Abstract
Minute virus of canines (MVC) is an autonomous parvovirus that replicates efficiently without helper viruses in Walter Reed/3873D (WRD) canine cells. We previously showed that MVC infection induces mitochondrion-mediated apoptosis and G2/M-phase arrest in infected WRD cells. However, the mechanism responsible for these effects has not been established. Here, we report that MVC infection triggers a DNA damage response in infected cells, as evident from phosphorylation of H2AX and RPA32. We discovered that both ATM (ataxia telangiectasia-mutated kinase) and ATR (ATM- and Rad3-related kinase) were phosphorylated in MVC-infected WRD cells and confirmed that ATM activation was responsible for the phosphorylation of H2AX, whereas ATR activation was required for the phosphorylation of RPA32. Both pharmacological inhibition of ATM activation and knockdown of ATM in MVC-infected cells led to a significant reduction in cell death, a moderate correction of cell cycle arrest, and most importantly, a reduction in MVC DNA replication and progeny virus production. Parallel experiments with an ATR-targeted small interfering RNA (siRNA) had no effect. Moreover, we identified that this ATM-mediated cell death is p53 dependent. In addition, we localized the Mre11-Rad50-Nbs1 (MRN) complex, the major mediator as well as a substrate of the ATM-mediated DNA damage response pathway to MVC replication centers during infection, and show that Mre11 knockdown led to a reduction in MVC DNA replication. Our findings are the first to support the notion that an autonomous parvovirus is able to hijack the host DNA damage machinery for its own replication and for the induction of cell death.
Bocavirus is a newly classified genus of the family Parvovirinae and includes human bocavirus (HBoV), minute virus of canines (MVC), and bovine parvovirus (BPV). HBoV was recently associated with acute respiratory wheezing and pneumonia (3, 44, 72) and is commonly detected in association with other respiratory viruses (44, 72). In addition to being linked to respiratory illnesses, HBoV has been associated with gastroenteritic diseases (2, 4, 50, 53, 85). Within their respective hosts, two closely related animal bocaviruses share these characteristics (12, 17, 42, 58, 66, 76). Although differentiated human airway epithelial cells were recently shown to support HBoV replication, the fact that this was at an extremely low level (31) makes this system a difficult one to study HBoV biology. MVC infection of Walter Reed/3873D (WRD) cells, however, has been proven much more efficient (11, 79). Using this system, we have shown that MVC infection induces mitochondrion-mediated apoptosis, that this effect is dependent on replication of the viral genome, and that the MVC genome per se is able to arrest the cell cycle at the G2/M phase (19).
Infection by many DNA viruses has been found to induce a cellular DNA damage response (DDR), which can either block or enhance viral DNA replication, as well as cell cycle arrest (in response to mild damage) or apoptosis (in response to irreparable damage), in infected cells (56). DNA damage rapidly activates conserved DDR pathways (41, 75) that involve three phosphatidylinositol 3-kinase-like kinases (PI3Ks): ATM (ataxia telangiectasia-mutated kinase), ATR (ATM- and Rad3-related kinase), and DNA-PK (DNA-dependent protein kinase) (7, 54, 65). ATM is activated primarily as a result of DNA double-strand breaks (DSBs) and is recruited to DSBs by the Mre11-Rad50-Nbs1 (MRN) complex. ATR, on the other hand, responds to the detection of single-stranded DNA (ssDNA) breaks and stalled DNA replication forks and is recruited to RPA-coated ssDNA by an ATR-interacting protein (ATRIP) (15, 41). Like ATM, DNA-PK is activated in response to DSBs, but it is recruited to the damage site in complex with Ku70 and Ku80. Once recruited to a site of damage, ATM, ATR, and DNA-PK phosphorylate a number of substrates (including H2AX, RPA, CHK1 and CHK2, p53, SMC1, Nbs1, and BRCA1) that in turn target other proteins, with the ultimate outcome being the silencing of cyclin-dependent kinases (CDKs) and an arrest of cell cycle progression to promote DNA repair or elimination of the potential hazardous cells by apoptosis (6, 41, 45).
Parvovirus contains a linear ssDNA genome with terminal repeat structures at both ends (24). Adeno-associated virus 2 (AAV2), a member of the genus Dependovirus of the family Parvovirinae, in the case of infection by (UV-inactivated) AAV2 alone, provokes a DDR that mimics stalled replication forks, with both ATM and ATR being activated, resulting in the phosphorylation of CHK1 and H2AX and G2-phase arrest (32, 43, 67). It is the p5 promoter sequence, rather than the AAV2 terminal repeats, that triggers the DDR (32). However, when AAV2 undergoes a productive infection in the presence of adenovirus, AAV2 DNA replication activates a DDR that is mediated primarily through the DNA-PK pathway and leads to phosphorylation of the downstream targets H2AX, RPA32, Nbs1, CHK1, CHK2, and SMC1 (22, 73) in the absence of the MRN complex (73). Replication of AAV2 requires degradation of the MRN complex, an upstream regulator essential for activation of the ATM pathway (74). For this, AAV2 requires the help of another virus, such as adenovirus. Adenovirus per se can induce a DDR and cell death (26). Therefore, a simple model for studying the relationships among parvovirus DNA replication, DDR, and induced cell death has not been established.
In the current study, we provide the first evidence that infection by MVC, an autonomous parvovirus, triggers a DDR that is represented by phosphorylation of both H2AX and RPA32. We show that both ATM- and ATR-mediated pathways are involved in the MVC infection-induced DDR but that only the ATM-mediated pathway, which is sensed by the MRN complex, is critical for replication of the MVC genome and MVC infection-induced cell death.
MATERIALS AND METHODS
Cell and virus.
WRD canine cells were maintained in Dulbecco's modified Eagle's medium with 10% fetal calf serum in 5% CO2 at 37°C. The MVC strain used in this study is the original strain, GA3, which was isolated at the College of Veterinary Science, Cornell University. MVC was cultured and quantified as previously described, and the virus titer was determined as the number of fluorescence focus-forming units (FFU) per ml (19). The WRD cell line and the MVC strain were obtained as gifts from Colin Parrish at Cornell University. WRD cells were infected with MVC at a multiplicity of infection (MOI) of 5.
Chemicals and treatment.
Hydroxyurea (HU) (Calbiochem) was diluted to deionized water as a stock solution at 250 mM. Inhibitors CGK733, KU55933, NU7441, and wortmannin were bought from Calbiochem and were diluted in dimethyl sulfoxide (DMSO) as stock solutions at 10 mM. Bromodeoxyuridine (BrdU) was purchased from Sigma and diluted in deionized water as a stock solution at 5 mM.
WRD cells were seeded on 60-mm dishes 1 day prior to chemical treatment. KU55933, CGK733, NU7441, and wortmannin were applied to cells at final concentrations of 20 μM, 2.5 μM, 10 μM, and 10 μM, respectively, 3 h prior to infection. DMSO (0.25%) was used as a control. HU was added to cells at a final concentration of 2.5 mM in parallel with MVC infection.
siRNA, plasmids, and transfection.
Small interfering RNA (siRNA) oligonucleotides were synthesized as dicer substrate RNA interference (RNAi) at Integrated DNA Technologies (IDT, Coralville, IA). The following siRNA sequences were chosen for targeting the genes of interest: siRNA specific to ATM (siATM), 5′-GUACUAGUUGCUUGUGUAACUGUA-3′; siRNA specific to ATR (siATR), 5′-AGAAAGGAUUGUAGGCUAAUGGAA-3′; siRNA specific to the DNA-PK catalytic subunit (siDNA-PKcs), 5′-CUAGGAAAUCCAUCGGUAUCAUUAA-3′; siRNA specific to Mre11 (siMre11), 5′-GGUCUUCUACUCUUAGGGUUGUUCCUU-3′; and siRNA specific to p53 (sip53), 5′-CCACCAUCCCUAAACUAAUGTG-3′. The following scrambled RNA (scrambled) was used as a siRNA control: 5′-CUUCCUCUCUUUCUCUCCCUUGUGA-3′. Transfection of all siRNAs was performed using Trifectin reagent (IDT) following the manufacturer's instructions. At 48 h posttransfection, the cells were fed with fresh medium and infected with MVC.
MVC plasmids pIMVC, pIMVCNS1(−), pIMVCNP1(−), pIMVCVP1/2(−), and pMVCNSCap and the method for transfection have been described previously (19, 79).
Antibodies.
Anti-MVC NS1 and anti-MVC NP1 antisera were produced previously (19, 79). Anti-phosphorylated H2AX (anti-γH2AX) (Millipore Corporation), anti-phosphorylated RAP32 (anti-p-RAP32) (Ser33) (Bethyl Laboratories, Inc.), anti-p-ATM (Ser1981) (Rockland Immunochemicals, Inc.), anti-Rad50 (GeneTex, Inc.), anti-p-SMC1 (Ser957) (Genscript USA, Inc.), and anti-ATM, anti-ATR, and anti-DNA-PKcs (Calbiochem, EMD Chemicals, Inc.) were used in this study. Both a monoclonal anti-Mre11 antibody (clone 12D7; GeneTex), which was generated by immunizing a truncated Mre11 from amino acids (aa) 182 to 582, and a polyclonal anti-Mre11 antibody (C-16; Santa Cruz Biotechnology, Inc.), which was raised against a peptide mapping near the C terminus of human Mre11, were used to detect Mre11. Anti-p-ATR (Ser428), anti-p-Nbs1 (Ser343), and anti-p-p53 (Ser15) were obtained from Cell Signaling, Inc. Antibody dilutions used for Western blotting and immunofluorescence analysis were those suggested in the manufacturers' instructions.
