Abstract
The HIV-1 protein Vpu counteracts the antiviral activity of the innate restriction factor BST-2/tetherin by a mechanism that partly depends on its interaction with β-TrCP, a substrate adaptor for an SCF (Skp-Cullin 1-F box) E3 ubiquitin ligase complex. This suggests that Vpu stimulates the ubiquitination of BST-2 and that this underlies the relief of restriction. Here, we show that Vpu stimulates ubiquitination of BST-2. Mutation of all potential ubiquitination sites in the cytoplasmic domain of BST-2, including lysines, cysteines, serines, and threonines, abrogates Vpu-mediated ubiquitination. However, a serine-threonine-serine sequence specifically mediates the downregulation of BST-2 from the cell surface and the optimal relief of restricted virion release. Serine-threonine ubiquitination of BST-2 is likely part of the mechanism by which Vpu counteracts innate defenses.
BST-2/tetherin is an interferon-induced transmembrane protein that captures newly formed, mature virions of HIV-1 and other enveloped viruses at the cell surface, preventing their spread (43). The counteraction of BST-2 by the HIV-1 accessory protein Vpu is associated with the downregulation of BST-2 from the cell surface, where BST-2 acts to restrict virion release (11, 38, 44). One mechanistic aspect of Vpu-mediated counteraction lies in an interaction between BST-2 and Vpu, which occurs via the transmembrane domains (TMDs) of the two proteins (15, 20, 31). The optimal downregulation of BST-2 from the cell surface and the counteraction of restricted virion release also depend on the interaction of Vpu with the Skp1-Cullin 1-β-TrCP E3 ubiquitin ligase complex and a ubiquitin-dependent pathway (8, 26, 33), and this pathway likely requires determinants of Vpu sensitivity in the cytosolic domain of BST-2. However, mutations within the cytosolic domain (CD) of BST-2 have neither revealed ubiquitin acceptor sites that are functionally relevant for counteraction by Vpu nor yielded a restrictive yet Vpu-resistant phenotype (19, 27, 35). Thus, the roles of the CD of BST-2 in ubiquitination induced by Vpu and in Vpu-mediated counteraction have been unclear.
In addition to its activity against HIV-1, BST-2 restricts the release of other enveloped viruses, including Ebola virus, Kaposi's sarcoma-associated herpesvirus (KSHV), and HIV-2, all of which lack Vpu. These viruses utilize other antagonist proteins to counteract BST-2, and all of these, with the exception of the Ebola glycoprotein, remove BST-2 from the cell surface (21, 22, 24, 27). KSHV encodes a ubiquitin ligase, the K5 protein, that ubiquitinates lysine residues in the CD of BST-2; this removes BST-2 from the cell surface via endosomal trafficking mediated by the ESCRT system, leading to lysosomal degradation (27, 37). A similar mechanism has been proposed for the action of Vpu (19, 33). However, the precise targets of the presumed ubiquitination induced by Vpu and the resulting trafficking of BST-2 are both unclear. Notably, decreased total cellular expression of BST-2 (that is, degradation per se) seems inessential for the removal of BST-2 from the cell surface by Vpu (9, 12, 33).
Protein ubiquitination leads to a variety of consequences, including degradation via the 26S proteasome, endocytosis, and the trafficking of membrane proteins to lysosomes, again for degradation (18). Viruses have co-opted these pathways to antagonize various host defenses, including host proteins involved in innate or adaptive immune responses (3). One advantage to viruses in co-opting ubiquitination pathways is the potential to antagonize several host proteins at once. For example, Vpu induces the degradation of the HIV-1 cell surface receptor CD4 by recruiting to the CD of CD4 the same Skp1-Cullin 1-β-TrCP E3 complex that it recruits to BST-2 (14). In the case of CD4, this occurs in the endoplasmic reticulum (ER), resulting in extraction of CD4 to the cytoplasm and proteasomal degradation. Remarkably, polyubiquitination of the CD of CD4 induced by Vpu occurs on not only lysine but also serine and threonine residues (25). Nonlysine residues, while noncanonical ubiquitin acceptors, are also utilized by the K3 and K5 proteins of KSHV to induce the degradation of major histocompatibility complex class I (MHC-I) (4, 5, 45), suggesting the possibility that viruses might favor such residues over the classical lysine as ubiquitination targets for the elimination of deleterious host proteins.
Here, we propose that HIV-1 Vpu uses such an approach to counteract BST-2. BST-2 is modified by ubiquitin, and Vpu increases its level of ubiquitination in a β-TrCP-dependent manner. Mutation of all potential ubiquitination sites in the CD of BST-2, including lysines, cysteines, serines, and threonines, is necessary to abrogate Vpu-mediated ubiquitination. However, an STS sequence is required for optimal Vpu-mediated downregulation of BST-2 and counteraction of virion release. We hypothesize that serine-threonine ubiquitination is a part of the mechanism by which Vpu counteracts BST-2.
MATERIALS AND METHODS
Plasmids, antibodies, and reagents.
For most transfections, pcDNA3.1 (Invitrogen, Carlsbad, CA) was used as a backbone vector, unless otherwise specified. pcDNA3.1-BST-2 as well as pcDNA3.1-BST-2/K18,21/R (where BST-2/K18,21/R is BST-2 with lysines 18 and 21 replaced with arginines, herein termed BST-2/KK) plasmid was obtained from Autumn Ruiz and Edward Stephens. Plasmids expressing ubiquitin-hemagglutinin (HA), β-TrCP-HA in the backbone vector pCS2-HA3, and enhanced green fluorescent protein (EGFP)-BST-2 have been described previously (10, 16, 28). The following cytosolic domain mutants of BST-2 were generated in pcDNA3.1-BST-2 using a QuikChange mutagenesis kit (Stratagene, La Jolla, CA): BST-2 with cysteines 9 and 20 replaced with alanines (BST-2/CC), BST-2 with the serine-threonine-serine sequence at positions 3 to 5 replaced with alanines (BST-2/STS), BST-2 with a combination of the CC mutation and KK mutation (BST-2/KK-CC), BST-2/CC-STS, BST-2/KK-STS, and BST-2 in which all potential ubiquitination sites were mutated (KCST-less). pNL4-3 was obtained from National Institutes of Health (NIH) AIDS Research and Reference Reagent Program (1). pNL4-3 mutants ΔVpu and Vpu with the mutation S52/56N (Vpu2/6), as well as pcDNA3.1-based plasmids expressing human codon-optimized Vpu, were provided by Klaus Strebel (36). The pcDNA3.1-based and codon-optimized Vpu2/6 mutant was constructed previously (33). The GFP-expressing plasmid used for flow cytometry (pCG-GFP) was provided by Jacek Skowronski (13). Murine monoclonal antibody to BST-2/HM1.24/CD317 was provided by Chugai Pharmaceutical Co. (Kanagawa, Japan). Rabbit polyclonal anti-HA antibody for detection of ubiquitin-HA was from Invitrogen, and mouse monoclonal anti-HA antibody for detection of β-TrCP was from Covance (Emeryville, CA). Monoclonal anti-ubiquitin P4D1 antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit antiserum to HIV-1 Vpu and rabbit anti-BST-2 were obtained from the NIH AIDS Research and Reference Reagent Program and contributed by Klaus Strebel. Monoclonal mouse antibody to p24 (clone 31-90-25) was obtained from ATCC (Manassas, VA). Monoclonal mouse antibody to actin and monoclonal mouse antibody to transferrin receptor (TfR) were from Sigma-Aldrich (St. Louis, MO). Mouse anti-CD63 conjugated to fluorescein isothiocyanate (FITC) was from BD Biosciences Pharmingen (San Diego, CA). The secondary antibodies for immunofluorescence experiments, donkey anti-mouse conjugated to rhodamine red-X and goat anti-rabbit conjugated to Cy5, were from Jackson Immunoresearch Laboratories (West Grove, PA). For flow cytometry, a mouse IgG2a isotope control and a goat anti-mouse IgG antibody conjugated to allophycocyanin (APC) were from BioLegend (San Diego, CA). Goat anti-mouse conjugated to horseradish peroxidase (HRP) was from Thermo Scientific (Rockford, IL), goat anti-rabbit-HRP was from Bio-Rad (Hercules, CA), and goat anti-mouse-HRP True Blot (used to avoid the detection of IgG on the immunoprecipitation [IP] and Western blotting) was from eBioscience (San Diego, CA). All other reagents were from Sigma-Aldrich (St. Louis, MO), unless specified otherwise.
Cells and transfections.
