Abstract
Background:
Measuring the quality of health care is a fundamental step toward improving health care and is increasingly used in pay-for-performance initiatives and maintenance of certification requirements. Measure development to date has focused on primary care and common conditions such as diabetes; thus, the number of measures that apply to neurologic care is limited. The American Academy of Neurology (AAN) identified the need for neurologists to develop measures of neurologic care and to establish a process to accomplish this.
Objective:
To adapt and test the feasibility of a process for independent development by the AAN of measures for neurologic conditions for national measurement programs.
Methods:
A process that has been used nationally for measure development was adapted for use by the AAN. Topics for measure development are chosen based upon national priorities, available evidence base from a systematic literature search, gaps in care, and the potential impact for quality improvement. A panel composed of subject matter and measure development methodology experts oversees the development of the measures. Recommendation statements and their corresponding level of evidence are reviewed and considered for development into draft candidate measures. The candidate measures are refined by the expert panel during a 30-day public comment period and by review by the American Medical Association for Current Procedural Terminology (CPT) II codes. All final AAN measures are approved by the AAN Board of Directors.
Results:
Parkinson disease (PD) was chosen for measure development. A review of the medical literature identified 258 relevant recommendation statements. A 28-member panel approved 10 quality measures for PD that included full specifications and CPT II codes.
Conclusion:
The AAN has adapted a measure development process that is suitable for national measurement programs and has demonstrated its capability to independently develop quality measures.
GLOSSARY
- AAN
= American Academy of Neurology;
- ABPN
= American Board of Psychiatry and Neurology;
- AMA
= American Medical Association;
- CPT II
= Current Procedural Terminology;
- PCPI
= Physician Consortium for Performance Improvement;
- PD
= Parkinson disease;
- PMAG
= Performance Measurement Advisory Group;
- PQRI
= Physician Quality Reporting Initiative;
- QMR
= Quality Measurement and Reporting Subcommittee.

Podcast
Health care stakeholders recognize the importance of measuring the quality of health care. Improvements in the quality of health care—such as the use of β-blockers after acute myocardial infarction1—have occurred shortly after programs to measure such care were implemented. Measuring the quality of health care is now central in the evaluation of health care plans for large corporations using Healthcare Effectiveness Data and Information Set measures,2 accreditation of hospitals by the Joint Commission,3 reimbursement of physicians through a pay-for-performance program run by Medicare,4–8 and maintenance of certification by specialty boards.9,10 Prior to measuring quality, the dominant measure of health care was cost. Measurement of quality permits evaluation of health care on its value, roughly defined as a ratio of quality to cost,11 and this is a step forward from evaluating health care solely on cost.
Programs that measure health care quality have focused on highly prevalent chronic conditions that are managed by primary care providers, such as asthma and diabetes, and have not focused on conditions treated by specialists. Although there is further work to be done in the science of measuring quality, there are consequences for delaying the development of quality measurement programs for specialty care. If the care delivered by specialists is not evaluated by these measurement programs, the value of health care delivered by specialists becomes difficult to quantify and can be underestimated. Furthermore, if the care delivered by specialists is measured by programs developed without the input of the specialists, the value of care may not be accurately measured.
Recognizing the potential impact of quality measurement on the practice of clinical neurology, the Board of Directors of the American Academy of Neurology (AAN) incorporated the development of quality measures (otherwise known as performance measures or quality indicators) for neurologic practice into their 2003 strategic plan12 and established the AAN Quality Measurement and Reporting (QMR) Subcommittee to carry out this task.13 A quality measure is a mechanism for assessing the degree to which a physician competently and safely delivers clinical services that are appropriate for the patient in the optimal time period.14,15 The measure specifications include a definition of the desired action or outcome and the patient population to whom the measure applies, which may include subpopulations that should be excluded. For example, a widely used quality measure is offering antiplatelet therapy to all patients presenting with acute ischemic stroke within 48 hours of hospital admission, excluding those patients who have contraindications to this therapy, such as active bleeding or allergies.16
In the past decade, the development of quality measures has been led by the American Medical Association (AMA)–convened Physician Consortium for Performance Improvement (PCPI), an organization consisting of over 170 representatives from key stakeholders such as medical specialty associations, including the AAN. The AAN was a lead organization in a PCPI activity to develop a set of quality measures for stroke and stroke rehabilitation,16 which is now part of Medicare's pay-for-reporting program.5 Because there is a backlog of measures to be developed by PCPI, the AAN developed a process to develop new quality measures independently or without the assistance of the PCPI. This report describes the development of quality measures for the care of PD, the first set of quality measures developed independently by the AAN.
