Abstract
Although stretches of serine and threonine are sometimes sites for O-linked carbohydrate attachment, specific sequence and structural determinants for O-linked attachment remain ill defined. The gp120 envelope protein of SIVmac239 contains a serine-threonine-rich stretch of amino acids at positions 128 to 139. Here we show that lectin protein from jackfruit seed (jacalin), which binds to non- and monosialylated core 1 O-linked carbohydrate, potently inhibited the replication of SIVmac239. Selection of a jacalin-resistant SIVmac239 variant population resulted in virus with specific substitutions within amino acids 128 to 139. Cloned simian immunodeficiency virus (SIV) variants with substitutions in the 128-to-139 region had infectivities equivalent to, or within 1 log unit of, that of SIVmac239 and were resistant to the inhibitory effects of jacalin. Characterization of the SIVmac239 gp120 O-linked glycome showed the presence of core 1 and core 2 O-linked carbohydrate; a 128-to-139-substituted variant gp120 from jacalin-resistant SIV lacked O-linked carbohydrate. Unlike that of SIVmac239, the replication of HIV-1 strain NL4-3 was resistant to inhibition by jacalin. Purified gp120s from four SIVmac and SIVsm strains bound jacalin strongly in an enzyme-linked immunosorbent assay, while nine different HIV-1 gp120s, two SIVcpz gp120s, and 128-to-139-substituted SIVmac239 gp120 did not bind jacalin. The ability or inability to bind jacalin thus correlated with the presence of the serine-threonine-rich stretch in the SIVmac and SIVsm gp120s and the absence of such stretches in the SIVcpz and HIV-1 gp120s. Consistent with sequence predictions, two HIV-2 gp120s bound jacalin, while one did not. These data demonstrate the presence of non- and monosialylated core 1 O-linked carbohydrate on the gp120s of SIVmac and SIVsm and the lack of these modifications on HIV-1 and SIVcpz gp120s.
Proteins in the cytoplasm, nucleus, and secretory pathway of the cell may be modified posttranslationally with O-linked carbohydrate. In all cases, carbohydrate is added to the hydroxyl group of serine (Ser) or threonine (Thr). There are no clear-cut rules that distinguish a glycosylated Ser or Thr from a nonglycosylated Ser or Thr in the primary protein sequence (15, 25, 33, 49). For O-linked glycosylation that occurs in the nucleus and in the cytoplasm, a single N-acetylglucosamine is added to Ser or Thr (16, 51). The addition of O-linked carbohydrate to proteins in the secretory pathway is commonly referred to as mucin-type O-linked carbohydrate attachment and is the most abundant type of O-linked carbohydrate attachment in humans (36, 43).
Mucin-type O-linked carbohydrate attachment initiates with the covalent attachment of N-acetylgalactosamine (GalNAc) to Ser or Thr in a protein substrate by a family of enzymes known as the UDP-GalNAc:polypeptide N-acetylgalactosamine transferases (7, 20, 46). Attachment of GalNAc to Ser or Thr forms the Tn antigen (9) (Fig. 1). The carbohydrate chain may then be elongated by the addition of galactose and N-acetylglucosamine in different combinations and linkages to form eight core mucin-type O-linked carbohydrate structures (25, 49). The Tn antigen, core 1, sialylated core 1, immature core 2, core 2, and sialylated core 2 structures are the most common mucin-type O-linked carbohydrate structures in humans (10, 25, 49, 52) (Fig. 1).
FIG. 1.
Common mucin-type O-linked carbohydrates. The Tn antigen, sialylated Tn antigen, nonsialylated and sialylated forms of core 1, immature core 2, and nonsialylated and sialylated forms of core 2 are the most common mucin-type carbohydrates. The main synthesis pathway of the common core types is indicated by black arrows from top to bottom. Initially, N-acetylgalactosamine is attached to serine or threonine to form the Tn antigen. Then attachment of galactose in a β-1,3 linkage forms the nonsialylated core 1 structure. An additional attachment of N-acetylglucosamine yields an immature core 2, which is not commonly modified with N-acetylneuraminic acid (sialic acid). Galactose is added to the immature core 2 to form nonsialylated core 2. N-Acetylneuraminic acid is attached to the Tn antigen, nonsialylated core 1, and nonsialylated core 2 structures. The sialylated forms of the common core structures are shown.
The gp120 glycoprotein of HIV type 1 (HIV-1) has been reported to be modified with mucin-type O-linked carbohydrate (3, 8, 17, 19, 45). Enzymatic deglycosylation of HIV-1 gp120 with O-glycosidase resulted in increased protein mobility, consistent with the presence of O-linked carbohydrate on the glycoprotein (3, 45). The presence of disaccharide/trisaccharide and monosaccharide O-linked carbohydrate on HIV-1 gp120 expressed by recombinant vaccinia virus was also consistent with gel filtration analysis of carbohydrate released by β-elimination (17). An antibody specific to the Tn antigen was found to neutralize the infectivity of HIV-1 (19); however, amino acid substitutions in the region of gp120 predicted to be the site of attachment did not alter the neutralization-sensitive phenotype (18).
In SIVmne-infected pigtail macaques (Macaca nemestrina), Ser and Thr amino acids were inserted into the V1 region of gp120 over the course of disease, introducing a potential O-linked carbohydrate attachment site (34). When the new Ser-Thr-rich strain, SIVmneC18, was transmitted to other pigtail macaques, the Ser-Thr-rich sequence in V1 remained (5). Chackerian et al. nicely showed that the alteration of three amino acids in SIVmneC18 V1 to Thr, Ser, and Thr in an existing Thr-dense region increased the mass of gp120 consistent with the addition of O-linked carbohydrate (6). The insertion of Thr, Ser, and Thr also increased the resistance of SIVmneC18 to neutralization by sera from infected monkeys (6).
Plant lectin proteins from jackfruit seeds (jacalin) and peanuts (peanut agglutinin [PNA]) are generally regarded to have primary specificity toward nonsialylated core 1 (Galβ-1,3GalNAc-α) O-linked carbohydrate (26, 31, 42). Jacalin also binds the Tn antigen and monosialylated core 1 carbohydrate (48, 53). PNA recognizes only nonsialylated, not sialylated, core 1 mucin-type O-linked carbohydrate (35, 47, 54). In this report, we identify an O-linked carbohydrate attachment region in gp120 whose mutation imparts high-level resistance to the inhibitory effects of jacalin to cloned SIVmac239. Modification of the gp120 of SIVmac239 with non- and monosialylated core 1 carbohydrate was confirmed by mass spectrometry. Furthermore, using a jacalin-binding enzyme-linked immunosorbent assay (ELISA), we show that assorted gp120s from SIVmac and SIVsm are consistently modified with core 1 mucin-type O-linked carbohydrate but that assorted HIV-1 and SIVcpz gp120s do not contain these jacalin-sensitive structures. These results demonstrate the consistent presence of non- and monosialylated core 1 mucin-type O-linked carbohydrate on the gp120s of simian immunodeficiency virus (SIV) from sooty mangabeys and macaques and the consistent absence of such structures on the gp120s of SIV from chimpanzees and of HIV-1.
MATERIALS AND METHODS
Cell culture and antibodies.
HEK293T and CEMx174 cells were obtained from the American Type Culture Collection. C8166-secreted alkaline phosphatase (SEAP) cells were generated previously (32). HEK293T cells were maintained in complete DMEM (Dulbecco's modified Eagle medium [DMEM] supplemented with 10% fetal bovine serum, 100 U/ml penicillin G sodium, and 100 μg/ml streptomycin [Gibco BRL, Rockville, MD]). CEMx174 and C8166-SEAP cells were maintained in complete RPMI medium (RPMI supplemented with 10% fetal bovine serum, 100 U/ml penicillin G sodium, and 100 μg/ml streptomycin [Gibco]). Cell lines expressing each rhesus monoclonal antibody (rhMAb) were maintained in RPMI medium supplemented with 20% fetal bovine serum and 100 μg/ml Primocin (InvivoGen, San Diego, CA).
Virus production.
Viral stocks were generated by transient transfection of a full-length SIV or a full-length HIV proviral vector in HEK293T cells. Briefly, 1.5 × 106 cells were plated in a T75 flask 24 h prior to transfection. For each viral stock produced, 5 μg of proviral DNA was transfected by the calcium phosphate method (Promega Corporation, Madison, WI). The medium was replaced 16 h after transfection. Two days later, the cell culture supernatant was passed through a 0.45-μm-pore-size polyethersulfone syringe filter. Virus production was measured with a capsid protein (p27 for SIV and p24 for HIV) antigen capture assay (Advanced Bioscience Laboratories, Inc., Kensington, MD).
Growth of SIVmac239 in the presence of jacalin.
