Abstract
Reduced frequencies of myeloid and plasmacytoid dendritic cell (DC) subsets (mDCs and pDCs, respectively) have been observed in the peripheral blood of HIV-1-infected individuals throughout the course of disease. Accumulation of DCs in lymph nodes (LNs) may partly account for the decreased numbers observed in blood, but increased DC death may also be a contributing factor. We used multiparameter flow cytometry to evaluate pro- and antiapoptotic markers in blood mDCs and pDCs from untreated HIV-1-infected donors, from a subset of infected donors before and after receiving antiretroviral therapy (ART), and from uninfected control donors. Blood mDCs, but not pDCs, from untreated HIV-1-infected donors expressed lower levels of antiapoptotic Bcl-2 than DCs from uninfected donors. A subset of HIV-1-infected donors had elevated frequencies of proapoptotic caspase-3+ blood mDCs, and positive correlations were observed between caspase-3+ mDC frequencies and plasma viral load and CD8+ T-cell activation levels. Caspase-3+ mDC frequencies, but not mDC Bcl-2 expression, were reduced with viral suppression on ART. Apoptosis markers on DCs in blood and LN samples from a cohort of untreated, HIV-1-infected donors with chronic disease were also evaluated. LN mDCs displayed higher levels of Bcl-2 and lower caspase-3+ frequencies than did matched blood mDCs. Conversely, LN pDCs expressed lower Bcl-2 levels than their blood counterparts. In summary, blood mDCs from untreated HIV-1-infected subjects displayed a proapoptotic profile that was partially reversed with viral suppression, suggesting that DC death may be a factor contributing to blood DC depletion in the setting of chronic, untreated HIV disease.
Dendritic cells (DCs) are antigen-presenting cells (APCs) found throughout the body that are central in bridging innate and adaptive immune responses (5, 6). Immature DCs typically detect the presence of microbes through the binding of conserved molecular patterns to pattern recognition receptors (PRRs) such as C-type lectins and Toll-like receptors (TLRs) (32, 55). DCs exposed to microbial products then undergo a regulated maturation process, migrate to lymph nodes (LNs) and stimulate naive T cells and other immune cells to induce appropriate adaptive immune responses (5, 6). Although shown to be the most potent of the APCs in activating naive T cells, DCs have also recently been shown to play a crucial role in reactivating memory T cells (43, 95, 99) and in maintaining peripheral tolerance to self-antigens (91, 92).
Human DCs can be divided into myeloid DC (mDC) and plasmacytoid DC (pDC) subsets that differ in ontogeny, phenotype, and function (19, 50, 51, 53, 54, 61, 77-79, 87, 94). Specifically, pDCs are considered to be involved in antiviral immunity, are capable of sensing viral single-stranded RNA (ssRNA) through TLR7 and bacterial CpG DNA through TLR9, and predominantly produce type I interferons (IFNs). Conversely, mDCs sense both bacterial and viral pattern motifs through a broader range of TLRs (TLR1 to TLR8) and are involved in the induction of T helper 1 (Th1)- and Th2-type responses through the production of cytokines such as interleukin-12 (IL-12) and IL-10.
Frequencies of both mDC and pDC subsets are decreased in peripheral blood and their APC functions altered in the setting of HIV-1 infection (9, 21, 25, 27, 28, 30, 36, 37, 65, 67, 75, 88). We and others have investigated possible mechanisms underlying the observed decrease in blood DCs. During acute and early chronic infection, DCs were found to accumulate in LNs (25, 64), and yet in late-stage disease, a dramatic depletion of LN DCs was observed (11). Decreased frequencies of LN mDCs and pDCs were also seen in a model of simian AIDS (17). However, in acute SIV infection, although increased migration of pDCs to peripheral LNs was detected, increased pDC death was also noted (18).
Apoptosis, a form of programmed cell death, results from a series of tightly controlled complex pathways (3, 29). These signaling cascades are typically initiated either extrinsically, through death receptors on the surfaces of cells, or intrinsically, through disruption in intracellular homeostasis, a process critically dependent on mitochondria. Although initiation of these pathways differs, there is significant cross talk and convergence between the two (3, 29, 49). Death receptors belong to the tumor necrosis factor receptor (TNFR) superfamily, including such members as Fas and TNFR1, and induce apoptosis signaling cascades after activation via specific ligands (e.g., Fas ligand [FasL] and TNF) (40, 85). Members of the Bcl-2 family are crucial in mediating apoptosis following intracellular disturbances and can either be proapoptotic (e.g., Bax and Bim) or antiapoptotic (e.g., Bcl-2 and Bcl-XL) (3, 22). It has been proposed that the antiapoptotic proteins interact with and inhibit the function of the proapoptotic proteins (3). The induction of either the extrinsic or intrinsic pathway results in the activation of specific initiator cysteine aspartate-specific proteases (caspases), ultimately leading to activation of effector caspases (e.g., caspase-3, -6, and -7) and cell death (3, 24). Effector caspases are common to both the extrinsic and intrinsic pathway and a characteristic of apoptosis that is, therefore, independent of the initial induction point (3, 24).
Early studies of T-cell dysfunction during HIV-1 infection showed increased susceptibility of blood CD4+ and CD8+ T cells to apoptosis in vitro (35). Subsequent in vivo studies demonstrated apoptotic T cells and B cells within the LNs of HIV-1-infected individuals (13, 74). Increased blood T-cell apoptosis has been associated with disease progression, (26, 34, 73, 98), and the expression of markers of apoptosis is predictive of adverse clinical events (96).
A number of studies have demonstrated a role for DCs in the induction of T-cell apoptosis (20, 45-47, 58, 68, 82, 89). However, despite multiple observations of decreased frequencies of blood DCs during the course of HIV-1 infection, investigations into DC apoptosis in the setting of HIV-1 disease have been limited. Increased HIV-1 replication in pDCs and pDC death has been shown to occur following CD40 ligation in vitro (84), and a recent study by Myers et al. demonstrated increased pDC apoptosis in response to exposure to HIV-1 in vitro, a process likely mediated through pDC-HIV-1 fusion (72). Few studies have addressed the impact of HIV-1 infection on DC survival in vivo. Brown et al. demonstrated that both increased pDC migration to LNs and increased pDC death within the LNs contributed to depletion of pDCs in a monkey model of acute SIV infection (18).
We hypothesized that reduced frequencies of blood DCs were, in part, related to increased DC apoptosis during HIV-1 infection. In this present study, we investigated whether blood DCs from HIV-1-infected individuals, evaluated directly ex vivo or after overnight in vitro culture, displayed altered levels of pro- and antiapoptotic markers compared to those of uninfected donor DCs. We also evaluated associations between expression of apoptotic markers and markers of clinical HIV-1 disease progression, including plasma viral load, CD4 count, and level of T-cell activation. Further, we investigated whether viral suppression with antiretroviral therapy (ART) modulated the apoptotic profile of blood DCs. We also compared blood and LN DC apoptotic profiles in HIV-1-infected individuals. Lastly, we evaluated how innate signaling contributed to the survival of blood DCs by stimulating total peripheral blood mononuclear cells (PBMC) with viral and bacterial TLR ligands (TLRLs) in vitro and assessing apoptotic markers on mDC and pDC subsets.
MATERIALS AND METHODS
Study participants.
For PBMC studies, blood samples were obtained from 23 HIV-1-infected subjects (Table 1) who were receiving care at the University of Colorado Infectious Disease Group Practice, University of Colorado Hospital (Aurora, CO). Inclusion criteria included being ART naive or on no ART for at least 6 months at the time of screening. Subjects coinfected with hepatitis C were excluded from the study. To assess the effect of ART, a second blood sample was obtained from 11 of these 23 HIV-1-infected donors after having received at least 3 months of ART. Blood samples were also obtained from 19 healthy adults, self-identifying as HIV-1-uninfected (seronegative), and used as controls.
TABLE 1.
Study subject characteristicsa
| Study type and parameter | HIV-1-infected donors | Seronegative donors |
|---|---|---|
| PBMC studies | ||
| Gender (no. male, no. female) | 19, 4 | 8, 11 |
| Age (yr) | 41 (24-60) | 30 (25-52) |
| CD4+ T-cell count (cells/mm3) | 312 (7-945) | |
| Viral load (HIV-1 RNA copies/ml of plasma) | 89,700 (6,390-220,000) | |
| Treatment follow-up studies | ||
| Gender (no. male, no. female) | 9, 2 | |
| Time on treatment (mo) | 12.5 (3-18.5) | |
| CD4+ T-cell count (cells/mm3) | 481 (90-975) | |
| Viral load (HIV-1 RNA copies/ml of plasma) | <48 (48-115) | |
| LN studies | ||
| Gender (no. male, no. female) | 2, 4 | |
| Age (yr) | 37 (25-45) | |
| CD4+ T-cell count (cells/mm3) | 663 (271-1,117) | |
| Viral load (HIV-1 RNA copies/ml of plasma) | 20,292 (1,820-50,119) |
Data are presented as the median (range) where applicable.
Inguinal LNs were collected from HIV-1-infected donors that were ART naive or not receiving ART for 3 months (n = 6; Table 1) as previously described in detail (25). Autologous blood samples were collected at the same time as collection of LNs.
All study subjects participated voluntarily and gave written, informed consent. The PBMC and LN studies were approved by the Colorado Multiple Institutional Review Board (COMIRB) at the University of Colorado Denver.
Isolation of PBMC and LN cells.
PBMC from seronegative and HIV-1-infected donors were isolated from heparinized blood by gradient density centrifugation using previously described standard techniques (25). To prevent unintended cell death, all blood samples were processed within 3 h after drawing of blood, and no statistical difference was found in the median time between blood draw and processing of the blood samples from seronegative or HIV-1-infected donors (data not shown).
Inguinal LNs from HIV-1-infected donors were processed as described previously (25). All LNs and matched PBMC samples were processed within 1 h. For these studies, disaggregated LN cells and matched PBMC were cryopreserved in freezing medium consisting of RPMI 1640 medium (Invitrogen, Carlsbad, CA) plus 1% penicillin-streptomycin-l-glutamine (Invitrogen), 45% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO), and 10% dimethyl sulfoxide (Fisher Scientific, Pittsburgh, PA) and stored in liquid nitrogen. LN cells and PBMC were thawed in RPMI 1640 medium, 1% penicillin-streptomycin-l-glutamine, 10% FBS, 10 μg of DNase I (Sigma-Aldrich)/ml prior to undergoing flow cytometry staining and analysis as detailed below.
Evaluation of activation and apoptosis levels of blood DCs.
Previous work evaluating apoptosis in total PBMC demonstrated minimal differences in apoptosis of PBMC from seronegative and HIV-1-infected donors directly ex vivo but, after overnight in vitro culture, significantly higher frequencies of apoptotic cells in PBMC from HIV-1-infected donors relative to seronegative donors were observed (76). Therefore, we also measured apoptosis markers on blood DCs in PBMC both directly ex vivo and after culturing total PBMC in vitro for 13 to 20 h. PBMC were immediately stained with monoclonal antibodies to enumerate DC subsets and determine their activation and apoptosis status and were also cultured overnight with or without various TLRLs and apoptosis states determined.
