Abstract
The HIV envelope (Env) protein uses a dense coat of glycans to mask conserved domains and evade host humoral immune responses. The broadly neutralizing antibody 2G12, which binds a specific cluster of high-mannose glycans on HIV Env, shows that the glycan shield can also serve as a target for neutralizing antibodies. We have described a triple mutant Saccharomyces cerevisiae strain that expresses high-mannose glycoproteins that bind to 2G12. When used to immunize rabbits, this yeast elicits antibodies that bind to gp120-associated glycans but fail to neutralize virus. Here we sought to determine the reason for these discordant results. Affinity purification of sera over columns conjugated with three 2G12-reactive yeast glycoproteins showed that these proteins could adsorb 80% of the antibodies that bind to gp120 glycans. Despite binding to monomeric gp120, these mannose-specific antibodies failed to bind cell surface-expressed trimeric Env. However, when Env was expressed in the presence of the mannosidase inhibitor kifunensine to force retention of high-mannose glycans at all sites, the purified antibodies gained the abilities to bind trimeric Env and to strongly and broadly neutralize viruses produced under these conditions. Combined, these data show that the triple mutant yeast strain elicits antibodies that bind to high-mannose glycans presented on the HIV envelope, but only when they are displayed in a manner not found on native Env trimers. This implies that the underlying structure of the protein scaffold used to present the high-mannose glycans may be critical to allow elicitation of antibodies that recognize trimeric Env and neutralize virus.
Despite the isolation of rare antibodies from human immunodeficiency virus (HIV)-infected patients that potently neutralize a broad range of HIV strains in vitro and are protective in macaque models (3, 13-15, 20-23, 30), a vaccine immunogen has yet to be designed that can efficiently elicit broadly neutralizing antibodies against HIV. Characterization of broadly neutralizing antibodies isolated from patients has revealed four conserved epitopes on the HIV envelope (Env) protein. These include the CD4 binding site (5, 9), a high-mannose glycan cluster on the outer domain of gp120 (31), the membrane-proximal external region of gp41 (24, 35), and a newly defined epitope composed of conserved elements in the V2 and V3 loops, including an essential glycan (32). These epitopes can serve as models for the design of vaccine scaffolds that attempt to mimic the desired epitope in an immunogenic context, with the hope of eliciting broadly neutralizing antibodies.
The monoclonal antibody (MAb) 2G12 recognizes terminal α1,2-linked mannose residues in the context of a cluster of conserved Man8 and Man9 glycans on the gp120 subunit, with no obvious recognition of the underlying polypeptide (6, 25, 27). An important characteristic of 2G12 is its unusual structure, which consists of domain-exchanged heavy chain F(ab′)2 arms that create a third antigen binding site between the two standard antigen binding regions (7). It has been hypothesized that this structure allows 2G12 to bind multiple high-mannose glycans on Env with high affinity and thus to neutralize virus. Crystal structures of 2G12 bound to free carbohydrates exist (7), but no structure of 2G12 bound to HIV Env has been achieved, leaving the exact details of 2G12 binding to Env unknown.
Several strategies have been used to create immunogens that mimic the 2G12 glycan epitope. In each case, the goal is to present a multivalent array of oligomannose glycans bearing terminal Manα1,2-Man moieties in an immunogenic context. One approach involves conjugation of oligomannose (Man4-9) carbohydrates, singly or as oligodendrons, to carrier proteins with multiple conjugation sites, such as bovine serum albumin (BSA), cyclic peptides, or viral capsid proteins (1, 2, 16, 17, 33, 34). An alternative approach employs modification of glycosylation in yeast or mammalian cells to force retention of high-mannose glycans on natural proteins (11, 18, 19, 28). We used the latter approach to produce a triple mutant (TM) Saccharomyces cerevisiae strain that expresses almost exclusively Man8 glycans on its surface. MAb 2G12 binds to several highly glycosylated TM yeast proteins, and immunization of rabbits with whole TM yeast elicits anti-mannose antibodies that efficiently cross-react with gp120 proteins from diverse HIV strains but fail to neutralize HIV virions (19).
In this study, we sought to determine why the anti-glycan antibodies elicited by TM yeast fail to neutralize HIV virions despite efficient binding to monomeric gp120. Glycan array analysis and Env binding assays showed that while α1,2-linked mannose residues are the primary targets of the antibodies, these antibodies recognize high-mannose glycans that lie outside the 2G12 epitope on monomeric gp120. These high-mannose glycans are either not present or exist in a different orientation on trimeric Env spikes, resulting in a lack of trimer binding and the inability to neutralize wild-type virus. However, when trimeric Env was forced to express high-mannose glycans at all N-linked sites, TM yeast-elicited antibodies gained the ability to bind trimeric envelope and to neutralize a broad array of HIV-1 strains. Rational deletion of glycans not essential for 2G12 binding to TM yeast glycoproteins (YGPs) may allow presentation of oligomannose glycans in a more relevant conformation and elicitation of antibodies that recognize wild-type trimeric HIV Env.
MATERIALS AND METHODS
Materials.
Monoclonal antibodies 2G12 and b12 were obtained from the IAVI Neutralizing Antibody Consortium repository. The AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, supplied the following reagents: U87.CD4.CCR5, U87.CD4.CXCR4, and TZM-BL cell lines, HIV immunoglobulin (HIV Ig), the protease inhibitor indinavir sulfate, and gp120 proteins from HIV-1 strains 96ZM651 and 93TH975. HIV-1 and simian immunodeficiency virus (SIV) gp120 proteins from all other strains were expressed in 293T cells by use of recombinant vaccinia virus vectors and were purified by Galanthus nivalis lectin-agarose (Vector Laboratories) affinity chromatography as previously described (29). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin G (IgG) antibody (111-035-144) and HRP-conjugated goat anti-human IgG antibody (709-035-149) were purchased from Jackson ImmunoResearch. A phycoerythrin (PE)-conjugated F(ab′)2 fragment of goat anti-rabbit IgG antibody (A10542) and a PE-conjugated F(ab′)2 fragment of goat anti-human IgG κ-specific antibody (H16104) were purchased from Invitrogen.
Immunizations.
Three groups of 4 to 6 New Zealand White rabbits were immunized with whole Δoch1 Δmnn1 Δmnn4 TM yeast cells at the following doses: group 1 received 3 × 107 heat-killed cells (70°C for 1 h) per 1-ml dose, group 2 received 1 × 108 heat-killed cells per 1-ml dose, and group 3 received 3 × 107 live cells per 1-ml dose. Rabbits were injected in the marginal ear vein with 1 ml of yeast cells in sterile 0.9% NaCl 3 times per week for 14 weeks. Rabbits were bled at weeks 0 (preimmunization), 3, 5, 7, 9, 11, and 13, 3 days after the last injection from the prior week, and at week 14, 7 days after the final injection. Sera were stored at −80°C in the absence of preservatives.
Column purification.
