Abstract
Measles virus (MV) vaccine effectively protects seronegative individuals against infection. However, inhibition of vaccine-induced seroconversion by maternal antibodies after vaccination remains a problem, as it leaves infants susceptible to MV infection. In cotton rats, passive transfer of MV-specific IgG mimics maternal antibodies and inhibits vaccine-induced seroconversion. Here, we report that immunization in the presence of passively transferred IgG inhibits the secretion of neutralizing antibodies but not the generation of MV-specific B cells. This finding suggested that MV-specific B cells require an additional stimulus to mature into antibody-secreting plasma cells. In order to provide such a stimulus, we generated a recombinant Newcastle disease virus (NDV) expressing the MV hemagglutinin (NDV-H). In contrast to MV, NDV-H induced high levels of type I interferon in plasmacytoid dendritic cells and in lung tissue. In cotton rats immunized with NDV-H, neutralizing antibodies were also generated in the presence of passively transferred antibodies. In the latter case, however, the level and kinetics of antibody generation were reduced. In vitro, alpha interferon stimulated the activation of MV-specific B cells from MV-immune spleen cells. NDV infection (which induces alpha interferon) had the same effect, and stimulation could be abrogated by antibodies neutralizing alpha interferon, but not interleukin 6 (IL-6). In vivo, coapplication of UV-inactivated MV with NDV led to increased MV-specific antibody production in the presence and absence of passively transferred antibodies. These data indicate that MV-specific B cells are being generated after immunization in the presence of maternal antibodies and that the provision of alpha interferon as an additional signal leads to antibody secretion.
Maternal antibodies are transferred from mother to child and confer protection against infections early in life. Over time, the titers of maternal antibodies decline to low, nonprotective levels. It is a serious problem that these nonprotective titers of maternal antibodies still inhibit seroconversion after vaccination, thus leaving a window of opportunity for pathogens. Although this problem arises for many pathogens, no effective immunization strategy for vaccination in the presence of maternal antibodies has been defined. One obvious example is acute measles, where early immunization is imperative and failure to do so results in approximately 200,000 deaths every year worldwide. In humans (12-14) (as well as in cotton rats [31]), it was shown that the development of neutralizing antibodies is impaired, whereas T-cell proliferation is still detectable after immunization in the presence of maternal antibodies. This is significant, because only neutralizing antibodies protect against infection (for a review, see reference 24). CD8 T cells help to clear viral infection but do not protect (28). CD4 T cells have no role in protection or clearance of virus from the respiratory tract (31) (although they might have a role in clearing virus infection from brain tissue through gamma interferon [IFN-γ] [10, 40]). Because of the inhibition of immunization by maternal antibodies, only seronegative children can be successfully immunized, which leaves a window of opportunity for wild-type (WT) virus infection (reviewed in reference 19). When children who were unsuccessfully immunized are reimmunized later in life, they develop good antibody responses, indicating that maternal antibodies do not induce immunological unresponsiveness (anergy). It is known that T cells are generated after immunization in the presence of maternal antibodies (12-14). However, whether B cells are being generated at all or whether antibody secretion by measles virus (MV)-specific B cells is inhibited remains an open question. B cells seem to require three signals to develop into antibody-secreting plasma cells: recognition of antigen through the B-cell receptor, B-cell-T-cell interaction through CD40-CD40 ligand, and soluble mediators. One of these mediators is alpha interferon, which has been shown to stimulate the secretion of antibody in vitro (16, 17, 33) and to support the maturation of B cells into plasma cells in vivo (4, 5). Measles virus is capable of interfering with type I interferon action through its V protein. It has been demonstrated that MV V protein binds to the interferon regulatory RNA helicases MDA5 and LGP2, as well as STAT-1 and STAT-2, to avoid cellular antiviral responses (3, 25). It can also block the induction of type I interferon by acting as a decoy substrate for IκB kinase α and thereby prevent Toll-like receptor 7 (TLR-7)/9-mediated interferon induction (29). As a consequence, infection of plasmacytoid dendritic cells (pDC) (which are the major source of type I interferon) does not induce interferon secretion (35). We hypothesized that providing a strong type I interferon signal would stimulate the B-cell response during immunization in the presence of maternal antibodies. Newcastle disease virus (NDV) induces high levels of type I interferon in dendritic cells in vitro and in animals in vivo (15, 26) and has been used as a vector system with markedly immune-activating functions in protection against infectious diseases (2, 8, 9, 23, 27). In order to utilize the ability of NDV to induce high levels of type I interferon, we produced an NDV vaccine vector that expresses MV hemagglutinin (H), a major target for neutralizing antibodies, and tested it in cotton rats in the presence of passively transferred human MV-specific IgG.
MATERIALS AND METHODS
Cell lines and viruses.
Vero (African green monkey) and CCRT (a cotton rat osteosarcoma cell line [37]) cells were grown in minimal essential medium (MEM)-10% fetal calf serum (FCS). MV strains Schwarz, a licensed vaccine strain, and HU2, a clinical isolate derived from the Schwarz strain, were grown, and titers were determined on Vero cells (30). Newcastle disease virus expressing the green fluorescent protein (NDV-GFP) has been described previously (20) and was grown and titrated like NDV expressing the MV hemagglutinin (NDV-H).
Cotton rats.
Inbred cotton rats were obtained from Harlan, Indianapolis, IN. Female animals from 6 to 10 weeks of age were used. The animals were purchased specific pathogen free, according to the breeder's specification, and were maintained in a barrier system. The animals were kept under controlled environmental conditions of 22 ± 1°C with a 12-h light cycle. All animals were euthanized by CO2 inhalation.
Cotton rat immunization and infection.
Cotton rats were injected intraperitoneally (i.p.) with human polyclonal MV-specific antibodies (IgG) with a neutralization titer (NT) of 320 (Carimune; CSL Behring). One day postinoculation, animals were immunized subcutaneously (s.c.) or intranasally (i.n.) with NDV-H or the MV vaccine strain Schwarz. Serum samples were collected weekly, and neutralization titers were measured. Six weeks postimmunization, the animals were challenged with 2 × 105 PFU of measles virus strain HU2. Four days postchallenge, the animals were euthanized, and the lungs and spleens were harvested to measure the lung viral load and immune responses, respectively.
Virus titration.