HEK293T cells were obtained from Ned Landau and maintained in Eagle's minimum essential medium (EMEM; Quality Biological, Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS; Gemini BioProducts, West Sacramento, CA) and l-glutamine (Invitrogen). To create 293T cells stably expressing BST-2, cells were transfected with pZeoSV (Invitrogen) linearized with EcoRI and with pcDNA3.1-BST-2 (encoding the wild-type [WT] or the mutants) linearized with BglII. Two days after transfection, the cells were exposed to 100 μM zeocin to select for integrants. Cells were serially diluted to obtain clonal lines. Cells from wells that appeared to contain singly transfected cells were analyzed by flow cytometry to assess the surface expression of BST-2, and clones were maintained that expressed similar surface levels of BST-2. HT-1080 cells were obtained from ATCC and were maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 10% fetal bovine serum. All transfections were done using Lipofectamine 2000 (Invitrogen), according to the manufacturer's protocol.
IP and Western blotting.
For IP, 90 to 95% confluent 293T cells were transfected with 2.5 μg of BST-2 and 5 μg of Vpu-expressing plasmids, as well as either 10 μg of ubiquitin-HA- or 10 μg of β-TrCP-HA-expressing plasmids, as specified in figure legends, in 10-cm2 dishes. Twenty-four hours later, the cells were collected and lysed in buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100) supplemented with protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN) for 30 min on ice and 30 min at room temperature (RT). Cell lysates were cleared by centrifugation at 16,000 × g at 4°C for 10 min and then incubated with anti-BST-2 antibody prebound to anti-mouse IgG-coated magnetic Dynabeads (Invitrogen Dynal, Oslo, Norway) for 2 h at 4°C. The ratio of the number of cells/anti-BST-2 antibody/magnetic beads was 10 × 106 to 20 × 106 cells/2 μg of anti-BST-2/200 × 105 beads. Beads were washed three times with buffer (lysis buffer supplemented with 250 mM NaCl), and bound proteins were eluted with 30 μl of Laemmli buffer, either in the absence or presence of β-mercaptoethanol. When specified in the figure legends, samples were additionally treated with peptide N-glycosidase F (PNGase F; Sigma-Aldrich), or reimmunoprecipitated with anti-BST-2 antibody, after which they were subjected to Western blot analysis.
Immunofluorescence microscopy.
HT-1080 cells were cotransfected with 80 ng of BST-2 and 670 ng of Vpu-expressing plasmids in a 24-well format. At 24 h posttransfection, the cells were stained with anti-BST-2 in cold phosphate-buffered saline (PBS) for 30 min at 4°C, washed with cold PBS, fixed with 4% paraformaldehyde (PFA) for 15 min, and permeabilized with 0.2% NP-40 in PBS for 5 min, both at RT. Next, cells were washed, blocked with 5% normal donkey and goat serum, stained with anti-BST-2 antibody and anti-Vpu antiserum for 1 h at RT, and washed three times. Cells were then incubated with the appropriate secondary antibody, blocked with 5% mouse serum, and incubated with mouse anti-CD63 antibody conjugated to FITC, all for 1 h at RT. The cells were washed three times, fixed again with 4% PFA for 15 min, mounted, and imaged using a fluorescence microscope (Olympus) and SlideBook, version 4.1, software (Intelligent Imaging Innovations, Denver, CO). For each field, a series of images along the Z axis was collected, the images were deconvolved using the nearest-neighbor method, and a projected, two-dimensional (2-D) image of the Z stacks was generated. Images were assembled using Adobe Photoshop software.
Flow cytometry.
For the analysis of cell surface BST-2, cells were transfected with the plasmids expressing BST-2 and Vpu or HIV-1 proviral plasmids, as indicated in the figure legends, along with a GFP-expressing plasmid (pCG-GFP) as a transfection marker. At 24 h posttransfection, cells were stained with anti-BST-2 or an IgG2a control in PBS containing 0.1% sodium azide and 2% FBS at 4°C. The cells were then washed in PBS, stained with anti-mouse IgG conjugated to APC, fixed in 1% formaldehyde, and analyzed by flow cytometry. Live cells were selected by forward and side scatter characteristics, gates for BST-2 were set using the IgG2a control, and gates for GFP were set using cells not expressing GFP. Dot plots and histograms were created using FlowJo software (Tree Star Inc., Ashland, OR).
Virion release.
293T cells were transfected in a six-well format with 75 ng of BST-2 and 3.75 μg of HIV-1 proviral DNA. For the parallel flow cytometric analyses, cells were also transfected with 0.5 μg of GFP-expressing plasmid. At 24 h posttransfection, the culture supernatants were collected and clarified by centrifugation at 400 × g, and the concentration of virion-associated p24 capsid protein was determined by enzyme-linked immunosorbent assay (ELISA; Perkin-Elmer) after centrifugation through a 20% sucrose cushion at 23,500 × g for 1 h. The amount of p55/p24, Vpu, BST-2, and actin in the cell lysates was determined by Western blotting using monoclonal anti-p24, polyclonal rabbit anti-Vpu and anti-BST-2, and monoclonal antiactin. For the infectivity assay, HeLa P4.R5 cells were plated in a 48-well format with 2 × 104 cells per well and infected in duplicate with serial dilutions of the above supernatants. Two days after infection, the cells were fixed and stained, and the number of infectious centers was measured by computer-assisted image analysis as described previously (7). Virion release in both the p24 ELISA and infectivity assays was expressed as a percentage of the release from cells not expressing BST-2 and transfected with ΔVpu proviral DNA.
RESULTS
HIV-1 Vpu induces β-TrCP-dependent ubiquitination of BST-2.
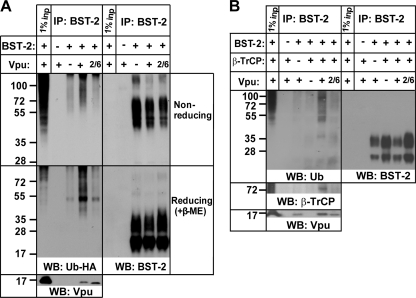
Because disruption of the interaction between β-TrCP and Vpu impairs the downregulation of BST-2 from the cell surface and the enhancement of virion release (8, 26, 33), we hypothesized that Vpu facilitates the ubiquitination of BST-2. To test this, we transiently expressed BST-2, ubiquitin-HA, and Vpu in 293T cells; we then immunoprecipitated BST-2 from cellular lysates using antibody recognizing the BST-2 ectodomain and analyzed the immunoprecipitates (IPs) by Western blotting. Precipitated proteins were eluted under nonreducing conditions to avoid potential deubiquitination of cysteine residues (5), as well as under reducing conditions to achieve better resolution of the protein bands.
Figure 1A shows that BST-2 is ubiquitinated under these conditions even in the absence of Vpu. The predominant band of approximately 50 kDa under reducing conditions, in addition to a number of other species, most likely represents oligo- and polyubiquitination of BST-2, as reported previously (27). The lower bands of this ubiquitin-HA ladder overlapped in size, with the higher bands on the BST-2 blot under both nonreducing and reducing conditions. However, the main ubiquitin-HA bands were not detectable with anti-BST-2 antibody and probably represent either a minor fraction of BST-2 or other ubiquitinated proteins that interact with BST-2.
FIG. 1.
HIV-1 Vpu induces the ubiquitination of proteins in BST-2 immunoprecipitates. (A) 293T cells were transfected to express transiently BST-2, ubiquitin (Ub)-HA, and either empty plasmid or a codon-optimized version of HIV-1NL4-3 Vpu. Where indicated, the mutant version of Vpu, Vpu-S52/56N (2/6) was used. At 24 h posttransfection, cells were lysed under nondenaturing conditions, and BST-2 was immunoprecipitated using a monoclonal antibody to the ectodomain of BST-2. The IPs were eluted in Laemmli buffer without β-mercaptoethanol ([β-ME] upper panel), which was added later to half of the eluates to produce reduced samples (lower panel). The IPs were analyzed by Western blotting (WB) using anti-BST-2 antibody for an internal loading control, anti-Vpu antibody to assess the interaction with Vpu, and anti-HA antibody for ubiquitin-HA. Cells not transfected with BST-2 were used as a negative control. One percent of the input was loaded in the first lane from the left. The results shown are representative of five independent experiments. (B) 293T cells were transfected to express transiently BST-2, β-TrCP-HA, and one of the following: empty plasmid, plasmid encoding Vpu, or plasmid encoding Vpu2/6. The IP was performed as described for panel A. Monoclonal anti-HA antibody was used to detect β-TrCP-HA, whereas monoclonal antibody to ubiquitin was used to detect ubiquitin. The results shown are representative of five independent experiments.