IgG was isolated from serum by affinity purification with either protein A or 2G12-reactive yeast glycoproteins, with a separate column used for each animal. For protein A purification, 2 ml of pooled sera from weeks 9, 11, 13, and 14 was applied to 0.5-ml protein A agarose columns (Invitrogen) for 1 h at room temperature (RT). For yeast glycoprotein column purification, three yeast glycoproteins (Ecm33, Gp38, and PstI) were purified from Δoch1 Δmnn1 Δmnn4 TM yeast and conjugated to AminoLink columns (Invitrogen) per the manufacturer's protocol. Thirty milliliters of pooled sera from weeks 9, 11, 13, and 14 was incubated overnight at 4°C on 1-ml yeast glycoprotein columns. Flowthrough, washes, and eluates were collected by gravity flow, and bound antibodies were eluted stepwise using 100 mM glycine at pH 3.0, 2.5, and 2.0. The antibody concentration was determined by measuring the optical density at 280 nm (OD280) (IgG concentration [mg/ml] = OD280/1.4), and samples were adjusted to a final IgG concentration of 1 mg/ml.
Immunoprecipitation and Western blotting.
One microgram of JRFL or R2 HIV-1 gp120 was mixed with 5 μg/ml protein A-purified prebleed or YGP-purified postimmunization serum from rabbit 1226 or 1238 or with 2 μg/ml 2G12 in phosphate-buffered saline (PBS). Samples were shaken overnight at 4°C. Twenty-five microliters of PBS-washed protein A-Sepharose 4B (Invitrogen) beads was mixed with the gp120-antibody sample and rotated at room temperature for 30 min. Following centrifugation at 14,000 rpm for 30 s, the supernatant was removed and the beads washed with Tris-buffered saline-Tween 20 (TBS-T) and PBS (twice each). Following the final centrifugation, the beads were resuspended in SDS sample buffer, boiled for 5 min, and loaded onto a 10% polyacrylamide gel for electrophoresis. Western blotting for detection of precipitated gp120 was conducted using rabbit anti-gp120 polyclonal antibody 3824 (1 μg/ml), which was raised against a synthetic peptide, or 2G12 at 0.5 μg/ml.
ELISA.
Enzyme-linked immunosorbent assay (ELISA) was used to measure binding of YGP-purified sera and 2G12 to monomeric gp120 proteins. Briefly, purified gp120 at 150 ng per well was adsorbed onto Immulon 2HB 96-well plates (Thermo Labsystems) in 100 μl ELISA capture buffer (0.15 M sodium carbonate, 0.35 M sodium bicarbonate in PBS, pH 9.6) and incubated overnight at 4°C. Wells were washed three times with 200 μl PBS-0.05% Tween and blocked with 200 μl 2% BSA in PBS for 1 h at RT. YGP-purified sera normalized for IgG content were added to the plate at 10 μg/ml and 100 μl/well and allowed to bind for 2 h at RT on an orbital shaker. After three washes, 100 μl HRP-conjugated goat anti-rabbit IgG secondary antibody (Jackson ImmunoResearch) was added at a 1:10,000 dilution in blocking buffer and allowed to bind for 1 h at RT. Following three final washes, 100 μl of 3,3′,5,5′-tetramethylbenzidine solution was added to each well and incubated for 2 min at 37°C. The reaction was stopped by adding 100 μl 1 M phosphoric acid per well, and the OD450 was measured using an MRX Revelation microplate reader (Dynex Technologies). Fifty percent effective concentrations (EC50s) were calculated using GraphPad Prism 4.0 (GraphPad Software, Inc.). Binding of 2G12 at 2 μg/ml to monomeric gp120 was assessed in parallel, using HRP-conjugated goat anti-human IgG secondary antibody at a 1:10,000 dilution for detection. Equal loading of gp120 proteins of each strain was confirmed using a gp120-specific polyclonal rabbit serum (1170) produced in our lab by immunization with recombinant JRFL gp120 protein.
To determine if YGP-purified rabbit sera could compete with 2G12 for binding to gp120, ELISA was performed as described above, with a few modifications. Wells were washed and blocked with a volume of 250 μl; 100-μl aliquots of protein A-purified preimmune and YGP-purified postimmune sera were added at 50 μg/ml and allowed to bind for 2 h at RT on an orbital shaker. MAb 2G12 or b12 (as a noncompeting control) was then added to wells at either 0.5 μg/ml or 0.1 μg/ml and allowed to bind for 20 min at RT on an orbital shaker. Polyclonal rabbit serum 1170 at a 1:100 dilution was used as a positive competing control. Wells were washed, and binding of 2G12 or b12 was detected using HRP-conjugated goat anti-human IgG antibody (Jackson ImmunoResearch) at a 1:10,000 dilution. Control wells were included to confirm binding of rabbit sera, which was detected using HRP-conjugated goat anti-rabbit IgG antibody (Jackson ImmunoResearch).
Glycan microarray analysis.
Glycan array analysis was used to characterize the glycan binding profiles of affinity-purified sera. Protein A-purified preimmune (week 0), protein A-purified postimmune (weeks 9 to 14), and YGP-purified postimmune (weeks 9 to 14) sera from 4 (protein A-purified preimmune and postimmune sera) or 6 (YGP-purified sera) rabbits were tested for binding to 465 synthetic and natural glycans (mammalian printed array, version 4.1; Consortium for Functional Glycomics [CFG; http://www.functionalglycomics.org/static/consortium/resources/resourcecoreh8.shtml]) printed on glass slides following a standard protocol as previously described (4). This work was done by Core H of the CFG at Emory University. Purified sera normalized for IgG concentration were tested at 10 μg/ml in PBS. Briefly, antibody samples were applied to the printed surface of the microarray and incubated in a humidified chamber for 1 h. The slide was then rinsed 4 times with PBS, followed by addition of fluorescently labeled (Alexa Fluor 488) anti-rabbit IgG, and was incubated for 1 h. The slide was then rinsed 4 times in PBS, and fluorescence was measured with a Perkin-Elmer microarray XL4000 scanner and analyzed using Imagene software (BioDiscovery).
Cell surface trimer binding assays.
QT6 (Japanese quail fibrosarcoma) cells were infected with recombinant vaccinia viruses expressing T7 polymerase (vTF1.1), JRFL gp160 (vCB28), or LAI-BH8 gp160ΔCt (vPE17), which contains a 104-amino-acid cytoplasmic tail truncation that increases cell surface expression levels (12), at a multiplicity of infection (MOI) of 10 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 2.5% fetal bovine serum (FBS), 60 μg/ml penicillin, and 100 μg/ml streptomycin for 1 h at 37°C. Vaccinia virus was removed and replaced with DMEM containing 10% FBS, 60 μg/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine (QT6 medium), and the cells were incubated at 37°C for 24 h. For retention of high-mannose glycans on oligomeric Env, cells were treated as described above, with the addition of 25 μg/ml kifunensine (CalBiochem) to the final incubation medium after vaccinia virus removal; this concentration of drug had no visible cytotoxic effect. The next day, cells were harvested by pipetting and washed with fluorescence-activated cell sorter (FACS) buffer (5% FBS, 0.05% sodium azide in PBS), followed by addition of antibodies. Antibodies were incubated with cells in FACS buffer at 10 μg/ml for 1 h at 4°C, washed with FACS buffer, and stained with phycoerythrin-conjugated goat anti-human IgG κ-specific F(ab′)2 (for detection of 2G12 and b12) or with phycoerythrin-conjugated goat anti-rabbit IgG F(ab′)2 (for detection of rabbit sera) in 100 μl for 30 min at 4°C. Binding was analyzed by flow cytometry on a FACSCalibur flow cytometer (Becton Dickinson), and data were analyzed using FlowJo software (Tree Star Inc.). CEMx174 cells chronically infected with HIV-1 strain R3A (and thus constitutively expressing Env) were kindly provided by James Hoxie (University of Pennsylvania) and were screened for serum binding as described above.