METHODS
The measure development process follows the QMR Subcommittee process manual for measure development (see appendix e-2 on the Neurology® Web site at www.neurology.org). This process is described below.
Topic selection for measurement development.
Topic selection is based on a literature review that demonstrates gaps in care (either room for improvement or unexplained variation in care), the availability of an evidence base to support the development of measures (existing evidence-based practice recommendations, consensus papers, or measures), and the potential impact of the topic area (prevalence, burden of illness [estimates of morbidity and mortality], cost, or the identification of the topic as a national clinical priority area).17 The QMR assigns one to two members to serve as facilitators and selects co-chairs for the measure development panel from disease- or condition-specific experts.
Literature search.
The co-chairs and facilitators, guided by a Master of Library and Information Science–level medical librarian, conduct a comprehensive search to identify published guidelines, measures, and consensus recommendations in the AAN guidelines, the National Guidelines Clearinghouse, the National Quality Measures Clearinghouse, PubMed, MEDLINE, EMBASE, and the Cochrane Library. Internet searches are also carried out on relevant Web sites.
Evaluation of the evidence base supporting development and writing of measures.
The AAN quality measures development process derives performance measures from the evidence base derived from clinical practice guidelines and consensus papers. Because guideline development methodology varies widely across guideline developers, the PCPI provides a framework to evaluate the acceptability of guidelines for measure development. AAN staff screen each selected full-text guideline or consensus paper against this framework to determine the acceptability of each guideline or other evidence review document.18 If the inclusion of an article based on eligibility criteria is unclear, the co-chairs and facilitators are consulted. From the eligible guidelines and consensus papers, AAN staff extract the recommendation statements and their corresponding level of evidence. The recommendation statements are reviewed and ranked by the co-chairs and facilitators based on validity, feasibility, and gap in care. This ranking narrows the recommendation statements considered for development into candidate measures.
Measure statements and specifications are carefully drafted with an experienced methodologist (a consultant with expertise in drafting measure specifications) to include a full measure description, a numerator (how to perform the measure), a denominator (patient population eligible for the measure), and appropriate exclusions from being included in the measure, with examples for medical, patient, or system reasons.
Panel formation.
The co-chairs independently select panelists from a group of disease- or condition-specific specialists who respond to a call for panelists. The selection is based on the nominee's experience in performance measures, quality improvement, and clinical activities. In addition, requests for nominations are sent to relevant physician organizations and patient advocacy groups. Large health care organizations or insurers are also invited to nominate one individual for the panel. All nominees must complete the AAN conflict of interest disclosure before they can be accepted as a panel member.
Selection of final measures.
The expert panel reviews the candidate measures at a face-to-face meeting. Panelists are given the opportunity to revise the measure statements and specifications or recommend that measures be dropped. The co-chairs and facilitators accommodate revisions to reach unanimous consent by the expert panel, but when unanimity cannot be achieved, the chairs call for a majority vote. The measures selected by the expert panel are then posted on the AAN Web site for a 30-day public comment period. Relevant stakeholders and stakeholder groups are notified and given the opportunity to post comments on the measures during this time period. After the public comment period, the expert panel reviews each comment and considers rewording measures to improve clarity or modify content. All public comments and responses are posted on the AAN Web site and remain a product of the measurement set. The final measures are submitted for AMA Current Procedural Terminology (CPT®) II code assignment by the AMA Performance Measurement Advisory Group (PMAG).