The ubiquitination of BST-2 was enhanced in the presence of Vpu, as observed under both reducing and nonreducing conditions (Fig. 1A). This effect was abolished when the Vpu2/6 mutant (in which serines 52 and 56 are replaced with asparagines, with the result that the mutant cannot bind β-TrCP) was expressed although Vpu2/6 efficiently interacted with BST-2. This ubiquitination is specific to BST-2 or BST-2-associated proteins since no ubiquitin was detected in IPs from cells not expressing BST-2. Notably, the amount of ubiquitinated proteins in the input lysates of 293T cells transiently overexpressing ubiquitin appeared to be slightly increased in the presence of Vpu (see Fig. 8A); this might represent BST-2 along with other Vpu-dependent ubiquitination targets, such as CD4 (25). To ensure that these effects were not dependent on the overexpression of ubiquitin-HA, we detected endogenous ubiquitin in the IPs of BST-2 obtained from 293T cells transiently expressing BST-2 and β-TrCP in the presence or absence of Vpu. Figure 1B shows that in addition to the enhanced appearance of bands also seen in the absence of Vpu, unique ubiquitin-positive species appeared in the IPs of BST-2 in the presence of both Vpu and β-TrCP. One of these species, around 35 kDa, also probed for BST-2 and might represent a monoubiquitinated, highly glycosylated form of BST-2. As expected, β-TrCP interacted with BST-2 only in the presence of Vpu and not Vpu2/6 although Vpu2/6 was efficiently recruited to BST-2. Notably, the amount of ubiquitinated proteins in the input samples under these conditions was equal (see Fig. 8B).
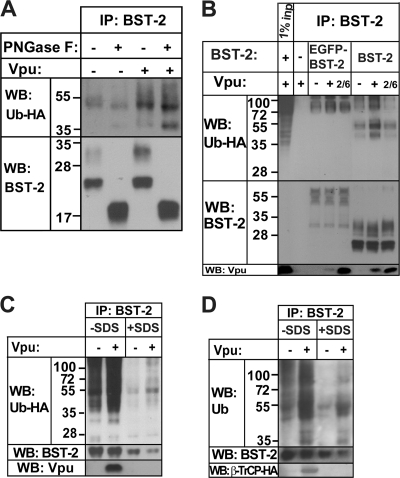
The ubiquitinated species observed in the IPs of BST-2 might be BST-2 itself or other proteins that are recruited to BST-2 and ubiquitinated in response to Vpu. To distinguish between these possibilities, we took two approaches: mobility shift of BST-2 and IP of BST-2 under denaturing conditions. The mobility of BST-2 was changed by treating BST-2 IPs with the glycosidase PNGase F as well as by using EGFP-tagged BST-2. The mobility of ubiquitinated species was assessed by detecting ubiquitin-HA. BST-2 acquires α-linked high-mannose glycosyl chains in the ER and N-linked glycosyl chains in the Golgi compartment. Upon treatment with PNGase F, all glycosyl groups are removed, which shifts the mobility of BST-2. Figure 2A shows that the immunoprecipitated glycosylated and nonglycosylated BST-2 bands that run at 30 and 25 kDa collapse into a doublet that runs at 19 kDa after PNGase F treatment, as reported previously (2). This treatment shifted the mobility of the ubiquitin-HA bands in BST-2 IPs and caused accumulation of a 40-kDa species. A minor shift was observed in the mobility of the prominent 50-kDa band, which might represent unglycosylated BST-2 that is polyubiquitinated. We also expressed EGFP-BST-2 and observed larger ubiquitinated species in the GFP-BST-2 IPs than in the native BST-2 IPs (Fig. 2B). This suggests that the ubiquitinated species are shifted in size along with BST-2 and are likely BST-2 itself. Despite the weak interaction between EGFP-BST-2 and Vpu, the Vpu-dependent increase in the ubiquitination of BST-2 was maintained, and this required serines 52 and 56 in Vpu (Fig. 2B).
FIG. 2.
The ubiquitinated species in BST-2 immunoprecipitates are likely BST-2 itself. (A) The transfection of 293T cells and IP was performed as described in the legend of Fig. 1A. Sodium phosphate (to a concentration of 50 mM) and NP-40 (to a concentration of 1%) were added directly to the IPs in Laemmli buffer before treatment with PNGase F. For each 10-cm2 dish, 2.5 units of PNGase F (Sigma) was added, followed by incubation at 37°C for 6 h. (B) The transfection of 293T cells and IP were performed as described in the legend of Fig. 1A, except that EGFP-BST-2 was used. Cells transfected with empty EGFP plasmid were used as a no-BST-2 negative control. (C) The transfection of 293T cells and IP were performed as described in the legend of Fig. 1A. IPs were eluted in Laemmli buffer, with boiling. Half of the sample was diluted 35 times with lysis buffer and reimmunoprecipitated using monoclonal anti-BST-2 antibody prebound to anti-mouse IgG magnetic beads. (D) The transfection of 293T cells was performed as described in the legend of Fig. 1B, and IP was performed as described in the legend of Fig. 2C, except that two-thirds of the sample was used for reimmunoprecipitation.
To further ensure the ubiquitination of BST-2, we reimmunoprecipitated BST-2 from the initial IPs after subjecting them to boiling with 1% SDS and either 10 mM dithiothreitol (DTT) or 10% β-mercaptoethanol, which we expected to disrupt protein complexes. Figure 2C shows that the Vpu-dependent increase in the ubiquitination of BST-2 IPs obtained from cells overexpressing ubiquitin-HA was maintained even after proteins were denatured and reduced. Similar results were obtained from cells expressing endogenous ubiquitin; the Vpu-dependent increase in ubiquitination as well as the appearance of the new ubiquitin-positive bands was maintained after proteins were denatured and reduced (Fig. 2D). The interaction between BST-2 and Vpu or between BST-2 and the Vpu/β-TrCP complex was abrogated under these conditions, indicated by the absence of Vpu and β-TrCP in the reimmunoprecipitated samples (Fig. 2C and D). This confirms that in these IPs protein complexes were disrupted, and all that is reimmunoprecipitated is probably BST-2. These data indicate that at least some of the ubiquitinated species that appear in the IPs of BST-2 in response to Vpu are, indeed, BST-2.
Expression of putative ubiquitin acceptor site mutants of BST-2.
We next sought to determine the targets of the ubiquitination in BST-2. Only the CD of BST-2 is exposed to the ubiquitination machinery, and we assumed this to be the region targeted by Vpu and the β-TrCP E3 ligase complex, at least in the absence of endoplasmic-reticulum associated degradation (ERAD). The CD of human BST-2 contains two lysines, two cysteines, and a serine-threonine-serine (STS) sequence (Fig. 3A). Both lysines and cysteine 20 are conserved between human and nonhuman primate BST-2 proteins, including those resistant to Vpu, and cysteine 9 is conserved in all but the rhesus macaque protein (summarized in Fig. 3A). The ST sequence of the uniquely human STS sequence correlates with the sensitivity of BST-2 to Vpu. We generated a series of BST-2 mutants in which these potential ubiquitination sites were replaced: lysines 18 and 21 with arginines (KK), cysteines 9 and 20 with alanines (CC), and the serine-threonine-serine sequence at positions 3 to 5 with alanines (STS); we also generated combinations including KK-CC and KK-CC-STS or KCST-less, in which all potential ubiquitination sites were mutated (Fig. 3A, highlighted residues).
FIG. 3.
Mutated residues in the cytoplasmic domain of BST-2 and the expression of potential ubiquitination site mutants. (A) Sequence alignment of the CDs of BST-2 proteins from humans, African green monkeys, pigtail macaques, and rhesus macaques. The conserved residues are indicated with dashes. The responsiveness of BST-2 proteins to HIV-1 Vpu as reported previously is indicated to the right (23, 39, 40). S, sensitive; R, resistant. The mutated residues are highlighted. (B) 293T cells were transfected in a six-well format with 3 μg of each BST-2- and Vpu-expressing plasmid. At 24 h posttransfection, the cells were lysed and cleared as described in Materials and Methods. The lysates were boiled in Laemmli buffer and analyzed by Western blotting for Vpu, actin, and BST-2.
First, we assessed the expression of the mutated BST-2 proteins by Western blotting. All the mutants were normally dimerized and glycosylated but appeared to be more stable than the wild-type protein (Fig. 3B). Reduction of the intracellular level of BST-2 by Vpu in these transiently expressing 293T cells was modest under these conditions (but see Fig. 7, where such reductions are more evident).
