The theoretical upper limit for the operational efficiency of plant photosynthesis has been estimated from a detailed stepwise analysis of the biophysical and biochemical subprocesses to be about 4.6% for C3 and 6.0% C4 plants (Zhu et al., 2008, 2010). (These estimates assume a leaf temperature of 30°C and an atmospheric [CO2] of 387 ppm and were calculated relative to the full solar spectrum at the earth’s surface. These efficiencies would be slightly more than double if calculated relative to only the photosynthetically active radiation [i.e. 400–700 nm; Zhu et al., 2008].) While estimates of the theoretical upper limit of photosynthetic efficiency in microalgae have not been conducted as systematically, the same considerations apply as for plants, although microalgae may have a lower respiratory rate that would raise the upper limit for the efficiency of net photosynthesis accordingly (Melis, 2009). Microalgae expressing bicarbonate transporters will mimic the higher efficiency of C4 plants due to suppression of photorespiration, while those that do not actively concentrate CO2 would have upper efficiency limits similar to C3 plants. The highest short-term efficiencies observed for plants in the field, assessed from maximum growth rates, are about 3.5% for C3 and 4.3% for C4 plants, and these drop further to 2.4% and 3.4% when calculated over a full growing season (Monteith, 1977; Piedade et al., 1991; Beale and Long, 1995). For crop plants, these highest full growing season efficiencies are those that define the yield potential (Fischer and Edmeades, 2010) or record yield of the crop and are at least twice greater than photosynthetic efficiencies observed under most commercial farming conditions.
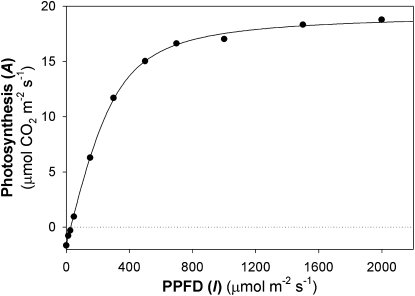
The primary reason why the highest observed photosynthetic efficiencies are 30% or more lower than theoretical efficiencies is light saturation of photosynthesis. Photosynthesis responds nonlinearly to increases in insolation. For example, C3 leaf photosynthesis is saturated by approximately 25% of maximum full sunlight, and light intercepted above this amount will lower photosynthetic efficiency in proportion to the excess light absorbed (Fig. 1). In plant canopies or microalgae mass cultures, this leads to a situation in which photosynthetic tissue directly exposed to bright sunlight is saturated and wastefully dissipates energy, while photosynthesis in shaded leaves or shaded microalgal cells in the culture interior are light limited. High radiation also engages photoprotective mechanisms (Li et al., 2009), which in dynamic light environments can further lower efficiency (Zhu et al., 2004a) or result in photodamage from which repair is required to restore efficiency (Melis, 1999). Greater light penetration beyond the top layers of photosynthetic tissue mitigates efficiency losses associated with light saturation. For example, canopy structure in which the upper leaves are vertically oriented enhances light distribution within the canopy and thereby improves the efficiency of photosynthetic carbon gain (Long et al., 2006), a strategy that has contributed to enhanced yield of super hybrid rice (Oryza sativa). Lowering the chlorophyll (Chl) content of photosynthetic tissue is another and potentially more robust strategy to improve light penetration into plant canopies and algal mass cultures. But hasn’t evolution already optimized Chl content in plants and microalgae? No doubt it has for competitive natural habitats where a large assembly of antenna pigments confers a selective advantage. That is, even when photosynthesis is already saturated, intercepting more light deprives a potential competitor that may have otherwise received the light. However, evolution has not optimized Chl content for achieving maximum carbon gain in dense crop monocultures or microalgal mass cultures where competition for resources including light is not a benefit (Anten, 2005). Here, we discuss the theory and physiological factors that need to be considered in optimizing Chl content for improved photosynthetic efficiency and maximum carbon gain by crops and microalgae.
Figure 1.
The light intensity dependence of net photosynthesis of a chamber-grown tobacco leaf. The measurement temperature was 25°C and the [CO2] in the measurement chamber (LI-COR 6400-40) was 400 ppm. The data are fit to Equation 1, for which ΦCO2 = 0.059, Asat = 21.03 μmol CO2 m−2 s−1, θ = 0.79, and R = 1.65 μmol CO2 m−2 s−1.