PD measurement set.
The PD topic was selected because it is a clinical priority for neurology and because the AAN had recently published a broad set of guidelines on this topic.19–23 The literature search identified 258 relevant recommendation statements from 8 guidelines24–31 and 1 consensus paper.32 The co-chair and facilitator rankings resulted in 12 recommendation statements that served as candidate measures for review by the full expert panel.
Twenty-two movement disorder specialists from the AAN movement disorders section responded to a call for serving on the panel. The final panel consisted of 28 members: 9 movement disorder specialists, 4 insurance plan representatives, 3 patient organization representatives, 2 physician coding specialists, 2 facilitators, 2 AAN staff, 1 methodologist, 1 psychiatrist, 1 neuropsychologist, 1 psychologist, 1 family practice physician, and 1 neurosurgeon. The in-person meeting was held in Orlando, FL, on January 17, 2009. The panel revised the draft measure statements and specifications and eliminated one measure. The remaining 11 candidate PD measures were posted for a 30-day public comment period on the AAN Web site. A total of 227 comments were reviewed and responded to by the expert panel, resulting in an additional measure being dropped. The remaining 10 PD measures were reviewed and approved for CPT II codes by the PMAG in November 2009. The final measurement set was approved by the AAN Board of Directors on December 21, 2009.
The short measure titles and measure statements for each of the 10 PD quality measures are listed in the table. The measure statement contains the denominator and numerator for each measure. The appropriate exclusions for each measure are found in the full measure specifications (appendix e-3).
Table Measure title and description of final 10 Parkinson disease measures approved by the American Academy of Neurology
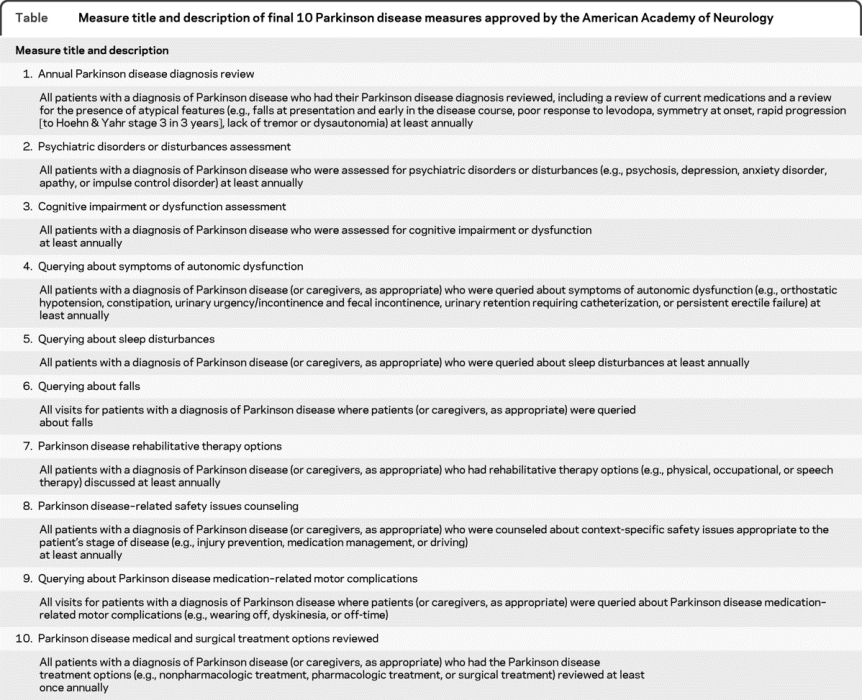
For example, for measure 7, “PD rehabilitative therapy options,” the eligible patient population (denominator) is all patients with a diagnosis of PD, as identified by the ICD-9 code 332.0 documented in the medical record. In order to complete the measure (numerator), the clinician needs to discuss rehabilitative therapy options (e.g., physical, occupational, or speech therapy) with the patient (or caregiver, as appropriate) at least once annually and document the discussion in the medical record or document the corresponding CPT II code 4400F. This measure has an applicable medical exclusion, as a patient may be unable to respond and no informant may be available to discuss rehabilitative therapy options. The exclusion may be documented in the medical record as 4400F-1P.
