Abstract
Adenovirus type 5 (Ad5) infection of macrophages results in rapid secretion of interleukin-1β (IL-1β) and is dependent on the inflammasome components NLRP3 and ASC and the catalytic activity of caspase-1. Using lentivirus-expressed short hairpin RNA (shRNA) and competitive inhibitors, we show that Ad-induced IL-1β release is dependent upon Toll-like receptor 9 (TLR9) sensing of the Ad5 double-stranded DNA (dsDNA) genome in human cell lines and primary monocyte-derived macrophages but not in mouse macrophages. Additionally, a temperature-sensitive mutant of Ad5 unable to penetrate endosomal membranes, ts1, is unable to induce IL-1β release in TLR2-primed THP-1 cells, suggesting that penetration of endosomal membranes is required for IL-1β release. Disruption of lysosomal membranes and the release of cathepsin B into the cytoplasm are required for Ad-induced NLRP3 activation. Ad5 cell entry also induces reactive oxygen species (ROS) production, and inhibitors of ROS prevent Ad-induced IL-1β release. Ad5 activation of NLRP3 also induces necrotic cell death, resulting in the release of the proinflammatory molecule HMGB1. This work further defines the mechanisms of virally induced inflammasome activation.
Viral infections are initially recognized via Rig-I-like receptors or Toll-like receptors (TLRs) (27). Signaling through these receptors triggers proinflammatory responses, which help to establish an antiviral state, recruit additional immune effector cells, and direct the adaptive immune response (30). A key component of the innate immune response to viruses has recently been shown to involve the Nod-like receptor NLRP3 (1, 25, 26, 48). NLRP3 senses a variety of stimuli and initiates the formation of a multiprotein complex known as the inflammasome (32, 39, 42). Previous studies have demonstrated that the NLRP3 inflammasome is “primed” when signaling through TLRs increases the levels of NLRP3 and the interleukin-1β precursor (pro-IL-1β) (4). An additional signal is then required to activate NLRP3, leading to the formation of the inflammasome (32). The inflammasome leads to the activation of caspase-1, which regulates the secretion of the proinflammatory cytokines IL-1β and IL-18 (32, 39, 42). Unlike other viruses which activate NLRP3 upon viral replication (1, 25, 26, 48), adenovirus type 5 (Ad5) activation of NLRP3 is independent of viral replication (35). The mechanisms of Ad5 activation of NLRP3 remain to be fully defined.
Adenoviruses are a family of nonenveloped dsDNA viruses commonly associated with acute upper respiratory, gastrointestinal, and ocular infections. Over 50 human serotypes of adenovirus (Ad) have been identified to date, which are currently split among seven subgroups based on hemagglutination properties and sequence homology (5). While Ad5 infections of the upper respiratory tract are typically self-limiting in healthy individuals, severe pneumonia and gastroenteritis in children and severe pneumonia in adult military populations are a common cause of significant morbidity and mortality (18). The recent emergence of a novel Ad14 genotype with hospitalization rates of 38% and mortality rates of 5% in otherwise healthy individuals highlights the importance of this common respiratory pathogen (2). Recent implementation of PCR-based detection techniques have shown that 5 to 20% of solid organ and bone marrow transplant patients suffer from complications related to Ad5 infections, with mortality rates of up to 50% observed in these patients (46). A key component of the Ad pathogenesis is the potent inflammatory response induced by Ad5 infections (17).
In spite of Ad5 pathogenicity, Ad-based vectors continue to be explored for use in gene therapies to treat cancer (44) and cardiovascular disease (41). However, the utility of Ad5-based vectors for gene therapy is severely limited by the potent inflammatory response to the virus (34). Additionally, Ad5 vectors are also being examined as vectors in genetic vaccines against HIV (40), influenza (21), and numerous microbial pathogens. The recent failure of the Merck Ad5-based HIV vaccine to prevent HIV infection or reduce viral loads in infected individuals highlights our need for a better understanding of the role that Ad5 vectors play in stimulating and directing an immune response against pathogens such as HIV (40).
The proinflammatory response to Ad5 infections is well documented (34). Adenovirus infection elicits the release of numerous cytokines, including IL-1, tumor necrosis factor alpha (TNF-α), IL-6, and type I interferons. Additionally, Ad5 infection results in the release of several chemokines, such as macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and IL-8. The bulk of the proinflammatory response to Ad5 appears to be evoked during the cell entry phase of infection, as evidenced by the potent inflammatory response observed in mice transduced with replication-defective Ad5 vectors (34). During Ad5 infection, resident macrophages of the lung or liver are implicated in releasing the majority of these cytokines and chemokines in response to the virus (43, 60). In fact, within minutes of intratracheal administration or intravenous (i.v.) administration, the majority of the virus appears to be associated with these phagocytic cells (43, 60). In mice, systemic release of IL-1β occurs within minutes of intravenous administration of Ad5 (43). This same study demonstrated that the rapid release of IL-1β was followed by the release of IL-6 and TNF-α within hours. However, the severity of the inflammatory response and tissue toxicity was significantly reduced in IL-1 receptor (IL-1R) knockout mice or upon preadministration of neutralizing IL-1 antibodies and less so upon preadministration of neutralizing TNF-α antibodies. Thus, IL-1β was shown to be not only a key mediator of the immune response to Ad5 but also a key molecule involved in the pathogenesis of Ad5 infection. The importance of IL-18 in Ad5 infection of the lung was reported with comparisons of Ad5 infections of the lungs of wild-type (WT) or granulocyte-macrophage colony-stimulating factor (GM-CSF) knockout mice (6). The production of IL-18 was severely reduced upon infection of GM-CSF knockout mice compared to that observed in WT mice, which greatly impaired the Th1 response evoked by Ad5 infection. This diminished Th1 response in GM-CSF knockout mice was later shown to correlate with reduced uptake of Ad5 virions by alveolar macrophages due to altered receptor expression (60). Thus, the proinflammatory cytokines, IL-1β, and IL-18 have an apparently crucial role in the immune response to Ad5 infections but also appear to contribute to the pathogenesis of Ad5 infection.
We report here the mechanism of human adenovirus type 5 (Ad5) activation of the NLRP3 inflammasome. In human monocyte-derived macrophages, Ad5 signaling through Toll-like receptor 9 (TLR9) induces the expression of NLRP3 and the apoptosis-related speckle-like protein containing a caspase recruitment domain (ASC) which controls the extent of inflammasome activation and IL-1β secretion. The induced expression of these genes is not observed in mouse bone marrow-derived macrophages (mBMMs), suggesting subtle differences in the recognition of human Ads between human and mouse macrophages. Activation of the NLRP3 inflammasome is dependent upon viral penetration of endosomal membranes and the release of the lysosomal protease cathepsin B into the cytoplasm. Adenovirus cell entry also induces the production of reactive oxygen species (ROS), which are required for NLRP3 activation. Finally, Ad5 infection of macrophages leads to necrotic cell death and the release of the proinflammatory mediator HMGB1. This necrotic cell death is dependent on cathepsin B activity and NLRP3 activation but is independent of caspase-1 or -3 activity. In summary, these results identify key signaling events initiated during adenovirus cell entry. A better understanding of these events could provide significant insight into the relative pathogenicity of different adenovirus serotypes and may aid in the design of improved adenoviral vectors for use in gene therapy or genetic vaccination.
MATERIALS AND METHODS
Cell lines and reagents.
The human monocytic cell line THP-1 and murine L929 fibroblasts were obtained from the ATCC. Histopaque, ATP, apocynin, diphenyliodonium bromide (DPI), phorbol-12-myristate-13-acetate (PMA), and N-acetylcysteine (NAC) were purchased from Sigma-Aldrich. RPMI 1640 and Dulbecco modified Eagle medium (DMEM) were obtained from Mediatech. PAM3CSK4, flagellin, ODN 2006, and (TTAGGG)4 were obtained from InvivoGen. Polyclonal rabbit anti-IL-1β (catalog no. 2022) and polyclonal rabbit anti-caspase-1 (catalog no. 2225) were purchased from Cell Signaling Technology. Polyclonal rabbit anti-mouse caspase-1 (catalog no. AB1871) was purchased from Chemicon. Horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Biomeda Ltd. The human IL-1β enzyme-linked immunosorbent assay (ELISA) Ready-SET-Go! kit was obtained from eBioscience. YVAD-fmk, DVED-fmk, and CA-074me were purchased from Enzo Life Sciences. All other chemical reagents were obtained from Fisher Chemicals.