CEMx174 cells at a density of 1 × 106 cells per milliliter were infected with 30 ng/ml SIVmac239 capsid protein (p27). The cultures were divided 24 h following infection, and the medium was replaced with a medium that contained Galanthus nivalis agglutinin (GNA) or Hippeastrum hybrid agglutinin (HHA) at 0, 0.1, 1, 10, 100, or 200 μg/ml. Every 3 to 4 days, the cell cultures were split one to two with a medium that contained the same concentration of jacalin. SIV p27 released into the supernatant was measured by an antigen capture assay (Advanced Bioscience Laboratories, Inc.).
Jacalin selection of SIVmac239.
CEMx174 cells at a density of 1 × 106 per milliliter were infected with 30 ng/ml SIVmac239 capsid protein (p27). The cultures were divided 24 h following infection, and the medium was replaced with a medium that contained 0, 1, 10, or 100 μg/ml of jacalin. Every 3 to 4 days, the cell cultures were split one to two with a medium that contained the same concentration of jacalin. SIV p27 released into the supernatant was measured by an antigen capture assay (Advanced Bioscience Laboratories, Inc.). For the second passage, CEMx174 cells were infected with 30 ng p27 of the virus population at day 14 from the culture with 100 μg/ml jacalin. The medium was changed 24 h later to one that contained 250 μg/ml jacalin. Every 3 to 4 days, the cell cultures were split one to two with a medium that contained 250 μg/ml jacalin. The virus that grew from selection at passage 2 was used for a new infection (passage 3) at the same concentration of jacalin (250 μg/ml). Virus was passaged in this manner until passage 5. As a control, SIVmac239 was passaged in CEMx174 cells concurrently with the lectin-selected population mentioned above.
Isolation of RNA and env from the jacalin-resistant population.
Viral RNA was isolated from the culture supernatant by using the MagMAX viral RNA isolation kit according to the manufacturer's recommendations (Applied Biosystems, Foster City, CA). RNA was isolated from 400 μl of culture supernatant with p27-containing SIV. The envelope sequence (env) was amplified from isolated viral RNA using the SuperScript III one-step RT-PCR system (Invitrogen, Carlsbad, CA). Primers used to amplify env were as follows: forward primer, 5′-GACGAGCGCTCTTCATGCATTTCAGAGG-3′; reverse primer, 5′-AAGCTTGCATGCTATAACACATGC-3′. The PCR product was then cloned with the TOPO XL PCR cloning kit (Invitrogen). Twenty env clones were sequenced (Retrogen Inc., San Diego, CA) and were then aligned with the SIVmac239 sequence.
Construction of O-linked SIV variant clones.
Mutant derivatives of the SIVmac239 proviral vector that contained codon substitutions (nucleotides [nt] 6979 to 7020) were constructed. The nucleotide numbers correspond to the original published sequence of SIVmac239 (39). To introduce changes to the desired codons, the 3′ half of the SIVmac239 genome was used as a template for PCR site-directed mutagenesis. For each mutant, complementary and reverse-oriented mutagenic primers containing the appropriate nucleotide changes from the SIVmac239 sequence were designed. Multiple-round PCR using PfuUltra II Fusion HS DNA polymerase (Stratagene, Cedar Creek, TX) incorporated the mutation from the primers into the 3′ SIVmac239 plasmid. Double mutants were generated by sequential PCRs. Following amplification, the entire coding region of the 3′ half of the SIVmac239 genome was sequenced to confirm that only the intended changes were introduced. Sequence-confirmed variants were then digested with SphI and XhoI and were ligated to the 5′ half of SIVmac239. Full-length clones were confirmed by restriction digestion.
Infectivity assay.
SIVmac239 and each O-linked SIV variant stock were used to infect an immortalized human T-cell line (C8166-45) with a stably integrated, Tat-inducible SEAP gene as described previously (32). Briefly, 25 ng p27 for each virus was serially diluted by 2-fold in RPMI complete medium. Virus from each dilution (100 μl) was added to 5,000 C8166-SEAP cells in 100 μl medium. Three days later, SIV infection of C8166-SEAP cells was measured by the production of SEAP in the culture medium. SEAP activity in the supernatant was measured using a Phospha-Light assay system (Applied Biosystems).
Codon-optimized SIV gp120 expression vectors.
The codon-optimized (c.o.) SIVmac239 Env expression vector (40) was modified using PCR mutagenesis to introduce two stop codons at the end of the gp120 coding sequence (arginine at nt 8176 to 8178) into the SIVmac239 genome such that gp120 was secreted into the culture medium. These changes were made in the c.o. expression vector with the forward primer 5′-GAACAAGCGGTGATAATTCGTCCTGG-3′ together with a reverse-oriented mutagenic primer (c.o.SIVmac239gp120). The SIV04 and Jacalin4 c.o. gp120 sequences were synthesized (GenScript, Piscataway, NJ) and cloned into the same vector that contained the SIVmac239 gp120. The c.o. PBj14 clone 6.6 was synthesized (GenScript, Piscataway, NJ) based on the genomic sequence (GenBank accession number L09212.1). The c.o. HIV-1 NL4-3 gp120 (GenBank accession number M19921) was synthesized and cloned into pcDNA3.1(+) (Invitrogen). The SIVsmE543.3 gp120 was cloned from the c.o. SIVsmE543.3 expression vector provided by Vanessa Hirsch. For SIVsmPBj14 6.6 and SIVsmE543.3, env was amplified with primers that contained the appropriate restriction site. The env was then cloned via the NheI and ApaI restriction sites into the cytomegalovirus (CMV)-driven mammalian expression vector pcDNA3.1(+) (Invitrogen).
Protein production.
The c.o. SIV gp120 constructs were used to transfect HEK293T cells using the GenJet method (SignaGen Laboratories, Ijamsville, MD). The medium was replaced 24 h later with serum-free DMEM. Two days later, the culture supernatant was passed through a 0.45-μm-pore-size polyethersulfone syringe filter. The filtered supernatant was incubated with GNA agarose beads (Sigma) for 24 h at 4°C. The beads were washed sequentially with 10 ml phosphate-buffered saline (PBS). The last wash was determined when the elutant reached an optical density less than 0.03. Then gp120 was eluted from the beads with 18 ml of 0.75 M methyl α-d-mannopyranoside in PBS. The samples were concentrated at 700 × g using Vivaspin 20 columns with a 100-kDa cutoff (Fisher, Pittsburgh, PA). Each sample was dialyzed against PBS. Following dialysis, protein was recovered, and the total-protein level was determined with a bicinchoninic acid assay (Pierce, Rockford, IL). The following histidine-tagged gp120s produced from human 293 cells and purified using the His tag were purchased from Immune Technology Corp., New York, NY: SIVmac239, SIVsmE660, HIV-2 Ben, HIV-2 UC1, HIV-2 NIH-Z, HIV-1 Bal, HIV-1 JRCSF, HIV-1 consensus B, HIV-1 p.i. clade A (92RW020), HIV-1 consensus A2, HIV-1 ZM53M.PB12, HIV-1 ZM214M.PL15, HIV-1 consensus C, SIVcpzEK505, and SIVcpzMB66.
Jacalin ELISA.
Normalized amounts of total protein were loaded into six wells of a high-protein-binding 96-well plate (Fisher). After two washes with 0.05% Tween 20 in PBS, the wells were blocked for 1 h at 37°C with Carbo-Free blocking reagent (Vector Laboratories, Burlingame, CA). Wells were washed 5 times with 0.05% Tween 20 in PBS. Then 50 μl of horseradish peroxidase-conjugated jacalin (jacalin-HRP) (United States Biological, Swampscott, MA) diluted 150 times in Carbo-Free blocking buffer was added to four of the test wells. For SIV and HIV proteins purchased from Immune Technology Corp., 50 μl of His-HRP antibody (Abcam, Cambridge, MA) was diluted 1:100 and added to duplicate wells of each sample as a normalization control. SIV gp120 proteins that did not include the His tag were normalized with polyclonal sera from SIVmac239-positive rhesus macaques. Serum IgGs were detected with an anti-rhesus-HRP antibody (Southern Biotech, Birmingham, AL). Following an hourlong incubation with the secondary antibody, wells were washed 10 times with 0.05% Tween 20 in PBS, and then 50 μl of fresh tetramethylbenzidine (TMB) solution (EMD Chemicals, Gibbstown, NJ) was added to each well. Wells were allowed to develop for 30 min before the reaction was stopped. Plates were read at 450 nm on an MRX Revelation absorbance reader (Dynex Technologies, Chantilly, VA).
Statistical significance of jacalin binding.
GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA) was used to run an unpaired t test to calculate the significance of the difference in the jacalin binding capacity between SIVmac/sm and HIV-1. The unpaired t test method tests the null hypothesis that the population means relating to two independent random samples from an approximately normal distribution are equal.
Release of O-linked glycans.