The left lung lobe was removed aseptically, and the tissue was minced using scissors and ground in a glass homogenizer. Serial 10-fold dilutions of virus-containing supernatant were assessed for the presence and levels of infectious virus in a 48-well microassay using Vero cells with cytopathic effect (CPE) as an endpoint after 7 days. The amount of virus in the inoculum was expressed as the quantity of virus that could infect 50% of the tissue culture monolayer (50% tissue culture infective dose [TCID50]). The TCID50 was calculated according to the methods described by Reed and Muench (32).
Neutralization assay.
Cotton rat serum samples were 2-fold serially diluted and incubated with 50 PFU MV strain NSE for 1 h at 37°C in a 96-well plate. One hour postincubation, 104 Vero cells were added per well. Five days postinfection, the CPE was determined microscopically. The titer was defined as the reciprocal of the last protective serum dilution, as calculated from duplicate measurements.
ELISA.
For enzyme-linked immunosorbent assay (ELISA), a plate was coated with 10 μg/ml of gradient-purified, UV-inactivated MV in 200 mM NaCO3 buffer (pH 9.6) at 4°C overnight, blocked with phosphate-buffered saline (PBS)-10% FCS-0.05% Tween 20, and incubated with dilutions of human serum at room temperature for 1 h. After being washed, the plate was incubated for 1 h at room temperature with a horseradish peroxidase-coupled goat serum specific for human IgG (Zymed) and subsequently developed with 0.5 mg of ortho-phenyldiamine/ml in buffer (35 mM citrate, 66 mM Na2HPO4 [pH 5.2], 0.01% H2O2). To test for MV-specific cotton rat IgG, MV-coated plates were incubated with dilutions of cotton rat serum at 4°C for 1 h. After being washed, the plates were incubated with rabbit serum specific for cotton rat IgG (Virion Systems) for 1 h at room temperature and subsequently with horseradish peroxidase-coupled goat serum specific for rabbit IgG (Zymed) for 45 min at room temperature and developed as described above.
B-cell enzyme-linked immunospot (ELISPOT) assay.
Ninety-six-well plates (Nunc-Immuno Polysorp; Thermo Fisher) were coated with 15 μg/ml gradient-purified, UV-inactivated MV antigen overnight at 4°C. Serially diluted splenocytes were plated in advanced RPMI 1640 with 10% FCS, and NDV-GFP and neutralizing sera specific for alpha interferon and interleukin 6 (IL-6) (R&D Systems) were added. The plates were cultured overnight at 37°C in an incubator and washed with PBS-0.05% Tween 20 and subsequently three times with PBS. The plates were incubated with rabbit serum specific for cotton rat IgG (Virion Systems) and subsequently with goat serum specific for rabbit IgG conjugated to alkaline phosphatase (Zymed). For development of the spots, the plates were washed three times with PBS, and 3% agarose containing AMP-BCIP (5-bromo-4-chloro-3-indolylphosphate) (Sigma) substrate was added for 30 min at 4°C. The plates were incubated at room temperature for 2 h, and the spots were counted microscopically.
T-cell proliferation assay.
For proliferation assays, 96-well plates were coated with 50 μl/well (10 μg/ml) of gradient purified, UV-inactivated MV in 200 mM NaCO3 buffer (pH 9.6) at 4°C overnight. After being washed twice with PBS, spleen cells were plated in triplicate at 5 × 105 cells/well in a 96-well plate in RPMI 1640 with 10% FCS in wells with or without (medium control) antigen. After 40 h, 0.5 μCi [3H]thymidine/well was added, and 16 to 20 h later, cells were harvested onto glass fiber filter mats and counted with a Betaplate Counter (Wallac, Turku, Finland). The stimulation index (SI) was calculated as the mean of proliferation of MV-stimulated cells in cpm/proliferation of cells in medium in cpm. To control for the specificity of the assay, spleen cells from infected animals were tested against Vero cell antigen and cells from naïve animals were tested against MV. In controls, proliferation did not exceed a stimulation index of 2, indicating the threshold of the assay.
Bone marrow-derived plasmacytoid dendritic cells.
Bone marrow cells were flushed from the femoral diaphyseal marrow cavity, and 8 × 106 cells were plated in 25-cm2 tissue culture flasks in advanced RPMI 1640 supplemented with 5% FCS and 5 × 10−5 M β-mercaptoethanol. The bone marrow cells were cultured for 7 days in medium supplemented with 100 ng mouse Flt-3 ligand (R&D Systems). Every 2 days, fresh medium supplemented with Flt-3 ligand was added. The presence of pDC was confirmed by their ability to secrete type I interferon after stimulation with type A oligonucleotides and Sendai virus. For these experiments, plasmacytoid dendritic cells were infected with NDV or measles virus, and 1 day later, the supernatants were harvested and analyzed.
Type I interferon bioassay.
Samples were acid treated with 0.1 M hydrochloric acid to inactivate IFN-γ, and the acid was neutralized with sodium bicarbonate. Serially diluted samples were added to CCRT cells. Recombinant cotton rat IFN-α (R&D Systems) was used to create a standard curve. The following day, CCRT cells were infected with 103 PFU of recombinant vesicular stomatitis virus expressing green fluorescent protein (rVSV-GFP) (6). After 48 h of incubation at 37°C, the plates were evaluated for the presence or absence of green fluorescence on an Olympus IX51 fluorescence microscope. IFN-α/β concentrations in samples were expressed as units, with 1 unit being the equivalent of 1 pg of IFN-α. The threshold of detection was 16 units.
Construction and rescue of recombinant NDV expressing MV H protein.