Virus preparation.
An E1/E3-deleted adenovirus encoding enhanced green fluorescent protein (EGFP) under the control of a cytomegalovirus promoter, Ad5gfp (29), or a temperature-sensitive mutant, Ad2ts1 (55), were propagated in HEK293 cells and twice purified by cesium chloride gradient centrifugation as previously described (55). For these studies, the ts1 virus was propagated at 39.5°C, resulting in viral particles incapable of membrane penetration (55). Viral concentrations were determined by the Bradford assay. A total of 1 μg of protein corresponded to 4 × 109 viral particles. The titers of the viruses were determined by serial dilution on HeLa cells, using flow cytometry to quantify GFP expression. The specific infectivity of the viral preparations used in these studies ranged from 100 to 200 viral particles per GFP-transducing unit (GTU). Since infection of myeloid cells frequently leads to considerable cell death in some cases, we report the infectious dose of adenoviruses used in all studies described below in terms of the number of GTUs by determining the titers of the viruses on HeLa cells.
Cell culture.
THP-1 cells were maintained in RPMI 1640 medium, supplemented with 10% fetal bovine serum (FBS), 100 IU/ml penicillin, 1 mg/ml streptomycin, 0.25 μg/ml amphotericin B, nonessential amino acids, 1 mM sodium pyruvate, 10 mM HEPES buffer, and 2 mM glutamine. THP-1 cells were differentiated by overnight stimulation with 100 nM phorbol-12-myristate-13-acetate (PMA) to macrophage-like cells.
Primary bone marrow-derived macrophages (BMMs) were obtained from 6- to 12-week-old C57BL/6 mice with IACUC approval. Bone marrow was harvested from mouse femurs, filtered through 100-mm cell strainers (BD Biosciences), and plated in 15-cm culture dishes. Cells were cultured in RPMI 1640 plus 10% FBS plus 20% conditioned medium from L929 cells for 7 days to obtain differentiated macrophages.
Human monocyte-derived macrophages were cultured from peripheral blood mononuclear cells (PBMCs) obtained from healthy donors. Briefly, PBMCs were isolated from fresh whole blood by centrifugation on Histopaque cell separation medium. Monocytes were selected by adherence to culture dishes for 1 h, washed, and cultured in Iscove's modified Eagle's medium plus 10% FBS plus 25 ng/ml recombinant human M-CSF (PeproTech) for 7 days to obtain differentiated macrophages.
Generation of THP-1 cells stably expressing shRNA.
Lentiviral vectors with an LKO.1 backbone and encoding short hairpin RNAs (shRNAs) for NLRP3 (Open Biosystems catalog no. RHS3979-9629911 and clone ID TRCN0000062727) or TLR9 (Open Biosystems catalog no. RHS3979-9624075 and clone ID TRCN0000056891) or control shRNAs recommended by the RNA interference (RNAi) consortium (Addgene catalog no. 10879) were generated by cotransfection of 293T cells with the packaging plasmids pHEF-VSVG (catalog no. 4693; NIH AIDS Research and Reagent Program), pRSV-REV (Addgene catalog no. 12253), and pMDLg/pRRE (Addgene catalog no. 12251). Supernatants collected after 48 h were passed through 0.2-μm filters and used to transduce THP-1 cells by spinoculation. Transduced cells were selected with puromycin and used in subsequent studies.
Quantification of IL-1β secretion by ELISA.
For primary BMMs or human monocyte-derived macrophages (hMDMs), 50,000 cells were plated per well in a 96-well plate overnight. Cells were then primed with 30 ng/ml PAM3CSK4 for 4 h. Cells were then infected with various amounts of Ad5gfp or ts1, or treated with 3 mM ATP, and transfected with 0.1 μg pEGFP-C1 plasmid using Lipofectamine 2000 per the manufacturer's protocol. For THP-1 cells and shRNA-expressing derivatives, 50,000 cells per well were differentiated with 100 nM PMA for 16 h. Cells were then washed, rested in serum-free medium for 2 h, and then treated as described above. When used, chemical inhibitors were added to cells 30 min prior to virus treatment at the concentrations indicated in the text and kept in the samples throughout the virus treatment period.
Western blotting for inflammasome components.
To determine the levels of inflammasome-related proteins present in cells after being primed with PAM3CSK4 or infection with Ad5gfp, 2 × 106 BMMs or hMDMs were plated in 6-well plates overnight. Cells were then washed and treated with 30 ng/ml PAM3CSK4 or 1,000 GTUs of Ad5gfp for 4 h. Cells were then lysed in 25 mM Tris at pH 8, 25 mM NaCl, 0.1 mM EDTA, 1% Triton X-100, 0.5% deoxycholate, and 15 mM beta-mercaptoethanol (solution B) with 1 mM phenylmethylsulfonyl fluoride (PMSF). Lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 15% acrylamide gels, transferred to nitrocellulose membranes, and immunoblotted for NLRP3 (catalog no. ALX-804-881; Enzo), ASC (catalog no. 04-147; Millipore), procaspase-1 (human, Santa Cruz catalog no. sc-514; mouse, Cell Signaling Technology catalog no. 2225), and pro-IL-1β (Santa Cruz catalog no. sc-7884). Immunoblotting for actin (Sigma catalog no. A5441) served as a loading control.
Measurement of cathepsin B release into cytoplasm.
PMA-differentiated THP-1 cells (2 × 106 cells/well) in 6-well plates were treated with medium alone or 1,000 GTUs of Ad5gfp or ts1 for 15 min. Cells were then permeabilized with 50 μg/ml ice-cold digitonin in phosphate-buffered saline (PBS), pH 7.4, and 1 mM PMSF for 15 min at 4°C, which selectively permeabilizes the plasma membrane while leaving the intracellular membranes intact (15). These cytosolic extracts were subjected to SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted for cathepsin B (Santa Cruz catalog no. sc-13985).
To assess the catalytic activity of cathepsin B in the cytoplasm, PMA-differentiated THP-1 cells (2 × 105 cells/well) plated on glass coverslips in 24-well plates were treated with medium alone or 1,000 GTUs of Ad5gfp or ts1 for 15 min, followed be the addition of the fluorogenic cathepsin B substrate Magic Red (ImmunoChemistry, Inc.) for 30 min. Cells were washed in PBS, counterstained with Hoechst 33242 for 15 min, and then visualized by epifluorescence microscopy.
Measurement of ROS production.
THP-1 cells were plated in black 96-well plates (200,000 cells/well), PMA differentiated overnight, washed, and then rested for 2 days in RPMI 1640 plus 10% FBS. Cells were then loaded with the ROS-sensitive fluorescent dye 2,7-dichlorofluorescein diacetate (DCFDA; Invitrogen) for 30 min by following the manufacturer's protocol, washed of unincorporated dye, and incubated with medium alone, 3 mM ATP, 1,000 GTUs of Ad5gfp, or an equivalent number of ts1 viral particles. Fluorescence emission was measured over the course of 6 h using a Polarstar fluorescence plate reader (BMG Labtech). Fluorescence intensity was normalized to values obtained immediately prior to the addition of stimuli.
Measurement of NLRP3-dependent necrotic cell death.
PMA-differentiated THP-1 cells stably transduced with control (THP-1cntrl) or NLRP3-specific shRNA (THP-1NLRP3KD) were rested in serum-free medium for 2 h, primed with 30 ng/ml PAM3CSK4 for 2 h, pretreated with 50 mM YVAD-fmk, DVED-fmk, or CA-074me for 30 min, and then treated with 1,000 GTUs of Ad5gfp for 1 h in the continued presence of the drug. Cells were then washed and stained with propidium iodide (PI) and DAPI (4′,6-diamidino-2-phenylindole) for 20 min. Fluorescence micrographs were then obtained, and the percentage of DAPI-positive nuclei staining positive for PI was determined. Treatment of cells with 50 μg/ml digitonin to permeabilize the plasma membrane served as a positive control. A minimum of 300 cells were counted per replicate.