We also determined the intracellular distribution of the BST-2 mutants as well as their colocalization with Vpu. First, we transfected HT-1080 cells, which do not express BST-2, with plasmids expressing BST-2 and stained the cells for BST-2 and transferrin receptor (TfR), a marker for early sorting and recycling endosomes. Figure 4 shows partial colocalization between BST-2 and TfR in both peripheral endosomes and the juxtanuclear region, as reported previously (30). A few of the cells expressing wild-type BST-2 displayed enlarged vesicular structures that contained BST-2 and were partly positive for TfR. The BST-2 mutants were distributed similarly to the wild-type protein, and they colocalized similarly with TfR (Fig. 4). Of note, enlarged vesicular structures were more apparent in the cells expressing the mutants, perhaps as a consequence of their greater levels of expression. Overall, these data indicated that the BST-2 CD mutants were distributed within the endosomal system similarly to the wild-type protein.
FIG. 4.
Subcellular distribution and colocalization of the BST-2 mutants with transferrin receptor (TfR). HT-1080 cells were transfected with a plasmid expressing either wild-type BST-2 (WT) or the BST-2 mutants. BST-2 and endogenous TfR were visualized as described in Materials and Methods, using polyclonal rabbit anti-BST-2 and monoclonal mouse anti-TfR antibodies. The distribution of the proteins in the same field is shown separately in black and white, as well as together as merged images in which BST-2 is red and TfR is green. Scale bar, 10 μm.
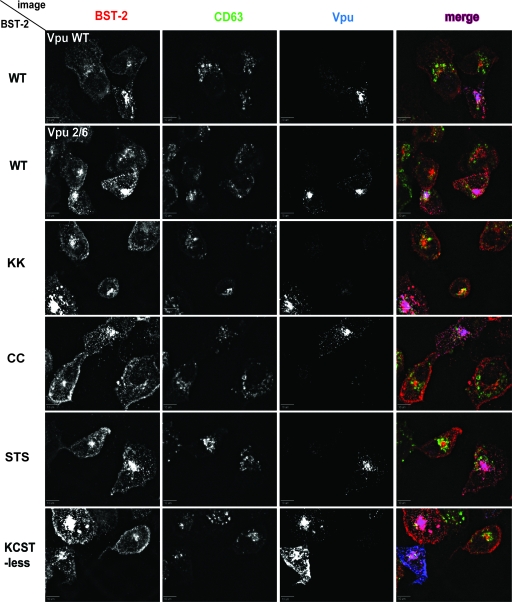
To assess the colocalization of the BST-2 mutants with Vpu, we cotransfected HT-1080 cells with plasmids expressing BST-2 and Vpu and stained the cells for BST-2, Vpu, and the lysosomal protein CD63 (Fig. 5). The effect of wild-type Vpu on the distribution of BST-2 was modest but consistent. In the case of wild-type BST-2, the amount of signal at the cell surface was decreased, while the intracellular signal was increased, presumably due to a redistribution of the protein by Vpu, as reported previously (9). Partial colocalization of BST-2 with CD63 was observed, but Vpu did not markedly increase this. The effect of Vpu2/6 on the surface expression of BST-2 was variable: the distribution of BST-2 ranged from similar to that of cells expressing wild-type Vpu to that observed in the cell at the center of the Vpu2/6 field, where large BST-2 puncta accumulated at the surface. Overall, the distributions of the BST-2 CD mutants overlapped similarly with the distribution of wild-type Vpu. Whether the BST-2 mutants were affected differentially by Vpu was difficult to assess although in some cells the surface downregulation was clearly impaired, such as is seen in the upper left cell in the KCST-less field in Fig. 5.
FIG. 5.
Colocalization of the BST-2 mutants with CD63 and Vpu. HT-1080 cells were transfected with a plasmid expressing either wild-type (WT) BST-2 or the BST-2 mutants, together with a plasmid expressing either wild-type Vpu or Vpu2/6. BST-2 mutant proteins were coexpressed only with wild-type Vpu. BST-2, Vpu, and endogenous CD63 were visualized as described in Materials and Methods, using monoclonal mouse anti-BST-2, rabbit antiserum to HIV-1 Vpu, and FITC-conjugated mouse monoclonal anti-CD63 antibodies. The distribution of the proteins in the same field is shown separately in black and white, as well as together as merged images in which BST-2 is red, CD63 is green, and Vpu is blue. Scale bar, 10 μm. The results shown are representative of two independent experiments.
The STS sequence in the CD of BST-2 is required for optimal Vpu-mediated downregulation of BST-2 from the cell surface and the counteraction of virion release.
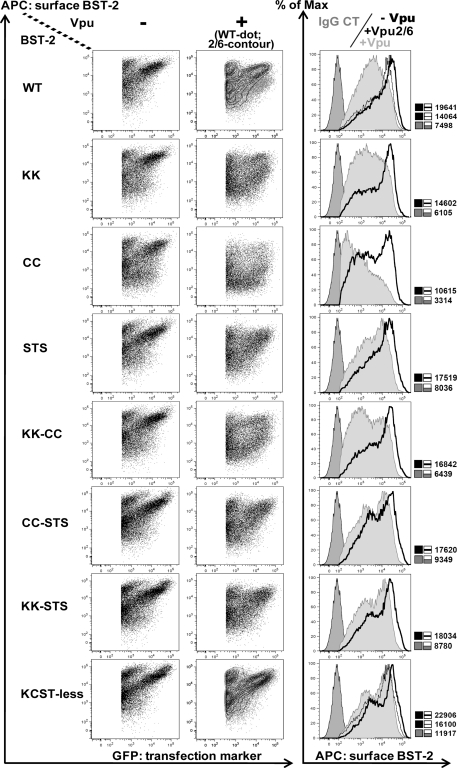
To quantitatively assess the surface expression of the BST-2 mutants in the absence and presence of Vpu, we analyzed transiently expressing 293T cells using flow cytometry. As shown in Fig. 6, the surface expression of the mutants in the absence of Vpu was similar to that of wild-type BST-2, with the exception of the CC mutant, whose mean fluorescence intensity was slightly lower. Vpu downregulated wild-type BST-2 by approximately 3-fold, whereas the activity of Vpu2/6 was markedly impaired, consistent with previous reports (8, 33). The downregulation by wild-type Vpu was slightly more pronounced in the case of BST-2/CC, possibly due to the modest defect in the surface expression of this BST-2 mutant even in the absence of Vpu.
FIG. 6.
The STS sequence in the CD of human BST-2 is required for optimal downregulation from the cell surface by Vpu. 293T cells were transfected with 60 ng of BST-2 and 500 ng of either empty plasmid or plasmid expressing Vpu, as well as 250 ng of plasmid expressing GFP as a transfection marker, in a 12-well format. At 24 h posttransfection, cells were stained for surface BST-2 using mouse monoclonal anti-BST-2 antibody and analyzed by two-color flow cytometry. Dot plots represent the amount of BST-2 on the cell surface versus the amount of GFP signal for the individual cells. Contour plots represent the distribution of cells expressing Vpu2/6. Histograms represent the relative cell number versus BST-2 fluorescence intensity for the population of cells shown in the dot plots. In the histograms, the far left peaks represent the IgG2a antibody isotype negative control, thick lines represent cells not expressing Vpu, and gray-shaded peaks represent cells expressing Vpu. Thin lines represent cells expressing Vpu2/6. The MFI values for each distribution are to the right of each histogram. The results shown are representative of three independent experiments. Max, maximum.
Strikingly, the STS mutant and all mutants containing the STS substitution were relatively resistant to downregulation by Vpu, indicating that the STS sequence confers responsiveness to Vpu. The combination of KCST-less BST-2 with Vpu2/6 did not further impair the phenotype of Vpu (mean fluorescence intensity [MFI] of 22,906 without Vpu versus 16,100 with Vpu2/6), relative to the combination of wild-type BST-2 with Vpu2/6 (MFI of 19,641 versus 14,064). This indicates that these mutations in the CDs of BST-2 and Vpu were not additive, consistent with their hypothesized involvement in the same pathway, the ubiquitination of BST-2.