Western blotting and immunofluorescence.
Western blotting and immunofluorescence assays were performed as previously described (19). Confocal images were taken at a magnification of ×100 (objective lens) with an Eclipse C1 Plus confocal microscope (Nikon) controlled by Nikon EZ-C1 software.
For BrdU incorporation, WRD cells were seeded on a chamber slide and infected with MVC at an MOI of 5. At 18 h postinfection (p.i.), BrdU was added into the cell culture medium at a final concentration of 5 μM. At 24 h p.i., cells were fixed and coimmunostained with rat anti-MVC NS1 and mouse anti-BrdU to mark the MVC replication centers.
Southern blotting.
Low-molecular-weight DNA (Hirt DNA) was extracted from WRD cells, and DpnI digestion and Southern blotting were performed using an MVC NSCap probe as described previously (79).
Virus titration assay.
WRD cells were transfected with siRNAs for 48 h or treated with inhibitors for 3 h prior to MVC infection (MOI of 5). At 48 h p.i., both the cells and the medium were collected and lysed by repeated freezing and thawing. After lysis, the samples were briefly centrifuged and the supernatants were collected for the virus titration assay.
WRD cells were seeded on 4-well chamber slides (Lab-Tek) 24 h prior to infection. Virus samples were serially diluted 10-fold and added to each well. At 24 h p.i., cells were fixed in 100% acetone, stained with anti-MVC NS1, and then processed for the immunofluorescence assays. The number of fluorescence-positive cells in each chamber was counted. The number of focus-forming units in each well was calculated by multiplying the number of fluorescence-positive cells per chamber by the dilution of the virus-containing supernatant. The viral titer is expressed as the average number of focus-forming units per ml of supernatant (FFU/ml).
Flow cytometry analysis.
Live/Dead Violet staining for detection of cell death and DAPI (4′,6-diamidino-2-phenylindole) staining for analysis of cell cycle were performed as described previously (19). All of the processed samples were analyzed on a three-laser flow cytometer (LSR II; BD Biosciences) at the Flow Cytometry Core of the University of Kansas Medical Center. All flow cytometry data were analyzed using FACSDiva software (BD Biosciences).
RESULTS
MVC infection causes a DDR in infected cells.
To examine whether a DNA damage response (DDR) is induced during MVC infection, we evaluated the phosphorylation status of H2AX and RPA32 in MVC-infected cells. First, we used BrdU incorporation to identify the MVC DNA replication centers and inspected whether anti-BrdU staining colocalizes with the MVC NS1 protein. As shown in Fig. 1A, NS1 was present at active replication foci, as indicated by anti-BrdU staining. In subsequent experiments, we used anti-NS1 staining as a marker for the MVC DNA replication centers.
FIG. 1.
MVC infection induces a DDR. (A) Detection of the MVC DNA replication foci using BrdU incorporation. Punctate staining by anti-NS1 (red) or anti-BrdU (green) antibody indicates the replication foci. (B and C) Immunofluorescence analysis of MVC infection-induced DDR. At 48 h p.i., MVC-infected cells were coimmunostained with anti-MVC NS1 (red) and anti-γH2AX (green) (B) or anti-MVC NS1 (red) and anti-p-RPA (Ser33) (green) (C). Cells treated with hydroxyurea (HU) were used as a positive control for an induced DDR (5, 86). Nuclei were marked by DAPI staining. (D) Western blotting of MVC infection-induced DDR. MVC-infected cells were harvested at the indicated time points and analyzed by Western blotting. In addition to the levels of the MVC NS1 protein, phosphorylation of the DDR markers H2AX and RPA32 was assessed using the appropriate antibodies. The same membrane was reprobed with each antibody. HU-treated cells served a positive control, and anti-β-actin was used as a loading control. The arrowhead shows phosphorylated RPA32 at serine 33; two bands of phosphorylated RPA32 were detected in HU-treated cells.
MVC-infected cells were coimmunostained with anti-NS1 and anti-phosphorylated H2AX (γH2AX) or with anti-NS1 and anti-RPA32 phosphorylated at serine 33 (p-RPA32). In parallel, we treated cells with hydroxyurea (HU), an agent known to induce a DDR (5, 86), as a positive control. At 48 h p.i., we found that MVC infection led to significant increases in the levels of both γH2AX and p-RPA32 in NS1-expressing cells (Fig. 1B and C, MVC-infected), with most of the NS1-expressing cells (red) also positive for anti-γH2AX or anti-p-RPA (green), respectively. Interestingly, p-RPA32 colocalized with NS1 at replication foci, but γH2AX did not (Fig. 1B and C, MVC-infected). In the HU-treated positive-control cells, γH2AX and p-RPA32 were also expressed in the nuclei (Fig. 1B and C, HU-treated). Thus, MVC infection specifically induces the phosphorylation of H2AX and RPA32. Western blot analysis revealed that H2AX and RPA32 were increasingly phosphorylated over time (Fig. 1D). Both H2AX and RPA32 were phosphorylated starting at 18 h p.i. and were maximally phosphorylated at 36 h p.i.; this correlated with the level of MVC replication as assessed by NS1 expression (Fig. 1D). Collectively, these findings show that MVC infection induces a significant DDR that is correlated with MVC replication.
Both ATM and ATR are activated in the MVC infection-induced DDR.
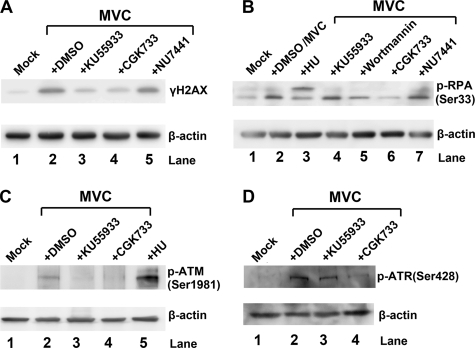
We examined which kinase pathway underlies the DDR induced during MVC infection. First, we used pharmacological inhibitors to ATM, ATR, and DNA-PK to block the phosphorylation of these kinases. Both the ATM-specific inhibitor KU55933 (38) and the ATR/ATM-specific inhibitor CGK733 (1, 10, 25, 35, 89, 92) reduced γH2AX levels approximately 5-fold compared with those in DMSO control cells (Fig. 2A, lanes 3 and 4 versus lane 2); this did not hold true for the DNA-PK inhibitor NU7441 (51) (Fig. 2A, lane 5). CGK733 also significantly interfered with the phosphorylation of RPA32, whereas KU55933 and NU7441 did not (Fig. 2B). The pan-PI3K inhibitor wortmannin (61) also inhibited the phosphorylation of RPA32 but not as potently as did CGK733 (Fig. 2B). Dephosphorylation of ATM by the inhibitors KU55933 and CGK733 was confirmed by anti-p-ATM staining (Fig. 2C, lanes 3 and 4), and ATR dephosphorylation by the inhibitor CGK733 was confirmed by anti-p-ATR staining (Fig. 2D, lane 4).
FIG. 2.
Treatment with pharmacological inhibitors of ATM and ATR reduces MVC infection-induced phosphorylation of H2AX and RPA32, respectively. WRD cells were treated with DMSO (0.25%) and inhibitors and then infected with MVC at 3 h posttreatment. MVC-infected cells were harvested at 48 h p.i. and were lysed for immunoblotting using anti-γH2AX (A), anti-p-RPA (Ser33) (B), anti-p-ATM (Ser1981) (C), and anti-p-ATR (Ser428) (D). In all blots, anti-β-actin was used to control for loading. Mock-infected and HU-treated cells were used as a negative and a positive control, respectively.
We next used siRNA molecules to specifically knock down ATM, ATR, and the DNA-PK catalytic subunit (DNA-PKcs). As shown in Fig. 3A, the ATM-specific siRNA inhibited ATM expression approximately 4-fold compared to that seen with the scrambled siRNA control (Fig. 3A). Consistent with an inhibition of ATM expression, the ATM siRNA reduced γH2AX more than 5-fold, to the background level (Fig. 3D, lane 3), but did not affect phosphorylation of RPA32 (Fig. 3E, lane 3). Similarly, the ATR-specific siRNA reduced ATR expression (Fig. 3B) and accordingly decreased the level of phosphorylated RPA32 to the background level in the mock control but not that of γH2AX (Fig. 3D and E, lane 4 versus lane 1). However, neither phosphorylation event was diminished in MVC-infected cells treated with the DNA-PKcs-specific siRNA (Fig. 3D and E, lane 5), in spite of the fact that the DNA-PKcs-specific siRNA inhibited nearly 90% of the DNA-PKcs (Fig. 3C, lane 3).
FIG. 3.