DISCUSSION
Quality measures have been developed for different frameworks of medical care. They address the structure, process, or outcome of medical care.33 They also address the system components of health care quality as outlined by the Institute of Medicine34: patient safety, timeliness, effectiveness, efficiency, equity, and patient-centeredness. Desirable physician-level measures address a gap in care, are evidence-based and linked to outcomes, are actionable, are feasible to collect, and have well-defined specifications.17 Measure developers are called and increasingly coordinated by the National Quality Forum to develop measures following the National Priorities Partnership's list of priorities: patient and family engagement, population health, safety, care coordination, palliative care and end of life care, and overuse.35
The PD measures are written using a lexicon that is intended to facilitate implementation by clinicians in practice. Each measure identifies the patient population eligible for the measure (e.g., all patients with a diagnosis of PD) and identifies the temporal application (e.g., at least annually). Once the clinician determines whether a patient is eligible, then the measure states how it is fulfilled (e.g., assessment for cognitive impairment or dysfunction). The practice group can then implement a method to identify the patients and determine how to conduct the assessment.
The quality measure development process for PD resulted in 10 measures, which can be grouped into 3 categories. Six measures (numbers 2, 3, 4, 5, 6, and 9) assess or query PD symptoms. Five of these pertain to nonmotor symptoms, as studies show gaps in assessing nonmotor symptoms36 even though these are often strongly associated with quality of life.37 The second category (measures 1, 7, and 10) reviews the patient's current diagnosis or treatment. The third category (measure 8) is a safety measure and involves counseling on preventable complications. The evidence base for safety measures is often sparse because large, randomized controlled trials cannot be ethically conducted. Therefore, measure developers and approving organizations accept safety measures based on lower levels of evidence than would be required in other areas of measurement.38 Note that these measures do not prescribe the use of specific medications, assessment tools, or treatment options. These measures leave clinicians with some flexibility within the evidence base in how the measures can be successfully completed.
The AAN quality measures for PD are an example of the increasing involvement of specialty organizations in measure development. Although the American College of Cardiology began work on measures in the early 1990s,39 most early measurement efforts were oriented toward primary care, and most specialty organizations did not become substantially involved until the formation of the PCPI.38 The involvement of specialty organizations in quality measure development has been restrained by several factors, such as lack of membership support, limited technical expertise, lack of financial resources, and the organizational roles as advocates of the specialty rather than regulators of the specialty.40 A recently published survey suggests that this is changing, with 35% of specialty organizations now actively involved in measure development.41
Two factors appear to be motivating more involvement in quality measure development. The first is concern that measures developed without physician specialist input will be incorporated into payment incentive programs, or worse, payment incentive programs will not include measures related to the services that specialists provide. One example of a payment incentive program is the Centers for Medicare & Medicaid Services Physician Quality Reporting Initiative, a pay-for-reporting program that currently pays a small bonus to participating physicians, but in 2015 will penalize physicians who do not participate.42 The other factor relates to changes in maintenance of certification programs. Specialty organizations are being asked to provide modules for measuring and improving performance in practice. For example, the AAN is developing performance in practice modules that can be used to satisfy the American Board of Psychiatry and Neurology maintenance of certification requirements for neurologists.43 Recently passed health care reform legislation contains provisions where 0.5% of Medicare billing payments in 2011 will be for clinicians who participate in maintenance of certification programs, establishing a link between certification and payment.42 Given these changes, quality measure development is likely to be an increasingly important initiative for specialty organizations such as the AAN.
