Abstract
Diverse brain insults, including traumatic brain injury, stroke, infections, tumors, neurodegenerative diseases, and prolonged acute symptomatic seizures, such as complex febrile seizures or status epilepticus (SE), can induce “epileptogenesis,” a process by which normal brain tissue is transformed into tissue capable of generating spontaneous recurrent seizures. Furthermore, epileptogenesis operates in cryptogenic causes of epilepsy. In view of the accumulating information about cellular and molecular mechanisms of epileptogenesis, it should be possible to intervene in this process before the onset of seizures and thereby either prevent the development of epilepsy in patients at risk or increase the potential for better long-term outcome, which constitutes a major clinical need. For identifying pharmacological interventions that prevent, interrupt or reverse the epileptogenic process in people at risk, two groups of animal models, kindling and SE-induced recurrent seizures, have been recommended as potentially useful tools. Furthermore, genetic rodent models of epileptogenesis are increasingly used in assessing antiepileptogenic treatments. Two approaches have been used in these different model categories: screening of clinically established antiepileptic drugs (AEDs) for antiepileptogenic or disease-modifying potential, and targeting the key causal mechanisms that underlie epileptogenesis. The first approach indicated that among various AEDs, topiramate, levetiracetam, carisbamate, and valproate may be the most promising. On the basis of these experimental findings, two ongoing clinical trials will address the antiepileptogenic potential of topiramate and levetiracetam in patients with traumatic brain injury, hopefully translating laboratory discoveries into successful therapies. The second approach has highlighted neurodegeneration, inflammation and up-regulation of immune responses, and neuronal hyperexcitability as potential targets for antiepileptogenesis or disease modification. This article reviews these areas of progress and discusses the challenges associated with discovery of antiepileptogenic therapies.
I. Introduction
Epilepsy, one of the most common disorders of the brain, is characterized by recurrent, usually unprovoked, epileptic seizures, and by the cognitive, psychosocial, and social consequences of this condition (Chang and Lowenstein, 2003; Engel and Pedley, 2008). Epilepsies can be divided into three major categories on the basis of etiology: idiopathic, symptomatic, and presumed symptomatic (also called “cryptogenic”). Idiopathic epilepsies are generally thought to arise from genetic abnormalities that lead to alteration of basic neuronal regulation. Symptomatic (or acquired) epilepsies arise from the effects of an epileptic lesion, whether that lesion is focal, such as a tumor, or a defect in metabolism causing widespread injury to the brain. Cryptogenic epilepsies involve a presumptive lesion that is otherwise difficult or impossible to uncover during evaluation. In approximately 40% of all epilepsy cases, the etiology is known, including brain insults such as traumatic brain injury (TBI1), ischemic stroke, intracerebral hemorrhage, infections, tumors, cortical dysplasia, several neurodegenerative diseases, and prolonged acute symptomatic seizures such as complex febrile seizures or status epilepticus (SE) (Banerjee et al., 2009). Thus, epilepsy is one of the only brain diseases known to man in which people at risk can be identified, but there is no prophylactic treatment to prevent the development of epilepsy in those at risk (Dichter, 2009a,b).
II. The Concept of Epileptogenesis and Antiepileptogenesis
Almost 130 years ago, Gowers (1881) first recognized that there is often a seizure-free interval lasting months to years between brain insults and the onset of symptomatic epilepsy. The interval between injury and the appearance of clinically obvious seizures suggests that an active, time-consuming process leads to changes that eventually cause epilepsy (Fig. 1). A widely accepted hypothesis holds that during this latent period, which characterizes many (if not all) cases of symptomatic epilepsy, there is a cascade of poorly understood changes that transform the nonepileptic brain into one that generates spontaneous recurrent seizures (Herman, 2002 Löscher, 2002c; Pitkänen, 2002, 2010; Stables et al., 2002; Walker et al., 2002; André et al., 2007; Pitkänen et al., 2007; Dichter, 2009a,b; Jacobs et al., 2009; Pitkänen and Lukasiuk, 2009). This insult-induced process, which is of variable length in different patients and ultimately leads to chronic epilepsy, is called epileptogenesis. In addition to symptomatic or acquired epilepsy, epileptogenesis also operates in cryptogenic causes of epilepsy, which are far more common than the acute symptomatic forms with identifiable disease processes or injuries. Furthermore, the latent period between gene mutations and first onset of spontaneous seizures in idiopathic epilepsies indicates that an epileptogenic process is induced by the mutation, which is substantiated by experimental data suggesting that early pharmacological intervention can prevent or modify the development of genetic epilepsies (see sections III.D and V).
Fig. 1.
Steps in the development and progression of temporal lobe epilepsy and possible therapeutic interventions. The term epileptogenesis includes processes that take place before the first spontaneous seizure occurs to render the epileptic brain susceptible to spontaneous recurrent seizures and processes that intensify seizures and make them more refractory to therapy (progression). It is important to note that the concept of a multistep process of epileptogenesis illustrated in this figure bears similarities to the multistep process of carcinogenesis with initiation (DNA damage), repair of damage or failure to repair, promotion to tumor, and progression to malignancy and metastasis (Löscher and Liburdy, 1998). See section II for further explanation and discussion. [Adapted from Löscher W, Gernert M, and Heinemann U (2008) Cell and gene therapies in epilepsy—promising avenues or blind alleys? Trends Neurosci 31:62–73. Copyright © 2008 Elsevier Science. Used with permission.]
Numerous possible mechanisms underlying this process of epileptogenesis have been suggested (Fig. 1), but no consensus has emerged about which of the observed changes is causal or consequential and how they interact. It is noteworthy that the concept of epileptogenesis illustrated in Fig. 1 bears similarities to the multistep process of carcinogenesis with initiation, repair or promotion, and progression (Löscher and Liburdy, 1998; Löscher, 2002c). It should be noted, however, that the concept of the latent period and epileptogenesis has been criticized (Sloviter, 2008; Dudek, 2009), which will be discussed in section III.C.3.c.
The most common type of localization-related epilepsy induced by brain insults is temporal lobe epilepsy (TLE), which develops on average 7.5 years after the initial insult, with a large variation among individuals (French et al., 1993), indicating that the severity, location, and spatial dimension of the injury, genetic and environmental factors, or a “second hit” during the latent period modify the risk of developing epilepsy (Walker et al., 2002). In this respect, it is important to note that estimating the latent period for development of TLE in patients is only possible for cases in which a symptomatic cause has been identified, but not for the many cryptogenic cases. TLE, the most frequent and medically refractory type of epilepsy in humans, is characterized by simple or complex partial seizures, originating from the medial or lateral temporal lobe (most often the hippocampus, parahippocampal areas, or amygdala) that may evolve to secondarily generalized seizures (Chang and Lowenstein, 2003). In addition to seizures, many patients with TLE suffer from behavioral alterations, such as depression, anxiety, and psychosis, and impairment of learning and memory, which may be consequences of the morphologic and functional alterations in the temporal lobe associated with TLE (Marcangelo and Ovsiew, 2007). Although the causes of TLE are widely varied, hippocampal sclerosis (Ammon's horn sclerosis) is a common pathologic finding. Classic hippocampal sclerosis involves a characteristic pattern of selective neuron loss in the CA1 and CA3 regions and the dentate hilus, whereas the CA2 and dentate granule cell layers of the hippocampal formation are relatively spared. In addition to neuronal damage, gliosis and mossy fiber sprouting, the growth of aberrant collaterals of granule cell axons, are also common and have been implicated in epileptogenesis (Sutula et al., 1992b). Neurodegeneration in TLE may also occur in other (extrahippocampal) temporal lobe structures such as parahippocampal areas (e.g., entorhinal cortex) and amygdala (i.e., anatomically linked limbic structures of the mesiotemporal lobe) (Yilmazer-Hanke et al., 2000). The only common pathologic condition present in all patients with TLEs (even in the absence of any detectable pathologic condition in the hippocampus) is neuron loss in the hilus of the dentate gyrus, which is called endfolium sclerosis (Sloviter, 1994). Dentate hilar neurons are presumed to govern dentate granule cell excitability, so that hilar neuron loss has been suggested as the common pathological denominator and primary network defect underlying development of a hippocampal seizure “focus” (Sloviter, 1994). However, the significance, if any, of neuronal death as the precipitant of epilepeptogenesis remains debated, which will be discussed later in this review.
It is important to note that the brain tries to repair itself after damage (Fig. 1), which may contribute to the fact that only a fraction of patients develop epilepsy after brain insults. It is thus vital to understand which of the molecular and cellular alterations induced by brain insults contribute directly to the development of epilepsy and which are involved in the attempt of the brain to repair the damage and recover lost function (Dichter, 2009b; Jacobs et al., 2009).
The latent period after brain insults may offer a window of opportunity in which an appropriate treatment may stop or modify the epileptogenic process induced by a brain insult (Pitkanen, 2004; Dichter, 2009a; Jensen, 2009). On the basis of this concept, several clinical trials have been carried out to evaluate whether prolonged prophylactic administration of an “antiepileptic” (anticonvulsant, anti-ictal) drug (AED) prevents the development of epilepsy after head trauma. In such clinical trials, administration of conventional AEDs, such as phenytoin, phenobarbital, carbamazepine, or valproate, after TBI has thus far failed to prevent epileptogenesis (Temkin, 2001, 2009). However, AEDs have been developed for symptomatic suppression of seizures and not for prevention of epilepsy or disease-modification. It is likely that antiepileptogenic drugs, if they exist, will have mechanisms of action distinct from traditional AEDs, because the molecular mechanisms underlying epileptogenesis and ictogenesis probably differ (Weaver, 2003). Better understanding the process of epileptogenesis, improved testing treatments that demonstrate antiepileptogenic effects in the laboratory, and performing thorough preclinical and clinical evaluations before attempting definitive trials should greatly improve the chance of identifying ways to prevent or modify epilepsy after brain insults (Temkin, 2009).
The ultimate goal of any prophylactic drug treatment after a brain insult is prevention of spontaneous recurrent seizures (i.e., a true antiepileptogenic effect). However, an alternative goal would be disease modification, a term employed to convey the concept that although a treatment may not prevent the occurrence of a disease, it may nevertheless modify the natural course of the disease. Disease modification after epileptogenic brain insults may affect the development of spontaneous seizures, in that the seizures, if not prevented, are less frequent, less severe, and less resistant to AED treatment, thus improving the patients' quality of life. In addition, the prevention of progression of epilepsy after first diagnosis would be a disease-modifying effect of treatment (Fig. 1). Furthermore, any beneficial effect on the neuronal damage developing after brain insults and the cognitive and behavioral disturbances associated with such damage would be desirable (Fig. 1). In this review, we will discuss the animal models that are commonly used in the search for antiepileptogenic or disease-modifying drugs. Furthermore, the numerous experimental studies that have been performed in this respect are critically reviewed with the aim to identify guiding principles for future translational research.
III. Animal Models for Epileptogenesis
Epileptogenesis can be studied in numerous rodent models of symptomatic epilepsy, including kindling, post-SE models of TLE, TBI, and stroke models, and models of febrile seizures (Walker et al., 2002; Stables et al., 2003; Pitkänen et al., 2007a). Furthermore, a number of genetic rodent models of generalized epilepsy, such as rats with spontaneously occurring absence seizures or the genetically epilepsy prone rat, can be used in this respect (Hosford, 1995; Löscher, 1999). In recent decades, animal models of epileptogenesis have greatly enhanced our understanding of the processes leading to epilepsy and thus of potential targets for antiepileptogenic therapies. However, not all models are suitable for testing antiepileptogenic or disease-modifying therapies (Stables et al., 2003). Reasons include a long latency period and low incidence of spontaneous seizures, which complicates drug studies. On the basis of such logistical considerations, a models workshop organized by the National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS) in 2002 thus recommended only two groups of models as potentially useful tools for antiepileptogenic treatment discovery: kindling and post-SE models of TLE (Stables et al., 2003). Therefore, the review concentrates predominantly on these two groups of TLE models but also compares data obtained in the latter models with data from other epileptogenesis models, including genetically epilepsy prone rodent strains.
A. The Kindling Model of Temporal Lobe Epilepsy
Kindling, which was described in 1969 by Graham Goddard and colleagues (Goddard et al., 1969), is a model in which repeated excitatory stimuli initially induce subconvulsive or partial seizures. The stimuli usually consist of electrical stimulation of a specific brain region, such as amygdala or hippocampus, via chronically implanted depth electrodes (McIntyre et al., 2002; Morimoto et al., 2004). Repetition of the same stimuli results in a progressive increase in the severity and duration of the seizures (i.e., acquisition of kindling). Fully kindled seizures resemble complex partial seizures with secondary generalization, so that amygdala or hippocampal kindling is considered a model of TLE that is substantiated by the anticonvulsant profile of AEDs in this model (Löscher, 1998a; McIntyre et al., 2002; Morimoto et al., 2004). Once an animal has been kindled, the heightened response to the stimulus seems to be permanent, indicating the development of chronic brain alterations (McIntyre et al., 2002). If daily kindling is repeated over many weeks and months (“overkindling”), spontaneous convulsive seizures develop in approximately half of the rats, indicating a very prolonged latent period (Coulter et al., 2002; McIntyre et al., 2002). The kindling model has been extensively evaluated by investigators worldwide and provides the opportunity for investigators to study the stepwise progression of various neurobiologic alterations that underlie the epileptogenic process (Stables et al., 2003).
However, several critical arguments have been raised. First, to what degree does kindling reproduce human epileptogenesis? Some indication of kindling in humans stems from anecdotal reports of seizures occurring in the setting of thalamic stimulation for treatment of chronic pain and of development of spontaneous seizures some time after repeated sessions of electroconvulsive therapy, but kindling is unlikely to be a ubiquitous explanation of epileptogenesis in partial epilepsy (Walker et al., 2002; Reisner, 2003). Second, most studies on kindling examine rats that do not exhibit spontaneous seizures, so that neurobiological alterations in such rats may differ from those underlying development of spontaneous seizures (Pitkänen and Halonen, 1998). Third, which type of epileptogenic brain insult, if any, is mimicked by kindling? Because electrical kindling needs long-term implantation of an electrode into a region of the temporal lobe such as amygdala or hippocampus, the brain injury caused by electrode implantation may play a role in the kindling process. Amygdala electrode implantation per se has been demonstrated to induce a prokindling effect (i.e., to enhance the susceptibility of rats to subsequent kindling) and to lead to epileptiform field potentials in the hippocampus (Löscher et al., 1995; Niespodziany et al., 1999). The mechanisms underlying these kindling-like changes observed after mere electrode implantation are not clear, but we have suggested that the functional consequences of electrode implantation into sensitive brain areas of rats resemble those of penetrating brain injury (Löscher, 2002a). Thus, kindling via depth electrodes may represent a model in which the consequences of TBI are facilitated by electrical stimulation. Fourth, for antiepileptogenic drug testing during the kindling acquisition phase, drugs are usually given before each electrical stimulus, so that the acute anticonvulsant effect of each drug administration alone could be sufficient to retard kindling, thus producing false positive data on the antiepileptogenic potential of a given drug (Dudek, 2009).
The latter argument, however, does not explain why some AEDs (i.e., carbamazepine and phenytoin) that exert anticonvulsant effects on kindled seizures did not retard kindling when animals were treated during kindling development (Löscher, 2002a). Vice versa, N-methyl-d-aspartate (NMDA) antagonists such as dizocilpine maleate (MK-801) are extremely potent in retarding kindling (Sutula et al., 1996) but do not suppress partial seizures in fully kindled rats, again arguing against a simple relationship between antiepileptogenic and anticonvulsant drug effects in this model (Table 1). Furthermore, Silver et al. (1991) demonstrated that the powerful antikindling effect of valproate really reflects an antiepileptogenic or disease-modifying activity of this drug. They were able to do so by using an experimental design that excluded the possibility that valproate simply masked the expression of kindled seizures through an anticonvulsant action. In this design, illustrated in Fig. 2, treatment of rats during kindling is followed by a wash-out phase without treatment and subsequent continuation of kindling in the absence of drug. Treatment with valproate during the first phase of kindling retarded subsequent kindling in the absence of drug (Silver et al., 1991). By using the same experimental design, phenobarbital and levetiracetam, but not several other AEDs, were shown to retard kindling after drug withdrawal, indicating plastic antiepileptogenic brain alterations in response to these drugs (Table 1). It is noteworthy that unlike any other currently available AED, treatment with levetiracetam during kindling resulted in a persistent reduction of electrographic seizure activity in kindled brain, even long after the termination of treatment (Löscher et al., 1998; Stratton et al., 2003). After our initial observation of an antiepileptogenic or disease-modifying potential of levetiracetam in the kindling model (Löscher et al., 1998), we used gene expression analysis to identify the mechanisms responsible for these effects (Gu et al., 2004). Previously described epilepsy-related genes, such as neuropeptide Y (NPY), thyrotropin-releasing hormone, and glial fibrillary acidic protein were up-regulated by kindling and partially normalized by levetiracetam treatment. In a subsequent study, Matveeva et al. (2008) showed that levetiracetam also inhibits the kindling-induced increase of the synaptic vesicle protein SV2a, which contains a specific binding site for this AED and is thought to be responsible, at least in part, for the anticonvulsant effect of levetiracetam (Lynch et al., 2004). Our approach illustrates how kindling can be used to identify potential drug targets for modifying epileptogenesis. On the basis of this finding and several other findings discussed later, levetiracetam may be a promising candidate for epilepsy prevention trials.
TABLE 1.
Effects of drugs in the amygdala-kindling model
| Drugs | Effects in the Kindling Model |
Reference | ||
|---|---|---|---|---|
| Suppression of Fully Kindled Seizures (Anticonvulsant Effect) | Retardation of Kindling Development (Antiepileptogenic or Disease-Modifying Effect) |
|||
| Kindling Acquisition Retarded when Drug Is Given before Each Stimulus | Further Retardation of Kindling Acquisition (or Less Severe Seizures) after Washout of Drug (Disease Modification) | |||
| Carbamazepine | + | N.E. | N.E. | Schmutz et al., 1988; Silver et al., 1991 |
| Phenytoin | + | N.E. | N.E. | Racine et al., 1975; Turner et al., 1977; Schmutz et al., 1988; Ebert et al., 1997 |
| Lamotrigine | + | + | N.E. | Stratton et al., 2003 |
| Lacosamide | + | + | N.E. | Brandt et al., 2006b |
| Phenobarbital | + | + | + | Turner et al., 1977; Silver et al., 1991 |
| Valproate | + | + | + | Silver et al., 1991 |
| Levetiracetam | + | + | + | Löscher et al., 1998; Stratton et al., 2003 |
| Benzodiazepines | + | + | N.D. | Schmutz et al., 1988 |
| Vigabatrin | + | + | N.D. | Shin et al., 1986 |
| Topiramate | + | + | N.D. | Amano et al., 1998; Mazarati et al., 2007 |
| NMDA antagonists (e.g., MK-801) | N.E. (only reduction of seizure severity) | + | N.D. | Gilbert, 1988; Löscher, 1998b |
+, effect is present; N.D., not determined; N.E., not effective.
Fig. 2.
Schematic illustration of an experimental protocol to evaluate drug effects on kindling acquisition. Note that three categories of drug effects are analyzed: 1) drug is administered before each stimulation and the effects on kindling acquisition are determined relative to vehicle controls; 2) kindling is continued after washout of drug; 3) anticonvulsant drug effects are studied in fully kindled rats.
One problem of testing effects of drugs on kindling acquisition as shown in Fig. 2 is that conventional once-daily stimulation experiments are time- and labor-intensive, so that this model is not convenient for antiepileptogenic treatment screening. Sankar and colleagues (Mazarati et al., 2006a,b, 2007, 2009) have therefore proposed a modification of the “rapid kindling” protocol for drug testing that was originally developed by Lothman et al. (1985). In contrast to conventional kindling, which requires weeks for full motor seizures to develop, epileptogenesis is compressed to several hours under conditions of rapid kindling but still bears key hallmarks of kindling: appearance and gradual progression of the severity of limbic seizures and enhanced seizure susceptibility (Mazarati et al., 2006a). However, a potential drawback of this protocol for testing drugs is that each elicited seizure induces a postictal rise in seizure threshold, which accumulates during frequent seizure initiation and may interact with the effects of the test drug, thus producing false positive data (Löscher and Hönack, 1990).
Although kindling has been crucial to our understanding of the epileptogenic process and is still the most widely used animal model of TLE, particularly during preclinical AED development, its use in the search for antiepileptogenic or disease-modifying drugs has declined, particularly because of the development of several post-SE models of TLE. These models are thought to be better suited than kindling for searching antiepileptogenic drugs, because the latent period between the SE and the first occurrence of spontaneous seizures allows testing drugs as a prophylactic treatment against epilepsy (Pitkänen and Halonen, 1998).
B. Post-Status Epilepticus Models of Temporal lobe Epilepsy
SE is a common, serious, potentially life-threatening, neurologic emergency characterized by prolonged seizure activity (Lowenstein, 1999). Epidemiologic studies indicate that epilepsy develops in up to 43% of patients with SE (Hesdorffer et al., 1998). For post-SE rodent models of TLE, a variety of different chemoconvulsants and intracerebral electrical stimulation patterns have been used to induce SE, which is followed, after a latent period of days to weeks, by spontaneous recurrent seizures (Goodman, 1998; Walker et al., 2002; Stables et al., 2003; Morimoto et al., 2004; Cavalheiro et al., 2006; Curia et al., 2008). Of the various systemic chemoconvulsants, kainate and pilocarpine have been the best characterized with regard to seizure phenomenology, electroencephalographic (EEG) features, cognitive outcome, and neuropathology. In both models, rats develop spontaneous recurrent partial and secondarily generalized seizures, hippocampal and extrahippocampal damage, and behavioral and cognitive alterations resembling the clinical characteristics of TLE (Morimoto et al., 2004).
Typically, in models with systemic administration of kainate or pilocarpine, SE is terminated after 60 to 90 min by AEDs (such as diazepam) or general anesthetics (such as pentobarbital) to reduce the otherwise high mortality associated with chemically induced SE. Furthermore, ramp-up dosing protocols, which allow for a more individual dosing than bolus injections, have been developed for kainate (Hellier et al., 1998) and pilocarpine (Glien et al., 2001) to increase the percentage of animals developing SE and to decrease mortality. In addition, lithium can be used to potentiate the convulsant activity of pilocarpine (Cavalheiro et al., 2006). An alternative to systemic administration of kainate or pilocarpine is unilateral focal injection into amygdala or hippocampus, which avoids the widespread brain damage associated with systemic administration, thus creating more realistic models of human TLE (Cavalheiro et al., 2006; Dudek et al., 2006).
A variety of direct brain electrical-stimulation patterns also have been used to induce SE and subsequent spontaneous seizures (Goodman, 1998; Walker et al., 2002; Stables et al., 2003; Mazarati et al., 2006b). Although this variation of the post-SE epilepsy model requires the surgical placement of an intracerebral electrode, no toxins are necessary.
Most of the SE models have the advantage of a latent period of days to weeks during which spontaneous seizures do not occur (but see section III.C.3.c). The duration of the latent period depends on the severity of the initial SE. After the latent period, spontaneous recurrent seizures typically escalate in frequency over time. The latent period offers an opportunity to introduce therapy and measure its effect on prevention. Post-SE models of TLE are frequently associated with cognitive impairment and behavioral psychopathology (Stafstrom, 2006). Another appealing feature of these models is their similarity to human TLE with partial seizures with or without secondary generalization (Stables et al., 2003).
The consequences of chemically and electrically induced SE differ in a number of important factors. First, although a SE duration of 60 to 90 min is sufficient to induce epilepsy in the majority of rats or mice with systemic administration of pilocarpine or kainate, 3 to 4 h of SE are needed in this respect in models in which SE is induced by focal electrical stimulation of amygdala or hippocampus (Brandt et al., 2003a; Pitkänen et al., 2005; Mazarati et al., 2006b). Chemically induced SE is more severe than SE induced by electrical stimulation and more difficult to terminate by AEDs such as diazepam (Bankstahl and Löscher, 2008). An additional difference from electrical models is that the neurotoxic effects of chemoconvulsant may add to the effects of SE. Thus, Navarro Mora et al. (2009) demonstrated that rats that did not develop SE after pilocarpine nevertheless developed spontaneous recurrent seizures after a latent period of several months. Another important difference between chemical and electrical SE models relates to inflammation. In humans, there are numerous causes of SE in nonepileptic patients, including anoxia, hemorrhage/stroke, tumors, and infectious diseases (Neligan and Shorvon, 2008). In contrast, chemical or electrical induction of SE is typically performed in healthy rodents. One exception is the lithium-pilocarpine model, in which lithium is given 24 h before pilocarpine to enhance the potency of the convulsant (Curia et al., 2008). Marchi et al. (2009) reported that lithium induces systemic inflammatory events and blood-brain barrier damage in rats before administration of pilocarpine and that blood-brain barrier damage and SE onset could be reduced by pretreatment with an interleukin (IL)-1β antagonist. Pilocarpine and SE itself are also known to induce neuroinflammatory responses (Voutsinos-Porche et al., 2004; Vezzani and Granata, 2005; Marchi et al., 2007), but the adaptive immune response to lithium clearly differentiates the lithium-pilocarpine model from all other SE models. However, with respect to the effects of inflammation on SE, it is also important to note that administration of the proinflammatory bacterial endotoxin lipopolysaccharide 72 h before pilocarpine did not potentiate its convulsant activity (Dmowska et al., 2010).