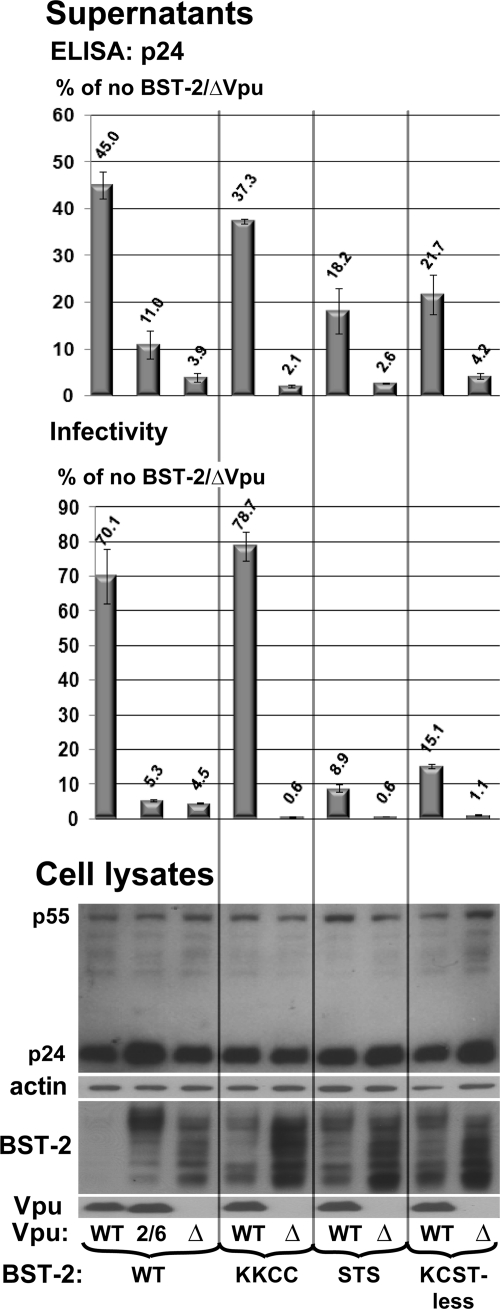
We next examined the KK-CC, STS, and KCST-less mutants in a virion release assay. 293T cells were transfected with proviral HIV-1 DNA together with BST-2 expression plasmids. The concentration of virion-associated p24 capsid antigen in the supernatants of these cultures was measured after centrifugation of the virions through 20% sucrose. The infectivity of the supernatants was also measured using an infectious center assay (Fig. 7). The amount of p55 Gag precursor was roughly the same in every sample, as assessed by Western blotting of the lysates of the cells, whereas the amount of BST-2 reflected the apparent increased stability of the mutants. In the absence of Vpu, cells expressing BST-2 released HIV-1 virions markedly less efficiently than cells without BST-2, and this restriction was counteracted when Vpu was expressed. The restriction of virion release by BST-2 was quantitatively similar when measured by p24 antigen concentration or by infectivity.
FIG. 7.
The STS sequence is required for optimal counteraction of restricted virion release by Vpu. 293T cells were transfected in duplicate to express transiently wild-type (WT), vpu-negative (Δ), or Vpu2/6-containing HIV-1 proviral DNA along with either an empty plasmid (not shown) or a plasmid expressing BST-2, as well as a plasmid expressing GFP as a transfection marker, as described in Materials and Methods. The supernatants of the cultures were collected 24 h posttransfection and analyzed by ELISA for p24 capsid antigen after centrifugation through a 20% sucrose cushion and by an infectious-center assay using HeLa cells that express CD4 as targets (infectivity). The values graphed are the percentages of the values obtained from the cells expressing HIV-1/ΔVpu and not expressing BST-2. The error bars are the average deviation obtained from two independent measurements of the duplicate transfections. The cell pellets were lysed in Laemmli buffer and subjected to Western blotting using anti-p24/55, anti-Vpu, anti-actin, and rabbit polyclonal anti-BST-2 antibodies. The results shown are representative of two independent experiments.
In the presence of BST-2 WT, HIV-1 WT virions were released more efficiently than ΔVpu virions (12-fold when measured by p24 and 16-fold when measured by infectivity), while Vpu2/6 virions exhibited an intermediate phenotype. The BST-2/KK-CC mutant was efficiently counteracted by Vpu. However, the release of HIV-1 WT virions from cells expressing the BST-2/STS mutant or the KCST-less BST-2 was substantially impaired. Interestingly, each of the mutants restricted ΔVpu virions slightly more efficiently than BST-2 WT; this was especially evident in the infectivity assay. This effect might be due to the higher levels of expression of these mutants, as detected by Western blotting. Nevertheless, the BST-2/KK-CC mutant was efficiently counteracted by Vpu, whereas both the BST-2/STS mutant and KCST-less BST-2 were not, despite the similar levels of expression of these mutants compared to each other in either the presence or absence of Vpu (Fig. 7). These results indicated that of each of these potential ubiquitination sites, STS is the key site involved in Vpu responsiveness. The results also indicated that virologic responsiveness to Vpu did not correlate with the total cellular level of BST-2.
To ensure consistency between the measurement of surface downregulation, in which Vpu was expressed from a codon-optimized expression construct (Fig. 6), and the measurement of virion release, in which Vpu was expressed from proviral DNA, we directly tested the cells used in the virion release assay for their surface expression of BST-2. Consistent with the findings shown in Fig. 6, BST-2/KK-CC responded to Vpu, whereas the responses of both BST-2/STS and KCST-less BST-2 to Vpu were impaired (see Fig. S1 in the supplemental material). Taken together, these surface expression and virion release data revealed a functional requirement for the STS sequence in the downregulation and counteraction of BST-2 by Vpu.
All potential acceptor sites in the CD of BST-2 contribute to Vpu-induced ubiquitination.
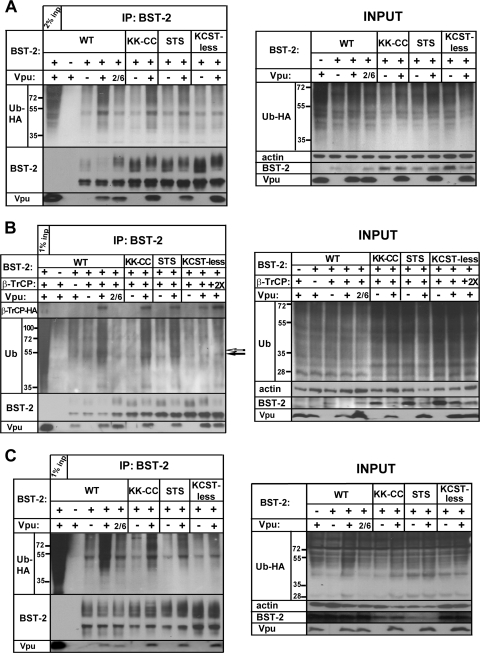
To determine whether these mutations affected the ubiquitination of BST-2, we used the two approaches presented in Fig. 1, transient expression of BST-2 in combination with either ubiquitin-HA (Fig. 8A) or β-TrCP overexpression (Fig. 8B). We also expressed BST-2 stably in 293T cells and combined this with transient expression of ubiquitin-HA (Fig. 8C).
FIG. 8.
All potential acceptor sites in the CD of BST-2 are ubiquitinated by Vpu. (A) 293T cells were transfected, and BST-2 was immunoprecipitated as described in the legend of Fig. 1A (coexpression of ubiquitin-HA), except that the BST-2 mutants KK-CC, STS, and KCST-less were tested in addition to wild-type BST-2. The input samples shown in the right panel are the cell lysates before IP. The amount of actin in the input samples was assessed as a loading control. The immunoprecipitated proteins were probed for BST-2, ubiquitin-HA, and Vpu. The results shown are representative of two independent experiments. (B) 293T cells were transfected, and BST-2 was immunoprecipitated as described in the legend of Fig. 1B (coexpression of β-TrCP-HA) except that the BST-2 mutants KK-CC, STS, and KCST-less were tested in addition to wild-type BST-2. The immunoprecipitated proteins were probed for BST-2, endogenous ubiquitin, β-TrCP-HA, and Vpu. A band that is induced by Vpu but disappears in the case of KCST-less BST-2 is indicated by an open arrow; a band that is increased by Vpu but remains detectable in the case of KCST-less BST-2 is indicated by a filled arrow. The results shown are representative of two independent experiments. (C) 293T cells stably expressing BST-2 WT or the BST-2 mutants KK-CC, STS, and KCST-less were transfected with empty plasmid or Vpu- or Vpu2/6-expressing plasmid, along with a plasmid expressing ubiquitin-HA, and BST-2 was immunoprecipitated as described in Materials and Methods. The input samples shown in the right panel are the cell lysates before IP. The amount of actin in the input samples was assessed as a loading control. The results shown are representative of two independent experiments.
Vpu stimulated the ubiquitination of BST-2 by 2- to 3-fold when BST-2 was expressed transiently (Fig. 8A and B). When BST-2 was expressed stably, this stimulation was about 6-fold (Fig. 8C). The Vpu-dependent increase in ubiquitination was decreased ∼40% by mutation of the KK-CC residues and ∼60% by mutation of the STS residues. In cells overexpressing ubiquitin-HA, no Vpu-dependent increase in ubiquitination was observed in the case of KCST-less BST-2 (Fig. 8A and C). In cells expressing endogenous ubiquitin but overexpressing β-TrCP, Vpu enhanced the overall levels of ubiquitinated proteins in the BST-2 IPs, and it induced the appearance of new ubiquitin-positive bands, in particular, a species of around 55 kDa, indicated by an open arrow in Fig. 8B. This band was diminished in the cases of BST-2/KK-CC and BST-2/STS, and it was absent in the case of KCST-less BST-2, even when we used twice as much β-TrCP-encoding DNA in the transfection in an attempt to further stimulate ubiquitination. The 55-kDa band might represent BST-2 that is oligoubiquitinated at K, C, S, and/or T residues. Notably, a clear shift in the mobility of this band was not observed when either the KK-CC or STS sequence alone was mutated. This suggests that the use of either or both sites yields ubiquitinated species of nearly the same sizes. Alternatively, the 55-kDa band could represent a protein other than BST-2, but the data of Fig. 2 weigh against this.