PD MEASURE DEVELOPMENT PANEL
Stewart A. Factor, DO, FAAN (Emory University, Co-Chair); William J. Weiner, MD, FAAN (University of Maryland, Co-Chair); Lisa Shulman, MD, FAAN (Maryland, Workgroup Member); Sotirios A. Parashos, MD, PhD (Minnesota, Workgroup Member); Helen Bronte-Stewart, MD, FAAN (Workgroup Member); Janis Miyasaki, MD, FAAN (Ontario, Workgroup Member); Marian Evatt, MD (Georgia, Workgroup Member); H. James Brownlee Jr., MD (Florida, Workgroup Member); Karl Sillay, MD (Wisconsin, Workgroup Member); Blair Ford, MD, FAAN (New York, Workgroup Member); Paul Moberg, PhD, ABPP/CN (Pennsylvania, Workgroup Member); Laura Marsh, MD (Texas, Workgroup Member); Daniel Tarsy, MD, FAAN (Massachusetts, Workgroup Member); Alexander Tröster, PhD (North Carolina, Workgroup Member); Marc Nuwer, MD, PhD, FAAN (California, Coding Specialist); Mustafa Saad Siddiqui, MD (North Carolina, Coding Specialist); Michele Popadynec, RN (New York, Workgroup Member); Joyce Oberdorf, MA (Florida, Workgroup Member); Jim Beck, PhD (New York, Workgroup Member); Robert M. Kropp, MD, MBA (Florida, Aetna Insurance Representative); Wesley B. Wong MD, MMM (Indiana, Anthem Blue Cross and Blue Shield Insurance Representative); Monte Masten, MD (Illinois, Humana Insurance Representative); David Stumpf, MD (Illinois, UnitedHealth Group Insurance Representative); Rebecca Kresowik (methodologist); Gina Gjorvad (St. Paul, AAN Staff); Rebecca Swain-Eng, MS (St. Paul, AAN Staff); Sarah T. Tonn, MPH (St. Paul, AAN Staff); Eric M. Cheng, MD, MS (VA Greater Los Angeles, QMR Facilitator); and Christopher T. Bever, Jr., MD, MBA, FAAN (VA Maryland Healthcare System, QMR Facilitator).
ACKNOWLEDGMENT
The authors thank the following organizations that provided representatives for the panel: American Academy of Neurology, Movement Disorders Society, American Academy of Family Physicians, American Association of Neurological Surgeons/Congress of Neurological Surgeons, American Neurological Association, National Academy of Neuropsychology, American Psychological Association, American Psychiatric Association, American PD Association, PD Foundation, National Parkinson Foundation, Aetna, Inc., Anthem Blue Cross and Blue Shield, Humana, Inc., and UnitedHealth Group, Inc.
DISCLOSURE
Dr. Cheng serves as a consultant for the National Parkinson Foundation and receives research support from the NIH/NINDS (K23NS058571 [PI]), the VA Parkinson's Disease Research, Education, and Clinical Center, the Department of Veterans Affairs, the California Office of Statewide Planning and Development, the National Multiple Sclerosis Society, and the American Heart Association. Ms. Tonn is a full-time employee of the American Academy of Neurology (AAN) and served as project director for AAN grants from Pfizer Inc. and the CDC. Ms. Swain-Eng is a full-time employee of the American Academy of Neurology. Dr. Factor has served on scientific advisory boards for Lundbeck Inc., Allergan, Inc., and UCB; serves as a section editor for Current Treatment Options in Neurology; receives royalties from the publication of Parkinson's Disease Diagnosis and Clinical Management (Demos, 2008) and Drug Induced Movement Disorders (Blackwell Futura, 2005); has given expert testimony, prepared affidavits, and served as a consultant for Boehringer Ingelheim; and receives research support from Teva Pharmaceutical Industries Ltd., Ipsen, UCB, and Schering-Plough Corp. Dr. Weiner has served on scientific advisory boards for Santhera Pharmaceuticals and Rexahn Pharmaceuticals, Inc.; serves on the editorial boards of Parkinsonism and Related Disorders and Neurological Reviews, and as Editor of Treatment Options in Neurology; receives royalties from the publication of Neurology for the Non-Neurologist (6th edition, Kluwer/Lippincott 2010), Parkinson's Disease: A Complete Guide for Patients and Family (Hopkins University Press 2nd edition, 2007), and Handbook of Clinical Neurology Hyperkinetic Disorders (Elsevier, 2011); has received honoraria from Santhera Pharmaceuticals and Novartis; has received research support from Novartis, Santhera Pharmaceuticals, Boehringer Ingelheim, and has provided expert testimony and served as a subject matter expert in legal proceedings. Dr. Bever serves on the editorial board of the MS Quarterly Report; is listed as a co-inventor on and receives royalties from Abraxis BioScience, Inc. for a pending patent re: Use of hematogenous stem cells in neuronal replacement therapy and gene delivery; receives royalties from the publication of Ambulatory Medicine (Lippincott Williams & Wilkins, 7th edition, 2006); and has received research support from the Department of Veterans Affairs and the National MS Society.