Over the last 15 years, post-SE models of TLE have been widely used in the search for antiepileptogenic or disease-modifying drugs. Respective studies used varying protocols for SE induction, different SE duration, different onset and duration of drug treatment after SE, and different outcome measures, thus allowing analysis of which experimental factors are important for antiepileptogenic or disease-modifying drug effects in these models.
C. Analysis of Antiepileptogenic Drug Studies in Post-Status Epilepticus Models of Temporal Lobe Epilepsy
In Tables 2 and 3, only studies in which drugs were administered after onset of SE are shown. In various other studies, not included in this review, drugs were given before induction of SE, which may attenuate the severity or shorten the duration of SE and thereby reduce the long-term consequences of the brain insult. However, only a drug capable of preventing epilepsy after an initial insult such as a SE would be clinically relevant (Löscher, 2002a). A schematic illustration of drug testing in post-SE models of TLE, as used by our and other groups, is shown in Fig. 3. For assessing antiepileptogenic or disease-modifying drug effects, it is important that spontaneous seizures are monitored after a washout phase after termination of drug treatment, because effects during treatment may simply reflect an anticonvulsant activity of the treatment.
TABLE 2.
Prophylactic effects of treatment with clinically used antiepileptic drugs on the long-term consequences of SE in rats
Only studies in which treatment started after onset of SE are included. If studies were performed in immature rats, this is indicated in the Model column.
| Drug | Model (Induction of SE) | SE duration (Limited by) | Beginning of Prophylactic Treatment with Test Drug | Duration of Prophylactic Treatment | Consequences of Prophylactic Drug Treatment |
Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Latency to SRS | Incidence of SRS | Frequency, Severity, or Duration of SRS | Neurodegeneration | Behavioral Alterations (Psychopathology) | Impairment of Learning and Memory | ||||||
| Carbamazepine | Kainate | Not limited | 1 day after SE | 56 days | N.D. | N.E. | ↓ | ↓ (Hippocampus) | N.D. | N.D. | Capella and Lemos, 2002 |
| Carbamazepine | Pilocarpine (in hippocampus) | 3 h (thiopental) | 1 h after 3 h SE | 4 days | N.D. | N.D. | N.D. | ↓ (CA1, CA3, hilus) | N.D. | ↓ | Cunha et al., 2009 |
| Carisbamate | Lithium-pilocarpine | 1 h (diazepam in controls) | 1 h after SE onset | 7 day | Increased | ↓ (motor SRS) | ↓ | ↓ (CA1, PC, EC, amygdala, thalamus) | N.D. | N.D. | François et al., 2005 (A) |
| Diazepam* | Amygdala stimulation | Not limited in controls | 2 or 3 h after SE onset | Second dose 6 h later | N.D. | ↓ | ↓ | ↓ (Hippocampus) | N.D. | N.D. | Pitkänen et al., 2005* |
| Diazepam | Pilocarpine (in hippocampus) | 3 h (thiopental) | 1 h after 3 h SE | 4 days | N.D. | N.D. | N.D. | ↓ (CA1, CA3, hilus) | N.D. | ↓ | Cunha et al., 2009 |
| Fluorofelbamate* | Perforant path stimulation | Not limited in controls | 10 or 40 min after onset of stimulation | 1 dose | N.D. | N.E. | ↓ | N.D. | N.D. | N.D. | Mazarati et al., 2002* |
| Gabapentin | Kainate (P35) | Not limited | 1 day after SE | 10 days | N.D. | N.D. | N.D. | ↓ (Hippocampus) | N.E. | N.E. | Cilio et al., 2001 |
| Lamotrigine | Perforant path stimulation | 2 h after end of PPS (diazepam) | 1 h after SE onset | 2 weeks | N.D. | N.D. | N.D. | ↓ (CA3, hilus) | N.D. | N.E. | Halonen et al., 2001b |
| Lamotrigine | Amygdala stimulation | Not limited in controls | 2 h after SE onset | 11 weeks | N.D. | N.E. | N.E. | N.E. | N.D. | N.D. | Nissinen et al., 2004 |
| Levetiracetam | Pilocarpine | 30 min (diazepam) | 30 min after SE onset | 21 days | N.D. | N.E. | N.D. | ↓(Hippocampus) | N.D. | N.D. | Klitgaard et al., 2001 (A) |
| Levetiracetam | Perforant path stimulation (PPS) | Not limited in controls | 1, 3, and 6 h after 30 min of PPS stimulation | 29 days | N.D. | N.E. | ↓ | N.D. | N.D. | N.D. | Mazarati et al., 2003 (A) |
| Levetiracetam | Amygdala stimulation | 4 h (by diazepam) in exp. 2 | 24 h after onset of stimulation (exp. 1) or 4 h after SE onset (exp. 2) | 5–8 weeks | N.D. | N.E. | N.E. | N.E. | N.E. | N.E. | Brandt et al., 2007 |
| Levetiracetam | Lithium-pilocarpine | Not limited | 24 h after SE onset | 2 weeks | N.D. | N.E. | N.D. | ↓ (CA1, CA3, hilus) | N.D. | N.E. | Zhou et al., 2007 |
| Phenobarbital | Kainate (P35) | Not limited | 1 day after SE | 97 days | N.D. | N.E. | N.E. | N.E. | Worsened | Worsened | Mikati et al., 1994 |
| Phenobarbital | Kainate (P35) | Not limited | 1 day after SE | 40 days | N.D. | N.E. | N.E. | N.E. | N.D. | N.E. | Bolanos et al., 1998 |
| Phenobarbital* | Hippocampal stimulation | Not limited in controls | 1, 2 or 4 h after SE onset | 1 dose | N.D. | ↓ (Only for the 1 h after SE onset group) | N.D. | N.D. | N.D. | N.D. | Prasad et al., 2002* |
| Phenobarbital | Lithium-Pilocarpine | 90 min (diazepam plus phenobarbital) | 90 min after SE onset | 2 weeks | Increased | N.E. | ↓ | N.E. (?) | N.E. | N.D. | Brandt et al., 2010 |
| Phenytoin | Hippocampal stimulation | Not limited | 1, 2, or 4 h after SE onset | 1 dose | N.D. | N.E. | N.D. | N.D. | N.D. | N.D. | Prasad et al., 2002 |
| Phenytoin | Pilocarpine (in hippocampus) | 3 h (thiopental) | 1 h after 3 h SE | 4 days | N.D. | N.D. | N.D. | ↓ (CA1, CA3, hilus) | N.D. | ↓ | Cunha et al., 2009 |
| Pregabalin* | Lithium-pilocarpine | 2 h (diazepam) | 20 min after pilocarpine | 55 days | Increased | N.D. | N.D. | ↓ (PC, EC) | N.D. | N.D. | André et al., 2003* |
| Retigabine | Kainate | 1.5 h (diazepam) | 1.5, 2.5 and 3.5 h after SE onset | 3 doses | N.D. | N.D. | N.D. | N.E. | N.D. | N.D. | Ebert et al., 2002 |
| Topiramate | Hippocampal stimulation | 140 min (termination of stimulation) | 140 min after onset of stimulation | 1 dose | N.D. | N.D. | N.D. | ↓ (CA1, CA3, hilus) | N.D. | N.D. | Niebauer and Gruenthal, 1999 |
| Topiramate | Lithium-pilocarpine | Not limited | 24 h after SE | 28 days | N.D. | N.D. | N.D. | ↓ (Hippocampus) | N.D. | ↓ | Cha et al., 2002 |
| Topiramate | Pilocarpine | 1 h (diazepam) | 1 h after SE onset | 4 days | N.D. | ↓ (3–6 months after SE) | N.D. | ↓ (CA1) | N.D. | N.D. | DeLorenzo et al., 2002 (A) |
| Topiramate | Lithium-pilocarpine | 1 h (diazepam) in controls) | 1 h after SE onset | 7 days | N.E. | N.E. | N.E. | ↓ (CA1, CA3) | N.D. | N.D. | Rigoulot et al., 2004 |
| Topiramate (plus diazepam) | Lithium-pilocarpine | 1 h (diazepam) in controls | Topiramate at SE onset, diazepam 2 h after SE onset | 7 days | N.E. | N.E. | N.E. | ↓ (CA1, hilus) | N.D. | N.D. | François et al., 2006 |
| Topiramate* | Lithium-pilocarpine (in P15 or P28 rats) | In controls atropine after 70 min SE | 20, 40, or 70 min after pilocarpine (together with atropine) | 1 dose | N.D. | ↓ (P15>P28) | ↓ | N.D. | N.D. | N.D. | Suchomelova et al., 2006* |
| Topiramate | Pilocarpine | 2 h (diazepam) in controls | 40 min after SE onset | N.D. | N.D. | N.D. | ↓ (CA1, CA3) | N.D. | ↓ | Frisch et al., 2007 | |
| Topiramate | Lithium-pilocarpine | 2 h (pentobarbital) | 1 h after 2 h SE | 6 weeks | N.D. | N.D. | N.D. | N.E. (Hippocampus) | N.D. | (↓) | Shatskikh et al., 2009 |
| Valproate | Kainate (P35) | Not limited | 1 day after SE | 40 days | N.D. | ↓ (During taper) | ↓ (During taper) | ↓ (CA1) | ↓ | ↓ | Bolanos et al., 1998 |
| Valproate | Pilocarpine | 30 min (diazepam) | 30 min after SE onset | 21 days | N.D. | N.E. | N.D. | N.E. | N.D. | N.D. | Klitgaard et al., 2001 (A) |
| Valproate | Amygdala stimulation | 4 h (diazepam) | 4 h after SE onset | 4 weeks | N.D. | N.E. | N.E. | ↓ (Hippocampus and hilus) | ↓ | N.E. | Brandt et al., 2006 |
| Valproate | Kainate | Not limited in controls | 5 h after SE onset | 1–5 weeks | N.D. | N.D. | N.D. | N.E. (Hippocampus and hilus) | N.D. | ↓ | Jessberger et al., 2007 |
| Vigabatrin* | Lithium-pilocarpine | Not limited in controls | 10 min after pilocarpine | 45 days | N.E. | N.E. | N.E. | ↓ (CA1, CA3 and hilus) | N.D. | N.D. | André et al., 2001* |
| Vigabatrin | Amygdala stimulation | Not limited | 2 days after SE | 10 weeks | N.E. | N.E. | N.E. | N.E. | N.D. | N.E. | Halonen et al., 2001a |
↓, A prophylactic (beneficial) effect; *, studies in which treatment effects were due to initial insult modification (i.e., reduction of SE duration or severity) rather than an antiepileptogenic effect (see text for discussion); (A), studies that are available only as abstracts.
EC, entorhinal cortex; N.D., not determined; N.E., no effect; P, postnatal day; PC, piriform cortex; PPS, perforant path stimulation; SRS, spontaneous recurrent seizures.
TABLE 3.
Prophylactic effects of treatment with various drug categories on the long-term consequences of SE in rats
Only studies in which treatment started after onset of SE are included. If studies were performed in immature rats, this is indicated in the Model column.
| Drug | Model (Induction of SE) | SE duration (Limited by) | Beginning of Prophylactic Treatment with Test Drug | Duration of Prophylactic Treatment | Consequences of Prophylactic Drug Treatment |
Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Latency to SRS | Incidence of SRS | Frequency, Severity, or Duration of SRS | Neurodegeneration | Behavioral Alterations (Psychopathology) | Impairment of Learning and Memory | ||||||
| Neuroprotective | |||||||||||
| Ketamin* (NMDA antagonist) | Pilocarpine | 2 h (Clonazepam) | 15 min (K15) or 120 min (K120) after SE onset | 1 dose | N.D. | ↓ (K15) | N.D. | ↓ (Ca1, Ca3) (K15>K120) | N.D. | ↓ (K15> K120) | Hort et al., 1999* |
| Ketamin | Pilocarpine (in hippocampus) | 3 h (Thiopental) | 1 h after 3 h SE | 4 days | N.D. | N.D. | N.D. | ↓ (CA1, CA3, hilus) | N.D. | ↓ | Cunha et al., 2009 |
| MK-801* (NMDA antagonist) | Hippocampal stimulation | Not limited in controls | 1, 2 or 4 h after SE onset | 1 dose | N.D. | ↓ (only for the 1 and 2 h after SE onset groups) | N.D. | N.D. | N.D. | N.D. | Prasad et al., 2001* |
| MK-801 | Kainate | 1.5 h (Diazepam) | 90 min after SE onset | 1 dose | N.D. | N.E. | N.E. | ↓ (CA1, CA3, PC, thalamus) | N.D. | N.D. | Brandt et al., 2003b |
| MK-801 | Lithium-pilocarpine | 1.5 h (Diazepam) | 90 min after SE onset | 1 dose | N.D. | N.D. | N.D. | ↓ (CA1, CA3, PC, SN) | N.D. | N.D. | Bankstahl et al., 2008 |
| NS1209* (AMPA antagonist) | Amygdala stimulation | Not limited in controls | 2–3 h after SE onset | 1 dose or infusion for 24 h | N.D. | N.D. | N.D. | ↓ (Hippocampus) | N.D. | N.D. | Pitkänen et al., 2007b* |
| DEVD (caspase-3 inhibitor) | Kainate | 1.5 h (Diazepam) | 1.5 h and 24 h after SE onset | 2 doses | N.D. | N.D. | N.D. | N.E. (Hippocampus) | N.D. | N.D. | Ebert et al., 2002 |
| z-DEVD-fmk (caspase-3 inhibitor) | Amygdala stimulation | 3 h (Diazepam) | 3 h after SE onset | 1 week | N.D. | ↓ (At 8–11 weeks after SE) | N.E. | ↓ (CA3 and hilus) | N.D. | N.E. | Narkilahti et al., 2003 |
| Erythropoietin | Pilocarpine | 2 h (Diazepam) | 0.5 h after SE | Additional doses at 1 and 3 days affter SE | N.D. | N.D. | N.D. | ↓ (CA1, CA3 and hilus) | N.D. | N.D. | Nadam et al., 2007 |
| Erythropoietin | Lithium-pilocarpine | 1 h (Diazepam) | 1 h after SE onset | 1 week | N.D. | N.E. | ↓ | ↓ (CA1, CA3, hilus) | N.D. | N.D. | Chu et al., 2008 |
| FGF-2 and BDNF gene therapy | Pilocarpine | 2 h (Diazepam) | 4 days after SE | 1 Unilateral injection into hippocampus | N.E. | ↓ (In 20% of rats) | ↓ | No neuroprotective effect, but partial repair by increased neurogenesis (hippocampus) | N.D. | N.D. | Paradiso et al., 2009 |
| Anti-inflammatory | |||||||||||
| Celecoxib (COX-2 inhibitor) | Lithium-pilocarpine | 1 h (Diazepam) | 1 day after SE | 2 weeks | N.D. | ↓ | ↓ | ↓ (CA1, CA3, hilus) | N.D. | N.D. | Jung et al., 2006 |
| SC58236 (COX-2 inhibitor) | Hippocampus stimulation | 4 h (Isoflurane) | 4 h after SE | 7 days | N.D. | N.E. | N.E. | N.E. (Hilus) | N.D. | N.D. | Holtman et al., 2009 |
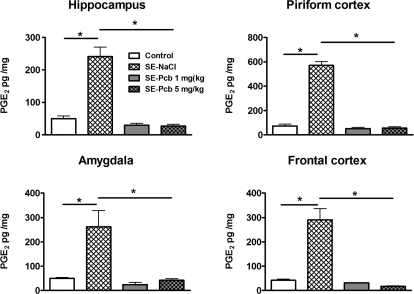
| Parecoxib | Lithium-pilocarpine | 1.5 h (Diazepam) | 1.5 h after SE onset | 18 days | N.D. | N.E. | ↓ | ↓ (CA1, PC) | (↓) | (↓) | Polascheck et al., 2010 (A) |
| α4 integrin specific monoclonal antibody | Pilocarpine (mice) | 2 h (Diazepam) | 1 h after SE | 20 days | N.E. | N.E. | ↓ | ↓ | ↓ | N.D. | Fabene et al., 2008 |
| Immunosuppressive | |||||||||||
| Rapamycin | Kainate | Not limited | 24 h after SE | 6 weeks | N.D. | N.E. | ↓ | N.E. (CA1, CA3, hilus) | N.D. | N.D. | Zeng et al., 2009 |
| Rapamycin | Pilocarpine | 2 h (Diazepam) | 1–8 h after termination of SE | 1–2 months | N.D. | N.D. | N.D. | N.E. (Hilus) | N.D. | N.D. | Buckmaster et al., 2009 |
| FK506 (tacrolimus) | Amygdala stimulation | Not indicated | 24 h after SE | 2 weeks | Decreased | Increased | Increased | N.D. | N.D. | N.D. | Lukasiuk and Sliwa, 2009 (A) |
| FK506 (tacrolimus) | Pilocarpine | Not limited | At time of generalized convulsive SE | 1 dose | N.D. | N.D. | N.D. | ↓ | N.D. | N.D. | Chwiej et al., 2010 |
| Neuromodulatory | |||||||||||
| Atipamezole (α2 antagonist) | Amygdala stimulation | Exp. 1: 3 h (diazepam); Exp. 2:not limited | 7 days after SE | 9 weeks | N.D. | N.E. | ↓ | ↓ (Hilus) | N.D. | N.E. | Pitkänen et al., 2004 |
| Rimonabant (CB1 antagonist) | Kainate | Not limited | Immediate after SE onset | 1 dose | N.E. | N.E. | N.E. | N.D. | N.D. | N.D. | Pouliot et al., 2009 (A) |
| Bumetanide | Lithium-Pilocarpine | 1.5 h (Diazepam + phenobarbital) | 90 min after SE onset | 5 days | N.E. | N.E. | N.E. | N.E. | N.E. | N.D. | Brandt et al., 2010 |
| Bumetanide + phenobarbital | Lithium-pilocarpine | 1.5 h (Diazepam + phenobarbital) | 90 min after SE onset | 5–14 days | Increased | N.E. | ↓ | N.E. (?) | ↓ | N.D. | Brandt et al., 2010 |
↓, A prophylactic (beneficial) effect; *, studies in which treatment effects were due to initial insult modification (i.e., reduction of SE duration or severity) rather than an antiepileptogenic effect (see text for discussion); (A), studies that are available only as abstracts.
EC, entorhinal cortex; FGF, fibroblast growth factor; K, ketamine; N.D., not determined; N.E., no effect; P, postnatal day; PC, piriform cortex; PPS, perforant path stimulation; SN, substantia nigra; SRS, spontaneous recurrent seizures; z-DEVD-fmk, N-benzyloxycarbonyl-Asp-Glu-Val-Asp-fluoromethyl ketone.
Fig. 3.
Schematic illustration of an experimental protocol to evaluate antiepileptogenic (or disease-modifying) drug effects by prophylactic drug treatment after a status epilepticus.
When comparing the different experimental studies in which drugs were given after onset of SE, it is important to differentiate between drug effects resulting from “initial insult modification” and effects representing “true” antiepileptogenic or disease-modifying and neuroprotective drug efficacy (Löscher, 2002a; Pitkänen, 2002). Initial insult modification means that the long-term consequences of the insult can be diminished by reducing the severity or duration of the initial brain insult, such as SE. This has been demonstrated, for instance, by reducing the duration of SE by phenobarbital, the NMDA antagonist MK-801 (dizocilpine), pregabalin, or diazepam in SE models in rats (Sutula et al., 1992a; Prasad et al., 2002; André et al., 2003; Pitkänen et al., 2005), thus substantiating that early termination of SE is a powerful means for preventing or limiting its consequences (Lowenstein, 2006). As discussed above, in post-SE models of TLE with electrical SE induction, a SE duration of at least 3 to 4 h is needed to induce epileptogenesis in the majority of rats, so that any reduction of this duration by anticonvulsant drugs will result in a modification of the long-term consequences of the SE in such a way that fewer rats develop epilepsy or that the epilepsy that develops is milder (Löscher, 2002a; Pitkänen, 2002; Pitkanen, 2004). Thus, in such SE models the antiepileptogenic or neuroprotective potential of a drug should be tested by administering this drug after a SE of at least 3- to 4-h duration (Löscher, 2002a). In chemical models of SE, such as the pilocarpine or kainate model, the critical duration of SE for induction of epileptogenesis and brain damage is considerably shorter (i.e., approximately 60–90 min) (Löscher, 2002a). Numerous studies have tested drugs after such critical duration of SE for effects on epileptogenesis, brain damage, and/or behavioral and cognitive alterations in rats (Tables 2 and 3). To our knowledge, however, no incontrovertible evidence supports the idea that any drug, including various novel AEDs, administered during the latent period after SE, prevents the development of epilepsy, although several studies indicated that development of epilepsy may be delayed or the severity of spontaneous seizures may be reduced by such treatment (Tables 2 and 3). Furthermore, several experimental trials found positive effects of prophylactic treatment on neurodegeneration and development of cognitive impairment after SE.
1. Prophylactic Effects of Antiepileptic Drugs in Post-Status Epilepticus Models of Temporal Lobe Epilepsy.
It is important to emphasize that clinical trials in antiepileptogenesis are a complex issue (Dichter, 2009a). Epilepsy prevention trials are more complex, lengthy, and costly than standard epilepsy treatment trials for many reasons (Herman, 2006). Issues revolve around selection of subjects, consent for participation, length of follow-up, and selection of an appropriate endpoint. As a consequence, only five drugs (phenytoin, phenobarbital, carbamazepine, valproate, and magnesium) have been rigorously tested for an antiepileptogenic effect in clinical trials, and none has been shown to exert any beneficial effect after TBI (Temkin, 2009). However, the clinical trials that have been performed to date have substantial limitations (Temkin, 2009). These include the lack of EEG monitoring to evaluate subclinical seizures, lack of compliance monitoring or drug concentration testing, high rates of loss to follow-up, and relatively short periods of observation after the drug was stopped (Temkin, 2009). Furthermore, the clinical studies that have been done had little laboratory work to inform their design. Therefore, decisions on when to start the drug, what dose to use, and what duration of treatment to use were made without benefit of knowing what worked best in the laboratory (Temkin, 2009). The range of drugs tested has been narrow, and only older AEDs (approved before 1980) have been tested so far. Thus, newer AEDs need to be evaluated in the laboratory at least and, if results are promising, then in clinical trials (Temkin, 2009).
As shown in Table 2, at least 30 experimental studies have examined whether clinically used AEDs exert antiepileptogenic or disease-modifying effects when administered after SE in different models. Almost all old and new AEDs were evaluated in this regard. Because these drugs differ widely in their mechanism of action (Rogawski and Löscher, 2004), including mechanisms that play a role in epileptogenesis, there was a relatively high chance to identify key mechanisms to prevent or modify epilepsy after brain insults. Three drugs, carisbamate, topiramate, and valproate, were found to reduce the incidence of rats with epilepsy, indicating a true antiepileptogenic effect.
a. Carisbamate.
Carisbamate (RWJ-333369) is a novel neuromodulator that has undergone clinical trials in patients with epilepsy (Novak et al., 2007), but the application for a marketing authorization for use in the treatment of partial-onset seizures in patients with epilepsy was withdrawn by the company because of inconsistent antiepileptic efficacy in two phase III trials. However, because carisbamate is possibly the first and so far the only proof of principle that epilepsy can be prevented in post-SE models of TLE, we will briefly discuss this drug. Its mechanism of action has not been elucidated, but data indicate that block of voltage-gated sodium channels contributes to its antiepileptic activity (Liu et al., 2009). In the study by François et al., 2005, as yet available only in abstract form, and in review articles (André et al., 2007; Nehlig, 2007), administration of carisbamate after lithium-pilocarpine induced SE markedly reduced the number of rats developing spontaneous recurrent seizures (only motor seizures were recorded by video monitoring) during several months of recording. In rats developing spontaneous seizures, the latency to such seizures was increased, and their frequency was decreased. Furthermore, carisbamate was able to protect all limbic brain regions that are damaged in the lithium-pilocarpine model (Table 2). André et al. (2007) pointed out that carisbamate is the most neuroprotective and only antiepileptogenic drug known so far, but their very promising data need independent replication.
b. Topiramate.