Neutralization assays.
Luciferase-reporter viral pseudotypes competent for a single round of infection were produced in 293T cells by cotransfection of the NL4-3-based luciferase vector pNL-luc (Env− Vpr+) with plasmids expressing the desired Env protein, as previously described (8), and were used to infect U87.CD4.CCR5 or U87.CD4.CXCR4 cells. Viruses produced in the presence of kifunensine were treated as described above, with the addition of 25 μg/ml kifunensine to the final incubation medium. Kifunensine treatment resulted in lower infectivity of pseudoviral stocks, which decreased 40 to 90%, in a strain-dependent manner. Replication-competent viruses produced in human peripheral blood mononuclear cells (PBMCs) were obtained from the University of Pennsylvania Center for AIDS Research (CFAR) viral isolate repository and were used to infect TZM-BL cells. Viral titers were normalized by p24 content (Cell BioLabs, Inc.), and a linear range of infection for each virus was determined. For neutralization assays, 5 ng of virus was incubated with monoclonal antibodies, human serum, or rabbit serum for 1 h at 37°C and then spin inoculated onto cells at 500 × g for 2 h at RT. Cells were transferred to 37°C and assayed for luciferase expression at 72 h postinfection, using Bright-Glo substrate (Promega) and an MLX Revelation microtiter plate luminometer (Dynex Technologies). Neutralization assays of replication-competent virus on TZM-BL cells were performed as described above, except that a 1 μM concentration of the protease inhibitor indinavir sulfate was included in the final incubation medium to prevent multiple rounds of infection, no spin inoculation was performed, and luciferase levels were measured at 48 h postinfection.
RESULTS
Purification of yeast-elicited gp120 cross-reactive antibodies.
In a previous study, we described the creation of a yeast strain lacking three carbohydrate processing enzymes (Δoch1 Δmnn1 Δmnn4) that expresses primarily Man8 glycans on its surface glycoproteins. Immunization of rabbits with whole TM yeast cells elicited antibodies that cross-reacted with high-mannose glycans on gp120 proteins derived from diverse HIV-1 and SIV strains but failed to neutralize virus (19). In the present study, we sought to determine why these antisera failed to neutralize virus despite binding efficiently to gp120 glycans. To increase the number of sera available for analysis, we completed a second immunization trial with three dose regimens. Three groups of 4 to 6 rabbits each were immunized intravenously, 3 times per week for 14 weeks, with 3 × 107 heat-killed TM yeast cells, 1 × 108 heat-killed TM yeast cells, or 3 × 107 live TM yeast cells per 1-ml dose. Serum samples collected at weeks 0 (prebleed), 3, 5, 7, 9, 11, 13, and 14 were tested for gp120 reactivity by ELISA, using recombinant gp120 proteins derived from the primary clade B HIV-1 strains JRFL, R2, and R3A. All postimmunization sera contained antibodies that bound specifically to each of these gp120 proteins, and pooled week 9 to 14 sera from the two animals in each group exhibiting the highest titers of anti-gp120 antibodies were chosen for further study (data not shown).
Our previous work showed that the broadly neutralizing antibody 2G12 specifically recognizes at least five endogenous TM yeast glycoproteins under denaturing Western blot conditions (18, 19). To determine if these glycoproteins could be used to purify mannose-specific antibodies that cross-react with HIV-1 gp120 glycans, we purified the three most highly 2G12-reactive YGPs, Ecm33, Gp38, and PstI, and conjugated them to a column. The pooled week 9 to 14 sera from each of the six animals were applied to the column, and the flowthrough and eluate fractions were examined for reactivity against gp120 proteins derived from the clade B HIV-1 strains LAI-BH8, JRFL, and R2 and the clade C HIV-1 strain DU179. Analysis of the input serum and flowthrough fractions showed that, on average, the YGP columns captured 80% or more of the gp120-reactive serum antibodies, regardless of the strain examined, indicating that the yeast glycoproteins Ecm33, Gp38, and PstI are able to adsorb the majority of antibodies that cross-react with HIV-1 gp120 (Fig. 1A). We confirmed the glycan specificity of the YGP-purified sera by finding a complete loss of gp120 binding following endo-β-N-acetylglucosaminidase H (endo H) digestion (data not shown). These results suggest that 2G12-reactive glycoproteins in TM yeast are the primary inducers of glycan-specific gp120-cross-reactive antibodies when used for immunization, justifying further evaluation of these individual yeast glycoproteins as immunogens.
FIG. 1.
2G12-reactive TM yeast glycoproteins capture the majority of gp120-reactive serum antibodies. (A) Sera (weeks 9 to 14) from each animal were passed over a column conjugated with the 2G12-reactive yeast glycoproteins Ecm33, Gp38, and PstI. Raw sera and the YGP column flowthrough were tested at a dilution of 1:100 for binding to LAI-BH8 gp120 in an ELISA to determine the fraction of gp120-reactive antibodies captured by the column. Data represent the averages for 3 independent experiments ± standard errors of the means. (B) The gp120-binding capacity of the purified antibodies was confirmed using immunoprecipitation of gp120 in solution. Lane 1, 100 ng JRFL gp120; lanes 2 and 4, gp120s precipitated with 5 μg/ml protein A-purified prebleed sera from rabbits 1226 and 1238, respectively; lanes 3 and 5, gp120s precipitated with 5 μg/ml YGP column-purified postimmunization sera from rabbits 1226 and 1238, respectively; lane 6, gp120 precipitated with 2 μg/ml 2G12. One-eighth of the final precipitated immunocomplex sample, equivalent to 125 ng gp120 starting material, was loaded per lane. The upper panel was probed with 1 μg/ml of rabbit anti-gp120 antibody 3824, which was raised with a synthetic peptide, and the lower panel was probed with 0.5 μg/ml 2G12. Binding to JRFL gp120 is shown, while similar results were obtained for binding to R2 gp120.
Elicited antibodies recognize monomeric gp120 in solution.
To confirm the gp120-binding capacity of the elicited antibodies after purification, we tested the ability of protein A-purified prebleed and YGP-purified postimmunization sera from rabbits 1226 and 1238 to bind gp120 in solution. Using immunoprecipitation followed by Western blotting, we found that prebleed samples were unable to pull gp120 out of solution (Fig. 1B, lanes 2 and 4), while postimmunization samples were able to bind gp120 in solution (lanes 3 and 5). 2G12 was used to show successful binding of gp120 in solution (lane 6). Based on the fraction of precipitate loaded on the gel, which would equal 125 ng gp120 if precipitation were 100% efficient, the postimmunization sera and 2G12 bound the soluble form of Env quite efficiently, since they gave a signal roughly equivalent to that of 100 ng gp120, which was loaded as a control (Fig. 1B, lane 1). These data demonstrate that purification of our sera by use of yeast glycoproteins successfully isolated the gp120-binding antibody fraction and that these antibodies efficiently bind to soluble gp120.