The release of the proinflammatory mediator HMGB1 from necrotic cells was assessed by Western blotting. Supernatants from the cells described above were collected and trichloroacetic acid (TCA) precipitated. The resulting pellets were subjected to SDS-PAGE, transferred to nitrocellulose, and immunoblotted for HMGB1 (Novus Biologicals catalog no. NB100-2322).
RESULTS
Adenovirus activates the inflammasome during cell entry.
Activation of the NLRP3 inflammasome involves priming of the inflammasome via upregulation of NLRP3, followed by triggering of inflammasome formation, which activates caspase-1, leading to secretion of IL-1β (4). Using the human monocytic cell line THP-1, we first examined the time course of IL-1β release upon infection with Ad5gfp. In THP-1 cells differentiated to a macrophage-like phenotype by PMA, significant Ad5gfp-induced IL-1β release was observed within 2 h and continued to increase for at least 8 h postinfection (Fig. 1A, closed circles), supporting previous observations that Ad5 activates NLRP3 during cell entry (35). Since the priming and activating signals for NLRP3 associated with Ad5 cell entry are unknown, we further defined the time course for the activating signal induced during Ad5 cell entry by first priming cells with a TLR2 ligand, PAM3CSK4, to increase the levels of NLRP3, ASC, and pro-IL-1β. When cells were first primed with PAM3CSK4 for 2 h, followed by infection with Ad5gfp, IL-1β release was observed within 30 min after infection (Fig. 1A, open circles). Together, these results led us to pursue a model in which Ad5 infection requires priming to increase levels of inflammasome components. The NLRP3 inflammasome is then activated by a signal that is initiated very early after infection, perhaps during endosomal trafficking/endosomal escape.
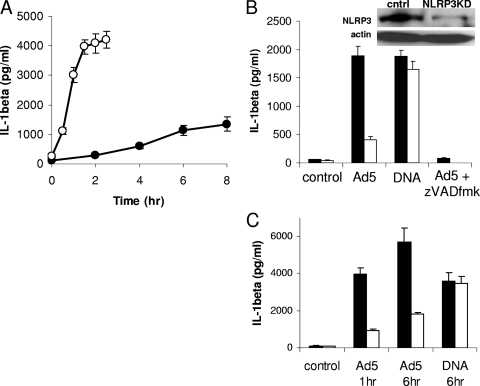
FIG. 1.
Kinetics of Ad5 activation of the NLRP3 inflammasome. (A) Cells were rested in serum-free medium for 2 h and either primed (open) or not (filled) with 30 ng/ml of the TLR2 agonist PAM3CSK4 for 2 h prior to infection with 1,000 GTUs of Ad5gfp. (B) Cells stably expressing either control (THP-1cntrl, filled) or NLRP3-specific (THP-1nlrp3KD, open) shRNA were rested in serum-free medium for 2 h and then treated with medium alone (control), Ad5gfp, or Lipofectamine/DNA for 6 h. THP-1cntrl cells were also pretreated with the pan-caspase inhibitor zVAD-fmk for 30 min before infection with Ad5gfp for 6 h. (Inset) Western blot for NLRP3 and actin loading control in THP-1cntrl and THP-1nlrp3KD cell lysates. (C) THP-1cntrl (filled) or THP-1nlrp3KD (open) cells were rested in serum-free medium for 2 h, primed with PAM3CSK4 for 2 h, and then treated with medium alone (control), Ad5gfp for 1 or 6 h, or Lipofectamine/DNA for 6 h. The release of IL-1β was quantified by ELISA. Data represent the means and standard errors from 3 replicates.
To confirm that Ad5gfp-induced IL-1β release is dependent on NLRP3 in human cells, PMA-differentiated THP-1 cells stably transduced with a lentivirus-expressing shRNA against NLRP3 or a control shRNA were treated with Ad5gfp for 6 h. Compared to control cells, THP-1nlrp3KD cells released ∼80% less IL-1β in response to Ad5gfp infection (Fig. 1B). This reduction in IL-1β release correlates with a ∼70% reduction in the levels of the NLRP3 protein in the THP-1nlrp3KD cells versus that in the control shRNA cells, as assessed by Western blotting (Fig. 1B, inset), thus confirming previous observations in primary mouse macrophages. To confirm that THP-1nlrp3KD cells are competent for secretion of IL-1β, cells were stimulated with transfected DNA, which requires AIM2, ASC, and caspase-1 but not NLRP3 for inflammasome activation (22, 35). The caspase-1 dependence of the Ad5gfp-induced IL-1β release was confirmed by treatment with the caspase inhibitor zVAD-fmk (Fig. 1B). A similar dependence of Ad5-induced IL-1β secretion on NLRP3 was observed when cells were first primed with PAM3CSK4, followed by infection with Ad5 for 1 h or 6 h (Fig. 1C).
TLR9 is required for Ad5 activation of the NLRP3 inflammasome.
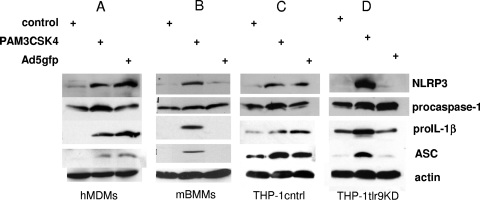
Previous work using BMMs from TLR9−/− mice suggested that TLR9 was not required for Ad5-induced NLRP3-dependent IL-1β release. These studies were confounded by the use of lipopolysaccharide (LPS) to prime cells via TLR4, however. Since Ad5gfp induced IL-1β from THP-1 cells in the absence of additional TLR priming (e.g., PAM3CSK4 in Fig. 1A), we next examined whether Ad5gfp-induced IL-1β release was dependent on TLR9 priming in human cells. To determine whether Ad5gfp infection leads to increased levels of inflammasome proteins, human monocyte-derived macrophages (hMDMs) or mouse bone marrow-derived macrophages (mBMMs) were infected with Ad5gfp for 4 h or treated with TLR2 agonist, and the levels of NLRP3, ASC, procaspase-1, and pro-IL-1β were examined. Compared to cells treated with medium alone, Ad5gfp and TLR2 agonist treatment enhanced the expression of NLRP3, ASC, and pro-IL-1β in hMDMs (Fig. 2A). However, Ad5gfp did not increase the levels of these proteins in mBMMs (Fig. 2B). To confirm that the Ad5-induced increase in the levels of inflammasome components in human cells depends upon TLR9, PMA-differentiated THP-1 cells stably transduced with control (THP-1cntrl) or TLR9-specific shRNA (THP-1tlr9KD) were similarly treated with PAM3CSK4 or Ad5gfp. While Ad5gfp increased the levels of NLRP3, ASC, and pro-IL-1β in THP-1cntrl cells, this effect was significantly reduced in THP-1tlr9KD cells (Fig. 2C and D), which expressed ∼70% less TLR9, as assessed by Western blotting (Fig. 3 C, inset). Significant changes in procaspase-1 levels were not observed in any case. Thus, human cells appear to sense Ad5gfp via TLR9 during cell entry, leading to the upregulation of inflammasome components and pro-IL-1β, while TLR9 recognition of Ad5 does not occur in vitro with mBMMs, as previously reported (35).
FIG. 2.
TLR9 is required for expression of inflammasome components in human but not mouse macrophages. hMDMs (A), mBMMs (B), PMA-differentiated THP-1cntrl cells (C), or THP-1tlr9KD cells (D) were treated with control medium, PAM3CSK4, or Ad5gfp for 4 h, and cell lysates were examined for NLRP3, ASC, and procaspase-1 expression levels by Western blotting. Blots were probed for actin as a loading control.
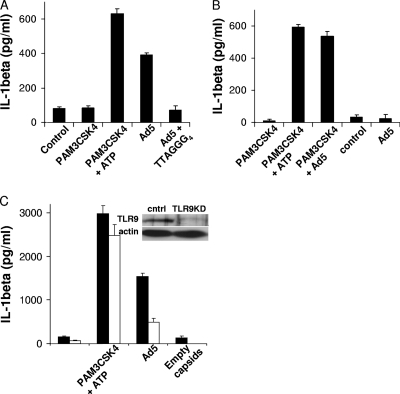
FIG. 3.