Topiramate is a structurally novel broad-spectrum AED with established efficacy in adult and pediatric patients (Lyseng-Williamson and Yang, 2007). Electrophysiological and biochemical studies have revealed a combination of pharmacologic properties of topiramate that include modulatory effects on Na+ channels, GABAA receptors, and glutamate receptors of the AMPA/kainate type (Rogawski and Löscher, 2004a). On the basis of evidence that some of the effects of topiramate on AMPA/kainate receptors are influenced by the phosphorylation state of the receptors, it has been postulated that topiramate may bind to these membrane channel complexes at phosphorylation sites in the inner loop and thereby allosterically modulate ionic conductance through the channels (Shank et al., 2000). By this combination of mechanisms, topiramate appeared to be an ideal candidate for antiepileptogenesis, and various studies have been performed in this respect (Table 2). Indeed, one of the first studies reported that administration of topiramate after a pilocarpine-induced SE was effective in reducing the number of rats that developed epilepsy by >60% compared with vehicle controls (DeLorenzo et al., 2002). A similar promising effect was reported by Suchomelova et al. (2006), although, at least in part, the effects of topiramate in this study were due to disease-modification rather than antiepileptogenesis. However, as shown in Table 2, several other studies did not confirm the antiepileptogenic effect of topiramate first reported by DeLorenzo et al. (2002), although a neuroprotective effect was determined in most studies. In some studies, topiramate partially prevented the impairment of cognitive functions, indicating a disease-modifying effect (Cha et al., 2002; Frisch et al., 2007). According to the ClinicalTrials.gov web site, which describes clinical trials listed for various neurologic disorders, a pilot clinical trial, conducted by Drs. Marc Dichter and Susan Herman (University of Pennsylvania), is currently testing the safety and feasibility of using topiramate to prevent epilepsy after TBI.
c. Valproate.
For several decades, valproate has been one of the most widely used broad-spectrum AEDs, but its mechanism of action is still not completely understood (Löscher, 2002b; Rogawski and Löscher, 2004a,b; Rosenberg, 2007). Similar to topiramate, it combines various mechanisms, including activation of GABA synthesis, modulation of ion channels and NMDA receptor-mediated glutamatergic excitation, alterations in cell signaling, and epigenetic actions by inhibition of histone deacetylases (HDACs) (Rogawski and Löscher, 2004a,b; Rosenberg, 2007). These various mechanisms most likely explain the broad clinical use of valproate in epilepsy and nonepileptic disorders, including migraine and bipolar disorders, but may also provide potential antiepileptogenic activity after brain insults. Thus, alterations in GABA and glutamatergic transmission are long thought to be critically involved in epileptogenesis (Dudek and Sutula, 2007), and histone modifications may have a crucial role in the development of epilepsy induced by SE (Taniura et al., 2006; Jessberger et al., 2007). Bolanos et al. (1998) reported that prolonged treatment of immature rats with valproate after a kainate-induced SE prevented the development of epilepsy, hippocampal damage, behavioral abnormalities, and deficits in visuospatial learning. However, video recordings to assess spontaneous seizures were performed after tapering valproate (i.e., when rats were still treated with a relatively low dose of this drug), so it is not clear whether the findings on spontaneous seizure occurrence represented an antiepileptogenic or anticonvulsant effect of valproate. In a subsequent study in our laboratory, using a model in which SE is induced by sustained electrical stimulation of the basolateral amygdala (BLA), prolonged treatment with valproate after SE exerted no antiepileptogenic or disease-modifying effect on the development of spontaneous seizures (Brandt et al., 2006a). However, the treatment prevented damage in the hippocampal formation, including the dentate hilus, and most of the behavioral alterations associated with epilepsy in this model. A lack of antiepileptogenic efficacy of valproate was also reported by Klitgaard et al. (2001) in the pilocarpine model, although seizure recording was limited to 72 h, so the sensitivity to detect any antiepileptogenic or disease-modifying potential of drug treatment was low. Epigenetic modulation of SE-induced neurogenesis and cognitive decline by treatment with valproate after a kainate-induced SE has been reported by Jessberger et al. (2007), effects that appeared to be mainly mediated by inhibiting HDACs and normalizing HDAC-dependent gene expression within the epileptic dentate area. The lack of any antiepileptogenic effect of valproate in SE models is in line with results from a clinical trial in patients with TBI in which valproate exerted no significant effect on development of epilepsy (Temkin et al., 1999). However, on the basis of the experimental studies discussed above, valproate may exert neuroprotective and disease-modifying effects on cognitive and behavioral dysfunctions developing after brain insults in patients. In the randomized double-blind valproate trial for prevention of post-traumatic epilepsy, in which patients were either treated for 1 month or 6 months after TBI with valproate and were followed up for 2 years (Temkin et al., 1999), valproate had no positive effects on cognition when patients were examined with a battery of neuropsychological measures at 1, 6, and 12 months after injury (Dikmen et al., 2000). Furthermore, psychopathology was assessed in this trial without finding any effect of valproate in the areas examined (depression and anxiety; N. Temkin, unpublished data). On the basis of the lack of benefit and the potentially higher mortality rate in the valproate group, the authors suggested that valproate should not be routinely used for the prevention of post-traumatic seizures (Temkin et al., 1999; Dikmen et al., 2000).
e. Levetiracetam.
Another interesting candidate for antiepileptogenesis is levetiracetam (Klitgaard and Pitkänen, 2003). Although levetiracetam's mechanism of action is still not fully elucidated, it appears to differ from that of other known AEDs (Klitgaard and Pitkänen, 2003; Rogawski and Löscher, 2004a; De Smedt et al., 2007; Rogawski and Bazil, 2008). Levetiracetam has a specific membrane binding site (i.e., the synaptic vesicle protein SV2a) within the brain that seems to act as positive modulator of synaptic transmission by increasing the available amount of secretory vesicles and thus release probability (De Smedt et al., 2007). Long-term exposure to levetiracetam inhibits presynaptic neurotransmitter release in a use-dependent fashion, which seems most consistent with an antagonizing rather than enhancing action of levetiracetam on SV2a (Yang et al., 2007). As discussed above, kindling increases the expression of SV2a, which is prevented by levetiracetam (Matveeva et al., 2008). In addition to interacting with SV2a, levetiracetam exerts several other cellular effects, including modulation of high-voltage activated Ca2+ currents, reversal of the inhibitory effects of the negative allosteric modulators zinc and β-carbolines on both GABAA and glycine receptor-mediated responses, and strengthening GABA inhibition of neuronal circuits by blocking the receptor run-down (Klitgaard and Pitkänen, 2003; Rogawski and Löscher, 2004a; Palma et al., 2007; De Smedt et al., 2007). Furthermore, we reported that levetiracetam alters GABA metabolism and turnover in the striatum and reduces neuronal activity in the substantia nigra pars reticulata, a brain region in which decrease of neuronal firing results in protection against various seizure types (Löscher et al., 1996). These numerous effects could explain the unique antiepileptogenic or disease-modifying activity of levetiracetam in the kindling model (Löscher et al., 1998; Stratton et al., 2003). However, as shown in Table 2, levetiracetam did not prevent epilepsy when administered in post-SE models of TLE, and only one study found indication for a disease-modifying effect. This negative outcome of levetiracetam studies in post-SE models was unexpected and gave rise to critical arguments on study design used for drug testing in the SE models (Dudek et al., 2008). It is noteworthy that Margineanu et al. (2008) reported that, although prophylactic treatment with levetiracetam after a pilocarpine-induced SE did not seem to prevent development of spontaneous seizures, it completely prevented the development of hippocampal hyperexcitability (i.e., increased amplitude of population spike recorded in the dentate gyrus and reduced paired-pulse inhibition in the CA1 area). Furthermore, levetiracetam has been found to exert antiepileptogenic or disease-modifying effects in spontaneously epileptic rats (Yan et al., 2005; Russo et al., 2009), indicating that studies on kindling acquisition may be more predictive for such effects than data from SE models (see also discussion in section IV). A clinical pilot trial directed by Dr. Pavel Klein (Washington, DC hospitals) currently determines the safety and feasibility of using levetiracetam to decrease the risk of post-traumatic epilepsy. The first data from this trial indicate that levetiracetam was tolerated and safe and that pharmacokinetics in patients with TBI did not diffr substantially from healthy subjects or patients with chronic epilepsy (Klein et al., 2008).
For other AEDs, some indication for a disease-modifying effect in post-SE models was found for carbamazepine, diazepam, phenobarbital, and phenytoin (Table 2). Furthermore, several AEDs exerted neuroprotective activity (Table 2), which will be discussed later.
2. Novel Approaches for Antiepileptogenesis in Post-Status Epilepticus Models of Temporal Lobe Epilepsy.
As pointed out in section II, it is likely that antiepileptogenic drugs, if they exist, will have mechanisms of action distinct from traditional AEDs, because the molecular mechanisms underlying epileptogenesis and ictogenesis probably differ. Thus, a rational strategy for discovery of antiepileptogenic drugs would be testing of experimental compounds that interfere with one or several of the mechanisms underlying epileptogenesis (Fig. 1). However, this approach is complicated by the possibility that several of the processes underlying epileptogenesis also underlie neuronal repair, physiological compensation, and endogenous anticonvulsant or even antiepileptogenic processes (Walker et al., 2002). One strategy for identifying key causative changes is to determine whether they are common to multiple animal models (Jacobs et al., 2009). This approach was used in a meta-analysis of global gene expression studies of SE- and TBI-induced epileptogenesis (Lukasiuk et al., 2006). It is noteworthy that an especially prominent up-regulation of immune response genes was seen at all time points, indicating that anti-inflammation or immunosuppression may be therapeutic approaches for antiepileptogenesis. Further approaches discussed in the following include neuroprotection and neuromodulation.
a. Neuroprotection.
Because hippocampal damage has long been thought to be critically involved in the development of TLE, one potentially promising strategy for antiepileptogenesis is administration of neuroprotective drugs after a brain insult (Fisher et al., 1998; Willmore, 2005; Walker, 2007; Acharya et al., 2008). Such a strategy is reasonable because at least part of the brain damage develops after the initial insult, as a result of delayed (“programmed”) types of cell death (Fujikawa, 2005). However, various studies indicated that neuroprotection in epilepsy is not a straightforward concept (Tables 2 and 3). To our knowledge, the first demonstration that hippocampal neurodegeneration is not needed for epileptogenesis in symptomatic TLE models came from our laboratory (Ebert et al., 2002; Brandt et al., 2003b). In this study, we found that a single administration of a low dose (0.1 mg/kg) of the NMDA antagonist MK-801 after a kainate-induced SE of 90 min was capable of preventing most of the hippocampal and parahippocampal damage occurring in this model, but this treatment did not prevent the development of spontaneous seizures (Brandt et al., 2003b). A similar finding was obtained by starting prolonged treatment with valproate after 4 h of an electrically induced SE, which prevented hippocampal damage, including cell loss in the hilus, but did not prevent development of spontaneous seizures (Brandt et al., 2006a). These data thus substantiate the findings with MK-801 that an epileptogenic cascade, resulting in altered network excitability, may be triggered by SE, even in the absence of discernible neuronal injury in the hippocampal formation. It is noteworthy that although treatment with valproate after SE did not exert an antiepileptogenic effect, it did prevent development of most of the behavioral alterations after SE in rats (Brandt et al., 2006a). We are currently evaluating the optimal therapeutic window and dosage protocol for these effects of valproate. Our data indicate that, at least in the SE models used, overt hippocampal damage is not critically involved in the development of spontaneous recurrent seizures, but may play a role in the psychopathology associated with epilepsy. We presently prove this hypothesis by experiments with the AMPA antagonist 8-methyl-5-(4-(N,N-dimethylsulfamoyl)phenyl)-6,7,8,9-tetrahydro-1H-pyrrolo[3,2-h]-iso-quinoline-2,3-dione-3-O-(4-hydroxybutyric acid-2-yl)oxime (NS-1209), which has been shown to exert pronounced neuroprotective effects when administered after SE in rats (Table 3). However, it is impossible to exclude damage of subtle degrees in studies such as our experiments with valproate or NS-1209, so that such studies can demonstrate only that epilepsy can be induced in the absence of overt damage.
Numerous other studies substantiated our initial finding that overt hippocampal damage can be prevented or minimized by administration of a neuroprotective agent after SE of sufficient length to induce epileptogenesis but that such damage is not a prerequisite for epileptogenesis (André et al., 2007; Nehlig, 2007; Table 3). However, most neuroprotective agents used in this regard did not completely protect degeneration of dentate hilus cells, indicating that neurodegeneration in the dentate hilus is more resistant to neuroprotective agents. Loss of neurons in the hilus is a characteristic finding in most rodent models of TLE, including post-SE models (Sloviter, 1987; Dudek and Sutula, 2007). Furthermore, in patients with TLE and other types of partial epilepsy, the most consistent cell loss occurs in the hilus of the dentate gyrus (Margerison and Corsellis, 1966; Sloviter, 1994; Blümcke et al., 2000; Lowenstein, 2001; Nadler, 2003; Thom et al., 2009). Hilar cell loss observed in patients with TLE and in models of acquired partial epilepsy involves both excitatory mossy cells and inhibitory peptide-containing interneurons (Sloviter, 1987). There are two controversial explanations for how this hilar cell loss may result in hyperexcitability of dentate granule cells, which could be causal for increased seizure susceptibility or the development of spontaneous seizures. One prominent theory of epileptogenesis was based on the assumption that loss of mossy cells results in reduction of afferent excitatory drive onto insult-resistant inhibitory basket cells, rendering them “dormant” and granule cells hyperexcitable (Sloviter, 1987, 1991), but this hypothesis has now been refuted by multiple direct recording methods in different physiological experiments in different laboratories. Alternatively, loss of inhibitory interneurons in the hilus may lead to a loss of inhibitory synaptic input to granule cells that could contribute to the abnormal recurrent excitation of granule cells found in epileptic rats (Sloviter, 1987; Kobayashi and Buckmaster, 2003; Ratzliff et al., 2004). However, accumulating evidence indicates that in individual rats, epileptogenesis may develop independent of hilar cell loss in post-SE models and other models of TLE (Pitkänen et al., 2002; Nehlig, 2007). Examples from our own experiments in three different TLE models are illustrated in Fig. 4. Although average density of hilus neurons is significantly decreased in rats developing spontaneous recurrent seizures after either kainate (Fig. 4A), an electrically induced SE (Fig. 4B), or overkindling (Fig. 4C), some rats with spontaneous seizures do not differ in hilar cell density from nonepileptic control rats. When comparing rats which do or do not develop epilepsy after SE (Fig. 4B) or overkindling (Fig. 4C), those with spontaneous seizures show lower hilar cell counts on average than rats without such seizures; again, however, there is an overlap between both groups, indicating that hilar cell loss contributes to epileptogenesis but is not an absolute requirement. This is also substantiated by our data on MK-801 (Fig. 4A), showing that this NMDA antagonist reduces SE-induced hilar cell loss, but all rats still develop spontaneous seizures. However, in view of the widespread extrahippocampal damage in models with convulsive SE, such as the kainate model used for the experiments illustrated in Fig. 4A, spontaneous seizures may arise from outside the hippocampal formation (Sloviter et al., 2007). For ultimately testing the role of hilar neuron loss in TLE, it will be important to use models with spontaneous hippocampal-onset seizures such as the model described by Norwood et al. (2010), which is discussed in more detail in section III.C.3.g.
Fig. 4.
Lack of direct relationship between loss of dentate hilus neurons and development of spontaneous recurrent seizures (SRS) in three rat models of temporal lobe epilepsy. In all models, the occurrence of SRS was monitored in the chronic epileptic phase, and rats with or without observed SRS were differentiated. Hilus neurons were quantified with counting frames in serial sections, using stereological methods (see Brandt et al., 2003b for details). Sham controls were used for comparison. Each symbol illustrates the neuronal density in the hilus of one rat. The median of the individual data are indicated by horizontal line. In A, SE was induced by systemic administration of kainate (10 mg/kg i.p.) and terminated after 90 min by diazepam. Six rats were treated with 0.1 mg/kg MK-801 immediately after diazepam. Data are from six sham controls, seven rats with SE plus vehicle, and six rats with SE plus MK-801. Analysis of data by nonparametric analysis of variance (Kruskal-Wallis test) indicated a significant difference between means (P = 0.0082). Post hoc analysis by Dunn's test indicated that only the SE-vehicle rats differed significantly from controls (P < 0.01), suggesting a neuroprotective effect of MK-801. However, all except one of the MK-801-treated rats developed SRS. Data were reanalyzed from the study of Brandt et al. (2003a). In B, SE was induced by sustained electrical stimulation of the BLA. Data are from 6 sham controls and 26 SE rats (18 with SRS and 8 without observed SRS). The asterisk indicates a significant difference between the two groups (P = 0.0003). When SE rats with SRS (median neuronal density 4223 neurons/mm3) and without SRS (8042 neurons/mm3) were compared with controls (10,598 neurons/mm3), only the group with SRS differed significantly from controls (P < 0.001). However, note that several rats with SRS had neuronal densities within control range, indicating no direct relationship between hilar cell loss and development of SRS. Data are from the study of Brandt et al. (2003b) and unpublished experiments. In C, rats were kindled via the BLA and then further stimulated twice daily for up to approximately 280 stimulations (“overkindling”) until SRS were observed in approximately 50% of rats. Data are from 10 sham controls and 21 overkindled rats (10 with SRS and 11 without observed SRS). The asterisk indicates a significant difference between the two groups (P = 0.0011). When overkindled rats with SRS (median neuronal density 6294 neurons/mm3) and without SRS (7693 neurons/mm3) were compared with controls (10,371 neurons/mm3), only the group with SRS differed significantly from controls (P < 0.01). Data were reanalyzed from the study of Brandt et al. (2004).
Limbic seizures have often been attributed to pathology in the hippocampus, such as the well described condition termed Ammon's horn sclerosis, in which many of the hippocampal principal cells have degenerated (Fisher et al., 1998; Sloviter, 2008; Pitkänen and Lukasiuk, 2009). However, several studies in both the clinical and basic literature indicate that the parahippocampal region, including the piriform and entorhinal cortices, may also play an important role (Gale, 1992; Löscher and Ebert, 1996; Coulter et al., 2002; Nehlig, 2007; McIntyre and Gilby, 2008). This region sustains a characteristic pattern of damage in most animal models of TLE that is similar to that identified in humans with intractable TLE. Furthermore, the amygdala and several thalamic nuclei are often damaged in TLE models and patients with TLE (Margerison and Corsellis, 1966; Roch et al., 2002). On the basis of their series of studies on epileptogenesis and antiepileptogenesis in the lithium-pilocarpine model, Nehlig and colleagues (André et al., 2007; Nehlig, 2007) have suggested that neurodegeneration in the piriform and entorhinal cortices is an important factor early in the epileptogenic process, whereas the involvement of the hippocampus is delayed. Only the simultaneous protection of Ammon's horn and the parahippocampal cortices (plus the amygdala and thalamic nuclei) by carisbamate was able to largely delay or totally prevent the occurrence of spontaneous seizures, whereas treatments protecting only CA1 or CA2 were not effective. However, even with drugs such as carisbamate, which completely protected hippocampal formation and parahippocampal cortices from damage, some rats developed epilepsy (André et al., 2007), so that neuroprotection in the narrow definition of protecting neurons from death, even if necessary, may not be sufficient for antiepileptogenic therapy (Sankar, 2005). Thus, neuroprotection after SE should encompass not only the prevention of neuronal death, but also preservation of neuronal and network function (Walker, 2007). Overall, the clear structural heterogeneity in the human condition and the fact that spontaneous seizures can be induced in normal animals presumably without any damage argue against damage in any “key” or “critical” brain areas as a prerequisite for epileptogenesis. Nevertheless, even if neuroprotection does not allow preventing epilepsy, it may modify or prevent the development of learning and memory deficits and behavioral alterations associated often associated with epilepsy.
Apart from neuroprotection, there are interesting strategies aimed to repair the consequences of neurodegeneration, including cell transplantation and gene therapy (Löscher et al., 2008). In this respect, it is interesting to note that intrahippocampal injection of a vector expressing fibroblast growth factor-2 and brain-derived neurotrophic factor (BDNF) 4 days after a pilocarpine-induced SE has been reported to increase neurogenesis, limit or partially repair the hippocampal damage, and exert antiepileptogenic and disease-modifying effects (Paradiso et al., 2009). However, whether BDNF exerts an epileptogenic or antiepileptogenic function remains controversial because contrasting effects of BDNF have been reported (Koyama and Ikegaya, 2005). Various studies have shown that BDNF increases neuronal excitability via tyrosine kinase receptor B (TrkB), the high-affinity receptor for BDNF, and may contribute to epileptogenesis (Binder et al., 2001; Koyama and Ikegaya, 2005). On the other hand, several reports demonstrate that intrahippocampal infusion of BDNF can attenuate (or retard) the development of epilepsy. This antiepileptogenic effect seems to be mediated mainly by an increase in the expression of NPY (Koyama and Ikegaya, 2005). Thus, inhibiting BDNF-TrkB signaling and reinforcing the NPY system in the adult hippocampus seem to be potential therapeutic strategies for TLE (Koyama and Ikegaya, 2005). In this respect, it is interesting to note that valproate has been shown to down-regulate BDNF and TrkB in the epileptogenic hippocampus of patients with TLE (Hou et al., 2010).
b. Anti-Inflammation.
Another rational strategy for preventing or reducing the long-term consequences of brain insults is anti-inflammation. There is accumulating preclinical and clinical evidence that different types of brain insults, including SE, induce inflammatory processes in the brain that may critically contribute to epileptogenesis (Vezzani and Granata, 2005). Thus, SE provoked experimentally in rodents triggers a prominent inflammatory response in brain areas recruited in the onset and propagation of epileptic activity (Vezzani and Granata, 2005; Vezzani and Baram, 2007). This seizure-induced brain inflammation involves both the innate and the adaptive immune systems and shares molecules and pathways also activated by systemic infection (Vezzani and Granata, 2005). Various pro-inflammatory mediators are induced by SE in the brain, including cytokines such as IL-1β, IL-6, or tumor necrosis factor-α; complement; and cyclooxygenase-2 (COX-2), which is responsible for generation of prostaglandins (PGs) from arachidonic acid (Vezzani and Granata, 2005). Increase of these pro-inflammatory mediators is thought to be involved in impairment of blood-brain barrier function, neurodegeneration, and the neuronal hyperexcitability developing after SE (Vezzani and Granata, 2005; Vezzani and Baram, 2007). Thus, prevention or reduction of the increase of inflammatory cytokines seems to be a plausible approach for antiepileptogenesis, but it should be kept in mind that cytokines are also prominent modulators of normal synaptic functions. On the basis of the potential role of inflammation in epileptogenesis, Jung et al. (2006) administered the COX-2 inhibitor, celecoxib, after a pilocarpine-induced SE in rats (Table 3). Compared with vehicle-treated controls, treatment with celecoxib prevented neuronal damage in the hippocampus and reduced the incidence and frequency of spontaneous recurrent seizures (i.e., a true antiepileptogenic effect). This important finding prompted us to perform a similar study with the more selective COX-2 inhibitor, parecoxib. Parecoxib was administered twice daily at 10 mg/kg over 18 days, starting immediately after a pilocarpine-induced SE of 90-min duration. This treatment reduced hippocampal damage and the impairment of learning and memory in the Morris water maze test but did not prevent the development of spontaneous seizures, although seizures were less severe compared with those in control rats (Polascheck et al., 2010). Thus, anti-inflammation by COX-2 inhibition seems to constitute an interesting novel approach for disease modification after brain insults such as SE. However, a study with the COX-2 inhibitor 4-(5-(4-chlorophenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzenesulfonamide (SC58236) found no disease-modifying or neuroprotective effect when rats were treated after an electrically induced SE (Holtman et al., 2009). The most likely explanation for this different outcome of studies with prophylactic administration of COX-2 inhibitors after SE is the duration of the initial brain insult. Jung et al. (2006) terminated SE after 60 min by diazepam and Polascheck et al. (2010) after 90 min by diazepam, whereas Holtman et al. (2009) terminated SE after 4 h by isoflurane anesthesia. However, SE was only transiently interrupted by anesthesia, and continued for several more hours thereafter, resulting in a total SE duration of approximately 9 to 10 h (Holtman et al., 2009). Holtman et al. (2009) suggested that the long duration of SE negatively interfered with the outcome of COX-2 inhibition that started within this period. Thus, such technical details are very important when comparing studies on prophylactic drug treatment after SE.