Treatment with siRNAs targeting ATM and ATR reduces MVC infection-induced phosphorylation of H2AX and RPA32, respectively. WRD cells were transfected with the indicated siRNA to silence ATM, ATR, or DNA-PK. The cells were infected with MVC at 48 h posttransfection. (A to C) To confirm the efficiency of knockdown, at 48 h p.i., cells were collected and lysed for immunoblotting using antibodies against ATM (A), ATR (B), and DNA-PKcs (C). A scrambled siRNA served as a negative control. (D and E) To assess the effect of the knockdown on the DDR, at 48 h p.i., cells were collected and lysed for immunoblotting using anti-γH2AX (D) and anti-p-RPA (Ser33) (E). Both blots were reprobed with anti-β-actin antibody. Mock-infected cells were used as a background control.
Together, these results show that the increase in γH2AX during MVC infection is mediated by ATM phosphorylation, whereas the increase in RPA32 phosphorylation is a consequence of ATR activation. Thus, the MVC infection-induced DDR appears to involve activation of both the ATM and the ATR pathway. Due to the lack of an antibody to detect phosphorylated canine DNA-PK, we were not able to test whether DNA-PK is phosphorylated during MVC infection. However, based on results using the DNA-PKcs-specific siRNA, we believe that DNA-PK is less likely to be involved in the DDR induced by MVC infection.
The ATM-mediated DDR plays an important role in inducing the cytopathic effects that occur during MVC infection.
We used the above-described pharmacological inhibitors and siRNAs to examine the effects of inhibiting ATM, ATR, and DNA-PK on the cell death that is triggered in WRD cells by MVC infection (19). At 24 and 48 h p.i., the cells were harvested for flow cytometry analysis with the cell death marker dye Live/Dead Violet and an anti-MVC NS1 antibody. NS1-expressing cells were selectively gated to determine the percentage of dead cells. We found that when either an ATM inhibitor (KU55933 or CGK733) or an ATM-specific siRNA was applied to MVC-infected cells, cell death at 48 h p.i. was significantly inhibited (Fig. 4). More specifically, treatment with KU55933 and CGK733 reduced cell death by 55% and 84%, respectively, over that seen in the DMSO control, and application of the ATM-specific siRNA reduced cell death by 70% compared to that achieved when the scrambled siRNA control was applied. Treatment of the cells with the ATR-specific siRNA resulted in only a slight inhibition of cell death, by approximately 11% (Fig. 4). In contrast, application of neither the DNA-PK inhibitor NU7441 nor DNA-PKcs-specific siRNA resulted in significant inhibition of the cell death induced by MVC infection at 48 h p.i. (Fig. 4). These results suggest that ATM activation is important to the cell death induced by MVC infection, whereas the ATR pathway contributes minimally. At 24 h p.i., cells in the DMSO control group did not undergo cell death at a significant level; therefore, inhibitory effects of the ATM inhibitor or of the siRNA could not easily be evaluated (Fig. 4). Moreover, application of the ATM-specific siRNA inhibited phosphorylation of p53 at serine 15 (Fig. 5A). Knockdown of p53 by a p53-specific siRNA reduced cell death by approximately 61% compared to that achieved with the treatment with the scrambled siRNA (Fig. 5B and C) but did not correct the cell cycle arrest (Fig. 5D). Thus, our results suggest that MVC infection induces an ATM-mediated and p53-dependent cell death.
FIG. 4.
Both inhibition of ATM phosphorylation and knockdown of ATM expression significantly reduce MVC infection-induced cell death. WRD cells were treated with inhibitors for 3 h prior to MVC infection or transfected with the indicated siRNAs 48 h prior to MVC infection. (A) At 24 or 48 h p.i., cells were collected and analyzed using Live/Dead Violet and anti-NS1 costaining by flow cytometry. The result shown is one representative from three independent experiments. Percentages of dead cells in NS1-expressing cells are shown in each histogram. (B) Statistical analysis of the percentages of dead cells in NS1-expressing cells from three independent experiments. Averages (numerical values) and standard deviations (error bars) are shown for each treatment group.
FIG. 5.
MVC infection-induced cell death is dependent on phosphorylation of p53. WRD cells were transfected with the indicated siRNAs 48 h prior to MVC infection. (A) At 48 h p.i., cells were collected and lysed for immunoblotting using antibodies against phosphorylated p53 at serine 15. The blot was reprobed with anti-β-actin. (B and C) Cells were collected and analyzed using Live/Dead Violet and anti-NS1 costaining by flow cytometry. (B) Results from a representative experiment are shown, with percentages of dead cells in NS1-expressing cells given in each histogram. (C) Averages (numerical values) and standard deviations (error bars) are indicated for each treatment group. (D) Cells were collected and analyzed for cell cycle using DAPI and anti-NS1 costaining by flow cytometry. A representative experiment is shown, with percentages of cells in each cell cycle.
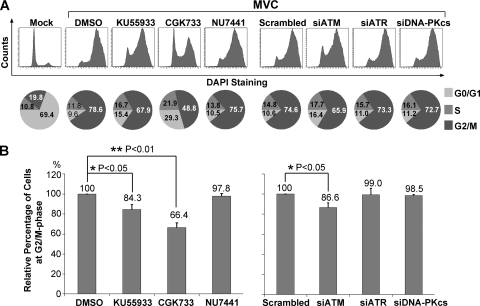
We reported previously that a G2/M cell cycle arrest occurs during late MVC infection (19). When KU55933 and CGK733 were applied to WRD cells prior to infection, the G2/M arrest, which normally occurs at 48 h p.i., was inhibited to some extent; only approximately 56% (CGK733) and 67% (KU55933) were in the G2/M phase, compared to 76% in the DMSO control sample (Fig. 6). In contrast, the DNA-PK inhibitor had no effect (Fig. 6). Consistent with these results, only the ATM siRNA reduced the MVC infection-induced G2/M arrest, by approximately 16% compared to that seen in the cells treated with the control scrambled siRNA (Fig. 6); neither the ATR- nor the DNA-PKcs-specific siRNA had an effect (Fig. 6). These results suggest that ATM activation is likely involved in the G2/M cell cycle arrest during MVC infection.
FIG. 6.
Both inhibition of ATM phosphorylation and knockdown of ATM attenuate MVC infection-induced G2/M arrest. (A) WRD cells were treated with inhibitors for 3 h prior to MVC infection or transfected with siRNAs 48 h prior to MVC infection. At 48 h p.i., the cells were subjected to flow cytometry for an assessment of G2/M arrest using DAPI and anti-NS1 costaining. The percentage of cells in each phase of the cell cycle was quantified and is shown at the bottom. Significant changes in the numbers of cells in the G2/M phase are shown. Mock-infected cells were analyzed as a normal cell cycle control. (B) Statistical analysis of the percentage of cells in the G2/M phase in NS1-expressing cells from three independent experiments. Averages (numerical values) and standard deviations (error bars) are shown for each treatment group.
The ATM-mediated DDR is required for replication of the MVC genome.
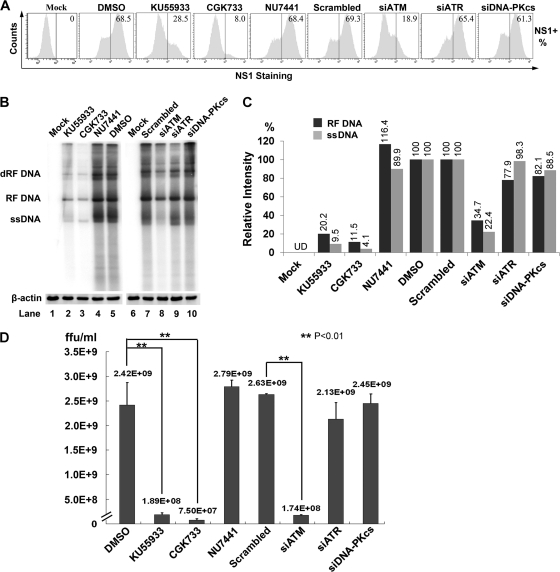
To test whether MVC replication was impaired by deactivation of any of the three DDR pathways, we evaluated the percentage of MVC-infected cells by intracellular staining using anti-NS1 antiserum. A typical experiment is shown in Fig. 7A, where, at 48 h p.i., approximately 70% of WRD cells were infected with MVC (Fig. 7A, DMSO and Scrambled). In cells treated with KU55933 or CGK733, NS1-expressing cells were decreased to 41.6% or 11.6%, respectively, of the numbers seen in the control, indicating that ATM inactivation reduced MVC infection. Furthermore, knockdown of ATM using an ATM-specific siRNA led to a 72% decrease in NS1-expressing cells compared to the number in the scrambled siRNA control group (Fig. 7A). However, treatment with either an ATR- or a DNA-PKcs-specific siRNA failed to reduce the number of NS1-expressing cells significantly (Fig. 7A). These results indicate that ATM activation may facilitate MVC replication.
FIG. 7.
The ATM pathway is required for MVC DNA replication and progeny virus production. WRD cells were treated with inhibitors as indicated 3 h prior to MVC infection or transfected with the indicated siRNAs 48 h prior to MVC infection. Mock-infected cells were analyzed as a negative control. (A) At 48 h p.i., the cells were assessed for MVC NS1 expression by flow cytometry. The percentage of NS1-expressing cells is shown in each histogram. (B) At 48 h p.i., one half of the cells were collected for extracting Hirt DNA, and the other half were collected and immunoblotted with anti-β-actin. Hirt DNA was normalized based on the level of β-actin in each sample and subjected to analysis by Southern blotting. dRF DNA, double replicative form of DNA. (C) Relative levels of the replicative-form (RF DNA) and single-stranded (ssDNA) MVC DNA in each group were quantified using Image Quant TL software (GE Health). UD, undetectable. (D) At 48 h p.i., progeny virus was isolated and quantified as FFU/ml, as described in Materials and Methods. The averages (numerical values) and standard deviations (error bars) are shown.
