Abstract
The immunologic mechanisms underlying the faster progression of hepatitis C virus (HCV) disease in the presence of human immunodeficiency virus (HIV) coinfection are not clearly understood. T-cell cross-reactivity between HCV and influenza virus-specific epitopes has been associated with rapid progression of HCV disease (S. Urbani, B. Amadei, P. Fisicaro, M. Pilli, G. Missale, A. Bertoletti, and C. Ferrari, J. Exp. Med. 201:675-680, 2005). We asked whether T-cell cross-reactivity between HCV and HIV could exist during HCV/HIV coinfection and affect pathogenesis. Our search for amino acid sequence homology between the HCV and HIV proteomes revealed two similar HLA-A2-restricted epitopes, HIV-Gag (SLYNTVATL [HIV-SL9]) and HCV-NS5b (ALYDVVSKL [HCV-AL9]). We found that 4 out of 20 HLA-A2-positive (HLA-A2+) HIV-infected individuals had CD8+ T cells that recognized both the HIV-SL9 and HCV-AL9 epitopes. However, the AL9 epitope was generally shown to be a weak agonist. Although HCV-monoinfected individuals in our study did not show AL9-specific responses, we found that about half of HCV/HIV-coinfected individuals had dual responses to both epitopes. High dual T-cell recognition among coinfected subjects was usually due to separate T-cell populations targeting each epitope, as determined by pentamer staining. The one individual demonstrating cross-reactive T cells to both epitopes showed the most advanced degree of liver disease. In coinfected individuals, we observed a positive correlation between the magnitudes of T-cell responses to both the SL9 and the AL9 epitopes, which was also positively associated with the clinical parameter of liver damage. Thus, we find that HIV infection induces T cells that can cross-react to heterologous viruses or prime for T cells that are closely related in sequence. However, the induction of cross-reactive T cells may not be associated with control of disease caused by the heterologous virus. This demonstrates that degeneracy of HIV-specific T cells may play a role in the immunopathology of HCV/HIV coinfection.
Hepatitis C virus (HCV)-related liver disease progresses faster in individuals coinfected with human immunodeficiency virus (HIV) type 1 (HIV-1) (8, 19); however, the underlying immunologic mechanisms are not clear. Many studies indicate that exposure to an infectious agent may alter the immune responses of the host to other infections, a phenomenon referred to as heterologous immunity (25). Several lines of evidence signify the extent of T-cell degeneracy and the role of molecular mimicry in provoking an immune response. The stimulating target for CD8+ T cells is the complex of a specific foreign peptide and a self major histocompatibility complex (MHC) class I molecule (20). During molecular mimicry, a variant of an original peptide ligand interacts with the cognate T-cell receptor (TCR), inducing total or partial T-cell activation. Only a few TCR contact residues on the peptide ligand are required for T-cell stimulation, and certain amino acid substitutions in the peptide sequence can still result in the activation of T cells (13). The ability of a TCR to cross-recognize multiple antigens is shown to have many implications for CD8+ T-cell functioning, including protective responses against pathogens (16), immunodominance (3), and maintenance of memory responses (14).
Activated memory T cells that are specific for a virus are shown to further restrain the responses from naive T cells specific for the same virus (4). Accordingly, in a primary infection, even relatively weak T-cell responses to nondominant epitopes may affect the immunodominance hierarchy of T-cell responses to a subsequent infection. This could result in an enhanced response to subdominant cross-reactive epitopes at the cost of responses to more immunodominant epitopes.
Cross-reactivity between HCV- and influenza virus-specific CD8+ T cells and an association with the severity of the clinical course of HCV disease have been previously demonstrated (21, 24). Urbani et al. identified individuals with a severe course of HCV infection who had a narrowly focused response to the HCV-NS3 (CVNGVCWTV) epitope, and these cells cross-reacted with the influenza A virus neuraminidase (FLU-NA) (CVNGSCFTV) epitope (21). This suggested that preexisting responses to the FLU-NA epitope may have adversely affected HCV responses by narrowing it to the NS3 epitope. In another recent study, although the FLU-NA response was not prevalently seen in the studied cohort, HCV-NS3-specific CD8+ T cells from HCV-infected individuals that cross-reacted with the FLU-NA epitope were identified (9). These authors showed that the cross-reactive T cells responded only weakly to the FLU-NA epitope, indicating that the FLU-NA peptide was a weak agonist and was mainly a consequence of the presence of a preexisting response to the HCV NS3 epitope. These studies show that cross-reactivity to heterologous viruses may occur. However, it is still unclear how such responses alter the subsequent responses to the heterologous viruses.
Individuals with HCV/HIV coinfection have high levels of circulating antigen to both viruses in their plasma, and given that HIV CD8+ T-cell responses are robust in most HIV-infected individuals, it is unclear what effect heterologous cross-reactivity may have on HCV-specific responses. In this study, we investigated if HIV-specific T cells could recognize heterologous HCV epitopes and if this cross-recognition could alter the immunopathological profile of HCV infection in HCV/HIV-coinfected individuals. We focused on two defined HLA-A2-restricted epitopes: HIV-Gag (SLYNTVATL [HIV-SL9]) and HCV-NS5b (ALYDVVSKL [HCV-AL9]). T-cell responses targeting HIV-SL9 are mainly prevalent during the chronic phase of HIV infection (7). HCV-AL9 is also a defined epitope that is associated with protection during acute infection (11). This study aimed to specifically address if the presence of HIV-SL9-specific T cells influences the responses toward HCV-AL9 and hypothesized that in HCV/HIV coinfection, individuals with SL9-specific T-cell responses weakly mount AL9-targeted responses.
MATERIALS AND METHODS
Study participants.
Blood samples were obtained from 20 HIV-monoinfected, 17 HCV/HIV-coinfected, and 5 HCV-monoinfected individuals and were cryopreserved until use. All studied individuals were HLA-A2 positive (HLA-A2+), as identified by HLA-A2 antibody staining (BD Pharmingen). Informed consent was obtained in accordance with the guidelines of the institutional ethics boards for the conduct of clinical research at the University of Toronto and St. Michael's Hospital. Peripheral blood mononuclear cells (PBMCs) were isolated as previously described (18). Clinical data for the studied individuals are presented in Table 1. None of the HCV-infected individuals were treated with interferon/ribavirin prior to sampling. All studied coinfected individuals had HCV infection prior to being infected with HIV.