Fabene et al. (2008) studied leukocyte-endothelial interaction as a potential target for the prevention and treatment of epilepsy. Using the pilocarpine mouse model of TLE, they showed that seizures induce elevated expression of vascular cell adhesion molecules and enhanced leukocyte rolling and arrest in brain vessels mediated by the leukocyte mucin P-selectin glycoprotein ligand-1 and leukocyte integrins α4β1 and αLβ2. Treatment of mice with an α4 integrin-specific monoclonal antibody after SE exerted disease-modifying effects, including reduced frequency of spontaneous seizures, less severe blood-brain barrier damage, and reduced neurodegeneration (Table 3). However, it remains to be determined whether the effect of treatment on frequency of spontaneous seizures also is maintained after termination of treatment.
Another strategy to interfere with inflammatory processes during epileptogenesis would be antagonizing the release or effects of IL-1β, which is a key player in the onset of insult-induced inflammation (Vezzani and Baram, 2007). Interesting drugs in this respect include the IL-1β antagonist anakinra and the caspase-1 inhibitor 1-(2-((1-(4-amino-3-chlorophenyl)methanoyl)amino)-3,3-dimethylbutanoyl)pyrrolidine-2-carboxylic acid (VX-765) (Vezzani et al., 2010). Studies are under way to test the antiepileptogenic potential of these drugs in different post-SE models of SE.
c. Immunosuppression.
Although numerous downstream mechanisms may mediate epileptogenesis, less is known about initial signaling pathways that trigger the subsequent changes in the brain causing epilepsy. The mammalian target of rapamycin (mTOR), a conserved serine/threonine protein kinase, is crucial for many forms of synaptic plasticity in the adult brain (Cao et al., 2009). Studies have revealed that mTOR activity is up-regulated or down-regulated in brain tumors, tuberous sclerosis complex, cortical dysplasia, traumatic brain injury, and several neurodegenerative disorders (Inoki et al., 2005; Hoeffer and Klann, 2010). Zeng et al. (2009) first demonstrated that the mTOR pathway was up-regulated after a kainate-induced SE in a biphasic manner, correlating with the development of chronic epileptogenesis in the hippocampus. When the mTOR inhibitor rapamycin, which is clinically used as an immunosuppressant, was administered after termination of SE, it blocked the chronic phase of mTOR activation and reduced mossy-fiber sprouting and the frequency of spontaneous seizures but not neurogenesis or neuronal death (Zeng et al., 2009). Suppression of SE-induced mossy-fiber sprouting by rapamycin has also been reported in the pilocarpine model of TLE (Buckmaster et al., 2009). However, suppression required continual treatment, and rapamycin treatment did not reverse already established axon reorganization (Buckmaster et al., 2009). Whether rapamycin exerted a disease-modifying effect after SE or simply an anticonvulsant effect in the study of Zeng et al. (2009) is not known, because spontaneous seizures were recorded only during the treatment phase. Likewise, Zeng et al. (2008) reported previously strong antiepileptogenic effects of rapamycin in a mouse model of tuberous sclerosis, but epileptic activity was assessed only during treatment, so that a direct anticonvulsant effect of this drug may explain the findings. In this respect, it is important to note that another immunosuppressant, FK506 (tacrolimus) exerted no disease-modifying or antiepileptogenic effects in a post-SE model of TLE but instead aggravated development and severity of seizures in this model (Lukasiuk and Sliwa, 2009). However, topographic and quantitative elemental analysis of rat brain tissue, with the use of multielemental analysis of thin tissue slices by the synchrotron-based X-ray fluorescence technique, indicated that FK506 exerted neuroprotective effects in the pilocarpine model (Chwiej et al., 2010).
d. Neuromodulation.
The fourth and possibly most promising rational strategy for preventing or modifying epileptogenesis and its consequences is to counteract the development of neuronal hyperexcitability after brain insults by administering neuromodulatory drugs (Table 3). It is noteworthy that a number of studies have shown that administration of different CNS-stimulating drugs, including the adenosine antagonist caffeine, the α2 receptor antagonist atipamezole, and the cannabinoid (CB)-1 receptor antagonist rimonabant (SR141716A) exert neuromodulatory and/or antiepileptogenic and neuroprotective effects in epilepsy models (Rigoulot et al., 2003; Pitkänen et al., 2004; Chen et al., 2007; Echegoyen et al., 2009; but see Pouliot et al., 2009). Paradoxically, all these compounds exert proconvulsant activity in normal animals, so that brain insults such as SE seem to change the pharmacology of these compounds. This is obviously a consequence of the molecular reorganization that develops after brain insults, resulting in alterations in the subunit composition and expression of receptors and ion channels and, thus, their functions and pharmacology (Coulter, 2001; Coulter et al., 2002; Stefan et al., 2006). Furthermore, brain insults seem to induce a shift from adult to neonatal receptor and ion channel functions, indicating that epileptogenesis recapitulates ontogenesis (Köhling, 2002; Ben-Ari and Holmes, 2005).
Such a shift in GABAergic response polarity from hyperpolarizing to depolarizing has been described in human epileptic neurons recorded in the subiculum of hippocampal slices obtained from resections in patients suffering from mesial TLE (Cohen et al., 2002). This shift is thought to be a result of increased intraneuronal Cl− levels, caused by increased neuronal expression of NKCC1, an inwardly directed Na+K+2Cl− cotransporter that facilitates the accumulation of intracellular Cl−, and down-regulation of KCC2, an outwardly directed K+Cl− cotransporter (Köhling, 2002; Ben-Ari and Holmes, 2005; Rivera et al., 2005; Palma et al., 2006). Up-regulation of NKCC1 and down-regulation of KCC2 in the hippocampus have been described both in patients with TLE and in the kindling and pilocarpine models of TLE (Okabe et al., 2002, 2003; Rivera et al., 2002; Palma et al., 2006; Huberfeld et al., 2007; Pathak et al., 2007; Li et al., 2008b). This prompted us to evaluate whether inhibition of NKCC1 after SE affects the development of epilepsy in rats (Brandt et al., 2010). The diuretic bumetanide was used for these experiments, administered either alone or in combination with phenobarbital. Because bumetanide is very rapidly eliminated by rats and does not penetrate very well into the brain, various dosing protocols of bumetanide were evaluated in our experiments. Our data indicated no beneficial effects of bumetanide alone, but a combination of bumetanide and phenobarbital retarded development of epilepsy and reduced frequency of spontaneous seizures (Table 3). However, this effect was not significantly different from treatment with phenobarbital alone. We currently test various prodrugs of bumetanide to enhance its penetration into the brain of adult rats and mice. Furthermore, on the basis of the observations with proconvulsant drugs (Table 3), we have started experiments in which we administer the GABAA receptor antagonist pentylenetetrazole at subconvulsant doses after SE to examine whether this treatment modifies epileptogenesis. Our concept of administering a GABAA receptor antagonist after SE is based on two apparently opposing hypotheses: 1) the shift from inhibitory to excitatory GABA actions that is believed to contribute to the development of neuronal hyperexcitability in the hippocampus (see above) and 2) enhanced GABAergic inhibition that may increase network synchronization and thus contribute to epileptogenesis (Khazipov and Holmes, 2003; Mann and Mody, 2008). It remains to be established whether moving beyond anticonvulsants—even to proconvulsants—will allow us to find the ideal preventative strategy for acquired epilepsy (Armstrong et al., 2009).
Another novel and promising neuromodulatory strategy for antiepileptogenesis is based on findings with the inhibitory neuromodulator and endogenous anticonvulsant adenosine, which is largely regulated by astrocytes and its key metabolic enzyme adenosine kinase (ADK; Boison, 2008). On the basis of findings in mouse models of epileptogenesis, Boison (2008) proposed a “ADK hypothesis of epileptogenesis”:
Mouse models of epileptogenesis suggest a sequence of events leading from initial down-regulation of ADK and elevation of ambient adenosine as an acute protective response, to changes in astrocytic adenosine receptor expression, to astrocyte proliferation and hypertrophy (i.e., astrogliosis), to consequential overexpression of ADK, reduced adenosine and—finally—to spontaneous focal seizure activity restricted to regions of astrogliotic overexpression of ADK.
Transgenic mice overexpressing ADK display increased sensitivity to brain injury and seizures.
Conversely, after pharmacological induction of an otherwise epileptogenesis-precipitating acute brain injury, transgenic mice with reduced forebrain ADK are resistant to subsequent epileptogenesis.
Intrahippocampal implants of stem cells engineered to lack ADK prevent epileptogenesis in mice in which epileptogenesis is induced by injection of kainate into the amygdala (Li et al., 2008a).
Thus, ADK emerges both as a diagnostic marker to predict epileptogenesis as well as a prime therapeutic target to prevent it (Boison, 2008). However, pharmacological approaches to manipulate the adenosine system in the brain by systemic administration of ADK inhibitors or adenosine receptor agonists and adenosine transport inhibitors are limited by strong systemic side effects, so that new strategies have been developed aimed at the local reconstitution of the inhibitory adenosinergic tone by focal brain implants of adenosine-releasing cells or by RNA interference-based gene therapies aimed at down-regulating the astrogliotic overexpression of ADK during disease progression (Boison, 2008). Such invasive procedures, of course, significantly limit their clinical use for prevention of epilepsy in patients at risk. In addition to adenosine, several other endogenous neuromodulators, including neuropeptide Y and galanin, have been used in in vivo gene therapy approaches to epilepsy (Löscher et al., 2008).
A further potentially interesting target for antiepileptogenesis is the T-type Ca2+ channel Cav3.2, which has been suggested as a central player in epileptogenesis (Becker et al., 2008). One of the most striking examples of intrinsic plasticity in SE-triggered epileptogenesis is the conversion of hippocampal pyramidal neurons from regular firing, which is the predominant spiking mode in normal conditions, to burst firing a few days after SE (Beck and Yaari, 2008). Detailed pharmacological experiments have suggested that aberrant bursting after SE is caused mainly by an up-regulation of T-type Ca2-channels in the apical dendrites (Beck and Yaari, 2008). The up-regulation of T-type Ca2-currents is the consequence of a transient transcriptional up-regulation of Cav3.2 channels after SE (Becker et al., 2008). Genetic deletion of Cav3.2 prevents this form of intrinsic plasticity, protects against SE-induced neuropathological hippocampal damage, and ameliorates the development of chronic epilepsy in the pilocarpine model in mice (Becker et al., 2008). Thus, development of selective blockers of Cav3.2 or of inhibitors of Cav3.2 up-regulation may provide a novel strategy to inhibit or modify epileptogenesis. However, the T-type calcium channel blocker ethosuximide is inactive in the pilocarpine model (Leite and Cavalheiro, 1995) and does not block epileptogenesis in other models such as kindling (Turner et al., 1977). Furthermore, development of aberrant bursting after SE seems to be a specific phenomenon of the pilocarpine model and does occur less markedly or not at all in other post-SE models of TLE (i.e., the kainate model and epilepsy developing after SE induced by electrical stimulation of the BLA) (Y. Yaari, C. Brandt, and W. Löscher, unpublished observations).
3. Problems Associated with Drug Testing in Post-Status Epilepticus Models of Temporal Lobe Epilepsy.
As shown in Tables 2 and 3, drug administration after SE has become a widely used model in the search for antiepileptogenic agents and is often considered more relevant in this respect than kindling or other models of symptomatic or genetic epilepsies. However, there are many conceptual, logistical, and experimental problems associated with the use of post-SE models, so that lack of antiepileptogenic efficacy in such models may have various explanations, including the model, the study design, or the compound used (Pitkänen, 2002). It is not easy to resolve such problems without knowing whether drug testing in post-SE models would identify an antiepileptogenic drug if it were to exist. Perhaps the only way to resolve this dilemma would be validation of the models by a drug that effectively prevents epilepsy in humans. Such a drug, however, does not yet exist or has not been identified so far.
a. Duration and Severity of Status Epilepticus.
Over the more than 15 years that SE models have been extensively used for assessing the effects of drugs on SE and its long-term consequences, we have learned a great deal about how to best use these models in the search for disease-modifying or antiepileptogenic therapeutics. As discussed above, when drugs are administered before or during the SE, any resulting reduction in the severity or duration of SE will reduce or prevent its long-term consequences, which is termed “initial insult modification” and should be differentiated from true antiepileptogenic or disease-modifying effects achieved when a drug is administered after an SE of sufficient length to fully induce the epileptogenic cascade (Fig. 1). Any clinical strategy for prevention, early interruption, or modification of an initial precipitating injury such as TBI, infection, or SE is a powerful means for forestalling the development of abnormal excitatory circuitry in the injured brain (Acharya et al., 2008). Examples for initial insult modification in SE models are given in Tables 2 and 3.
Different experimental protocols have been used in the search for drugs that are capable of improving the long-term consequences of a brain insult when administered at delayed time points after the insult (Tables 2 and 3). One is to terminate SE by an AED (e.g., diazepam) in both vehicle control and drug groups and then start treatment with the investigational drug immediately thereafter. The advantage of this approach is that SE duration should be the same in all animals, thus eliminating the bias of varying SE duration on long-term outcome. However, particularly in chemical SE models, it is very difficult to terminate SE. High doses of diazepam, other AEDs, or general anesthetics typically suppress the motor seizures and reduce or suppress paroxysmal EEG discharges, but electrographic and clinical seizures often recur later. Thus, when combining the drug used for SE interruption with the investigational drug, SE termination may be more effective than in control subjects, which may affect the long-term consequences of SE. It is therefore very important to use video and EEG monitoring during SE and for up to at least 24 h after termination of SE to document any differences in SE duration between groups. For instance, in our study on MK-801 in the kainate model, we demonstrated by video-EEG monitoring that the neuroprotective activity of this treatment was not secondary to more efficacious SE termination (Brandt et al., 2003b). It is noteworthy that the drug used for terminating SE may also affect its long-term consequences. Thus, after we found that a combination of diazepam and phenobarbital was much more effective in terminating a pilocarpine-induced SE than either drug alone (Bankstahl and Löscher, 2008), we used this combination in antiepileptogenesis studies (e.g., Brandt et al., 2010) and determined that neurodegeneration in rats in which SE was terminated by diazepam plus phenobarbital is much less severe compared with rats in which SE was terminated by diazepam alone. We are currently investigating whether this is a result of more effective SE termination or of a neuroprotective effect of phenobarbital in combination with diazepam. In this respect, it is also important to note that a combination of diazepam and the NMDA antagonist ketamine has been reported as an effective means for terminating SE in the pilocarpine and kainate models (Martin and Kapur, 2008; Vermoesen et al., 2010). This, however, is not a suitable strategy for SE termination when testing drugs for antiepileptogenic effects, because a single dose of an NMDA antagonist such as ketamine and MK-801 is capable of altering the long-term consequences of SE (Table 3).
Compared with chemical SE models, electrically induced SE is easier to suppress by drugs such as diazepam, at least in part because SE induced by systemic administration of pilocarpine or kainate is typically more severe than electrically induced SE (Bankstahl and Löscher, 2008). Another experimental protocol often used in antiepileptogenesis studies involves no cessation of SE duration, and the test drug is administered at delayed time-points after the SE (Tables 2 and 3). However, Gao et al. (2007) reported that periodic hippocampal paroxysmal discharges occur for up to 24 to 72 h after onset of lithium-pilocarpine induced SE, so that onset of drug treatment after 24 h, for example, may induce alterations in SE duration compared with untreated SE control subjects. Furthermore, the large interindividual variation in SE duration forms a bias for antiepileptogenesis studies, which is a further argument for terminating SE at the same time in all animals per group. A third experimental protocol used in some studies involves SE that is terminated by an AED such as diazepam in SE control subjects, but only the investigational drug (e.g., topiramate) is used in the antiepileptogenesis group. The potential bias in this scenario is that the investigational drug may be more effective in blocking SE than the AED used in the control group, resulting in modification of the initial insult.
Another problem related to severity or duration of SE is the use of different SE models in antiepileptogenesis studies. As discussed above, SE induced by systemic administration of pilocarpine and kainate is typically more severe and associated with strikingly higher mortality and brain damage than electrically induced SE. As a result, it is possibly much more difficult to modify the consequences of chemically than electrically induced SE. Two studies on valproate constitute one example. In our study (Brandt et al., 2006a), in which we administered valproate 4 h after onset of a self-sustained SE induced by prolonged stimulation of the BLA, valproate completely prevented neuronal damage in the hippocampal formation, including the hilus. In the subsequent study by Jessberger et al. (2007), with administration of valproate 5 h after onset of a kainate-induced SE, no neuroprotective effect was observed in the hippocampal formation. In addition to differences in the induction and severity of SE, both groups used different dosing protocols for valproate. Furthermore, starting treatment at 5 h after onset of kainate-induced SE may be too late for effective neuroprotection in this model, because neurodegeneration starts within a few hours after induction of SE by kainate or pilocarpine (Cavalheiro et al., 2006; Dudek et al., 2006).
The impact of SE induction and severity on neuroprotection or antiepileptogenesis was also demonstrated by Cunha et al. (2009), who used intrahippocampal injection of pilocarpine to induce SE. SE was interrupted after 3 h by thiopental, followed 1 h later by treatment with different AEDs (diazepam, carbamazepine, phenytoin) or the NMDA antagonist ketamine. All drugs reduced cell loss in the hippocampal formation, including the hilus, and reduced SE-induced impairment of learning and memory (Cunha et al., 2009). These effects were clearly more marked than respective effects found after systemic administration of chemoconvulsants (Tables 2 and 3), substantiating the idea that the SE model used has a striking effect on the outcome of antiepileptogenesis studies.
b. Convulsive versus Nonconvulsive Status Epilepticus.
Except for the study by Cunha et al. (2009), which involved focal intrahippocampal injection of pilocarpine, all antiepileptogenesis studies have used systemic administration of either pilocarpine or kainate, which induces a severe generalized convulsive SE (summarized in Tables 2 and 3). Furthermore, the different electrical stimulation-based SE models have typically used stimulation protocols that induce a self-sustained convulsive SE that continues for hours after termination of stimulation. As discussed by Sloviter and colleagues (Sloviter, 2005, 2008, 2009; Sloviter et al., 2007), rats subjected to prolonged convulsive SE exhibit several inherent problems as models of TLE. Such rats often exhibit variable hippocampal damage in both hemispheres, and extensive bilateral extrahippocampal neurodegeneration (Sloviter, 2005; Sloviter et al., 2007). Spontaneous seizures in these animals appear to arise from various locations, possibly as a result of widespread and extensive brain damage, and the hippocampus may be involved only secondarily (Harvey and Sloviter, 2005; Sloviter et al., 2007), because convulsive SE that is initiated chemically or electrically is often allowed to continue for hours in a “self-sustained” manner, so that seizure activity propagates in different pathways in different animals, frequently bypasses the hippocampus, and results in significant interanimal variability in the location and extent of brain damage (Sloviter et al., 2007). The severity and extent of the damage and variability seen after convulsive SE makes it difficult to show that any treatment has a statistically significant antiepileptogenic or disease-modifying protective effect (Sloviter et al., 2007). Furthermore, after a prolonged severe convulsive SE, rats often exhibit only a very short latent period (or no latent period at all) before onset of spontaneous seizures (Bumanglag and Sloviter, 2008; Sloviter, 2008; Dudek, 2009), which would explain why almost all attempts to prevent epileptogenesis after convulsive SE have failed (see section III.C.3.g). As discussed in section III.C.3.g, this problem can be reduced by restricting the duration of the convulsive SE by AEDs or general anesthetics, and by using ramp-up dosing protocols for chemoconvulsants, which allow for a more individual dosing than bolus injections, but animals still show high interindividual variability in the duration of the latent period and the extent of brain damage.
Most of the problems of models with sustained generalized SE induced by chemical or electrical means can be resolved by focal (intra-amygdalar or intrahippocampal) unilateral injection of convulsants such as kainate or pilocarpine, which typically causes a nonconvulsive (limbic or focal) type of SE that induces much less widespread brain damage (Cavalheiro et al., 2006; Dudek et al., 2006; Curia et al., 2008). Furthermore, Sloviter and colleagues (Sloviter et al., 2007; Norwood et al., 2010) have developed new models in which prolonged focal, nonconvulsive SE is induced by perforant path stimulation. The minimal interindividual variability in brain damage and increased duration of the latent period in such models are excellent prerequisites for studies on antiepileptogenesis and disease-modification (see section III.C.3.g).
c. Window of Opportunity in Post-Status Epilepticus Models of Temporal Lobe Epilepsy.
The fact that various clinically used AEDs (Table 2) as well as several investigational drugs (Table 3) exerted neuroprotective and disease-modifying effects in SE models when administered after an SE of sufficient length to induce epileptogenesis clearly argues in favor of a window of opportunity during which the long-term consequences of the initiating brain insult can be prevented or at least modified. However, it is not sufficiently clear when to start treatment and how long to continue it. On the basis of studies with the CB1 receptor antagonist rimonabant, Armstrong et al. (2009) have argued that the time-window during which potential antiepileptogenic agents are able to act may be much smaller—on the order of minutes rather than hours or days—than has been investigated in previous clinical and most experimental studies on antiepileptogenesis. Soltesz and colleagues reported that rimonabant prevents the development of increased seizure susceptibility when administered during or shortly after a brain insult in models of prolonged febrile seizures (Chen et al., 2007) or TBI (Echegoyen et al., 2009), but such treatment was not effective in modifying the development of spontaneous recurrent seizures in the kainate SE model (Pouliot et al., 2009). This indicates either that the same drug exhibits varying effects in different models of epileptogenic brain insults or, as argued by Dudek (2009), that determining seizure threshold as a measure of increased neuronal hyperexcitability and hence epileptogenesis (as done in the studies by Soltesz and colleagues) is highly susceptible to errors and is indirect, at best.
In apparent contrast to the assumption of Armstrong et al. (2009) that the window of opportunity after a brain insult may be very short, Pitkänen et al. (2004) found that administration of the α2 receptor antagonist atipamezole exerted impressive disease-modifying effects when treatment was started 7 days after an electrically induced SE (Table 3). Thus, the time-window for antiepileptogenesis or disease modification may depend on the severity or nature of the initiating brain insult. On the basis of current evidence in post-SE models, treatment in such models should start early and should be continued for at least 1 to 2 weeks.
The concept of an extended “therapeutic window” or latent period after brain injury has been questioned (Sloviter, 2008; Dudek, 2009). The duration of the latent period (i.e., the time from brain insult to the first spontaneous electrographic or motor seizure) has traditionally been considered to be a measure of epileptogenesis, which formed the conceptual basis for the design and interpretation of antiepileptogenesis studies in post-SE models of TLE (Sloviter, 2008; Dudek, 2009). However, studies of rats monitored continuously after a pilocarpine-induced convulsive SE (which was terminated after 3 h by urethane) have reported that spontaneous seizures may begin immediately after convulsive SE, coincident with the initial injury, indicating that epileptogenesis may just be a network excitability change due to cell death (Harvey and Sloviter, 2005; Bumanglag and Sloviter, 2008). Similar observations have been reported from experiments in which induction of convulsive SE was performed by bilateral perforant path stimulation for 3 h (Bumanglag and Sloviter, 2008). On the basis of their observations, Sloviter and colleagues (Bumanglag and Sloviter, 2008; Sloviter, 2008; Norwood et al., 2010) suggested that the latent period, when it exists, is simply a kindling or kindling-like process that changes initially subclinical focal epileptiform events to clinically detectable seizures. From this network perspective, epilepsy, defined at minimum as a state of abnormal focal discharges, develops at the time of the initial injury, with the latent period involving a progressive process that gradually lowers the seizure threshold (“kindling”), rather than a “silent” pre-epileptic period after injury that awaits the development of a distinct secondary process (Sloviter, 2008).
An alternative view was presented by Dudek (2009). On the basis of data from nearly continuous surface cortical and bilateral hippocampal recordings with radiotelemetry and semiautomated seizure detection to monitor the onset and frequency of seizures after kainate-induced SE in adult rats, Williams et al. (2009) have suggested that the latent period is the first of many long interseizure intervals and a poor measure of the time frame of epileptogenesis.