Elicited antibodies recognize epitopes on monomeric gp120 that are distinct from the 2G12 epitope.
We tested the ability of the YGP-purified sera as well as MAb 2G12 to recognize monomeric gp120 proteins from 9 clade B, 4 clade C, 1 clade A, 1 clade E, and 1 SIV strain by ELISA. We found that the YGP-purified sera recognized all clade B gp120 proteins efficiently, with some slight differences in reactivity compared to that of MAb 2G12. In addition, YGP-purified sera recognized many non-clade B gp120 proteins nearly as well as clade B gp120s, while 2G12 exhibited preferential binding to clade B gp120s (Fig. 2A). The broader reactivity of TM yeast sera than that of 2G12 is accounted for at least in part by recognition of additional glycan structures. For example, sequence analysis of clade B strains R2 and YU2 showed that they lack 2 of 5 glycans that form the 2G12 epitope (N339 and N392 [HxB numbering system]), but they did not show a loss of serum binding. Sequence analysis of clade C strains DU179 and IN012 showed that these strains both lack an essential glycan for 2G12 binding (N295), which explains their low reactivity with 2G12. However, the YGP-purified antibodies recognized these strains efficiently, suggesting that the elicited antibodies recognize glycans that lie outside the 2G12 epitope. This was further confirmed by the strong reactivity of YGP-purified antibodies with HIV-1 clade A and E and SIV gp120s.
FIG. 2.
Elicited antibodies display different gp120 strain preferences and bind clade B gp120s with lower affinity than 2G12. (A) YGP-purified serum IgG at 10 μg/ml (green bars) and 2G12 at 2 μg/ml (blue bars) were tested for binding to gp120s from various strains in ELISA. YGP-purified serum from each rabbit was tested for binding to each strain. For each individual serum, the strain that was recognized most efficiently was set as 100%, and the relative percent binding to all other strains was calculated. Green bars represent the average percent gp120 binding across the 6 animals for each strain. Error bars represent the variability between animals for each gp120, indicating the maximum and minimum relative percent binding across all 6 rabbit sera as a measure of strain preference. Data represent 3 independent experiments. (B) The ability of YGP-purified serum IgG to prevent binding of MAb 2G12 or b12 to LAI-BH8 gp120 was measured using ELISA, with percent inhibition of MAb binding indicated. Data represent the averages for 3 independent experiments ± standard errors of the means. (C) Relative affinities of YGP-purified sera and 2G12 for 4 gp120s were measured using 2-fold dilutions of antibody in ELISA. EC50s are indicated in the table. Data represent the averages for 3 independent experiments ± standard errors of the means.
To more directly test the ability of TM sera to recognize the N-linked glycans that are most important for 2G12 recognition of HIV-1 Env, we performed competition ELISAs with 2G12. Monomeric gp120s from strains LAI-BH8, JRFL, and R2 were captured on plastic and then incubated with protein A-purified preimmune sera or YGP-purified postimmune sera at 50 μg/ml. After binding for 120 min, 2G12 was added to each well (without washing) at a concentration of 0.5 or 0.1 μg/ml for 20 min. After washing of the plates, the amount of bound 2G12 was assessed. We found that none of the sera inhibited subsequent binding of 2G12 to any of the gp120 proteins tested (Fig. 2B), suggesting that TM yeast-elicited antibodies do not bind to glycans forming the 2G12 epitope or that they bind to these glycans in a way that does not prevent subsequent 2G12 binding.
To measure the efficiency of gp120 binding by YGP-purified sera relative to that by 2G12, EC50s were measured by ELISA with four gp120s: those from clade B strains LAI-BH8, JRFL, and R2 and clade C strain DU179. On average, TM sera demonstrated roughly 100- to 1,000-fold lower affinities than 2G12 for clade B gp120s but exhibited EC50s similar to that of 2G12 for the clade C strain (Fig. 2C). The broader reactivity of TM sera with diverse gp120s than that of 2G12, coupled with the lower apparent affinities, indicates that while immunization with TM yeast elicits antibodies that efficiently bind gp120, much of the response is directed against glycans that either are not recognized by 2G12 or bind to the 2G12 epitope with low affinity.
TM yeast-elicited antibodies preferentially bind to high-mannose glycans with exposed Manα1,2-Manα1,2-Man trisaccharides.
To characterize the fine specificities of TM yeast-induced antibodies to oligosaccharides, a glycan binding profile analysis was conducted with 11 synthetic mannose-containing glycans that can be divided into three groups based on the linkage of the terminal Man residues, with group 1 glycans containing no terminal Manα1,2 structures, group 2 glycans containing terminal Manα1,2-Man disaccharides, and group 3 glycans containing terminal Manα1,2-Manα1,2-Man trisaccharides (Fig. 3A). MAb 2G12 efficiently recognizes all glycans in group 3 but does not recognize group 1 or group 2 glycan structures (19). We tested protein A-purified prebleed and YGP-purified sera from all six rabbits. Prebleed sera did not react with any of the glycans. In contrast, YGP-purified sera from rabbits 1226 and 1229 exhibited broad binding to all high-mannose glycans in group 3, with very similar relative fluorescence unit (RFU) values (Fig. 3B). In contrast, samples from rabbits 1234, 1237, 1238, and 1242 exhibited a narrower pattern of reactivity, with high RFUs only for the group 3 glycan 212 and relatively lower RFUs for glycan 205 (Fig. 3B). These two glycans contain an exposed Manα1,2-Manα1,2-Man trisaccharide structure without the additional bulky branches found in glycans 313 and 314, which could allow for tighter packing of Manα1,2-Manα1,2-Man trisaccharides on the array. Considering that TM yeast cells express primarily Man8GlcNAc2 N-linked glycans, it is plausible that the narrow binding range exhibited by this second subset of animals was due to antibodies that bound only to tight clusters of terminal Manα1,2-Manα1,2-Man trisaccharides from the D1 arm of Man8GlcNAc2. In summary, the YGP-purified antibodies recognize similar terminal mannose linkages to those recognized by 2G12 but likely bind to different high-mannose N-linked glycans on gp120 from those bound by 2G12 or bind with a lower affinity.
FIG. 3.
TM yeast-elicited antibodies preferentially bind terminal Manα1,2-Manα1,2 trisaccharides. (A) Schematic representation of the synthetic mannose-containing carbohydrate structures present on the CFG-printed glycan array, version 4.1. (B) YGP-purified serum IgG from each animal was tested at 10 μg/ml for binding to the various mannose-containing synthetic carbohydrates. Fluorescent antibodies were used to detect serum antibody binding, which was measured in relative light units.
Elicited antibodies bind trimeric Env poorly.