TABLE 1.
Characteristics of HIV-monoinfected and HCV/HIV-coinfected individualsa
| Group and participant no. | OM no. | HIV infection status | HIV loadb (no. of copies/ml) | CD4 count (no. of cells/mm3) | HAART | HCV infection status | HCV loadb (IU/ml) | HCV genotype | ALT concn (IU/liter) | APRI score |
|---|---|---|---|---|---|---|---|---|---|---|
| HIV monoinfected | ||||||||||
| 1 | 18 | Chronic | <50 | 770 | Yes | |||||
| 2 | 54 | LTNP | 4,950 | NA | No | |||||
| 3 | 77 | LTNP | <50 | 872 | No | |||||
| 4 | 115 | LTNP | 53 | 670 | No | |||||
| 5 | 119 | Chronic | 45,700 | 720 | No | |||||
| 6 | 125 | LTNP | 200,600 | 350 | No | |||||
| 7 | 136 | Chronic | 62,986 | 640 | No | |||||
| 8 | 150 | Chronic | 2,187 | 430 | No | |||||
| 9 | 189 | Acute | 459,640 | 520 | No | |||||
| 10 | 194 | LTNP | 57 | 1,200 | No | |||||
| 11 | 266 | Acute | 332,257 | 800 | No | |||||
| 12 | 267 | Chronic | <50 | 400 | Yes | |||||
| 13 | 275 | Chronic | <50 | 840 | Yes | |||||
| 14 | 288 | Chronic | <50 | 950 | Yes | |||||
| 15 | 358 | LTNP | 29,562 | 852 | No | |||||
| 16 | 365 | Chronic | 180 | 490 | Yes | |||||
| 17 | 374 | LTNP | <50 | 990 | No | |||||
| 18 | 380 | Chronic | 17,873 | 550 | No | |||||
| 19 | 383 | Chronic | 12,000 | 420 | No | |||||
| 20 | 387 | Chronic | 933 | 660 | No | |||||
| HCV/HIV coinfected | ||||||||||
| 1 | 142 | Chronic | <50 | 400 | Yes | Chronic | 601 | 1a | 81 | 0.355 |
| 2 | 178 | Chronic | 8,827 | 340 | No | Chronic | NA | 1 | 26 | 0.371 |
| 3 | 249 | Chronic | 9,060 | 280 | No | Chronic | NA | NA | 37 | 0.25 |
| 4 | 256 | Chronic | 21,000 | 120 | No | Chronic | 8.51 × 105 | 1b | 28 | 0.393 |
| 5 | 305 | Acute | N/A | N/A | No | Acute | NA | NA | NA | NA |
| 6 | 405 | Chronic | 22,000 | 820 | Yes | Chronic | 1.34 × 106 | 1a | 29 | 0.561 |
| 7 | 414 | Chronic | 100,000 | 468 | No | Chronic | 9.94 × 106 | 1a | 92 | 0.264 |
| 8 | 453 | Chronic | <50 | 434 | Yes | Cleared | Undetectable | NA | 20 | 0.155 |
| 9 | 456 | Chronic | <50 | 350 | Yes | Acute | 1.33 × 106 | 1 | 72 | 0.283 |
| 10 | 491 | Chronic | 11,758 | 400 | No | Acute | 2.01 × 107 | NA | 246 | 0.821 |
| 11 | 499 | Chronic | 2,997 | 1,112 | Yes | Chronic | 6.18 × 105 | 1a | 122 | 0.419 |
| 12 | 501 | Chronic | 300,000 | 316 | No | Chronic | 9.94 × 106 | NA | 36 | 0.38 |
| 13 | 502 | Chronic | <50 | 600 | Yes | Acute | 600 | 1 | 33 | 0.135 |
| 14 | 503 | Chronic | 1,000 | 354 | No | Chronic | 2.07 × 105 | 4 | 66 | 0.222 |
| 15 | 530 | Chronic | 12,000 | 325 | No | Acute | 9.4 × 106 | 1a | 56 | 0.265 |
| 16 | 531 | Chronic | 50 | 300 | Yes | Chronic | 1.4 × 106 | 1a | 73 | 0.391 |
| 17 | 539 | Chronic | 5,890 | 346 | No | Chronic | NA | 1a | 115 | 0.826 |
OM, participant code; ALT, alanine aminotransferase; LTNP, long-term nonprogressor; NA, not available.
The viral loads are those in plasma.
Identifying amino acid sequence homology between HIV and HCV by BLAST proteomic search.
A detailed search for amino acid sequence homology between the HCV and HIV proteomes was conducted using the Basic Local Alignment Search Tool (BLAST) from the National Center for Biotechnology Information (NCBI) database. The BLAST search was conducted in two different ways. First, a general search for short, nearly exact amino acid matches between HCV (H77 strain) polyprotein and different parts of the HIV (HXB2 strain) proteome was performed. We found four similar hits, as defined by relative similarities in short amino acid sequences: two within the HIV-Gag region, one within the HIV-Pol region, and one within the HIV-Vif region. In the second approach, we conducted a BLAST search with sequences within identified HCV cytotoxic T-lymphocyte epitopes to find amino acid sequence matches within the whole HIV proteome. We found seven hits, as defined by relative amino acid sequence similarities within the HCV core epitopic region, with none being identified HIV epitopes. However, our search within the HCV-NS5b epitope map resulted in the identification of a relatively high degree of amino acid sequence similarity between HIV (HXB2)-Gag p17 (SL9 positions 76 to 84, SLYNTVATL) and HCV (H77)-NS5b (AL9 positions 2594 to 2602, ALYDVVSKL), with 4 out of 9 amino acids and HLA anchorage residues being similar and with both being defined HLA-A2-restricted epitopes.
IFN-γ ELISPOT assay screen.