We noted the residual ubiquitination of KCST-less BST-2 in the absence of Vpu in all three systems. However, the extent of this ubiquitination was similar to that of the wild-type BST-2 in the absence of Vpu. This basal level of ubiquitination is Vpu independent and apparently occurs at the residues other than K, C, S, and T in the CD of BST-2.
Vpu coimmunoprecipitated with each BST-2 mutant as well as or better than it did with wild-type BST-2 (Fig. 8). Furthermore, in the presence of Vpu, β-TrCP coimmunoprecipitated with each BST-2 mutant as well as it did with wild-type BST-2 (Fig. 8B). These findings weighed against the possibility that the mutants were inefficiently ubiquitinated due to impaired recruitment of the ubiquitination machinery.
Overall, these data indicate that three Vpu-dependent effects—stimulation of the ubiquitination of BST-2, the downregulation of BST-2 from the cell surface, and the enhancement of virion release—are connected by the role of the STS sequence. Vpu enhanced the ubiquitination of all potential sites in the CD of BST-2, but the STS sequence was the key Vpu-responsive element. The data suggest that the surface downregulation of BST-2 by Vpu and the relief of restricted virion release occur in a manner dependent on serine-threonine ubiquitination.
DISCUSSION
Previously, we reported the involvement of the β-TrCP E3 ligase complex in the downregulation of BST-2 and the counteraction of restricted virion release by Vpu (33), findings confirmed in two follow-up studies (8, 26). Here, we show that the ubiquitination of proteins in IPs of BST-2 is increased when Vpu is expressed. This increase includes the appearance of new ubiquitin-positive species and is absent when a Vpu mutant unable to recruit the β-TrCP subunit of the E3 complex (Vpu2/6) is expressed. Vpu cannot stimulate ubiquitination when all the potential ubiquitin acceptors in the CD of BST-2 are mutated (KCST-less BST-2), even though this mutant interacts normally with Vpu and Vpu/β-TrCP complex. Although K, C, S, and T residues in the BST-2 CD can apparently each contribute to Vpu-mediated ubiquitination, only the STS sequence is required for optimal downregulation of BST-2 and the optimal enhancement of virion-release by Vpu.
These data support the hypothesis that the BST-2 mutants herein have lost Vpu responsiveness due to mutation of an STS ubiquitin acceptor site. Considerable data weigh against alternative hypotheses. For example, BST-2/STS appears to be more stable than wild-type BST-2; in principle this might render BST-2 less responsive to Vpu. However, the model in which Vpu downregulates the intracellular level of BST-2 to counteract restricted virion release has been challenged (9, 12, 33, 34, 41). Furthermore, two lines of evidence herein weigh against this possibility. First, the levels of the expression at the cell surface and total cellular level of BST-2/STS in the absence of Vpu are similar to those of BST-2/KK-CC; yet BST-2/KK-CC is efficiently downregulated from the cell surface by Vpu, whereas BST-2/STS is not. Second, the restriction of virion release by BST-2/KK-CC is efficiently counteracted by Vpu, whereas the restriction by BST-2/STS is not. In principle, BST-2/STS might be less responsive to Vpu because of altered trafficking. However, unlike BST-2 with a deleted CD, which is trapped in the ER (35), the mutants described here localize normally within the endosomal system. All of the mutants colocalize well with Vpu, and they interact well with Vpu and Vpu/β-TrCP complex in the co-IP assays. These data suggest that the inability of BST-2/STS to respond normally to Vpu is caused neither by increased levels of expression nor by hampered delivery to Vpu-positive endosomal compartments.
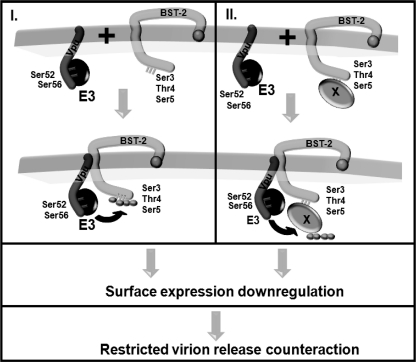
We propose that the ubiquitination of the STS sequence by the Vpu/β-TrCP E3 ligase complex enables optimal removal of BST-2 from the cell surface and the relief of restricted virion release (Fig. 9, model I). The mutation of the BST-2 STS sequence results in a Vpu2/6-like phenotype in the surface downregulation and virion release assays, a similarity consistent with the notion that the STS sequence is the key ubiquitination target. Mutating the lysines and the cysteines in addition to the serines and threonines did not produce a greater defect in Vpu responsiveness, suggesting that although these residues appear to be ubiquitinated by Vpu, they do not contribute to the surface downregulation of BST-2 or to the counteraction of restricted virion release.
FIG. 9.
Models for the ubiquitin-mediated downregulation of BST-2 by Vpu and the role of the STS sequence. In model I, Vpu recruits the β-TrCP-containing E3 ubiquitin ligase complex to directly ubiquitinate the BST-2 CD at S and T residues. This triggers the removal of BST-2 from the cell surface and the consequent relief of the restricted virion release. In model II, Vpu recruits the β-TrCP-containing E3 ubiquitin ligase complex to ubiquitinate factor X, which interacts with the STS sequence in the CD of BST-2. The ubiquitination of factor X triggers the downregulation of BST-2 from the cell surface and the consequent relief of the restricted virion release.
We considered, however, an alternative hypothesis: the STS sequence might be required for the interaction of BST-2 with an unidentified factor that is either itself ubiquitinated (Fig. 9, model II) or is a cofactor for ubiquitination elsewhere on the BST-2 protein. In these scenarios, the Vpu/β-TrCP E3 ligase complex ubiquitinates either factor “X” or BST-2 residues other than the cytosolic K, C, S, and T of BST-2.
We favor the first model, in which BST-2 is directly ubiquitinated, for several reasons based on the data herein. First, species of the same molecular mass probe for both BST-2 and ubiquitin in BST-2 IPs are detected when Vpu is expressed. Second, the glycosidase PNGase F shifts the mobility of ubiquitinated species in IPs of BST-2, indicating that some of these species are glycosylated and are probably BST-2. Third, the mobility of ubiquitinated species is shifted appropriately when EGFP-BST-2 is used in place of native BST-2; this would not occur if the ubiquitinated species were BST-2 interactors rather than BST-2 itself. Fourth, ubiquitinated species were successfully reimmunoprecipitated after boiling under denaturing and reducing conditions, which disrupts protein complexes and, indeed, dissociated Vpu from BST-2. All of the preceding observations suggest that BST-2 is the protein that is ubiquitinated in these IPs. In contrast, the evidence supporting the model involving a factor X or ubiquitination of BST-2 elsewhere is primarily the persistence of ubiquitinated species in the IPs of KCST-less BST-2. However, constitutive ubiquitination at levels similar to that of KCST-less BST-2 in the presence of Vpu was evident in the case of wild-type BST-2 in the absence of Vpu. Thus, we conclude that constitutive, Vpu-independent ubiquitination of BST-2 likely occurs elsewhere on the protein. In contrast, the ubiquitination of K, C, S, and T residues in the CD of BST-2 occurs in a Vpu-dependent manner. Another argument against the involvement of factor X is that mutating the STS sequence is sufficient to nearly abolish the counteraction of BST-2 by Vpu but not the ubiquitination of proteins in the BST-2 IPs. If mutating the K and C residues compromises the interaction between BST-2 and factor X, why does it not compromise Vpu counteraction? These observations support functionally important ubiquitination at the STS sequence of BST-2 and weigh against the involvement of a factor X that is recruited by the STS sequence.
Ultimately, mass spectrometry of the BST-2 IPs might be necessary to definitively document the ubiquitination of BST-2 at serine and threonine residues. Moreover, this approach might identify cellular factors recruited to BST-2 and/or ubiquitinated in a Vpu-dependent manner. Such proteins would function as cofactors for the downregulation of BST-2 and might be components of clathrin adaptor complexes, such as Eps15, or ESCRT pathway components, such as Hrs or TSG101, all of which are modified by or recognize ubiquitin (29, 32).