Monoclonal antibodies and apoptotic markers.
The following antibodies were used to identify blood or LN DCs directly ex vivo or blood DCs after overnight in vitro culture: fluorescein isothiocyanate (FITC)-labeled anti-lineage (Lin) cocktail CD3/CD14/CD16/CD19/CD20/CD56), FITC-labeled anti-CD34 and allophycocyanin (APC)-Cy7-labeled anti-HLA-DR, phycoerythrin (PE)-Cy5- or PE-labeled CD11c (all BD Biosciences, San Jose, CA) and APC-labeled CD123 (IL-3R; Miltenyi Biotec, Auburn, CA). DC subsets were defined as follows: mDCs were Lin− CD34− HLA-DR+ CD123low CD11c+, and pDCs were Lin− CD34− HLA-DR+ CD11c− CD123high. The surface expression of CD40 was assessed by using biotinylated CD40 (Ancell, Bayport, MN) with streptavidin-PE Texas Red (ECD; Beckman Coulter, Fullerton, CA) used as the secondary antibody, and nonspecific binding was controlled for by using a matched isotype control antibody (biotinylated mouse IgG1). Surface Fas (CD95) expression was detected with Pacific Blue-labeled Fas (eBioscience, San Diego, CA) and matched isotype control (Pacific Blue-labeled mouse IgG1). Intracellular expression of Fas ligand (FasL; CD178), Bcl-2, and active caspase-3 (hereafter referred to as caspase-3) was performed using biotinylated FasL (eBioscience), streptavidin-ECD, and PE-labeled Bcl-2 and PE-labeled caspase-3 (both from BD Biosciences). Matched isotype controls were used for FasL (biotin-labeled mouse IgG1) and Bcl-2 (PE-labeled Armenian hamster IgG2). Pacific Blue annexin V and 7-amino-actinomycin D (7-AAD; both from BD Biosciences) were used to assess early (annexin V+ 7-AAD−) and late (annexin V+ 7-AAD+) apoptosis.
T-cell activation was assessed by measuring CD38 and HLA-DR expression on T cells using ECD-labeled CD3 (Beckman Coulter) and APC-labeled CD4 and FITC-labeled CD8 (both from BD Biosciences), PE-Cy5-labeled CD38 (eBioscience), and APC-Cy7-labeled HLA-DR. Matched isotype controls were used for CD38 (PE-Cy5-labeled mouse IgG1) and HLA-DR (APC-Cy7-labeled mouse IgG2a).
Surface and intracellular staining.
Freshly isolated PBMC or thawed LN cells and matched PBMC samples were washed with 4 ml of cold DPBS (Invitrogen), 1% bovine serum albumin (Sigma-Aldrich), and 2 mM EDTA (fluorescence-activated cell sorting [FACS] buffer) and centrifuged at 4°C. To determine the surface expression of CD40 and Fas, cells were incubated with biotinylated CD40 or matched isotype for 20 min at 4°C, washed twice in cold FACS buffer, and surface stained with the appropriate DC-identification antibodies and with Fas or matched isotype for an additional 20 min at 4°C. Cells were washed in FACS buffer and fixed in 1% paraformaldehyde (PFA; Sigma-Aldrich) and held overnight at 4°C prior to acquisition on a FACSAria (BD Biosciences) flow cytometer.
To determine intracellular FasL, Bcl-2, and caspase-3 expression, the intracellular staining protocol from BD Biosciences was performed. Briefly, cells were surface stained with DC identification antibodies as described above, washed with cold FACS buffer, and fixed in Fix/Perm solution (BD Biosciences) for 15 min, 4°C. Cells were washed with cold FACS buffer, resuspended in FACS buffer, and held overnight at 4°C. The next day, the FACS buffer was removed, and the cells were resuspended in Perm/Wash buffer (BD Biosciences) for 15min at 4°C. Cells were washed in Perm/Wash buffer, stained first with FasL or isotype in Perm/Wash buffer (20 min, 4°C), washed twice, and stained with Bcl-2 or isotype control in Perm/Wash buffer for 30 min for 4°C. For caspase-3 staining, cells were resuspended in Perm/Wash buffer containing caspase-3, followed by incubation for 30 min at room temperature. Cells were resuspended in FACS buffer and acquired. For assessment of Bcl-2 and caspase-3 expression after overnight in vitro culture of PBMC, similar staining procedures were followed except the cells were surface stained, fixed in Fix/Perm, washed twice with Perm/Wash buffer, stained with Bcl-2 or caspase-3, and acquired immediately on the FACSAria.
For assessment of T-cell activation, freshly isolated PBMC were surface stained with CD3, CD4, CD8, and CD38 and HLA-DR or matched isotype controls, washed in FACS buffer, and fixed in 1% PFA. The cells were held overnight at 4°C prior to acquisition on the FACSAria.
FcR blocking reagent (Miltenyi Biotec) was included in all initial incubations to limit nonspecific antibody binding through Fc receptors.
Annexin V and 7-AAD staining.
After overnight culture, total PBMC were collected, washed, and stained with DC identification markers as described above. Cells were then washed twice in cold phosphate-buffered saline (PBS) and resuspended in annexin binding buffer (BD Biosciences) containing annexin V and 7-AAD. Cells were gently vortexed and incubated 15 min at room temperature. The cells were then washed in annexin binding buffer plus 20 μg of actinomycin D (Sigma-Aldrich)/ml and subsequently fixed in 1% PFA prepared in annexin binding buffer plus 20 μg of actinomycin D/ml. The addition of nonfluorescent actinomycin D to the wash buffer and fixative prevents further 7-AAD staining within fixed cells. The cells were acquired on the FACSAria within 1 h.
In vitro cultures and TLRL stimulation of total PBMC.
PBMC were isolated as described above and resuspended in RPMI 1640 medium with 10% human AB serum (Gemini Bioproducts, West Sacramento, CA) at 2 × 106 cells/ml. PBMC were cultured at 37°C, 5% CO2 without stimuli or with either lipopolysaccharide (LPS) from Salmonella enterica serotype Minnesota (10 μg/ml; Sigma-Aldrich), with Imiquimod (TLR7L; 10 μg/ml; InvivoGen, San Diego, CA) or with CLO97, a derivative of the imidazoquinoline compound R848 (TLR7/8L; 5 μg/ml, InvivoGen). After 13 to 20 h, PBMC were collected, and the assessment of DC activation and the expression of apoptotic markers was performed as described above.
Flow cytometry acquisition and analysis.
To control for the accuracy and precision of measurements taken over the course of the study, routine quality control was performed on the FACSAria using the Cytometer Setup & Tracking (CS&T) feature within BD FACSDiva software version 6.1.2 (BD Biosciences). Voltage, laser delay, and area scaling were determined using standardized CS&T beads (BD Biosciences), and the settings were tracked over time. To verify the laser delay and area scaling determined by CS&T, a manual quality control using rainbow beads was performed daily. To further control for quantitative comparisons of levels of expression of surface and intracellular molecules, the collection of data from seronegative donors was interspersed throughout the study period with the acquisition of data from HIV-1-infected donors.
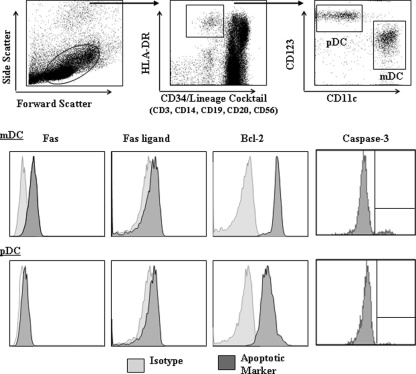
Up to seven color flow cytometry was performed to identify mDCs (Lineage− CD34− HLA-DR+ CD123low CD11c+) and pDCs (Lineage− HLA-DR+ CD11c− CD123+) within PBMC or LN cells (25). Intracellular expression of Bcl-2 and caspase-3 was assessed within each of the DC subsets. In addition, the expression of the death receptor Fas, and its corresponding ligand (FasL), was determined on each DC subset. Membrane-bound FasL is cleaved into soluble FasL by matrix metalloproteinase (56), making detecting surface expression of FasL by flow cytometry difficult. Thus, intracellular FasL expression was evaluated (82). A representative example of DC gating strategy and expression of apoptosis markers by DC subsets in fresh PBMC is shown in Fig. 1. Most apoptosis markers were assessed on DCs in PBMC stained directly ex vivo, as well as stained after culturing PBMC in vitro, without exogenous stimulation, for 13 to 20 h. The expression of DCs undergoing early and late apoptosis, as defined by annexin V and 7-AAD staining, were only evaluated after overnight in vitro culture, and Fas/FasL levels were only measured on DCs in PBMC directly ex vivo. To assess the CD4+ and CD8+ T-cell activation state, the levels of expression of CD38 on CD4+ or CD8+ blood T cells, as well as the percentage coexpressing HLA-DR and CD38, was assessed directly ex vivo.
FIG. 1.
Gating strategy used to identify DC subsets and expression of markers involved in apoptosis. An initial gate was selected based on forward- and side-scatter properties that eliminated the majority of dead cells and cellular debris. Multiparameter flow cytometry techniques were then used to identify total DCs defined as Lineage/CD34− HLA-DR+ and then further subdivided into myeloid DCs (mDCs; CD11chigh CD123lo) and plasmacytoid DCs (pDCs; CD11c− CD1123high). Surface expression of Fas and Fas ligand and intracellular expression of Bcl-2 and caspase-3 by mDCs and pDCs was then assessed. Isotype controls were used for all apoptotic markers. Profiles shown are representative of seronegative donors.
Data analysis was performed using BD FACSDiva software version 6.1.2 on total cells. The average numbers of events collected for mDCs and pDCs from seronegative donors were 2,591 for mDCs and 2,115 for pDCs, and the fewest events collected were 776 for mDCs and 278 for pDCs. On average, 1,713 mDC and 702 pDC events were collected from HIV-1-infected donors (minimum numbers: mDCs, 361; pDCs, 155). Values were typically expressed as mean fluorescence intensity (MFI) minus the background isotype staining (net MFI). Due to the distinctive bimodal expression of caspase-3 by DCs (Fig. 1), caspase-3 expression is shown as the fraction of DCs within the total DC population that are positive for this apoptotic marker. In addition, the frequency of T cells coexpressing CD38 and HLA-DR within the total T-cell population are shown with background staining removed (net %).
Statistical analysis.