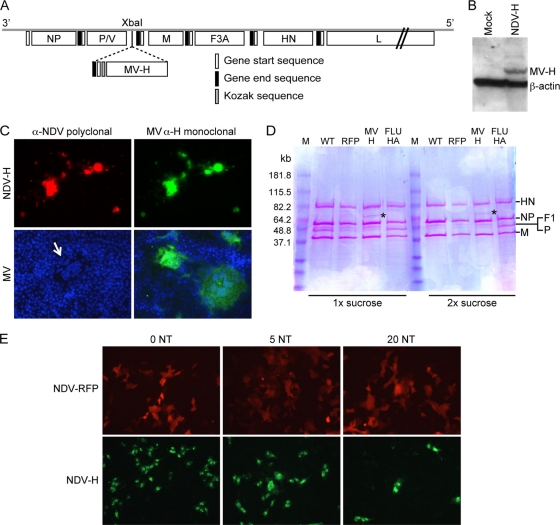
MV RNA from the Schwarz vaccine strain was isolated from infected Vero cell lysates (QIAmp viral RNA minikit; Qiagen). Viral cDNA was synthesized at 37°C with Moloney murine leukemia virus reverse transcriptase (Invitrogen) using oligo(dT) primer. The open reading frame (ORF) of the MV Schwarz H gene was amplified by PCR and cloned between the P and M genes of the NDV Hitchner B1 cDNA, in which the cleavage site of the F protein contains three amino acid changes (NDV/F3aa). This new engineered cleavage site can be activated by ubiquitously expressed proteases of the furin family (27). PCR was performed using the sense primer (5′AATTACTAGT TTAGAAAAAATACGGGTAGAACCGCCACCATGTCACCACAACGAGACCGGATAAATGCC-3′) containing the SpeI restriction site (underlined), the NDV gene stop codon (boldface), the intergenic region (T), the transcription start site (italics), and the Kozak consensus sequence (CCGCCACC), and the antisense primer (5′-AATTACTAGTCCTATCTGCGATTGGTTCCATCTTC-3′) containing the SpeI restriction site (underlined) and 1 extra C nucleotide after the stop codon (boldface) to comply with the rule of six. Amplification was performed using high-fidelity Platinum Taq DNA polymerase (Invitrogen) with 32 cycles as follows: denaturation at 94°C for 30 s, primer annealing at 60°C for 30 s, and extension at 68°C for 2 min. The PCR product was cloned into pGEM-T (pMVszHA) and confirmed by sequencing. MV H was subcloned (SpeI) into pNDV/F3A (XbaI) (see Fig. 2A). The virus was rescued from cDNA as previously described (23). The presence of the MV H ORF in the viral genome was confirmed by reverse transcription (RT)-PCR and sequencing (data not shown), and MV H protein expression was confirmed by Western blotting and immunofluorescence.
The NDV-H virus was grown in 10-day-old embryonated chicken eggs. Virus titers were determined by immunofluorescence using a rabbit polyclonal antibody against NDV, as previously described (23).
Immunofluorescence assay.
Confluent monolayers of Vero cells were infected with NDV-H or measles virus (Schwarz strain) for 1 h. At 24 h postinfection, the cells were fixed in 2.5% paraformaldehyde and permeabilized with 0.1% Triton X-100. Viral antigens were detected with a rabbit polyclonal antibody specific for NDV (1:100) and two mouse monoclonal antibodies against MV H protein (K17 and K71) (38). These antibodies were not cross-reactive. After 1 h of incubation at room temperature, the cells were washed twice with PBS and incubated with rhodamine red-labeled goat serum specific for rabbit IgG (Jackson laboratories) to detect NDV antigens (red), and fluorescein isothiocyanate-labeled donkey serum specific for mouse IgG (Dako) was used to detect MV H protein (green). Nuclear staining was performed with 4′-6-diamidino-2-phenylindole (DAPI) (Sigma). Cells were visualized under a fluorescence microscope at ×20 magnification.
Western blotting.
Cell lysates were prepared by infecting confluent Vero cells with the recombinant NDV-H at a multiplicity of infection of 1 and harvesting cell pellets in RIPA buffer (0.15 mM NaCl, 0.05 mM Tris-HCl, pH 7.2, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS) 36 h postinfection. For virus purification, 10-day-old eggs were infected with WT or recombinant NDV expressing the red fluorescent protein (RFP), measles virus hemagglutinin, or influenza A virus hemagglutinin (27). Two days postinfection, viruses were harvested from allantoic fluids and clarified (1,000 rpm for 5 min at 4°C). A further clarification step (10,000 rpm for 30 min at 4°C) was performed prior to one- or two-step 30% sucrose cushion virus purification in NTE buffer (100 mM NaCl, 10 mM Tris-HCl, 1 mM EDTA) at 25,000 rpm for 2.5 h. The pelleted virus was resuspended in 100 μl of PBS. Equal amounts of protein were separated by SDS-PAGE and stained with Coomassie blue stain or transferred to nitrocellulose membranes. The blots were blocked in 10% fat-free milk powder in PBS. To detect expression of MV H protein, membranes were stained with Carimune, a human antiserum (1:1,000) with a known anti-MV neutralizing antibody titer (CSL Behring). An anti-actin (1:1,000) monoclonal antibody was used as a loading control. Both primary antibodies were diluted in 5% fat-free milk powder in PBS at 4°C overnight on a rotating platform. After overnight incubation, the membranes were washed three times for 5 min each time in 0.5% Tween 20-PBS and incubated for 1 to 2 h with anti-human and anti-mouse horseradish peroxidase-linked antibodies (1:2,000). After three 5-min washes and one wash with PBS, antibody-protein complexes were detected using a Western lighting chemiluminescence system (Perkin Elmer).
Antibody blocking assay.
A mixture of mouse monoclonal antibodies specific for MV H (K17 and K71; 1:1 ratio) was incubated with 50 PFU/well of NDV-RFP or NDV-H in a volume of 50 μl of MEM without FCS. Following a 1-h incubation at 37°C, 104 Vero cells were added to each well. The plates were further incubated for 48 h at 37°C and subsequently analyzed by immunofluorescence as described above.
RESULTS
MV-specific B cells but no neutralizing antibodies are generated after immunization in the presence of passively transferred MV-specific IgG.
Studies in both cotton rats and humans have demonstrated that immunization in the presence of MV-specific IgG (maternal antibodies) results in the induction of MV-specific T cells, whereas the generation of neutralizing antibodies is inhibited (12-14, 31). Suppression of the antibody response could be due to inhibition of the generation of MV-specific B cells or to a lack of antibody secretion after immunization in the presence of maternal antibodies. In order to clarify this question, cotton rats were immunized in the presence and absence of human MV-specific IgG, and after 7 weeks (when the passively transferred antibody was metabolized), their immune response was analyzed. Neutralizing antibody titers were determined from serum, the number of MV-specific B cells was measured from spleen cells by ELISPOT assay, and the T-cell response was measured by [3H]thymidine incorporation after stimulation with MV. As reported previously (31), T-cell proliferation did not differ after immunization in the absence or presence of MV-specific IgG, whereas the secretion of neutralizing antibodies was severely inhibited after immunization in the presence of MV-specific IgG (Table 1). The numbers of MV-specific B cells were comparable independent of the route of immunization (subcutaneous, intraperitoneal, or intranasal) and whether the animals were immunized in the presence or absence of passively transferred MV-specific IgG at the time of immunization (Table 1). These data led to the conclusion that MV-specific B cells are being generated in the presence of passively transferred MV-specific IgG but that antibody secretion is either inhibited or not sufficiently stimulated. The current model of B-cell activation postulates three signals for the generation of B cells and antibody secretion (5): interaction of B-cell receptor with antigen, B-cell-T-cell interaction through CD40-CD40 ligand, and soluble mediators (e.g., IL-6 or type I interferon). Based on our current data, the first two signals seem to be present during immunization in the presence of maternal antibodies and lead to the generation of MV-specific B cells. It has been shown, however, that MV is a poor inducer of type I interferon (reviewed in reference 11). We tested the hypothesis that the induction of a stronger third signal might be capable of overcoming the inhibitory effect of passively transferred MV-specific IgG by stimulating type I interferon, which in turn would lead to antibody secretion by B cells.