Purified SIVmac239 gp120, Jacalin4 gp120, and SIV04 gp120 were freeze-dried in 30-μg aliquots prior to the release of O-glycans by reductive elimination. This was performed by incubation of the samples in 200 μl of a solution comprising 55 mg/ml potassium borohydride (KBH4) in 0.1 M potassium hydroxide for 24 h at 45°C. The reaction was terminated by dropwise addition of glacial acetic acid. Released O-glycans were purified using an ion-exchange 50W-X8 Dowex column, previously equilibrated in 5% (vol/vol) acetic acid. The sample was loaded, eluted with 3 ml of 5% (vol/vol) acetic acid, collected, and dried on a Savant Speed-Vac concentrator. Excess borates resulting from the reductive elimination were removed by successive coevaporations using a solution of 10% (vol/vol) acetic acid in methanol. Released and purified O-glycans were finally dried under a stream of nitrogen and were lyophilized prior to methylation.
Permethylation of O-linked carbohydrate.
O-glycan permethylation was performed using the sodium hydroxide procedure (24). Briefly, sodium hydroxide pellets were crushed with dimethyl sulfoxide (DMSO) to form a slurry. A 1-ml aliquot of this slurry was added to the dried glycans, followed by the addition of 1 ml of methyl iodide (ICH3). The mixture was vigorously mixed on an automatic shaker for 10 min at room temperature. The reaction was terminated by the addition of 1 ml of water, and permethylated glycans were recovered by chloroform extraction. The chloroform layer was washed several times with water in order to remove impurities and was then dried under a stream of nitrogen. Permethylated O-glycans were purified using a reverse-phase C18 Sep-Pak cartridge. The reverse phase was conditioned successively with methanol, water, acetonitrile (ACN), and water. The sample was dissolved in 1:1 (vol/vol) methanol-water, loaded onto the cartridge, washed with water and 15% (vol/vol) aqueous ACN solution, and then eluted using a 75% (vol/vol) aqueous ACN solution. The organic solvent was removed on a Savant Speed-Vac concentrator, and samples were lyophilized prior to matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) analyses.
MALDI mass spectrometry.
MALDI-MS data on permethylated samples were acquired in the reflector positive-ion mode using a 4800 MALDI-TOF/TOF (Applied Biosystems, Foster City, CA) mass spectrometer. The instrument was calibrated externally using the Calmix 4700 calibration standard, containing des-Arg1-bradykinin (molecular mass, 904.46 Da), angiotensin I (molecular mass, 1,296.68 Da), human [Glu1]-fibrinopeptide B (molecular mass, 1,570.67 Da), adrenocorticotropin (ACTH) fragment 1-17 (molecular mass, 2,093.08 Da), ACTH fragment 18-39 (molecular mass, 2,465.19 Da), and ACTH fragment 7-38 (molecular mass, 3,657.92 Da). Samples were dissolved in 20 μl of methanol, and 1 μl was mixed at a 1:1 ratio (vol/vol) with 2,5-dihydrobenzoic acid (20 mg/ml in 50% [vol/vol] methanol in water) as a matrix. Then samples were spotted onto a 100-well sample plate and were dried under a vacuum. Data were acquired using 4000 Series Explorer instrument control software and were processed using Data Explorer MS processing software. MS spectra were assigned and annotated with the help of the GlycoWorkbench software (4).
Neutralization assay.
The sensitivities of infection with O-linked SIV variants, SIVmac239, and SIV316 to neutralization by sera from SIVmac239-infected rhesus macaques, soluble CD4 (sCD4), and monoclonal antibodies that bind SIV Env were measured using C8166-SEAP cells as previously described (32).
RESULTS
Selection of a jacalin-resistant population derived from SIVmac239.
Lectin protein from jackfruit seeds (jacalin) binds with a primary specificity to non- and monosialylated core 1 (Galβ-1,3GalNAc-α) O-linked carbohydrate (26, 42, 48, 53). To determine if SIVmac239 is sensitive to jacalin, the replication of SIVmac239 was assayed at a low multiplicity of infection under conditions of a spreading infection. In the absence of lectin, SIVmac239 had a peak height of virus production 8 days after infection, as determined by quantitation of the capsid protein (p27) from the culture supernatant. Similar replication kinetics were observed for SIVmac239 in the presence of 1 μg/ml jacalin (Fig. 2A). Progressively lower levels of viral replication were observed with increasing concentrations of lectin. Virus replication was delayed for SIVmac239 in the presence of 10 μg/ml jacalin (Fig. 2A). In the presence of 100 μg/ml jacalin, production of SIVmac239 remained at low levels (8 to 10 ng/ml p27) for 10 days and was detected at day 14 after infection at approximately 200 ng/ml p27 (Fig. 2A), consistent with the virus growing out of lectin inhibition.
FIG. 2.
Selection of jacalin-resistant SIVmac239. (A) Representative replication of SIVmac239 in the absence of jacalin (squares) and in the presence of jacalin at 1 μg/ml (circles), 10 μg/ml (triangles), or 100 μg/ml (diamonds). (B) Replication of SIVmac239 and the jacalin-selected virus population in the absence of jacalin. (C) Replication of SIVmac239 and the jacalin-selected population in the presence of 250 μg/ml jacalin. CEMx174 cells were infected with SIVmac239 containing 5 ng of p27 or the jacalin-selected virus containing 5 ng p27. The cultures were divided in half 24 h later, and the medium was replaced with a medium that contained either no lectin or the indicated concentration of jacalin. SIV capsid protein (p27) that was released into the culture medium was measured on the days indicated in order to follow virus production.
To select for a population of SIV that replicated in the presence of jacalin, SIVmac239 was passaged in CEMx174 cells in the presence of escalating concentrations of lectin. After passage 5, the lectin-selected virus population replicated similarly to a parallel, unselected virus population in the absence of lectin (Fig. 2B). However, in the presence of 250 μg/ml jacalin, only the lectin-selected virus had a resistant phenotype (Fig. 2C).
SIVmac239 variants cloned from the jacalin-selected, lectin-resistant virus population.
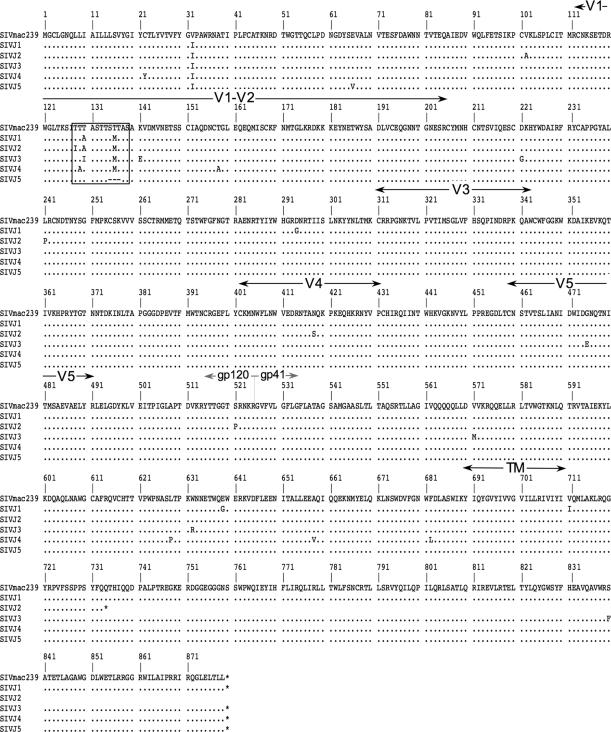
Sequences of env were amplified from the viral genomes of the SIV population that replicated in the presence of 250 μg/ml jacalin, and 20 clones were sequenced. All 20 clones had sequence changes that clustered to the potential O-linked glycosylation site in variable domain 1 (V1) of gp120 (amino acids 128 to 139). Five sequences with representative changes in amino acids 128 to 139 of the jacalin-selected population were aligned to the sequence of the parental SIVmac239 Env (Fig. 3). The amino acid substitutions of SIVJ1 were the most prevalent in the population (30%). SIVJ5 was present as 25% of the population. SIVJ2 was a minor part of the population (5%), while the changes observed in SIVJ3 and SIVJ4 were each 20%.
FIG. 3.
Substitutions of amino acids in the jacalin-selected SIV population cluster in the potential O-linked carbohydrate attachment site. Envelope sequences were amplified from viral RNA, and 20 clones were sequenced. Five clones representative of the population are shown. The predicted O-linked glycosylation site (amino acids 128 to 139) in gp120 is boxed. The gp120 variable regions (V1 to V5), the junction between gp120 and gp41, and the transmembrane domain (TM) are also indicated. Amino acids in the jacalin-selected virus population that are the same as those in the parental SIVmac239 strain are shown as periods. Dashes indicate deletions. Asterisks represent stop codons.