However, correct measurement of the latent period, if it exists, is affected by a number of factors. First, if SE is not effectively terminated, periodic paroxysmal discharges may recur over several days, as reported by Gao et al. (2007). Second, as with other epileptogenic brain insults, such as TBI or stroke (Beghi et al., 2010), insult-associated acute symptomatic seizures may occur in the first 1 to 2 days after SE, which should not be confounded with spontaneous seizures. Third, the severity and duration of SE determines the duration of the latency until the first spontaneous seizures. For instance, using continuous video-EEG recordings (with depth electrodes in the dentate gyrus) in the repeated low-dose pilocarpine model that we used in most of our antiepileptogenesis studies, latency to spontaneous seizures is approximately 1 week after SE in most rats (M. Rattka and C. Brandt, unpublished observations) and similar or longer latent periods have been reported by several other studies with continuous monitoring in different post-SE models of TLE (Williams et al., 2007; Sloviter, 2008). In this respect, it is important to note that the duration of the latent period depends on the type of induction and severity of the initial SE, ranging from approximately 7 days in the pilocarpine model (Goffin et al., 2007) to >30 days in models with electrical induction of SE (Nissinen et al., 2000). The fact that drug treatment during this period affects the long-term consequences of SE (Tables 2–3) clearly argues in favor of the concept that the latent period offers a window of opportunity to modify epileptogenesis.
d. Dosing Protocols for Studying Antiepileptogenic or Disease-Modifying Drugs in Post-Status Epilepticus Models.
Another unresolved question is which doses of a drug are best suited to interfere with epileptogenesis in post-SE models. Because antiepileptogenesis trials in such models are extremely time- and labor-expensive, it is practically not possible to test several doses of a drug in parallel. Thus, negative findings in drug trials may be due to the use of too high or too low doses. An example is the study on topiramate in the lithium-pilocarpine model by Suchomelova et al. (2006), in which a dose of 50 mg/kg was less effective than 10 mg/kg to prevent the development of epilepsy. Thus, if possible, the optimal drug dosing should be determined in preliminary experiments. An example is shown in Fig. 5, illustrating dose-finding experiments with the COX-2 inhibitor parecoxib.
Fig. 5.
Illustration of a dose-finding experiment for an experimental trial with the COX-2 inhibitor parecoxib (Pcb) in the pilocarpine model of TLE in rats. A lithium-pilocarpine–induced SE was terminated after 60 min by diazepam plus phenobarbital. Twenty-four hours after SE induction, PGE2 levels were significantly increased in hippocampus, piriform cortex, amygdala, and frontal cortex. Additional groups of rats were treated with Pcb at either 1 or 5 mg/kg. Pcb was administered 1, 7, and 22 h after onset of SE. Both dosing protocols completely prevented the increase in PGE2 after SE, thus allowing rational selection of a dosing protocol for an epilepsy prevention trial. Data are means ± S.E.M. of four to six rats per group; statistical analysis was performed by analysis of variance with post hoc Bonferroni test (*, P < 0.05). Data are from unpublished experiments (N. Polascheck, M. Bankstahl, W. Löscher).
In addition to the choice of an adequate dose, the dosing interval is important for the effects of the treatment. Rodents such as rats and mice eliminate most drugs much more rapidly than humans (Löscher, 2007). Thus, knowledge about elimination rate of a test drug in a laboratory species is essential for development of a treatment paradigm that allows maintaining adequate drug levels in the system over the period of treatment. This is often not dealt with in antiepileptogenesis studies and may be involved in the negative outcome of such studies. Different technologies for continuous drug delivery, including implantable osmotic minipumps, can be used to resolve this problem (Löscher, 2007).
e. Outcome Measures in Post-Status Epilepticus Models of Temporal Lobe Epilepsy.
Targets include the prevention or modification of epilepsy, improved behavioral or cognitive outcomes, the prevention of pharmacoresistance, or neuronal loss (Stables et al., 2003). Incidence and frequency of spontaneous seizures is the most frequently used outcome measure in antiepileptogenesis trials (Tables 2 and 3). However, the frequency of spontaneous seizures in post-SE models is highly variable between individual rats and even in the same rats, and several rats exhibit long interseizure intervals and large interval differences. Thus, these features substantially increase the time intervals over which animals must be monitored to avoid false-negative or false-positive conclusions (Dudek et al., 2008). However, most studies summarized in Tables 2 and 3 used seizure monitoring over only 1 to 2 weeks, and some studies did not use continuous video-EEG recording, but animals were recorded only some days per week or some hours per day. Continuous video-EEG recording in groups of rats, typically 8 to 10 control rats and 8 to 10 drug-treated animals, is technically difficult, time consuming, and expensive, necessitating adequate equipment for parallel 24-h monitoring of so many rats. The use of historical controls should be avoided, because seizure frequency may markedly vary among different batches of rats and may also be affected by the season. A further problem is the lack of adequate programs that allow automatic seizure detection in the rats' EEG, so that most groups performing antiepileptogenesis studies analyze the EEG visually, resulting in an enormous commitment of personnel. The available commercial programs that have been developed for patients with epilepsy are not suited for animal experiments, because they are not optimized for analysis of long-term (i.e., months long) recordings of continuous EEG data from small electrode arrays acquired in animal models of epileptogenesis and chronic epilepsy (White et al., 2006). Some algorithms for automatic EEG detection of seizures in rodents have been reported (White et al., 2006; de Araujo Furtado et al., 2009), but these programs are not yet validated and generally available. It should be noted that even when EEG is obtained in an optimal recording paradigm, sampling errors with recording result in considerable limitations of EEG as a tool for seizure quantification.
Surrogate markers for epileptogenesis include alterations in seizure threshold (Dudek et al., 2008), interictal spikes (Staley et al., 2005; White et al., 2010), high-frequency oscillations (“ripples”) in depth EEG recordings (Engel et al., 2009), and electrophysiological correlates of hippocampal hyperexcitability such as extracellular field potentials (Armstrong et al., 2009; Gorji and Speckmann, 2009). To our knowledge, however, none of these measures has been shown to correlate with an effect of drug treatment on development of spontaneous seizures after SE. For instance, in the study by Margineanu et al. (2008), administration of levetiracetam after a pilocarpine-induced SE was shown to prevent alterations in hippocampal field responses, but the same group (Klitgaard et al., 2001) reported that this treatment did not reduce the incidence of rats developing epilepsy, which was subsequently confirmed by our group in another SE model (Brandt et al., 2007). Dudek et al. (2008) criticized our study because we monitored the animals for only 1 week by video-EEG. However, in both control and drug-treated groups, seven of eight rats exhibited spontaneous seizures during this period, excluding any antiepileptogenic effect of levetiracetam (Brandt et al., 2007). It is difficult to understand how longer video-EEG monitoring would have changed this conclusion, but we may have missed a disease-modifying effect (e.g., reduced seizure frequency) by the short seizure monitoring used for our study.
Typically, rats are killed at the end of an antiepileptogenesis trial to investigate histological brain alterations (Fig. 3). In recent years, small animal magnetic resonance imaging (μMRI) has increasingly been used to determine brain alterations at different times after SE (André et al., 2007; Gröhn and Pitkänen, 2007; Pitkänen et al., 2007a). Several studies have demonstrated that μMRI can be used to associate the progressive development of brain pathology with the evolution of clinical phenotype (Gröhn and Pitkänen, 2007), and researchers have started to include this technology in studies on antiepileptogenesis (François et al., 2006; André et al., 2007).
An interesting novel idea is that, although administration of a neuroprotective drug after SE does not prevent development of spontaneous seizures (see section III.C.2.a), it may improve the prognosis of treatment of such seizures. Two findings of our group formed the backbone for this hypothesis. First, we found that rats developing epilepsy after an SE induced by sustained electrical BLA stimulation differ strikingly in their response to phenobarbital (Brandt et al., 2004b). In approximately 30% of the rats, the spontaneous seizures did not respond to treatment, although seizures were suppressed in the other rats, resulting in two subgroups (i.e., responders and nonresponders) (Brandt et al., 2004b). This finding was confirmed in several subsequent studies (Volk et al., 2006; Bethmann et al., 2007). Second, nonresponders exhibited hippocampal damage, whereas most responders did not, indicating a causal relationship between neuronal damage and pharmacoresistance (Volk et al., 2006; Bethmann et al., 2008). This finding in our rat model of TLE is in line with the clinical observation that hippocampal sclerosis is associated with poor prognosis of AED treatment in patients with TLE but is a good indicator for a positive outcome to surgery (Schmidt and Löscher, 2005). Our previous finding that prophylactic treatment with valproate after a BLA-induced SE protects against hippocampal damage initiated a study in which we will evaluate whether such treatment prevents pharmacoresistance of spontaneous seizures. In preliminary experiments, we evaluated the optimal therapeutic window and dosage protocol for the neuroprotective effect of valproate, indicating that continuous i.v. infusion over 5 days after SE is as effective as twice-daily treatment for 4 weeks (M. Langer and C. Brandt, unpublished experiments).
f. Impact of Rat Strains.
In most studies detailed in Tables 2 and 3, outbred strains such as Sprague-Dawley or Wistar have been used. Only a few studies examined potential interstrain and intergender differences when the same protocol for SE induction was used, but overall results from different studies have not shown major differences in the development of behavioral and electrographic alterations (Curia et al., 2008). We directly compared the characteristics and long-term consequences of SE induced by sustained stimulation of the BLA in male and female Wistar and Sprague-Dawley rats (Brandt et al., 2003a). Female Sprague-Dawley rats were most sensitive to BLA stimulation in terms of SE induction and development of epilepsy after SE, so that we used this strain and gender for all subsequent experiments. However, in recent years, the sensitivity of Sprague-Dawley rats to BLA stimulation and SE has undergone a marked reduction, so that fewer rats develop a self-sustained SE, the development of epilepsy after SE is retarded, and neurodegeneration is lessened (Langer et al., 2010). These differences to previous experiments are most likely a consequence of a genetic drift that occurred at the breeder (Harlan). We originally received our rats from Harlan-Winkelmann in Germany, but this colony was closed, and Harlan in the Netherlands now provides Sprague-Dawley rats. We also experienced similar alterations between previous and more recent batches of rats in sensitivity to SE induction and consequences of SE in the pilocarpine model (Bankstahl et al., 2009). This prompted us to perform a series of experiments in which we compared the sensitivity of Sprague-Dawley and Wistar rats from different breeders to BLA stimulation and pilocarpine, resulting in marked substrain differences (Bankstahl et al., 2009; Langer et al., 2010). These data indicate that genetic differences in outbred rat strains may contribute to variations between experimental data in post-SE models of SE. Similar differences between strains and substrains have also been reported for TLE models in mice (Schauwecker, 2002; Borges et al., 2003; Müller et al., 2009c).
It is noteworthy that, similar to our observations with systemic administration of pilocarpine (Bankstahl et al., 2009), Portelli et al. (2009) reported intrastrain differences in seizure susceptibility to focal (intrahippocampal) administration of pilocarpine in Wistar rats from two breeding locations of Charles River and Harlan and even rats obtained at different times from the same breeding location. On the basis of these data, Portelli et al. (2009) concluded that intrastrain differences can have a substantial impact on the outcome of SE models, so that scientists should pay attention to this issue when designing studies. Such intrastrain differences may be one major reason why certain results cannot be reproduced from one laboratory to another, or even within the same laboratory.
g. Consequences for Drug Testing Protocols in Post-Status Epilepticus Models.
In view of the lack of positive validation of such model by a drug that prevents or modifies epilepsy in patients, it is difficult to recommend any “best-of protocol.” However, many of the data summarized in Tables 2 and 3 are promising and seem to indicate that discovery of disease-modifying therapeutics is not an unrealistic goal. The main risk is that interesting therapeutics are missed, because of the various problems associated with drug testing in post-SE models of TLE. In addition to the problems discussed above, it is important to consider that the long duration of SE commonly used in such models results in the development of epilepsy in the majority of rats, so that less potent treatments may be missed. On the other hand, models that have a high incidence of rats developing spontaneous seizures allow us to develop uniform outcome measures and to increase the statistical power for identifying effective treatments (Stables et al., 2003).
We currently try to improve the chance of discovering disease-modifying therapeutics in the lithium-pilocarpine model by using a protocol with the following characteristics:
The duration of SE should be as short as possible but induce epilepsy in the majority of rats; according to previous experiments in the repeated low-dose pilocarpine model, 60-min SE is sufficient for this goal (Glien et al., 2001).
SE should be terminated by a protocol that suppresses both clinical and electrographic seizures and prevents or minimizes seizure recurrence in the hours after SE termination; in our hands, a combination of diazepam and phenobarbital is better suited to this goal than any drug alone (Bankstahl and Löscher, 2008). The anticholinergic drug scopolamine can be administered together with diazepam and phenobarbital, so that both the self-sustained SE and the CNS stimulatory effects of pilocarpine are terminated. This cocktail also results in a much better and more rapid recovery of the rats after termination of SE (C. Brant and K. Töllner, unpublished data). Video/EEG recording is used during and up to 24 h after termination of SE to document any differences between experimental groups. It is important to note that experiments with kainate have indicated that subclinical EEG seizures alone (i.e., without clinical seizures) do not induce epilepsy (White et al., 2010), so that complete suppression of clinical (focal and convulsive) seizures may be sufficient for effective termination of SE.
Treatment with an investigational drug (or combinations of different drugs) should start immediately after termination of SE and last for at least 2 weeks (to cover the latent period, which ranges from 6 to 10 days in our hands); dosing intervals should be based on pharmacokinetics and/or duration of action of test compound(s) in rats.
In view of the time commitment of personnel and the high costs associated with antiepileptogenesis trials, we will use as many outcome measures as possible, which will also minimize the risk that a disease-modifying drug effect is missed; outcome measures will include the recording of preictal spikes and ripples during the latent period and thereafter, video/EEG monitoring of spontaneous seizures for at least 2 weeks at one or two intervals after the latent period, repeated μMRI imaging during and after the latent period, characterization of behavioral alterations by a behavioral test battery (see Brandt et al., 2006a, 2007) in the chronic epilepsy state, analysis of cognitive alterations, determination of hippocampal field potentials, and, finally, histological assessment of neurodegeneration in the hippocampus and parahippocampal areas. Furthermore, we have started to study whether determination of seizure threshold in the latent period by the timed i.v. pentylenetetrazole infusion method (cf., Löscher, 2009) can be used to predict the development of neuronal hyperexcitability and spontaneous seizures.
The most interesting compounds identified by the protocol described above will be tested in at least one other post-SE model of TLE [i.e., SE induced by sustained BLA stimulation (Brandt et al., 2003a) or intrahippocampal administration of kainate (Raedt et al., 2009)]. In both models, the SE and its consequences are less severe compared with systemic administration of pilocarpine (Brandt et al., 2003a; Dudek et al., 2006), thus enhancing the chance of identifying potentially useful antiepileptogenic therapies. A comparison of how the three post-SE TLE models will be used in an optimized fashion for antiepileptogenic drug discovery in the authors' laboratory is shown in Table 4.
TABLE 4.
Model parameters and outcome measures of three post-SE models of TLE that are currently used for antiepileptogenic drug discovery in the authors' laboratory
See section III.C.3.g for details.
| Post-SE TLE Model in Rats |
|||
|---|---|---|---|
| Lithium-Pilocarpine (Systemic Administration of Pilocarpine with Ramping Design for Individual Dosing) | Unilateral Electrical Stimulation of Basolateral Amygdala | Unilateral Intrahippocampal Injection of Kainate | |
| Duration of SE for inducing SRS | ≥60 min | ≥4 h | ≥4 h |
| Effective interruption of SE by | Diazepam + phenobarbital | Diazepam | Diazepam |
| Latent period | ∼7 days | ∼30 days | ? |
| Rats with spontaneous recurrent seizures | >90% | >90% | >90% |
| Neuropathology | Bilateral, widespread | Mostly unilateral, less widespread | Mostly unilateral, less widespread |
| Antiepileptogenic drug testing | |||
| Start of drug administration | Immediately after termination of SE | ||
| Duration of drug administration | At least 2 weeks | ||
| Dosing interval | Depends on elimination rate of test drug in rats | ||
| Outcome measures | Latent period to first spontaneous seizure; Incidence, frequency, severity, and duration of spontaneous seizures; Surrogate measures, including preictal and interictal spikes, ripples, seizure threshold; Behavioral alterations; Learning and memory; Hippocampal field potentials; Brain imaging (μMRI); Postmortem (immuno)histology | ||
SRS, spontaneous recurrent seizures.
An interesting alternative to models with convulsive SE is the TLE model described by Norwood et al. (2010), in which prolonged bilateral perforant path stimulation in awake rats with a relative moderate stimulus intensity that does not induce convulsive SE (but focal, nonconvulsive SE) produces the extensive neuronal injury that defines classic hippocampal sclerosis and, after a latent period of ∼3 weeks, spontaneous hippocampal-onset seizures, but avoids the massive extra-hippocampal damage in models with prolonged convulsive SE. As outlined by Norwood et al. (2010), the primary value of this model lies in its similarity to the human neurological condition. That is, the human pattern of hippocampal sclerosis with limited temporal cortical damage is produced in rats with negligible variability and no lethality, and every animal develops hippocampal-onset epilepsy, features that are needed in any model of hippocampal epileptogenesis. These interesting features should encourage antiepileptogenesis studies in this model.
In addition to using rats for antiepileptogenesis trials, we plan to use mice for such experiments, because the availability of numerous spontaneous and engineered mouse mutants allows studying the effects of genetics on epileptogenesis and its pharmacological modulation. We have established different SE models in both out- and inbred mouse strains in our laboratory (Gröticke et al., 2007, 2008; Müller et al., 2009a,b) and demonstrated that substrain differences and mutations affect the pilocarpine model of TLE (Müller et al., 2009a). Likewise, the consequences of kainate differ among mouse strains (Schauwecker and Steward, 1997), thus allowing elucidation of the genetic influences contributing to susceptibility to seizure disorders.
D. Models of Traumatic Brain Injury- and Stroke-Induced Epilepsy
Epidemiologic data indicate that the leading etiologies for symptomatic epilepsies in adults are TBI, stroke, and brain infections, whereas SE as a sole cause of epileptogenesis is rare in adults (Hauser, 1997; Pitkänen et al., 2007a). On the basis of epidemiologic studies, depending on severity of the brain insult, epilepsy develops in up to 53% of patients with TBI, up to 5% with ischemic stroke, and up to 43% of patients with SE. Prospective follow-up studies indicate that approximately 80% of patients developing epilepsy after TBI or ischemic stroke do so within 2 years and 90% of patients with SE who develop epilepsy do so within 7 years (Pitkänen et al., 2007a). In rat models of TBI, stroke, and SE, there are also clear differences in the percentage of animals developing epilepsy and the latent period to the first spontaneous seizure (Pitkänen et al., 2007a). Typically, depending on the severity of the initial insult, the latent period ranges from 1 to 4 weeks in post-SE models of TLE, and 90 to 100% of rats develop spontaneous seizures, whereas the latent period is several months in TBI and stroke models, and only 50% (TBI) or 10 to 20% (stroke) of rats develop spontaneous seizures. This explains the apparent paradox that experimental antiepileptogenesis studies are typically performed in post-SE models of TLE, although SE is only a relatively rare cause of symptomatic epilepsy in humans. The short latent period and high percentage of rats developing epilepsy after SE is an important advantage for drug studies, whereas the logistical problems associated with a latent period of several months, and particularly the low percentage of animals developing epilepsy in TBI and stroke models, make drug studies even more laborious and statistically challenging than studies in SE models. Thus, to our knowledge, no antiepileptogenesis study using TBI or stroke models is available. Surrogate markers of epileptogenesis, such as decreased seizure threshold for drug studies in TBI models (e.g., Echegoyen et al., 2009) have been tried, but, as pointed out by Dudek (2009), induction of a change in seizure threshold is not exactly the same as altering epileptogenesis and, regardless of how it is done, seems indirect and susceptible to bias.
The most commonly used TBI and stroke models for inducing epileptogenesis in rats are lateral fluid-percussion and controlled cortical impact for TBI and cortical photothrombosis and endothelin-1-induced occlusion of the middle cerebral artery for ischemic stroke (Karhunen et al., 2005; Pitkänen et al., 2007a; Kharatishvili and Pitkänen, 2010). Gene expression studies after potentially epileptogenic events such as TBI, ischemia, and SE in rats suggest that groups of functionally related genes but also several individual genes change similarly after such brain insults, and therefore might be of particular relevance for the development of epilepsy due to different etiologies (Lukasiuk et al., 2006; Pitkänen et al., 2007a). However, whether data from experimental antiepileptogenesis trials in post-SE models of TLE can be extrapolated to other etiologies is not yet known, because of the lack of respective data from TBI and stroke models. There are, however, two promising examples in which a drug provided similar disease-modifying effects in SE and TBI models of TLE. Thus, the CB-1 receptor antagonist rimonabant, a clinically approved antiobesity drug, counteracted neuronal hyperexcitability when tested for antiepileptogenic activity in two different models (i.e., epileptogenesis induced by hyperthermia-induced SE and TBI) (Chen et al., 2007; Echegoyen et al., 2009). Likewise, the mTOR inhibitor rapamycin was reported to have disease-modifying properties in the kainate and pilocarpine models of TLE (Buckmaster et al., 2009; Zeng et al., 2009) and in the weight-drop model of TBI (Erlich et al., 2007). This may indicate that, because of common molecular and cellular alterations underlying epileptogenesis in different etiologies, antiepileptogenic or disease-modifying effects of a drug discovered in one model can be extrapolated to other models and eventually to the clinic. Whether this assumption is true depends on further comparisons between models and, ultimately, clinical trials with promising antiepileptogenic drug candidates. The improvement of TBI models, resulting in shorter latent period and higher yield of epileptic animals (Kharatishvili and Pitkänen, 2010), will greatly facilitate comparison of antiepileptogenic treatments between TBI and SE models of TLE.
E. Genetic Animal Models of Epilepsy
Disease-susceptibility genes play an important role in epileptogenesis. Whether the brain develops seizures after an insult may be partially genetically determined. The 2002 NINDS/NIH Models Workshop on therapy discovery for pharmacoresistant epilepsy and for disease-modifying therapeutics therefore recommended that investigators take advantage of the progress in rodent genomics and existing genetically prone strains such as the genetically epilepsy prone rat in assessing antiepileptogenic treatments (Stables et al., 2003). Furthermore, because of genetic differences among rodent strains, models of epileptogenesis should not be confined to one strain of mouse or rat.
Various genetic animal models of epilepsy have been characterized over recent decades (Löscher and Meldrum, 1984; Löscher, 1984, 1999; Jobe et al., 1991). Genetic animal models of epilepsy comprise genetically predisposed animal species in which seizures occur either spontaneously or in response to sensory stimulation. The major advantage of these naturally occurring epilepsies in animals as models of human epilepsy is that they simulate the clinical situation more closely than any other experimental epilepsy. Models with idiopathic spontaneous recurrent seizures include epileptic dogs, tottering mice, and rats with spike-wave absence seizures. Models with reflex seizures comprise photosensitive baboons (Papio papio) and fowl, audiogenic seizure-susceptible mice and rats, and gerbils with seizures in response to different sensory stimuli. Genetic animal models of epilepsy offer unique approaches to the evaluation of drugs, but the use of such models for antiepileptogenic drug discovery has just begun (see section V).
IV. Comparison of Efficacy in Animal Models and Clinical Trials
As one of the overall recommendations of the NIH/NINDS models workshop in 2002, Stables et al. (2003) suggested that models used for discovery of antiepileptogenic or disease-modifying therapeutics should verify the lack of antiepileptogenic efficacy reported in human trials of epilepsy prevention. Table 5 compares data from kindling and post-SE models with those from clinical trials. For this comparison, it is important to note that the available clinical trials examined whether spontaneous seizures developed after TBI in the placebo and drug-treated groups; however, it was not examined whether prophylactic drug treatment altered the frequency, severity, progression, or prognosis of the seizures. Furthermore, except for the valproate trial, it was not examined whether AED treatment after TBI modified psychopathology or impaired cognitive functions developing after TBI, and none of the trials included MRI imaging of brain damage for determining potential neuroprotective effects of the treatment. Consequently, it is not possible to exclude the possibility that prophylactic treatment with AEDs exerted disease-modifying effects in patients as seen in several of the preclinical studies (Tables 2 and 4).