BST-2/STS is the first described human BST-2 variant that loses responsiveness to Vpu due to a mutation in its CD. BST-2/K18,21R (here, BST-2/KK) was reported as resistant to Vpu-mediated degradation and impaired for ubiquitination in response to Vpu, but these effects were not associated with resistance to surface downregulation or to Vpu-mediated counteraction of restriction (12, 37; also this study). Lysines 18 and 21 could be responsible for a degradation component of a multifaceted mechanism of Vpu action, but these ubiquitination sites might be unnecessary under certain conditions, such as relatively low levels of expression of BST-2. In contrast to the case of Vpu, BST-2/KK is not susceptible to downregulation by the KSHV ubiquitin ligase K5 (27, 37). These data raise the question of why Vpu responsiveness would specifically require ubiquitination of the STS sequence; we wonder whether the cellular proteins that recognize ubiquitinated BST-2 might be different in the cases of lysine or serine/threonine ubiquitination.
How does ubiquitination of the STS sequence fit with the clear role of the BST-2 TMD in the susceptibility to Vpu? BST-2 proteins from certain nonhuman primates are rendered responsive to Vpu by insertion of the human TMD, despite lacking the STS sequence (20, 31). However, close examination reveals that the TMD of human BST-2 provides an incomplete rescue of responsiveness (20). Apparently, the CD of BST-2 contains additional determinants of Vpu sensitivity, and we suggest that the STS sequence is such a determinant. Notably, we did not assess the contribution of single residues within the STS sequence. However, the BST-2 proteins of gorillas and chimpanzees contain an STL sequence together with a TMD that is nearly identical to that of the human protein. Chimpanzee BST-2 has a methionine at position 35, whereas human and gorilla BST-2s have valines. The fact that these BST-2 proteins are responsive to Vpu might narrow the region determining Vpu sensitivity to the first serine and threonine (23, 40).
The Vpu/β-TrCP E3 ligase complex joins the K3 and K5 RING-finger ubiquitin ligases as mediators of virus-induced ubiquitination of nonlysine residues (4, 5, 25, 45). Lysine-independent ubiquitination by cellular ubiquitin ligases has been suggested (42, 46), but the human β-TrCP-SCF E3 ligase complex provides the first described cellular example of such ubiquitination (25; also this study). We speculate that nonlysine ubiquitination might be more attractive to viruses than lysine ubiquitination, but why this would be is not obvious. Perhaps the thioester and ester bonds of ubiquitin-modified C, S, and T residues are differentially labile in comparison to the isopeptide bond of modified lysines, or perhaps deubiquitinating enzymes recognize these bonds differentially. Interestingly, whereas ubiquitination of C and K residues in MHC-I by KSHV K3 (kK3) and kK5 induces the endocytosis and lysosomal degradation of MHC-I, the ubiquitination of S, T, and K residues in MHC-I by murine K3 (mK3) induces ER dislocation and proteosomal degradation (3a, 6). The ubiquitination of BST-2 by Vpu at all possible ubiquitin acceptors consequently provides the potential for a multifaceted mechanism of Vpu activity that involves both ER-associated proteosomal degradation and defective trafficking within the endosomal system.
A specific Vpu-induced defect in the trafficking of BST-2 is not identified here although we confirm that decreased surface levels rather than decreased total cellular levels of BST-2 correlate with the relief or restriction. Presumably, the ubiquitination of BST-2 contributes to its downregulation from the cell surface. Ubiquitin is an endocytic signal, but the rate of endocytosis of BST-2 appears unaffected by Vpu (9, 33). We favor the hypothesis that the ubiquitination of BST-2 modifies the postendocytic trafficking of the protein, potentially by blocking its recycling to the plasma membrane, but the precise alteration in trafficking induced by Vpu and the ubiquitin interacting proteins involved remain to be identified.
In summary, we show that the STS sequence in the CD of human BST-2 is required for the efficient downregulation of BST-2 from the cell surface by Vpu and consequently for the counteraction of restricted virion release. The data support a model in which Vpu recruits the β-TrCP-containing E3 ubiquitin ligase complex to directly ubiquitinate these serine and threonine residues as part of the mechanism by which HIV-1 counteracts this innate antiviral factor.
Supplementary Material
Acknowledgments
We thank Parris Jordan, Demetrius dela Cruz, Pokman Cheng, Neal Sekiya, Judy Nordberg, and Sherri Rostami for technical assistance provided by the University of California, San Diego, CFAR, David Lau for making the 293T cell line stably expressing BST-2, Klaus Strebel for the ΔVpu- and Vpu2/6-expressing proviral plasmids as well as the plasmid expressing codon-optimized Vpu, Paul Lehner for pcDNA3.1-ubiquitin-HA, Paula Cannon for EGFP-BST-2, Autumn Ruiz and Edward Stephens for pcDNA3.1-BST-2-WT and K18,21R, Florence Margottin-Goguet for pCS2-β-TrCP-HA, and Jacek Skowronski for pCG-GFP. Antibody to Vpu and rabbit anti-BST-2 was originated by Klaus Strebel and obtained from the National Institutes of Health AIDS Research and Reference Reagent Program. We thank Chugai Pharmaceuticals for the antibody to HM1.24/BST-2.
This work was supported by grants from the California AIDS Research Program of the University of California to A.A.T. and from the National Institutes of Health to J.C.G. (AI081668 and RO1AI081668-O1S1).
Footnotes
Published ahead of print on 27 October 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrew, A. J., E. Miyagi, S. Kao, and K. Strebel. 2009. The formation of cysteine-linked dimers of BST-2/tetherin is important for inhibition of HIV-1 virus release but not for sensitivity to Vpu. Retrovirology 6:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barry, M., and K. Fruh. 2006. Viral modulators of cullin RING ubiquitin ligases: culling the host defense. Sci. STKE 2006:pe21. [DOI] [PubMed]
- 3a.Boname, J. M., and P. G. Stevenson. 2001. MHC class I ubiquitination by a viral PHD/LAP finger protein. Immunity 15:627-636. [DOI] [PubMed] [Google Scholar]
- 4.Cadwell, K., and L. Coscoy. 2008. The specificities of Kaposi's sarcoma-associated herpesvirus-encoded E3 ubiquitin ligases are determined by the positions of lysine or cysteine residues within the intracytoplasmic domains of their targets. J. Virol. 82:4184-4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cadwell, K., and L. Coscoy. 2005. Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. Science 309:127-130. [DOI] [PubMed] [Google Scholar]
- 6.Coscoy, L., and D. Ganem. 2000. Kaposi's sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc. Natl. Acad. Sci. U. S. A. 97:8051-8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Day, J. R., L. E. Martinez, R. Sasik, D. L. Hitchin, M. E. Dueck, D. D. Richman, and J. C. Guatelli. 2006. A computer-based, image-analysis method to quantify HIV-1 infection in a single-cycle infectious center assay. J. Virol. Methods 137:125-133. [DOI] [PubMed] [Google Scholar]
- 8.Douglas, J. L., K. Viswanathan, M. N. McCarroll, J. K. Gustin, K. Fruh, and A. V. Moses. 2009. Vpu directs the degradation of the human immunodeficiency virus restriction factor BST-2/tetherin via a βTrCP-dependent mechanism. J. Virol. 83:7931-7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dube, M., B. B. Roy, P. Guiot-Guillain, J. Binette, J. Mercier, A. Chiasson, and E. A. Cohen. 2010. Antagonism of tetherin restriction of HIV-1 release by Vpu involves binding and sequestration of the restriction factor in a perinuclear compartment. PLoS Pathog. 6:e1000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duncan, L. M., S. Piper, R. B. Dodd, M. K. Saville, C. M. Sanderson, J. P. Luzio, and P. J. Lehner. 2006. Lysine-63-linked ubiquitination is required for endolysosomal degradation of class I molecules. EMBO J. 25:1635-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzpatrick, K., M. Skasko, T. J. Deerinck, J. Crum, M. H. Ellisman, and J. Guatelli. 2010. Direct restriction of virus release and incorporation of the interferon-induced protein BST-2 into HIV-1 particles. PLoS Pathog. 6:e1000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goffinet, C., S. Homann, I. Ambiel, N. Tibroni, D. Rupp, O. T. Keppler, and O. T. Fackler. 2010. Antagonism of CD317 restriction of human immunodeficiency virus type 1 (HIV-1) particle release and depletion of CD317 are separable activities of HIV-1 Vpu. J. Virol. 84:4089-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenberg, M. E., S. Bronson, M. Lock, M. Neumann, G. N. Pavlakis, and J. Skowronski. 1997. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 16:6964-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guatelli, J. C. 2009. Interactions of viral protein U (Vpu) with cellular factors. Curr. Top. Microbiol. Immunol. 339:27-45. [DOI] [PubMed] [Google Scholar]
- 15.Gupta, R. K., S. Hue, T. Schaller, E. Verschoor, D. Pillay, and G. J. Towers. 2009. Mutation of a single residue renders human tetherin resistant to HIV-1 Vpu-mediated depletion. PLoS Pathog. 5:e1000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauser, H., L. A. Lopez, S. J. Yang, J. E. Oldenburg, C. M. Exline, J. C. Guatelli, and P. M. Cannon. 2010. HIV-1 Vpu and HIV-2 Env counteract BST-2/tetherin by sequestration in a perinuclear compartment. Retrovirology 7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reference deleted.
