompromised hosts. Rev. Med. Virol. 7:181-192. [DOI] [PubMed] [Google Scholar]
- 40.Schneider, K., A. Loewendorf, C. De Trez, J. Fulton, A. Rhode, H. Shumway, S. Ha, G. Patterson, K. Pfeffer, S. A. Nedospasov, C. F. Ware, and C. A. Benedict. 2008. Lymphotoxin-mediated crosstalk between B cells and splenic stroma promotes the initial type I interferon response to cytomegalovirus. Cell Host Microbe 3:67-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharpe, A. H., and G. J. Freeman. 2002. The B7-CD28 superfamily. Nat. Rev. Immunol. 2:116-126. [DOI] [PubMed] [Google Scholar]
- 42.Snyder, C. M., K. S. Cho, E. L. Bonnett, S. van Dommelen, G. R. Shellam, and A. B. Hill. 2008. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity 29:650-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sylwester, A. W., B. L. Mitchell, J. B. Edgar, C. Taormina, C. Pelte, F. Ruchti, P. R. Sleath, K. H. Grabstein, N. A. Hosken, F. Kern, J. A. Nelson, and L. J. Picker. 2005. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 202:673-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thale, R., P. Lucin, K. Schneider, M. Eggers, and U. H. Koszinowski. 1994. Identification and expression of a murine cytomegalovirus early gene coding for an Fc receptor. J. Virol. 68:7757-7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tyznik, A. J., E. Tupin, N. A. Nagarajan, M. J. Her, C. A. Benedict, and M. Kronenberg. 2008. Cutting edge: the mechanism of invariant NKT cell responses to viral danger signals. J. Immunol. 181:4452-4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vescovini, R., C. Biasini, F. F. Fagnoni, A. R. Telera, L. Zanlari, M. Pedrazzoni, L. Bucci, D. Monti, M. C. Medici, C. Chezzi, C. Franceschi, and P. Sansoni. 2007. Massive load of functional effector CD4+ and CD8+ T cells against cytomegalovirus in very old subjects. J. Immunol. 179:4283-4291. [DOI] [PubMed] [Google Scholar]
- 47.Wikby, A., F. Ferguson, R. Forsey, J. Thompson, J. Strindhall, S. Lofgren, B. O. Nilsson, J. Ernerudh, G. Pawelec, and B. Johansson. 2005. An immune risk phenotype, cognitive impairment, and survival in very late life: impact of allostatic load in Swedish octogenarian and nonagenarian humans. J. Gerontol. A Biol. Sci. Med. Sci. 60:556-565. [DOI] [PubMed] [Google Scholar]