Knowing that our TM yeast-elicited antibodies bound monomeric gp120 efficiently and recognized the same glycan linkages as 2G12, we next sought to determine whether they could bind trimeric HIV-1 Env. It is generally believed that HIV-1 neutralizing antibodies bind Env trimers efficiently, while nonneutralizing antibodies do not (10, 26). We hypothesized that a lack of efficient trimer binding may be one cause for the low neutralization capacity of elicited sera despite their efficient recognition of monomeric gp120. To evaluate binding to oligomeric Env, we transiently expressed gp160 in quail QT6 cells by infecting them with recombinant vaccinia virus vectors expressing LAI-BH8 or JRFL Env and measured antibody binding to the cell surface by flow cytometry. In this system, all cells express high levels of Env. MAbs 2G12 and b12, a broadly neutralizing antibody that recognizes the CD4 binding site on gp120, bound efficiently to cells expressing either LAI-BH8 or JRFL Env and did not bind to cells infected with a vaccinia virus vector that expresses T7 RNA polymerase (Env-negative cells) (Fig. 4A). Env-negative and Env-positive cells were then probed with protein A-purified prebleed and YGP-purified postimmune sera from all 6 animals. In each case, prebleed sera showed no increase in binding to Env-positive cells, while YGP-purified sera recognized Env-positive cells poorly, with a maximum 2-fold increase in mean fluorescence intensity (MFI) (Fig. 4B). These results stand in marked contrast to the 3-log MFI shift seen with 2G12 and b12, antibodies that are known to recognize the trimeric Env protein efficiently. Thus, while immunization with TM yeast elicits antibodies that cross-react with monomeric gp120, only a very small fraction of serum antibodies are able to bind Env expressed on the cell surface. This low level of binding could be due to a small population of the polyclonal antibodies binding to trimeric Env or to binding of nonfunctional forms of Env, such as uncleaved gp160 or trimers from which one or more gp120 subunits have dissociated. The YGP-purified sera also failed to efficiently bind CEMx174 cells chronically infected with HIV-1 R3A, suggesting that serum antibodies fail to bind Env glycans even when they are processed in human lymphocytes (data not shown).
FIG. 4.
Serum antibodies bind cell surface oligomeric Env poorly. (A) MAbs b12 and 2G12 were tested at 10 μg/ml for binding to LAI-BH8 Env expressed on the surfaces of quail fibroblast cells (red) to determine envelope expression levels. Binding to cells not expressing Env (blue) was measured in parallel. (B) Protein A-purified prebleed sera (PB) or YGP-purified postimmune sera were tested at 10 μg/ml for binding to Env-negative and Env-positive cells. Data are from a single representative experiment.
Retention of high-mannose glycans on Env increases serum antibody binding to Env trimers.
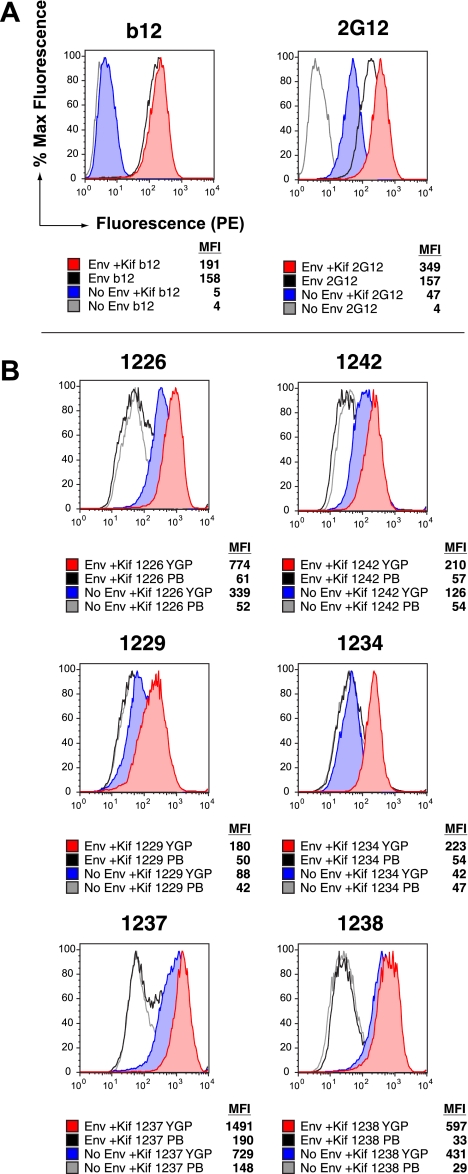
The failure of YGP-purified sera to bind trimeric Env could be due to differential packing, orientation, or occlusion of high-mannose glycans on monomeric gp120 versus trimeric Env. To differentiate between these possibilities, we expressed gp160 in the presence of the mannosidase I inhibitor kifunensine to force retention of high-mannose Man9GlcNac2 moieties at all N-linked glycosylation sites, allowing modified orientation and packing of surface glycans. Cell surface trimer binding FACS analysis was performed as described above, with the exception that kifunensine was included in the medium following vaccinia virus infection. Binding by antibody b12, which is not glycan dependent, was unchanged when Env was expressed in the presence of kifunensine (Fig. 5A). However, Env-specific binding by antibody 2G12 was increased on kifunensine-treated Env, and 2G12-binding sites were created on Env-negative cells, as has been reported previously (28) (Fig. 5A). Endo H digestion and gp120-specific Western blotting of lysates from Env-positive cells grown in the absence and presence of kifunensine confirmed that only high-mannose glycans were retained on gp120 after kifunensine treatment (data not shown).
FIG. 5.
Retention of Man9 N-linked glycans on Env increases serum antibody recognition of Env trimers. (A) Binding of MAbs b12 and 2G12 to Env-negative and Env-positive cells grown in the absence or presence of 25 μg/ml kifunensine (Kif). (B) Binding of protein A-purified prebleed sera (PB) and YGP-purified antibodies to Env-negative and Env-positive cells produced in the presence of 25 μg/ml kifunensine. Data are from one representative experiment.
Env-negative and Env-positive cells grown in the absence or presence of kifunensine were then probed for binding by protein A-purified prebleed sera and YGP-purified postimmune sera from all 6 animals. As expected, prebleed sera showed no binding to kifunensine-treated cells, whether they expressed Env or not (Fig. 5B). However, YGP-purified sera showed a dramatic increase in trimer binding when all glycans on Env were retained in a high-mannose form (Fig. 5B). In addition, just like 2G12, the YGP-purified sera gained the ability to bind kifunensine-treated Env-negative cells, though not to the same extent as Env-positive cells. Thus, it appears that elicited antibodies fail to bind to native trimeric Env, perhaps due to a combination of differences in packing and orientation of high-mannose glycans on Env trimers versus monomers.
Elicited sera are able to neutralize virions that retain high-mannose glycans at all N-glycosylation sites.