TABLE 5.
Antiepileptogenic and disease-modifying drug effects in chronic rat models of epilepsy and post-traumatic epilepsy trials in humans
Data for animal models are from Tables 1 and 2. Data for post-traumatic epilepsy are from Temkin (2009).
| Drug | Electrical Kindling |
Post-SE Models of TLE |
Post-Traumatic Epilepsy in Patients |
|||
|---|---|---|---|---|---|---|
| Retardation of Kindling during Treatment | Disease Modification after Termination of Treatment | Prevention of Epilepsy | Disease Modification after Termination of Treatment | Prevention of Epilepsy | Disease-Modification | |
| Carbamazepine | N.E. | N.E. | N.E. | + | N.E. | ? |
| Phenytoin | N.E. | N.E. | N.E. | + | N.E. | ? |
| Phenobarbital | + | + | N.E. | +/− | N.E. | ? |
| Valproate | + | + | N.E. | + | N.E. | N.E. |
| Levetiracetam | + | + | N.E. | +/− | ? | ? |
| Topiramate | + | ? | +/− | +/− | ? | ? |
| Benzodiazepines | + | ? | N.E. | + | ? | ? |
| Vigabatrin | + | ? | N.E. | N.E. | ? | ? |
| Lamotrigine | + | N.E. | N.E. | + | ? | ? |
| NMDA antagonists | + | ? | N.E. | + | ? | ? |
+, effective; +/−, inconsistent data; N.E., not effective; ?, no data available.
The lack of antiepileptogenic efficacy of carbamazepine, phenytoin, phenobarbital, and valproate in the clinical trials was verified by post-SE models (Table 5). It is noteworthy that carbamazepine and phenytoin also did not retard the acquisition of kindling. AEDs that modified kindling development after termination of treatment also exhibited disease-modifying effects in post-SE studies, although often not in all studies performed in this respect. Valproate, which exerted disease-modifying effects in kindling and post-SE models, did not exert such effects in clinical trials, although only effects on psychopathology and cognition were studied in this regard. Ideally, future clinical trials should include additional outcome measures such as seizure frequency and severity and serial EEGs and MRIs as planned in the pilot clinical trial on topiramate (see section III.C.1.b). Only then will it be possible to truly compare drug effects between preclinical and clinical trials in a more comprehensive fashion. Furthermore, epilepsy prevention trials should not be restricted to civilian or military brain trauma, but also address other brain insults known to bear a risk of developing epilepsy in the next several years after a given event. These include SE, intracerebral hemorrhage, known cortical dysplasia (e.g., tuberous sclerosis), ischemic stroke, CNS infection, brain tumors, children with prolonged febrile seizures, and some forms of chronic neurodegeneration. Each of these has a definable risk, and the subsequent epilepsy may be preventable (Dichter, 2009a,b).
V. Which Animal Model Is Best Suited for Antiepileptogenesis Studies?
A number of models have been suggested as appropriate for the study of mechanisms underlying epileptogenesis and its prevention, but there is no general agreement about which models may be most appropriate and relevant to the human condition. More importantly, none of the available models has been clinically validated. The latent period between the induction of SE and the beginning of spontaneous seizures has attracted the attention of many investigators to SE models as a tool for therapy discovery. Although the concept of such a latent period has been questioned (Sloviter, 2008; Dudek, 2009), as discussed in section III.C.3.c, the use of post-SE models has greatly enhanced our understanding of the processes involved in epileptogenesis and its pharmacological modification. A very prolonged latent period, which most likely reflects the epileptogenic process, has also been described in the kindling model, in which spontaneous seizures develop after several months of daily electrical stimulation (overkindling) in rats, cats, dogs, and primates (Coulter et al., 2002). One important advantage of kindling in this respect is that it identifies which variables are not essential for the spontaneous seizures (Coulter et al., 2002). For example, amygdala-kindled rats exhibiting spontaneous seizures show no significant damage in CA1 and CA3 sectors of the hippocampus, the amygdala, parahippocampal regions, or thalamus (Brandt et al., 2004a). This implies that the gross forebrain damage that is routinely apparent in all SE models is not a prerequisite for spontaneous seizures but likely a bystander effect in their development (Coulter et al., 2002), which is substantiated by the findings with neuroprotective drugs in post-SE models (Tables 2 and 3). The data on drug testing summarized in Table 5 indicate that kindling provides an interesting approach for identifying disease-modifying effects of drugs when using the protocol illustrated in Fig. 2. However, kindling is possibly not useful to test drugs for antiepileptogenic efficacy, because the latency to development of spontaneous seizures is too long for such studies. It is noteworthy that a similarly long latency between brain insult and onset of spontaneous seizures has been described for TBI and stroke models in rats (Pitkänen et al., 2007a). As discussed in section III.A, we have hypothesized previously that kindling via depth electrodes may represent a model of TBI in which the consequences of TBI are facilitated by electrical stimulation (Löscher, 2002a).
Apart from kindling or SE models, only a few other models have been used for antiepileptogenesis experiments. Echegoyen et al. (2009) used a TBI model for studying the effects of the CB1 antagonist rimonabant on seizure susceptibility caused by head injury, but the potential consequences of this treatment for development of spontaneous seizures were not investigated in this study. The same group used also a model of fever induced (febrile) seizures to study the effects of rimonabant on limbic hyperexcitability (Chen et al., 2007); again, however, potential effects on spontaneous seizures were not analyzed. Various other experimental studies have used TBI and stroke models in the search for drugs that improve the outcome (e.g., neurodegeneration, loss of memory) after such insults, but, to our knowledge, the development of late spontaneous seizures has never been an outcome measure in such preclinical trials (Hossmann, 2009; Pitkänen et al., 2009). The long latent period and low incidence of spontaneous seizures in such models will make such studies extremely difficult and laborious (Pitkänen et al., 2007a).
Three studies that have used genetic models of epilepsy for testing antiepileptogenic drug potential (Yan et al., 2005; Blumenfeld et al., 2008; Russo et al., 2009) demonstrate the value of such models in this respect. Yan et al. (2005) used an epileptic double mutant rat (SER; zi/zi, tm/tm) that exhibits recurring tonic and absence-like seizures in response to mild sensory stimulation (such as air blast), starting at approximately 7 to 8 weeks of age. These rats were treated with levetiracetam over three weeks (weeks 5–8) before the onset of spontaneous seizures, followed by seizure recording for 5 weeks after termination of treatment. Prophylactic treatment with this AED resulted in a significant decrease in the incidence of both tonic and absence-like seizures, indicating a disease-modifying effect (Yan et al., 2005). Blumenfeld et al. (2008) used WAG/Rij rats with spontaneously occurring spike-wave discharges in the EEG, an established model of human absence epilepsy. Oral ethosuximide was given from postnatal day 21 to 5 months of age, covering the usual period in which seizures develop in this model. Early treatment with ethosuximide blocked changes in the expression of ion channels Nav1.1, Nav1.6, and HCN1 normally associated with epilepsy in this model. In addition, the treatment led to a persistent suppression of seizures, even over several months after therapy was discontinued. These findings suggest that early treatment during development may provide a new strategy for preventing epilepsy in susceptible persons (Blumenfeld et al., 2008). Russo et al. (2009) confirmed the antiepileptogenic or disease-modifying effect of ethosuximide in WAG/Rij and demonstrated a similar effect for levetiracetam.
In the context of this review, it is important to note that a disease-modifying effect of levetiracetam has been demonstrated in three models, kindling (Löscher et al., 1998), SER rats (Yan et al., 2005), and WAG/Rij rats (Russo et al., 2009), whereas most experimental trials in post-SE models were negative (Table 2). This seems to indicate that, depending on the specific drug tested, drug trials in post-SE models alone may yield false negative data, but that a battery of models, including genetic models, should be used for identifying antiepileptogenic or disease-modifying therapies. Whether kindling and genetic models are more predictive in the search for such therapies than post-SE models must await clinical trials with drugs such as levetiracetam, including trials in patients with certain genetic forms of epilepsy who have not yet become symptomatic (Dichter, 2009a).
Model validation is complex and is dependent on the identification of a clinically effective therapy for epileptogenesis (Stables et al., 2002). To give guidance for future antiepileptogenic therapy, the utility of specific mechanisms of action in preventing or modifying epileptogenesis must be studied in multiple model systems. As outlined in this review, the failure of a particular compound with a defined mechanism of action may not be related to the mechanism of action; rather, it may be due to various technical problems associated with drug testing in a given model.
VI. Conclusions
Prevention of epilepsy in people at risk is one of the major U.S. NINDS/NIH Epilepsy Research Benchmarks (Kelley et al., 2009) and also a research priority of the European scientific community involved in epilepsy research (Baulac and Pitkänen, 2009). Trauma and stroke are the most common brain injuries that result in epilepsy (Pitkänen et al., 2007a). However, studies on the mechanisms of epileptogenesis and its prevention have so far primarily been conducted in animal models in which SE triggers the development of epilepsy, which is not a major cause of epilepsy in humans. We do not know yet to any sufficient extent whether the mechanisms leading to epilepsy differ after different brain insults, which, of course, would affect the development of therapies for preventing epilepsy. Furthermore, we need better understanding of the genetic, environmental, and other variables that predispose to the development of epilepsy after brain injury (Baulac and Pitkänen, 2009). The ultimate goal is to identify therapeutic interventions that prevent, interrupt, or reverse the epileptogenic process. As shown in this review, such an intervention has yet to be identified, but new promising data with neuroprotective, anti-inflammatory, and neuromodulatory drugs seem to indicate that this goal is not unrealistic. However, because of the numerous pathologic alterations that occur simultaneously during the epileptogenic cascade (Fig. 1), it will most certainly not be possible to halt epileptogenesis by targeting only one of these processes. Instead, cocktails of drugs that target different epileptogenic alterations should be administered after brain insults, and we have started to explore this strategy. Furthermore, the search for antiepileptogenic drugs should not rely solely on post-SE models of TLE, but other approaches, including kindling, genetic animal models of epilepsy, TBI models, and novel approaches, such as the TLE model described by Norwood et al. (2010), should be included. The experiments with ethosuximide and levetiracetam in genetic models (see section V) seem to demonstrate that epilepsy prevention is possible. However, any experimental strategy for identifying antiepileptogenic therapies needs positive validation by a drug with such activity in patients at risk. The outcome of the pilot clinical trials with topiramate and levetiracetam (see sections III.C.1.b and III.C.1.e) will be important in this respect. In the end, only clinical trials can determine whether a drug possesses antiepileptogenic or disease-modifying potential. However, defining the clinical paradigm and selecting appropriate outcomes to detect such potential effects present challenges to clinicians studying the antiepileptogenic or neuroprotective properties of drugs (Sankar, 2005; Willmore, 2005). The emerging preclinical and clinical research bases are poised for pilot and larger scale clinical trials on epilepsy prevention (Jensen, 2009), but only interdisciplinary research and communication between basic and clinical scientists will identify treatment strategies that provide a real progress in the prevention or modification of epilepsy.
Acknowledgments.
We thank Drs. Astrid Nehlig and Robert S. Sloviter for critical reading of the manuscript and helpful suggestions; Dr. Nancy Temkin for providing unpublished clinical data on valproate; Drs. Marc A. Dichter and Pavel Klein for providing information on their pilot trials with topiramate and levetiracetam in patients with TBI; Nadine Polascheck and Dr. Marion Bankstahl for the PGE2 data shown in Fig. 5; and Dr. Keun-Hwa Jung for additional information on his study on celecoxib (Jung et al., 2006). The authors' own work was supported by the Deutsche Forschungsgemeinschaft [Grant Lo 274/11-1] and the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant R21-NS049592].
This article is available online at http://pharmrev.aspetjournals.org.
doi:10.1124/pr.110.003046.
- ADK
- adenosine kinase
- AED
- antiepileptic drug
- AMPA
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- BDNF
- brain-derived neurotrophic factor
- BLA
- basolateral nucleus of the amygdala
- CA
- cornu ammonis
- CB
- cannabinoid
- COX-2
- cyclooxygenase-2
- EEG
- electroencephalographic/-graphy
- FK506
- tacrolimus
- HDAC
- histone deacetylase
- IL
- interleukin
- MK-801
- dizocilpine maleate
- μMRI
- small animal magnetic resonance imaging
- mTOR
- mammalian target of rapamycin
- NIH
- National Institutes of Health
- NINDS
- National Institute of Neurological Disorders and Stroke
- NMDA
- N-methyl-d-aspartate
- NPY
- neuropeptide Y
- NS-1209
- 8-methyl-5-(4-(N,N-dimethylsulfamoyl)phenyl)-6,7,8,9-tetrahydro-1H-pyrrolo[3,2-h]-iso-quinoline-2,3-dione-3-O-(4-hydroxybutyric acid-2-yl)oxime
- PG
- prostaglandin
- RWJ-333369
- carisbamate
- SC58236
- 4-(5-(4-chlorophenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzenesulfonamide
- SE
- status epilepticus
- SR141716A
- rimonabant
- TBI
- traumatic brain injury
- TLE
- temporal lobe epilepsy
- TrkB
- tyrosine kinase receptor B
- VX-765
- 1-(2-((1-(4-amino-3-chlorophenyl)methanoyl)amino)-3,3-dimethylbutanoyl)pyrrolidine-2-carboxylic acid.
References
- Acharya MM, Hattiangady B, Shetty AK. (2008) Progress in neuroprotective strategies for preventing epilepsy. Prog Neurobiol 84:363–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano K, Hamada K, Yagi K, Seino M. (1998) Antiepileptic effects of topiramate on amygdaloid kindling in rats. Epilepsy Res 31:123–128 [DOI] [PubMed] [Google Scholar]
- André V, Dubé C, François J, Leroy C, Rigoulot MA, Roch C, Namer IJ, Nehlig A. (2007) Pathogenesis and pharmacology of epilepsy in the lithium-pilocarpine model. Epilepsia 48 (Suppl 5):41–47 [DOI] [PubMed] [Google Scholar]
- André V, Ferrandon A, Marescaux C, Nehlig A. (2001) Vigabatrin protects against hippocampal damage but is not antiepileptogenic in the lithium-pilocarpine model of temporal lobe epilepsy. Epilepsy Res 47:99–117 [DOI] [PubMed] [Google Scholar]
- André V, Rigoulot MA, Koning E, Ferrandon A, Nehlig A. (2003) Long-term pregabalin treatment protects basal cortices and delays the occurrence of spontaneous seizures in the lithium-pilocarpine model in the rat. Epilepsia 44:893–903 [DOI] [PubMed] [Google Scholar]
- Armstrong C, Morgan RJ, Soltesz I. (2009) Pursuing paradoxical proconvulsant prophylaxis for epileptogenesis. Epilepsia 50:1657–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee PN, Filippi D, Allen Hauser W. (2009) The descriptive epidemiology of epilepsy-a review. Epilepsy Res 85:31–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankstahl M, Brandt C, Löscher W. (2009) Differences in pilocarpine-induced epileptogenesis and behavioral alterations of Sprague-Dawley rats from two different breeders. Naunyn-Schmiedebergs Arch Pharmacol 379 (Suppl 1):48–49 [Google Scholar]
- Bankstahl JP, Löscher W. (2008) Resistance to antiepileptic drugs and expression of P-glycoprotein in two rat models of status epilepticus. Epilepsy Res 82:70–85 [DOI] [PubMed] [Google Scholar]
- Baulac M, Pitkänen A. (2009) Research priorities in epilepsy for the next decade—a representative view of the European scientific community. Epilepsia 50:571–578 [Google Scholar]
- Beck H, Yaari Y. (2008) Plasticity of intrinsic neuronal properties in CNS disorders. Nat Rev Neurosci 9:357–369 [DOI] [PubMed] [Google Scholar]
- Becker AJ, Pitsch J, Sochivko D, Opitz T, Staniek M, Chen CC, Campbell KP, Schoch S, Yaari Y, Beck H. (2008) Transcriptional upregulation of Cav3.2 mediates epileptogenesis in the pilocarpine model of epilepsy. J Neurosci 28:13341–13353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beghi E, Carpio A, Forsgren L, Hesdorffer DC, Malmgren K, Sander JW, Tomson T, Hauser WA. (2010) Recommendation for a definition of acute symptomatic seizure. Epilepsia 51:671–675 [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Holmes GL. (2005) The multiple facets of gamma-aminobutyric acid dysfunction in epilepsy. Curr Opin Neurol 18:141–145 [DOI] [PubMed] [Google Scholar]
- Bethmann K, Brandt C, Löscher W. (2007) Resistance to phenobarbital extends to phenytoin in a rat model of temporal lobe epilepsy. Epilepsia 48:816–826 [DOI] [PubMed] [Google Scholar]
- Bethmann K, Fritschy JM, Brandt C, Löscher W. (2008) Antiepileptic drug resistant rats differ from drug responsive rats in GABAA-receptor subunit expression in a model of temporal lobe epilepsy. Neurobiol Dis 31:169–187 [DOI] [PubMed] [Google Scholar]
- Binder DK, Croll SD, Gall CM, Scharfman HE. (2001) BDNF and epilepsy: too much of a good thing? Trends Neurosci 24:47–53 [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Klein JP, Schridde U, Vestal M, Rice T, Khera DS, Bashyal C, Giblin K, Paul-Laughinghouse C, Wang F, et al. (2008) Early treatment suppresses the development of spike-wave epilepsy in a rat model. Epilepsia 49:400–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blümcke I, Suter B, Behle K, Kuhn R, Schramm J, Elger CE, Wiestler OD. (2000) Loss of hilar mossy cells in Ammon's horn sclerosis. Epilepsia 41 (Suppl 6):S174–S180 [DOI] [PubMed] [Google Scholar]
- Boison D. (2008) The adenosine kinase hypothesis of epileptogenesis. Prog Neurobiol 84:249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanos AR, Sarkisian M, Yang Y, Hori A, Helmers SL, Mikati M, Tandon P, Stafstrom CE, Holmes GL. (1998) Comparison of valproate and phenobarbital treatment after status epilepticus in rats. Neurology 51:41–48 [DOI] [PubMed] [Google Scholar]
- Borges K, Gearing M, McDermott DL, Smith AB, Almonte AG, Wainer BH, Dingledine R. (2003) Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp Neurol 182:21–34 [DOI] [PubMed] [Google Scholar]
- Brandt C, Ebert U, Löscher W. (2004a) Epilepsy induced by extended amygdala-kindling in rats: lack of clear association between development of spontaneous seizures and neuronal damage. Epilepsy Res 62:135–156 [DOI] [PubMed] [Google Scholar]
- Brandt C, Gastens AM, Sun M, Hausknecht M, Löscher W. (2006a) Treatment with valproate after status epilepticus: effect on neuronal damage, epileptogenesis, and behavioral alterations in rats. Neuropharmacology 51:789–804 [DOI] [PubMed] [Google Scholar]
- Brandt C, Glien M, Gastens AM, Fedrowitz M, Bethmann K, Volk HA, Potschka H, Löscher W. (2007) Prophylactic treatment with levetiracetam after status epilepticus: Lack of effect on epileptogenesis, neuronal damage, and behavioral alterations in rats. Neuropharmacology 53:207–221 [DOI] [PubMed] [Google Scholar]
- Brandt C, Glien M, Potschka H, Volk H, Löscher W. (2003a) Epileptogenesis and neuropathology after different types of status epilepticus induced by prolonged electrical stimulation of the basolateral amygdala in rats. Epilepsy Res 55:83–103 [DOI] [PubMed] [Google Scholar]
- Brandt C, Heile A, Potschka H, Stoehr T, Löscher W. (2006b) Effects of the novel antiepileptic drug lacosamide on the development of amygdala kindling in rats. Epilepsia 47:1803–1809 [DOI] [PubMed] [Google Scholar]
- Brandt C, Nozadze M, Heuchert N, Rattka M, Löscher W. (2010) Disease-modifying effects of phenobarbital and the NKCC1 inhibitor bumetanide in the pilocarpine model of temporal lobe epilepsy. J Neurosci 30:8602–8612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C, Potschka H, Löscher W, Ebert U. (2003b) N-methyl-D-aspartate receptor blockade after status epilepticus protects against limbic brain damage but not against epilepsy in the kainate model of temporal lobe epilepsy. Neuroscience 118:727–740 [DOI] [PubMed] [Google Scholar]
- Brandt C, Volk HA, Löscher W. (2004b) Striking differences in individual anticonvulsant response to phenobarbital in rats with spontaneous seizures after status epilepticus. Epilepsia 45:1488–1497 [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Ingram EA, Wen X. (2009) Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J Neurosci 29:8259–8269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumanglag AV, Sloviter RS. (2008) Minimal latency to hippocampal epileptogenesis and clinical epilepsy after perforant pathway stimulation-induced status epilepticus in awake rats. J Comp Neurol 510:561–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Li A, Cho HY. (2009) mTOR signaling in epileptogenesis: too much of a good thing? J Neurosci 29:12372–12373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capella HM, Lemos T. (2002) Effect on epileptogenesis of carbamazepine treatment during the silent period of the pilocarpine model of epilepsy. Epilepsia 43 (Suppl 5):110–111 [DOI] [PubMed] [Google Scholar]
- Cavalheiro EA, Naffah-Mazacoratti MG, Mello LE, Leite JP. (2006) The pilocarpine model of seizures, in Models of Seizures And Epilepsy (Pitkänen A, Schwartzkroin PA, Moshé SL. eds) pp 433–448, Elsevier, Amsterdam [Google Scholar]
- Cha BH, Silveira DC, Liu X, Hu Y, Holmes GL. (2002) Effect of topiramate following recurrent and prolonged seizures during early development. Epilepsy Res 51:217–232 [DOI] [PubMed] [Google Scholar]
- Chang BS, Lowenstein DH. (2003) Epilepsy. N Engl J Med 349:1257–1266 [DOI] [PubMed] [Google Scholar]
- Chen K, Neu A, Howard AL, Földy C, Echegoyen J, Hilgenberg L, Smith M, Mackie K, Soltesz I. (2007) Prevention of plasticity of endocannabinoid signaling inhibits persistent limbic hyperexcitability caused by developmental seizures. J Neurosci 27:46–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K, Jung KH, Lee ST, Kim JH, Kang KM, Kim HK, Lim JS, Park HK, Kim M, Lee SK, et al. (2008) Erythropoietin reduces epileptogenic processes following status epilepticus. Epilepsia 49:1723–1732 [DOI] [PubMed] [Google Scholar]