PBMCs from each individual (2 × 105/well) were incubated in 96-well, polyvinylidene plates precoated with capture anti-human gamma interferon (IFN-γ) monoclonal antibody and stimulated with 10 μg/ml of either the HIV-Gag p17 (SL9) or HCV-NS5b (AL9) peptide (Poimmune PEPscreen custom peptide libraries) or dimethyl sulfoxide (DMSO) control. An irrelevant HLA-A2-restricted melanoma-associated antigen (MAGE) peptide (positions 271 to 279, FLWGPRALV) was used as a control peptide for the screen of monoinfected individuals. HLA-A2-restricted peptide HCV-NS3 (positions 1073 to 1081, CINGVCWTV; HCV-CI9), whose amino acid sequence has no similarity to the amino acid sequence of HIV-SL9, was used as a random control for the coinfected screen. Staphylococcus enterotoxin B (SEB; 3 μg/ml) was used as a positive-control stimulant. The frequency of responses was measured using an automated enzyme-linked immunospot (ELISPOT) assay counter, and a positive response was defined as one that was 2-fold higher than the background response (obtained with DMSO) with a minimum of 50 spots per 106 PBMCs and is presented as the number of spot-forming units (SFUs) per million PBMCs.
CD8+ enrichment of T cells.
CD8+-enriched T cells were isolated from PBMCs using a StemSep magnetic bead negative selection system (StemCell Technologies). Briefly, PBMCs were resuspended in separation medium (phosphate-buffered saline [PBS] with 2% fetal bovine serum [FBS]) and incubated with enrichment cocktail antibodies directed against CD4, CD14, CD16, CD19, and CD56; further incubated with magnetic colloids; and passed through a gravity feed column. Unlabeled CD8+ T cells were collected, washed, and resuspended in RPMI 1640 (Gibco Laboratories) for further analysis.
Generation of short-term T-cell lines.
Antigen-specific T cells were expanded from either the CD8+ T-cell enriched compartment or whole PBMCs in 24-well plates. Cultures of 105 CD8+-enriched cells and 5 × 105 autologous irradiated (3,000 rads) feeder PBMCs were stimulated with synthetic SL9 or AL9 peptide (10 μg/ml) in RPMI 1640 supplemented with 10% heat-inactivated human type AB serum, l-glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml) in the presence of anti-CD28 and anti-CD49 antibodies. Recombinant interleukin-7 (rIL-7; 10 ng/ml) and recombinant IL-15 (10 ng/ml) (R&D Systems) were added. rIL-2 (50 IU/ml; Invitrogen Inc.) was added on day 3, and the cells were harvested for experiments after 7 to 10 days.
Pentamer staining.
Pentamer staining for the SL9 or AL9 sequence was performed prior to peptide stimulation at room temperature for 15 min (fluorescent-labeled Pro5 MHC class I pentamers; Proimmune). Pentamer-stained cells were then washed with 2% FBS in PBS and stimulated with each SL9 or AL9 peptide (10 μg/ml), followed by intracellular staining (ICS) and flow cytometry analysis.
Ex vivo CD8+ T-cell stimulation and ICS.
One million PBMCs from each individual were stimulated with 10 μg/ml of either SL9 or AL9 peptide or DMSO control for 6 h in the presence of costimulatory antibodies (CD28 and CD49d, 1 μg/ml; BD Biosciences), as well as monensin (0.7 μl/ml; BD Biosciences) and brefeldin A (10 μg/ml; Sigma-Aldrich). CD107a antibody (BD Pharmingen) was also added during the time of stimulation. Following the stimulation, the cells were washed and stained with cell surface antibodies against CD3 and CD8 (BD Biosciences) and then fixed, permeabilized, and stained for the intracellular cytokine IFN-γ (BD Biosciences). The percentage of responding antigen-specific CD8+ T cells was measured by flow cytometry, using either a FACSAria flow cytometer or a FACSCalibur instrument (BD Biosciences). Further analysis was performed using FlowJo (version 7.5) software (Tree Star Inc.).
Statistical analysis.
Data were analyzed by performing a two-tailed Student's t test using Prism (version 4.00) software (GraphPad Software Inc.). The Mann-Whitney test was used for nonparametric data. Correlations were tested for significance by Spearman rank analysis. P values of <0.05 were considered significant.
RESULTS
Subject characteristics.
The two groups of studied individuals are described in Table 1: HIV-monoinfected individuals (n = 20; of whom 15 were untreated and 5 were receiving highly active antiretroviral therapy [HAART] for greater than 1 year at the time of evaluation) and HCV/HIV-coinfected individuals (n = 17; 7 were receiving HAART). HIV-monoinfected individuals had negative HCV serology and undetectable HCV loads by branched DNA assay. In addition, we examined 5 HCV-monoinfected individuals. None of the coinfected individuals received prior pegylated interferon/ribavirin treatment for HCV infection. All the coinfected individuals were infected with HCV first. HIV-monoinfected individuals had a median HIV load of 1,560 copies/ml (± standard error [SE], 28,143 copies/ml) and a median CD4 T-cell count of 670/mm3 (± SE, 52.7/mm3). HCV/HIV-coinfected individuals had a median HIV load of 7,359 copies/ml (± SE, 18,942 copies/ml), a median CD4 T-cell count of 352/mm3 (± SE, 59 mm3), and a median HCV load of 1,330,000 IU/ml (± SE, 1,722,000 IU/ml). Between the two cohorts, HIV-monoinfected subjects had higher CD4 counts (P = 0.0008). There was no significant difference in HIV loads between the two cohorts.
Identifying individuals with T-cell responses to both HIV-SL9 and HCV-AL9 antigens.