In the jacalin-selected population, five of the 20 sequences had stop codons that truncated SIV gp41. Truncation of the cytoplasmic tail of SIV gp41 has been noted previously when SIV was serially passaged in human cells (21, 30). Truncation of the cytoplasmic tail occurred at multiple amino acids and was not linked to any one sequence change observed in amino acids 128 to 139. Two of the five sequences were truncated at amino acid 734, as seen in SIVJ2 (Fig. 3).
Infectivities of V1 variants of SIVmac239.
Cloned SIV variants based on amino acid changes observed in the jacalin-selected population and cloned SIV variants that disrupt the V1 serine/threonine stretch of SIVmac239 gp120 were constructed in the context of the SIVmac239 proviral genome (Fig. 4). To determine if the cloned 128-to-139-substituted SIV variants were infectious in the context of the SIVmac239 genome, virus stocks of SIVmac239 and of each of the SIV variants (Jacalin1 to Jacalin8; SIV01 to SIV07) were produced by transfection of HEK293T cells. C8166-secreted alkaline phosphatase (C8166-SEAP) cells were infected with input virus normalized for the amount of SIV capsid protein (p27) present in the virus stock. Upon infection, C8166-SEAP cells secrete alkaline phosphatase into the culture medium such that the levels of phosphatase activity correlate directly with the amount of input p27. SEAP activity in the cell-free supernatant was measured 3 days after infection, a time that primarily reflects the first round of infection prior to appreciable spread of secondary progeny virions. The infectivities of Jacalin3, Jacalin4, Jacalin7, Jacalin8, and SIV05 were similar to that of SIVmac239 (Fig. 5A and B). SIV01 and SIV02 were severely reduced (4 log units or more) in infectivity compared to SIVmac239 (Fig. 5B). The remaining cloned SIV variants had infectivities 0.8 to 1 log unit lower per ng of input p27 than that of SIVmac239 (Fig. 5A and B).
FIG. 4.
Schematic representation of cloned SIV variants. Combinations of amino acid substitutions in the potential O-linked site in gp120 were introduced into the SIVmac239 proviral backbone. SIV variants Jacalin1 to Jacalin8 were based on changes present in the jacalin-selected population. Amino acid substitutions introduced in SIV01 to SIV07 were designed to disrupt the serine-threonine-rich stretch (amino acids 128 to 139).
FIG. 5.
Comparative infectivities of SIV variants that lack the O-linked carbohydrate attachment site. (A) SIV variants with V1 sequences that mirror the jacalin-selected virus population were within 0.8 log unit as infectious as the parental SIVmac239 strain. (B) SIV variants that were noninfectious or had an infectivity within 1 log unit of that of SIVmac239. Virus stocks for each cloned SIV variant and the cloned SIVmac239 were produced by transient transfection of HEK293T cells. Virus containing normalized amounts of p27 was used to infect C8166-secreted alkaline phosphatase (SEAP) cells, which contain a stably integrated, Tat-inducible SEAP reporter gene. Viral infectivity is directly correlated to the amount of SEAP released into the culture supernatant. At 72 h after infection, SEAP activity was measured in triplicate with a Tropix Phospha-Light kit. The mean SEAP activity (± standard deviation) is shown for each viral input (ng p27) indicated.
Replication of the cloned SIV variants determined in the absence and presence of jacalin.
The spread of virus through human CEMx174 cells in culture was determined for each cloned 128-to-139-substituted SIV variant and was compared to that of the parental cloned SIVmac239. In the absence of lectin, the time of peak virus production for the majority of the SIV variants was similar to that for SIVmac239 (Fig. 6A and B). For SIV03, replication was delayed significantly even in the absence of jacalin, precluding further analysis (Fig. 6B). In the presence of 250 μg/ml jacalin, SIVmac239 replication remained at background levels, indicative of an infection that could not overcome the inhibition due to this concentration of jacalin (Fig. 6C and D). Resistance to the inhibitory effects of jacalin was conferred on all O-linked SIV variants tested (Fig. 6C and D). The production of resistant virus variants (as indicated by p27 production) in the presence of jacalin was 4- to 5-fold lower than that in the absence of jacalin (Fig. 6); it is possible that this difference reflects the effects of jacalin on the cells (1, 11, 37).
FIG. 6.
Jacalin resistance is conferred by sequence variations in the V1 domain of SIV gp120. (A) SIV variants with V1 sequences that mirror the jacalin-selected virus population were replication competent in the absence of jacalin. (B) SIV V1 variants replicated similarly to SIVmac239. (C) SIV variants with V1 sequences that mirror the jacalin-selected virus population were more resistant to jacalin than the sensitive SIVmac239 strain. (D) SIV V1 variants were more resistant to jacalin than the sensitive SIVmac239 strain. Virus stocks for each cloned SIV variant and cloned SIVmac239 were produced by transient transfection of HEK293T cells. CEMx174 cells were infected with SIV variant virus or SIVmac239 virus, each containing 5 ng p27. One day following infection, the culture was split, and the medium was replaced with a medium that did not contain lectin or with a medium that contained jacalin (250 μg/ml). SIV capsid protein (p27) released into the culture medium was measured on the days indicated in order to follow virus production.
Jacalin binds to SIVmac239 gp120 but not to jacalin-resistant SIV variant gp120s.
The capacity of jacalin to bind SIV gp120 was measured by an ELISA. SIV gp120 proteins purified from HEK293T cell supernatants were normalized for total protein bound to each well. The gp120 proteins were probed in quadruplicate with HRP-conjugated jacalin (black) or in duplicate with sera from SIVmac239-positive macaques (gray) (Fig. 7A). The gp120s of SIV04 and Jacalin4, two jacalin-resistant variants, exhibited jacalin binding capacities >95% lower than that of the gp120 of the parental SIVmac239 (Fig. 7B).
FIG. 7.
Jacalin binds O-linked carbohydrate attached to the V1 domain of SIVmac239. (A) SIV04 and Jacalin4 gp120 proteins have markedly lower jacalin binding capacities than that of SIVmac239. HEK293T cells were transfected with no DNA (mock) or with the gp120 eukaryotic expression vector for SIVmac239, SIV04, or Jacalin4. Protein released into serum-free medium was purified with the lectin from Galanthus nivalis. Equivalent amounts of protein from each sample were loaded into six wells of a high-protein-binding plate. Jacalin-HRP was used to probe each sample in quadruplicate. Filled bars represent mean jacalin signals; error bars, standard deviations. Sera from SIV-positive rhesus macaques were used to probe each sample in duplicate. Shaded bars represent signals from sera binding gp120. (B) Normalized jacalin binding to gp120 from SIV04 or Jacalin4. The mean jacalin signal was normalized to the mean signal from sera. Filled bars represent normalized jacalin signals; error bars, standard deviations.
SIVmac239 gp120, but not jacalin-resistant SIV variant gp120s, is modified with non- and monosialylated core 1 O-linked carbohydrate.
To confirm the jacalin ELISA data, we determined the O-linked glycomes of purified SIVmac239 gp120, SIV04 gp120, and Jacalin4 gp120. O-linked carbohydrate was released from gp120, permethylated, and analyzed by MALDI-MS. Carbohydrate structures were assigned based on monosaccharide composition and fragmentation analysis.
The most abundant O-linked carbohydrates of SIVmac239 gp120 were of the core 2 mucin type (m/z 983.5, 1,344.8, and 1,705.9, representing 35%, 27%, and 6% of the O-glycan population, respectively) (Fig. 8A). Immature core 2 (m/z 779.5) and disialylated core 1 (m/z 1,256.8) carbohydrates were also detected at relative intensities of 10% and 5%, respectively (Fig. 8A). The SIVmac239 gp120 O-glycome also contained, at about 10%, both non- and monosialylated core 1 O-linked carbohydrates, structures to which jacalin binds (m/z 534.3 and m/z 895.6) (Fig. 8A). In contrast, the SIV04 mass spectrum had only traces of O-glycans and no evidence of jacalin-sensitive structures (non- and monosialylated core 1) (Fig. 8B). The Jacalin4 gp120 also lacked jacalin-sensitive structures but retained the more abundant core 2 structures at m/z 983.5 and 1,344.8 (Fig. 8C). The spectrum for protein purified from mock-transfected cell supernatant proteins was devoid of any signal consistent with O-linked carbohydrate (Fig. 8D). The only signals in the mock-transfected sample were common to all samples (Fig. 8A, B, C, and D) and can be attributed to hexose oligomers.
FIG. 8.