We next wanted to determine if binding of YGP-purified serum antibodies to high-mannose structures on trimeric Env results in virus neutralization. To test this, we produced a panel of clade B pseudoviruses expressing a luciferase reporter gene in 293T cells in either the absence or presence of kifunensine and used these to infect U87.CD4.CCR5/CXCR4 cells. This panel included prototypic as well as primary Env proteins, which ranged from generally neutralization sensitive (tier 1) to moderately neutralization resistant (tier 2), and a negative-control virus bearing vesicular stomatitis virus glycoprotein (VSV-G). These viruses were first tested for neutralization by protein A-purified preimmune sera and YGP-purified postimmune sera at a concentration of 50 μg/ml. Pseudovirions bearing wild-type Env protein showed little to no neutralization by TM yeast-elicited antibodies, while control antibodies 2G12, b12, and pooled HIV-positive human serum (HIV Ig) neutralized the virions as expected (Fig. 6A). YGP-purified sera also failed to neutralize various strains of replication-competent virus produced in human PBMCs (ADA, BaL, JRFL, SF162, YU2, 89.6, IIIB, and HxB) when tested on TZM-BL cells (data not shown). However, when serum samples were tested for neutralization of pseudovirions produced in the presence of kifunensine, YGP-purified antibodies from each rabbit were able to neutralize most or all virus strains by at least 75%, while pseudovirions bearing kifunensine-treated VSV-G were not neutralized (Fig. 6B). As expected, 2G12 gained neutralization potency against several strains of kifunensine-treated viruses, and interestingly, HIV Ig also gained potency against several kifunensine-treated virus strains. This could be due to the presence of mannose-specific antibodies in patient sera or to exposure of viral epitopes normally hidden by complex sugars on Env. Antibody b12 showed little change in its neutralization pattern, with the largest changes associated with lower potency against certain kifunensine-treated virions; this could be caused by steric occlusion of the CD4 binding site by the additional high-mannose glycans.
FIG. 6.
Serum antibodies are able to neutralize virions produced in the presence of kifunensine. (A) Pseudovirions expressing the indicated Envs on a luciferase viral core were tested for neutralization by protein A-purified prebleed sera, yeast glycoprotein-purified postimmune sera, MAb 2G12, pooled HIV-positive human serum (HIV Ig), and MAb b12 at 50 μg/ml. Percent inhibition by YGP-purified sera relative to prebleed sera is indicated in the top panel, while percent inhibition by the control antibodies and human serum is indicated in the bottom panel. (B) Pseudovirions produced in the presence of 25 μg/ml kifunensine were tested for neutralization as described above. (C to F) To measure the neutralization potency of each rabbit serum relative to that of 2G12, serial antibody dilutions were tested for neutralization of clade B pseudovirions bearing the envelopes of HIV strains JRFL (C), LAI (D), PVO (E), and R2 (F). Data represent the averages for 3 independent experiments ± standard errors of the means.
To determine the potency of kifunensine-treated pseudovirus neutralization by YGP-purified antibodies relative to that by 2G12, 2-fold serial dilutions of YGP-purified sera and 2G12 were tested for the ability to neutralize JRFL, LAI, PVO, and R2 pseudoviruses produced in the presence of kifunensine on U87.CD4.CCR5/CXCR4 cells. We found that 2G12 neutralized LAI and JRFL 1 to 2 log more efficiently than any of the YGP-purified sera, while neutralization of R2 and PVO by YGP-purified sera was largely similar to that by 2G12. Thus, antibodies elicited by TM yeast recognize high-mannose glycans on HIV gp120 but not on intact trimers. When trimeric Env was forced to express only high-mannose glycans, TM yeast-elicited antibodies bound to trimeric Env and exhibited broad neutralizing activity.
DISCUSSION
Among the monoclonal antibodies identified to date that are capable of neutralizing a broad array of primary HIV-1 isolates, the human MAb 2G12 is unique in that its epitope is entirely carbohydrate dependent (7, 25, 27). An unusual domain-exchanged structure enables it to bind to multiple Manα1,2-Man-linked moieties presented by a cluster of high-mannose oligosaccharides on the surface of gp120, likely accounting for the high affinity displayed by 2G12 for HIV-1 Env (7). While these carbohydrates are technically self antigens, the vast majority of N-linked glycans present on the surfaces of mammalian cells are processed to more-complex forms, and we know of no mammalian proteins expressed on the cell surface that have numerous high-mannose oligosaccharides. Consistent with this, 2G12 fails to bind cell surface glycoproteins of mammalian cells, except in the presence of mannosidase inhibitors that result in the artificial presentation of high-mannose glycans (28). As a result, high-affinity binding of 2G12 appears to be quite specific for HIV-1 Env.
The broad neutralizing activity of 2G12, coupled with its ability to protect rhesus macaques from mucosal challenge with virus (14), has led to attempts to recapitulate the 2G12 epitope in a variety of contexts. While several immunogens displaying multivalent high-mannose glycans have been developed that support 2G12 binding, their affinity is typically much lower than that of 2G12 for gp120, and thus far none have elicited antibodies that cross-react with glycans present on HIV-1 Env, let alone neutralize virus (1, 2, 11, 16, 34). However, genetic modification of the S. cerevisiae N-linked glycan processing pathway, such that endogenous glycoproteins express exclusively high-mannose glycans like those recognized by 2G12, resulted in a yeast strain (TM yeast) that mimics at least some aspects of the 2G12 epitope. 2G12 binds to several highly glycosylated proteins expressed by TM yeast, and immunization of rabbits with whole TM yeast generates mannose-specific antibodies that cross-react with the glycans present on gp120 proteins from numerous HIV-1 clades as well as SIV strains—something not yet accomplished with synthetically produced glycans (18, 19). Unfortunately, these immune sera do not exhibit significant neutralizing activity, suggesting that antibodies with 2G12 specificity are not elicited in these animals.
The most important finding of this study is that despite efficient binding to gp120, antibodies elicited by TM yeast fail to recognize trimeric HIV-1 Env, accounting for the absence of virus neutralization. Clearly, monomeric gp120 protein binding by itself cannot be used as a surrogate for binding the 2G12 epitope. There are several possibilities that could account for this discrepancy between robust binding of monomeric gp120 and a lack of trimeric Env binding. It is possible, though unlikely, that antibodies elicited by TM yeast bind to glycans that are presented in high-mannose forms on monomeric gp120 but that are processed to more complex glycans in the context of trimeric Env, and thus no longer support antibody binding. In support of this hypothesis is the finding that trimeric Env expressed in the presence of kifunensine not only supports binding by TM yeast-elicited antibodies but also results in the production of virus particles that are efficiently neutralized by these mannose-specific antibodies. However, it is also possible that use of this mannosidase inhibitor generates entirely new binding sites for TM yeast-elicited antibodies on trimeric Env. In fact, kifunensine treatment enables 2G12 to bind to at least one additional epitope on HIV-1 monomeric gp120 (28). Regardless, these data show that it is possible to design an immunogen that under some conditions can target the glycan shield of HIV-1 in a manner that results in efficient virus neutralization.