TABLE 1.
Generation of MV-specific B cells and T cells but no neutralizing antibodies after MV (Schwarz) immunization in the presence of MV-specific IgGa
| Route of Immunization | MV-specific human IgG | Neutralizing antibody titer | No. of MV- specific B cells/106 spleen cells | T-cell proliferation (stimulation index) |
|---|---|---|---|---|
| I.p. | None | 100 ± 95 | 163 ± 37 | 5 ± 4.4 |
| NT 320 | <10 | 161 ± 42 | 3.4 ± 1 | |
| I.n. | None | 95 ± 30 | 155 ± 44 | 5.5 ± 1.3 |
| NT 320 | <10 | 154 ± 45 | 3.2 ± 0.5 | |
| S.c. | None | 130 ± 76 | 144 ± 38 | 4.4 ± 1.7 |
| NT 320 | 12 ± 6 | 108 ± 27 | 2 ± 0.4 |
Cotton rats were immunized with 105 PFU of MV (Schwarz) i.n., s.c., or i.p. in the absence or presence of passively transferred human MV-specific IgG (NT, 320). After 7 weeks (when human IgG was metabolized), actively induced neutralizing antibody responses were measured by ELISA, the numbers of MV-specific B cells were measured by ELISPOT assay, and the stimulation index of T-cell proliferation was measured by [3H]thymidine incorporation assay. The results are expressed as means (4 animals per group) ± SD. A neutralizing antibody titer of <10 is below the threshold of detection and is considered negative. Whereas the titers of neutralizing antibodies differed significantly between groups (P < 0.001; ANOVA), the numbers of B cells and T-cell proliferation did not differ between groups.
Induction of type I interferon through a recombinant Newcastle disease virus vector expressing MV hemagglutinin.
In order to induce high levels of type I interferon secretion, we chose the NDV vector system (for a review, see reference 1). In this vector system, MV H was expressed, because it is the major target for MV neutralizing antibodies. After insertion of the MV H gene of the Schwarz strain between the phosphoprotein and matrix genes of rNDV/F3aa, a recombinant virus was obtained from the molecular clone via reverse genetics (NDV-H). The expression of MV H by NDV-H was confirmed by Western blotting and immunofluorescence (Fig. 1). MV H was found to be incorporated into virions in a 5-fold-smaller amount than NDV-H (Fig. 1). Antibodies against measles virus hemagglutinin inhibited infection of Vero cells with NDV-H in vitro, indicating that MV H was incorporated into the NDV envelope and was able to function as a receptor-binding protein (Fig. 1).
FIG. 1.
Construction and characterization of a recombinant NDV vector expressing MV H. (A) Schematic representation of the NDV-H cDNA construct. The hemagglutinin gene from the MV Schwarz vaccine strain (MV H) was inserted between the P and M genes of the molecular clone of rNDV/F3aa as described in Materials and Methods. (B) Expression of MV H protein in infected cells. Vero cells mock infected or infected with NDV-H were harvested 48 h postinfection, and the cell lysates were analyzed for MV H protein expression by Western blotting. (C) MV H expression by immunostaining of infected Vero cells. Vero cells were infected with NDV-H or measles virus; 24 h after infection, the cells were fixed and stained with anti-NDV polyclonal antibodies (red) and MV H monoclonal antibodies (green). DAPI staining revealed nuclear chromatin (blue). The arrow indicates a measles virus-infected syncytium. (D) Measles virus hemagglutinin is incorporated into the NDV virion. Wild-type NDV or recombinant viruses expressing RFP, MV H, or influenza A virus hemagglutinin were purified once or twice over a sucrose gradient. Virus proteins were separated by SDS-PAGE and stained with NDV- and MV-specific antisera (not shown) or Coomassie blue. The positions of the NDV hemagglutinin/neuraminidase (HN), nucleoprotein (NP), fusion protein (F1), polymerase (P), and matrix protein (M) are indicated on the right. Measles virus hemagglutinin is indicated by an asterisk. (E) MV H-specific antibodies block NDV-H expression. NDV-RFP and NDV-H were preincubated with neutralizing antibodies specific for MV H at different neutralizing titers and used to infect Vero cells. Virus-infected cells were visualized 48 h later by immunofluorescence.
In order to test the ability of NDV-H to induce type I interferon and compare it to MV Schwarz, bone marrow-derived pDC, which are the major source of type I interferon, were infected with both viruses. As reported previously for human pDC (35), MV Schwarz did not induce type I interferon. In contrast, NDV-H induced the secretion of type I interferon in a dose-dependent manner (Table 2). Neither virus induced IL-6 in bone marrow-derived dendritic cells as assessed by RT-PCR (data not shown). In order to evaluate the level of type I interferon induction after intranasal inoculation, bronchoalveolar lavage (BAL) fluids from cotton rats infected with NDV-H and MV Schwarz were compared. NDV-H immunization led to high levels of type I interferon in vivo on days 1 and 2, with declining amounts on days 3 and 4 and background levels on day 7 (Fig. 2). In contrast, MV Schwarz induced only low levels of type I interferon on days 1 through 7 (Fig. 2). IL-6 was undetectable by RT-PCR from BAL cells irrespective of the virus used for immunization (data not shown).
TABLE 2.