Mass spectra of SIVmac239, SIV04, and Jacalin4 gp120 permethylated O-glycans released by reductive elimination. The spectra were acquired in positive-ion mode using the reflectron. Molecular ions of m/z 534, 895, and 1,256 correspond to non-, mono-, and disialylated core 1 structures, respectively, whereas molecular ions of m/z 779, 983, 1,344, and 1,705 were defined as core 2 O-linked glycans. Signals from hexose oligomers are indicated by X's. All molecular ions are [M + Na]+, and each panel is normalized to the most abundant signal (100% intensity) in the raw data. The abundance reported in the text for each O-glycan corresponds to its percentage of the total observed O-glycome (based on peak heights). The O-glycome value was obtained by summing the peak heights of the molecular ions attributable to O-glycans. These are noted in each of the panels. Structural assignments are based on monosaccharide composition, fragmentation analyses, and knowledge of the glycan biosynthetic pathways. The sugar symbols used are those employed by the Consortium for Functional Glycomics (www.functionalglycomics.org). (A) SIVmac239; (B) SIV04; (C) Jacalin4; (D) mock transfection.
The replication of HIV-1 strain NL4-3 is resistant to inhibition by jacalin.
To determine if the replication of HIV-1 is inhibited by jacalin, CEMx174 cells were infected with HIV-1 strain NL4-3 containing 10 ng/ml p24. The culture was divided, and the virus was allowed to replicate in the absence and presence of jacalin. SIVmac239 was included in parallel as a control. In the absence of lectin, the peaks of virus production were similar for HIV-1 NL4-3 and SIVmac239 (Fig. 9A). In the presence of jacalin, HIV-1 NL4-3 had a resistant phenotype while SIVmac239 was sensitive to the inhibitory effects of jacalin (Fig. 9B).
FIG. 9.
HIV-1 NL4-3 is resistant to jacalin inhibition. (A) Replication of HIV-1 NL4-3 and SIVmac239 in the absence of lectin. (B) Replication of HIV-1 NL4-3 and SIVmac239 in the presence of 250 μg/ml jacalin. CEMx174 cells were infected with SIVmac239 containing 5 ng p27 or with HIV-1 NL4-3 containing 5 ng of p24. One day following infection, the culture was split, and the medium was replaced either with a medium that did not contain lectin or with a medium that contained jacalin (250 μg/ml). The amount of SIV or HIV capsid protein that was released into the culture medium was measured on the days indicated in order to follow virus production.
SIVmac/sm gp120 has a much higher content of core 1 mucin-type O-linked carbohydrate than HIV-1 gp120.
To compare the core 1 mucin-type O-linked carbohydrate contents, the jacalin binding capacities of HIV-1 and SIVmac/sm gp120 proteins were determined. Eight independent commercially available His-tagged HIV-1 gp120 proteins and two commercially available His-tagged SIVmac/sm gp120 proteins were bound to a high-binding ELISA plate. Subsequently, the jacalin signal was normalized to the average signal from the His tag (Fig. 10A and B). The gp120s of two additional SIVsm isolates and one additional HIV-1 isolate were produced from HEK293T cells (see Materials and Methods), normalized for total protein, and probed with jacalin-HRP (Fig. 10C). The gp120s of the four independent SIVsm/SIVmac isolates all bound jacalin with similar capacities (Fig. 10A, B, and C). However, all of the HIV-1 isolates had >95% lower capacities for gp120 to bind jacalin than the SIVsm/SIVmac isolates (Fig. 10A, B, and C). The difference between the jacalin binding capacities of the nine unrelated isolates of HIV-1 and those of the four SIVmac/sm isolates was statistically significant (P, <0.0001) in an unpaired t test.
FIG. 10.
The jacalin and PNA binding capacities of SIVmac/sm, HIV-2 Ben, and HIV-2 UC1 gp120s differ from those of HIV-2 NIH-Z, HIV-1, and SIVcpz gp120 proteins. (A) Jacalin binding capacities of SIVmac, SIVsm, HIV-2, HIV-1, and SIVcpz gp120 proteins. Equivalent amounts of histidine-tagged gp120 proteins were each loaded into six wells of a high-protein-binding plate. Jacalin-HRP was used to probe each sample in quadruplicate. A histidine-HRP antibody was used to probe each sample in duplicate. The mean jacalin signal was normalized to the mean signal from the histidine tag. The normalized jacalin signal (± standard deviation) is shown. (B) Same as panel A but without normalization. (C) Comparison of the jacalin binding capacities of SIVmac, SIVsm, and HIV-1 gp120s. HEK293T cells were transiently transfected either with no DNA (mock) or with a gp120 eukaryotic expression vector for SIVmac, SIVsm, or HIV-1. Env was purified from the cell culture supernatant with lectin from Galanthus nivalis. Equivalent amounts of total protein were loaded into the wells of a high-protein-binding plate. Jacalin-HRP was used to probe each sample in quadruplicate. The mean jacalin signal (± standard deviation) is shown. (D) Lectin from peanut (PNA) binds to the gp120 of SIVmac/sm but not to the gp120 of SIVcpz or HIV-1. Equivalent amounts of histidine-tagged gp120 proteins were each loaded into six wells of a high-protein-binding plate. PNA-HRP was used to probe each sample in quadruplicate. A histidine-HRP antibody was used to probe each sample in duplicate. The mean PNA signal was normalized to the mean signal from the histidine tag. The normalized PNA signal (± standard deviation) is shown.
Since HIV-1 is thought to have arisen by the transmission of SIVcpz to humans (23, 28) and HIV-2 is thought to have arisen by the transmission of SIVsm to humans (14, 22, 41), we investigated the jacalin binding capacities of the SIVcpz and HIV-2 gp120 proteins. Like those of HIV-1, two independent SIVcpz gp120s had extremely low jacalin binding capacities (Fig. 10A and B). Jacalin bound HIV-2 Ben and HIV-2 UC1 gp120 proteins, consistent with the modification of core 1 mucin-type O-linked carbohydrate (Fig. 10A and B). HIV-2 NIH-Z gp120 did not bind jacalin.
Thus, all four SIVmac/sm strains and two of the three HIV-2 strains tested were modified with non- and/or monosialylated core 1 mucin-type O-linked carbohydrate. All nine HIV-1 strains, one HIV-2 strain, and two SIVcpz strains lacked non- and/or monosialylated core 1 mucin-type carbohydrate.
To support the jacalin binding data, we probed gp120s from SIVmac/sm, HIV-1, and SIVcpz with HRP-conjugated lectin from peanut (PNA), which binds primarily to nonsialylated core 1 mucin-type O-linked carbohydrate. SIVmac/sm gp120 proteins had a >10-fold greater capacity to bind PNA than gp120s of HIV-1 or SIVcpz (Fig. 10D).
Primate immunodeficiency viruses that contain potential O-linked carbohydrate attachment sites in the V1 domain of gp120 are modified with core 1 mucin-type O-linked carbohydrate.
Env sequences of SIV and HIV strains tested in the jacalin-binding ELISA were analyzed with NetOGlyc 3.1, a program that predicts sites of O-linked carbohydrate attachment (27). The gp120s of SIVmac239, three SIVsm strains, and two HIV-2 strains, to which jacalin bound, had serine and threonine amino acids in the V1 region that were predicted to be modified with O-linked carbohydrate (Table 1). None of the strains that jacalin failed to bind (HIV-2 NIH-Z, nine different HIV-1 strains, and two SIVcpz strains) contained any predicted sites in the V1 domain of gp120 (Table 1).
TABLE 1.
Predicted O-linked sites in the V1 domain of the gp120s for SIVsm, SIVmac, HIV-2, HIV-1, and SIVcpz
| Strain | V1 domaina | Predicted no. of O-linked sites in V1 |
|---|---|---|
| SIVmac239 | CNKSETDRWGLTKSITTTASTTSTTASAKVDMVNETSSC | 2 |
| SIVsmE660 | CNKTETDRWGLTRNAGTTTTTTTTTTAATPSVAENVINESNPC | 11 |
| SIVsmE543.3 | CNKTETDRWGLTGRAETTTTAKSTTSTTTTTVTPKVINEGDSC | 12 |
| SIVsmPBj14 | CNKSETDRWGLTGTPAPTTTQTTTTQASTTPTSPITAKVVNDSDPC | 15 |
| HIV-2 Ben | CSRVQGNTTTPNPRTSSSTTSRPPTSAASIINETSNC | 11 |
| HIV-2 UC1 | CNNTGTNTTTKPITTPITTTKPSENLLNDTSPC | 5 |
| HIV-2 NIH-Z | CTRNMTTWTGRTDTQNITIINDTSHARADNC | 0 |
| HIV-1 NL4-3 | CTDLKNDTNTNSSSGRMIMEKGEIKNC | 0 |
| HIV-1 Bal | CTDLRNATNGNDTNTTSSSRGMVGGGEMKNC | 0 |
| HIV-1 JRCSF | CKDVNATNTTSSSEGMMERGEIKNC | 0 |
| HIV-1 consensus B | CTDLMNATNTNTTIIYRWRGEIKNC | 0 |
| HIV-1 92RW020 | CNATASNVTNEMRNC | 0 |
| HIV-1 consensus A2 | CSNANTTNNSTMEEIKNC | 0 |
| HIV-1 ZM53 M.PB12 | CSKLNNATDGEMKNC | 0 |
| HIV-1 ZM214 M.PL15 | CSNVNINETSIDFNVTSNISMKEEMKNC | 0 |
| HIV-1 consensus C | CTNATNATNTMGEIKNC | 0 |
| SIVcpzEK505 | CSSWRSVNNSVNQTNHVQMQNC | 0 |
| SIVcpzMB66 | CSLFKCIKENGNTTNCTVQISTGNDSTANNITVGTIDMYNC | 0 |
Amino acids predicted to have an O-linked carbohydrate attached are shown in boldface. Predicted N-linked attachment site (NXS or NXT, where X is any amino acid except P) are underlined.