A second possibility to account for the lack of TM yeast-elicited antibody binding to trimeric Env is that the conformation or packing of the high-mannose cluster recognized by 2G12 is impacted by Env oligomerization. Perhaps antibodies elicited by TM yeast bind to the high-mannose glycans that constitute the 2G12 epitope but fail to recognize the conformation of these glycans in the context of trimeric Env. An argument against this hypothesis is that TM yeast-elicited antibodies failed to prevent subsequent binding of 2G12 to gp120, suggesting that cross-reaction with monomeric gp120 occurs through recognition of high-mannose epitopes other than that recognized by 2G12 or through binding of glycans on different gp120 monomers that are arrayed in an irrelevant conformation on an ELISA plate. If TM yeast-elicited antibodies bind to glycans recognized by 2G12, they must do so with a very low affinity or bind in a manner that does not sterically prevent subsequent 2G12 binding. With regard to the former possibility, it is possible that the domain-exchanged structure of 2G12 is essential for high-affinity binding as well as neutralizing activity and that the antibodies elicited in rabbits by TM yeast do not possess this unusual structure. It remains unknown whether antibodies with normal Fab arms are able to recognize the spacing of high-mannose glycans on Env with a high enough avidity to allow binding of native Env trimers and virus neutralization. If they are not, then a greater understanding of the factors that lead to domain exchange will be needed. Perhaps the use of different adjuvants or lengthier immunization schedules in different animal species will allow for the affinity maturation events needed to force the generation of domain-exchanged antibodies capable of binding to multiple high-mannose glycans present on the surfaces of HIV-1 Env trimers.
Another key observation from this study is that the yeast glycoproteins Ecm33, Gp38, and PstI can adsorb approximately 80% of the antibodies elicited by TM yeast that are capable of recognizing high-mannose glycans on HIV-1 gp120. Thus, one or more of these yeast glycoproteins could provide a genetic scaffold that can be used to optimize 2G12 binding and hopefully allow elicitation of 2G12-like antibodies. All three of these yeast glycoproteins are heavily glycosylated, with 13 to 15 N-linked consensus sites. While each protein supports 2G12 binding, we do not know how many 2G12 epitopes exist on each protein or what roles the fine differences in protein structure and glycan presentation play in 2G12 binding to each protein. By genetically eliminating glycans that are not involved in 2G12 binding on each protein, it may be possible to focus the immune response on high-mannose structures that most efficiently recapitulate the 2G12 epitope. Additionally, once the 2G12 epitope on each TM yeast glycoprotein is defined, it may be possible to study differences in glycan presentation by each protein to determine which provides the best underlying scaffold structure to elicit 2G12-like antibodies. The ease with which yeast proteins can be modified and expressed also raises the possibility that an iterative process of mutagenesis combined with 2G12 binding studies could allow enhanced 2G12 binding that could result in improved immunogenicity and elicitation of broadly neutralizing antibodies.
In conclusion, despite elicitation of antibodies that recognize the same high-mannose glycan structures as 2G12, binding of the 2G12 epitope on trimeric Env has not been achieved. While the antibodies elicited by TM yeast recognize terminal α1,2-linked mannose residues, they appear to recognize these glycans in the context of tightly packed high-mannose branches, which may present terminal mannose residues in an orientation not found on trimeric HIV Env. This has implications for vaccine design concepts that use dense arrays of high-mannose glycans for recapitulation of the 2G12 epitope, as targeting the correct glycan structures is essential but not sufficient for induction of neutralizing antibodies. The packing of glycans on the native Env protein is important, too, and future scaffolds will have to take glycan density into consideration to allow the presentation of high-mannose glycans in an orientation found on the native HIV envelope. Following this line of reasoning, a crystal structure of 2G12 bound to gp120 would be extremely enlightening for the design of scaffolds that accurately mimic the 2G12 epitope.
Acknowledgments
The authors thank David Smith, Emory University for help with the glycan microarray.
Footnotes
Published ahead of print on 20 October 2010.
REFERENCES
- 1.Astronomo, R. D., E. Kaltgrad, A. K. Udit, S. K. Wang, K. J. Doores, C. Y. Huang, R. Pantophlet, J. C. Paulson, C. H. Wong, M. G. Finn, and D. R. Burton. 2010. Defining criteria for oligomannose immunogens for HIV using icosahedral virus capsid scaffolds. Chem. Biol. 17:357-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Astronomo, R. D., H. K. Lee, C. N. Scanlan, R. Pantophlet, C. Y. Huang, I. A. Wilson, O. Blixt, R. A. Dwek, C. H. Wong, and D. R. Burton. 2008. A glycoconjugate antigen based on the recognition motif of a broadly neutralizing human immunodeficiency virus antibody, 2G12, is immunogenic but elicits antibodies unable to bind to the self glycans of gp120. J. Virol. 82:6359-6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. [DOI] [PubMed] [Google Scholar]
- 4.Blixt, O., S. Head, T. Mondala, C. Scanlan, M. E. Huflejt, R. Alvarez, M. C. Bryan, F. Fazio, D. Calarese, J. Stevens, N. Razi, D. J. Stevens, J. J. Skehel, I. van Die, D. R. Burton, I. A. Wilson, R. Cummings, N. Bovin, C. H. Wong, and J. C. Paulson. 2004. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc. Natl. Acad. Sci. U. S. A. 101:17033-17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton, D. R., J. Pyati, R. Koduri, S. J. Sharp, G. B. Thornton, P. W. Parren, L. S. Sawyer, R. M. Hendry, N. Dunlop, P. L. Nara, et al. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024-1027. [DOI] [PubMed] [Google Scholar]
- 6.Calarese, D. A., H. K. Lee, C. Y. Huang, M. D. Best, R. D. Astronomo, R. L. Stanfield, H. Katinger, D. R. Burton, C. H. Wong, and I. A. Wilson. 2005. Dissection of the carbohydrate specificity of the broadly neutralizing anti-HIV-1 antibody 2G12. Proc. Natl. Acad. Sci. U. S. A. 102:13372-13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calarese, D. A., C. N. Scanlan, M. B. Zwick, S. Deechongkit, Y. Mimura, R. Kunert, P. Zhu, M. R. Wormald, R. L. Stanfield, K. H. Roux, J. W. Kelly, P. M. Rudd, R. A. Dwek, H. Katinger, D. R. Burton, and I. A. Wilson. 2003. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science 300:2065-2071. [DOI] [PubMed] [Google Scholar]