LIGHT DEPENDENCE OF PHOTOSYNTHETIC CO2 UPTAKE RATES—THEORY
The light response of the photosynthetic CO2 uptake rate by a suspension of microalgae or a leaf per unit area (A) is well described by a nonrectangular hyperbola:
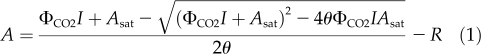 |
where ΦCO2 is the maximal quantum efficiency of photosynthetic CO2 fixation, θ is the convexity of the hyperbola, Asat is the light-saturated rate of photosynthetic CO2 uptake rate, I is the photosynthetic photon flux density (PPFD), and R is the rate CO2 efflux from mitochondrial respiration. As Figure 1 depicts for a tobacco (Nicotiana tabacum) leaf, initially A increases nearly linearly with I and after an inflection A approaches a plateau. The initial slope of A versus PPFD represents the quantum yield of CO2 uptake, i.e. the fractional number of CO2 molecules that can be fixed with absorption of one photon.
The attenuation of PPFD with depth in a uniform suspension of microalgae can be inferred to a first approximation from the Beer-Lambert law:
where a is the absorbance of light from the surface to the particular depth; e is the molar absorbtivity of algal pigments with units of L mol−1 cm−1; b is the path length of the sample, in this case, the depth into the microalgae suspension from the surface (cm); and c is the concentration of the microalgae pigments in each depth layer (mol L−1). The absorbance at the ith depth (Ii) is calculated based on the difference of a between the ith depth and the (i − 1)th depth:
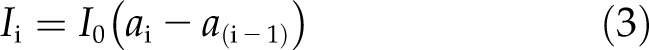 |
where ai represents the absorbance by the ith layer and above and a(i − 1) represents the absorbance by the (i − 1)th layer and above. I0 is the incident light level above the suspension. The daily integral gross CO2 uptake of the microalgae suspension (Ata) is then calculated as:
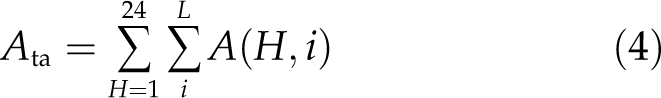 |
where A(H, i) is the net CO2 uptake for all algae cells at the ith depth at the Hth hour, calculated based on the light response curve of Equation 1 and a, and L is the total number of depth layers of the microalgae suspension.
In a crop canopy, gross photosynthesis (Ac) is the integral of photosynthetic CO2 uptake rate of all leaves in the canopy, as shown in Equation 5.
 |
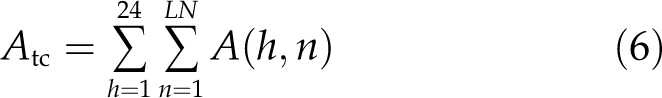 |
Total canopy CO2 uptake rate (Atc) is correspondingly the integral of the leaf photosynthetic CO2 uptake for all the leaves over the course of an entire day (Eq. 6).
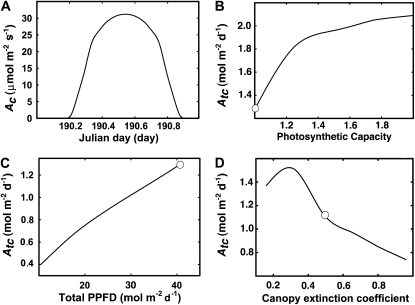
In this equation, h represents time, while A(n) represents the photosynthetic CO2 uptake rate for leaf number n. Because it is experimentally challenging to measure all the relevant microclimatic parameters within a canopy, in practice numerous simplified models have been developed to simulate microclimatic conditions inside canopies to estimate canopy photosynthetic CO2 uptake rates. These models include the big-leaf models, the sun-shade models, and multilayer models (Farquhar et al., 1980; Forseth and Norman, 1991; Humphries and Long, 1995). The big-leaf model assumes that the entire canopy can be effectively represented by a single leaf of specified leaf area index (LAI) and photosynthetic properties. The sunlit-shade model treats all leaves of a canopy as two dynamic populations, i.e. those that are directly sun exposed and those that are shaded. Following the formulations of Forseth and Norman (1991), the total canopy photosynthetic CO2 uptake rate is computed as the integral of the photosynthetic CO2 uptake rates of these two dynamic leaf populations. The multilayer model further paramaterizes the canopy by dividing it into multiple layers of leaves, each with different photosynthetic properties and microclimatic conditions. Using the sunlit-shaded model as implemented in the WIMOVAC (for Windows Intuitive MOdel of Vegetation response to Atmosphere and Climate change) model (Humphries and Long, 1995), we have simulated the response of Ac and Atc to changes in light levels, photosynthetic properties, and canopy extinction coefficient (Fig. 2). The detailed equations used to predict the light environments inside the canopy in WIMOVAC are the same as in Zhu et al. (2004b) and for convenience are provided here in Supplemental Appendices S1 and S2. Over the course of a day, instantaneous Ac is described by a bell-shape curve that follows the diurnal changes in light level incident on the top of the canopy (Fig. 2A). The inability of the upper layer leaves in a canopy to utilize all the available light energy can be directly demonstrated by the increase of Atc upon gradual increase of both Vcmax and Jmax at the top of the canopy, i.e. in the sunlit leaves (Fig. 2B). We simulated the total available light and also Atc for different days of a year, illustrating the relationship between the total available light and Atc (Fig. 2C).