Neutralization of O-linked SIV variants by soluble CD4, SIVmac239-positive macaque sera, and a panel of anti-SIV monoclonal antibodies.
The sensitivities of O-linked SIV variants to neutralization by pooled SIV-positive rhesus macaque sera, by sCD4, and by a panel of monoclonal antibodies directed toward SIV gp120 were examined. Several variants (Jacalin5, SIV05, SIV06, and SIV07) were more sensitive than SIVmac239 to neutralization by SIVmac239-positive rhesus macaque sera (Fig. 11A and B; Table 2). All had similar sensitivities to inhibition by sCD4 and monoclonal antibodies directed toward multiple epitopes of gp120 (Fig. 11C, D, E, and F; Table 2).
FIG. 11.
Comparative neutralization of SIVmac239 and jacalin-resistant SIV V1 variants. (A and B) Neutralization of infectivity by pooled SIV-positive sera. (C and D) Neutralization of infectivity with soluble CD4 (sCD4). (E and F) Representative neutralization of infectivity by rhesus monoclonal antibody 1.9C. HEK293T-produced virus stocks for each cloned SIV variant and the parental SIVmac239 strain were incubated for 1 h with either pooled sera from SIVmac239-positive rhesus macaques, sCD4, or a rhesus monoclonal antibody. C8166-SEAP cells, which contain a stably integrated, Tat-inducible secreted alkaline phosphatase (SEAP) reporter gene, were then added. SEAP activity was measured 72 h later with a Tropix Phospha-Light kit. A lower percentage of SEAP activity is indicative of neutralization, while 100% SEAP activity indicates the lack of neutralization of virus infectivity.
TABLE 2.
Relative sensitivities of SIV variants containing amino acid substitutions in the potential O-linked glycosylation site to neutralization of infectivity by SIVmac239-positive rhesus macaque sera, soluble CD4, or a panel of rhesus monoclonal antibodiesa
| Viral strain | Potential O-linked sequence (aa 128-139) | Serum IC50 (inverse dilution) | sCD4 IC80 (μg/ml) | rhMAb IC50 (μg/ml) |
|---|---|---|---|---|
| SIVmac239 | TTTASTTSTTAS | <80 | 3 | >10 |
| SIV316 | TTTASTTSTTAS | >10,240 | 0.05 | >10 to <0.078 |
| Jacalin1 | TTTASTT---AS | <80 | 1.75 | >10 |
| Jacalin2 | TTTASTTSMTAS | <80 | 3 | >10 |
| Jacalin3 | TTTASTTSGTAS | <80 | 3 | >10 |
| Jacalin4 | ITAASTTSMTAS | <80 | 1.75 | >10 |
| Jacalin5 | GTGASTTSGTAS | 120 | 3 | >10 |
| Jacalin6 | TATASTTSMTAS | <80 | 3 | >10 |
| Jacalin7 | TTAASTTSMTAS | <80 | 3 | >10 |
| Jacalin8 | TTIASTTSMTAS | <80 | 3 | >10 |
| SIV04 | VVVAAVTSTTAS | <80 | 2 | >10 |
| SIV05 | TTTASTVAVVAA | 300 | 1.75 | >10 |
| SIV06 | TVTAAVTAVTAA | 400 | 2 | >10 |
| SIV07 | VAVASVAAVVAS | 2,560 | 1.75 | >10 |
sCD4, soluble CD4; rhMAb, rhesus monoclonal antibodies.
DISCUSSION
Our results unambiguously demonstrate the presence of non- and monosialylated core 1 mucin-type O-linked carbohydrates on the gp120s of 4 of 4 SIVmac and SIVsm isolates and the absence of such structures on the gp120s of 11 of 11 SIVcpz and HIV-1 isolates. Here we also demonstrate the utility of using jacalin, a lectin from jackfruit (Artocarpus integrifolia) seeds, as an inhibitior of viral replication, to identify mucin-type O-linked carbohydrate attachment via selection of lectin-resistant variants, as well as the utility of a lectin-binding ELISA to demonstrate the presence or absence of non- and monosialylated core 1 mucin-type O-linked carbohydrates on gp120.
The absence of specific types of O-linked carbohydrate (the Tn antigen, nonsialylated core 1, and monosialylated core 1) on the gp120s of HIV-1 in the data presented here is an apparent discrepancy with some reports in the literature. Hansen et al. found that an antibody directed toward the Tn antigen broadly neutralized the infectivity of HIV-1 (19). This group later used β-elimination on the HIV-1 gp120 expressed in the context of the vaccinia virus to calculate four monomeric O-linked carbohydrate structures, presumably the Tn antigen, per gp120 molecule (17). Subsequently, Corbeau et al. used a lectin-binding ELISA to demonstrate that jacalin bound HIV-1 gp160 (8). These data were in disagreement with the results of Favero et al., who conducted a dot blot analysis showing that jacalin bound immunoglobulin A and soluble CD4 but not recombinant HIV-1 gp120 (11). Our findings are in agreement with those of Favero et al. and expand on them to show that jacalin does not bind multiple isolates of clade A, clade B, or clade C HIV-1 gp120 proteins.
Is HIV-1 gp120 modified with mucin-type O-linked carbohydrate? It remains possible that HIV-1 gp120 is modified with mucin-type O-linked carbohydrate to which jacalin and PNA do not bind. Jacalin binds the Tn antigen, core 1, and monosialylated core 1 mucin-type carbohydrate (26, 42, 48, 53). PNA binds to nonsialylated core 1 carbohydrate (31, 35, 47, 54). There are eight core mucin-type O-linked carbohydrate structures, of which core 1 and core 2 are the most common (25, 49) (Fig. 1). If HIV-1 gp120 is modified with O-linked carbohydrate, it is most likely to be disialylated core 1 or core 2 mucin-type carbohydrate based on the data presented here. The potential modification of HIV-1 gp120 with disialylated core 1 or core 2 mucin-type carbohydrate would be consistent with the findings of Bernstein et al. and Stein and Engleman, who used enzymatic digestion to detect the presence of O-linked carbohydrate (3, 45). Sophisticated chemical analysis will be needed to determine unequivocally whether HIV-1 gp120 contains O-linked carbohydrate and, if so, its nature.
Our results shed light on the sequence requirements for the O-linked carbohydrate attachment to gp120 of SIVmac239. A single amino acid substitution of Thr136 in the V1 Ser-Thr-rich stretch imparted a significant degree of partial resistance to the inhibitory effects of jacalin. It is interesting that 100% of the jacalin-selected variants replaced or deleted Thr136 in SIVmac239 gp120. Thr136 is also one of the amino acids predicted to be an O-linked carbohydrate attachment site in NetOGlyc 3.1 analysis (Table 1). Based on these data, it is likely that Thr136 of SIVmac239 gp120 is one site in V1 that is consistently modified with mucin-type O-linked carbohydrate. The jacalin resistance data and the mass spectrometric data provide strong evidence that the serine-threonine run in V1 is the only site of O-linked carbohydrate attachment in the gp120 of SIVmac239.
The finding that SIVsm and SIVmac consistently retain a mucin-type O-linked carbohydrate attachment site in the V1 domain of gp120, while SIVcpz and HIV-1 appear to consistently lack O-linked carbohydrate attachment sites in the V1 domain of gp120, could conceivably reflect virus-host evolutionary dynamics in viral carbohydrate composition. Factors that influence core 1 mucin-type O-linked carbohydrate attachment are the substrate specificity of the initiating GalNAc transferase (38, 55) and the neighboring carbohydrate (12, 13). The difference in O-linked carbohydrate content between the SIVsm and SIVmac gp120s and the HIV-1 and SIVcpz gp120s might relate to recently elucidated differences between these viral lineages in the proportional contents of high-mannose versus complex N-linked carbohydrate (44).