To confirm the role of ATM activation in MVC DNA replication, we treated WRD cells with our panel of kinase inhibitors and siRNAs and then analyzed MVC DNA replication by Southern blotting. As shown in Fig. 7B, treatment of cells with either KU55933 or CGK733 reduced the level of the replicative form (RF DNA) of the MVC DNA approximately 5-fold (Fig. 7B, lanes 2 and 3 versus lane 5), whereas treatment with the DNA-PK inhibitor NU7441 did not (Fig. 7B, lane 4). Notably, both KU55933 and CGK733 significantly blocked synthesis of the MVC ssDNA, approximately 10-fold (Fig. 7B, lanes 2 and 3 versus lane 5); this effect of ATM inhibition was more pronounced when the ATM-specific siRNA was applied (Fig. 7B, lane 8 versus lane 7). In contrast, ATR- and DNA-PKcs-specific siRNAs failed to inhibit synthesis of both the RF DNA and the ssDNA of MVC (Fig. 7B, lanes 9 and 10). The inhibition of ssDNA synthesis was confirmed by measuring progeny virus production from MVC-infected cells subjected to each treatment. As expected, the virus titers in the ATM-inhibited groups (KU55933, CGK733, and siATM treated) were reduced more than 12-fold compared with those in their respective control groups (Fig. 7D, Mock and Scrambled). Consistent with results from Southern blotting, CGK733 treatment reduced progeny virus production 32-fold; however, the inhibition of ATR alone using an ATR-specific siRNA did not significantly decrease the production of progeny virus (Fig. 7D, siATR). Likewise, treatment of cells with a DNA-PK-specific inhibitor or siRNA did not affect the production of progeny virus compared to that seen in the respective controls (Fig. 7D, NU7441 and siDNA-PKcs).
Taken together, these results show that the ATM-mediated DDR is essential to MVC DNA replication and that this particular DDR is the most important with respect to synthesis of the MVC ssDNA genome during infection. This is the first time to demonstrate that an autonomous parvovirus is able to hijack the cellular DNA damage response machinery for its productive replication.
The MRN complex facilitates MVC DNA replication.
To examine whether the MRN complex is involved in MVC DNA replication, we first evaluated whether this complex forms in early infection. At 24 h p.i., Mre11 in infected cells colocalized with the replication foci (punctate patterns) as well as with Rad50 and phosphorylated Nbs1 (p-Nbs1) (Fig. 8A); in uninfected cells, Mre11, Rad50, and p-Nbs1 were broadly distributed throughout the nuclei without forming bright foci (data not shown). As MVC infection proceeded, Mre11 was degraded, as evident from a decrease in Mre11 in the MVC replication foci at 48 h p.i. (Fig. 8A). Importantly, Rad50 and p-Nbs1 remained at the same level in these locations as at 48 h p.i. (Fig. 8A). Immunoblotting revealed a clear transition of Mre11 expression between 24 and 36 h p.i. (Fig. 8B, Mre11); this period corresponds to the time point that is critical for replication of the MVC DNA (Fig. 8B, NS1). Notably, starting at 18 h p.i., we observed a smaller band of approximately 70 kDa (Fig. 8B, Mre11a). This small Mre11 band reached a maximal level at 24 h p.i. and decreased in late infection; this correlates with the timing of MVC DNA replication, as indicated by anti-NS1 staining (Fig. 8B, NS1). It was not expressed in HU-treated cells (Fig. 8B) and was confirmed not to be a viral protein (data not shown). We speculate that it is an active form of Mre11, which may play the important role of sensing DSBs and recruiting p-Nbs1 and Rad50 to the MVC replication foci. We used another anti-Mre11 antibody to confirm that Mre11 is degraded. Indeed, a slight reduction of the Mre11 band was observed, and only in infected cells, at 24 and 36 h p.i. (Fig. 8B, Mre11b). In contrast, the level of Rad50 remained constant throughout MVC infection (Fig. 8B, Rad50).
FIG. 8.
The MRN complex is an upstream regulator of the ATM pathway and facilitates replication of the MVC genome. (A) Immunofluorescence analysis of colocalization of the MRN complex in the MVC replication center. At 24 or 48 h p.i. as indicated, Mre11, phosphorylated Nbs1 at serine 343 (p-Nbs1), and Rad50 were examined for colocalization with MVC NS1 by use of their respective antibodies. A monoclonal anti-Mre11 antibody (clone 12D7) was used to detect Mre11. (B) Western blot analysis of MVC-infected cells. MVC-infected cells were collected at various times postinfection as indicated and were analyzed by immunoblotting for the level of Mre11. Mre11 was examined using an anti-Mre11 monoclonal antibody (Mre11a) and a polyclonal antibody (Mre11b). The blot was reprobed using anti-Rad50 (Rad50), anti-NS1 (NS1), and anti-β-actin (β-actin) antibodies, sequentially. (C and D) WRD cells were transfected with the Mre11-specific siRNA (siMre11) and a scrambled siRNA as a control 48 h prior to MVC infection. Mock-infected cells were used as a negative control. At 48 h p.i., cells were collected and immunoblotted with anti-Mre11 and reprobed using anti-β-actin (C); Hirt DNA was prepared from infected cells, normalized based on the level of β-actin in each sample, and analyzed by Southern blotting (D). (E) WRD cells were transfected with siRNAs as indicated 48 h prior to MVC infection. At 48 h p.i., the cells were collected and immunoblotted with anti-Mre11 (C-16; Santa Cruz) and reprobed sequentially with anti-p-ATM (Ser1918), anti-p-SMC1 (Ser957), anti-p-Nbs1 (Ser343), and anti-β-actin. Mock-infected cells were used as a background control.
To further explore the role of Mre11 in MVC DNA replication, we knocked down Mre11 and then measured MVC DNA replication. Unlike the loss of MRN complex function, which occurs during replication of AAV2 when it is coinfected with adenovirus (74), Mre11 knockdown led to a significant decrease, approximately 3-fold, in MVC DNA replication (Fig. 8C and D). We speculated that this was caused by the failure to activate ATM, since the MRN complex acts as an upstream regulator of the ATM pathway (52, 63, 81). Indeed, we found that silencing of Mre11 reduced ATM phosphorylation significantly, to a level similar to that produced by ATM knockdown (Fig. 8E). Moreover, phosphorylation of the ATM substrate SMC1 (47, 71, 93) was reduced in this context (Fig. 8E). In addition, we found that in contrast to the results we obtained by silencing Mre11, knockdown of ATM reduced Nbs1 phosphorylation only slightly, indicating that a low level of activated ATM is sufficient to phosphorylate Nbs1. As Nbs1 is essential for DSB repair and genome stability (29), the persistent presence of p-Nbs1 in the MVC replication foci (Fig. 8A) suggests that this activated form may play a role in replication of the MVC genome.
These results show that the MRN complex localizes to the MVC replication foci, indicating that MVC genomes are sensed as DNA damage, likely as DSBs (20), by the MRN complex at the replication foci. On the other hand, colocalization of p-Nbs1 and Rad50 in the MRN complex within the MVC DNA replication foci may facilitate replication of the MVC genome.
Replication of the MVC genome induces the DDR.
We next sought to explore which viral components are involved in the MVC infection-induced DDR. We transfected the MVC infectious clone pIMVC, as well as its derivatives pIMVCNS1(−), pIMVCNP1(−), pIMVCVP1/2(−), and the NSCap-expressing construct pMVCNSCap, in which both terminal repeats were deleted, into WRD cells, separately (Fig. 9C). At 48 h p.i., we analyzed transfected cells for anti-γH2AX staining and costained all samples except pIMVCNS1(−)-transfected cells with anti-NS1; pIMVCNS1(−)-transfected cells were costained with anti-NP1. Transfection of pIMVC induced phosphorylation of H2AX in ∼80% of NS1-expressing cells at 48 h p.i. (Fig. 9A). H2AX was not phosphorylated in NP1-expressing cells or NS1-expressing cells transfected with the replication-defective construct pIMVCNS1(−) or pMVCNSCap, respectively (19, 79) (Fig. 9A). Transfection of the NP1 knockout construct pIMVCNP1(−), which replicates poorly (79), also failed to induce significant phosphorylation of H2AX, whereas transfection of pIMVCVP1/2(−) induced phosphorylation of H2AX in ∼40% of NS1-expressing cells (Fig. 9A). Consistent with this result, transfection of the replicative constructs pIMVC and pIMVCVP1/2(−) phosphorylated p53, in ∼10% and 5% of NS1-expressing cells, respectively, but transfection of the nonreplicative MVC constructs did not (Fig. 9B), suggesting that activation of the death signaling cascade requires a high level of DDR. In the context of knockout of both VP1 and VP2, in pIMVCVP1/2(−)-transfected cells, the MVC genome was replicated at a level approximately 2 times lower than that measured in pIMVC-transfected cells (Fig. 9C).
FIG. 9.