Type I interferon secretion by plasmacytoid dendritic cells after infection with NDV-H or MV (Schwarz)a
| Multiplicity of infection | Type I interferon (units/ml) |
|
|---|---|---|
| NDV-H | MV (Schwarz) | |
| 0.1 | 64 | <16 |
| 1 | 128 | <16 |
| 10 | 256 | <16 |
Bone marrow-derived plasmacytoid dendritic cells were infected at different multiplicities of infection with NDV-H or MV (Schwarz); 24 h later, the supernatants were removed and tested for the presence of type I interferon by bioassay. The threshold of the assay was 16 units/ml.
FIG. 2.
NDV-H is a potent inducer of type I interferon in the cotton rat lung. An IFN-α/β bioassay was performed with BAL fluid to determine the levels of induction at days 1, 2, 3, 4, and 7 after intranasal inoculations with 105 PFU NDV-H or MV Schwarz. Differences at days 1, 2, and 3 were statistically significant (analysis of variance [ANOVA]). The results reflect the means of four animals per group plus standard deviations (SD).
NDV-H induces neutralizing antibodies after immunization in the presence of maternal antibodies.
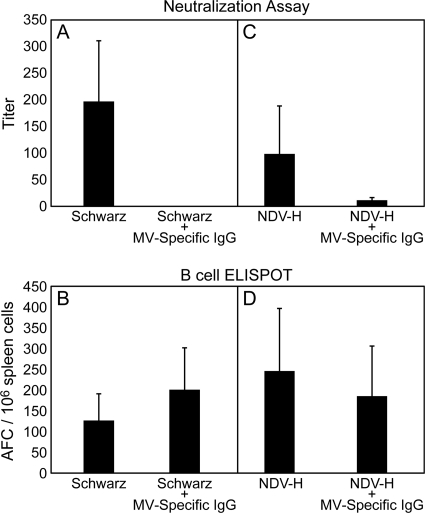
To test the ability of NDV-H to stimulate the secretion of neutralizing antibodies after immunization in the presence of MV-specific antibodies, cotton rats were immunized with NDV-H both subcutaneously and intranasally for comparison to MV Schwarz. After subcutaneous immunization, neither MV Schwarz nor NDV-H induced neutralizing antibodies in the presence of MV-specific IgG (data not shown). Additionally, after i.n. immunization with MV Schwarz in the presence of passively transferred MV-specific IgG, no induction of neutralizing antibodies was observed (Fig. 3). In contrast, i.n. immunization with NDV-H led to the induction of low levels of protective neutralizing antibodies (Fig. 3), and protection increased with increasing doses of NDV-H (Table 3). Although the levels of neutralizing antibody induced were protective (36), the peak titers were not as high as after immunization in the absence of passively transferred MV-specific IgG (Fig. 3). To investigate this difference in antibody production, the kinetics of antibody generation after i.n. immunization with NDV-H in the absence and presence of passively transferred MV-specific IgG were compared. After immunization in the presence of passively transferred MV-specific IgG, antibody production was delayed by 2 weeks and reached lower total titers than after immunization in the absence of passively transferred MV-specific IgG (Fig. 4).
FIG. 3.
Comparison of the generation of MV-specific B cells and the induction of neutralizing antibodies. Cotton rats were immunized i.n. with 5 × 105 PFU of MV Schwarz (A and B) or NDV-H (C and D) in the presence or absence of human MV-specific IgG (NT, 320). From serum, neutralizing antibody titers were determined (A and C), and from spleen cells, B-cell antibody-forming cells (AFC) were determined (B and D) in cotton rats immunized with MV Schwarz (A and B) and NDV-H (C and D). The results reflect the means of four animals per group plus SD. The difference in induction of neutralizing antibodies in the presence of human MV-specific IgG between NDV-H and MV is statistically significant (P < 0.002).
TABLE 3.
Dose-dependent protection against MV challenge correlates with MV-specific neutralizing antibodies in cotton rats inoculated with NDV-H in the presence of passively transferred MV-specific IgGa
| Immunization with NDV-H (PFU) | Titer |
P | |
|---|---|---|---|
| Neutralizing antibody | Virus (log10 TCID50/g lung tissue) | ||
| None | None | 5 ± 0.3 | |
| 1 × 105 | 11.3 ± 2.5 | 4.3 ± 0.5 | <0.05 |
| 5 × 105 | 12 ± 6 | 3.4 ± 0.5 | <0.001 |
| 2.5 × 106 | 17 ± 12 | ND | |
| 5 × 106 | 21 ± 14.3 | <1 | <0.001 |
Cotton rats were inoculated with human MV-specific IgG (NT, 320) intraperitoneally and 1 day later with increasing doses of NDV-H intranasally. Seven weeks later (when human antibody had been metabolized), serum was obtained for a neutralization assay and the animals were challenged with MV. The results are expressed as means (4 animals per group) ± SD (ND, not done). A neutralizing antibody titer of <10 is below the threshold of detection and is considered negative. The neutralizing antibody titers of immunized animals were significantly higher than the neutralizing antibody titers of nonimmunized animals (a P value of at least <0.05). The virus titers of immunized animals were significantly lower than the titers of nonimmunized animals (P <0.05 or P <0.001) and increased with a vaccine dose of NDV-H.
FIG. 4.
Antibody generation is delayed in cotton rats immunized in the presence of MV-specific IgG. Cotton rats were immunized i.n. with 5 × 105 PFU NDV-H in the presence and absence of human MV-specific IgG (NT, 320). Human antibodies declined over time (as measured by ELISA) (open triangles). In cotton rats immunized with NDV-H in the absence of human MV-specific IgG, MV-specific cotton rat antibodies were found as early as 1 week after infection, with an increase in antibody titers over time (open diamonds). In animals immunized in the presence of passively transferred MV-specific antibody, the development of MV-specific cotton rat antibodies was delayed by 2 weeks (closed squares). All time points reflect the means of four animals per group ± SD. The dashed line indicates the threshold of the assay (optical density [O.D.] = 0.14).
When the numbers of MV-specific B cells were compared after intranasal immunization with MV Schwarz versus NDV-H, no difference was detected (independent of which virus was used or whether immunization took place in the presence or absence of maternal antibodies) (Fig. 3). Similarly, no difference in T-cell proliferation was detected (the stimulation indices ranged from 2 to 5.5). These data confirm that the first and second signals for B-cell development (antigen and T cells) are present and lead to generation of MV-specific B cells. These data also suggest that the induction of type I interferon by NDV-H immunization induces B cells to secrete neutralizing antibodies after immunization in the presence of passively transferred MV-specific IgG antibodies.