Why is HIV-2 diverse with regard to mucin-type O-linked carbohydrate attachment? The HIV-2 isolates that bound jacalin, Ben and UC1, have gp120 sequences that phylogenetically appear to represent a more recent introduction into the human population than the gp120 sequence of the NIH-Z isolate, to which jacalin did not bind (2, 29, 50). It appears that the O-linked carbohydrate attachment site in the middle of V1 for HIV-2 Ben and HIV-2 UC1 has been replaced with an N-linked attachment site for HIV-2 NIH-Z (Table 1). This is consistent with the idea that a host factor in the sooty mangabey interacts with SIVsm in a way that favors the maintenance of the presence of an O-linked carbohydrate attachment site in the V1 domain of the virus gp120, while in the human, the virus favors N-linked glycosylation sites in V1. Further studies will be needed to understand whether host cell factors contribute to maintaining the presence or absence of these O-linked carbohydrate structures among the individual SIVs and HIVs.
Acknowledgments
We thank George Pavlakis for the expression-optimized SIVmac239 gp160 cassette. We thank Elizabeth MacKenzie, Jacqueline Bixby, Olga Laur, and Anusha Anukanth for technical assistance. We thank Eloisa Yuste and the members of the Desrosiers lab for helpful discussions and support.
The International AIDS Vaccine Initiative (IAVI) and the National Institutes of Health (R01-AI025328 and P51-RR000168) supported this work. E. Stansell was supported in part by an NIH institutional NRSA T32-A1007245 grant. This work was also supported by the Biotechnology and Biological Sciences Research Council (BBSRC), grant BBF0083091 (to A.D. and S.M.H.).
Footnotes
Published ahead of print on 20 October 2010.
REFERENCES
- 1.Baba, M., B. Yong Ma, M. Nonaka, Y. Matsuishi, M. Hirano, N. Nakamura, N. Kawasaki, and T. Kawasaki. 2007. Glycosylation-dependent interaction of jacalin with CD45 induces T lymphocyte activation and Th1/Th2 cytokine secretion. J. Leukoc. Biol. 81:1002-1011. [DOI] [PubMed] [Google Scholar]
- 2.Barnett, S. W., M. Quiroga, A. Werner, D. Dina, and J. A. Levy. 1993. Distinguishing features of an infectious molecular clone of the highly divergent and noncytopathic human immunodeficiency virus type 2 UC1 strain. J. Virol. 67:1006-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernstein, H. B., S. P. Tucker, E. Hunter, J. S. Schutzbach, and R. W. Compans. 1994. Human immunodeficiency virus type 1 envelope glycoprotein is modified by O-linked oligosaccharides. J. Virol. 68:463-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ceroni, A., K. Maass, H. Geyer, R. Geyer, A. Dell, and S. M. Haslam. 2008. GlycoWorkbench: a tool for the computer-assisted annotation of mass spectra of glycans. J. Proteome Res. 7:1650-1659. [DOI] [PubMed] [Google Scholar]
- 5.Chackerian, B., W. R. Morton, and J. Overbaugh. 1994. Persistence of simian immunodeficiency virus Mne variants upon transmission. J. Virol. 68:4080-4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chackerian, B., L. M. Rudensey, and J. Overbaugh. 1997. Specific N-linked and O-linked glycosylation modifications in the envelope V1 domain of simian immunodeficiency virus variants that evolve in the host alter recognition by neutralizing antibodies. J. Virol. 71:7719-7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng, L., K. Tachibana, H. Iwasaki, A. Kameyama, Y. Zhang, T. Kubota, T. Hiruma, T. Kudo, J. M. Guo, and H. Narimatsu. 2004. Characterization of a novel human UDP-GalNAc transferase, pp-GalNAc-T15. FEBS Lett. 566:17-24. [DOI] [PubMed] [Google Scholar]
- 8.Corbeau, P., J. L. Pasquali, and C. Devaux. 1995. Jacalin, a lectin interacting with O-linked sugars and mediating protection of CD4+ cells against HIV-1, binds to the external envelope glycoprotein gp120. Immunol. Lett. 47:141-143. [DOI] [PubMed] [Google Scholar]
- 9.Dahr, W., G. Uhlenbruck, and G. W. Bird. 1974. Cryptic A-like receptor sites in human erythrocyte glycoproteins: proposed nature of Tn-antigen. Vox Sang. 27:29-42. [DOI] [PubMed] [Google Scholar]
- 10.David, S., and A. Veyieres. 1975. The synthesis of 3,6-di-O-(2-acetamido-2-deoxy-β-d-glucopyranosyl)-d-galactose, a branched trisaccharide reported as a hydrolysis product of blood-group substances. Carbohydr. Res. 40:23-29. [DOI] [PubMed] [Google Scholar]
- 11.Favero, J., P. Corbeau, M. Nicolas, M. Benkirane, G. Trave, J. F. Dixon, P. Aucouturier, S. Rasheed, J. W. Parker, J. P. Liautard, et al. 1993. Inhibition of human immunodeficiency virus infection by the lectin jacalin and by a derived peptide showing a sequence similarity with gp120. Eur. J. Immunol. 23:179-185. [DOI] [PubMed] [Google Scholar]
- 12.Gerken, T. A. 2004. Kinetic modeling confirms the biosynthesis of mucin core 1 (β-Gal(1-3) α-GalNAc-O-Ser/Thr) O-glycan structures are modulated by neighboring glycosylation effects. Biochemistry 43:4137-4142. [DOI] [PubMed] [Google Scholar]
- 13.Gerken, T. A., C. L. Owens, and M. Pasumarthy. 1998. Site-specific core 1 O-glycosylation pattern of the porcine submaxillary gland mucin tandem repeat. Evidence for the modulation of glycan length by peptide sequence. J. Biol. Chem. 273:26580-26588. [DOI] [PubMed] [Google Scholar]
- 14.Gojobori, T., E. N. Moriyama, Y. Ina, K. Ikeo, T. Miura, H. Tsujimoto, M. Hayami, and S. Yokoyama. 1990. Evolutionary origin of human and simian immunodeficiency viruses. Proc. Natl. Acad. Sci. U. S. A. 87:4108-4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gooley, A. A., and K. L. Williams. 1994. Towards characterizing O-glycans: the relative merits of in vivo and in vitro approaches in seeking peptide motifs specifying O-glycosylation sites. Glycobiology 4:413-417. [DOI] [PubMed] [Google Scholar]
- 16.Hanover, J. A., C. K. Cohen, M. C. Willingham, and M. K. Park. 1987. O-linked N-acetylglucosamine is attached to proteins of the nuclear pore. Evidence for cytoplasmic and nucleoplasmic glycoproteins. J. Biol. Chem. 262:9887-9894. [PubMed] [Google Scholar]
- 17.Hansen, J. E., H. Clausen, S. L. Hu, J. O. Nielsen, and S. Olofsson. 1992. An O-linked carbohydrate neutralization epitope of HIV-1 gp120 is expressed by HIV-1 env gene recombinant vaccinia virus. Arch. Virol. 126:11-20. [DOI] [PubMed] [Google Scholar]
- 18.Hansen, J. E., B. Jansson, G. J. Gram, H. Clausen, J. O. Nielsen, and S. Olofsson. 1996. Sensitivity of HIV-1 to neutralization by antibodies against O-linked carbohydrate epitopes despite deletion of O-glycosylation signals in the V3 loop. Arch. Virol. 141:291-300. [DOI] [PubMed] [Google Scholar]
- 19.Hansen, J. E., C. Nielsen, M. Arendrup, S. Olofsson, L. Mathiesen, J. O. Nielsen, and H. Clausen. 1991. Broadly neutralizing antibodies targeted to mucin-type carbohydrate epitopes of human immunodeficiency virus. J. Virol. 65:6461-6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hearn, V. M., S. D. Goodwin, and W. M. Watkins. 1970. Biosynthesis of blood group active glycoproteins: a peptidyl: α-N-acetylgalactosaminyltransferase from human submaxillary gland and stomach mucosal tissue. Biochem. Biophys. Res. Commun. 41:1279-1286. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch, V. M., P. Edmondson, M. Murphey-Corb, B. Arbeille, P. R. Johnson, and J. I. Mullins. 1989. SIV adaptation to human cells. Nature 341:573-574. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch, V. M., R. A. Olmsted, M. Murphey-Corb, R. H. Purcell, and P. R. Johnson. 1989. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature 339:389-392. [DOI] [PubMed] [Google Scholar]
- 23.Huet, T., R. Cheynier, A. Meyerhans, G. Roelants, and S. Wain-Hobson. 1990. Genetic organization of a chimpanzee lentivirus related to HIV-1. Nature 345:356-359. [DOI] [PubMed] [Google Scholar]
- 24.Jang-Lee, J., S. J. North, M. Sutton-Smith, D. Goldberg, M. Panico, H. Morris, S. Haslam, and A. Dell. 2006. Glycomic profiling of cells and tissues by mass spectrometry: fingerprinting and sequencing methodologies. Methods Enzymol. 415:59-86. [DOI] [PubMed] [Google Scholar]