Figure 2.
Simulations of the diurnal photosynthesis. Section A simulates a typical diurnal profile of the canopy photosynthetic CO2 uptake rate (Ac) for day of year 190 at 52°N latitude for a LAI of 3. The changes of the photon flux density were achieved in WIMOVAC by changing the day of year used in the simulation. The modeled responses of the total daily integral gross canopy photosynthetic CO2 uptake rate (Atc) are shown for changes in the photosynthetic capacities (i.e. Vcmx and Jmax relative to the default value; B), total PPFD (C), and canopy extinction coefficient (D). For A to C, the default LAI is 3, canopy extinction coefficient is 0.5, and total leaf reflectance and transmittance is 0.2 (Humphries and Long, 1995). For D, the assumed Chl concentration is 0.5 mmol Chl m−2. The default values in B to D are shown by white circles. The canopy extinction coefficient was assumed to change proportionally with the changes in the leaf Chl concentration. We used the relationship between Chl concentration and the initial slope of the light response curve Φ from Hikosaka and Terashima (1995). The relationship between Chl concentration and leaf absorbance follows Evans (1993). In all these simulations, the light environments were predicted using WIMOVAC as done by Zhu et al. (2004b). In B and C, the daily total photosynthesis was calculated following Zhu et al. (2004b). In D, the daily total canopy photosynthesis was calculated based on Equations 1, 5, 6, and the predicted light environments using WIMOVC (with Amax 25 μmol m−2 s−1 and θ as 0.7). The detailed equations used in these WIMOVAC simulations are reproduced here in Supplemental Appendices S1 and S2.
We further simulated decreases in leaf Chl content by decreased canopy extinction coefficient, which inherently incorporated higher transmittance through the canopy. With the decrease in Chl content, there is a concurrent decrease in the initial slope of the light response curve (Hikosaka and Terashima, 1995). Assuming a Chl content of 0.5 mmol m−2 (Evans, 1989), the initial slope of the light response curve was estimated based an empirical equation (Hikosaka and Terashima, 1995). We further assumed that with decreased Chl content, there was a proportional decrease in the canopy extinction coefficient and a proportional increase in the leaf transmittance and reflectance. This assumed proportional changes between leaf Chl content and canopy extinction coefficient needs to be improved since the leaf absorbance and leaf Chl concentration has a strong nonlinear relationship (Evans, 1993). A more mechanistic model of canopy light environment is needed to better define the relationship between leaf Chl concentration and canopy extinction coefficient. The simulation results suggested that with increase in canopy extinction coefficient, i.e. increase in the leaf Chl concentration, the Atc first increases and then gradually decreases (Fig. 2D). This gradual increase of Atc with decrease of leaf Chl content arises from the fact that leaves at the top of canopies frequently receive more solar energy over the course of a day than they can efficiently utilize, while at the same time leaves in the lower layers of the canopy are light limited. As expected, when the Chl content is decreased below a certain threshold, then further decrease in leaf Chl content results in a decrease in Atc. In principle, for a given LAI the canopy extinction coefficient could be manipulated and optimized for maximum Atc by altering the leaf Chl content. However, to date, no systematic manipulation of Chl concentration to optimize Atc has been undertaken in crop plants, possibly because low Chl mutations frequently carry agronomically undesirable pleiotropic effects (e.g. Pettigrew et al., 1989) and because Chl accumulation in Chl-deficient mutants is frequently dependent on developmental conditions and/or stage of growth (e.g. Droppa et al., 1988).