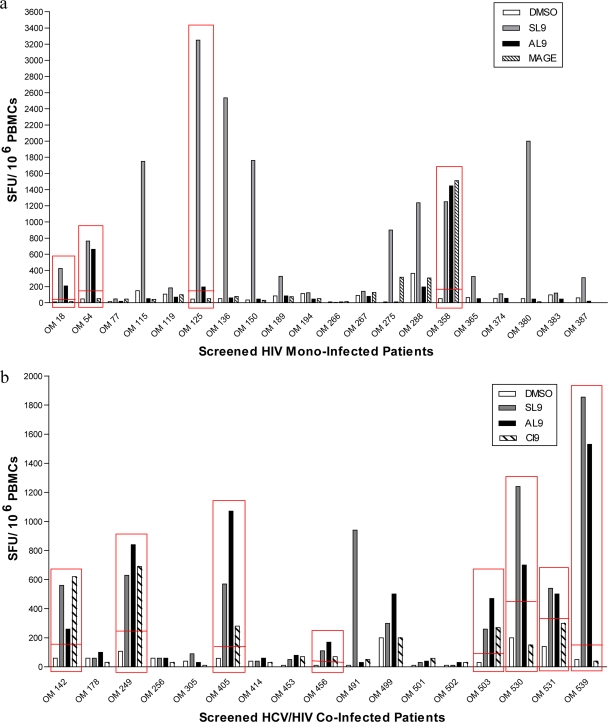
In order to examine if HIV-monoinfected individuals with an SL9-specific T-cell response would also respond to the HCV-AL9 antigen, 20 HLA-A2+, HIV-monoinfected individuals were screened for responses to both the SL9 and AL9 peptides, using IFN-γ ELISPOT assay. Of the HLA-A2+ HIV-monoinfected individuals, 15 subjects (75%) showed a response to SL9 peptide stimulation (more than 2-fold of the background). Of these individuals, 4 (26%) showed significant responses to the AL9 peptide as well, despite not being HCV infected (HCV antibody negative, HCV load negative). We refer to these individuals as HIV-infected dual responders. No AL9 responses were detectable in the absence of SL9 in these individuals. One individual (participant OM 358) also responded to a MAGE self-antigen peptide and could not be studied further due to a lack of additional samples (Fig. 1a). These findings suggest that the presence of an HCV-specific AL9 response in HIV-monoinfected individuals may be a result of cross-reaction of T cells specific to the HIV-SL9 epitope.
FIG. 1.
IFN-γ ELISPOT cross-reactivity screening assay. PBMCs from HLA-A2+, HIV-monoinfected (a) and HCV/HIV-coinfected (b) individuals were screened for their response to the SL9 and/or AL9 peptide by direct ex vivo IFN-γ ELISPOT assay. An irrelevant HLA-A2-restricted MAGE peptide (FLWGPRALV; FV9) was used as a control for the HIV monoinfection screen. HLA-A2-restricted peptide HCV-CI9 was used as a random control for the coinfection screen. A positive response was defined as one that was 2-fold higher than that for the DMSO background (presented by the red horizontal line) with a minimum of 50 spots per 106 PBMCs and is presented as the number of SFUs/million PBMCs.
We examined the prevalence and the magnitude of dual recognition of SL9 and AL9 antigens in HCV/HIV coinfection by screening 17 HLA-A2+ coinfected individuals by ELISPOT assay. Overall responses to SL9 peptide were weaker in magnitude among the screened coinfected individuals than among the HIV-monoinfected cohort. A total of 10 out of 17 coinfected individuals (59%) responded to SL9 antigen. Responses to AL9 peptide were observed in a total of 9 out of 17 coinfected individuals (53%). Of the coinfected individuals, 8 out of 17 (47%) had responses to both antigens (we refer to these individuals as HCV/HIV-coinfected dual responders) (Fig. 1b). Only one of the screened HIV/HCV-coinfected subjects (6%) showed an AL9-specific response in the absence of an SL9 response.
In a similar screening of a limited number of HLA-A2+ HCV-monoinfected individuals (n = 5), we observed no response to either the AL9 or SL9 peptide (data not shown). Our observation of a lack of HCV-AL9 response among the HCV-monoinfected subjects studied was consistent with the findings of a previous study demonstrating infrequent HCV-NS5b responses in HLA-A2+ individuals with chronic HCV infection (11). Lechner et al. demonstrated that 0/10 studied individuals who were chronically infected with HCV had CD8+ T cells detecting the HCV-NS5b (position 2594; AL9) epitope. We observed a greater frequency of responses to the HCV-AL9 epitope in HCV/HIV coinfection than in HCV monoinfection. The AL9 response was predominantly associated with the presence of the HIV-SL9 response, as shown by correlation analysis (see below).
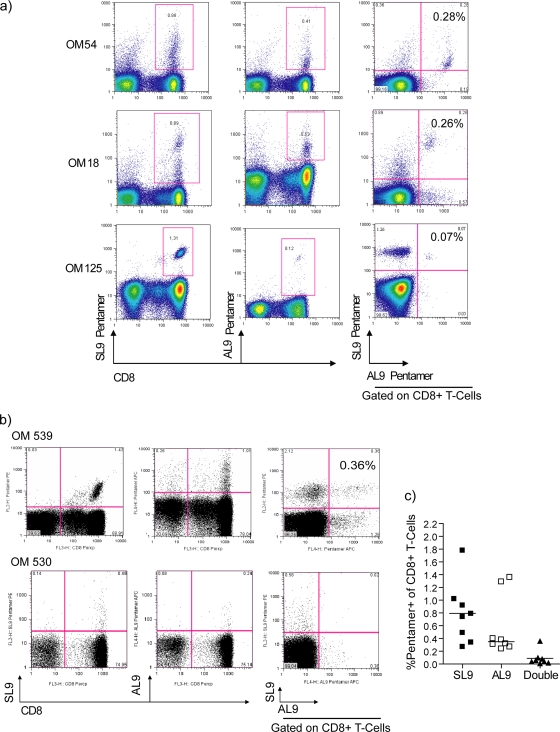
Detection of cross-reactive CD8+ T cells in identified dual responders.
In order to determine whether dual responses in the HIV-monoinfected group were due to true cross-reactivity by SL9-specific CD8+ T cells, PBMCs from the identified dual responders were directly stained ex vivo with the corresponding pentamers. All three HIV-monoinfected dual responders showed CD8+ T cells distinctly double stained for both pentamers, indicating the presence of a cross-reactive T-cell population (Fig. 2a). Similar pentamer staining was also conducted with PBMCs from HCV/HIV-coinfected dual responders. In contrast, only 1 of the 8 coinfected dual responders (OM 539) was identified to have T cells cross-recognizing both antigens. This individual had two populations identifying AL9 or SL9, with a small population being dual stained with both pentamers, thus being cross-reactive to both epitopes (Fig. 2b, upper plots). The majority of coinfected dual responders demonstrated separate, non-cross-reactive recognition of T cells directed against either epitope (Fig. 2b, lower panels). Compiled data of the percentages of pentamer-positive (pentamer+)/CD8+ T cells from all coinfected dual responders indicate that the cross-reactive T-cell population recognizing both HIV-SL9 and HCV-AL9 among HCV/HIV-coinfected individuals is infrequent, although populations recognizing either epitope are common (Fig. 2c). This indicates that in HCV/HIV coinfection, the majority of SL9- and AL9-specific CD8+ T cells are not cross-reactive to each other but mainly comprise T cells directed toward separate epitopes.