Nonparametric statistics were used due to small sample sizes. No adjustments were made for multiple comparisons due to the exploratory nature of the present study. Comparisons between independent groups were made by using the Mann-Whitney t test, and paired analysis was performed using the Wilcoxon signed-rank test. A P value of <0.05 was considered to be of exploratory significance. The Spearman test was used to determine associations between two variables with correlations considered to be of exploratory significance when r > 0.3 and P < 0.05. All statistical analyses were performed by using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA).
RESULTS
Untreated, HIV-1-infected donors have reduced frequencies of blood mDCs and pDCs compared to seronegative donors.
In agreement with our previous studies (9, 25) and those of others (28, 30, 37, 57, 75, 88), fewer mDCs and pDCs were observed in PBMC from 23 untreated, HIV-1-infected donors (mDCs: median, 0.26% of total cells; range, 0.11 to 0.47%; pDCs: 0.10%; range, 0.05 to 0.28%) compared to 19 seronegative donors (mDCs: 0.41%; range, 0.17 to 0.72%, P = 0.006; pDCs: 0.31%; range, 0.12 to 0.57%, P < 0.0001).
Blood mDCs from untreated, HIV-1-infected donors express lower levels of Bcl-2 than mDCs from seronegative donors.
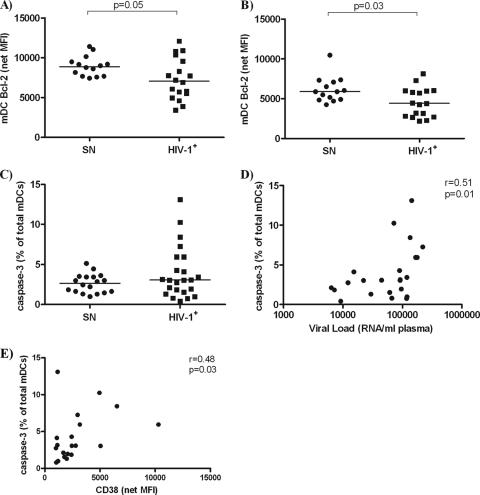
In fresh PBMC, mDCs from HIV-1-infected donors (n = 18) had statistically lower expression levels of Bcl-2 than did mDCs from seronegative donors (n = 14) (Fig. 2A). No significant associations of blood mDC Bcl-2 expression with viral load (r = −0.21, P = 0.4) or with CD4 T-cell count (r = −0.34, P = 0.17) were noted among HIV-1-infected donors. Evaluation of Bcl-2 expression by blood mDCs after overnight in vitro culture also showed that HIV-1-infected donors (n = 17) expressed statistically lower levels of Bcl-2 compared to mDCs from seronegative donors (n = 14) (Fig. 2B). Similar to observations made with Bcl-2 expression directly ex vivo, no significant correlations with viral load or CD4 T-cell count were identified for Bcl-2 expression among HIV-1-infected donors after overnight in vitro culture (viral load: r = −0.07, P = 0.78; CD4 T-cell count: r = −0.30, P = 0.25).
FIG. 2.
Expression of Bcl-2 and caspase-3 by blood mDCs. Intracellular expression of Bcl-2 by blood mDCs from seronegative (SN; n = 14) or HIV-1-infected (HIV-1+; n = 17 to 18) donors was assessed either directly ex vivo (A) or after overnight in vitro culture (B). Values are shown as the mean fluorescence intensity (MFI) with background isotype staining removed (net MFI). Intracellular caspase-3 expression by blood mDCs was assessed directly ex vivo (C) from seronegative (n = 18) or HIV-1-infected (n = 20 to 23) donors and expressed as the fraction of mDCs positive for caspase-3 within the total mDC population (%). Viral load (D) and CD8+ T-cell activation, defined by CD38 expression (E), positively correlate with caspase-3 expression by blood mDCs directly ex vivo. Statistical analysis was performed by using the Mann-Whitney t test to compare Bcl-2 and caspase-3 expression by blood mDCs between seronegative and HIV-1-infected donors, and the Spearman test was performed for plasma viral load and CD8+ T-cell correlations.
Expression of caspase-3 by blood mDCs correlates with viral load.
The median percentage of caspase-3+ mDCs from HIV-1-infected donors (n = 23; median net caspase-3+ mDCs within total mDCs 3.1%; range, 0.4 to 13.1%) was only slightly higher than that of seronegative donors (n = 18; median 2.7%; range, 1.0 to 5.1%; P = 0.43) (Fig. 2C). However, a greater range of caspase-3+ mDC frequencies was noted among HIV-1-infected donors, with >25% of subjects having higher caspase-3+ mDC percentages than the highest percentage observed among seronegative donors (Fig. 2C). Among HIV-1-infected donors, a significant positive correlation was observed between the percentages of caspase-3+ mDCs and plasma viral load (r = 0.51, P = 0.01; Fig. 2D). No significant association between caspase-3+ mDC frequency and CD4 count was noted (r = −0.30, P = 0.16). When percentages of caspase-3+ mDCs were evaluated after overnight in vitro culture, no statistical differences were noted between HIV-1-infected (n = 22; median, 11.4%; range, 2.7 to 35.2%) and seronegative donors (n = 18; median, 11.7%; range, 3.6 to 22.7%).
Frequency of caspase-3+ mDCs from untreated, HIV-1-infected donors is associated with CD8+ T-cell activation levels.
Increased levels of CD4+ and CD8+ T-cell activation, defined as either (i) total CD38 expression (assessed as MFI) or (ii) as the percentage of each T-cell subset coexpressing CD38 and HLA-DR, were observed in HIV-1-infected donors compared to seronegative donors (data not shown). CD4 count correlated inversely with both measures of CD4+ and CD8+ T-cell activation and a positive correlation with plasma viral load was observed for CD4+ CD38+ HLA-DR+ T cells (data not shown).
We then determined if associations existed between percentages of caspase-3+ blood mDCs or Bcl-2 expression by blood mDCs and CD38 expression (MFI) on CD8+ and on CD4+ T cells, and with the percentage of CD8+ T cells and CD4+ T cells coexpressing HLA-DR and CD38. A statistically significant association was observed between the percentage of caspase-3+ mDCs and the level of CD38 on CD8+ T cells (r = 0.48, P = 0.03; Fig. 2E). No statistically significant correlation was observed between caspase-3+ blood mDC percentages and the level of CD38 expression on CD4+ T cells (r = 0.37, P = 0.11) or with coexpression of CD38 and HLA-DR on either CD4+ (r = 0.30, P = 0.16) or CD8+ (r = 0.02, P = 0.93) T cells.
Despite lower Bcl-2 expression by blood mDCs from HIV-1-infected donors, no statistically significant associations were observed between blood mDC Bcl-2 expression and CD38 expression (MFI) on CD8+ (r = −0.01, P = 0.98) and CD4+ (r = 0.13, P = 0.65) T cells and the percentages of CD8+ T cells and CD4+ T cells coexpressing CD38 and HLA-DR (CD8+ T cells: r = 0.22, P = 0.39; CD4+ T cells: r = 0.39, P = 0.11).
Blood pDCs from untreated, HIV-1-infected donors do not statistically differ in expression of Bcl-2 or caspase-3 compared to pDCs from seronegative donors.
Assessment of Bcl-2 expression in pDCs measured directly ex vivo or after overnight in vitro culture showed no significant differences between seronegative and untreated, HIV-1-infected donors (Table 2). Similarly, no statistical differences were observed in the percentages of caspase-3-expressing blood pDCs from HIV-1-infected and seronegative donors, assessed directly ex vivo or after overnight in vitro culture (Table 2).
TABLE 2.
Expression of Bcl-2 and caspase-3 by blood pDCs from seronegative and untreated, HIV-1-infected donors assessed directly ex vivo or after overnight in vitro culture
| Analysis | Median (range) |
P | |
|---|---|---|---|
| Seronegative donors | HIV-1-infected donors | ||
| Bcl-2 expression (net MFI): | |||
| Directly ex vivo (seronegative donors, n = 14; HIV-1-infected donors, n = 18) | 4,779 (3,542-6,646) | 4,871 (2,074-8,484) | 0.95 |
| After overnight in vitro culture (seronegative donors, n = 14; HIV-1-infected donors, n = 17) | 5,189 (3,239-8,319) | 4,841 (2,292-7,984) | 0.49 |
| Caspase-3 expression (%): | |||
| Directly ex vivo (seronegative donors, n = 18; HIV-1-infected donors, n = 23) | 0.7 (0-2.4) | 0.6 (0-2.2) | 0.59 |
| After overnight in vitro culture (seronegative donors, n = 18; HIV-1-infected donors, n = 22) | 30.5 (13.3-53.7) | 22.5 (10.7-55.0) | 0.19 |
No statistically significant differences are observed in spontaneous apoptosis of blood mDCs or pDCs based on annexin V and 7-AAD staining between seronegative and untreated, HIV-1-infected donors.
Two different stages of apoptosis can be assessed based on the use of the surface apoptosis marker annexin V and a nonvital dye (7-AAD). Cells undergoing early apoptosis stain positive for annexin V but not with 7-AAD, whereas late apoptotic cells costain with both annexin V and 7-AAD (90). PBMC were cultured overnight in vitro, and the percentage of DCs undergoing either early or late apoptosis was evaluated by flow cytometry. Although a trend toward higher frequencies of blood mDCs from HIV-1-infected donors undergoing late apoptosis (P = 0.07) was observed, no statistically significant differences were found in the percentages of mDCs or pDCs in early or late apoptosis after overnight culture between seronegative and untreated, HIV-1-infected donors (Table 3).
TABLE 3.
Percentages of blood mDCs and pDCs undergoing early and late apoptosis after overnight in vitro culture
| DC type and stage | Median % (range) |
P | |
|---|---|---|---|
| Seronegative donors (n = 17) | HIV-1-infected donors (n = 18) | ||
| mDCs | |||
| Early apoptosis | 11.9 (2.4-24.3) | 8.0 (2.8-34.3) | 0.63 |
| Late apoptosis | 0.83 (0-4.5) | 1.6 (0.03-14.1) | 0.07 |
| pDCs | |||
| Early apoptosis | 19.6 (5.7-38.8) | 21.1 (3.8-46.0) | 0.92 |
| Late apoptosis | 6.3 (2.4-17.8) | 4.3 (0-19.8) | 0.30 |
No statistically significant differences are observed in Fas and FasL expression on blood mDCs and pDCs between seronegative and untreated, HIV-1-infected individuals.
Surface Fas expression and intracellular expression of FasL was evaluated on blood mDCs and pDCs from seronegative and HIV-1-infected donor PBMC directly ex vivo. No significant differences were observed in expression of Fas by mDCs from seronegative (n = 13; median net MFI, 44; range, 27 to 92) versus HIV-1-infected donors (n = 13; median net MFI, 42; range, 0 to 131; P = 0.29). Fas expression was low on blood pDCs from both seronegative (n = 13; median net MFI, 6; range, 0 to 13) and HIV-1-infected donors (n = 13; median net MFI, 4; range, 0 to 27) with no statistical difference in expression between the two cohorts (P = 0.42).