The optimal Chl concentration for maximal Atc will depend on several factors. First, the optimal photosynthetic pigment concentration is influenced by the spatial and temporal heterogeneities of light inside a canopy (Zhu et al., 2004b) and correspondingly by the canopy architecture. For example, for a given LAI the Atc of canopies with more erect leaf orientation would be expected to benefit less with a given Chl reduction than canopies with horizontal leaf orientation. Similarly, due to differing solar angle, canopies at different latitudes will experience different ambient light levels and the optimal Chl concentrations would therefore be expected to differ with latitude. Different LAI will also influence the optimal leaf Chl content for maximal Atc. Second, as transmittance of photosynthetically active radiation is increased, there will be an increase in amount of incident light that is reflected, potentially lowering the total PPFD absorbed by the canopy. In our simulation, we assumed that reflectance increased by the same amount that transmittance increased (Knipling, 1970). Had the reabsorption of a portion of the reflected light been included in our simulation, it would have had the effect of further reducing the Chl threshold at which reducing Chl will benefit Atc. Third, the optimal Chl concentration will also depend on the rate of canopy development. The rate of canopy development determines how quickly self-shading develops and therefore how quickly into the growing season that reduced Chl levels become an asset versus a detriment to maximizing Atc. Additionally, leaves at different layers within a canopy often acquire different physiological and photosynthetic properties, e.g. leaves adapted to shade environments showed increased light-harvesting complex compared to leaves adapted to high light (Evans, 1989; Hikosaka and Terashima, 1995) and leaves of different crops can have different physiological properties, such as longevity (Wright et al., 2004) and leaf optical properties (e.g. reflectance), all of which will influence the optimal Chl concentration. Fourth, the optimal Chl distribution may also depend on a number of other environmental factors that will influence the threshold between light limitation and light saturation. For example, many abiotic stresses lower photosynthetic capacity and therefore the irradiance at which photosynthesis becomes saturated (e.g. Ortiz-Lopez et al., 1990).
PROOF OF CONCEPT IN MICROALGAE MASS CULTURES
Current interest in the potential application of microalgae as feedstock for synthetic chemistry and biofuels (Dismukes et al., 2008; Melis, 2009; Sun et al., 2009; Radakovits et al., 2010; Stephens et al., 2010) has lead to significant public and private investment in improving the efficiency of mass culture technology and the photosynthetic efficiency of dense microalgal cultures for which the wasteful dissipation of light energy by cells on the surface is a dominating issue. Cyanobacteria (e.g. Synechocystis, Synechococcus, and Arthrospira spp.) and red algae (e.g. Porphyridium spp.) are especially subject to surface light saturation and attendant shading of cells lower in the water column as, in addition to their Chl pigment beds, these algae possess phycobilisomes containing hundreds of light-harvesting bilin antenna pigments. Green microalgae (e.g. Chlamydomonas, Dunaliella, and Haematococcus spp.), and diatoms (Cylindrotheca and Nitzschia spp.), as well as brown algae (Macrocystis pyrifera), have smaller Chl antenna sizes that are comparable to those in C3 plants (Melis, 1991).
Reducing the size of the light-harvesting pigment arrays in the photosynthetic apparatus has proven to be an effective strategy to mitigate photosynthetic inefficiency associated with the overabsorption of sunlight at the surface of microalgae mass cultures (Melis, 1999; Polle et al., 2003). Microalgae with truncated light-harvesting Chl antenna (abbreviated as Tla) have been shown to improve solar-to-biomass conversion efficiencies in mass cultures (Nakajima and Ueda, 1997, 1999; Mitra and Melis, 2008; Beckmann et al., 2009; Melis, 2009). Specifically, Tla microalgae required a higher light intensity for the saturation of photosynthesis and in mass culture showed overall greater productivity on a per Chl basis. Within the structural constrains permitted for Chl antenna size (Melis, 2009), productivity was found to be inversely proportional to the photosystem antenna size (Polle et al., 2003). It was shown that Tla strains substantially reduced overabsorption and the consequent wasteful dissipation of excess incident sunlight, reduced photodamage and photoinhibition of photosynthesis, and minimized mutual cell shading that lowers productivity in cells deeper in the culture water column (Mitra and Melis, 2008).