TLR9 recognition of Ad5 DNA contributes to IL-1β release from human but not mouse macrophages. (A) hMDMs were treated with control medium, primed with PAM3CSK4 for 4 h before medium with or without ATP was replaced for 1 h, or treated with Ad5gfp in the presence or absence of 20 μM of the TLR9 inhibitor (TTAGGG)4 for 8 h. (B) mBMMs were either primed with PAM3CSK4 for 4 h before being treated with control medium (4 h), ATP (1 h), or Ad5gfp (4 h) or left unprimed and treated with control medium or Ad5gfp for 8 h. (C) PMA-differentiated THP-1cntrl (closed) or THP-1tlr9KD (open) cells were treated with control medium for 6 h, primed with PAM3CSK4 before treatment with ATP for 1 h, or treated with Ad5gfp alone or an equivalent amount of empty capsids for 6 h, as described in Materials and Methods. The release of IL-1β was quantified by ELISA. Data represent the means and standard errors from 3 replicates. (Inset) Western blot for TLR9 and the actin loading control in THP-1cntrl and THP-1tlr9KD cell lysates.
The TLR9-dependent priming observed in Fig. 2 was found to be necessary for Ad5gfp-induced IL-1β secretion in hMDMs since Ad5gfp induced IL-1β release in the absence of additional TLR priming (Fig. 3A). When hMDMs were infected with Ad5gfp in the presence of the competitive TLR9 antagonist (TTAGGG)4, a significant reduction in IL-1β release was observed (Fig. 3A). Priming with a TLR agonist (PAM3CSK4) was necessary for Ad5gfp-induced IL-1β release from mBMMs as previously reported, however (Fig. 3B). Infection of THP-1-tlr9KD cells with Ad5gfp resulted in the release of ∼70% less IL-1β than that released by THP-1cntrl cells (Fig. 3C). The release of IL-1β in response to PAM3CSK4 plus ATP was unaffected by TLR9 knockdown. In further support of a role for TLR9 signaling in Ad5 priming of the inflammasome, differentiated THP-1cntrl cells infected with an equivalent amount of empty capsids, which are competent for endosomal membrane penetration but which do not possess a packaged DNA genome, resulted in no significant release of IL-1β (Fig. 3C).
Ad5 membrane penetration is required for NLRP3 activation.
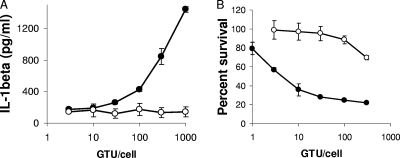
While our data suggest that Ad5 primes the inflammasome in human macrophages by activation of TLR9, the Ad5-related signal which activates the inflammasome remains undefined. Initial studies suggested that Ad5 DNA was required for NLRP3 activation, based on the inability of empty capsids to induce IL-1β release (35). However, additional studies describing particulate activation of the NLRP3 inflammasome have suggested that destabilization of phagosomal membranes provides the necessary trigger for NLRP3 activation of caspase-1 (23). Since Ad5 cell entry involves the destruction of endosomal membranes (29, 55), we examined whether membrane penetration was required for NLRP3 activation. PMA-differentiated THP-1 cells were primed with PAM3CSK4 to upregulate pro-IL-1β, NLRP3, and ASC and then infected with increasing multiplicities of infection (MOIs) of Ad5gfp or a temperature-sensitive mutant of Ad2, ts1, which possesses a hyperstable capsid when produced at the nonpermissive temperature and is incapable of rupturing endosomes (9, 19, 54, 55). After 1 h, supernatants were analyzed for IL-1β release by ELISA (Fig. 4A). This approach minimizes complications arising from differences in TLR9-dependent priming between Ad5 and ts1. While Ad5gfp induces a dose-dependent increase in IL-1β release, significant release of IL-1β is not observed for any MOI for ts1. To confirm the defect in ts1 disruption of endosomal membranes, PMA-differentiated THP-1 cells were infected with increasing MOIs of Ad5gfp or ts1 in the presence of the membrane-impermeable ribosomal toxin saporin (29). Saporin-dependent cytotoxicity was assessed 20 h later by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. Compared to Ad5gfp, which demonstrated a dose-dependent ability to facilitate cytosolic translocation of saporin, ts1 was significantly defective in this capacity, as previously reported (Fig. 4B) (55).
FIG. 4.
Ad5 membrane penetration is required for NLRP3 inflammasome activation. (A) PMA-differentiated THP-1 cells were rested in serum-free medium for 2 h, primed with PAM3CSK4 for 2 h, and treated with increasing GFP-transducing units of Ad5gfp (filled) or equivalent particle numbers of the temperature-sensitive mutant ts1 (open) for 1 h. The release of IL-1β was quantified by ELISA. Data represent the means and standard errors of 3 replicates. (B) The efficiency of Ad5 membrane penetration was assessed by measuring the residual percent cell viability after infecting PMA-differentiated THP-1 cells with increasing numbers of GTUs/cell of Ad5gfp (filled) or equivalent numbers of ts1 (open) particles in the presence of the ribosomal toxin saporin. After 24 h, cell viability was assessed by MTT assay. Values obtained were normalized to uninfected controls to calculate the percent cell viability. Data represent the means and standard errors from 3 replicates.
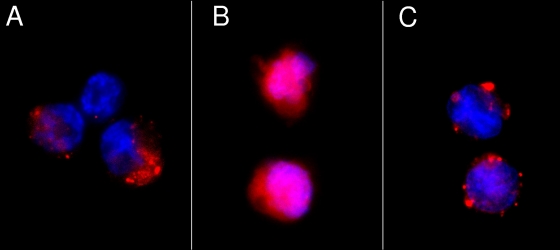
Disruption of lysosomal membranes leading to NLRP3 activation was previously reported to depend upon the release of catalytically active cathepsin B into the cytoplasm (23). Therefore, cathepsin B release into the cytoplasm was assessed in PMA-differentiated THP-1 cells infected with Ad5gfp or ts1 for 1 h. By staining for catalytically active cathepsin B using the Magic Red cathepsin B assay kit, Magic Red puncta were observed in the cytoplasm of uninfected cells (Fig. 5A). Ad5gfp infection resulted in diffuse Magic Red staining throughout the cell, suggesting that Ad5gfp had released catalytically active cathepsin B from lysosomal compartments (Fig. 5B). Infection with ts1 did not alter the punctate Magic Red staining observed in uninfected cells, suggesting that Ad5gfp membrane penetration was required for this redistribution (Fig. 5C).
FIG. 5.
Ad5 membrane penetration releases cathepsin B into the cytoplasm. PMA-differentiated THP-1 cells were treated with control medium (A), Ad5gfp (B), or an equivalent amount of ts1 (C) for 1 h before staining live cells with Magic Red cathepsin B and counterstaining with Hoechst dye, as described in Materials and Methods. Cells were visualized by epifluorescence microscopy, and representative images are shown.
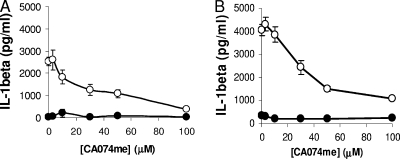
To determine whether the release of lysosomal cathepsin B into the cytoplasm is required for NLRP3 activation, PMA-differentiated THP-1 cells were treated with increasing concentrations of the cathepsin B inhibitor CA-074me for 30 min before being infected with Ad5gfp for 1 h. Ad5 cell entry was not inhibited by CA-074me (data not shown). The release of IL-1β was inhibited in cells pretreated with CA-074me in a dose-dependent manner, suggesting that the catalytic activity of cathepsin B was required for NLRP3 activation by Ad5gfp (Fig. 6A). Similar results were obtained in cells which were first primed with PAM3CSK4 before Ad5gfp transduction (Fig. 6B).
FIG. 6.