In addition, no statistical differences were observed in FasL expression by mDCs between seronegative (n = 14; median net MFI, 422; range, 138 to 827) and HIV-1-infected donors (n = 16; median net MFI, 479; range, 171 to 1,024; P = 0.29). Similarly, no statistical differences were observed in FasL expression by pDCs from seronegative (n = 14; median net MFI, 238; range, 81 to 724) and HIV-1-infected donors (n = 16; median net MFI, 356; range, 88 to 838; P = 0.2). Due to limited differences in expression of Fas and FasL by either DC subset between seronegative and HIV-1-infected donors directly ex vivo, no further analysis of Fas or FasL was undertaken.
Percentage of caspase-3+ blood mDCs decreases after initiation of ART.
To determine whether ART impacted the specific differences in mDC subset survival characteristics noted above, a subset of 11 donors (Tables 1 and 4) from the untreated, HIV-1-infected cohort were further evaluated for changes in mDC Bcl-2 (n = 7) and caspase-3 (n = 11) expression in PBMC after starting ART (Fig. 3). Within this cohort, there was a significant increase in the median CD4 T-cell count and a significant decrease in plasma viral load after initiation of ART, with 10 of 11 subjects (91%) achieving complete virologic suppression (Table 4) at the time of the post-ART measurement. The level of CD38 expressed by of CD4+ and CD8+ T cells and the percentage of CD4+ and CD8+ T cells coexpressing CD38 and HLA-DR were also significantly reduced after treatment (Table 4). The frequencies of blood pDCs significantly increased, whereas no significant change was noted in mDC frequency after ART. The median CD40 expression on both pDCs and mDCs was reduced on ART, although this only reached statistical significance for pDCs (Table 4).
TABLE 4.
Changes in clinical characteristics and in T-cell and DC activation states after the initiation of ART
| Category and parameter | Median (range) |
|
|---|---|---|
| Pre-ART | Post-ARTa | |
| CD4 T-cell count and VL | ||
| CD4 T-cell count (cells/mm3) | 306 (36-700) | 481 (90-975)* |
| VL (HIV-1 RNA copies/ml of plasma) | 91,600 (7,200-143,000) | <48 (48-115)* |
| CD3+ CD4+ T cells | ||
| CD38 (net MFI) | 3,632 (1,390-9,772) | 1,386 (911-2,193)† |
| CD38+ HLA-DR+ (%) | 6.8 (0.78-22.01) | 1.83% (0.56-7.81)* |
| CD3+ CD8+ T cells | ||
| CD38 (net MFI) | 2,228 (1,036-6,530) | 500 (106-1,293)† |
| CD38+ HLA-DR+ (%) | 13.5 (5.10-29.10) | 2.53 (0.22-7.06)* |
| Blood DCs | ||
| mDCs (% total cells) | 0.26 (0.12-0.47) | 0.22 (0.12-0.57) |
| pDCs (% total cells) | 0.10 (0.08-0.20) | 0.13 (0.07-0.33)‡ |
| mDCs CD40 expression (net MFI) | 2,578 (1,328-5,320) | 1,805 (929-3,530) |
| pDCs CD40 expression (net MFI) | 1,398 (822-2,841) | 855 (227-1,263)§ |
Statistical analysis was performed by using a Wilcoxon paired test(*, P = 0.001; †, P = 0.002; ‡, P = 0.03; §, P = 0.02).
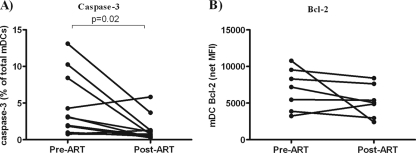
FIG. 3.
Expression of caspase-3 and Bcl-2 by blood mDCs following ART. To determine the effects of ART on expression of caspase-3 (A) and Bcl-2 (B) by blood mDCs directly ex vivo, a subset of donors (Table 1; Pre-ART) from the untreated, HIV-1-infected cohort were further evaluated for changes in caspase-3 (n = 11) and Bcl-2 (n = 7) expression at least 3 months after starting treatment (Post-ART). Values are expressed as either the fraction of mDCs positive for caspase-3 within the total mDC population (%) (A) or as the mean fluorescence intensity (MFI) with background isotype staining removed (net MFI) for Bcl-2 expression (B). Statistical analysis was performed by using the Wilcoxon signed-rank test.
A statistically significant reduction in the percentage of caspase-3+ mDCs was observed post-ART relative to pre-ART (Fig. 3A). Although blood mDCs from untreated, HIV-1-infected donors displayed lower Bcl-2 expression directly ex vivo compared to seronegative donors, mDC Bcl-2 expression did not significantly change on ART (Fig. 3B).
Differential expression of Bcl-2 and caspase-3 in DC subsets in blood versus the LNs of HIV-1-infected individuals.
The LN is a site of active HIV-1 replication (41) and an environment in which DCs interact closely with T cells (15). To determine whether this specific tissue environment influenced DC survival profiles, the expression of Bcl-2 and caspase-3 by mDCs and pDCs from the blood was compared to the expression detected in matched LN samples from an additional cohort of six untreated, HIV-1-infected donors with a median peripheral blood CD4 count of 663 cells/mm3 and a median plasma viral load 20,292 copies HIV-1 RNA/ml (Table 1).
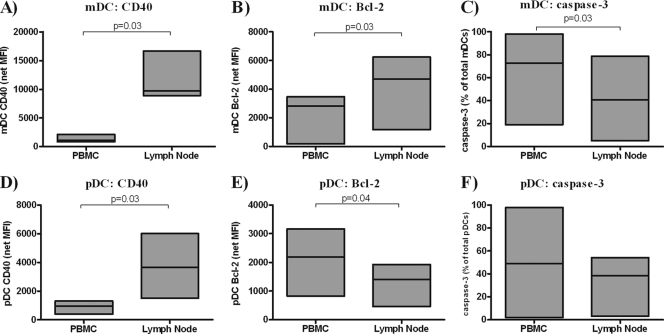
In agreement with our previously published study (25), CD40 expression on mDCs and pDCs in LNs from these HIV-infected subjects was higher than that on mDCs and pDCs from autologous peripheral blood (Fig. 4A and D), suggesting a higher activation state of LN DCs. LN mDCs expressed higher levels of Bcl-2 (Fig. 4B) and had lower percentages of caspase-3+ (Fig. 4C) than their counterparts in peripheral blood. Conversely, LN pDCs expressed significantly lower levels of Bcl-2 than did blood pDCs (Fig. 4E). The percentage of caspase-3+ pDCs was also lower in LN than PBMC, although this difference did not achieve statistical significance (Fig. 4F).
FIG. 4.
Comparison of expression of CD40, Bcl-2, and caspase-3 by DC subsets from PBMC and matched lymph node samples from HIV-1-infected donors. Expression of CD40 (A and D), Bcl-2 (B and E), and caspase-3 (C and F) were evaluated in mDCs (A to C) or pDCs (D to E) from matched PBMC and lymph node samples from HIV-1-infected donors (n = 6). Values are expressed as the mean fluorescence intensity (MFI) with background isotype staining removed (net MFI) for CD40 and Bcl-2 expression or as the fraction of mDCs or pDCs positive for caspase-3 within the total mDC or pDC population (%). Statistical analysis was performed by using the Wilcoxon signed-rank test.
TLR-specific signaling induces increased Bcl-2 and decreased caspase-3 expression in blood DCs in vitro.
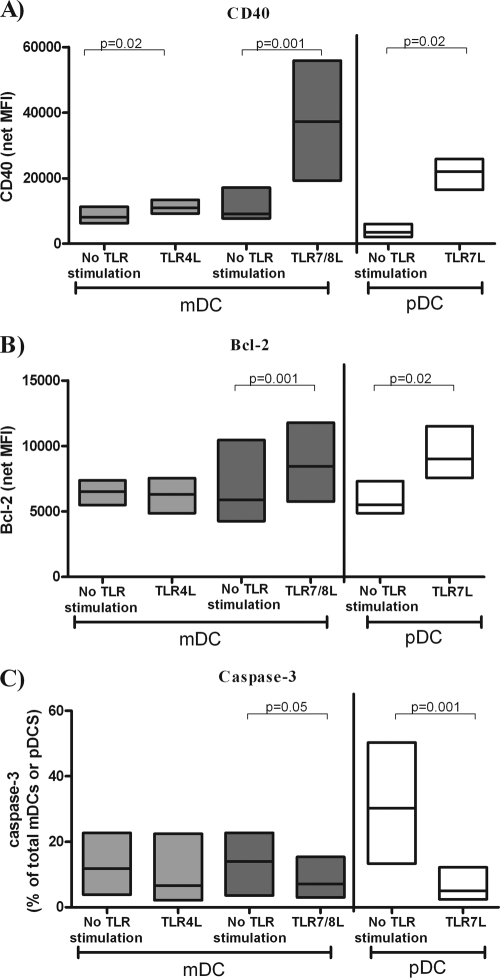
HIV-1 ssRNA encodes for numerous ligands that bind and activate DCs via TLR7/8 in vitro, (10, 44, 70, 71) and TLR8 triggering by HIV-1 ssRNA, in combination with DC-SIGN, a c-type lectin expressed by DCs, was shown to be required for productive infection of DCs (39). In addition, microbial products such as LPS, a known TLR4L, have been shown to be elevated in the blood of HIV-1-infected individuals (7, 16, 52). Blood mDCs express a range of TLRs that include TLR4 and TLR8, whereas blood pDCs only express TLR7 and TLR9 (51, 54). To determine whether HIV-1-related TLRLs modulated DC survival profiles, total PBMC from seronegative individuals (n = 7 to 12) were stimulated in vitro with viral and bacterial TLR ligands and apoptosis markers on mDCs or pDCs evaluated. PBMC were stimulated with LPS (TLR4L) and with TLR7/8L or TLR7L to mimic innate signals that might be delivered by microbial products or by HIV-1 to blood mDCs or pDCs. The effect of these ligands on the activation state (CD40) and apoptotic profile (Bcl-2, caspase-3) of each DC subset was evaluated after overnight in vitro culture (13 to 20 h) in a TLR expression-specific manner, with mDCs assessed in LPS- and TLR78/L-stimulated cultures and pDCs evaluated in cultures stimulated with TLR7L.
Although both TLR4L and TLR7/8L significantly increased CD40 expression on blood mDCs, the impact of TLR7/8L was much greater at the stimulating doses used. TLR7 stimulation also significantly increased CD40 expression on blood pDCs (Fig. 5A). Bcl-2 expression in mDCs was significantly increased by stimulation with TLR7/8L but not with TLR4L (Fig. 5B). Similarly, TLR7L stimulation induced statistically higher levels of Bcl-2 expression in pDCs (Fig. 5B). TLR7/8L stimulation of PBMC resulted in significantly decreased percentages of caspase-3+ mDCs, whereas TLR4L stimulation only trended toward significance (P = 0.08) (Fig. 5C). TLR7L stimulation also significantly reduced the percentage of caspase-3+ pDCs (Fig. 5C).