A number of important criteria must be met to ensure that truncated antenna mutants are not photosynthetically handicapped in ways other than reduced Chl antenna size. To ensure that Tla strains will operate with improved solar energy conversion efficiency, the following analytical tests are useful: (1) the functional PSII and PSI Chl antenna size should be determined to assess the extent of reduction in antenna size, (2) the titer of functional PSII and PSI reaction centers per cell should be determined to ensure that the density of the photosynthetic electron-transport chains remains unaffected, (3) the quantum yield of photosynthesis must remain high to ensure that the efficiency of photosynthesis under limiting light remains unchanged, and (4) the light-saturated rate of photosynthesis should be inversely proportional to the measured Chl antenna size. With these criteria met, Tla strains will very likely operate with improved solar energy conversion efficiency and productivity under mass culture and bright sunlight conditions. The utility of this verification approach was demonstrated in the screening of 6,500 Chlamydomonas reinhardtii transformants from a DNA insertional mutagenesis library that initially resulted in the isolation of 129 putative Tla transformants. From this screen, only a single strain, tla1 (Polle et al., 2003), proved to be a truly useful truncated antenna mutant.
FINE-TUNING ANTENNA SIZE OF CROP CANOPIES
Under clear skies, the daily integral of photosynthesis of dense crop canopies is limited by the amount of light that reaches the lower leaves. The optimum situation for a crop monoculture would seemingly be to have a minimum antenna size in upper canopy leaves with progressively larger antenna as light intensity decreases within the canopy. In theory, this should lead to increased photosynthetic efficiencies of crop canopies, but there are counteracting considerations. For example, early in the growing season, reduced antenna size would prolong the period in which canopy density is insufficient to absorb all of the photosynthetically active radiation. While incomplete interception of light would not reduce the efficiency of canopy photosynthesis, it could reduce early season canopy carbon gain compared to a canopy of normally green leaves due to lower overall absorption of the available photosynthetically active radiation.
The hypothesis that a constitutive smaller antenna size will improve canopy photosynthetic efficiency and season long carbon gain has yet to be rigorously tested in crops, but there are promising indications that this may be so. Mutants of soybean (Glycine max) cultivars Clark Y9 and Y11 contain about one-half the Chl of the wild type. These mutant isolines are Chl b deficient, and in consequence the antenna size of PSII is reduced from approximately 250 Chl in the wild type to approximately 150 and for PSI from approximately 210 Chl to approximately 160 in the mutants. Furthermore, these Chl b-deficient mutant isolines compensated for a disproportionately large decrease in the PSII absorption cross section by increasing the PSII/PSI ratio by approximately 40% (Ghirardi and Melis, 1988). Yet, even with these ad hoc alterations to the photosynthetic apparatus, mature canopies of these low Chl soybean mutants are capable of a substantially higher daily integral of photosynthesis than wild type. More sunlight penetrating to the lower leaves in these Chl-depleted canopies increased the amount of leaf area positively contributing to canopy, resulting in greater than 30% increases in the daily integral of photosynthesis in some cases (Pettigrew et al., 1989). A secondary potential benefit is that increased light penetration could alter the canopy temperature regime with cooler temperatures in the upper portion of the canopies and warmer temperatures near the soil; if so, lowering temperature in warmest part of the canopy would be predicted to militate for improved yield (Ainsworth and Ort, 2010).