- Chwiej J, Janeczko K, Marciszko M, Czyzycki M, Rickers K, Setkowicz Z. (2010) Neuroprotective action of FK-506 (tacrolimus) after seizures induced with pilocarpine: quantitative and topographic elemental analysis of brain tissue. J Biol Inorg Chem 15:283–289 [DOI] [PubMed] [Google Scholar]
- Cilio MR, Bolanos AR, Liu Z, Schmid R, Yang Y, Stafstrom CE, Mikati MA, Holmes GL. (2001) Anticonvulsant action and long-term effects of gabapentin in the immature brain. Neuropharmacology 40:139–147 [DOI] [PubMed] [Google Scholar]
- Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. (2002) On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science 298:1418–1421 [DOI] [PubMed] [Google Scholar]
- Coulter DA. (2001) Epilepsy-associated plasticity in gamma-aminobutyric acid receptor expression, function, and inhibitory synaptic properties. Int Rev Neurobiol 45:237–252 [DOI] [PubMed] [Google Scholar]
- Coulter DA, McIntyre DC, Löscher W. (2002) Animal models of limbic epilepsies: what can they tell us? Brain Pathol 12:240–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha AO, Mortari MR, Liberato JL, dos Santos WF. (2009) Neuroprotective effects of diazepam, carbamazepine, phenytoin and ketamine after pilocarpine-induced status epilepticus. Basic Clin Pharmacol Toxicol 104:470–477 [DOI] [PubMed] [Google Scholar]
- Curia G, Longo D, Biagini G, Jones RS, Avoli M. (2008) The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods 172:143–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo Furtado M, Zheng A, Sedigh-Sarvestani M, Lumley L, Lichtenstein S, Yourick D. (2009) Analyzing large data sets acquired through telemetry from rats exposed to organophosphorous compounds: an EEG study. J Neurosci Methods 184:176–183 [DOI] [PubMed] [Google Scholar]
- DeLorenzo RJ, Morris A, Blair RE, Wallace M, Razvi B. (2002) Topiramate is both neuroprotective and antiepileptogenic in the pilocarpine model of status epilepticus. Epilepsia 43 (Suppl 7):15 [Google Scholar]
- De Smedt T, Raedt R, Vonck K, Boon P. (2007) Levetiracetam: the profile of a novel anticonvulsant drug-part I: preclinical data. CNS Drug Rev 13:43–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter MA. (2009a) Posttraumatic epilepsy: the challenge of translating discoveries in the laboratory to pathways to a cure. Epilepsia 50 (Suppl 2):41–45 [DOI] [PubMed] [Google Scholar]
- Dichter MA. (2009b) Emerging concepts in the pathogenesis of epilepsy and epileptogenesis. Arch Neurol 66:443–447 [DOI] [PubMed] [Google Scholar]
- Dikmen SS, Machamer JE, Winn HR, Anderson GD, Temkin NR. (2000) Neuropsychological effects of valproate in traumatic brain injury: a randomized trial. Neurology 54:895–902 [DOI] [PubMed] [Google Scholar]
- Dmowska M, Cybulska R, Schoenborn R, Piersiak T, Jaworska-Adamu J, Gawron A. (2010) Behavioural and histological effects of preconditioning with lipopolysaccharide in epileptic rats. Neurochem Res 35:262–272 [DOI] [PubMed] [Google Scholar]
- Dudek FE. (2009) Commentary: a skeptical view of experimental gene therapy to block epileptogenesis. Neurotherapeutics 6:319–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek FE, Clark S, Williams PA, Grabenstatter HL. (2006) Kainate-induced status epilepticus: A chronic model of acquired epilepsy, in Models of Seizures And Epilepsy (Pitkänen A, Schwartzkroin PA, Moshé SL. eds) pp 415–432, Elsevier, Amsterdam [Google Scholar]
- Dudek FE, Bertram EH, Staley KJ. (2008) Antiepileptogenesis therapy with levetiracetam: data from kindling versus status epilepticus models. Epilepsy Curr 8:28–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek FE, Sutula TP. (2007) Epileptogenesis in the dentate gyrus: a critical perspective. Prog Brain Res 163:755–773 [DOI] [PubMed] [Google Scholar]
- Ebert U, Brandt C, Löscher W. (2002) Delayed sclerosis, neuroprotection, and limbic epileptogenesis after status epilepticus in the rat. Epilepsia 43 (Suppl 5):86–95 [DOI] [PubMed] [Google Scholar]
- Ebert U, Cramer S, Löscher W. (1997) Phenytoin's effect on the spread of seizure activity in the amygdala kindling model. Naunyn-Schmiedebergs Arch Pharmacol 356:341–347 [DOI] [PubMed] [Google Scholar]
- Echegoyen J, Armstrong C, Morgan RJ, Soltesz I. (2009) Single application of a CB1 receptor antagonist rapidly following head injury prevents long-term hyperexcitability in a rat model. Epilepsy Res 85:123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, Jr, Bragin A, Staba R, Mody I. (2009) High-frequency oscillations: what is normal and what is not? Epilepsia 50:598–604 [DOI] [PubMed] [Google Scholar]
- Engel JJ, Pedley TA. (2008) Epilepsy: A Comprehensive Textbook. Lippincott Williams & Wilkins, Philadelphia [Google Scholar]
- Erlich S, Alexandrovich A, Shohami E, Pinkas-Kramarski R. (2007) Rapamycin is a neuroprotective treatment for traumatic brain injury. Neurobiol Dis 26:86–93 [DOI] [PubMed] [Google Scholar]
- Fabene PF, Navarro Mora G, Martinello M, Rossi B, Merigo F, Ottoboni L, Bach S, Angiari S, Benati D, Chakir A, et al. (2008) A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat Med 14:1377–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PD, Sperber EF, Moshé SL. (1998) Hippocampal sclerosis revisited. Brain Dev 20:563–573 [DOI] [PubMed] [Google Scholar]
- François J, Ferrandon A, Koning E, Nehlig A. (2005) Epileptic outcome is correlated with protection in temporal cortices: evidence from neuroprotection studies with a new drug, RWJ333369, in the lithium-pilocarpine model. Epilepsia 46 (Suppl 6):62–63 [Google Scholar]
- François J, Koning E, Ferrandon A, Nehlig A. (2006) The combination of topiramate and diazepam is partially neuroprotective in the hippocampus but not antiepileptogenic in the lithium-pilocarpine model of temporal lobe epilepsy. Epilepsy Res 72:147–163 [DOI] [PubMed] [Google Scholar]
- French JA, Williamson PD, Thadani VM, Darcey TM, Mattson RH, Spencer SS, Spencer DD. (1993) Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol 34:774–780 [DOI] [PubMed] [Google Scholar]
- Frisch C, Kudin AP, Elger CE, Kunz WS, Helmstaedter C. (2007) Amelioration of water maze performance deficits by topiramate applied during pilocarpine-induced status epilepticus is negatively dose-dependent. Epilepsy Res 73:173–180 [DOI] [PubMed] [Google Scholar]
- Fujikawa DG. (2005) Prolonged seizures and cellular injury: understanding the connection. Epilepsy Behav 7 (Suppl 3):S3–11 [DOI] [PubMed] [Google Scholar]
- Gale K. (1992) Subcortical structures and pathways involved in convulsive seizure generation. J Clin Neurophysiol 9:264–277 [DOI] [PubMed] [Google Scholar]
- Gao XG, Liu Y, Liu XZ. (2007) Treatment of late lithium-pilocarpine-induced status epilepticus with diazepam. Epilepsy Res 74:126–130 [DOI] [PubMed] [Google Scholar]
- Gilbert ME. (1988) The NMDA-receptor antagonist, MK-801, suppresses limbic kindling and kindled seizures. Brain Res 463:90–99 [DOI] [PubMed] [Google Scholar]
- Glien M, Brandt C, Potschka H, Voigt H, Ebert U, Löscher W. (2001) Repeated low-dose treatment of rats with pilocarpine: low mortality but high proportion of rats developing epilepsy. Epilepsy Res 46:111–119 [DOI] [PubMed] [Google Scholar]
- Goddard GV, McIntyre DC, Leech CK. (1969) A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol 25:295–330 [DOI] [PubMed] [Google Scholar]
- Goffin K, Nissinen J, Van Laere K, Pitkänen A. (2007) Cyclicity of spontaneous recurrent seizures in pilocarpine model of temporal lobe epilepsy in rat. Exp Neurol 205:501–505 [DOI] [PubMed] [Google Scholar]
- Goodman JH. (1998) Experimental models of status epilepticus, in Neuropharmacology Methods in Epilepsy Research (Peterson SL, Albertson TE. eds) pp 95–125, CRC Press, Boca Raton [Google Scholar]
- Gorji A, Speckmann EJ. (2009) Epileptiform EEG spikes and their functional significance. Clin EEG Neurosci 40:230–233 [DOI] [PubMed] [Google Scholar]
- Gowers WR. (1881) Epilepsy and Other Chronic Convulsive Diseases. Wood, London [Google Scholar]
- Gröhn O, Pitkänen A. (2007) Magnetic resonance imaging in animal models of epilepsy-noninvasive detection of structural alterations. Epilepsia 48 (Suppl 4):3–10 [DOI] [PubMed] [Google Scholar]
- Gröticke I, Hoffmann K, Löscher W. (2007) Behavioral alterations in the pilocarpine model of temporal lobe epilepsy in mice. Exp Neurol. 207:329–349 [DOI] [PubMed] [Google Scholar]
- Gröticke I, Hoffmann K, Löscher W. (2008) Behavioral alterations in a mouse model of temporal lobe epilepsy induced by intrahippocampal injection of kainate. Exp Neurol 213:71–83 [DOI] [PubMed] [Google Scholar]
- Gu J, Lynch BA, Anderson D, Klitgaard H, Lu S, Elashoff M, Ebert U, Potschka H, Löscher W. (2004) The antiepileptic drug levetiracetam selectively modifies kindling-induced alterations in gene expression in the temporal lobe of rats. Eur J Neurosci 19:334–345 [DOI] [PubMed] [Google Scholar]
- Halonen T, Nissinen J, Pitkänen A. (2001a) Chronic elevation of brain GABA levels beginning two days after status epilepticus does not prevent epileptogenesis in rats. Neuropharmacology 40:536–550 [DOI] [PubMed] [Google Scholar]
- Halonen T, Nissinen J, Pitkänen A. (2001b) Effect of lamotrigine treatment on status epilepticus-induced neuronal damage and memory impairment in rat. Epilepsy Res 46:205–223 [DOI] [PubMed] [Google Scholar]
- Harvey BD, Sloviter RS. (2005) Hippocampal granule cell activity and c-Fos expression during spontaneous seizures in awake, chronically epileptic, pilocarpine-treated rats: implications for hippocampal epileptogenesis. J Comp Neurol 488:442–463 [DOI] [PubMed] [Google Scholar]
- Hauser WA. (1997) Incidence and prevalence, in Epilepsy: A Comprehensive Textbook (Engel JJ, Pedley TA. eds) pp 47–57, Lippincott-Raven, Philadelphia [Google Scholar]
- Hellier JL, Patrylo PR, Buckmaster PS, Dudek FE. (1998) Recurrent spontaneous motor seizures after repeated low-dose systemic treatment with kainate: assessment of a rat model of temporal lobe epilepsy. Epilepsy Res 31:73–84 [DOI] [PubMed] [Google Scholar]
- Herman ST. (2002) Epilepsy after brain insult: targeting epileptogenesis. Neurology 59:S21–S26 [DOI] [PubMed] [Google Scholar]
- Herman ST. (2006) Clinical trials for prevention of epileptogenesis. Epilepsy Res 68:35–38 [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. (1998) Risk of unprovoked seizure after acute symptomatic seizure: effect of status epilepticus. Ann Neurol 44:908–912 [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. (2010) mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci 33:67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtman L, van Vliet EA, van Schaik R, Queiroz CM, Aronica E, Gorter JA. (2009) Effects of SC58236, a selective COX-2 inhibitor, on epileptogenesis and spontaneous seizures in a rat model for temporal lobe epilepsy. Epilepsy Res 84:56–66 [DOI] [PubMed] [Google Scholar]
- Hort J, Broźek G, Mares P, Langmeier M, Komárek V. (1999) Cognitive functions after pilocarpine-induced status epilepticus: changes during silent period precede appearance of spontaneous recurrent seizures. Epilepsia 40:1177–1183 [DOI] [PubMed] [Google Scholar]
- Hosford DA. (1995) Models of primary generalized epilepsy. Curr Opin Neurol 8:121–125 [DOI] [PubMed] [Google Scholar]
- Hossmann KA. (2009) Pathophysiological basis of translational stroke research. Folia Neuropathol 47:213–227 [PubMed] [Google Scholar]
- Hou X, Wang X, Zhang L. (2010) Conditional downregulation of brain- derived neurotrophic factor and tyrosine kinase receptor B blocks epileptogenesis in the human temporal lobe epilepsy hippocampus. Neurol India 58:29–34 [DOI] [PubMed] [Google Scholar]
- Huberfeld G, Wittner L, Clemenceau S, Baulac M, Kaila K, Miles R, Rivera C. (2007) Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci 27:9866–9873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Corradetti MN, Guan KL. (2005) Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet 37:19–24 [DOI] [PubMed] [Google Scholar]
- Jacobs MP, Leblanc GG, Brooks-Kayal A, Jensen FE, Lowenstein DH, Noebels JL, Spencer DD, Swann JW. (2009) Curing epilepsy: progress and future directions. Epilepsy Behav 14:438–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen FE. (2009) Introduction. Posttraumatic epilepsy: treatable epileptogenesis. Epilepsia 50 (Suppl 2):1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Nakashima K, Clemenson GD, Jr., Mejia E, Mathews E, Ure K, Ogawa S, Sinton CM, Gage FH, Hsieh J. (2007) Epigenetic modulation of seizure-induced neurogenesis and cognitive decline. J Neurosci 27:5967–5975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe PC, Mishra PK, Ludvig N, Dailey JW. (1991) Scope and contribution of genetic models to an understanding of the epilepsies. Crit Rev Neurobiol 6:183–220 [PubMed] [Google Scholar]
- Jung KH, Chu K, Lee ST, Kim J, Sinn DI, Kim JM, Park DK, Lee JJ, Kim SU, Kim M, et al. (2006) Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiol Dis 23:237–246 [DOI] [PubMed] [Google Scholar]
- Karhunen H, Jolkkonen J, Sivenius J, Pitkänen A. (2005) Epileptogenesis after experimental focal cerebral ischemia. Neurochem Res 30:1529–1542 [DOI] [PubMed] [Google Scholar]
- Kelley MS, Jacobs MP, Lowenstein DH.and NINDS Epilepsy Benchmark Stewards (2009) The NINDS epilepsy research benchmarks. Epilepsia 50:579–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharatishvili I, Pitkänen A. (2010) Posttraumatic epilepsy. Curr Opin Neurol 23:183–188 [DOI] [PubMed] [Google Scholar]
- Khazipov R, Holmes GL. (2003) Synchronization of kainate-induced epileptic activity via GABAergic inhibition in the superfused rat hippocampus in vivo. J Neurosci 23:5337–5341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P, Herr D, Pearl PL, Sandoval F, Trzcinski S, Nogay C, Heffron A, Tsuchida T, Vanden-Anker J, Natale J, et al. (2008) Safety, tolerability and pharmacokinetics of levetiracetam in patients with acute traumatic brain injury with high risk for developing post-traumatic brain injury. Epilepsia 49 (Suppl 7):81 [Google Scholar]
- Klitgaard H, Pitkänen A. (2003) Antiepileptogenesis, neuroprotection, and disease modification in the treatment of epilepsy: focus on levetiracetam. Epileptic Disord 5 (Suppl 1):S9–S16 [PubMed] [Google Scholar]
- Klitgaard HV, Matagne AC, Vanneste-Goemaere J, Margineanu DG. (2001) Effects of prolonged administration of levetiracetam on pilocarpine-induced epileptogenesis in rats. Epilepsia 42 (Suppl 7):114–115 [Google Scholar]
- Kobayashi M, Buckmaster PS. (2003) Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J Neurosci 23:2440–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhling R. (2002) Neuroscience. GABA becomes exciting. Science 298:1350–1351 [DOI] [PubMed] [Google Scholar]
- Koyama R, Ikegaya Y. (2005) To BDNF or not to BDNF: that is the epileptic hippocampus. Neuroscientist 11:282–287 [DOI] [PubMed] [Google Scholar]
- Langer M, Brandt C, Löscher W. (2010) Comparison of rats from different breeders in a model for temporal lobe epilepsy after induction of status epilepticus by prolonged electrical stimulation of the basolateral amygdala. FENS Abstr 5:106.26 [Google Scholar]
- Leite JP, Cavalheiro EA. (1995) Effects of conventional antiepileptic drugs in a model of spontaneous recurrent seizures in rats. Epilepsy Res 20:93–104 [DOI] [PubMed] [Google Scholar]
- Li T, Ren G, Lusardi T, Wilz A, Lan JQ, Iwasato T, Itohara S, Simon RP, Boison D. (2008a) Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Invest 118:571–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhou J, Chen Z, Chen S, Zhu F, Zhou L. (2008b) Long-term expressional changes of Na+-K+-Cl− co-transporter 1 (NKCC1) and K+-Cl− co-transporter 2 (KCC2) in CA1 region of hippocampus following lithium-pilocarpine induced status epilepticus (PISE). Brain Res 1221:141–146 [DOI] [PubMed] [Google Scholar]
- Liu Y, Yohrling GJ, Wang Y, Hutchinson TL, Brenneman DE, Flores CM, Zhao B. (2009) Carisbamate, a novel neuromodulator, inhibits voltage-gated sodium channels and action potential firing of rat hippocampal neurons. Epilepsy Res 83:66–72 [DOI] [PubMed] [Google Scholar]
- Löscher W. (1984) Genetic animal models of epilepsy as a unique resource for the evaluation of anticonvulsant drugs. A review. Methods Find Exp Clin Pharmacol 6:531–547 [PubMed] [Google Scholar]
- Löscher W. (1998a) New visions in the pharmacology of anticonvulsion. Eur J Pharmacol 342:1–13 [DOI] [PubMed] [Google Scholar]
- Löscher W. (1998b) Pharmacology of glutamate receptor antagonists in the kindling model of epilepsy. Prog Neurobiol 54:721–741 [DOI] [PubMed] [Google Scholar]
- Löscher W. (1999) Animal models of epilepsy and epileptic seizures. Handb Exp Pharmacol 138:19–62 [DOI] [PubMed] [Google Scholar]
- Löscher W. (2002a) Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res 50:105–123 [DOI] [PubMed] [Google Scholar]
- Löscher W. (2002b) Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs 16:669–694 [DOI] [PubMed] [Google Scholar]
- Löscher W. (2002c) Current status and future directions in the pharmacotherapy of epilepsy. Trends Pharmacol Sci 23:113–118 [DOI] [PubMed] [Google Scholar]
- Löscher W. (2007) The pharmacokinetics of antiepileptic drugs in rats: consequences for maintaining effective drug levels during prolonged drug administration in rat models of epilepsy. Epilepsia 48:1245–1258 [DOI] [PubMed] [Google Scholar]
- Löscher W. (2009) Preclinical assessment of proconvulsant drug activity and its relevance for predicting adverse events in humans. Eur J Pharmacol 610:1–11 [DOI] [PubMed] [Google Scholar]
- Löscher W, Ebert U. (1996) The role of the piriform cortex in kindling. Prog Neurobiol 50:427–481 [DOI] [PubMed] [Google Scholar]
- Löscher W, Gernert M, Heinemann U. (2008) Cell and gene therapies in epilepsy—promising avenues or blind alleys? Trends Neurosci 31:62–73 [DOI] [PubMed] [Google Scholar]
- Löscher W, Hönack D. (1990) The effect of interstimulation interval on the assessment of anticonvulsant drug potency in fully kindled rats. Epilepsy Res 7:182–196 [DOI] [PubMed] [Google Scholar]
- Löscher W, Hönack D, Bloms-Funke P. (1996) The novel antiepileptic drug levetiracetam (ucb L059) induces alterations in GABA metabolism and turnover in discrete areas of rat brain and reduces neuronal activity in substantia nigra pars reticulata. Brain Res 735:208–216 [DOI] [PubMed] [Google Scholar]
- Löscher W, Hönack D, Rundfeldt C. (1998) Antiepileptogenic effects of the novel anticonvulsant levetiracetam (ucb L059) in the kindling model of temporal lobe epilepsy. J Pharmacol Exp Ther 284:474–479 [PubMed] [Google Scholar]
- Löscher W, Liburdy RP. (1998) Animal and cellular studies on carcinogenic effects of low frequency (50/60-Hz) magnetic fields. Mutation Res 410:185–220 [DOI] [PubMed] [Google Scholar]
- Löscher W, Meldrum BS. (1984) Evaluation of anticonvulsant drugs in genetic animal models of epilepsy. Fed Proc 43:276–284 [PubMed] [Google Scholar]
- Löscher W, Wahnschaffe U, Hönack D, Rundfeldt C. (1995) Does prolonged implantation of depth electrodes predispose the brain to kindling? Brain Res 697:197–204 [DOI] [PubMed] [Google Scholar]
- Lothman EW, Hatlelid JM, Zorumski CF, Conry JA, Moon PF, Perlin JB. (1985) Kindling with rapidly recurring hippocampal seizures. Brain Res 360:83–91 [DOI] [PubMed] [Google Scholar]
- Lowenstein DH. (1999) Status epilepticus: An overview of the clinical problem. Epilepsia 40 (Suppl 1):S3–S8 [DOI] [PubMed] [Google Scholar]
- Lowenstein DH. (2001) Structural reorganization of hippocampal networks caused by seizure activity. Int Rev Neurobiol 45:209–236 [DOI] [PubMed] [Google Scholar]
- Lowenstein DH. (2006) The management of refractory status epilepticus: an update. Epilepsia 47:35–40 [DOI] [PubMed] [Google Scholar]
- Lukasiuk K, Sliwa A. (2009) FK506 aggravates development and severity of disease in the rat model of temporal lobe epilepsy. Epilepsia 50 (Suppl 4):93–9419298435 [Google Scholar]
- Lukasiuk K, Dabrowski M, Adach A, Pitkänen A. (2006) Epileptogenesis-related genes revisited. Prog Brain Res 158:223–241 [DOI] [PubMed] [Google Scholar]
- Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, Fuks B. (2004) The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA 101:9861–9866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyseng-Williamson KA, Yang LP. (2007) Topiramate: a review of its use in the treatment of epilepsy. Drugs 67:2231–2256 [DOI] [PubMed] [Google Scholar]
- Mann EO, Mody I. (2008) The multifaceted role of inhibition in epilepsy: seizure-genesis through excessive GABAergic inhibition in autosomal dominant nocturnal frontal lobe epilepsy. Curr Opin Neurol 21:155–160 [DOI] [PubMed] [Google Scholar]
- Marcangelo MJ, Ovsiew F. (2007) Psychiatric aspects of epilepsy. Psychiatr Clin North Am 30:781–802 [DOI] [PubMed] [Google Scholar]
- Marchi N, Fan Q, Ghosh C, Fazio V, Bertolini F, Betto G, Batra A, Carlton E, Najm I, Granata T, et al. (2009) Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol Dis 33:171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Oby E, Batra A, Uva L, De Curtis M, Hernandez N, Van Boxel-Dezaire A, Najm I, Janigro D. (2007) In vivo and in vitro effects of pilocarpine: relevance to ictogenesis. Epilepsia 48:1934–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margerison JH, Corsellis JA. (1966) Epilepsy and the temporal lobes. A clinical, electroencephalographic and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain 89:499–530 [DOI] [PubMed] [Google Scholar]
- Margineanu DG, Matagne A, Kaminski RM, Klitgaard H. (2008) Effects of chronic treatment with levetiracetam on hippocampal field responses after pilocarpine-induced status epilepticus in rats. Brain Res Bull 77:282–285 [DOI] [PubMed] [Google Scholar]
- Martin BS, Kapur J. (2008) A combination of ketamine and diazepam synergistically controls refractory status epilepticus induced by cholinergic stimulation. Epilepsia 49:248–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matveeva EA, Vanaman TC, Whiteheart SW, Slevin JT. (2008) Levetiracetam prevents kindling-induced asymmetric accumulation of hippocampal 7S SNARE complexes. Epilepsia 49:1749–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati A, Lundström L, Sollenberg U, Shin D, Langel U, Sankar R. (2006a) Regulation of kindling epileptogenesis by hippocampal galanin type 1 and type 2 receptors: The effects of subtype-selective agonists and the role of G-protein-mediated signaling. J Pharmacol Exp Ther 318:700–708 [DOI] [PubMed] [Google Scholar]
- Mazarati A, Shin D, Auvin S, Sankar R. (2007) Age-dependent effects of topiramate on the acquisition and the retention of rapid kindling. Epilepsia 48:765–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati A, Shin D, Sankar R. (2009) Bumetanide inhibits rapid kindling in neonatal rats. Epilepsia 50:2117–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati AM, Baldwin RA, Klitgaard H, Matagne A, Wasterlain CG. (2003) Treatment with levetiracetam during the latent period after experimental status epilepticus reduces chronic spontaneous recurrent seizures. Epilepsia 44 (Suppl 9):223 [Google Scholar]