FIG. 5.
Changes in expression of CD40, Bcl-2, and caspase-3 expression by mDCs and pDCs from seronegative donors in response to TLR ligation. Surface expression of CD40 (A) and intracellular expression of Bcl-2 (B) and caspase-3 expression (C) was assessed on mDCs and pDCs in PBMC from seronegative donors, after overnight (13 to 20 h) in vitro culture with or without TLR ligand (TLRL) stimulation. TLR-specific stimulation of mDCs was assessed using a TLR4L (LPS; 10 μg/ml, n = 7 to 11) or a TLR7/8L (CL097, a derivative of the imidazoquinoline compound R848; 5 μg/ml, n = 11). TLR-specific stimulation of pDCs was assessed using a TLR7L (Imiquimod, [R837], 10 μg/ml, n = 7 to 11). Values are expressed as the mean fluorescence intensity (MFI) minus background isotype staining (net MFI) for CD40 (A) and Bcl-2 (B) or as the fraction of mDCs or pDCs positive for caspase-3 as a percentage of total mDCs or pDCs (C). Statistical analysis was performed comparing the change in expression with or without TLR stimulation using the Wilcoxon signed-rank test.
We next evaluated whether DCs from a subset of the untreated HIV-1-infected donors (n = 7 to 14), potentially already exposed to viral and bacterial TLRLs in vivo, could respond to in vitro stimulation with TLRLs in a similar manner to DCs from uninfected donors. CD40 and Bcl-2 expression levels by blood mDCs and pDCs from HIV-infected donors were upregulated to a similar extent following exposure to TLR4/7/8L as DCs from uninfected donors, and the percentages of caspase-3+ mDCs or pDCs induced by TLRL stimulation of total PBMC were likewise reduced in a manner similar to those observed in seronegative donor DCs (data not shown).
DISCUSSION
Despite the wealth of data demonstrating reduced frequencies of circulating DC subsets in the blood of HIV-1-infected donors (9, 25, 28, 30, 37, 75, 88), a limited number of studies have addressed possible mechanisms responsible for this depletion. During acute and chronic HIV-1 infection, it is likely that recruitment to LNs partly accounts for decreased blood DC frequencies (25, 64). Conversely, in late-stage disease there is a reduced frequency of DCs within the LN (11), suggesting that LN recruitment alone does not fully account for the deficit in circulating DCs throughout the course of HIV-1 disease.
We hypothesized that another possible contributor to HIV-1-associated depletion of circulating DCs is increased DC death. We show that HIV-1 infection induced a proapoptotic profile in blood mDCs, defined as reduced Bcl-2 expression and increased frequencies of caspase-3+ mDCs in a subset of subjects that was associated with viral replication. Higher frequencies of caspase-3+ mDCs were also associated with increased levels of T-cell activation, as defined by CD38 expression on CD8+ T cells. Elevated CD38 expression on CD8+ T cells has been associated with shorter survival in advanced HIV-1 disease (33) and is considered by some groups to be a better indicator of disease progression than frequencies of CD8+ T cells coexpressing CD38 and HLA-DR (63). Since mDC-T-cell interactions form a critical step in initiation of adaptive immune responses, it is possible that T-cell activation status may directly contribute to the increased levels of apoptotic mDCs observed during untreated HIV-1 infection. An alternative hypothesis is that mDC survival and T-cell activation are independently influenced by HIV-associated factors.
The HIV-associated reduction in mDC Bcl-2 expression reported in the present study concurs with findings of reduced Bcl-2 expression described in T cells during HIV-1 infection (1, 14, 26, 80, 83, 100), raising the possibility that a common mechanism may be responsible for predisposing both mDCs and T cells to apoptosis. Most studies addressing the effect of ART on levels of T-cell Bcl-2 expression have noted increased expression of Bcl-2 with therapy (1, 4, 93), although one study found that Bcl-2 expression by T cells was not altered during 6 months of ART (38). In agreement with this latter study, we also did not observe any significant change in blood mDC Bcl-2 expression in a subset of HIV-1-infected donors that initiated ART during the study. Given that we did not observe a direct association between Bcl-2 expression and viral load in untreated, HIV-1-infected individuals, this lack of ART effect on Bcl-2 expression was not surprising and further supports the idea that HIV-associated factors other than viral load must be contributing to the decrease in mDC Bcl-2 expression. Conversely, caspase-3+ mDC frequencies were elevated above normal levels in a subset of HIV-1-infected subjects, were significantly associated with viral load, and were reduced after viral suppression with ART. The fact that we did not observe statistically greater percentages of caspase-3+ mDCs between HIV-1-infected and uninfected subject groups is possibly related to small sample size, which may be considered a limitation of the present study. These observed differences in the association of pro- and antiapoptotic protein expression with viral load suggests that multiple mechanisms are likely responsible for the observed HIV-associated changes in blood mDC apoptosis profiles.
In a recent study using direct ex vivo annexin V and 7-AAD staining to evaluate apoptosis of blood mDCs, Meera et al. reported a higher percentage of apoptotic mDCs in AIDS patients compared to controls (69). Although we also observed HIV-associated, proapoptotic changes in Bcl-2 and caspase-3 expression in blood mDCs directly ex vivo, we did not observe HIV-associated significant increases in DCs undergoing apoptosis using annexin V/7-AAD staining on cultured PBMC. However, a trend toward higher frequencies of blood mDCs from HIV-1-infected donors undergoing late apoptosis was noted (P = 0.07). Blood DCs have been reported to survive poorly in vitro (59, 66), and we noted high frequencies of mDCs and pDCs undergoing spontaneous apoptosis in both subject groups after overnight PBMC culture (Table 3). High levels of spontaneous apoptosis and a large variability in apoptotic DC frequencies between donors, as defined by their expression of annexin V and 7-AAD, may have prevented detection of subtle differences between the subject groups. It is also possible that the lack of differences using this measure of apoptosis simply reflects a poor correlation between assays assessing different stages of the apoptosis pathway or in those carried out on cells directly ex vivo versus following a culture period. Indeed, in an early study of T-cell apoptosis during HIV-1 infection, lower Bcl-2 expression of CD4+ and CD8+ T cells measured directly ex vivo did not correlate with the percentage of cells induced to undergo apoptosis in vitro (26).
In contrast to our findings on blood mDCs, our study found that levels of blood pDC markers of apoptosis were not markedly altered in the setting of untreated HIV-1 infection, suggesting that survival of blood DC subsets may be differentially influenced by HIV-1. Meera et al. noted an increased frequency of blood pDCs undergoing apoptosis directly ex vivo in individuals with early and advanced HIV infection (69). The lack of similar observations of decreased survival tendencies of blood pDCs based on Bcl-2 and caspase-3 expression in our study may reflect analysis of different cohorts of HIV-1-infected donors in addition to differences in the assays used to detect apoptosis.
Since lymphoid tissue is a site of active viral replication and a tissue compartment in which DCs might interact most closely with both HIV-1 and T cells, we hypothesized that in HIV-infected donors the apoptotic profiles of LN DC subsets would differ from those of circulating DCs and perhaps more accurately reflect HIV-associated changes. The increase in CD40 and Bcl-2 expression and decrease in caspase-3 expression by LN relative to blood mDCs that we observed in HIV-1-infected donors suggests that mDCs may be receiving both activation and survival signals in HIV-infected lymphoid tissue. A number of studies have demonstrated a positive relationship between CD40 ligation and increased expression of the antiapoptotic Bcl-2 family members (12, 42, 48, 81), although expression of these “survival” signals may depend on the activation state of the DCs at the time of CD40 ligation (23). Induction of spontaneous apoptosis of total DCs from patients with breast cancer was prevented by inducing increased Bcl-2 expression with CD40 ligation (81). The role for CD40-induced survival may be most notable in the LNs where close interactions between T cells and DCs occur (15), thereby increasing the likelihood of CD40-CD40L interactions. The small sample size of LN donors in our study prevented accurate determination of associations between CD40 expression and these apoptotic markers. However, given the parallel observation of higher expression of CD40 by the same LN mDCs relative to blood mDCs, it is tempting to speculate that LN mDCs are indeed receiving survival signals within the LN of HIV-1-infected infected donors that may, in part, be due to increased CD40 signaling resulting in increased Bcl-2 expression and/or decreased caspase-3 expression. These results might also provide a mechanism to explain the observation of our group that mDCs accumulate in the LNs of chronically HIV-infected subjects (25). Enhanced DC survival by IL-10-induced reversal of DC susceptibility to NK cell-mediated elimination (2) has also been reported to provide a mechanism to explain accumulation of mDCs in LNs. The survival signals received by mDCs in the LN, or a lack of “death-inducing” signals, may help to offset the proapoptotic tendencies of circulating blood mDCs in HIV-1 infection, although this hypothesis needs to be confirmed with additional studies.
Stimulation of mDCs in vitro with a TLRL mimicking HIV-1 ssRNA induced strong activation and an antiapoptotic profile, whereas stimulation with a bacterial TLRL only moderately activated mDCs and did not significantly alter their apoptotic status. Thus, in addition to the possibility of increased CD40 activation inducing mDC survival within the LN, these data suggest that signaling via TLR7/8 by HIV-1 may also aid in the survival of LN mDCs during chronic infection. We, and others, have reported that DCs in LNs of HIV-1-infected donors are in a state of partial activation (25, 60). Thus, LN mDCs may receive survival signals via viral TLRs and CD40 ligation that allow them to persist in this state of partial activation, thereby potentially contributing to the state of chronic immune activation through nonspecific activation of T cells. In addition, survival of these “semimature” DCs may contribute further to T-cell dysfunction in the context of HIV-1 infection through the induction of regulatory T cells (60).
Conversely, pDCs from LNs of HIV-1-infected donors had reduced expression of Bcl-2 in combination with increased CD40 expression compared to circulating pDCs from the same donors. Thus, the LN environment during HIV-1 infection may provide both activating and proapoptotic signals to pDCs in contrast to our findings for LN mDCs and highlighting further functional differences between these two DC subsets. Indeed, in vitro studies have shown activation of pDCs through CD40-CD40L in HIV-1 infection models increased viral replication, productive infection, pDC death, and increased transmission of HIV-1 to T cells (31, 84). This suggests that the LN is a site of increased pDC death in HIV-1 infection, which is likely related, at least in part, to the increased expression of CD40 by LN pDCs. A recent study by Lehmann et al. provides further evidence for an HIV-associated increase in LN pDC death (62). They report that pDCs that had homed to the LNs of HIV-1-infected subjects underwent apoptosis, based on annexin V staining, at a greater rate than LN pDCs from uninfected donors, and this increased pDC apoptosis was associated with high pDC IFN-α production (62). Similarly, a study investigating pDC accumulation and death in the LNs of acutely SIV-infected monkeys demonstrated increased pDC death and SIV-infection rates of the pDCs similar to that of CD4+ T cells (18). The accumulation of pDCs in the LN of HIV-1-infected donors as reported by our group (25) and others (62), and the similar observations noted during acute SIV infection (8, 18) suggests that a balance exists between recruitment of pDCs to the LN and their subsequent death.