The way in which the antenna is reduced may also be important to determining the extent to which photosynthetic efficiency is improved. In particular, decreasing the antenna size by dramatically reducing Chl b synthesis, as is the case for the Clark Y9 and Y11 soybean mutants, would imbalance the antenna size of PSI and PSII, resulting in the increase in PSII/PSI ratio mentioned above. In addition, since Chl b binding is required to stabilize the accessory pigment proteins (Flachmann and Kühlbrandt, 1995), these mutations might be expected to create a respiratory drag due to continuous synthesis and degradation of pigment proteins. Finally, the Chl b-containing light-harvesting pigment proteins are critical to excitation energy transfer among PSII centers (Melis and Homann, 1976), whose absence would comprise efficiency as the likelihood of excitation into a closed PSII center increases at higher irradiance levels. These potential nonbenefical alterations to the photosynthetic apparatus along with other pleiotropic effects of the mutations (e.g. changes in time to maturity; Pettigrew et al., 1989) may underlie the variable yield response of these Chl-deficient soybean mutants, which in some years showed yield increases (Pettigrew et al., 1989) and other years not (Hesketh et al., 1981). Down-regulating Chl synthesis early in the pathway might avoid some of these issues and be a better option for optimizing antenna size. For example, suppressing the rate of 5-aminolevulinate synthesis, the first committed step in tetrapyrrole biosynthesis, can drastically reduce leaf Chl content (e.g. Härtel et al., 1997; Goslings et al., 2004), and at least in the case of reduced Glu 1-semialdehyde aminotransferase activity, the titer of PSI and PSII units decreased but without a change in antenna size (Härtel et al., 1997). Intervening further upstream in the pathway(s) that regulates light-harvesting antenna assembly (e.g. Huq et al., 2004; Waters et al., 2009) has the potential to coordinately down-regulate the entire process and thereby avoid undesirable imbalances in energy distribution discussed above. For example, changes in antenna size in C. reinhardtii due to a mutation affecting the expression of the TLA1 gene down-regulates LHC protein expression in tandem with the reduction in Chl synthesis (Mitra and Melis, 2010), thereby avoiding the respiratory penalty associated with excessive LHC turnover.
PERSPECTIVES
For the purposes of agriculture and the commercial application of microalgae mass culture, implementing truncated antenna to mitigate surface saturation of photosynthesis and improve light penetration is among the most promising strategies to improve photosynthetic efficiency in the near term. Nevertheless, given the complexity of factors influencing the determination of the optimal Chl concentration for maximizing Atc, particularly for crop canopies, a primarily empirical approach to determining the optimum antenna size is impractical. The development of more sophisticated models than we have employed here will need to play a central role in dealing with the complexity and optimizing Chl content for different crops with differing canopy architectures, differing planting dates, and at different geographical locations.
Use of Tla genetically modified organisms, whether plants or algae, inevitably would raise issues about release and the potential environmental impact of these organisms. Experience in the field with crop plants, and in the laboratory with microalgae, has consistently demonstrated that, when left on their own, Tla strains cannot effectively compete with their wild-type counterparts. Accordingly, Tla isolines, when cultivated under controlled and protective conditions, can afford substantial productivity improvements under high-density conditions, with little environmental risk of escape. But these facts also represent a limitation of the truncated antenna approach, i.e. organisms with truncated antenna would unavoidably be less competitive than their full antenna counterparts. For crop plants this means mostly reduced competitiveness against weeds. Current advanced weed control may have to be intensified for truncated antenna crops particularly with the prospect of herbicide resistance. For microalgal mass cultures, competition is a more serious issue and may limit the use of truncated antenna organisms to closed bioreactors to prevent airborne contamination by full antenna organisms.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Appendix S1. Equations used to simulate leaf and canopy net photosynthetic carbon uptake.
Supplemental Appendix S2. Definition of symbols.
Supplementary Material
Acknowledgments
We thank Dr. John Evans and two anonymous referees for their valuable input into this article. We also thank Dr. David Rosenthal for the data presented in Figure 1 and Dr. Aleel Grennan for her assistance with the manuscript.