Replication of the MVC genome is required to trigger a DNA damage response. WRD cells were transfected with pIMVC, its mutants pIMVCNP1(−), pIMVCNS1(−), and pIMVCVP1/2(−), and an MVC NSCap-expressing construct, as indicated. Untransfected cells were set up as the control. (A and B) At 48 h p.i., cells were fixed and coimmunostained with anti-γH2AX, anti-NS1/NP1, and DAPI (A) or anti-p-p53 (Ser15), anti-NS1/NP1, and DAPI (B). (C) Hirt DNA was isolated from transfected cells, digested with DpnI, and analyzed by Southern blotting. The arrowhead indicates a faint DpnI-resistant band (19). (D) Transfected plasmids are illustrated, and conclusions derived from experiments with results shown in panels A to C are given. An asterisk denotes a very low level of replication. LTR, left terminal repeat; RTR, right terminal repeat; Rep, replication.
These results indicate that expression of the MVC protein(s) [NS1 in pIMVCNP1(−)-transfected cells, NP1 in pIMVCNS1(−)-transfected cells, or NS1, NP1, and VP1/2 in pMVCNSCap-transfected cells] is not sufficient to induce H2AX or p53 phosphorylation. Instead, H2AX and p53 phosphorylation is tightly associated with replication status of the MVC genome. These results suggest that at least replication of the MVC genome is required to induce a DDR (Fig. 9D).
DISCUSSION
In this study, we have demonstrated that MVC infection leads to phosphorylation of H2AX and RPA32, hallmarks of the DDR (13, 16, 34, 60, 68, 86, 91), and that the ATM- and ATR-mediated pathways are both activated. In some respects, the DDR induced by MVC infection is beneficial to virus infection, i.e., facilitating replication of viral DNA, especially synthesis of ssDNA, as well as inducing cell death, which is essential for virus egress. On the other hand, the MVC infection-induced DDR is detrimental to the host in that it leads to activation of cell cycle checkpoints and apoptosis of infected cells. Notably, the DDR was not triggered by expression of viral proteins and the delivery of plasmids containing the nonreplicative MVC DNA but rather by replication of the MVC DNA. Thus, we provide convincing evidence that a DDR induced by autonomous parvovirus plays critical roles in the virus life cycle and virus infection-induced cytopathic effects.
MVC infection induced a DDR mediated by both the ATM and the ATR pathway.
ATR and its downstream effector RPA32 were phosphorylated during MVC infection, and phosphorylated RPA32 colocalized with MVC NS1 in the replication centers (Fig. 1B). Interestingly, RPA32 is an ssDNA binding protein that is essential for replication of both the minute virus of mice (MVM) (21) and the AAV2 (62) genome. Activation of ATR and subsequent phosphorylation of RPA32 have been shown to play a pivotal role in the DDR induced by infection with UV-inactivated AAV2 (32, 43). Moreover, AAV2 DNA replication activates DNA-PK, which then phosphorylates RPA32 at multiple sites (73). RPA32 phosphorylation also has been shown to increase as infection by Epstein-Barr virus (EBV) progresses (48). However, in our study, we found that inhibition of RPA32 phosphorylation by silencing ATR did not affect MVC DNA replication. In fact, it has been reported that RPA32 phosphorylation appears to occur outside the cellular replication sites (33, 84). CGK733 inhibits both ATM and ATR activation (1, 10, 25, 35, 89, 92) and was the most effective inhibitor of DDR-mediated cell death and cell cycle arrest in MVC-infected cells. We believe that these potent inhibitory effects are due to the fact that CGK733 is a more potent inhibitor of ATM than is the ATM-specific inhibitor KU55933 in WRD cells, rather than due to its additional inhibition of ATR phosphorylation.
The DDR that is induced during simian virus 40 (SV40) infection has been suggested to be activated by the large T antigen via Bub1 binding (37). The large T antigen also interacts with the MRN complex (30, 49, 90). The human papillomavirus (HPV) E7 protein directly binds to ATM, and this induces an ATM-mediated DDR upon HPV infection of differentiated epithelia (59). In parvoviruses, both AAV2 Rep78 and parvovirus H-1 NS1 have been implicated in the phosphorylation of H2AX (9, 39), which was hypothesized to occur as a response by either nonspecific nicking of the cellular DNA by Rep78 (9) or NS1-induced reactive oxygen species (ROS) as a DNA damage agent (39). However, Rep78 accounts for only a small portion of the DDR that is induced during AAV2 replication (73). Notably, our results suggest that neither NS1 nor a stalled replication fork (Fig. 9), which would potentially be assembled in the region of the MVC replication origin to activate ATR (32, 43), is responsible for the DDR induced during MVC infection. The low level of DNA replication achieved by transfection of the NP1-deficient infectious clone [pIMVCNP1(−)] in WRD cells failed to induce a clear DDR. Given that a moderate level of genome replication is absolutely required for the MVC-induced DDR, we hypothesize that specific nicking of the replicative form (RF) of the MVC genome by the helicase activity of NS1 may create lesions that mimic DSBs (Fig. 10, c) and that a DDR is triggered when this signal accumulates to a certain level. In fact, ATM is a prime candidate for mediating the cellular damage response to DSBs (52), as DSBs are sensed by the MRN complex, which triggers ATM-mediated H2AX phosphorylation (52, 64). How ATM is activated during virus infection is not clearly understood. Based on studies of herpes simplex virus type 1 (HSV-1), it was proposed that DSBs may arise as a consequence of replication fork collapse at sites of oxidative damage (57, 82), possibly due to cleavage of the viral α sequences by endonuclease G during genome isomerization (40, 88). Parvovirus NS1 nicks only the positive strand of the RF DNA at the terminal resolution site (23), and it has been shown that opening of the DNA helix is required for MRN complex stimulation of functional ATM (52, 63). Self-complementary recombinant AAV2 (scAAV), of which the genome is an RF DNA, contains palindromic hairpin-structured terminal repeats, which resemble a repair intermediate of DSBs (20). Thus, NS1-nicked RF DNA and unpaired (replication) intermediates (Fig. 10, d and e) might be perfectly opened DNA helices that function as DSBs and trigger ATM activation.
FIG. 10.
Proposed DNA damage response pathways induced during MVC infection. The proposed pathways are described in detail in the Discussion. The model of MVC DNA replication refers to DNA replication of the minute virus of mice (23). Bax translocalization and caspase activation have been shown previously (19) to induce apoptotic cell death during MVC infection, and upregulation of cyclin B/CDK1 was confirmed to be responsible for the G2/M arrest that is induced during MVC infection (19). L, left terminal repeat; R, right terminal repeat.
In addition, delivery of the AAV2 genome by UV-inactivated AAV2 has been proven to induce an ATM/ATR-mediated DDR (32, 43, 67), which differs from the DNA-PK-mediated DDR induced by AAV2 DNA replication (22, 73). In our study, we observed that the extent of DDR induced by MVC DNA replication somehow correlated with the replication efficiency and the accumulation of the ssDNA genome during infection. Interestingly, H2AX was significantly phosphorylated when cells were inoculated with UV-inactivated MVC at a high MOI of 40 but not at a low MOI of 5 (data not shown). Thus, we speculate that the accumulated ssDNA genome of MVC may also contribute to the DDR induced during infection (Fig. 10, g), which warrants further investigation.
Based on the information summarized above, we hypothesize that during the virus life cycle, replication of the MVC genome leads to an accumulation of strand breaks and that these are registered as DSBs and thus trigger ATM activation. RPA-coated ssDNA breaks, on the other hand, could potentially trigger ATR activation (Fig. 10, a and f) during replication (95). Notably, MVM infection also induced an ATM-activated DDR that helps MVM DNA replication in MVM-permissive cells (David Pintel, personal communication). Both MVC and MVM are autonomous parvoviruses, meaning that replication of their genomes does not require the function from a helper virus. Thus, the MVC and MVM infection systems both provide simple models in which to study the DDR induced by the ssDNA genome of parvovirus. It is now clear that, like other DNA viruses (27, 56), the autonomous parvovirus hijacks the cellular DDR machinery to facilitate replication of its genome.
Only the ATM activation-mediated DDR facilitates MVC DNA replication and elicits cell death in MVC-infected cells.
Although MVC infection induces activation of both ATM and ATR, we found that only the ATM-mediated DDR contributes to MVC DNA replication and cell death. In response to DNA damage, cells activate a complex network of factors (6, 45) that silence CDKs and thereby arrest the cell cycle, promoting DNA repair (8). Interestingly, MVC infection impaired cell proliferation and disturbed the cell cycle, allowing a transition from the S-phase accumulation to the G2/M arrest as the infection progresses (19). Thus, the MVC infection-induced DDR supports replication of the viral DNA by first arresting cell cycle progression at the S phase and then impairing the cell cycle at the G2/M phase to prevent mitosis, which would lead to apoptotic cell death. On the other hand, if the cell sustains DNA damage that cannot be repaired, the DDR triggers a cascade of apoptotic cell death, through either a p53-dependent or a p53-independent pathway (69). We found that p53 was phosphorylated upon MVC infection and that it was dephosphorylated in the context of ATM inactivation (Fig. 5). Furthermore, replication of transfected MVC RF DNA induced phosphorylation of p53, albeit at a low level (Fig. 9), which presumably is due to the low level of DNA replication by transfection compared with that during MVC infection. These findings suggest that phosphorylated ATM activates apoptosis in a p53-dependent manner (Fig. 10). Bax translocalization and caspase activation have been shown to occur during cell death triggered by MVC infection (19). We hypothesize that phosphorylated p53 may activate the BH3-only molecules, e.g., tBID, BIM, and PUMA, which further activate Bax/Bak (46).