Stimulation with a viral TLRL induced both activation and an antiapoptotic profile in blood pDCs in vitro, a finding consistent with a study demonstrating increased pDC survival following in vitro stimulation with HIV-1-derived ssRNAs (70). However, these observations contrasted with the decreased survival profile observed in LN pDCs in HIV-1-infected donors. This difference implies that factors other than TLR7 activation of pDCs via HIV-1 ssRNA in vivo, such as other HIV-1 proteins (including Env, Net, Tat, and Vpr) that have been shown to induce apoptosis (86) or direct infection of pDCs (18, 84), likely account for the observed LN pDC survival patterns in HIV-1-infected subjects.
Although the intrasubject comparisons of apoptosis markers in blood versus LN DCs are informative, the absence of LN samples from an uninfected control group limits the interpretation of our findings. For instance, the relative antiapoptotic profile of LN mDCs that we observed may also reflect physiologic tissue-specific profiles that are unrelated to HIV-1 infection. In an acute SIV infection model, LN mDCs following SIV infection displayed increased caspase-3 expression compared to preinfection LN mDCs, although no alteration in Bcl-2 expression was observed after infection (97). Thus, it is possible that our observations of apoptotic markers indicative of an increased survival pattern by LN mDCs relative to blood mDCs may still reflect reduced survival of LN mDCs compared to LN mDCs from an uninfected individual.
In conclusion, we believe our study to be the first to show that circulating mDCs from untreated HIV-1-infected donors exhibit a proapoptotic profile characterized by lower Bcl-2 expression compared to seronegative donor mDCs and with frequencies of caspase-3+ mDCs that correlated with viral load and levels of CD8+ T-cell activation. Furthermore, we show that in the setting of untreated HIV-1 infection, LN mDCs appear to be receiving survival signals, whereas LN pDCs display a more apoptotic profile than their counterparts in blood. These findings underscore the role that DC survival may play in HIV-1 pathogenesis and may help to explain the patterns of blood DC depletion and LN accumulation that have been previously reported in several studies. Given the exploratory nature of these preliminary findings, larger studies are necessary to confirm the results generated in our study.
Acknowledgments
We thank the physicians, staff, and patients in the Infectious Diseases Group Practice at the University of Colorado Health Sciences Center and the University of Colorado Hospital and also the seronegative donors for their assistance and participation in our study. We acknowledge the Colorado Center for AIDS Research (CFAR) Immunology Core for assistance with flow cytometry and the Clinical Investigation Core for assistance with recruiting of subjects.
This study was supported by the National Institute of Health grants R01 AI065275 (C.C.W.), K24 AI07434 (C.C.W.), R21 HD051450 (E.C.), and P01 AI55356 (E.C.) and was facilitated by the infrastructure and resources provided by the Colorado Center for AIDS Research (AI054907).
Footnotes
Published ahead of print on 20 October 2010.
REFERENCES
- 1.Airo, P., C. Torti, M. C. Uccelli, F. Malacarne, L. Palvarini, G. Carosi, and F. Castelli. 2000. Naive CD4+ T lymphocytes express high levels of Bcl-2 after highly active antiretroviral therapy for HIV infection. AIDS Res. Hum. Retrovir. 16:1805-1807. [DOI] [PubMed] [Google Scholar]
- 2.Alter, G., D. Kavanagh, S. Rihn, R. Luteijn, D. Brooks, M. Oldstone, J. van Lunzen, and M. Altfeld. 2010. IL-10 induces aberrant deletion of dendritic cells by natural killer cells in the context of HIV infection. J. Clin. Invest. 120:1905-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashe, P. C., and M. D. Berry. 2003. Apoptotic signaling cascades. Prog. Neuropsychopharmacol. Biol. Psychiatry. 27:199-214. [DOI] [PubMed] [Google Scholar]
- 4.Balestrieri, E., S. Grelli, C. Matteucci, A. Minutolo, G. d'Ettorre, F. Di Sora, F. Montella, V. Vullo, S. Vella, C. Favalli, B. Macchi, and A. Mastino. 2007. Apoptosis-associated gene expression in HIV-infected patients in response to successful antiretroviral therapy. J. Med. Virol. 79:111-117. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 7.Baroncelli, S., C. M. Galluzzo, M. F. Pirillo, M. G. Mancini, L. E. Weimer, M. Andreotti, R. Amici, S. Vella, M. Giuliano, and L. Palmisano. 2009. Microbial translocation is associated with residual viral replication in HAART-treated HIV+ subjects with <50 copies/ml HIV-1 RNA. J. Clin. Virol. 46:367-370. [DOI] [PubMed] [Google Scholar]
- 8.Barratt-Boyes, S. M., V. Wijewardana, and K. N. Brown. 2010. In acute pathogenic SIV infection plasmacytoid dendritic cells are depleted from blood and lymph nodes despite mobilization. J. Med. Primatol. 39:235-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barron, M. A., N. Blyveis, B. E. Palmer, S. MaWhinney, and C. C. Wilson. 2003. Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1-infected individuals. J. Infect. Dis. 187:26-37. [DOI] [PubMed] [Google Scholar]
- 10.Beignon, A. S., K. McKenna, M. Skoberne, O. Manches, I. DaSilva, D. G. Kavanagh, M. Larsson, R. J. Gorelick, J. D. Lifson, and N. Bhardwaj. 2005. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J. Clin. Invest. 115:3265-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biancotto, A., J. C. Grivel, S. J. Iglehart, C. Vanpouille, A. Lisco, S. F. Sieg, R. Debernardo, K. Garate, B. Rodriguez, L. B. Margolis, and M. M. Lederman. 2007. Abnormal activation and cytokine spectra in lymph nodes of people chronically infected with HIV-1. Blood 109:4272-4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjorck, P., J. Banchereau, and L. Flores-Romo. 1997. CD40 ligation counteracts Fas-induced apoptosis of human dendritic cells. Int. Immunol. 9:365-372. [DOI] [PubMed] [Google Scholar]
- 13.Bofill, M., W. Gombert, N. J. Borthwick, A. N. Akbar, J. E. McLaughlin, C. A. Lee, M. A. Johnson, A. J. Pinching, and G. Janossy. 1995. Presence of CD3+ CD8+ Bcl-2low lymphocytes undergoing apoptosis and activated macrophages in lymph nodes of HIV-1+ patients. Am. J. Pathol. 146:1542-1555. [PMC free article] [PubMed] [Google Scholar]
- 14.Boudet, F., H. Lecoeur, and M. L. Gougeon. 1996. Apoptosis associated with ex vivo down-regulation of Bcl-2 and up-regulation of Fas in potential cytotoxic CD8+ T lymphocytes during HIV infection. J. Immunol. 156:2282-2293. [PubMed] [Google Scholar]
- 15.Bousso, P. 2008. T-cell activation by dendritic cells in the lymph node: lessons from the movies. Nat. Rev. Immunol. 8:675-684. [DOI] [PubMed] [Google Scholar]
- 16.Brenchley, J. M., D. A. Price, T. W. Schacker, T. E. Asher, G. Silvestri, S. Rao, Z. Kazzaz, E. Bornstein, O. Lambotte, D. Altmann, B. R. Blazar, B. Rodriguez, L. Teixeira-Johnson, A. Landay, J. N. Martin, F. M. Hecht, L. J. Picker, M. M. Lederman, S. G. Deeks, and D. C. Douek. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12:1365-1371. [DOI] [PubMed] [Google Scholar]
- 17.Brown, K. N., A. Trichel, and S. M. Barratt-Boyes. 2007. Parallel loss of myeloid and plasmacytoid dendritic cells from blood and lymphoid tissue in simian AIDS. J. Immunol. 178:6958-6967. [DOI] [PubMed] [Google Scholar]
- 18.Brown, K. N., V. Wijewardana, X. Liu, and S. M. Barratt-Boyes. 2009. Rapid influx and death of plasmacytoid dendritic cells in lymph nodes mediate depletion in acute simian immunodeficiency virus infection. PLoS Pathog. 5:e1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cella, M., F. Facchetti, A. Lanzavecchia, and M. Colonna. 2000. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat. Immunol. 1:305-310. [DOI] [PubMed] [Google Scholar]
- 20.Chehimi, J., E. Papasavvas, C. Tomescu, B. Gekonge, S. Abdulhaqq, A. Raymond, A. Hancock, K. Vinekar, C. Carty, G. Reynolds, M. Pistilli, K. Mounzer, J. Kostman, and L. J. Montaner. Inability of plasmacytoid dendritic cells to directly lyse HIV-infected autologous CD4+ T cells despite induction of tumor necrosis factor-related apoptosis-inducing ligand. J. Virol. 84:2762-2773. [DOI] [PMC free article] [PubMed]
- 21.Chehimi, J., S. E. Starr, I. Frank, A. D'Andrea, X. Ma, R. R. MacGregor, J. Sennelier, and G. Trinchieri. 1994. Impaired interleukin 12 production in human immunodeficiency virus-infected patients. J. Exp. Med. 179:1361-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cory, S., and J. M. Adams. 2002. The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2:647-656. [DOI] [PubMed] [Google Scholar]
- 23.de Goer de Herve, M. G., D. Durali, T. A. Tran, G. Maigne, F. Simonetta, P. Leclerc, J. F. Delfraissy, and Y. Taoufik. 2005. Differential effect of agonistic anti-CD40 on human mature and immature dendritic cells: the Janus face of anti-CD40. Blood 106:2806-2814. [DOI] [PubMed] [Google Scholar]
- 24.Degterev, A., M. Boyce, and J. Yuan. 2003. A decade of caspases. Oncogene 22:8543-8567. [DOI] [PubMed] [Google Scholar]