FIG. 2.
Pentamer binding analysis of antigen-specific CD8+ T cells from identified dual responders. PBMCs from individuals identified from the ELISPOT assay screen were stained with HLA-A2-restricted class I pentamers HIV-SL9 and HCV-AL9 and analyzed for the frequencies of dual-stained CD8+ T cells. (a) Flow cytometry plots from the three HIV-monoinfected dual responders. (b) Representative flow cytometry plots of two coinfected screened dual responders. The last plot on each row is gated on CD8+ T cells. (c) Compiled data from the percentages of single pentamer (stain with either SL9 or AL9 pentamer) and dual pentamer+/CD8+ T cells from all coinfected dual responders.
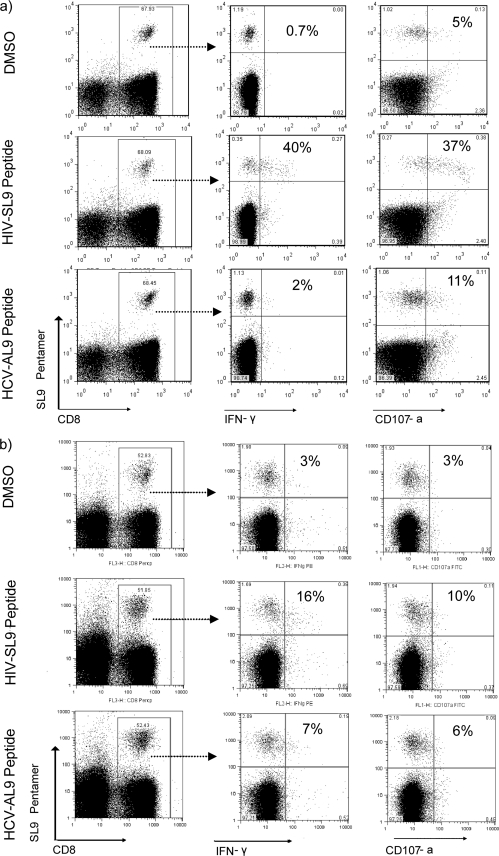
SL9-specific CD8+ T cells weakly respond to stimulation with the heterologous AL9 antigen.
To examine whether SL9-specific T cells could be activated by AL9 antigen, SL9 pentamer-stained CD8+ T cells from dual responders were assessed for IFN-γ and CD107a production by flow cytometry. Direct ex vivo stimulation of HIV-monoinfected PBMCs with either SL9 or AL9 peptide demonstrated that SL9 pentamer-positive CD8+ T cells responded to stimulation with not only their cognate peptide but also the heterologous HCV-AL9 peptide, as shown by IFN-γ and CD107a production (Fig. 3a). However, these cross-reactive responses to AL9 peptide were much weaker than those from SL9 peptide stimulation (percent CD8+/pentamer+ T cells in one representative [OM 125] for IFN-γ production, 40.8% for SL9 versus 1.9% for AL9; for CD107a production, 37.9% for SL9 versus 11.1% for AL9). Similar functional properties were observed in the only coinfected individual with a dual pentamer-stained population (OM 539), showing that SL9-specific CD8+ T cells are weakly responding to cross-reactive AL9 stimulation (percent CD8+/pentamer+ T cells for IFN-γ production, 15.59% for SL9 versus 7.14% for AL9) (Fig. 3b).
FIG. 3.
Functional properties of the cross-reactive T cells. PBMCs stained with HIV-SL9 pentamer were analyzed for IFN-γ and CD107a production after a 6-h stimulation with either the AL9 or SL9 peptide. Shown are representative flow cytometry plots of functional analysis of SL9-specific CD8+ T cells from blood of HIV-monoinfected dual responder OM 125 (a) and HCV/HIV-coinfected dual responder OM 539 (b). The last two columns were gated on SL9 pentamer+/CD8+ T cells. DMSO-treated PBMCs were used as a negative control, and responses greater than twice the DMSO response were considered positive.
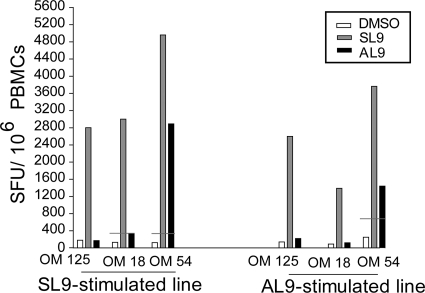
Substantial heterogeneity in expansion of the cross-reactive CD8+ T-cell population within SL9-generated T-cell lines from HIV-monoinfected blood.
In order to determine if HCV-AL9-specific CD8+ T cells could be expanded among HIV-monoinfected dual responders after in vitro peptide expansion, antigen-specific short-term T-cell lines were generated. ELISPOT assay analysis of the responses demonstrated that in one individual (OM 54), both SL9- and AL9-generated T-cell lines produced IFN-γ in response to stimulation with either peptide (Fig. 4). In the other two individuals, we could not expand functional AL9-specific T cells with either the cognate SL9 peptide or the cross-reactive AL9 antigen to a similar degree as we could with cells taken from OM 54. The compiled data analysis of the IFN-γ responses by antigen-specific T-cell lines of the three HIV-monoinfected dual responders demonstrated the presence of functional cross-reactive T cells in only one of these individuals (Fig. 4). The SL9-generated line from OM 18 showed a borderline-positive IFN-γ response to AL9 peptide stimulation. These individual variations depict the heterogeneity between individuals in the ability of T cells to expand in response to a cross-reactive epitope.
FIG. 4.
Analysis of T-cell cross-recognition after in vitro antigen-specific expansion. Shown are the compiled ELISPOT assay data on the frequencies of T cells producing IFN-γ in response to either the SL9 or AL9 peptide restimulation in short-term T-cell lines from the three HIV-monoinfected responders. The horizontal red lines show the threshold of positive response to both antigens.
SL9- and AL9-specific T-cell responses positively correlate with each other and with the clinical parameter of liver injury in HCV/HIV-coinfected responders.