Supplementary Material
Address correspondence and reprint requests to American Academy of Neurology, 1080 Montreal Avenue, St. Paul, MN 55116 quality@aan.com
Supplemental data at www.neurology.org
Parkinson disease measurement set approved by the AAN Parkinson Disease Measure Development Panel on December 11, 2009; by the Quality Measurement and Reporting Subcommittee on December 15, 2009; by the Practice Committee on December 15, 2009; and by the AAN Board of Directors on December 21, 2009.
Quality Measurement and Reporting Subcommittee members are listed in appendix e-1 on the Neurology® Web site at www.neurology.org.
Disclosure: Author disclosures are provided at the end of the article.
Received May 24, 2010. Accepted in final form September 10, 2010.
REFERENCES
- 1.Lee TH. Eulogy for a quality measure. N Engl J Med 2007;357:1175–1177. [DOI] [PubMed] [Google Scholar]
- 2.Harman JS, Scholle SH, Ng JH, et al. Association of Health Plans' Healthcare Effectiveness Data and Information Set (HEDIS) performance with outcomes of enrollees with diabetes. Med Care 2010;48:217–223. [DOI] [PubMed] [Google Scholar]
- 3.Chassin MR, Loeb JM, Schmaltz SP, Wachter RM. Accountability measures: using measurement to promote quality improvement. N Engl J Med 2010;363:68–688. [DOI] [PubMed] [Google Scholar]
- 4.Roland M, Campbell S, Bailey N, Whalley D, Sibbald B. Financial incentives to improve the quality of primary care in the U.K.: predicting the consequences of change. Prim Health Care Res Dev 2006;7:18–26. [Google Scholar]
- 5.Centers for Medicare and Medicaid Services. Overview: Physician Quality Reporting Initiative. Available at: http://www.coms.gov/pqri/01_overview.asp? Accessed May 3, 2010.
- 6.Doran T, Fullwood C, Gravelle H, et al. Pay-for-performance programs in family practices in the United Kingdom. N Engl J Med 2006;355:375–384. [DOI] [PubMed] [Google Scholar]
- 7.Bridges to Excellence: About Us. Available at: www.bridgestoexcellence.org/about_us/home.htm. Accessed May 3, 2010.
- 8.Endsley S, Kirkegaard M, Baker G, Murko AC. Getting rewards for your results: pay-for-performance programs. March 2004. Available at: www.aafp.org/fpm/20040300/45gett.html. Accessed May 3, 2010. [PubMed]
- 9.Faulkner LR, Tivnan PW, Johnston MV, et al. Invited article: The ABPN maintenance of certification program for neurologists: past, present, and future. Neurology 2008;71:599–604. [DOI] [PubMed] [Google Scholar]
- 10.Horowitz SD. Invited article: Maintenance of certification: the next phase in assessing and improving physician performance. Neurology 2008;71:605–609. [DOI] [PubMed] [Google Scholar]
- 11.Donabedian A, Wheeler JR, Wyszewianski L. Quality, cost, and health: an integrative model. Med Care 1982;20:975–992. [DOI] [PubMed] [Google Scholar]
- 12.American Academy of Neurology. Strategic plan 2003, practice and patient care. Available at: http://www.aan.com/globals/axon/assets/2284.pdf. Accessed May 24, 2010.