- 25.Dillon, S. M., K. B. Robertson, S. C. Pan, S. Mawhinney, A. L. Meditz, J. M. Folkvord, E. Connick, M. D. McCarter, and C. C. Wilson. 2008. Plasmacytoid and myeloid dendritic cells with a partial activation phenotype accumulate in lymphoid tissue during asymptomatic chronic HIV-1 infection. J. Acquir. Immune Defic. Syndr. 48:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobmeyer, T. S., S. A. Klein, J. M. Dobmeyer, B. Raffel, S. Findhammer, D. Hoelzer, E. B. Helm, R. Rossol, and D. Kabelitz. 1998. Differential expression of bcl-2 and susceptibility to programmed cell death in lymphocytes of HIV-1-infected individuals. Clin. Immunol. Immunopathol. 87:230-239. [DOI] [PubMed] [Google Scholar]
- 27.Donaghy, H., B. Gazzard, F. Gotch, and S. Patterson. 2003. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood 101:4505-4511. [DOI] [PubMed] [Google Scholar]
- 28.Donaghy, H., A. Pozniak, B. Gazzard, N. Qazi, J. Gilmour, F. Gotch, and S. Patterson. 2001. Loss of blood CD11c+ myeloid and CD11c− plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood 98:2574-2576. [DOI] [PubMed] [Google Scholar]
- 29.Elmore, S. 2007. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 35:495-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldman, S., D. Stein, S. Amrute, T. Denny, Z. Garcia, P. Kloser, Y. Sun, N. Megjugorac, and P. Fitzgerald-Bocarsly. 2001. Decreased interferon-alpha production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin. Immunol. 101:201-210. [DOI] [PubMed] [Google Scholar]
- 31.Fong, L., M. Mengozzi, N. W. Abbey, B. G. Herndier, and E. G. Engleman. 2002. Productive infection of plasmacytoid dendritic cells with human immunodeficiency virus type 1 is triggered by CD40 ligation. J. Virol. 76:11033-11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geijtenbeek, T. B., and S. I. Gringhuis. 2009. Signalling through C-type lectin receptors: shaping immune responses. Nat. Rev. Immunol. 9:465-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giorgi, J. V., L. E. Hultin, J. A. McKeating, T. D. Johnson, B. Owens, L. P. Jacobson, R. Shih, J. Lewis, D. J. Wiley, J. P. Phair, S. M. Wolinsky, and R. Detels. 1999. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J. Infect. Dis. 179:859-870. [DOI] [PubMed] [Google Scholar]
- 34.Gougeon, M. L., H. Lecoeur, C. Callebaut, E. Jacotot, A. Dulioust, R. Roue, L. Montagnier, and A. G. Hovanessian. 1996. Selective loss of the CD4+/CD26+ T-cell subset during HIV infection. Res. Immunol. 147:5-8. [DOI] [PubMed] [Google Scholar]
- 35.Gougeon, M. L., and L. Montagnier. 1999. Programmed cell death as a mechanism of CD4 and CD8 T-cell deletion in AIDS. Molecular control and effect of highly active anti-retroviral therapy. Ann. N. Y. Acad. Sci. 887:199-212. [DOI] [PubMed] [Google Scholar]
- 36.Granelli-Piperno, A., A. Golebiowska, C. Trumpfheller, F. P. Siegal, and R. M. Steinman. 2004. HIV-1-infected monocyte-derived dendritic cells do not undergo maturation but can elicit IL-10 production and T-cell regulation. Proc. Natl. Acad. Sci. U. S. A. 101:7669-7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grassi, F., A. Hosmalin, D. McIlroy, V. Calvez, P. Debre, and B. Autran. 1999. Depletion in blood CD11c-positive dendritic cells from HIV-infected patients. AIDS 13:759-766. [DOI] [PubMed] [Google Scholar]
- 38.Grelli, S., S. Campagna, M. Lichtner, G. Ricci, S. Vella, V. Vullo, F. Montella, S. Di Fabio, C. Favalli, A. Mastino, and B. Macchi. 2000. Spontaneous and anti-Fas-induced apoptosis in lymphocytes from HIV-infected patients undergoing highly active anti-retroviral therapy. AIDS 14:939-949. [DOI] [PubMed] [Google Scholar]
- 39.Gringhuis, S. I., M. van der Vlist, L. M. van den Berg, J. den Dunnen, M. Litjens, and T. B. Geijtenbeek. HIV-1 exploits innate signaling by TLR8 and DC-SIGN for productive infection of dendritic cells. Nat. Immunol. 11:419-426. [DOI] [PubMed]
- 40.Guicciardi, M. E., and G. J. Gores. 2009. Life and death by death receptors. FASEB J. 23:1625-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haase, A. T. 1999. Population biology of HIV-1 infection: viral and CD4+ T-cell demographics and dynamics in lymphatic tissues. Annu. Rev. Immunol. 17:625-656. [DOI] [PubMed] [Google Scholar]
- 42.Haenssle, H., T. Buhl, S. Knudsen, U. Krueger, A. Rosenberger, K. Reich, and C. Neumann. 2008. CD40 ligation during dendritic cell maturation reduces cell death and prevents interleukin-10-induced regression to macrophage-like monocytes. Exp. Dermatol. 17:177-187. [DOI] [PubMed] [Google Scholar]
- 43.Heath, W. R., and F. R. Carbone. 2009. Dendritic cell subsets in primary and secondary T-cell responses at body surfaces. Nat. Immunol. 10:1237-1244. [DOI] [PubMed] [Google Scholar]
- 44.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303:1526-1529. [DOI] [PubMed] [Google Scholar]
- 45.Herbeuval, J. P., A. Boasso, J. C. Grivel, A. W. Hardy, S. A. Anderson, M. J. Dolan, C. Chougnet, J. D. Lifson, and G. M. Shearer. 2005. TNF-related apoptosis-inducing ligand (TRAIL) in HIV-1-infected patients and its in vitro production by antigen-presenting cells. Blood 105:2458-2464. [DOI] [PubMed] [Google Scholar]
- 46.Herbeuval, J. P., A. W. Hardy, A. Boasso, S. A. Anderson, M. J. Dolan, M. Dy, and G. M. Shearer. 2005. Regulation of TNF-related apoptosis-inducing ligand on primary CD4+ T cells by HIV-1: role of type I IFN-producing plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 102:13974-13979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herbeuval, J. P., J. Nilsson, A. Boasso, A. W. Hardy, M. J. Kruhlak, S. A. Anderson, M. J. Dolan, M. Dy, J. Andersson, and G. M. Shearer. 2006. Differential expression of IFN-α and TRAIL/DR5 in lymphoid tissue of progressor versus nonprogressor HIV-1-infected patients. Proc. Natl. Acad. Sci. U. S. A. 103:7000-7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hou, W. S., and L. Van Parijs. 2004. A Bcl-2-dependent molecular timer regulates the lifespan and immunogenicity of dendritic cells. Nat. Immunol. 5:583-589. [DOI] [PubMed] [Google Scholar]
- 49.Igney, F. H., and P. H. Krammer. 2002. Death and anti-death: tumour resistance to apoptosis. Nat. Rev. Cancer 2:277-288. [DOI] [PubMed] [Google Scholar]
- 50.Ito, T., H. Kanzler, O. Duramad, W. Cao, and Y. J. Liu. 2006. Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood 107:2423-2431. [DOI] [PubMed] [Google Scholar]
- 51.Jarrossay, D., G. Napolitani, M. Colonna, F. Sallusto, and A. Lanzavecchia. 2001. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur. J. Immunol. 31:3388-3393. [DOI] [PubMed] [Google Scholar]
- 52.Jiang, W., M. M. Lederman, P. Hunt, S. F. Sieg, K. Haley, B. Rodriguez, A. Landay, J. Martin, E. Sinclair, A. I. Asher, S. G. Deeks, D. C. Douek, and J. M. Brenchley. 2009. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J. Infect. Dis. 199:1177-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ju, X., G. Clark, and D. N. Hart. 2010. Review of human DC subtypes. Methods Mol. Biol. 595:3-20. [DOI] [PubMed] [Google Scholar]
- 54.Kadowaki, N., S. Ho, S. Antonenko, R. W. Malefyt, R. A. Kastelein, F. Bazan, and Y. J. Liu. 2001. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaisho, T., and S. Akira. 2003. Regulation of dendritic cell function through Toll-like receptors. Curr. Mol. Med. 3:759-771. [DOI] [PubMed] [Google Scholar]
- 56.Kayagaki, N., A. Kawasaki, T. Ebata, H. Ohmoto, S. Ikeda, S. Inoue, K. Yoshino, K. Okumura, and H. Yagita. 1995. Metalloproteinase-mediated release of human Fas ligand. J. Exp. Med. 182:1777-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Killian, M. S., S. H. Fujimura, F. M. Hecht, and J. A. Levy. 2006. Similar changes in plasmacytoid dendritic cell and CD4 T-cell counts during primary HIV-1 infection and treatment. AIDS 20:1247-1252. [DOI] [PubMed] [Google Scholar]
- 58.Kim, N., A. Dabrowska, R. G. Jenner, and A. Aldovini. 2007. Human and simian immunodeficiency virus-mediated upregulation of the apoptotic factor TRAIL occurs in antigen-presenting cells from AIDS-susceptible but not from AIDS-resistant species. J. Virol. 81:7584-7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kohrgruber, N., N. Halanek, M. Groger, D. Winter, K. Rappersberger, M. Schmitt-Egenolf, G. Stingl, and D. Maurer. 1999. Survival, maturation, and function of CD11c− and CD11c+ peripheral blood dendritic cells are differentially regulated by cytokines. J. Immunol. 163:3250-3259. [PubMed] [Google Scholar]
- 60.Krathwohl, M. D., T. W. Schacker, and J. L. Anderson. 2006. Abnormal presence of semimature dendritic cells that induce regulatory T cells in HIV-infected subjects. J. Infect. Dis. 193:494-504. [DOI] [PubMed] [Google Scholar]
- 61.Langenkamp, A., M. Messi, A. Lanzavecchia, and F. Sallusto. 2000. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat. Immunol. 1:311-316. [DOI] [PubMed] [Google Scholar]
- 62.Lehmann, C., M. Lafferty, A. Garzino-Demo, N. Jung, P. Hartmann, G. Fatkenheuer, J. S. Wolf, J. van Lunzen, and F. Romerio. 2010. Plasmacytoid dendritic cells accumulate and secrete interferon alpha in lymph nodes of HIV-1 patients. PLoS One 5:e11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu, Z., W. G. Cumberland, L. E. Hultin, H. E. Prince, R. Detels, and J. V. Giorgi. 1997. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 16:83-92. [DOI] [PubMed] [Google Scholar]
- 64.Lore, K., A. Sonnerborg, C. Brostrom, L. E. Goh, L. Perrin, H. McDade, H. J. Stellbrink, B. Gazzard, R. Weber, L. A. Napolitano, Y. van Kooyk, and J. Andersson. 2002. Accumulation of DC-SIGN+CD40+ dendritic cells with reduced CD80 and CD86 expression in lymphoid tissue during acute HIV-1 infection. Aids 16:683-692. [DOI] [PubMed] [Google Scholar]