References
- Ainsworth EA, Ort DR. (2010) How do we improve crop production in a warming world? Plant Physiol 154: 526–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anten NPR. (2005) Optimal photosynthetic characteristics of individual plants in vegetation stands and implications for species coexistence. Ann Bot (Lond) 95: 495–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale CV, Long SP. (1995) Can perennial C4 grasses attain high efficiencies of radiant energy-conversion in cool climate. Agric For Meteorol 96: 103–115 [Google Scholar]
- Beckmann K, Messinger J, Badger MR, Wydrzynski T, Hillier W. (2009) On-line mass spectrometry: membrane inlet sampling. Photosynth Res 102: 511–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dismukes GC, Carrieri D, Bennette N, Ananyev GM, Posewitz MC. (2008) Aquatic phototrophs: efficient alternatives to land-based crops for biofuels. Curr Opin Biotechnol 19: 235–240 [DOI] [PubMed] [Google Scholar]
- Droppa M, Ghirardi ML, Horvath G, Melis A. (1988) Chlorophyll b deficiency in soybean mutants. II. Thylakoid membrane development and differentiation. Biochim Biophys Acta 92: 138–145 [Google Scholar]
- Evans JR. (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78: 9–19 [DOI] [PubMed] [Google Scholar]
- Evans JR. (1993) Photosynthetic acclimation of nitrogen partitioning within a lucerne canopy. II. Stability through time and comparison with a theoretical optimum. Aust J Plant Physiol 20: 69–82 [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149: 78–90 [DOI] [PubMed] [Google Scholar]
- Fischer RA, Edmeades GO. (2010) Breeding and cereal yield progress. Crop Sci 50: S85–S98 [Google Scholar]
- Flachmann R, Kühlbrandt W. (1995) Accumulation of plant antenna complexes is regulated by post-transcriptional mechanisms in tobacco. Plant Cell 7: 149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forseth IN, Norman JM. (1991) Modelling of solar irradiance, leaf energy budget, and canopy photosynthesis. Hall DO, Scurlock JMO, Bolhar-Nordenkampf HR, Leegood RC, Long SP, , Techniques in Photosynthesis and Productivity Research for a Changing Environment. Chapman and Hall, London, pp 207–219 [Google Scholar]
- Ghirardi ML, Melis A. (1988) Chlorophyll b deficiency in soybean mutants. I. Effects on the photosystem stoichiometry and chlorophyll antenna size. Biochim Biophys Acta 92: 130–137 [Google Scholar]
- Goslings D, Meskauskiene R, Kim C, Lee KP, Nater M, Apel K. (2004) Concurrent interactions of heme and FLU with Glu tRNA reductase (HEMA1), the target of metabolic feedback inhibition of tetrapyrrole biosynthesis, in dark- and light-grown Arabidopsis plants. Plant J 40: 957–967 [DOI] [PubMed] [Google Scholar]
- Härtel H, Kruse E, Grimm B. (1997) Restriction of chlorophyll synthesis due to expression of glutamate 1-semialdehyde aminotransferase antisense RNA does not reduce the light-harvesting antenna size in tobacco. Plant Physiol 113: 1113–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh JD, Ogren WL, Hageman ME, Peters DB. (1981) Correlations among leaf CO2-exchange rates, areas and enzyme activities among soybean cultivars. Photosynth Res 2: 21–30 [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Terashima I. (1995) A model of the acclimation of photosynthesis in the leaves of C-3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ 18: 605–618 [Google Scholar]
- Humphries SW, Long SP. (1995) WIMOVAC: a software package for modelling the dynamics of plant leaf and canopy photosynthesis. Comput Appl Biosci 11: 361–371 [DOI] [PubMed] [Google Scholar]
- Huq E, Al-Sady B, Hudson M, Kim C, Apel K, Quail PH. (2004) Phytochrome-interacting factor 1 is a critical bHLH regulator of chlorophyll biosynthesis. Science 305: 1937–1941 [DOI] [PubMed] [Google Scholar]
- Knipling EB. (1970) Physical and physiological basis for the reflectance of visible and near infrared radiation from vegetation. Remote Sens Environ 1: 155–159 [Google Scholar]
- Li Z, Wakao S, Fischer BB, Niyogi KK. (2009) Sensing and responding to excess light. Annu Rev Plant Biol 60: 239–260 [DOI] [PubMed] [Google Scholar]
- Long SP, Zhu XG, Naidu SL, Ort DR. (2006) Can improvement in photosynthesis increase crop yields? Plant Cell Environ 29: 315–330 [DOI] [PubMed] [Google Scholar]