- 13.Bever CT, Dubinsky R, Tonn S, Swain-Eng R, for the Quality Measures and Reporting Subcommittee. Quality Measures Process Manual: 2008 Edition. St. Paul, MN: American Academy of Neurology; Approved by the AAN Board of Directors December 18, 2008.
- 14.Center for Health Policy Studies, Harvard School of Public Health, Center for Quality of Care Research and Education. Understanding and choosing clinical performance measures for quality improvement: development of typology: final report. Rockville, MD: Agency for Healthcare Research and Quality (AHRQ); 1995. [Google Scholar]
- 15.Lawthers AW, Palmer H. In search of a few good performance measures. In: Seltzer J, Nash DB, eds. Models for Measuring Quality in Managed Care: Analysis and Impact (Medical Outcomes & Practice Guidelines Library II). New York: Faulkner & Gray's Healthcare Information Center; 1997:121–150. [Google Scholar]
- 16.American Academy of Neurology/American College of Radiology/Physician Consortium for Performance Improvement/National Committee for Quality Assurance. Stroke and Stroke Rehabilitation: Physician Performance Measure Set. September 2006. Updated February 2009. Available at: http://www.ama-assn.org/ama1/pub/upload/mm/370/stroke-measures.pdf. Accessed May 3, 2010.
- 17.Performance Measurement Coordinating Council. Desirable Attributes of Performance Measures: A Consensus Document from the AMA, JCAHO, and NCQA. 1999.
- 18.Physician Consortium for Performance Improvement Position Statement: The evidence base required for measure development. Available at: http://www.ama-assn.org/ama1/pub/upload/mm/370/pcpi-evidence-based-statement.pdf. Accessed May 10, 2010.
- 19.Miyasaki J, Martin W, Suchowersky O, Weiner W, Lang A. Practice parameter: initiation of treatment for Parkinson's disease: an evidence-based review: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2002;58:11–17. [DOI] [PubMed] [Google Scholar]
- 20.Suchowersky O, Reich S, Perlmutter J, Zesiewicz T, Gronseth G, Weiner W. Practice Parameter: diagnosis and prognosis of new onset Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:968–975. [DOI] [PubMed] [Google Scholar]
- 21.Pahwa R, Factor S, Lyons K, et al. Practice parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:983–995. [DOI] [PubMed] [Google Scholar]
- 22.Miyasaki J, Shannon K, Voon V, et al. Practice parameter: evaluation and treatment of depression, psychosis, and dementia in Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:996–1002. [DOI] [PubMed] [Google Scholar]
- 23.Suchowersky O, Gronseth G, Perlmutter J, Reich S, Zesiewicz T, Weiner W. Practice parameter: neuroprotective strategies and alternative therapies for Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:976–982. [DOI] [PubMed] [Google Scholar]
- 24.National Collaborating Centre for Primary Care. National Collaborating Centre for Chronic Conditions. Parkinson's Disease: National Clinical Guideline for Management in Primary and Secondary Care. London: Royal College of Physicians; 2006. [PubMed] [Google Scholar]
- 25.Horstink M, Tolosa E, Bonuccelli U, et al, European Federation of Neurological Societies, Movement Disorder Society-European Section. Review of the therapeutic management of Parkinson's disease: report of a joint task force of the European Federation of Neurological Societies, the Movement Disorder Society-European Section: part I: early (uncomplicated) Parkinson's disease. Eur J Neurol 2006;13:1170–1185.17038031 [Google Scholar]
- 26.Horstink M, Tolosa E, Bonuccelli U, et al, European Federation of Neurological Societies, Movement Disorder Society-European Section. Review of the therapeutic management of Parkinson's disease: report of a joint task force of the EFNS, the MDS-ES: part II: late (complicated) Parkinson's disease. Eur J Neurol 2006;13:1186–1202.17038032 [Google Scholar]