- 65.Louis, S., C. A. Dutertre, L. Vimeux, L. Fery, L. Henno, S. Diocou, S. Kahi, C. Deveau, L. Meyer, C. Goujard, and A. Hosmalin. 2010. IL-23 and IL-12p70 production by monocytes and dendritic cells in primary HIV-1 infection. J. Leukoc. Biol. 87:645-653. [DOI] [PubMed] [Google Scholar]
- 66.MacDonald, K. P., D. J. Munster, G. J. Clark, A. Dzionek, J. Schmitz, and D. N. Hart. 2002. Characterization of human blood dendritic cell subsets. Blood 100:4512-4520. [DOI] [PubMed] [Google Scholar]
- 67.Majumder, B., M. L. Janket, E. A. Schafer, K. Schaubert, X. L. Huang, J. Kan-Mitchell, C. R. Rinaldo, Jr., and V. Ayyavoo. 2005. Human immunodeficiency virus type 1 Vpr impairs dendritic cell maturation and T-cell activation: implications for viral immune escape. J. Virol. 79:7990-8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Majumder, B., N. J. Venkatachari, E. A. Schafer, M. L. Janket, and V. Ayyavoo. 2007. Dendritic cells infected with vpr-positive human immunodeficiency virus type 1 induce CD8+ T-cell apoptosis via upregulation of tumor necrosis factor alpha. J. Virol. 81:7388-7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meera, S., T. Madhuri, G. Manisha, and P. Ramesh. 2010. Irreversible loss of pDCs by apoptosis during early HIV infection may be a critical determinant of immune dysfunction. Viral Immunol. 23:241-249. [DOI] [PubMed] [Google Scholar]
- 70.Meier, A., G. Alter, N. Frahm, H. Sidhu, B. Li, A. Bagchi, N. Teigen, H. Streeck, H. J. Stellbrink, J. Hellman, J. van Lunzen, and M. Altfeld. 2007. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-like receptor ligands. J. Virol. 81:8180-8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meier, A., A. Bagchi, H. K. Sidhu, G. Alter, T. J. Suscovich, D. G. Kavanagh, H. Streeck, M. A. Brockman, S. LeGall, J. Hellman, and M. Altfeld. 2008. Upregulation of PD-L1 on monocytes and dendritic cells by HIV-1 derived TLR ligands. AIDS 22:655-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meyers, J. H., J. S. Justement, C. W. Hallahan, E. T. Blair, Y. A. Sun, M. A. O'Shea, G. Roby, S. Kottilil, S. Moir, C. M. Kovacs, T. W. Chun, and A. S. Fauci. 2007. Impact of HIV on cell survival and antiviral activity of plasmacytoid dendritic cells. PLoS One 2:e458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moretti, S., S. Marcellini, A. Boschini, G. Famularo, G. Santini, E. Alesse, S. M. Steinberg, M. G. Cifone, G. Kroemer, and C. De Simone. 2000. Apoptosis and apoptosis-associated perturbations of peripheral blood lymphocytes during HIV infection: comparison between AIDS patients and asymptomatic long-term non-progressors. Clin. Exp. Immunol. 122:364-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muro-Cacho, C. A., G. Pantaleo, and A. S. Fauci. 1995. Analysis of apoptosis in lymph nodes of HIV-infected persons: intensity of apoptosis correlates with the general state of activation of the lymphoid tissue and not with stage of disease or viral burden. J. Immunol. 154:5555-5566. [PubMed] [Google Scholar]
- 75.Pacanowski, J., S. Kahi, M. Baillet, P. Lebon, C. Deveau, C. Goujard, L. Meyer, E. Oksenhendler, M. Sinet, and A. Hosmalin. 2001. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood 98:3016-3021. [DOI] [PubMed] [Google Scholar]
- 76.Patki, A. H., D. L. Georges, and M. M. Lederman. 1997. CD4+-T-cell counts, spontaneous apoptosis, and Fas expression in peripheral blood mononuclear cells obtained from human immunodeficiency virus type 1-infected subjects. Clin. Diagn. Lab. Immunol. 4:736-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patterson, S. 2000. Flexibility and cooperation among dendritic cells. Nat. Immunol. 1:273-274. [DOI] [PubMed] [Google Scholar]
- 78.Penna, G., M. Vulcano, A. Roncari, F. Facchetti, S. Sozzani, and L. Adorini. 2002. Cutting edge: differential chemokine production by myeloid and plasmacytoid dendritic cells. J. Immunol. 169:6673-6676. [DOI] [PubMed] [Google Scholar]
- 79.Penna, G., M. Vulcano, S. Sozzani, and L. Adorini. 2002. Differential migration behavior and chemokine production by myeloid and plasmacytoid dendritic cells. Hum. Immunol. 63:1164-1171. [DOI] [PubMed] [Google Scholar]
- 80.Petrovas, C., Y. M. Mueller, I. D. Dimitriou, P. M. Bojczuk, K. C. Mounzer, J. Witek, J. D. Altman, and P. D. Katsikis. 2004. HIV-specific CD8+ T cells exhibit markedly reduced levels of Bcl-2 and Bcl-xL. J. Immunol. 172:4444-4453. [DOI] [PubMed] [Google Scholar]
- 81.Pinzon-Charry, A., T. Maxwell, M. A. McGuckin, C. Schmidt, C. Furnival, and J. A. Lopez. 2006. Spontaneous apoptosis of blood dendritic cells in patients with breast cancer. Breast Cancer Res. 8:R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Quaranta, M. G., B. Mattioli, L. Giordani, and M. Viora. 2004. HIV-1 Nef equips dendritic cells to reduce survival and function of CD8+ T cells: a mechanism of immune evasion. FASEB J. 18:1459-1461. [DOI] [PubMed] [Google Scholar]
- 83.Re, M., D. Gibellini, R. Aschbacher, M. Vignoli, G. Furlini, E. Ramazzotti, L. Bertolaso, and M. La Placa. 1998. High levels of HIV-1 replication show a clear correlation with downmodulation of Bcl-2 protein in peripheral blood lymphocytes of HIV-1-seropositive subjects. J. Med. Virol. 56:66-73. [DOI] [PubMed] [Google Scholar]
- 84.Schmidt, B., I. Scott, R. G. Whitmore, H. Foster, S. Fujimura, J. Schmitz, and J. A. Levy. 2004. Low-level HIV infection of plasmacytoid dendritic cells: onset of cytopathic effects and cell death after PDC maturation. Virology 329:280-288. [DOI] [PubMed] [Google Scholar]
- 85.Sharma, K., R. X. Wang, L. Y. Zhang, D. L. Yin, X. Y. Luo, J. C. Solomon, R. F. Jiang, K. Markos, W. Davidson, D. W. Scott, and Y. F. Shi. 2000. Death the Fas way: regulation and pathophysiology of CD95 and its ligand. Pharmacol. Ther. 88:333-347. [DOI] [PubMed] [Google Scholar]
- 86.Shedlock, D. J., D. Hwang, A. Y. Choo, C. W. Chung, K. Muthumani, and D. B. Weiner. 2008. HIV-1 viral genes and mitochondrial apoptosis. Apoptosis 13:1088-1099. [DOI] [PubMed] [Google Scholar]
- 87.Siegal, F. P., N. Kadowaki, M. Shodell, P. A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y. J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science 284:1835-1837. [DOI] [PubMed] [Google Scholar]
- 88.Soumelis, V., I. Scott, F. Gheyas, D. Bouhour, G. Cozon, L. Cotte, L. Huang, J. A. Levy, and Y. J. Liu. 2001. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood 98:906-912. [DOI] [PubMed] [Google Scholar]
- 89.Stary, G., I. Klein, S. Kohlhofer, F. Koszik, T. Scherzer, L. Mullauer, H. Quendler, N. Kohrgruber, and G. Stingl. 2009. Plasmacytoid dendritic cells express TRAIL and induce CD4+ T-cell apoptosis in HIV-1 viremic patients. Blood 114:3854-3863. [DOI] [PubMed] [Google Scholar]
- 90.Steensma, D. P., M. Timm, and T. E. Witzig. 2003. Flow cytometric methods for detection and quantification of apoptosis. Methods Mol. Med. 85:323-332. [DOI] [PubMed] [Google Scholar]
- 91.Thomson, A. W., and P. D. Robbins. 2008. Tolerogenic dendritic cells for autoimmune disease and transplantation. Ann. Rheum. Dis. 67(Suppl. 3):iii90-iii96. [DOI] [PubMed] [Google Scholar]
- 92.Turley, S. J. 2002. Dendritic cells: inciting and inhibiting autoimmunity. Curr. Opin. Immunol. 14:765-770. [DOI] [PubMed] [Google Scholar]
- 93.Uccelli, M. C., C. Torti, E. Quiros-Roldan, C. Tinelli, A. Patroni, F. Castelli, G. Carosi, and P. Airo. 2003. Bcl-2 expression is moderately correlated with long-term variability of CD4 T-cell increase under successful highly active antiretroviral therapy. AIDS 17:141-143. [DOI] [PubMed] [Google Scholar]
- 94.Vieira, P. L., E. C. de Jong, E. A. Wierenga, M. L. Kapsenberg, and P. Kalinski. 2000. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J. Immunol. 164:4507-4512. [DOI] [PubMed] [Google Scholar]
- 95.Wakim, L. M., J. Waithman, N. van Rooijen, W. R. Heath, and F. R. Carbone. 2008. Dendritic cell-induced memory T-cell activation in nonlymphoid tissues. Science 319:198-202. [DOI] [PubMed] [Google Scholar]
- 96.Wasmuth, J. C., K. H. Klein, F. Hackbarth, J. K. Rockstroh, T. Sauerbruch, and U. Spengler. 2000. Prediction of imminent complications in HIV-1-infected patients by markers of lymphocyte apoptosis. J. Acquir. Immune Defic. Syndr. 23:44-51. [DOI] [PubMed] [Google Scholar]
- 97.Wijewardana, V., K. N. Brown, X. Liu, and S. M. Barratt-Boyes. 2009. Caspase-mediated apoptosis of myeloid dendritic cells during acute simian immunodeficiency virus infection. J. Immunol. 182:81.15. [Google Scholar]
- 98.Yue, F. Y., C. M. Kovacs, R. C. Dimayuga, X. X. Gu, P. Parks, R. Kaul, and M. A. Ostrowski. 2005. Preferential apoptosis of HIV-1-specific CD4+ T cells. J. Immunol. 174:2196-2204. [DOI] [PubMed] [Google Scholar]
- 99.Zammit, D. J., L. S. Cauley, Q. M. Pham, and L. Lefrancois. 2005. Dendritic cells maximize the memory CD8 T-cell response to infection. Immunity 22:561-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zaunders, J. J., L. Moutouh-de Parseval, S. Kitada, J. C. Reed, S. Rought, D. Genini, L. Leoni, A. Kelleher, D. A. Cooper, D. E. Smith, P. Grey, J. Estaquier, S. Little, D. D. Richman, and J. Corbeil. 2003. Polyclonal proliferation and apoptosis of CCR5+ T lymphocytes during primary human immunodeficiency virus type 1 infection: regulation by interleukin (IL)-2, IL-15, and Bcl-2. J. Infect. Dis. 187:1735-1747. [DOI] [PubMed] [Google Scholar]