We have demonstrated that the ATM-mediated DDR is involved to some extent in cell cycle arrest, which is p53 independent. We believe that CHK2 (checkpoint kinase 2) likely signals to activate this ATM-mediated G2/M arrest (6). Notably, we did not observe a clear DDR in cells transfected with nonreplicative and poorly replicating MVC constructs (Fig. 9). The cell cycle of these transfected cells, however, was arrested at G2/M phase (19). We think that the DDR-induced replication of the MVC genome may not fully account for the cell cycle arrest during MVC infection and that an unknown mechanism may contribute to the cell cycle arrest induced by the viral genome, specifically the terminal repeats (19). Actually, both inhibition of ATM activation and knockdown of ATM only moderately rescued the cell cycle arrest. It could also be that a low level of DDR induced by the MVC genome, which is able to induce cell cycle arrest but not cell death (19), is not sufficient to induce a significant increase of γH2AX. Therefore, in the context of the DDR induced during MVC infection, it may be easier to prevent cell death than to prevent arrest of the cell cycle.
The MRN complex localizes to the MVC replication center.
The MRN complex is involved in the initial processing of DSBs as a sensor and is required for ATM activation by DNA damage (52, 63, 81, 83). It is also critical to the repair of DNA damage (28, 78). The MRN complex is required to signal DDR induction during HSV-1 infection (55), mutant adenovirus infection (18), and HPV infection (59). In contrast, the MRN complex has to be destroyed during adenovirus (77), AAV2 (74), and SV40 (94) infections. During MVC infection, the MRN complex was assembled at early stages of infection, but Mre11 was slightly degraded at later stages, while the virus was actively replicating. A loss of Mre11 at later times following HSV-1 infection has also been reported (36). However, the MRN complex was colocalized to the MVC replication center during the course of MVC infection. Further evidence that Mre11 knockdown reduced MVC replication approximately 3-fold (Fig. 8) strongly supports the notion that the MRN complex is required for replication of the MVC genome (Fig. 10) and that its role may be to recruit DNA repair factors to the replication center (14, 37), as p-Nbs1 is essential to DSB repair (29). On the other hand, the MRN complex senses ATM activation, which in turn may mediate proteasome-dependent degradation of the MRN subunit (94) at later stages of infection.
HSV-1, SV40, and HPV have all been shown to induce the ATM-mediated DDR, whereby a number of repair factors are recruited to the replication center (59, 80, 87, 94). Exactly how the DDR microenvironment helps viral DNA replication is largely unknown. DSBs can be repaired by either of two distinct repair pathways: nonhomologous end joining (NHEJ) or homologous recombination (HR) (70). Components of these repair machineries have been shown to support viral DNA replication (56). For example, DDR-induced Rad51 facilitates replication of the EBV and SV40 genomes (14, 48). Studying the MVC replication-induced DDR and how this response feeds back to help MVC DNA replication will likely help us to understand the mechanism underlying the virus infection-induced DDR.
In conclusion, MVC infection-caused cytopathic effects are unique and are mediated by the DDR induced by replication of the viral genome. We believe that the DDR is induced during parvovirus infection and that the ensured cell death and cell cycle arrest may be common and potentially synergistic mechanisms underlying parvovirus infection-induced cytopathic effects. Understanding the mechanism underlying the MVC-induced DDR and the DDR-induced cell death and cell cycle arrest pathways will potentially elucidate the molecular pathogenesis of Bocavirus infection, as well as unravel the mechanism underlying the regulatory DDR pathways.
Acknowledgments
This work was supported by PHS grant 1R21AI085236 from NIAID and grant P20 RR016443 from the NCRR COBRE program.
We thank Colin Parrish at the James A. Baker Institute, Cornell University, for valuable reagents. We are indebted to Mary Ashley Rimmer for initiating the experiment identifying the MVC replication center and to Fang Cheng for technical help.
Footnotes
Published ahead of print on 3 November 2010.
REFERENCES
- 1.Alao, J. P., and P. Sunnerhagen. 2009. The ATM and ATR inhibitors CGK733 and caffeine suppress cyclin D1 levels and inhibit cell proliferation. Radiat. Oncol. 4:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albuquerque, M. C., L. N. Rocha, F. J. Benati, C. C. Soares, A. G. Maranhao, M. L. Ramirez, D. Erdman, and N. Santos. 2007. Human bocavirus infection in children with gastroenteritis, Brazil. Emerg. Infect. Dis. 13:1756-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allander, T., T. Jartti, S. Gupta, H. G. Niesters, P. Lehtinen, R. Osterback, T. Vuorinen, M. Waris, A. Bjerkner, A. Tiveljung-Lindell, B. G. van den Hoogen, T. Hyypia, and O. Ruuskanen. 2007. Human bocavirus and acute wheezing in children. Clin. Infect. Dis. 44:904-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold, J. C., K. K. Singh, S. A. Spector, and M. H. Sawyer. 2006. Human bocavirus: prevalence and clinical spectrum at a children's hospital. Clin. Infect. Dis. 43:283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balajee, A. S., and C. R. Geard. 2004. Replication protein A and gamma-H2AX foci assembly is triggered by cellular response to DNA double-strand breaks. Exp. Cell Res. 300:320-334. [DOI] [PubMed] [Google Scholar]
- 6.Bartek, J., C. Lukas, and J. Lukas. 2004. Checking on DNA damage in S phase. Nat. Rev. Mol. Cell Biol. 5:792-804. [DOI] [PubMed] [Google Scholar]
- 7.Bartek, J., and J. Lukas. 2003. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3:421-429. [DOI] [PubMed] [Google Scholar]
- 8.Bartek, J., and J. Lukas. 2006. Cell biology. Balancing life-or-death decisions. Science 314:261-262. [DOI] [PubMed] [Google Scholar]
- 9.Berthet, C., K. Raj, P. Saudan, and P. Beard. 2005. How adeno-associated virus Rep78 protein arrests cells completely in S phase. Proc. Natl. Acad. Sci.U. S. A. 102:13634-13639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhattacharya, S., R. M. Ray, and L. R. Johnson. 2009. Role of polyamines in p53-dependent apoptosis of intestinal epithelial cells. Cell. Signal. 21:509-522. [DOI] [PubMed] [Google Scholar]
- 11.Binn, L. N., E. C. Lazar, G. A. Eddy, and M. Kajima. 1970. Recovery and characterization of a minute virus of canines. Infect. Immun. 1:503-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binn, L. N., R. H. Marchwicki, E. H. Eckermann, and T. E. Fritz. 1981. Viral antibody studies of laboratory dogs with diarrheal disease. Am. J. Vet. Res. 42:1665-1667. [PubMed] [Google Scholar]
- 13.Binz, S. K., A. M. Sheehan, and M. S. Wold. 2004. Replication protein A phosphorylation and the cellular response to DNA damage. DNA Repair (Amst.) 3:1015-1024. [DOI] [PubMed] [Google Scholar]
- 14.Boichuk, S., L. Hu, J. Hein, and O. V. Gjoerup. 2010. Multiple DNA damage signaling and repair pathways deregulated by simian virus 40 large T antigen. J. Virol. 84:8007-8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Branzei, D., and M. Foiani. 2008. Regulation of DNA repair throughout the cell cycle. Nat. Rev. Mol. Cell Biol. 9:297-308. [DOI] [PubMed] [Google Scholar]
- 16.Burma, S., B. P. Chen, M. Murphy, A. Kurimasa, and D. J. Chen. 2001. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J. Biol. Chem. 276:42462-42467. [DOI] [PubMed] [Google Scholar]
- 17.Carmichael, L. E., D. H. Schlafer, and A. Hashimoto. 1991. Pathogenicity of minute virus of canines (MVC) for the canine fetus. Cornell Vet. 81:151-171. [PubMed] [Google Scholar]
- 18.Carson, C. T., R. A. Schwartz, T. H. Stracker, C. E. Lilley, D. V. Lee, and M. D. Weitzman. 2003. The Mre11 complex is required for ATM activation and the G2/M checkpoint. EMBO J. 22:6610-6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen, A. Y., Y. Luo, F. Cheng, Y. Sun, and J. Qiu. 2010. Bocavirus infection induces a mitochondrion-mediated apoptosis and cell cycle arrest at G2/M phase. J. Virol. 84:5615-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi, V. W., D. M. McCarty, and R. J. Samulski. 2006. Host cell DNA repair pathways in adeno-associated viral genome processing. J. Virol. 80:10346-10356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christensen, J., and P. Tattersall. 2002. Parvovirus initiator protein NS1 and RPA coordinate replication fork progression in a reconstituted DNA replication system. J. Virol. 76:6518-6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collaco, R. F., J. M. Bevington, V. Bhrigu, V. Kalman-Maltese, and J. P. Trempe. 2009. Adeno-associated virus and adenovirus coinfection induces a cellular DNA damage and repair response via redundant phosphatidylinositol 3-like kinase pathways. Virology 392:24-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cotmore, S. F., and P. Tattersall. 2005. A rolling-haipin strategy: basic mechanisms of DNA replication in the parvoviruses, p. 171-181. In J. Kerr, S. F. Cotmore, M. E. Bloom, R. M. Linden, and C. R. Parrish (ed.), Parvoviruses. Hoddler Arond, London, United Kingdom.