Inhibition of cathepsin B attenuates Ad5-induced IL-1β release. (A) PMA-differentiated THP-1 cells were rested in serum-free medium for 2 h and pretreated with increasing concentrations of the cathepsin B inhibitor CA074me for 30 min before being infected with Ad5gfp (open) or medium alone (filled) for 6 h. (B) PMA-differentiated THP-1 cells were rested in serum-free medium for 2 h, primed with PAM3CSK4 for 2 h, and pretreated with increasing concentrations of CA074me for 30 min before being infected with Ad5gfp (open) or medium alone (filled) for 1 h. The release of IL-1β was quantified by ELISA. Data represent the means and standard errors from 3 replicates.
Ad5-induced ROS are required for NLRP3 activation.
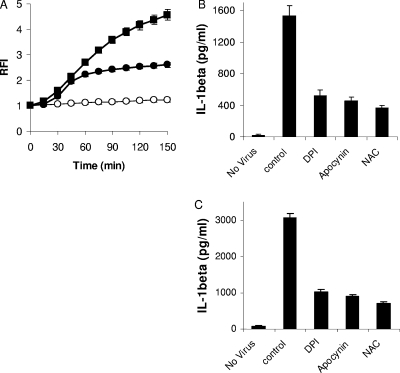
Since activation of the NLRP3 inflammasome by numerous stimuli depends on the production of ROS, we first assessed whether Ad5 infection induced ROS. PMA-differentiated THP-1 cells where first rested in serum-free medium for 3 h to reduce basal ROS levels. Cells were then briefly loaded with the redox-sensitive fluorophore DCFDA and then infected with 300 GTUs/cell of Ad5gfp. Ad5gfp induced ROS within 15 min, similar to induction with the positive control ATP (Fig. 7A). A requirement for ROS in Ad5gfp-induced NLRP3 activation is suggested by experiments which demonstrate the reduced release of IL-1β from PMA-differentiated THP-1 cells infected with Ad5gfp in the presence of inhibitors of NADPH oxidase (100 μM diphenyliodonium bromide [DPI], 1 mM apocynin) or the oxygen scavenger N-acetylcysteine (NAC) (Fig. 7B). These inhibitors did not significantly affect cell viability or viral infectivity for the time periods examined (data not shown). Similar data were also observed in PAM3CSK4-primed cells (Fig. 7C). These data suggest that production of ROS in response to Ad5 infection is required for NLRP3 inflammasome activation.
FIG. 7.
Inhibition of ROS production attenuates Ad5-induced IL-1β release. (A) PMA-differentiated THP-1 cells were rested in serum-free medium, loaded with the ROS-sensitive fluorophore DCFDA, and treated with control medium (open circles), ATP (filled squares), or Ad5gfp (filled circles). Relative fluorescence intensity (RFI) was measured as a function of time, as described in Materials and Methods. Data represents the means and standard errors from 3 replicates. (B) PMA-differentiated THP-1 cells were rested in serum-free medium for 2 h, pretreated with inhibitors of NADPH oxidase (DPI and apocynin) or the radical scavenger N-acetylcysteine (NAC) for 30 min, and then infected with Ad5gfp for 6 h in the continued presence of drug. (C) PMA-differentiated THP-1 cells were rested in serum-free medium for 2 h, primed with PAM3CSK4 for 2 h, pretreated with DPI, apocynin, or N-acetylcysteine (NAC) for 30 min, and then infected with Ad5gfp for 1 h in the continued presence of drug. The release of IL-1β was quantified by ELISA. Data represent the means and standard errors from 3 replicates.
NLRP3 activation leads to macrophage necrotic cell death and the release of proinflammatory mediators.
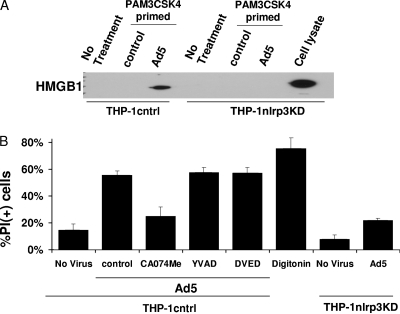
Previous reports have indicated that infection-induced inflammasome activation leads to necrotic cell death of macrophages, accompanied by the release of proinflammatory molecules, including HMGB1 (11, 56). To determine whether Ad5gfp infection induces inflammatory cell death in macrophage-like cells, we examined the loss of plasma membrane integrity and release of HMGB1 upon Ad5gfp infection of PMA-differentiated THP-1 cells. To ensure a more synchronous response to the activating signal induced by Ad5gfp membrane penetration, cells were first primed with PAM3CSK4 for 2 h and then infected with Ad5gfp for 1 h. Supernatants were collected and analyzed for HMGB1 release by Western blotting. Cells were washed and incubated with propidium iodide (PI) for 20 min before washing away unincorporated dye and counterstaining with DAPI to quantify the total nuclei present by fluorescence microscopy. Compared to uninfected cells, Ad5gfp-infected cells released HMGB1, as assessed by Western blotting (Fig. 8A). The release of HMGB1 corresponded with the loss of plasma membrane integrity, as indicated by a Ad5gfp-dependent increase in the percentage of PI cells (Fig. 8B).
FIG. 8.
Ad5 induces caspase-1-independent but NLRP3-dependent inflammatory cell necrosis. (A) PMA-differentiated THP-1cntrl or THP-1nlrp3KD cells were rested in serum-free medium for 2 h, primed with PAM3CSK4 for 2 h or not, and treated with control medium or infected with Ad5gfp for 1 h. HMGB1 was detected in the supernatants by Western blotting. Lysate from THP-1nlrp3KD cells served as a positive control for immunoblotting. (B) PMA-differentiated THP-1cntrl or THP-1nlrp3KD cells were rested in serum-free medium for 2 h, primed with PAM3CSK4 for 2 h, and preincubated with inhibitors of caspase-1 (YVAD-fmk), caspase-3 (DVED-fmk), or cathepsin B (CA074me) for 30 min before treatment with medium alone or infection with Ad5gfp for 1 h in the continued presence of drug. Cells were stained with propidium iodide (PI) and counterstained with DAPI, and the percentage of PI+ cells was determined by fluorescence microscopy. Disruption of plasma membrane integrity by 50 μg/ml digitonin was used as a positive control. Data represent the means and standard errors from 3 replicates.
To determine whether this Ad5gfp-induced cell death was dependent on cathepsin B catalytic activity or NLRP3 and independent of caspase-1 or -3 activity, similar studies were conducted in PMA-differentiated THP-1cntrl or THP-1nlrp3KD cells pretreated with cathepsin B inhibitor (CA-074me), caspase-1 inhibitor (YVAD-fmk), and caspase-3 inhibitor (DVED-fmk). Inhibition of cathepsin B but not caspase-1 or caspase-3 reduced the amount of Ad5gfp-induced HMGB1 released from cells as well as the percentage of PI-positive cells (Fig. 8B). Also, in agreement with previous observations of pathogen-dependent necrotic cell death of macrophages, knockdown of NLRP3 reduced the amount of the Ad5gfp-induced HMGB1 (Fig. 8A) release and the percentage of cells staining positive for PI (Fig. 8B). Thus, Ad5 cell membrane penetration appears to induce NLRP3- and cathepsin B-dependent but caspase-1-independent cell death in myeloid cells.
DISCUSSION
During Ad5 cell entry, multiple pattern recognition receptors and innate signaling pathways are reportedly activated (3, 8, 10, 12, 24, 34, 35, 37, 49, 59). And in many cases, there are conflicting descriptions of the exact molecules involved in the recognition of Ad5 infection. At least some of this discrepancy comes from studies involving cells from different species, different culture conditions, or different routes if in vivo administration of the virus took place. Results from our study confirm that Ad5-induced IL-1β release from human macrophages is dependent upon NLRP3, as was previously reported for mouse macrophages (35). In this previous study, the ability of Ad5 to activate the NLRP3 inflammasome was shown to occur in the absence of interactions with specific cell surface receptors, since inflammasome activation was also observed for Ad serotypes which use non-coxsackievirus B and adenovirus receptor (CAR) attachment receptors or a mutant Ad5 in which a penton base-encoded integrin-interacting RGD motif was deleted. Our results shed additional light on the role of endocytosis and membrane penetration in Ad5 activation of the NLRP3 inflammasome.