Type I interferon induces MV-specific antibody secretion in vitro and in vivo.
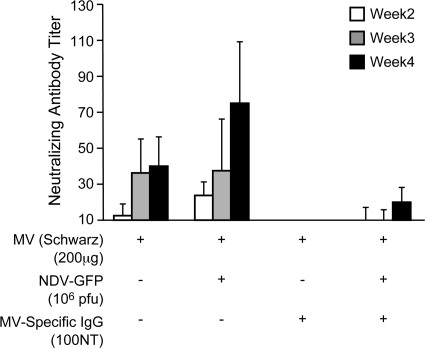
In order to verify the role of type I interferon in stimulating B-cell responses, bone marrow cells from MV-immune cotton rats were tested by ELISPOT assay (Fig. 5). As shown for human B cells (33), the addition of IFN-α increased the number of MV-specific B cells. After infection with NDV-GFP (which expressed GFP instead of hemagglutinin), the number of MV-specific B cells was also increased, and this stimulation was abrogated by the addition of IFN-α neutralizing serum but not by the addition of IL-6 neutralizing serum (Fig. 5). These data indicated that the induction of IFN-α by NDV infection was the primary costimulatory signal (in conjunction with MV antigen). In consequence, it should be possible to detach the antigen-producing function of NDV-H from type I interferon induction. For this reason, cotton rats were immunized intranasally in the absence or presence of passively transferred MV-specific IgG with gradient-purified UV-inactivated MV antigen with and without NDV-GFP. After immunization in the absence of passively transferred MV-specific IgG, the neutralizing antibody response increased 2-fold after coimmunization with NDV-GFP and MV compared to immunization with MV alone (Fig. 6). After immunization in the presence of passively transferred MV-specific IgG, only the group immunized with NDV-GFP and MV developed neutralizing antibodies (Fig. 6).
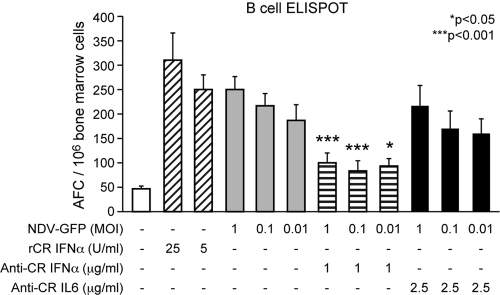
FIG. 5.
IFN-α induced by NDV infection stimulates MV-specific B-cell responses. Bone marrow cells from MV-immune cotton rats were stimulated with gradient-purified, UV-inactivated MV antigen. In addition, cotton rat IFN-α, NDV-GFP, and neutralizing sera against IFN-α or IL-6 were added, and 24 h later, the plates were developed and the MV-specific B cells were counted. After the addition of neutralizing serum against IFN-α to NDV-GFP-infected bone marrow cells, the number of MV-specific B cells was significantly reduced compared to NDV-GFP infection only and was not statistically significantly different from stimulation with MV antigen alone. The error bars indicate SD.
FIG. 6.
Coinfection with NDV increases neutralizing antibody response against MV antigen. Cotton rats were inoculated intranasally with gradient-purified, UV-inactivated MV antigen alone or together with NDV-GFP in the absence or presence of human MV-specific IgG (NT, 100). After 2, 3, and 4 weeks, serum was obtained and tested for the presence of neutralizing antibodies. The difference in neutralizing antibody titers after immunization in the presence of passively transferred MV-specific IgG was statistically significant (P < 0.003; t test). The error bars indicate SD.
DISCUSSION
The development of antigen-specific B cells relies on three signals (5): (i) binding of antigen by the B-cell receptor, (ii) T-cell help in the form of CD40-CD40 ligand interaction, and (iii) soluble mediators (e.g., IL-6 and type I interferon) to induce secretion of antibodies. The fact that MV-specific B cells and T cells are being generated after immunization in the presence of MV-specific IgG argues for the presence of the first and second signals. However, as no antibodies are being secreted, the third signal seems to be insufficient to overcome the inhibitory effect of passively transferred MV-specific IgG. In this report, we focused on type I interferon secretion because of its importance as a third signal in the formation of antigen-specific B cells. In vitro, type I interferon has been shown to stimulate IgG and IgM secretion of mitogen-stimulated B cells (33). After in vitro culture of peripheral blood mononuclear cells from influenza A virus-immune patients, the stimulation of pDC to produce type I interferon resulted in secretion of influenza virus-specific antibodies, whereas depletion of pDC abrogated the antibody response (16). In mice, the importance of type I interferon in stimulating the B-cell response against influenza A virus has been shown by using mice with a deletion of the type I IFN receptor (5) and a transcriptional mapping approach that identified type I interferon as the main innate stimulus for B cells within 48 h after infection (4).
Measles virus is capable of interfering with type I interferon action through its V protein. It has been demonstrated that MV V protein binds to the interferon regulatory RNA helicases MDA5 and LGP2, as well as STAT-1 and STAT-2, to avoid cellular antiviral responses (3, 25). It can also block the induction of type I interferon by acting as a decoy substrate for IκB kinase α and thereby preventing Toll-like receptor 7/9-mediated interferon induction (29). In consequence, infection of plasmacytoid dendritic cells (which are the major source of type I interferon) does not induce interferon secretion (35). Without a V protein, MV is not able to block type I interferon (7) but is severely restricted in its in vivo growth (7, 39). In spite of poor in vivo growth, it induces antibody levels comparable to those of parental MV, indicating that in the absence of type I interferon inhibition the antibody response is improved (7). Similar to findings with human pDC, MV infection of cotton rat pDC induces no type I interferon, and in MV-infected cotton rat lungs, very little type I interferon is detectable. These low levels of type I interferon in vivo apparently are still sufficient for the generation of MV-specific B cells and antibodies after immunization. However, these low levels do not seem to be sufficient to induce secretion of antibodies in the presence of inhibitory (maternal) IgG.
In contrast to that of MV, the V protein of NDV is not able to interact with cellular partners in mammalian cells to inhibit type I interferon induction (26). Due to its interaction with TLR-7 and the RIG-I pathway, it induces type I interferon in plasmacytoid dendritic cells in vivo (21, 34), thus supporting the production of neutralizing antibodies. Similar to its immunostimulatory capability in other animal models, NDV-H induced high levels of type I interferon in both cotton rat pDC and lung tissue and induced neutralizing antibodies after immunization in the presence of passively transferred MV-specific antibody. The fact that the induction of type I interferon by NDV-H is the major stimulatory effect was demonstrated by separating the antigen (MV H) and NDV infection for stimulation of B cells in vitro (ELISPOT assay) and in vivo after intranasal inoculation.