- Mazarati AM, Sofia RD, Wasterlain CG. (2002) Anticonvulsant and antiepileptogenic effects of fluorofelbamate in experimental status epilepticus. Seizure 11:423–430 [DOI] [PubMed] [Google Scholar]
- Mazarati AM, Thompson KW, Suchomelova L, Sankar R, Shirasaka Y, Nissinen J, Pitkänen A, Bertram E, Wasterlain C. (2006b) Status epilepticus: electrical stimulation models, in Models of Seizures and Epilepsy (Pitkänen M, Schwartzkroin PA, Moshé SL. eds) pp 449–464, Elsevier, Amsterdam [Google Scholar]
- McIntyre DC, Gilby KL. (2008) Mapping seizure pathways in the temporal lobe. Epilepsia 49 (Suppl 3):23–30 [DOI] [PubMed] [Google Scholar]
- McIntyre DC, Poulter MO, Gilby K. (2002) Kindling: some old and some new. Epilepsy Res 50:79–92 [DOI] [PubMed] [Google Scholar]
- Mikati MA, Holmes GL, Chronopoulos A, Hyde P, Thurber S, Gatt A, Liu Z, Werner S, Stafstrom CE. (1994) Phenobarbital modifies seizure-related brain injury in the developing brain. Ann Neurol 36:425–433 [DOI] [PubMed] [Google Scholar]
- Morimoto K, Fahnestock M, Racine RJ. (2004) Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol 73:1–60 [DOI] [PubMed] [Google Scholar]
- Müller CJ, Bankstahl M, Gröticke I, Löscher W. (2009a) Pilocarpine vs. lithium-pilocarpine for induction of status epilepticus in mice: development of spontaneous seizures, behavioral alterations and neuronal damage. Eur J Pharmacol 619:15–24 [DOI] [PubMed] [Google Scholar]
- Müller CJ, Gröticke I, Bankstahl M, Löscher W. (2009b) Behavioral and cognitive alterations, spontaneous seizures, and neuropathology developing after a pilocarpine-induced status epilepticus in C57BL/6 mice. Exp Neurol 219:284–297 [DOI] [PubMed] [Google Scholar]
- Müller CJ, Gröticke I, Hoffmann K, Schughart K, Löscher W. (2009c) Differences in sensitivity to the convulsant pilocarpine in substrains and sublines of C57BL/6 mice. Genes Brain Behav 8:481–492 [DOI] [PubMed] [Google Scholar]
- Nadam J, Navarro F, Sanchez P, Moulin C, Georges B, Laglaine A, Pequignot JM, Morales A, Ryvlin P, Bezin L. (2007) Neuroprotective effects of erythropoietin in the rat hippocampus after pilocarpine-induced status epilepticus. Neurobiol Dis 25:412–426 [DOI] [PubMed] [Google Scholar]
- Nadler JV. (2003) The recurrent mossy fiber pathway of the epileptic brain. Neurochem Res 28:1649–1658 [DOI] [PubMed] [Google Scholar]
- Narkilahti S, Nissinen J, Pitkänen A. (2003) Administration of caspase 3 inhibitor during and after status epilepticus in rat: effect on neuronal damage and epileptogenesis. Neuropharmacology 44:1068–1088 [DOI] [PubMed] [Google Scholar]
- Navarro Mora G, Bramanti P, Osculati F, Chakir A, Nicolato E, Marzola P, Sbarbati A, Fabene PF. (2009) Does pilocarpine-induced epilepsy in adult rats require status epilepticus? PLoS One 4:e5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlig A. (2007) What is animal experimentation telling us about new drug treatments of status epilepticus? Epilepsia 48 (Suppl 8):78–81 [DOI] [PubMed] [Google Scholar]
- Neligan A, Shorvon S. (2008) Refractory versus non-refractory status epilepticus: frequency, differentiating clinical features, and outcome, in Drug-Resistant Epilepsies. Progress in Epileptic Disorders (Kahane P, Berg A, Löscher W, Nordli D, Perucca E. eds) vol. 7, pp 29–46, John Libbey, Montrouge, France [Google Scholar]
- Niebauer M, Gruenthal M. (1999) Topiramate reduces neuronal injury after experimental status epilepticus. Brain Res 837:263–269 [DOI] [PubMed] [Google Scholar]
- Niespodziany I, Klitgaard H, Margineanu DG. (1999) Chronic electrode implantation entails epileptiform field potentials in rat hippocampal slices, similarly to amygdala kindling. Epilepsy Res 36:69–74 [DOI] [PubMed] [Google Scholar]
- Nissinen J, Halonen T, Koivisto E, Pitkänen A. (2000) A new model of chronic temporal lobe epilepsy induced by electrical stimulation of the amygdala in rat. Epilepsy Res 38:177–205 [DOI] [PubMed] [Google Scholar]
- Nissinen J, Large CH, Stratton SC, Pitkänen A. (2004) Effect of lamotrigine treatment on epileptogenesis: an experimental study in rat. Epilepsy Res 58:119–132 [DOI] [PubMed] [Google Scholar]
- Norwood BA, Bumanglag AV, Osculati F, Sbarbati A, Marzola P, Nicolato E, Fabene PF, Sloviter RS. (2010) Classic hippocampal sclerosis and hippocampal-onset epilepsy produced by a single “cryptic” episode of focal hippocampal excitation in awake rats. J Comp Neurol 518:3381–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak GP, Kelley M, Zannikos P, Klein B. (2007) Carisbamate (RWJ-333369). Neurotherapeutics 4:106–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe A, Ohno K, Toyoda H, Yokokura M, Sato K, Fukuda A. (2002) Amygdala kindling induces upregulation of mRNA for NKCC1, a Na(+), K(+)-2Cl(−) cotransporter, in the rat piriform cortex. Neurosci Res 44:225–229 [DOI] [PubMed] [Google Scholar]
- Okabe A, Yokokura M, Toyoda H, Shimizu-Okabe C, Ohno K, Sato K, Fukuda A. (2003) Changes in chloride homeostasis-regulating gene expressions in the rat hippocampus following amygdala kindling. Brain Res 990:221–226 [DOI] [PubMed] [Google Scholar]
- Palma E, Amici M, Sobrero F, Spinelli G, Di Angelantonio S, Ragozzino D, Mascia A, Scoppetta C, Esposito V, Miledi R, et al. (2006) Anomalous levels of Cl- transporters in the hippocampal subiculum from temporal lobe epilepsy patients make GABA excitatory. Proc Natl Acad Sci USA 103:8465–8468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma E, Ragozzino D, Di Angelantonio S, Mascia A, Maiolino F, Manfredi M, Cantore G, Esposito V, Di Gennaro G, Quarato P, et al. (2007) The antiepileptic drug levetiracetam stabilizes the human epileptic GABAA receptors upon repetitive activation. Epilepsia 48:1842–1849 [DOI] [PubMed] [Google Scholar]
- Paradiso B, Marconi P, Zucchini S, Berto E, Binaschi A, Bozac A, Buzzi A, Mazzuferi M, Magri E, Navarro Mora G, et al. (2009) Localized delivery of fibroblast growth factor-2 and brain-derived neurotrophic factor reduces spontaneous seizures in an epilepsy model. Proc Natl Acad Sci USA 106:7191–7196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak HR, Weissinger F, Terunuma M, Carlson GC, Hsu FC, Moss SJ, Coulter DA. (2007) Disrupted dentate granule cell chloride regulation enhances synaptic excitability during development of temporal lobe epilepsy. J Neurosci 27:14012–14022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A. (2002) Drug-mediated neuroprotection and antiepileptogenesis: animal data. Neurology 59:S27–S33 [DOI] [PubMed] [Google Scholar]
- Pitkanen A. (2004) New pharmacotherapy for epilepsy. IDrugs 7:471–477 [PubMed] [Google Scholar]
- Pitkänen A. (2010) Therapeutic approaches to epileptogenesis—hope on the horizon. Epilepsia 51 (Suppl 3):2–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A, Halonen T. (1998) Prevention of epilepsy. Trends Pharmacol Sci 19:253–255 [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Immonen RJ, Grohn OH, Kharatishvili I. (2009) From traumatic brain injury to posttraumatic epilepsy: what animal models tell us about the process and treatment options. Epilepsia 50 (Suppl 2):21–29 [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Kharatishvili I, Karhunen H, Lukasiuk K, Immonen R, Nairismagi J, Grohn O, Nissinen J. (2007a) Epileptogenesis in experimental models. Epilepsia 48 (Suppl 2):13–20 [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Kharatishvili I, Narkilahti S, Lukasiuk K, Nissinen J. (2005) Administration of diazepam during status epilepticus reduces development and severity of epilepsy in rat. Epilepsy Res 63:27–42 [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Lukasiuk K. (2009) Molecular and cellular basis of epileptogenesis in symptomatic epilepsy. Epilepsy Behav 14 (Suppl 1):16–25 [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Mathiesen C, Rønn LC, Møller A, Nissinen J. (2007b) Effect of novel AMPA antagonist, NS1209, on status epilepticus. An experimental study in rat. Epilepsy Res 74:45–54 [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Narkilahti S, Bezvenyuk Z, Haapalinna A, Nissinen J. (2004) Atipamezole, an alpha(2)-adrenoceptor antagonist, has disease modifying effects on epileptogenesis in rats. Epilepsy Res 61:119–140 [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Nissinen J, Nairismägi J, Lukasiuk K, Gröhn OH, Miettinen R, Kauppinen R. (2002) Progression of neuronal damage after status epilepticus and during spontaneous seizures in a rat model of temporal lobe epilepsy. Prog Brain Res 135:67–83 [DOI] [PubMed] [Google Scholar]
- Polascheck N, Bankstahl M, Löscher W. (2010) The COX-2 inhibitor parecoxib is neuroprotective but not antiepileptogenic in the pilocarpine model of temporal lobe epilepsy. Exp Neurol 224:219–233 [DOI] [PubMed] [Google Scholar]
- Portelli J, Aourz N, De Bundel D, Meurs A, Smolders I, Michotte Y, Clinckers R. (2009) Intrastrain differences in seizure susceptibility, pharmacological response and basal neurochemistry of Wistar rats. Epilepsy Res 87:234–246 [DOI] [PubMed] [Google Scholar]
- Pouliot WA, Staley KJ, Dudek FE. (2009) Quantitative analysis of the effects of the cannabinoid-receptor antagonist, SR 141716, on the development of spontaneous recurrent seizures after kainate-induced status epilepticus. Soc Neurosci Abstr 35:638.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A, Williamson JM, Bertram EH. (2002) Phenobarbital and MK-801, but not phenytoin, improve the long-term outcome of status epilepticus. Ann Neurol 51:175–181 [DOI] [PubMed] [Google Scholar]
- Racine R, Livingston K, Joaquin A. (1975) Effects of procaine hydrochloride, diazepam, and diphenylhydantoin on seizure development in cortical and subcortical structures in rats. Electroenceph Clin Neurophysiol 38:355–365 [DOI] [PubMed] [Google Scholar]
- Raedt R, Van Dycke A, Van Melkebeke D, De Smedt T, Claeys P, Wyckhuys T, Vonck K, Wadman W, Boon P. (2009) Seizures in the intrahippocampal kainic acid epilepsy model: characterization using long-term video-EEG monitoring in the rat. Acta Neurol Scand 119:293–303 [DOI] [PubMed] [Google Scholar]
- Ratzliff AH, Howard AL, Santhakumar V, Osapay I, Soltesz I. (2004) Rapid deletion of mossy cells does not result in a hyperexcitable dentate gyrus: implications for epileptogenesis. J Neurosci 24:2259–2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner AD. (2003) The electroconvulsive therapy controversy: evidence and ethics. Neuropsychol Rev 13:199–219 [DOI] [PubMed] [Google Scholar]
- Rigoulot MA, Koning E, Ferrandon A, Nehlig A. (2004) Neuroprotective properties of topiramate in the lithium-pilocarpine model of epilepsy. J Pharmacol Exp Ther 308:787–795 [DOI] [PubMed] [Google Scholar]
- Rigoulot MA, Leroy C, Koning E, Ferrandon A, Nehlig A. (2003) Prolonged low-dose caffeine exposure protects against hippocampal damage but not against the occurrence of epilepsy in the lithium-pilocarpine model in the rat. Epilepsia 44:529–535 [DOI] [PubMed] [Google Scholar]
- Rivera C, Li H, Thomas-Crusells J, Lahtinen H, Viitanen T, Nanobashvili A, Kokaia Z, Airaksinen MS, Voipio J, Kaila K, et al. (2002) BDNF-induced TrkB activation down-regulates the K+-Cl− cotransporter KCC2 and impairs neuronal Cl− extrusion. J Cell Biol 159:747–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Kaila K. (2005) Two developmental switches in GABAergic signalling: the K+-Cl− cotransporter KCC2 and carbonic anhydrase CAVII. J Physiol 562:27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roch C, Leroy C, Nehlig A, Namer IJ. (2002) Magnetic resonance imaging in the study of the lithium-pilocarpine model of temporal lobe epilepsy in adult rats. Epilepsia 43:325–335 [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Bazil CW. (2008) New molecular targets for antiepileptic drugs: alpha(2)delta, SV2A, and K(v)7/KCNQ/M potassium channels. Curr Neurol Neurosci Rep 8:345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA, Löscher W. (2004a) The neurobiology of antiepileptic drugs. Nat Rev Neurosci 5:553–564 [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Löscher W. (2004b) The neurobiology of antiepileptic drugs for the treatment of nonepileptic conditions. Nat Med 10:685–692 [DOI] [PubMed] [Google Scholar]
- Rosenberg G. (2007) The mechanisms of action of valproate in neuropsychiatric disorders: can we see the forest for the trees? Cell Mol Life Sci 64:2090–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo E, Citraro R, Scicchitano F, De Fazio S, Di Paola ED, Constanti A, De Sarro G. (2009) Comparison of the antiepileptogenic effects of an early long-term treatment with ethosuximide or levetiracetam in a genetic animal model of absence epilepsy. Epilepsia 11:1560–1569 [DOI] [PubMed] [Google Scholar]
- Sankar R. (2005) Neuroprotection in epilepsy: the Holy Grail of antiepileptogenic therapy. Epilepsy Behav 7 (Suppl 3):S1–S2 [DOI] [PubMed] [Google Scholar]
- Schauwecker PE. (2002) Complications associated with genetic background effects in models of experimental epilepsy. Prog Brain Res 135:139–148 [DOI] [PubMed] [Google Scholar]
- Schauwecker PE, Steward O. (1997) Genetic determinants of susceptibility to excitotoxic cell death: implications for gene targeting approaches. Proc Natl Acad Sci USA 94:4103–4108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D, Löscher W. (2005) Drug resistance in epilepsy: putative neurobiologic and clinical mechanisms. Epilepsia 46:858–877 [DOI] [PubMed] [Google Scholar]
- Schmutz M, Klebs K, Baltzer V. (1988) Inhibition or enhancement of kindling evolution by antiepileptics. J Neural Transm 72:245–257 [DOI] [PubMed] [Google Scholar]
- Shank RP, Gardocki JF, Streeter AJ, Maryanoff BE. (2000) An overview of the preclinical aspects of topiramate: pharmacology, pharmacokinetics, and mechanism of action. Epilepsia 41 (Suppl 1):S3–S9 [PubMed] [Google Scholar]
- Shatskikh T, Zhao Q, Zhou JL, Holmes GL. (2009) Effect of topiramate on cognitive function and single units from hippocampal place cells following status epilepticus. Epilepsy Behav 14:40–47 [DOI] [PubMed] [Google Scholar]
- Shin C, Rigsbee LC, McNamara JO. (1986) Anti-seizure and anti-epileptogenic effect of gamma-vinyl gamma-aminobutyric acid in amygdaloid kindling. Brain Res 398:370–374 [DOI] [PubMed] [Google Scholar]
- Silver JM, Shin C, McNamara JO. (1991) Antiepileptogenic effects of conventional anticonvulsants in the kindling model of epilepsy. Ann Neurol 29:356–363 [DOI] [PubMed] [Google Scholar]
- Sloviter RS. (1987) Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science 235:73–76 [DOI] [PubMed] [Google Scholar]
- Sloviter RS. (1991) Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the “dormant basket cell” hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus 1:41–66 [DOI] [PubMed] [Google Scholar]
- Sloviter RS. (1994) The functional organization of the hippocampal dentate gyrus and its relevance to the pathogenesis of temporal lobe epilepsy. Ann Neurol 35:640–654 [DOI] [PubMed] [Google Scholar]
- Sloviter RS. (2005) The neurobiology of temporal lobe epilepsy: too much information, not enough knowledge. C R Biol 328:143–153 [DOI] [PubMed] [Google Scholar]
- Sloviter RS. (2008) Hippocampal epileptogenesis in animal models of mesial temporal lobe epilepsy with hippocampal sclerosis: the importance of the “latent period” and other concepts. Epilepsia 49 (Suppl 9):85–92 [DOI] [PubMed] [Google Scholar]
- Sloviter RS. (2009) Experimental status epilepticus in animals: What are we modeling? Epilepsia 50 (Suppl 12):11–13 [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Zappone CA, Bumanglag AV, Norwood BA, Kudrimoti H. (2007) On the relevance of prolonged convulsive status epilepticus in animals to the etiology and neurobiology of human temporal lobe epilepsy. Epilepsia 48 (Suppl 8):6–10 [DOI] [PubMed] [Google Scholar]
- Stables JP, Bertram E, Dudek FE, Holmes G, Mathern G, Pitkanen A, White HS. (2003) Therapy discovery for pharmacoresistant epilepsy and for disease-modifying therapeutics: summary of the NIH/NINDS/AES models II workshop. Epilepsia 44:1472–1478 [DOI] [PubMed] [Google Scholar]
- Stables JP, Bertram EH, White HS, Coulter DA, Dichter MA, Jacobs MP, Loscher W, Lowenstein DH, Moshe SL, Noebels JL, et al. (2002) Models for epilepsy and epileptogenesis: report from the NIH workshop, Bethesda, Maryland. Epilepsia 43:1410–1420 [DOI] [PubMed] [Google Scholar]
- Stafstrom CE. (2006) Behavioral and cognitive testing procedures in animal models of epilepsy, in Models of Seizures and Epilepsy (Pitkänen A, Schwartzkroin PA, Moshé SL. eds) pp 613–628, Elsevier, Amsterdam [Google Scholar]
- Staley K, Hellier JL, Dudek FE. (2005) Do interictal spikes drive epileptogenesis? Neuroscientist 11:272–276 [DOI] [PubMed] [Google Scholar]
- Stefan H, Lopes da Silva FH, Löscher W, Schmidt D, Perucca E, Brodie MJ, Boon PA, Theodore WH, Moshé SL. (2006) Epileptogenesis and rational therapeutic strategies. Acta Neurol Scand 113:139–155 [DOI] [PubMed] [Google Scholar]
- Stratton SC, Large CH, Cox B, Davies G, Hagan RM. (2003) Antiepileptogenic-like effects of lamotrigine in a rat amygdala kindling model. Epilepsy Res 53:95–106 [DOI] [PubMed] [Google Scholar]
- Suchomelova L, Baldwin RA, Kubova H, Thompson KW, Sankar R, Wasterlain CG. (2006) Treatment of experimental status epilepticus in immature rats: dissociation between anticonvulsant and antiepileptogenic effects. Pediatr Res 59:237–243 [DOI] [PubMed] [Google Scholar]
- Sutula T, Cavazos J, Golarai G. (1992a) Alteration of long-lasting structural and functional effects of kainic acid in the hippocampus by brief treatment with phenobarbital. J Neurosci 12:4173–4187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutula T, Koch J, Golarai G, Watanabe Y, McNamara JO. (1996) NMDA receptor dependence of kindling and mossy fiber sprouting: evidence that the NMDA receptor regulates patterning of hippocampal circuits in the adult brain. J Neurosci 16:7398–7406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutula TP, Golarai G, Cavazos J. (1992b) Assessing the functional significance of mossy fiber sprouting. Epilepsy Res Suppl 7:251–259 [PubMed] [Google Scholar]
- Taniura H, Sng JC, Yoneda Y. (2006) Histone modifications in status epilepticus induced by kainate. Histol Histopathol 21:785–791 [DOI] [PubMed] [Google Scholar]
- Temkin NR. (2001) Antiepileptogenesis and seizure prevention trials with antiepileptic drugs: meta-analysis of controlled trials. Epilepsia 42:515–524 [DOI] [PubMed] [Google Scholar]
- Temkin NR. (2009) Preventing and treating posttraumatic seizures: the human experience. Epilepsia 50:10–13 [DOI] [PubMed] [Google Scholar]
- Temkin NR, Dikmen SS, Anderson GD, Wilensky AJ, Holmes MD, Cohen W, Newell DW, Nelson P, Awan A, Winn HR. (1999) Valproate therapy for prevention of posttraumatic seizures: a randomized trial. J Neurosurg 91:593–600 [DOI] [PubMed] [Google Scholar]
- Thom M, Martinian L, Catarino C, Yogarajah M, Koepp MJ, Caboclo L, Sisodiya SM. (2009) Bilateral reorganization of the dentate gyrus in hippocampal sclerosis: a postmortem study. Neurology 73:1033–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner IM, Newman SM, Louis S, Kutt H. (1977) Pharmacological prophylaxis against the development of kindled amygdaloid seizures. Ann Neurol 2:221–224 [DOI] [PubMed] [Google Scholar]
- Vermoesen K, Smolders I, Massie A, Michotte Y, Clinckers R. (2010) The control of kainic acid-induced status epilepticus. Epilepsy Res 90:164–166 [DOI] [PubMed] [Google Scholar]
- Vezzani A, Balosso S, Maroso M, Zardoni D, Noé F, Ravizza T. (2010) ICE/caspase 1 inhibitors and IL-1beta receptor antagonists as potential therapeutics in epilepsy. Curr Opin Investig Drugs 11:43–50 [PubMed] [Google Scholar]
- Vezzani A, Baram TZ. (2007) New roles for interleukin-1 Beta in the mechanisms of epilepsy. Epilepsy Curr 7:45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Granata T. (2005) Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia 46:1724–1743 [DOI] [PubMed] [Google Scholar]
- Volk HA, Arabadzisz D, Fritschy JM, Brandt C, Bethmann K, Löscher W. (2006) Antiepileptic drug-resistant rats differ from drug-responsive rats in hippocampal neurodegeneration and GABA(A) receptor ligand binding in a model of temporal lobe epilepsy. Neurobiol Dis 21:633–646 [DOI] [PubMed] [Google Scholar]
- Voutsinos-Porche B, Koning E, Kaplan H, Ferrandon A, Guenounou M, Nehlig A, Motte J. (2004) Temporal patterns of the cerebral inflammatory response in the rat lithium-pilocarpine model of temporal lobe epilepsy. Neurobiol Dis 17:385–402 [DOI] [PubMed] [Google Scholar]
- Walker M. (2007) Neuroprotection in epilepsy. Epilepsia 48 (Suppl 8):66–68 [DOI] [PubMed] [Google Scholar]
- Walker MC, White HS, Sander JW. (2002) Disease modification in partial epilepsy. Brain 125:1937–1950 [DOI] [PubMed] [Google Scholar]
- Weaver DF. (2003) Epileptogenesis, ictogenesis and the design of future antiepileptic drugs. Can J Neurol Sci 30:4–7 [DOI] [PubMed] [Google Scholar]
- White A, Williams PA, Hellier JL, Clark S, Edward Dudek F, Staley KJ. (2010) EEG spike activity precedes epilepsy after kainate-induced status epilepticus. Epilepsia 51:371–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Williams PA, Ferraro DJ, Clark S, Kadam SD, Dudek FE, Staley KJ. (2006) Efficient unsupervised algorithms for the detection of seizures in continuous EEG recordings from rats after brain injury. J Neurosci Methods 152:255–266 [DOI] [PubMed] [Google Scholar]
- Williams PA, Hellier JL, White AM, Staley KJ, Dudek FE. (2007) Development of spontaneous seizures after experimental status epilepticus: implications for understanding epileptogenesis. Epilepsia 48 (Suppl 5):157–163 [DOI] [PubMed] [Google Scholar]
- Williams PA, White AM, Clark S, Ferraro DJ, Swiercz W, Staley KJ, Dudek FE. (2009) Development of spontaneous recurrent seizures after kainate-induced status epilepticus. J Neurosci 29:2103–2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmore LJ. (2005) Antiepileptic drugs and neuroprotection: current status and future roles. Epilepsy Behav 7:S25–S28 [DOI] [PubMed] [Google Scholar]
- Yan HD, Ji-qun C, Ishihara K, Nagayama T, Serikawa T, Sasa M. (2005) Separation of antiepileptogenic and antiseizure effects of levetiracetam in the spontaneously epileptic rat (SER). Epilepsia 46:1170–1177 [DOI] [PubMed] [Google Scholar]
- Yang XF, Weisenfeld A, Rothman SM. (2007) Prolonged exposure to levetiracetam reveals a presynaptic effect on neurotransmission. Epilepsia 48:1861–1869 [DOI] [PubMed] [Google Scholar]
- Yilmazer-Hanke DM, Wolf HK, Schramm J, Elger CE, Wiestler OD, Blümcke I. (2000) Subregional pathology of the amygdala complex and entorhinal region in surgical specimens from patients with pharmacoresistant temporal lobe epilepsy. J Neuropathol Exp Neurol 59:907–920 [DOI] [PubMed] [Google Scholar]
- Zeng LH, Rensing NR, Wong M. (2009) The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci 29:6964–6972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Xu L, Gutmann DH, Wong M. (2008) Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol 63:444–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JL, Zhao Q, Holmes GL. (2007) Effect of levetiracetam on visual-spatial memory following status epilepticus. Epilepsy Res 73:65–74 [DOI] [PubMed] [Google Scholar]