Unlike previous studies in mouse macrophages, our data suggest that Ad5-induced IL-1β release from hMDMs is dependent upon TLR9 recognition of viral DNA. This discrepancy would appear to be related to differences in TLR9 activation by Ad5 during entry of human versus mouse macrophages, although additional studies are required to elucidate the mechanism behind this observation. The role for TLR9 in Ad5 inflammasome activation appears to be related to the increased expression of NLRP3, ASC, and pro-IL-1β. In mBMMs, priming with a TLR agonist such as LPS or PAM3CSK4 is therefore required in the absence of Ad5 activation of TLR9 to prime the inflammasome by inducing expression of inflammasome components. This is indeed the case with most agents which activate NLRP3 (4). In some cases, alternative means of NF-κB activation, such as TNF-α signaling, can replace TLR signaling (4, 14). Given the uncertain role of TLR9 signaling in Ad5-induced proinflammatory responses in mice, alternative pathways such as TNF-α should be considered.
Activation of NLRP3 occurs in response to cell-associated danger signals elicited by numerous, seemingly disparate pathogens, toxins, or particulates (32, 42). Our data demonstrate that Ad5 induces ROS production during cell entry and that inhibition of ROS production attenuates Ad5-induced IL-1β release. Although inhibition of ROS production prevents NLRP3 inflammasome activation, the mechanism of ROS production by many NLRP3 stimuli has yet to be fully elucidated. While NADPH oxidase inhibitors, including DPI and apocynin, appear to inhibit NLRP3 activation by many stimuli, including Ad5, genetic approaches have suggested that loss of function of numerous subunits of NADPH oxidase, including gp91phox, p47phox, and p22phox, either enhances or does not affect IL-1β release in response to NLRP3 stimuli (33, 51). Nonetheless, ROS are known to be produced in response to these NLRP3 stimuli, and NLRP3 activation can be prevented by treatments which prevent ROS production. The source for this ROS remains unknown in many instances, including during Ad5 cell entry.
Further studies have implicated the release of lysosomal cathepsins into the cytoplasm as an additional trigger for NLRP3 inflammasome activation (11, 23, 56). Initial studies demonstrated that phagocytosed particulates such as silica particles or asbestos destabilized lysosomal membrane integrity, which lead to the release of catalytically active cathepsin B into the cytoplasm (23). Pharmacological inhibition of cathepsin B or genetic depletion of cathepsin B or L in macrophages led to reduced inflammasome activation by these particulates. Subsequently, numerous studies have identified a role for lysosomal cathepsins in inflammasome activation in response to bacterial rupture of membrane integrity (11, 56) or RNA virus replication (1). Our studies support a role for cathepsin B in inflammasome activation, since rupture of endosomal membranes and cytosolic cathepsin B catalytic activity are required for Ad5gfp-induced NLRP3 activation. Beyond a requirement for cytosolic release of catalytically active cathepsin B, little information is available regarding the role of cathepsins in NLRP3 activation. One possibility is that cathepsin B is upstream of ROS production in response to many stimuli. A known role for cathepsin B in caspase-independent mitochondrial membrane destabilization and subsequent oxidative stress has been reported (15, 16, 47, 50, 58), although not necessarily in the context of the prosurvival NF-κB activation.
Previous studies have indicated that Ad5 genomic DNA is recognized by cytosolic pattern recognition receptors leading the activation of IRF3 (36, 37, 59). The absent in melanoma 2 (AIM2) protein was recently implicated in activation of the inflammasome by cytosolic DNA from herpesviruses, poxviruses, and bacteria (7, 13, 22, 53). Whether AIM2 recognizes Ad5 genomic DNA during cell entry remains to be determined. Our preliminary data suggest that interferons attenuate Ad5-induced IL-1β release (our unpublished observation). Since AIM2 is an interferon-inducible gene (13), our preliminary observation would argue against AIM2 recognition of Ad5 genomic DNA.
Several previous reports have demonstrated that the intravenous (i.v.) administration of Ad5 vectors to mice leads to the rapid necrotic death of macrophages in the liver and spleen (10, 31, 45). At least one report has suggested that this cell death was dependent upon Ad5 penetration of endosomal membranes (45). The necrotic death of these macrophages is thought to contribute to the proinflammatory response to i.v.-administered Ad5. We have demonstrated that necrotic cell death by Ad5 involves caspase-1-independent but cathepsin B- and NLRP3-dependent processes. This necrotic cell death leads to the release of inflammatory mediators such as HMGB1. Recently, it was demonstrated that TLR2 signaling contributes to hepatic inflammation observed after i.v. administration of Ad5 to mice, although at times significantly after initial infection (3). Since TLR2 has been implicated in mediating some of the proinflammatory signaling by HMGB1 released from necrotic cells (38, 52, 57), our data support a model in which Ad5 penetration of endosomal membranes leads to necrotic cell death which can propagate inflammatory processes, possibly through TLR2. A role for other putative receptors for HMGB1, such as the receptor for advanced glycation end products (RAGE), have not yet been implicated in Ad5-mediated inflammation.
The physiological relevance of Ad5 activation of the inflammasome has yet to be fully elucidated. Initial studies demonstrated that intraperitoneal infection of NLRP3−/− mice with replication-defective Ad5 vectors leads to reduced peritoneal recruitment of neutrophils, a phenomenon attributed to IL-1β secretion, and secretion of other proinflammatory cytokines (35). A more recent study suggested that splenic marginal zone macrophages were responsible for much of the Ad5-induced proinflammatory response (10). However, by using NLRP3−/−, caspase-1−/−, and IL-1β−/− mice, those authors concluded that the inflammasome and IL-1β did not contribute significantly to the inflammatory response to Ad5 vectors after i.v. administration (10). Another recent study concluded that TLRs and Nod-like receptors served redundant roles in the innate immune response to Ad5 vectors based on the lack of differences in the adaptive immune responses to intramuscularly administered Ad5 vaccine vectors among control, MyD88−/−, or NLRP3−/− mice (28). Since mBMMs did not appear to upregulate NLRP3 and ASC in response to Ad5 infection in our studies, it is not surprising that NLRP3 was not found to be involved in the rapid inflammatory response observed after i.v. Ad5 vector administration. Phenotypic differences in myeloid cells from different anatomical environments are likely and could possibly contribute to differential involvement of NLRP3 in Ad5-induced proinflammatory responses. This view is supported by observations that the activation of NLRP3 can be primed in a TLR-independent manner in vivo and that the NLRP3 inflammasome is attenuated through cell-cell interactions involving members of the TNF superfamily receptors on macrophages and cognate ligands on T cells (20). Thus, whether proinflammatory responses to Ad5 involve NLRP3 inflammasome activation when administered to alternative sites in mice, such as mucosal surfaces, remains to be determined.
Overall, our observations suggest that Ad5 is sensed by multiple pattern recognition receptors during cell entry. Exposure of viral genomes to TLR9 in endosomes and subsequent rupture of cathepsin-enriched lysosomes by protein VI during Ad5 cell entry lead the priming and activation of the NLRP3 inflammasome. Given the defined subcellular localization of TLR9 and cathepsins, it is worth considering whether enhancing or attenuating the degree to which Ad5 vectors localize to compartments containing these cellular molecules could be exploited to more precisely control the proinflammatory response. Targeting Ad5 vectors away from lysosomes could potentially reduce the proinflammatory response to vectors being developed for gene therapy, while targeting the virus to lysosomes could potentially augment the proinflammatory response in a manner beneficial for vectors to be used for genetic vaccination.
Acknowledgments
We acknowledge Joseph Conway for his assistance with assays of virus-induced necrosis.
C.M.W. acknowledges financial assistance from the NIH (grant AI082430 to C.M.W.; subcontract from HL054352 to Glen R. Nemerow) and the American Heart Association (grant 2261306). K.A.M. acknowledges financial support from the NIH (grant AI007508).
Footnotes
Published ahead of print on 27 October 2010.