In cotton rat lung tissue, a difference in the infection pattern and its functional consequences could explain differences in vaccination efficacy between NDV-H and MV Schwarz. Recent publications have suggested that, depending on the virus infecting the respiratory tract, different cell types are the main producers of type I interferon. Whereas influenza A virus seems to induce most of its interferon in epithelial cells (18), alveolar macrophages have been described as the major source of interferon during NDV infection (22). The histological analysis of cotton rat lung tissue demonstrated that both viruses (MV Schwarz and NDV-H) infect epithelial cells and macrophages to the same degree (data not shown). However, plasmacytoid and myeloid dendritic cells cannot be distinguished histologically, and no functional analysis of dendritic cell populations purified ex vivo has been performed.
In summary, our data indicate that passively transferred IgG antibodies block the secretion of antibody rather than the generation of antigen-specific B cells and that increased type I interferon induction is able to provide an additional stimulus for antibody secretion, thus overcoming inhibition by maternal antibodies.
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Disease (5R01AI064744-03 and P01AI082325-01). T.C. was supported by NIH/NIAID award 1-T32-AI-065411, an NRSA training grant administered by the Center for Microbial Interface Biology (CMIB) at the Ohio State University.
We thank Mary Carsillo and Devra Huey for technical assistance.
Footnotes
Published ahead of print on 20 October 2010.
REFERENCES
- 1.Bukreyev, A., and P. L. Collins. 2008. Newcastle disease virus as a vaccine vector for humans. Curr. Opin. Mol. Ther. 10:46-55. [PubMed] [Google Scholar]
- 2.Bukreyev, A., Z. Huang, L. Yang, S. Elankumaran, M. St Claire, B. R. Murphy, S. K. Samal, and P. L. Collins. 2005. Recombinant Newcastle disease virus expressing a foreign viral antigen is attenuated and highly immunogenic in primates. J. Virol. 79:13275-13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caignard, G., M. Bourai, Y. Jacob, F. Tangy, and P. O. Vidalain. 2009. Inhibition of IFN-alpha/beta signaling by two discrete peptides within measles virus V protein that specifically bind STAT1 and STAT2. Virology 383:112-120. [DOI] [PubMed] [Google Scholar]
- 4.Chang, W. L., E. S. Coro, F. C. Rau, Y. Xiao, D. J. Erle, and N. Baumgarth. 2007. Influenza virus infection causes global respiratory tract B cell response modulation via innate immune signals. J. Immunol. 178:1457-1467. [DOI] [PubMed] [Google Scholar]
- 5.Coro, E. S., W. L. Chang, and N. Baumgarth. 2006. Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J. Immunol. 176:4343-4351. [DOI] [PubMed] [Google Scholar]
- 6.Dalton, K. P., and J. K. Rose. 2001. Vesicular stomatitis virus glycoprotein containing the entire green fluorescent protein on its cytoplasmic domain is incorporated efficiently into virus particles. Virology 279:414-421. [DOI] [PubMed] [Google Scholar]
- 7.Devaux, P., G. Hodge, M. B. McChesney, and R. Cattaneo. 2008. Attenuation of V- or C-defective measles viruses: infection control by the inflammatory and interferon responses of rhesus monkeys. J. Virol. 82:5359-5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiNapoli, J. M., A. Kotelkin, L. Yang, S. Elankumaran, B. R. Murphy, S. K. Samal, P. L. Collins, and A. Bukreyev. 2007. Newcastle disease virus, a host range-restricted virus, as a vaccine vector for intranasal immunization against emerging pathogens. Proc. Natl. Acad. Sci. U. S. A. 104:9788-9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiNapoli, J. M., L. Yang, A. Suguitan, Jr., S. Elankumaran, D. W. Dorward, B. R. Murphy, S. K. Samal, P. L. Collins, and A. Bukreyev. 2007. Immunization of primates with a Newcastle disease virus-vectored vaccine via the respiratory tract induces a high titer of serum neutralizing antibodies against highly pathogenic avian influenza virus. J. Virol. 81:11560-11568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finke, D., and U. G. Liebert. 1994. CD4+ T cells are essential in overcoming experimental murine measles encephalitis. Immunology 83:184-189. [PMC free article] [PubMed] [Google Scholar]
- 11.Fontana, J. M., B. Bankamp, and P. A. Rota. 2008. Inhibition of interferon induction and signaling by paramyxoviruses. Immunol. Rev. 225:46-67. [DOI] [PubMed] [Google Scholar]
- 12.Gans, H., R. DeHovitz, B. Forghani, J. Beeler, Y. Maldonado, and A. M. Arvin. 2003. Measles and mumps vaccination as a model to investigate the developing immune system: passive and active immunity during the first year of life. Vaccine 21:3398-3405. [DOI] [PubMed] [Google Scholar]
- 13.Gans, H., L. Yasukawa, M. Rinki, R. DeHovitz, B. Forghani, J. Beeler, S. Audet, Y. Maldonado, and A. M. Arvin. 2001. Immune responses to measles and mumps vaccination of infants at 6, 9, and 12 months. J. Infect. Dis. 184:817-826. [DOI] [PubMed] [Google Scholar]
- 14.Gans, H. A., A. M. Arvin, J. Galinus, L. Logan, R. DeHovitz, and Y. Maldonado. 1998. Deficiency of the humoral immune response to measles vaccine in infants immunized at age 6 months. JAMA 280:527-532. [DOI] [PubMed] [Google Scholar]
- 15.Huang, Z., S. Krishnamurthy, A. Panda, and S. K. Samal. 2003. Newcastle disease virus V protein is associated with viral pathogenesis and functions as an alpha interferon antagonist. J. Virol. 77:8676-8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jego, G., A. K. Palucka, J. P. Blanck, C. Chalouni, V. Pascual, and J. Banchereau. 2003. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity 19:225-234. [DOI] [PubMed] [Google Scholar]
- 17.Jego, G., V. Pascual, A. K. Palucka, and J. Banchereau. 2005. Dendritic cells control B cell growth and differentiation. Curr. Dir. Autoimmun. 8:124-139. [DOI] [PubMed] [Google Scholar]