- 24.Cotmore, S. F., and P. Tattersall. 2005. Structure and organization of the viral genome, p. 73-94. In J. Kerr, S. F. Cotmore, M. E. Bloom, R. M. Linden, and C. R. Parrish (ed.), Parvoviruses. Hodder Arnold, London, United Kingdom.
- 25.Cruet-Hennequart, S., M. T. Glynn, L. S. Murillo, S. Coyne, and M. P. Carty. 2008. Enhanced DNA-PK-mediated RPA2 hyperphosphorylation in DNA polymerase eta-deficient human cells treated with cisplatin and oxaliplatin. DNA Repair (Amst.) 7:582-596. [DOI] [PubMed] [Google Scholar]
- 26.Cuconati, A., C. Mukherjee, D. Perez, and E. White. 2003. DNA damage response and MCL-1 destruction initiate apoptosis in adenovirus-infected cells. Genes Dev. 17:2922-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dahl, J., J. You, and T. L. Benjamin. 2005. Induction and utilization of an ATM signaling pathway by polyomavirus. J. Virol. 79:13007-13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D'Amours, D., and S. P. Jackson. 2002. The Mre11 complex: at the crossroads of DNA repair and checkpoint signalling. Nat. Rev. Mol. Cell Biol. 3:317-327. [DOI] [PubMed] [Google Scholar]
- 29.Difilippantonio, S., and A. Nussenzweig. 2007. The NBS1-ATM connection revisited. Cell Cycle 6:2366-2370. [DOI] [PubMed] [Google Scholar]
- 30.Digweed, M., I. Demuth, S. Rothe, R. Scholz, A. Jordan, C. Grotzinger, D. Schindler, M. Grompe, and K. Sperling. 2002. SV40 large T-antigen disturbs the formation of nuclear DNA-repair foci containing MRE11. Oncogene 21:4873-4878. [DOI] [PubMed] [Google Scholar]
- 31.Dijkman, R., S. M. Koekkoek, R. Molenkamp, O. Schildgen, and L. van der Hoek. 2009. Human bocavirus can be cultured in differentiated human airway epithelial cells. J. Virol. 83:7739-7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fragkos, M., M. Breuleux, N. Clement, and P. Beard. 2008. Recombinant adeno-associated viral vectors are deficient in provoking a DNA damage response. J. Virol. 82:7379-7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francon, P., J. M. Lemaitre, C. Dreyer, D. Maiorano, O. Cuvier, and M. Mechali. 2004. A hypophosphorylated form of RPA34 is a specific component of pre-replication centers. J. Cell Sci. 117:4909-4920. [DOI] [PubMed] [Google Scholar]
- 34.Furuta, T., H. Takemura, Z. Y. Liao, G. J. Aune, C. Redon, O. A. Sedelnikova, D. R. Pilch, E. P. Rogakou, A. Celeste, H. T. Chen, A. Nussenzweig, M. I. Aladjem, W. M. Bonner, and Y. Pommier. 2003. Phosphorylation of histone H2AX and activation of Mre11, Rad50, and Nbs1 in response to replication-dependent DNA double-strand breaks induced by mammalian DNA topoisomerase I cleavage complexes. J. Biol. Chem. 278:20303-20312. [DOI] [PubMed] [Google Scholar]
- 35.Goldstein, M., W. P. Roos, and B. Kaina. 2008. Apoptotic death induced by the cyclophosphamide analogue mafosfamide in human lymphoblastoid cells: contribution of DNA replication, transcription inhibition and Chk/p53 signaling. Toxicol. Appl. Pharmacol. 229:20-32. [DOI] [PubMed] [Google Scholar]
- 36.Gregory, D. A., and S. L. Bachenheimer. 2008. Characterization of mre11 loss following HSV-1 infection. Virology 373:124-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hein, J., S. Boichuk, J. Wu, Y. Cheng, R. Freire, P. S. Jat, T. M. Roberts, and O. V. Gjoerup. 2009. Simian virus 40 large T antigen disrupts genome integrity and activates a DNA damage response via Bub1 binding. J. Virol. 83:117-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hickson, I., Y. Zhao, C. J. Richardson, S. J. Green, N. M. Martin, A. I. Orr, P. M. Reaper, S. P. Jackson, N. J. Curtin, and G. C. Smith. 2004. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 64:9152-9159. [DOI] [PubMed] [Google Scholar]
- 39.Hristov, G., M. Kramer, J. Li, N. El-Andaloussi, R. Mora, L. Daeffler, H. Zentgraf, J. Rommelaere, and A. Marchini. 2010. Through its nonstructural protein NS1, parvovirus H-1 induces apoptosis via accumulation of reactive oxygen species. J. Virol. 84:5909-5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang, K. J., B. V. Zemelman, and I. R. Lehman. 2002. Endonuclease G, a candidate human enzyme for the initiation of genomic inversion in herpes simplex type 1 virus. J. Biol. Chem. 277:21071-21079. [DOI] [PubMed] [Google Scholar]
- 41.Jackson, S. P. 2009. The DNA-damage response: new molecular insights and new approaches to cancer therapy. Biochem. Soc. Trans. 37:483-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jarplid, B., H. Johansson, and L. E. Carmichael. 1996. A fatal case of pup infection with minute virus of canines (MVC). J. Vet. Diagn. Invest. 8:484-487. [DOI] [PubMed] [Google Scholar]
- 43.Jurvansuu, J., K. Raj, A. Stasiak, and P. Beard. 2005. Viral transport of DNA damage that mimics a stalled replication fork. J. Virol. 79:569-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kahn, J. 2008. Human bocavirus: clinical significance and implications. Curr. Opin. Pediatr. 20:62-66. [DOI] [PubMed] [Google Scholar]
- 45.Kastan, M. B., and J. Bartek. 2004. Cell-cycle checkpoints and cancer. Nature 432:316-323. [DOI] [PubMed] [Google Scholar]
- 46.Kim, H., H. C. Tu, D. Ren, O. Takeuchi, J. R. Jeffers, G. P. Zambetti, J. J. Hsieh, and E. H. Cheng. 2009. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol. Cell 36:487-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kitagawa, R., C. J. Bakkenist, P. J. McKinnon, and M. B. Kastan. 2004. Phosphorylation of SMC1 is a critical downstream event in the ATM-NBS1-BRCA1 pathway. Genes Dev. 18:1423-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kudoh, A., S. Iwahori, Y. Sato, S. Nakayama, H. Isomura, T. Murata, and T. Tsurumi. 2009. Homologous recombinational repair factors are recruited and loaded onto the viral DNA genome in Epstein-Barr virus replication compartments. J. Virol. 83:6641-6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lanson, N. A., Jr., D. B. Egeland, B. A. Royals, and W. C. Claycomb. 2000. The MRE11-NBS1-RAD50 pathway is perturbed in SV40 large T antigen-immortalized AT-1, AT-2 and HL-1 cardiomyocytes. Nucleic Acids Res. 28:2882-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lau, S. K., C. C. Yip, T. L. Que, R. A. Lee, R. K. Au-Yeung, B. Zhou, L. Y. So, Y. L. Lau, K. H. Chan, P. C. Woo, and K. Y. Yuen. 2007. Clinical and molecular epidemiology of human bocavirus in respiratory and fecal samples from children in Hong Kong. J. Infect. Dis. 196:986-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leahy, J. J., B. T. Golding, R. J. Griffin, I. R. Hardcastle, C. Richardson, L. Rigoreau, and G. C. Smith. 2004. Identification of a highly potent and selective DNA-dependent protein kinase (DNA-PK) inhibitor (NU7441) by screening of chromenone libraries. Bioorg. Med. Chem. Lett. 14:6083-6087. [DOI] [PubMed] [Google Scholar]
- 52.Lee, J. H., and T. T. Paull. 2005. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science 308:551-554. [DOI] [PubMed] [Google Scholar]
- 53.Lee, J. I., J. Y. Chung, T. H. Han, M. O. Song, and E. S. Hwang. 2007. Detection of human bocavirus in children hospitalized because of acute gastroenteritis. J. Infect. Dis. 196:994-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li, J., and D. F. Stern. 2005. Regulation of CHK2 by DNA-dependent protein kinase. J. Biol. Chem. 280:12041-12050. [DOI] [PubMed] [Google Scholar]
- 55.Lilley, C. E., C. T. Carson, A. R. Muotri, F. H. Gage, and M. D. Weitzman. 2005. DNA repair proteins affect the lifecycle of herpes simplex virus 1. Proc. Natl. Acad. Sci.U. S. A. 102:5844-5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lilley, C. E., R. A. Schwartz, and M. D. Weitzman. 2007. Using or abusing: viruses and the cellular DNA damage response. Trends Microbiol. 15:119-126. [DOI] [PubMed] [Google Scholar]
- 57.Milatovic, D., Y. Zhang, S. J. Olson, K. S. Montine, L. J. Roberts, J. D. Morrow, T. J. Montine, T. S. Dermody, and T. Valyi-Nagy. 2002. Herpes simplex virus type 1 encephalitis is associated with elevated levels of F2-isoprostanes and F4-neuroprostanes. J. Neurovirol. 8:295-305. [DOI] [PubMed] [Google Scholar]