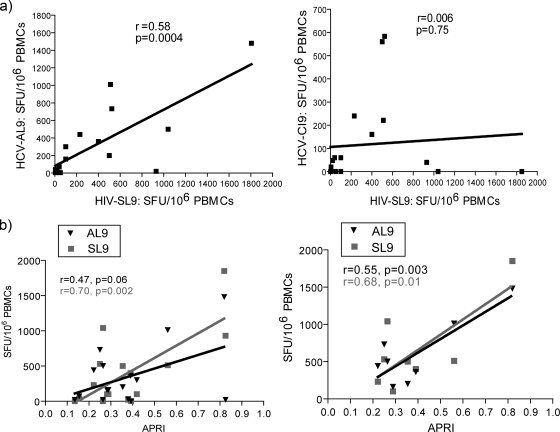
To understand whether the degree of T-cell responses to HIV-SL9 antigen is associated with the responses to HCV-AL9 peptide, correlation analysis of the direct ex vivo IFN-γ ELISPOT assay responses from all studied coinfected individuals was performed. A positive correlation between these two types of responses was observed (r = 0.58, P = 0.0004). No correlation between responses to HIV-SL9 antigen and to a less similar HLA-A2-restricted HCV epitope, the HCV-CI9 peptide, was found (Fig. 5a). Among dual-responding coinfected individuals, no correlations between either the SL9- or AL9-specific response and the HIV or HCV load were observed, although HCV loads were substantially high in all coinfected individuals.
FIG. 5.
Analysis of correlation among different antigen-specific responses and their association with parameter of disease progression in HCV/HIV-coinfected individuals. (a) Correlation analyses between the magnitudes of IFN-γ responses specific for HIV-SL9 and HCV-AL9 (left panel) and HCV-CI9 and HIV-SL9 (right panel) among all studied coinfected individuals. All data are presented after subtraction of the background. (b) Correlation analyses between antigen-specific IFN-γ responses and APRI scores among all studied coinfected individuals (left panel) and coinfected dual responders (right panel). Correlations were analyzed using the Spearman rank test, and the APRI score was calculated according to the following formula (23): [(AST level/ULN)/platelet count] × 100, where ULN is the upper level of normal of the AST level, or 56 IU/liter.
To investigate the clinical relevance of our findings, we examined if there is a correlation between the magnitude of SL9- and AL9-specific T-cell responses in coinfected individuals with their corresponding aspartate aminotransferase (AST)-to-platelet ratio index (APRI) score as a clinical indicator of the stage of liver fibrosis (2), in which a higher APRI score correlates with greater hepatic fibrosis. We first looked at this correlation among all the studied coinfected individuals. The magnitude of IFN-γ responses specific for HIV-SL9 correlated positively with the corresponding APRI score of each individual (r = 0.70, P = 0.002). A trend toward a positive correlation was observed for HCV-AL9-specific responses (Fig. 5b, left panel). We then looked at this correlation among coinfected dual responders only and observed that responses specific for both HIV-SL9 and HCV-AL9 correlated positively with the APRI score of each individual (r = 0.68, P = 0.01 for SL9; r = 0.55, P = 0.03 for AL9) (Fig. 5b, right panel).
DISCUSSION
The overall impact of T-cell cross-recognition of heterologous human infections is not clearly defined but has been suggested to skew the immunopathogenesis outcome (5, 21). Several mechanisms that largely explain the ability of T-cell receptors to cross-recognize different unrelated antigens are currently identified (26). Molecular mimicry, where ligands share key structural features, is one of the first mechanisms proposed for T-cell cross-reactivity (15).
The present study started out with a focus on the role of molecular mimicry in T-cell cross-recognition. We elaborated on T-cell cross-recognition of two well-defined HIV and HCV epitopes during HCV/HIV coinfection. We provided evidence that in HIV infection and in the absence of HCV infection, CD8+ T cells that are specific for the immunogenic HIV epitope SL9 recognize the sequentially similar HCV-AL9 antigenic peptide. However, this interaction seems to be of low affinity, as shown by limited production of cytokine IFN-γ and degranulation marker CD107a by these cells in response to the heterologous HCV-AL9 peptide. This was further supported by functional analysis of antigen-stimulated lines generated from PBMCs from HIV-infected individuals, in which the AL9 peptide weakly expanded the number of cross-reactive CD8+ T cells. Although pentamer staining could have interfered with the ability to stimulate stained T cells with the heterologous epitopes used in our assays, we had confirmed those findings by demonstrating weak induction of heterologous responses with independent peptide stimulations without pentamer costaining, hence ruling out the effect of potential pentamer interference. Our findings are also in line with those recently described by Kasprowicz et al., in which CD8+ T cells specific to an HCV-NS3 epitope weakly cross-reacted with an influenza virus neuraminidase epitope, producing low-affinity and weak responses to the latter epitope (9).
Contrary to our original hypothesis, our data suggest that coinfected individuals with higher HIV-SL9 T-cell responses mount higher HCV-AL9 responses as well, and these were more frequent than the responses observed in individuals with HCV monoinfection. This may suggest that the coexistence of the SL9 response did not prevent the acquisition of an AL9 response but, rather, helped its induction. Surprisingly, these apparently cross-reactive responses to AL9 in coinfected individuals were more often due to separate T-cell populations, determined by pentamer staining, although truly cross-reactive T cells could also be seen. It is possible that the separate T-cell population identified by the AL9 pentamer could represent a cross-reactive SL9 population with such poor avidity to the AL9 epitope as to abrogate binding to the AL9 pentamer. In this regard, Kasprowicz et al. were able to identify such populations using MHC class I tetramers (CD8hi tetramers) with an enhanced capacity to bind (9) to certain cross-reactive epitopes. We, however, did not see any improvement in the avidity of the SL9 staining with CD8hi tetramers directed to the AL9 epitope (data not shown). In addition, Kasprowicz et al. (9) also reported no difference in the staining and appearance of AL9-specific CD8+ T cells between normal and CD8hi tetramers, which supports the findings that T-cell responses to the HCV-AL9 epitope are defined as high-affinity responses (11). Given that we identified two separate populations in some individuals, it is possible that HIV epitopes other than SL9 could induce AL9 responses through alternative recognition of different TCR determinants in this situation. Further exploration is warranted to examine this possibility. Nevertheless, our findings suggest that exposure to the SL9 epitope expands SL9-specific T cells with TCRs that may cross-react with the AL9 antigen but may also expand T cells with low to no affinity for SL9 but with affinity to AL9, hence generating two populations responding to separate pentamers. These findings are consistent with previous observations noted with Epstein-Barr virus (EBV) infection in humans, in which acute EBV infection is shown to alter the T-cell-receptor repertoire of the memory T-cell population (5, 13). Upon infection of a heterologous virus, these previously expanded cells would then be recruited to respond to the heterologous virus (5, 13). Further studies with our population would have to be performed to determine if similar V or J beta recombination is used by SL9- and AL9-specific T cells in our cohort.