- 18.Jewell, N. A., N. Vaghefi, S. E. Mertz, P. Akter, R. S. Peebles, Jr., L. O. Bakaletz, R. K. Durbin, E. Flano, and J. E. Durbin. 2007. Differential type I interferon induction by respiratory syncytial virus and influenza a virus in vivo. J. Virol. 81:9790-9800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz, M. 1995. Clinical spectrum of measles. Curr. Top. Microbiol. Immunol. 191:1-12. [DOI] [PubMed] [Google Scholar]
- 20.Kochs, G., A. Garcia-Sastre, and L. Martinez-Sobrido. 2007. Multiple anti-interferon actions of the influenza A virus NS1 protein. J. Virol. 81:7011-7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumagai, Y., H. Kumar, S. Koyama, T. Kawai, O. Takeuchi, and S. Akira. 2009. Cutting edge: TLR-dependent viral recognition along with type I IFN positive feedback signaling masks the requirement of viral replication for IFN-{alpha} production in plasmacytoid dendritic cells. J. Immunol. 182:3960-3964. [DOI] [PubMed] [Google Scholar]
- 22.Kumagai, Y., O. Takeuchi, H. Kato, H. Kumar, K. Matsui, E. Morii, K. Aozasa, T. Kawai, and S. Akira. 2007. Alveolar macrophages are the primary interferon-alpha producer in pulmonary infection with RNA viruses. Immunity 27:240-252. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Sobrido, L., N. Gitiban, A. Fernandez-Sesma, J. Cros, S. E. Mertz, N. A. Jewell, S. Hammond, E. Flano, R. K. Durbin, A. Garcia-Sastre, and J. E. Durbin. 2006. Protection against respiratory syncytial virus by a recombinant Newcastle disease virus vector. J. Virol. 80:1130-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naniche, D. 2009. Human immunology of measles virus infection. Curr. Top. Microbiol. Immunol. 330:151-171. [DOI] [PubMed] [Google Scholar]
- 25.Parisien, J. P., D. Bamming, A. Komuro, A. Ramachandran, J. J. Rodriguez, G. Barber, R. D. Wojahn, and C. M. Horvath. 2009. A shared interface mediates paramyxovirus interference with antiviral RNA helicases MDA5 and LGP2. J. Virol. 83:7252-7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park, M. S., A. Garcia-Sastre, J. F. Cros, C. F. Basler, and P. Palese. 2003. Newcastle disease virus V protein is a determinant of host range restriction. J. Virol. 77:9522-9532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park, M. S., J. Steel, A. Garcia-Sastre, D. Swayne, and P. Palese. 2006. Engineered viral vaccine constructs with dual specificity: avian influenza and Newcastle disease. Proc. Natl. Acad. Sci. U. S. A. 103:8203-8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Permar, S. R., S. A. Klumpp, K. G. Mansfield, W. K. Kim, D. A. Gorgone, M. A. Lifton, K. C. Williams, J. E. Schmitz, K. A. Reimann, M. K. Axthelm, F. P. Polack, D. E. Griffin, and N. L. Letvin. 2003. Role of CD8(+) lymphocytes in control and clearance of measles virus infection of rhesus monkeys. J. Virol. 77:4396-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfaller, C. K., and K. K. Conzelmann. 2008. Measles virus V protein is a decoy substrate for IkappaB kinase alpha and prevents Toll-like receptor 7/9-mediated interferon induction. J. Virol. 82:12365-12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfeuffer, J., K. Püschel, V. ter Meulen, J. Schneider-Schaulies, and S. Niewiesk. 2003. Extent of measles virus spread and immune suppression differentiates between wild-type and vaccine strains in the cotton rat model (Sigmodon hispidus). J. Virol. 77:150-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pueschel, K., A. Tietz, M. Carsillo, M. Steward, and S. Niewiesk. 2007. Measles virus-specific CD4 T-cell activity does not correlate with protection against lung infection or viral clearance. J. Virol. 81:8571-8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 33.Rodriguez, M. A., W. A. Prinz, W. L. Sibbitt, A. D. Bankhurst, and R. C. Williams, Jr. 1983. Alpha-interferon increases immunoglobulin production in cultured human mononuclear leukocytes. J. Immunol. 130:1215-1219. [PubMed] [Google Scholar]
- 34.Schirrmacher, V., and P. Fournier. 2009. Newcastle disease virus: a promising vector for viral therapy, immune therapy, and gene therapy of cancer. Methods Mol. Biol. 542:565-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlender, J., V. Hornung, S. Finke, M. Gunthner-Biller, S. Marozin, K. Brzozka, S. Moghim, S. Endres, G. Hartmann, and K. K. Conzelmann. 2005. Inhibition of toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J. Virol. 79:5507-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlereth, B., L. Buonocore, A. Tietz, V. ter Meulen, J. K. Rose, and S. Niewiesk. 2003. Successful mucosal immunization of cotton rats in the presence of measles virus-specific antibodies depends on degree of attenuation of vaccine vector and virus dose. J. Gen. Virol. 84:2145-2151. [DOI] [PubMed] [Google Scholar]
- 37.Steel, J. C., B. J. Morrison, P. Mannan, M. S. Abu-Asab, O. Wildner, B. K. Miles, K. C. Yim, V. Ramanan, G. A. Prince, and J. C. Morris. 2007. Immunocompetent syngeneic cotton rat tumor models for the assessment of replication-competent oncolytic adenovirus. Virology 369:131-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.ter Meulen, V., S. Löffler, M. J. Carter, and J. R. Stephenson. 1981. Antigenic characterization of measles and SSPE virus hemagglutinin by monoclonal antibodies. J. Gen. Virol. 57:357-364. [DOI] [PubMed] [Google Scholar]
- 39.Tober, C., M. Seufert, H. Schneider, M. A. Billeter, I. C. D. Johnston, S. Niewiesk, V. ter Meulen, and S. Schneider-Schaulies. 1998. Expression of measles virus V protein is associated with transcriptional control and pathogenicity. J. Virol. 72:8124-8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weidinger, G., G. Henning, V. ter Meulen, and S. Niewiesk. 2001. Inhibition of major histocompatibility complex class II-dependent antigen presentation by neutralization of gamma interferon leads to breakdown of resistance against measles virus-induced encephalitis. J. Virol. 75:3059-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]