- 8.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 9.Corti, D., J. P. Langedijk, A. Hinz, M. S. Seaman, F. Vanzetta, B. M. Fernandez-Rodriguez, C. Silacci, D. Pinna, D. Jarrossay, S. Balla-Jhagjhoorsingh, B. Willems, M. J. Zekveld, H. Dreja, E. O'Sullivan, C. Pade, C. Orkin, S. A. Jeffs, D. C. Montefiori, D. Davis, W. Weissenhorn, A. McKnight, J. L. Heeney, F. Sallusto, Q. J. Sattentau, R. A. Weiss, and A. Lanzavecchia. 2010. Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV-1-infected individuals. PLoS One 5:e8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crooks, E. T., P. Jiang, M. Franti, S. Wong, M. B. Zwick, J. A. Hoxie, J. E. Robinson, P. L. Moore, and J. M. Binley. 2008. Relationship of HIV-1 and SIV envelope glycoprotein trimer occupation and neutralization. Virology 377:364-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunlop, D. C., C. Bonomelli, F. Mansab, S. Vasiljevic, K. J. Doores, M. R. Wormald, A. de Sa Palma, T. Feizi, D. J. Harvey, R. A. Dwek, M. Crispin, and C. N. Scanlan. 2010. Polysaccharide mimicry of the epitope of the broadly neutralising anti-HIV antibody, 2G12, induces enhanced antibody responses to self oligomannose glycans. Glycobiology 20:812-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Earl, P. L., S. Koenig, and B. Moss. 1991. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J. Virol. 65:31-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hessell, A. J., P. Poignard, M. Hunter, L. Hangartner, D. M. Tehrani, W. K. Bleeker, P. W. Parren, P. A. Marx, and D. R. Burton. 2009. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat. Med. 15:951-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hessell, A. J., E. G. Rakasz, P. Poignard, L. Hangartner, G. Landucci, D. N. Forthal, W. C. Koff, D. I. Watkins, and D. R. Burton. 2009. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 5:e1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hessell, A. J., E. G. Rakasz, D. M. Tehrani, M. Huber, K. L. Weisgrau, G. Landucci, D. N. Forthal, W. C. Koff, P. Poignard, D. I. Watkins, and D. R. Burton. 2010. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J. Virol. 84:1302-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joyce, J. G., I. J. Krauss, H. C. Song, D. W. Opalka, K. M. Grimm, D. D. Nahas, M. T. Esser, R. Hrin, M. Feng, V. Y. Dudkin, M. Chastain, J. W. Shiver, and S. J. Danishefsky. 2008. An oligosaccharide-based HIV-1 2G12 mimotope vaccine induces carbohydrate-specific antibodies that fail to neutralize HIV-1 virions. Proc. Natl. Acad. Sci. U. S. A. 105:15684-15689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krauss, I. J., J. G. Joyce, A. C. Finnefrock, H. C. Song, V. Y. Dudkin, X. Geng, J. D. Warren, M. Chastain, J. W. Shiver, and S. J. Danishefsky. 2007. Fully synthetic carbohydrate HIV antigens designed on the logic of the 2G12 antibody. J. Am. Chem. Soc. 129:11042-11044. [DOI] [PubMed] [Google Scholar]
- 18.Luallen, R. J., H. Fu, C. Agrawal-Gamse, I. Mboudjeka, W. Huang, F. H. Lee, L. X. Wang, R. W. Doms, and Y. Geng. 2009. A yeast glycoprotein shows high-affinity binding to the broadly neutralizing human immunodeficiency virus antibody 2G12 and inhibits gp120 interactions with 2G12 and DC-SIGN. J. Virol. 83:4861-4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luallen, R. J., J. Lin, H. Fu, K. K. Cai, C. Agrawal, I. Mboudjeka, F. H. Lee, D. Montefiori, D. F. Smith, R. W. Doms, and Y. Geng. 2008. An engineered Saccharomyces cerevisiae strain binds the broadly neutralizing human immunodeficiency virus type 1 antibody 2G12 and elicits mannose-specific gp120-binding antibodies. J. Virol. 82:6447-6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura, Y., T. Igarashi, N. Haigwood, R. Sadjadpour, R. J. Plishka, A. Buckler-White, R. Shibata, and M. A. Martin. 2002. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. J. Virol. 76:2123-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parren, P. W., P. A. Marx, A. J. Hessell, A. Luckay, J. Harouse, C. Cheng-Mayer, J. P. Moore, and D. R. Burton. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75:8340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purtscher, M., A. Trkola, G. Gruber, A. Buchacher, R. Predl, F. Steindl, C. Tauer, R. Berger, N. Barrett, A. Jungbauer, et al. 1994. A broadly neutralizing human monoclonal antibody against gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses 10:1651-1658. [DOI] [PubMed] [Google Scholar]
- 25.Sanders, R. W., M. Venturi, L. Schiffner, R. Kalyanaraman, H. Katinger, K. O. Lloyd, P. D. Kwong, and J. P. Moore. 2002. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J. Virol. 76:7293-7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sattentau, Q. J., and J. P. Moore. 1995. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J. Exp. Med. 182:185-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scanlan, C. N., R. Pantophlet, M. R. Wormald, E. Ollmann Saphire, R. Stanfield, I. A. Wilson, H. Katinger, R. A. Dwek, P. M. Rudd, and D. R. Burton. 2002. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1→2 mannose residues on the outer face of gp120. J. Virol. 76:7306-7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scanlan, C. N., G. E. Ritchie, K. Baruah, M. Crispin, D. J. Harvey, B. B. Singer, L. Lucka, M. R. Wormald, P. Wentworth, Jr., N. Zitzmann, P. M. Rudd, D. R. Burton, and R. A. Dwek. 2007. Inhibition of mammalian glycan biosynthesis produces non-self antigens for a broadly neutralising, HIV-1 specific antibody. J. Mol. Biol. 372:16-22. [DOI] [PubMed] [Google Scholar]
- 29.Selvarajah, S., B. Puffer, R. Pantophlet, M. Law, R. W. Doms, and D. R. Burton. 2005. Comparing antigenicity and immunogenicity of engineered gp120. J. Virol. 79:12148-12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shibata, R., T. Igarashi, N. Haigwood, A. Buckler-White, R. Ogert, W. Ross, R. Willey, M. W. Cho, and M. A. Martin. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 5:204-210. [DOI] [PubMed] [Google Scholar]
- 31.Trkola, A., M. Purtscher, T. Muster, C. Ballaun, A. Buchacher, N. Sullivan, K. Srinivasan, J. Sodroski, J. P. Moore, and H. Katinger. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 70:1100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker, L. M., S. K. Phogat, P. Y. Chan-Hui, D. Wagner, P. Phung, J. L. Goss, T. Wrin, M. D. Simek, S. Fling, J. L. Mitcham, J. K. Lehrman, F. H. Priddy, O. A. Olsen, S. M. Frey, P. W. Hammond, S. Kaminsky, T. Zamb, M. Moyle, W. C. Koff, P. Poignard, and D. R. Burton. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, J., H. Li, G. Zou, and L. X. Wang. 2007. Novel template-assembled oligosaccharide clusters as epitope mimics for HIV-neutralizing antibody 2G12. Design, synthesis, and antibody binding study. Org. Biomol. Chem. 5:1529-1540. [DOI] [PubMed] [Google Scholar]
- 34.Wang, S. K., P. H. Liang, R. D. Astronomo, T. L. Hsu, S. L. Hsieh, D. R. Burton, and C. H. Wong. 2008. Targeting the carbohydrates on HIV-1: interaction of oligomannose dendrons with human monoclonal antibody 2G12 and DC-SIGN. Proc. Natl. Acad. Sci. U. S. A. 105:3690-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zwick, M. B., A. F. Labrijn, M. Wang, C. Spenlehauer, E. O. Saphire, J. M. Binley, J. P. Moore, G. Stiegler, H. Katinger, D. R. Burton, and P. W. Parren. 2001. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 75:10892-10905. [DOI] [PMC free article] [PubMed] [Google Scholar]