We also observed heterogeneity in the functional nature of cross-reactive T cells in coinfected individuals. For example, in only one of three individuals tested were we able to show successful in vitro expansion of cross-reactive CD8+ T cells. The expanded population from this individual showed similar functionality in response to stimulation with either the HIV-SL9 or HCV-AL9 peptide. These findings suggest that depending on the private specificity of the TCR of an individual, cross-reactive T cells with various avidities to the heterologous epitope and with various functionalities can be induced.
The main question, however, is whether the consequences of this T-cell cross-recognition in HCV/HIV coinfection affect the HCV disease outcome in the host. T-cell cross-reactivity could result in two scenarios: (i) the T-cell responses in coinfected individuals could become skewed, as described for “original antigenic sin” (6, 10), leading to T-cell responses mainly to the initial pathogen. Hypothetically, this would lead to a loss of responses to either the HCV-AL9 or HIV-SL9 antigen. (ii) HIV infection could prime for the induction of heterologous HCV responses that would not have occurred without exposure to HIV by recruiting TCRs that could potentially cross-react with HCV. This may lead to the generation of functionally weaker T-cell responses toward the heterologous HCV-AL9 in those coinfected individuals with a detectable HIV-SL9 response due to recruitment of TCRs of weaker specificities for AL9 compared to the responses in those who do not mount an SL9-specific T-cell response.
Original antigenic sin, as referred to in the first scenario, was first described as a strong humoral response to an original influenza virus strain in humans but a modest response to a following influenza virus infection with a heterologous strain (6). Later, Klenerman and Zinkernagel demonstrated this phenomenon in cytotoxic T lymphocytes in mouse lymphocytic choriomeningitis virus infection (10). According to this phenomenon, the immune response to a current infection may be dominated by T cells targeting epitopes of a previously encountered pathogen. By virtue of the fact that this has been shown in other viral coinfections (21), this could result in a loss of breadth of responses toward HCV after an encounter with HIV and lead to progression of HCV disease. However, we did not observe this for the AL9 and SL9 epitopes and, in fact, found a positive correlation between the magnitudes of responses against the two described antigens.
The second scenario could potentially be advantageous if the presence of HIV infection would have primed T cells to respond to similar HCV epitopes but could be disadvantageous if the resulting responses to the new virus were of low avidity. The generation of weak heterologous responses mentioned in the second scenario could hypothetically result in an opportunity for HCV to escape and also lead to more severe HCV disease.
To further address the above scenarios, we used APRI scoring for validation of the clinical significance of our findings. APRI scores have been used as a noninvasive means for estimating liver fibrosis and reportedly have high sensitivities and specificities and prognostic values similar to those of the established model of end-stage liver disease (MELD) in HCV infection, with or without HIV coinfection (1, 2, 12, 17). Our observation that among the studied coinfected subjects the highest APRI score, which was detected for the liver belonging to the only individual with dual, cross-recognizing CD8+ T cells, may suggest a negative role that the presence of these cross-reactive T cells may play in advancing HCV-related liver disease in HCV/HIV coinfection. Antigen-specific lines generated from PBMCs of the same individual were shown to mount the highest magnitude of IFN-γ response to both the SL9 and AL9 antigens. We have previously shown that functional HIV-specific T cells accumulate in the liver in HCV/HIV coinfection (22). Accordingly, the presence of these cross-reactive CD8+ T cells in the blood may not be strong enough to facilitate HCV clearance, but the trafficking of these T cells to the liver and their dual response to both HIV and HCV antigens may negatively affect the cytokine milieu of the liver and add to the initial tissue damage. How exactly the cross talk between these two viral epitopes may affect HCV-related liver pathogenesis requires additional investigation with a much larger cohort of HLA-A2+ coinfected individuals.
In conclusion, our findings provide evidence for some degree of T-cell cross-reactivity between two immunogenic HIV and HCV epitopes. Although cross-recognition by the same T cells seems to be an infrequent event in HCV/HIV coinfection, it might be partially responsible for the clinical outcome of HCV disease in coinfection with HIV. It would be an underestimation to consider only structural similarities of epitopes to be responsible for T-cell cross-reactivity between HIV and HCV. A thorough analysis is warranted to identify the extent of this phenomenon in these two viral infections and its impact on disease outcome.
Acknowledgments
This research was supported by funds from the Canadian Institute for Health Research (CIHR). M.A.O. has salary support from the Ontario HIV Treatment Network (OHTN). B.V. acknowledges scholarship funding from the National Canadian Research Training Program (NCRTP) in Hepatitis C.
We are grateful to the patients who contributed their time and effort to this study.
We have no competing interests.
Footnotes
Published ahead of print on 27 October 2010.