- 25.Jensen, P. H., D. Kolarich, and N. H. Packer. 2010. Mucin-type O-glycosylation—putting the pieces together. FEBS J. 277:81-94. [DOI] [PubMed] [Google Scholar]
- 26.Jeyaprakash, A. A., P. Geetha Rani, G. Banuprakash Reddy, S. Banumathi, C. Betzel, K. Sekar, A. Surolia, and M. Vijayan. 2002. Crystal structure of the jacalin-T-antigen complex and a comparative study of lectin-T-antigen complexes. J. Mol. Biol. 321:637-645. [DOI] [PubMed] [Google Scholar]
- 27.Julenius, K., A. Molgaard, R. Gupta, and S. Brunak. 2005. Prediction, conservation analysis, and structural characterization of mammalian mucin-type O-glycosylation sites. Glycobiology 15:153-164. [DOI] [PubMed] [Google Scholar]
- 28.Keele, B. F., F. Van Heuverswyn, Y. Li, E. Bailes, J. Takehisa, M. L. Santiago, F. Bibollet-Ruche, Y. Chen, L. V. Wain, F. Liegeois, S. Loul, E. M. Ngole, Y. Bienvenue, E. Delaporte, J. F. Brookfield, P. M. Sharp, G. M. Shaw, M. Peeters, and B. H. Hahn. 2006. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science 313:523-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirchhoff, F., K. D. Jentsch, B. Bachmann, A. Stuke, C. Laloux, W. Luke, C. Stahl-Hennig, J. Schneider, K. Nieselt, M. Eigen, et al. 1990. A novel proviral clone of HIV-2: biological and phylogenetic relationship to other primate immunodeficiency viruses. Virology 177:305-311. [DOI] [PubMed] [Google Scholar]
- 30.Kodama, T., D. P. Wooley, Y. M. Naidu, H. W. Kestler III, M. D. Daniel, Y. Li, and R. C. Desrosiers. 1989. Significance of premature stop codons in env of simian immunodeficiency virus. J. Virol. 63:4709-4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lotan, R., E. Skutelsky, D. Danon, and N. Sharon. 1975. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea). J. Biol. Chem. 250:8518-8523. [PubMed] [Google Scholar]
- 32.Means, R. E., T. Greenough, and R. C. Desrosiers. 1997. Neutralization sensitivity of cell culture-passaged simian immunodeficiency virus. J. Virol. 71:7895-7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nehrke, K., K. G. Ten Hagen, F. K. Hagen, and L. A. Tabak. 1997. Charge distribution of flanking amino acids inhibits O-glycosylation of several single-site acceptors in vivo. Glycobiology 7:1053-1060. [DOI] [PubMed] [Google Scholar]
- 34.Overbaugh, J., and L. M. Rudensey. 1992. Alterations in potential sites for glycosylation predominate during evolution of the simian immunodeficiency virus envelope gene in macaques. J. Virol. 66:5937-5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pereira, M. E., E. A. Kabat, R. Lotan, and N. Sharon. 1976. Immunochemical studies on the specificity of the peanut (Arachis hypogaea) agglutinin. Carbohydr. Res. 51:107-118. [DOI] [PubMed] [Google Scholar]
- 36.Pigny, P., V. Guyonnet-Duperat, A. S. Hill, W. S. Pratt, S. Galiegue-Zouitina, M. C. d'Hooge, A. Laine, I. Van-Seuningen, P. Degand, J. R. Gum, Y. S. Kim, D. M. Swallow, J. P. Aubert, and N. Porchet. 1996. Human mucin genes assigned to 11p15.5: identification and organization of a cluster of genes. Genomics 38:340-352. [DOI] [PubMed] [Google Scholar]
- 37.Pineau, N., P. Aucouturier, J. C. Brugier, and J. L. Preud'homme. 1990. Jacalin: a lectin mitogenic for human CD4 T lymphocytes. Clin. Exp. Immunol. 80:420-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pratt, M. R., H. C. Hang, K. G. Ten Hagen, J. Rarick, T. A. Gerken, L. A. Tabak, and C. R. Bertozzi. 2004. Deconvoluting the functions of polypeptide N-α-acetylgalactosaminyltransferase family members by glycopeptide substrate profiling. Chem. Biol. 11:1009-1016. [DOI] [PubMed] [Google Scholar]
- 39.Regier, D. A., and R. C. Desrosiers. 1990. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res. Hum. Retroviruses 6:1221-1231. [DOI] [PubMed] [Google Scholar]
- 40.Rosati, M., A. von Gegerfelt, P. Roth, C. Alicea, A. Valentin, M. Robert-Guroff, D. Venzon, D. C. Montefiori, P. Markham, B. K. Felber, and G. N. Pavlakis. 2005. DNA vaccines expressing different forms of simian immunodeficiency virus antigens decrease viremia upon SIVmac251 challenge. J. Virol. 79:8480-8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santiago, M. L., F. Range, B. F. Keele, Y. Li, E. Bailes, F. Bibollet-Ruche, C. Fruteau, R. Noe, M. Peeters, J. F. Brookfield, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 2005. Simian immunodeficiency virus infection in free-ranging sooty mangabeys (Cercocebus atys atys) from the Tai Forest, Cote d'Ivoire: implications for the origin of epidemic human immunodeficiency virus type 2. J. Virol. 79:12515-12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sastry, M. V., P. Banarjee, S. R. Patanjali, M. J. Swamy, G. V. Swarnalatha, and A. Surolia. 1986. Analysis of saccharide binding to Artocarpus integrifolia lectin reveals specific recognition of T-antigen (β-d-Gal(1-3)d-GalNAc). J. Biol. Chem. 261:11726-11733. [PubMed] [Google Scholar]
- 43.Slayter, H. S., G. Lamblin, A. Le Treut, C. Galabert, N. Houdret, P. Degand, and P. Roussel. 1984. Complex structure of human bronchial mucus glycoprotein. Eur. J. Biochem. 142:209-218. [DOI] [PubMed] [Google Scholar]
- 44.Stansell, E., and R. C. Desrosiers. 2010. Fundamental difference in the content of high-mannose carbohydrate in the HIV-1 and HIV-2 lineages. J. Virol. 84:8998-9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stein, B. S., and E. G. Engleman. 1990. Intracellular processing of the gp160 HIV-1 envelope precursor. Endoproteolytic cleavage occurs in a cis or medial compartment of the Golgi complex. J. Biol. Chem. 265:2640-2649. [PubMed] [Google Scholar]
- 46.Strous, G. J. 1979. Initial glycosylation of proteins with acetylgalactosaminylserine linkages. Proc. Natl. Acad. Sci. U. S. A. 76:2694-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swamy, M. J., D. Gupta, S. K. Mahanta, and A. Surolia. 1991. Further characterization of the saccharide specificity of peanut (Arachis hypogaea) agglutinin. Carbohydr. Res. 213:59-67. [DOI] [PubMed] [Google Scholar]
- 48.Tachibana, K., S. Nakamura, H. Wang, H. Iwasaki, K. Maebara, L. Cheng, J. Hirabayashi, and H. Narimatsu. 2006. Elucidation of binding specificity of jacalin toward O-glycosylated peptides: quantitative analysis by frontal affinity chromatography. Glycobiology 16:46-53. [DOI] [PubMed] [Google Scholar]
- 49.Tian, E., and K. G. Ten Hagen. 2009. Recent insights into the biological roles of mucin-type O-glycosylation. Glycoconj. J. 26:325-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tolle, T., H. Petry, B. Bachmann, G. Hunsmann, and W. Luke. 1994. Variability of the env gene in cynomolgus macaques persistently infected with human immunodeficiency virus type 2 strain ben. J. Virol. 68:2765-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torres, C. R., and G. W. Hart. 1984. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J. Biol. Chem. 259:3308-3317. [PubMed] [Google Scholar]
- 52.Trottein, F., L. Schaffer, S. Ivanov, C. Paget, C. Vendeville, A. Cazet, S. Groux-Degroote, S. Lee, M. A. Krzewinski-Recchi, C. Faveeuw, S. R. Head, P. Gosset, and P. Delannoy. 2009. Glycosyltransferase and sulfotransferase gene expression profiles in human monocytes, dendritic cells and macrophages. Glycoconj. J. 26:1259-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu, A. M., J. H. Wu, L. H. Lin, S. H. Lin, and J. H. Liu. 2003. Binding profile of Artocarpus integrifolia agglutinin (jacalin). Life Sci. 72:2285-2302. [DOI] [PubMed] [Google Scholar]
- 54.Young, N. M., R. A. Johnston, and D. C. Watson. 1991. The amino acid sequence of peanut agglutinin. Eur. J. Biochem. 196:631-637. [DOI] [PubMed] [Google Scholar]
- 55.Young, W. W., Jr., D. R. Holcomb, K. G. Ten Hagen, and L. A. Tabak. 2003. Expression of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase isoforms in murine tissues determined by real-time PCR: a new view of a large family. Glycobiology 13:549-557. [DOI] [PubMed] [Google Scholar]