REFERENCES
- 1.Allen, I. C., M. A. Scull, C. B. Moore, E. K. Holl, E. McElvania-TeKippe, D. J. Taxman, E. H. Guthrie, R. J. Pickles, and J. P. Ting. 2009. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 30:556-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. 2007. Acute respiratory disease associated with adenovirus serotype 14—four states, 2006-2007. MMWR Morb. Mortal. Wkly. Rep. 56:1181-1184. [PubMed] [Google Scholar]
- 3.Appledorn, D. M., S. Patial, A. McBride, S. Godbehere, N. Van Rooijen, N. Parameswaran, and A. Amalfitano. 2008. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J. Immunol. 181:2134-2144. [DOI] [PubMed] [Google Scholar]
- 4.Bauernfeind, F. G., G. Horvath, A. Stutz, E. S. Alnemri, K. Macdonald, D. Speert, T. Fernandes-Alnemri, J. Wu, B. G. Monks, K. A. Fitzgerald, V. Hornung, and E. Latz. 2009. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183:787-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benko, M., and B. Harrach. 2003. Molecular evolution of adenoviruses. Curr. Top. Microbiol. Immunol. 272:3-35. [DOI] [PubMed] [Google Scholar]
- 6.Berclaz, P. Y., Y. Shibata, J. A. Whitsett, and B. C. Trapnell. 2002. GM-CSF, via PU. 1, regulates alveolar macrophage Fcgamma R-mediated phagocytosis and the IL-18/IFN-gamma-mediated molecular connection between innate and adaptive immunity in the lung. Blood 100:4193-4200. [DOI] [PubMed] [Google Scholar]
- 7.Burckstummer, T., C. Baumann, S. Bluml, E. Dixit, G. Durnberger, H. Jahn, M. Planyavsky, M. Bilban, J. Colinge, K. L. Bennett, and G. Superti-Furga. 2009. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 10:266-272. [DOI] [PubMed] [Google Scholar]
- 8.Cerullo, V., M. P. Seiler, V. Mane, N. Brunetti-Pierri, C. Clarke, T. K. Bertin, J. R. Rodgers, and B. Lee. 2007. Toll-like receptor 9 triggers an innate immune response to helper-dependent adenoviral vectors. Mol. Ther. 15:378-385. [DOI] [PubMed] [Google Scholar]
- 9.Cotten, M., and J. M. Weber. 1995. The adenovirus protease is required for virus entry into host cells. Virology 213:494-502. [DOI] [PubMed] [Google Scholar]
- 10.Di Paolo, N. C., E. A. Miao, Y. Iwakura, K. Murali-Krishna, A. Aderem, R. A. Flavell, T. Papayannopoulou, and D. M. Shayakhmetov. 2009. Virus binding to a plasma membrane receptor triggers interleukin-1 alpha-mediated proinflammatory macrophage response in vivo. Immunity 31:110-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan, J. A., X. Gao, M. T. Huang, B. P. O'Connor, C. E. Thomas, S. B. Willingham, D. T. Bergstralh, G. A. Jarvis, P. F. Sparling, and J. P. Ting. 2009. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J. Immunol. 182:6460-6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fejer, G., L. Drechsel, J. Liese, U. Schleicher, Z. Ruzsics, N. Imelli, U. F. Greber, S. Keck, B. Hildenbrand, A. Krug, C. Bogdan, and M. A. Freudenberg. 2008. Key role of splenic myeloid DCs in the IFN-alphabeta response to adenoviruses in vivo. PLoS Pathog. 4:e1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandes-Alnemri, T., J. W. Yu, P. Datta, J. Wu, and E. S. Alnemri. 2009. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458:509-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franchi, L., T. Eigenbrod, and G. Nunez. 2009. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J. Immunol. 183:792-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghavami, S., A. Asoodeh, T. Klonisch, A. J. Halayko, K. Kadkhoda, T. J. Kroczak, S. B. Gibson, E. P. Booy, H. Naderi-Manesh, and M. Los. 2008. Brevinin-2R(1) semi-selectively kills cancer cells by a distinct mechanism, which involves the lysosomal-mitochondrial death pathway. J. Cell. Mol. Med. 12:1005-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghavami, S., M. Eshraghi, K. Kadkhoda, M. M. Mutawe, S. Maddika, G. H. Bay, S. Wesselborg, A. J. Halayko, T. Klonisch, and M. Los. 2009. Role of BNIP3 in TNF-induced cell death—TNF upregulates BNIP3 expression. Biochim. Biophys. Acta 1793:546-560. [DOI] [PubMed] [Google Scholar]
- 17.Ginsberg, H. S., and G. A. Prince. 1994. The molecular basis of adenovirus pathogenesis. Infect. Agents Dis. 3:1-8. [PubMed] [Google Scholar]
- 18.Gray, G. C., T. McCarthy, M. G. Lebeck, D. P. Schnurr, K. L. Russell, A. E. Kajon, M. L. Landry, D. S. Leland, G. A. Storch, C. C. Ginocchio, C. C. Robinson, G. J. Demmler, M. A. Saubolle, S. C. Kehl, R. Selvarangan, M. B. Miller, J. D. Chappell, D. M. Zerr, D. L. Kiska, D. C. Halstead, A. W. Capuano, S. F. Setterquist, M. L. Chorazy, J. D. Dawson, and D. D. Erdman. 2007. Genotype prevalence and risk factors for severe clinical adenovirus infection, United States 2004-2006. Clin. Infect. Dis. 45:1120-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greber, U. F., P. Webster, J. Weber, and A. Helenius. 1996. The role of the adenovirus protease on virus entry into cells. EMBO J. 15:1766-1777. [PMC free article] [PubMed] [Google Scholar]
- 20.Guarda, G., C. Dostert, F. Staehli, K. Cabalzar, R. Castillo, A. Tardivel, P. Schneider, and J. Tschopp. 2009. T cells dampen innate immune responses through inhibition of NLRP1 and NLRP3 inflammasomes. Nature 460:269-273. [DOI] [PubMed] [Google Scholar]
- 21.Hoelscher, M. A., N. Singh, S. Garg, L. Jayashankar, V. Veguilla, A. Pandey, Y. Matsuoka, J. M. Katz, R. Donis, S. K. Mittal, and S. Sambhara. 2008. A broadly protective vaccine against globally dispersed clade 1 and clade 2 H5N1 influenza viruses. J. Infect. Dis. 197:1185-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hornung, V., A. Ablasser, M. Charrel-Dennis, F. Bauernfeind, G. Horvath, D. R. Caffrey, E. Latz, and K. A. Fitzgerald. 2009. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458:514-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hornung, V., F. Bauernfeind, A. Halle, E. O. Samstad, H. Kono, K. L. Rock, K. A. Fitzgerald, and E. Latz. 2008. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9:847-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iacobelli-Martinez, M., and G. R. Nemerow. 2007. Preferential activation of Toll-like receptor nine by CD46-utilizing adenoviruses. J. Virol. 81:1305-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichinohe, T., H. K. Lee, Y. Ogura, R. Flavell, and A. Iwasaki. 2009. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J. Exp. Med. 206:79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanneganti, T. D., M. Body-Malapel, A. Amer, J. H. Park, J. Whitfield, L. Franchi, Z. F. Taraporewala, D. Miller, J. T. Patton, N. Inohara, and G. Nunez. 2006. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J. Biol. Chem. 281:36560-36568. [DOI] [PubMed] [Google Scholar]
- 27.Kawai, T., and S. Akira. 2008. Toll-like receptor and RIG-I-like receptor signaling. Ann. N. Y. Acad. Sci. 1143:1-20. [DOI] [PubMed] [Google Scholar]
- 28.Lindsay, R. W., P. A. Darrah, K. M. Quinn, U. Wille-Reece, L. M. Mattei, A. Iwasaki, S. P. Kasturi, B. Pulendran, J. G. Gall, A. G. Spies, and R. A. Seder. 2010. CD8+ T cell responses following replication-defective adenovirus serotype 5 immunization are dependent on CD11c+ dendritic cells but show redundancy in their requirement of TLR and nucleotide-binding oligomerization domain-like receptor signaling. J. Immunol. 185:1513-1521. [DOI] [PubMed] [Google Scholar]