- 18.Hicke, L., and R. Dunn. 2003. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu. Rev. Cell Dev. Biol. 19:141-172. [DOI] [PubMed] [Google Scholar]
- 19.Iwabu, Y., H. Fujita, M. Kinomoto, K. Kaneko, Y. Ishizaka, Y. Tanaka, T. Sata, and K. Tokunaga. 2009. HIV-1 accessory protein Vpu internalizes cell-surface BST-2/tetherin through transmembrane interactions leading to lysosomes. J. Biol. Chem. 284:35060-35072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia, B., R. Serra-Moreno, W. Neidermyer, A. Rahmberg, J. Mackey, I. B. Fofana, W. E. Johnson, S. Westmoreland, and D. T. Evans. 2009. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 5:e1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaletsky, R. L., J. R. Francica, C. Agrawal-Gamse, and P. Bates. 2009. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc. Natl. Acad. Sci. U. S. A. 106:2886-2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Tortorec, A., and S. J. Neil. 2009. Antagonism to and intracellular sequestration of human tetherin by the human immunodeficiency virus type 2 envelope glycoprotein. J. Virol. 83:11966-11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim, E. S., H. S. Malik, and M. Emerman. 2010. Ancient adaptive evolution of tetherin shaped the functions of Vpu and Nef in human immunodeficiency virus and primate lentiviruses. J. Virol. 84:7124-7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez, L. A., S. J. Yang, H. Hauser, C. M. Exline, K. G. Haworth, J. Oldenburg, and P. M. Cannon. 2010. Ebola virus glycoprotein counteracts BST-2/tetherin restriction in a sequence-independent manner that does not require tetherin surface removal. J. Virol. 84:7243-7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magadan, J. G., F. J. Perez-Victoria, R. Sougrat, Y. Ye, K. Strebel, and J. S. Bonifacino. 2010. Multilayered mechanism of CD4 downregulation by HIV-1 Vpu involving distinct ER retention and ERAD targeting steps. PLoS Pathog. 6:e1000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mangeat, B., G. Gers-Huber, M. Lehmann, M. Zufferey, J. Luban, and V. Piguet. 2009. HIV-1 Vpu neutralizes the antiviral factor Tetherin/BST-2 by binding it and directing its beta-TrCP2-dependent degradation. PLoS Pathog. 5:e1000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansouri, M., K. Viswanathan, J. L. Douglas, J. Hines, J. Gustin, A. V. Moses, and K. Fruh. 2009. Molecular mechanism of BST2/tetherin downregulation by K5/MIR2 of Kaposi's sarcoma-associated herpesvirus. J. Virol. 83:9672-9681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Margottin-Goguet, F., J. Y. Hsu, A. Loktev, H. M. Hsieh, J. D. Reimann, and P. K. Jackson. 2003. Prophase destruction of Emi1 by the SCF(betaTrCP/Slimb) ubiquitin ligase activates the anaphase promoting complex to allow progression beyond prometaphase. Dev. Cell 4:813-826. [DOI] [PubMed] [Google Scholar]
- 29.Martin-Serrano, J. 2007. The role of ubiquitin in retroviral egress. Traffic 8:1297-1303. [DOI] [PubMed] [Google Scholar]
- 30.Masuyama, N., T. Kuronita, R. Tanaka, T. Muto, Y. Hirota, A. Takigawa, H. Fujita, Y. Aso, J. Amano, and Y. Tanaka. 2009. HM1.24 is internalized from lipid rafts by clathrin-mediated endocytosis through interaction with alpha-adaptin. J. Biol. Chem. 284:15927-15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNatt, M. W., T. Zang, T. Hatziioannou, M. Bartlett, I. B. Fofana, W. E. Johnson, S. J. Neil, and P. D. Bieniasz. 2009. Species-specific activity of HIV-1 Vpu and positive selection of tetherin transmembrane domain variants. PLoS Pathog. 5:e1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miranda, M., and A. Sorkin. 2007. Regulation of receptors and transporters by ubiquitination: new insights into surprisingly similar mechanisms. Mol. Interv. 7:157-167. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell, R. S., C. Katsura, M. A. Skasko, K. Fitzpatrick, D. Lau, A. Ruiz, E. B. Stephens, F. Margottin-Goguet, R. Benarous, and J. C. Guatelli. 2009. Vpu antagonizes BST-2-mediated restriction of HIV-1 release via beta-TrCP and endo-lysosomal trafficking. PLoS Pathog. 5:e1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyagi, E., A. J. Andrew, S. Kao, and K. Strebel. 2009. Vpu enhances HIV-1 virus release in the absence of Bst-2 cell surface down-modulation and intracellular depletion. Proc. Natl. Acad. Sci. U. S. A. 106:2868-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neil, S. J., T. Zang, and P. D. Bieniasz. 2008. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451:425-430. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen, K. L., M. llano, H. Akari, E. Miyagi, E. M. Poeschla, K. Strebel, and S. Bour. 2004. Codon optimization of the HIV-1 vpu and vif genes stabilizes their mRNA and allows for highly efficient Rev.-independent expression. Virology 319:163-175. [DOI] [PubMed] [Google Scholar]
- 37.Pardieu, C., R. Vigan, S. J. Wilson, A. Calvi, T. Zang, P. Bieniasz, P. Kellam, G. J. Towers, and S. J. Neil. 2010. The RING-CH ligase K5 antagonizes restriction of KSHV and HIV-1 particle release by mediating ubiquitin-dependent endosomal degradation of tetherin. PLoS Pathog. 6:e1000843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez-Caballero, D., T. Zang, A. Ebrahimi, M. W. McNatt, D. A. Gregory, M. C. Johnson, and P. D. Bieniasz. 2009. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell 139:499-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruiz, A., D. Lau, R. S. Mitchell, M. S. Hill, K. Schmitt, J. C. Guatelli, and E. B. Stephens. 2010. BST-2 mediated restriction of simian-human immunodeficiency virus. Virology 406:312-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sauter, D., M. Schindler, A. Specht, W. N. Landford, J. Munch, K. A. Kim, J. Votteler, U. Schubert, F. Bibollet-Ruche, B. F. Keele, J. Takehisa, Y. Ogando, C. Ochsenbauer, J. C. Kappes, A. Ayouba, M. Peeters, G. H. Learn, G. Shaw, P. M. Sharp, P. Bieniasz, B. H. Hahn, T. Hatziioannou, and F. Kirchhoff. 2009. Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe 6:409-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schindler, M., D. Rajan, C. Banning, P. Wimmer, H. Koppensteiner, A. Iwanski, A. Specht, D. Sauter, T. Dobner, and F. Kirchhoff. 2010. Vpu serine 52 dependent counteraction of tetherin is required for HIV-1 replication in macrophages, but not in ex vivo human lymphoid tissue. Retrovirology 7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tait, S. W., E. de Vries, C. Maas, A. M. Keller, C. S. D'Santos, and J. Borst. 2007. Apoptosis induction by Bid requires unconventional ubiquitination and degradation of its N-terminal fragment. J. Cell Biol. 179:1453-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tokarev, A., M. Skasko, K. Fitzpatrick, and J. Guatelli. 2009. Antiviral activity of the interferon-induced cellular protein BST-2/tetherin. AIDS Res. Hum. Retroviruses 25:1197-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Damme, N., D. Goff, C. Katsura, R. L. Jorgenson, R. Mitchell, M. C. Johnson, E. B. Stephens, and J. Guatelli. 2008. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3:245-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, X., R. A. Herr, W. J. Chua, L. Lybarger, E. J. Wiertz, and T. H. Hansen. 2007. Ubiquitination of serine, threonine, or lysine residues on the cytoplasmic tail can induce ERAD of MHC-I by viral E3 ligase mK3. J. Cell Biol. 177:613-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu, H., and R. R. Kopito. 1999. The role of multiubiquitination in dislocation and degradation of the alpha subunit of the T cell antigen receptor. J. Biol. Chem. 274:36852-36858. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.