- 58.Mochizuki, M., M. Hashimoto, T. Hajima, M. Takiguchi, A. Hashimoto, Y. Une, F. Roerink, T. Ohshima, C. R. Parrish, and L. E. Carmichael. 2002. Virologic and serologic identification of minute virus of canines (canine parvovirus type 1) from dogs in Japan. J. Clin. Microbiol. 40:3993-3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moody, C. A., and L. A. Laimins. 2009. Human papillomaviruses activate the ATM DNA damage pathway for viral genome amplification upon differentiation. PLoS Pathog. 5:e1000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mukherjee, B., C. Kessinger, J. Kobayashi, B. P. Chen, D. J. Chen, A. Chatterjee, and S. Burma. 2006. DNA-PK phosphorylates histone H2AX during apoptotic DNA fragmentation in mammalian cells. DNA Repair (Amst.) 5:575-590. [DOI] [PubMed] [Google Scholar]
- 61.Nakanishi, S., S. Kakita, I. Takahashi, K. Kawahara, E. Tsukuda, T. Sano, K. Yamada, M. Yoshida, H. Kase, and Y. Matsuda. 1992. Wortmannin, a microbial product inhibitor of myosin light chain kinase. J. Biol. Chem. 267:2157-2163. [PubMed] [Google Scholar]
- 62.Ni, T. H., W. F. McDonald, I. Zolotukhin, T. Melendy, S. Waga, B. Stillman, and N. Muzyczka. 1998. Cellular proteins required for adeno-associated virus DNA replication in the absence of adenovirus coinfection. J. Virol. 72:2777-2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paull, T. T., and J. H. Lee. 2005. The Mre11/Rad50/Nbs1 complex and its role as a DNA double-strand break sensor for ATM. Cell Cycle 4:737-740. [DOI] [PubMed] [Google Scholar]
- 64.Petrini, J. H., and T. H. Stracker. 2003. The cellular response to DNA double-strand breaks: defining the sensors and mediators. Trends Cell Biol. 13:458-462. [DOI] [PubMed] [Google Scholar]
- 65.Pommier, Y., J. N. Weinstein, M. I. Aladjem, and K. W. Kohn. 2006. Chk2 molecular interaction map and rationale for Chk2 inhibitors. Clin. Cancer Res. 12:2657-2661. [DOI] [PubMed] [Google Scholar]
- 66.Pratelli, A., D. Buonavoglia, M. Tempesta, F. Guarda, L. Carmichael, and C. Buonavoglia. 1999. Fatal canine parvovirus type-1 infection in pups from Italy. J. Vet. Diagn. Invest. 11:365-367. [DOI] [PubMed] [Google Scholar]
- 67.Raj, K., P. Ogston, and P. Beard. 2001. Virus-mediated killing of cells that lack p53 activity. Nature 412:914-917. [DOI] [PubMed] [Google Scholar]
- 68.Rao, V. A., A. M. Fan, L. Meng, C. F. Doe, P. S. North, I. D. Hickson, and Y. Pommier. 2005. Phosphorylation of BLM, dissociation from topoisomerase IIIalpha, and colocalization with gamma-H2AX after topoisomerase I-induced replication damage. Mol. Cell. Biol. 25:8925-8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roos, W. P., and B. Kaina. 2006. DNA damage-induced cell death by apoptosis. Trends Mol. Med. 12:440-450. [DOI] [PubMed] [Google Scholar]
- 70.Sancar, A., L. A. Lindsey-Boltz, K. Unsal-Kacmaz, and S. Linn. 2004. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 73:39-85. [DOI] [PubMed] [Google Scholar]
- 71.Schar, P., M. Fasi, and R. Jessberger. 2004. SMC1 coordinates DNA double-strand break repair pathways. Nucleic Acids Res. 32:3921-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schildgen, O., A. Muller, T. Allander, I. M. Mackay, S. Volz, B. Kupfer, and A. Simon. 2008. Human bocavirus: passenger or pathogen in acute respiratory tract infections? Clin. Microbiol. Rev. 21:291-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwartz, R. A., C. T. Carson, C. Schuberth, and M. D. Weitzman. 2009. Adeno-associated virus replication induces a DNA damage response coordinated by DNA-dependent protein kinase. J. Virol. 83:6269-6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schwartz, R. A., J. A. Palacios, G. D. Cassell, S. Adam, M. Giacca, and M. D. Weitzman. 2007. The Mre11/Rad50/Nbs1 complex limits adeno-associated virus transduction and replication. J. Virol. 81:12936-12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shiloh, Y. 2003. ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Cancer 3:155-168. [DOI] [PubMed] [Google Scholar]
- 76.Spahn, G. J., S. B. Mohanty, and F. M. Hetrick. 1966. Experimental infection of calves with hemadsorbing enteric (HADEN) virus. Cornell Vet. 56:377-386. [PubMed] [Google Scholar]
- 77.Stracker, T. H., C. T. Carson, and M. D. Weitzman. 2002. Adenovirus oncoproteins inactivate the Mre11-Rad50-NBS1 DNA repair complex. Nature 418:348-352. [DOI] [PubMed] [Google Scholar]
- 78.Stracker, T. H., J. W. Theunissen, M. Morales, and J. H. Petrini. 2004. The Mre11 complex and the metabolism of chromosome breaks: the importance of communicating and holding things together. DNA Repair (Amst.) 3:845-854. [DOI] [PubMed] [Google Scholar]
- 79.Sun, Y., A. Y. Chen, F. Cheng, W. Guan, F. B. Johnson, and J. Qiu. 2009. Molecular characterization of infectious clones of the minute virus of canines reveals unique features of bocaviruses. J. Virol. 83:3956-3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taylor, T. J., and D. M. Knipe. 2004. Proteomics of herpes simplex virus replication compartments: association of cellular DNA replication, repair, recombination, and chromatin remodeling proteins with ICP8. J. Virol. 78:5856-5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Uziel, T., Y. Lerenthal, L. Moyal, Y. Andegeko, L. Mittelman, and Y. Shiloh. 2003. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J. 22:5612-5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Valyi-Nagy, T., S. J. Olson, K. Valyi-Nagy, T. J. Montine, and T. S. Dermody. 2000. Herpes simplex virus type 1 latency in the murine nervous system is associated with oxidative damage to neurons. Virology 278:309-321. [DOI] [PubMed] [Google Scholar]
- 83.van den Bosch, M., R. T. Bree, and N. F. Lowndes. 2003. The MRN complex: coordinating and mediating the response to broken chromosomes. EMBO Rep. 4:844-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vassin, V. M., M. S. Wold, and J. A. Borowiec. 2004. Replication protein A (RPA) phosphorylation prevents RPA association with replication centers. Mol. Cell. Biol. 24:1930-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vicente, D., G. Cilla, M. Montes, E. G. Perez-Yarza, and E. Perez-Trallero. 2007. Human bocavirus, a respiratory and enteric virus. Emerg. Infect. Dis. 13:636-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ward, I. M., and J. Chen. 2001. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J. Biol. Chem. 276:47759-47762. [DOI] [PubMed] [Google Scholar]
- 87.Wilkinson, D. E., and S. K. Weller. 2004. Recruitment of cellular recombination and repair proteins to sites of herpes simplex virus type 1 DNA replication is dependent on the composition of viral proteins within prereplicative sites and correlates with the induction of the DNA damage response. J. Virol. 78:4783-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wohlrab, F., S. Chatterjee, and R. D. Wells. 1991. The herpes simplex virus 1 segment inversion site is specifically cleaved by a virus-induced nuclear endonuclease. Proc. Natl. Acad. Sci. U. S. A. 88:6432-6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Won, J., M. Kim, N. Kim, J. H. Ahn, W. G. Lee, S. S. Kim, K. Y. Chang, Y. W. Yi, and T. K. Kim. 2006. Small molecule-based reversible reprogramming of cellular lifespan. Nat. Chem. Biol. 2:369-374. [DOI] [PubMed] [Google Scholar]
- 90.Wu, X., D. Avni, T. Chiba, F. Yan, Q. Zhao, Y. Lin, H. Heng, and D. Livingston. 2004. SV40 T antigen interacts with Nbs1 to disrupt DNA replication control. Genes Dev. 18:1305-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu, X., S. M. Shell, and Y. Zou. 2005. Interaction and colocalization of Rad9/Rad1/Hus1 checkpoint complex with replication protein A in human cells. Oncogene 24:4728-4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang, X. H., B. Shiotani, M. Classon, and L. Zou. 2008. Chk1 and Claspin potentiate PCNA ubiquitination. Genes Dev. 22:1147-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yazdi, P. T., Y. Wang, S. Zhao, N. Patel, E. Y. Lee, and J. Qin. 2002. SMC1 is a downstream effector in the ATM/NBS1 branch of the human S-phase checkpoint. Genes Dev. 16:571-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao, X., R. J. Madden-Fuentes, B. X. Lou, J. M. Pipas, J. Gerhardt, C. J. Rigell, and E. Fanning. 2008. Ataxia telangiectasia-mutated damage-signaling kinase- and proteasome-dependent destruction of Mre11-Rad50-Nbs1 subunits in simian virus 40-infected primate cells. J. Virol. 82:5316-5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zou, L., and S. J. Elledge. 2003. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300:1542-1548. [DOI] [PubMed] [Google Scholar]