- 29.Maier, O., D. L. Galan, H. Wodrich, and C. M. Wiethoff. 2010. An N-terminal domain of adenovirus protein VI fragments membranes by inducing positive membrane curvature. Virology 402:11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manicassamy, S., and B. Pulendran. 2009. Modulation of adaptive immunity with Toll-like receptors. Semin. Immunol. 21:185-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manickan, E., J. S. Smith, J. Tian, T. L. Eggerman, J. N. Lozier, J. Muller, and A. P. Byrnes. 2006. Rapid Kupffer cell death after intravenous injection of adenovirus vectors. Mol. Ther. 13:108-117. [DOI] [PubMed] [Google Scholar]
- 32.Martinon, F., A. Mayor, and J. Tschopp. 2009. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27:229-265. [DOI] [PubMed] [Google Scholar]
- 33.Meissner, F., R. A. Seger, D. Moshous, A. Fischer, J. Reichenbach, and A. Zychlinsky. 2010. Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood 116:1570-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muruve, D. A. 2004. The innate immune response to adenovirus vectors. Hum. Gene Ther. 15:1157-1166. [DOI] [PubMed] [Google Scholar]
- 35.Muruve, D. A., V. Petrilli, A. K. Zaiss, L. R. White, S. A. Clark, P. J. Ross, R. J. Parks, and J. Tschopp. 2008. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452:103-107. [DOI] [PubMed] [Google Scholar]
- 36.Nociari, M., O. Ocheretina, M. Murphy, and E. Falck-Pedersen. 2009. Adenovirus induction of IRF3 occurs through a binary trigger targeting Jun N-terminal kinase and TBK1 kinase cascades and type I interferon autocrine signaling. J. Virol. 83:4081-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nociari, M., O. Ocheretina, J. W. Schoggins, and E. Falck-Pedersen. 2007. Sensing infection by adenovirus: Toll-like receptor-independent viral DNA recognition signals activation of the interferon regulatory factor 3 master regulator. J. Virol. 81:4145-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park, J. S., F. Gamboni-Robertson, Q. He, D. Svetkauskaite, J. Y. Kim, D. Strassheim, J. W. Sohn, S. Yamada, I. Maruyama, A. Banerjee, A. Ishizaka, and E. Abraham. 2006. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am. J. Physiol. Cell Physiol. 290:C917-C924. [DOI] [PubMed] [Google Scholar]
- 39.Petrilli, V., C. Dostert, D. A. Muruve, and J. Tschopp. 2007. The inflammasome: a danger sensing complex triggering innate immunity. Curr. Opin. Immunol. 19:615-622. [DOI] [PubMed] [Google Scholar]
- 40.Priddy, F. H., D. Brown, J. Kublin, K. Monahan, D. P. Wright, J. Lalezari, S. Santiago, M. Marmor, M. Lally, R. M. Novak, S. J. Brown, P. Kulkarni, S. A. Dubey, L. S. Kierstead, D. R. Casimiro, R. Mogg, M. J. DiNubile, J. W. Shiver, R. Y. Leavitt, M. N. Robertson, D. V. Mehrotra, and E. Quirk. 2008. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin. Infect. Dis. 46:1769-1781. [DOI] [PubMed] [Google Scholar]
- 41.Rissanen, T. T., and S. Yla-Herttuala. 2007. Current status of cardiovascular gene therapy. Mol. Ther. 15:1233-1247. [DOI] [PubMed] [Google Scholar]
- 42.Schroder, K., R. Zhou, and J. Tschopp. 2010. The NLRP3 inflammasome: a sensor for metabolic danger? Science 327:296-300. [DOI] [PubMed] [Google Scholar]
- 43.Shayakhmetov, D. M., Z. Y. Li, S. Ni, and A. Lieber. 2005. Interference with the IL-1-signaling pathway improves the toxicity profile of systemically applied adenovirus vectors. J. Immunol. 174:7310-7319. [DOI] [PubMed] [Google Scholar]
- 44.Shirakawa, T. 2008. The current status of adenovirus-based cancer gene therapy. Mol. Cells 25:462-466. [PubMed] [Google Scholar]
- 45.Smith, J. S., Z. Xu, J. Tian, S. C. Stevenson, and A. P. Byrnes. 2008. Interaction of systemically delivered adenovirus vectors with Kupffer cells in mouse liver. Hum. Gene Ther. 19:547-554. [DOI] [PubMed] [Google Scholar]
- 46.Suparno, C., D. W. Milligan, P. A. Moss, and V. Mautner. 2004. Adenovirus infections in stem cell transplant recipients: recent developments in understanding of pathogenesis, diagnosis and management. Leuk. Lymphoma 45:873-885. [DOI] [PubMed] [Google Scholar]
- 47.Thibodeau, M., C. Giardina, and A. K. Hubbard. 2003. Silica-induced caspase activation in mouse alveolar macrophages is dependent upon mitochondrial integrity and aspartic proteolysis. Toxicol. Sci. 76:91-101. [DOI] [PubMed] [Google Scholar]
- 48.Thomas, P. G., P. Dash, J. R. Aldridge, Jr., A. H. Ellebedy, C. Reynolds, A. J. Funk, W. J. Martin, M. Lamkanfi, R. J. Webby, K. L. Boyd, P. C. Doherty, and T. D. Kanneganti. 2009. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 30:566-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tibbles, L. A., J. C. Spurrell, G. P. Bowen, Q. Liu, M. Lam, A. K. Zaiss, S. M. Robbins, M. D. Hollenberg, T. J. Wickham, and D. A. Muruve. 2002. Activation of p38 and ERK signaling during adenovirus vector cell entry lead to expression of the C-X-C chemokine IP-10. J. Virol. 76:1559-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tran, T. M., V. Temkin, B. Shi, L. Pagliari, S. Daniel, C. Ferran, and R. M. Pope. 2009. TNFalpha-induced macrophage death via caspase-dependent and independent pathways. Apoptosis 14:320-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Bruggen, R., M. Y. Koker, M. Jansen, M. van Houdt, D. Roos, T. W. Kuijpers, and T. K. van den Berg. 2010. Human NLRP3 inflammasome activation is Nox1-4 independent. Blood 115:5398-5400. [DOI] [PubMed] [Google Scholar]
- 52.van Zoelen, M. A., H. Yang, S. Florquin, J. C. Meijers, S. Akira, B. Arnold, P. P. Nawroth, A. Bierhaus, K. J. Tracey, and T. van der Poll. 2009. Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock 31:280-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vilaysane, A., and D. A. Muruve. 2009. The innate immune response to DNA. Semin. Immunol. 21:208-214. [DOI] [PubMed] [Google Scholar]
- 54.Weber, J. 1976. Genetic analysis of adenovirus type 2 III. Temperature sensitivity of processing viral proteins. J. Virol. 17:462-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wiethoff, C. M., H. Wodrich, L. Gerace, and G. R. Nemerow. 2005. Adenovirus protein VI mediates membrane disruption following capsid disassembly. J. Virol. 79:1992-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Willingham, S. B., D. T. Bergstralh, W. O'Connor, A. C. Morrison, D. J. Taxman, J. A. Duncan, S. Barnoy, M. M. Venkatesan, R. A. Flavell, M. Deshmukh, H. M. Hoffman, and J. P. Ting. 2007. Microbial pathogen-induced necrotic cell death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe 2:147-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu, M., H. Wang, A. Ding, D. T. Golenbock, E. Latz, C. J. Czura, M. J. Fenton, K. J. Tracey, and H. Yang. 2006. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock 26:174-179. [DOI] [PubMed] [Google Scholar]
- 58.Zhang, H., C. Zhong, L. Shi, Y. Guo, and Z. Fan. 2009. Granulysin induces cathepsin B release from lysosomes of target tumor cells to attack mitochondria through processing of bid leading to necroptosis. J. Immunol. 182:6993-7000. [DOI] [PubMed] [Google Scholar]
- 59.Zhu, J., X. Huang, and Y. Yang. 2007. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J. Virol. 81:3170-3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zsengeller, Z., K. Otake, S. A. Hossain, P. Y. Berclaz, and B. C. Trapnell. 2000. Internalization of adenovirus by alveolar macrophages initiates early proinflammatory signaling during acute respiratory tract infection. J. Virol. 74:9655-9667. [DOI] [PMC free article] [PubMed] [Google Scholar]