- Melis A. (1991) Dynamics of photosynthetic membrane composition and function. Biochim Biophys Acta 1058: 87–106 [Google Scholar]
- Melis A. (1999) Photosystem-II damage and repair cycle in chloroplasts: what modulates the rate of photodamage? Trends Plant Sci 4: 130–135 [DOI] [PubMed] [Google Scholar]
- Melis A. (2009) Solar energy conversion efficiencies in photosynthesis: minimizing the chlorophyll antennae to maximize efficiency. Plant Sci 177: 272–280 [Google Scholar]
- Melis A, Homann PH. (1976) Heterogeneity of the photochemical centers in system II of chloroplasts. Photochem Photobiol 23: 343–350 [DOI] [PubMed] [Google Scholar]
- Mitra M, Melis A. (2008) Optical properties of microalgae for enhanced biofuels production. Opt Express 16: 21807–21820 [DOI] [PubMed] [Google Scholar]
- Mitra M, Melis A. (2010) Genetic and biochemical analysis of the TLA1 gene in Chlamydomonas reinhardtii. Planta 231: 729–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteith JL. (1977) Climate and the efficiency of crop production in Britain. Philos Trans R Soc Lond B Biol Sci 281: 277–294 [Google Scholar]
- Nakajima Y, Ueda R. (1997) Improvement of photosynthesis in dense microalgal suspension by reduction of light harvesting pigments. J Appl Phycol 9: 503–510 [Google Scholar]
- Nakajima Y, Ueda R. (1999) Improvement of microalgal photosynthetic productivity by reducing the content of light harvesting pigment. J Appl Phycol 11: 195–201 [Google Scholar]
- Ortiz-Lopez A, Nie GY, Ort DR, Baker NE. (1990) The involvement of the photoinhibition of photosystem II and impaired membrane energization in the reduced quantum yield of carbon assimilation in chilled maize. Planta 181: 78–84 [DOI] [PubMed] [Google Scholar]
- Pettigrew WT, Hesketh JD, Peters DB, Woolley JT. (1989) Characterization of canopy photosynthesis of chlorophyll-deficient soybean isolines. Crop Sci 29: 1025–1029 [Google Scholar]
- Piedade MTF, Junk WJ, Long SP. (1991) The productivity of the C4 grass Echinochloa polystachya on the Amazon floodplain. Ecology 72: 1456–1463 [Google Scholar]
- Polle JEW, Kanakagiri SD, Melis A. (2003) tla1, a DNA insertional transformant of the green alga Chlamydomonas reinhardtii with a truncated light-harvesting chlorophyll antenna size. Planta 217: 49–59 [DOI] [PubMed] [Google Scholar]
- Radakovits R, Jinkerson RE, Darzins A, Posewitz MC. (2010) Genetic engineering of algae for enhanced biofuel production. Eukaryot Cell 9: 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens E, Ross IL, King Z, Mussgnug JH, Kruse O, Posten C, Borowitzka MA, Hankamer B. (2010) An economic and technical evaluation of microalgal biofuels. Nat Biotechnol 28: 126–128 [DOI] [PubMed] [Google Scholar]
- Sun JD, Yang LX, Wang YL, Ort DR. (2009) FACE-ing global change: opportunities for improvement in photosynthetic radiation use efficiency and crop yield. Plant Sci 177: 511–522 [Google Scholar]
- Waters MT, Wang P, Korkaric M, Capper RG, Saunders NJ, Langdale JA. (2009) GLK transcription factors coordinate expression of the photosynthetic apparatus in Arabidopsis. Plant Cell 21: 1109–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, et al. (2004) The worldwide leaf economics spectrum. Nature 428: 821–827 [DOI] [PubMed] [Google Scholar]
- Zhu XG, Long SP, Ort DR. (2008) What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr Opin Biotechnol 19: 153–159 [DOI] [PubMed] [Google Scholar]
- Zhu XG, Long SP, Ort DR. (2010) Improving photosynthetic efficiency for greater yield. Annu Rev Plant Biol 61: 235–261 [DOI] [PubMed] [Google Scholar]
- Zhu XG, Ort DR, Whitmarsh J, Long SP. (2004a) The slow reversibility of photosystem II thermal energy dissipation on transfer from high to low light may cause large losses in carbon gain by crop canopies: a theoretical analysis. J Exp Bot 55: 1167–1175 [DOI] [PubMed] [Google Scholar]
- Zhu XG, Portis AR, Jr, Long SP. (2004b) Would transformation of C3 crop plants with foreign Rubisco increase productivity? A computational analysis extrapolating from kinetic properties to canopy photosynthesis. Plant Cell Environ 27: 155–165 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.