REFERENCES
- 1.Al-Mohri, H., C. Cooper, T. Murphy, and M. B. Klein. 2005. Validation of a simple model for predicting liver fibrosis in HIV/hepatitis C virus-coinfected patients. HIV Med. 6:375-378. [DOI] [PubMed] [Google Scholar]
- 2.Borsoi Viana, M. S., K. Takei, D. C. Collarile Yamaguti, B. Guz, and E. Strauss. 2009. Use of AST platelet ratio index (APRI score) as an alternative to liver biopsy for treatment indication in chronic hepatitis C. Ann. Hepatol. 8:26-31. [PubMed] [Google Scholar]
- 3.Brehm, M. A., A. K. Pinto, K. A. Daniels, J. P. Schneck, R. M. Welsh, and L. K. Selin. 2002. T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat. Immunol. 3:627-634. [DOI] [PubMed] [Google Scholar]
- 4.Chen, W., K. A. Masterman, S. Basta, S. M. Haeryfar, N. Dimopoulos, B. Knowles, J. R. Bennink, and J. W. Yewdell. 2004. Cross-priming of CD8+ T cells by viral and tumor antigens is a robust phenomenon. Eur. J. Immunol. 34:194-199. [DOI] [PubMed] [Google Scholar]
- 5.Clute, S. C., L. B. Watkin, M. Cornberg, Y. N. Naumov, J. L. Sullivan, K. Luzuriaga, R. M. Welsh, and L. K. Selin. 2005. Cross-reactive influenza virus-specific CD8+ T cells contribute to lymphoproliferation in Epstein-Barr virus-associated infectious mononucleosis. J. Clin. Invest. 115:3602-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fazekas de St. Groth and R. G. Webster. 1966. Disquisitions of original antigenic sin. I. Evidence in man. J. Exp. Med. 124:331-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goulder, P. J., M. A. Altfeld, E. S. Rosenberg, T. Nguyen, Y. Tang, R. L. Eldridge, M. M. Addo, S. He, J. S. Mukherjee, M. N. Phillips, M. Bunce, S. A. Kalams, R. P. Sekaly, B. D. Walker, and C. Brander. 2001. Substantial differences in specificity of HIV-specific cytotoxic T cells in acute and chronic HIV infection. J. Exp. Med. 193:181-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham, C. S., L. R. Baden, E. Yu, J. M. Mrus, J. Carnie, T. Heeren, and M. J. Koziel. 2001. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin. Infect. Dis. 33:562-569. [DOI] [PubMed] [Google Scholar]
- 9.Kasprowicz, V., S. M. Ward, A. Turner, A. Grammatikos, B. E. Nolan, L. Lewis-Ximenez, C. Sharp, J. Woodruff, V. M. Fleming, S. Sims, B. D. Walker, A. K. Sewell, G. M. Lauer, and P. Klenerman. 2008. Defining the directionality and quality of influenza virus-specific CD8+ T cell cross-reactivity in individuals infected with hepatitis C virus. J. Clin. Invest. 118:1143-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klenerman, P., and R. M. Zinkernagel. 1998. Original antigenic sin impairs cytotoxic T lymphocyte responses to viruses bearing variant epitopes. Nature 394:482-485. [DOI] [PubMed] [Google Scholar]
- 11.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nunes, D., C. Fleming, G. Offner, D. Craven, O. Fix, T. Heeren, M. J. Koziel, C. Graham, S. Tumilty, P. Skolnik, S. Stuver, C. Robert Horsburgh, Jr., and D. Cotton. 2010. Noninvasive markers of liver fibrosis are highly predictive of liver-related death in a cohort of HCV-infected individuals with and without HIV infection. Am. J. Gastroenterol. 105:1346-1353. [DOI] [PubMed] [Google Scholar]
- 13.Selin, L. K., M. A. Brehm, Y. N. Naumov, M. Cornberg, S. K. Kim, S. C. Clute, and R. M. Welsh. 2006. Memory of mice and men: CD8+ T-cell cross-reactivity and heterologous immunity. Immunol. Rev. 211:164-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selin, L. K., M. Y. Lin, K. A. Kraemer, D. M. Pardoll, J. P. Schneck, S. M. Varga, P. A. Santolucito, A. K. Pinto, and R. M. Welsh. 1999. Attrition of T cell memory: selective loss of LCMV epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity 11:733-742. [DOI] [PubMed] [Google Scholar]
- 15.Selin, L. K., S. R. Nahill, and R. M. Welsh. 1994. Cross-reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses. J. Exp. Med. 179:1933-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selin, L. K., S. M. Varga, I. C. Wong, and R. M. Welsh. 1998. Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J. Exp. Med. 188:1705-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shastry, L., T. Wilson, S. Lascher, and J. A. Nord. 2007. The utility of aspartate aminotransferase/platelet ratio index in HIV/hepatitis C-co-infected patients. AIDS 21:2541-2543. [DOI] [PubMed] [Google Scholar]
- 18.Sheth, P. M., S. Sunderji, L. Y. Shin, A. Rebbapragada, S. Huibner, J. Kimani, K. S. Macdonald, E. Ngugi, J. J. Bwayo, S. Moses, C. Kovacs, M. Loutfy, and R. Kaul. 2008. Coinfection with herpes simplex virus type 2 is associated with reduced HIV-specific T cell responses and systemic immune activation. J. Infect. Dis. 197:1394-1401. [DOI] [PubMed] [Google Scholar]
- 19.Thein, H. H., Q. Yi, G. J. Dore, and M. D. Krahn. 2008. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS 22:1979-1991. [DOI] [PubMed] [Google Scholar]
- 20.Townsend, A. R., J. Rothbard, F. M. Gotch, G. Bahadur, D. Wraith, and A. J. McMichael. 1986. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell 44:959-968. [DOI] [PubMed] [Google Scholar]
- 21.Urbani, S., B. Amadei, P. Fisicaro, M. Pilli, G. Missale, A. Bertoletti, and C. Ferrari. 2005. Heterologous T cell immunity in severe hepatitis C virus infection. J. Exp. Med. 201:675-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vali, B., F. Y. Yue, R. B. Jones, P. M. Sheth, R. Kaul, M. R. Betts, D. Wong, C. Kovacs, M. Loutfy, A. Common, R. Halpenny, and M. A. Ostrowski. 2008. HIV-specific T-cells accumulate in the liver in HCV/HIV infection. PLoS One 3:e3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wai, C. T., J. K. Greenson, R. J. Fontana, J. D. Kalbfleisch, J. A. Marrero, H. S. Conjeevaram, and A. S. Lok. 2003. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 38:518-526. [DOI] [PubMed] [Google Scholar]
- 24.Wedemeyer, H., E. Mizukoshi, A. R. Davis, J. R. Bennink, and B. Rehermann. 2001. Cross-reactivity between hepatitis C virus and influenza A virus determinant-specific cytotoxic T cells. J. Virol. 75:11392-11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welsh, R. M., and L. K. Selin. 2002. No one is naive: the significance of heterologous T-cell immunity. Nat. Rev. Immunol. 2:417-426. [DOI] [PubMed] [Google Scholar]
- 26.Yin, Y., and R. A. Mariuzza. 2009. The multiple mechanisms of T cell receptor cross-reactivity. Immunity 31:849-851. [DOI] [PubMed] [Google Scholar]