- 27.National Collaborating Centre for Chronic Conditions. Parkinson's Disease: National Clinical Guideline for Management in Primary and Secondary Care. London: Royal College of Physicians; 2006. [Google Scholar]
- 28.Horstink M, Tolosa E, Bonuccelli U, et al. Review of the therapeutic management of Parkinson's disease: report of a joint task force of the European Federation of Neurological Societies (EFNS) and the Movement Disorder Society-European Section (MDS-ES): part II: late (complicated) Parkinson's disease. Eur J Neurol 2006;13:1186–1202.17038032 [Google Scholar]
- 29.Horstink M, Tolosa E, Bonuccelli U, et al. Review of the therapeutic management of Parkinson's disease: report of a joint task force of the European Federation of Neurological Societies and the Movement Disorder Society-European Section: part I: early (uncomplicated) Parkinson's disease. Eur J Neurol 2006;13:1170–1185.17038031 [Google Scholar]
- 30.Goetz CG, Koller WC, Poewe W, et al. Management of Parkinson's disease: an evidence-based review. Mov Disord 2002;17(suppl 4):S1–166. [DOI] [PubMed] [Google Scholar]
- 31.Goetz CG, Poewe W, Rascol O, Sampaio C. Evidence-based medical review update: pharmacological and surgical treatments of Parkinson's disease: 2001 to 2004. Mov Disord 2005;20:523–539. [DOI] [PubMed] [Google Scholar]
- 32.Cheng EM, Siderowf A, Swarztrauber K, et al. Development of quality of care indicators for Parkinson's disease. Mov Disord 2004;19:136–150. [DOI] [PubMed] [Google Scholar]
- 33.Donabedian A. The quality of care: how can it be assessed? JAMA 1988;260:1743–1748. [DOI] [PubMed] [Google Scholar]
- 34.Institute of Medicine. Crossing the Quality Chasm: A New Health Care System for the 21st Century. Washington, DC: National Academy Press; 2001. [Google Scholar]
- 35.National Priorities Partnership. National Priorities and Goals. November 2008. Available at: http://www.nationalprioritiespartnership.org/uploadedFiles/NPP/08-253-NQF%20ReportLo[6].pdf. Accessed August 3, 2010.
- 36.Zesiewicz TA, Sullivan KL, Arnulf I, et al. Practice parameter: treatment of nonmotor symptoms of Parkinson disease: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2010;74:924–931. [DOI] [PubMed] [Google Scholar]
- 37.Chrischilles EA, Rubbenstein LM, Voelker MD, Wallace RB, Rodnitzky RL. Linking clinical variables to health-related quality of life in Parkinson disease. Parkinsonism Related Disord 2002;8:199–209. [DOI] [PubMed] [Google Scholar]
- 38.Kmetik K. PCPI: what you should know about Consortium performance measures. J Fam Pract 2007;56(suppl A): 8A–12A. [PubMed] [Google Scholar]
- 39.Physician Consortium for Performance Improvement. Principles for Performance Measurement in Health Care: A Consensus Statement from the American Medical Association, The Joint Commission on Accreditation of Healthcare Organizations and the National Committee for Quality Assurance. Chicago, IL: American Medical Association; 2000. [Google Scholar]
- 40.Eagle KA, Garson AJ, Beller GA, Sennett C. Closing the gap between science and practice: the need for professional leadership. Health Aff 2003;2:196–201. [DOI] [PubMed] [Google Scholar]
- 41.Ferris TG, Vogell C, Marder J, Sennett CS, Campbell EG. Physician specialty societies and the development of physician performance measures. Health Aff 2007;26:1712–1719. [DOI] [PubMed] [Google Scholar]
- 42.Congressional legislation H.R. 3590. Patient protection and affordable care act. Public law no. 111–148; March 23, 2010.
- 43.American Board of Psychiatry and Neurology. ABPN Maintenance of certification requirement: 26 July 2009. Available at: http://www.abpn.com/downloads/moc/MOC_web_doc.pdf. Accessed May 4, 2010.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


