Abstract
Study Objectives:
Hypocapnia is an important mediator of sleep-dependent respiratory instability. Positive pressure-associated ventilatory control instability results in poor control of sleep apnea and persistent sleep fragmentation. We tested the adjunctive efficacy of low volumes of dead space (enhanced expiratory rebreathing space [EERS]) using a non-vented mask to minimize sleep hypocapnia.
Design:
Retrospective chart review.
Setting:
American Academy of Sleep Medicine accredited sleep center and laboratory.
Intervention:
Enhanced expiratory rebreathing space
Measurements and Results:
204 patients diagnosed with continuous positive pressure (CPAP)-refractory sleep apnea between 1/1/04 and 7/1/06 were included in this retrospective review. All patients had in-lab attended polysomnography for diagnosis, conventional CPAP titration, and further assessments of added EERS. EERS volume was titrated to control of disease, which was typically obtained when end-tidal (ET) CO2 during sleep was 1-2 mm Hg above wake eupneic CO2 levels. The clinic records were reviewed for clinical outcomes. Poor laboratory response to, and initial clinical abandonment of CPAP, was very common (89.2%) in this group of patients, who as a group demonstrated mild resting wake hypocapnia (ETCO2 = 38.1 ± 3.1 mm Hg). Minimizing sleep hypocapnia by adding 100-150 mL EERS (mean ETCO2 at optimal therapy 38.6 ± 2.9 mm Hg) markedly improved polysomnographic control of sleep apnea, without inducing tachypnea or tachycardia. Follow-up (range 30-1872 days) showed improved clinical tolerance, compliance, and sustained clinical improvement. Leak and sleep fragmentation modified clinical outcomes.
Conclusions:
EERS is a potentially useful adjunctive therapy for positive pressure-associated respiratory instability and salvage of some CPAP treatment failures.
Citation:
Gilmartin G; McGeehan B; Vigneault K; Daly RW; Manento M; Weiss JW; Thomas RJ. Treatment of positive airway pressure treatment-associated respiratory instability with enhanced expiratory rebreathing space (EERS). J Clin Sleep Med 2010;6(6):529-538.
Keywords: Respiratory chemoreflex, positive airway pressure, dead space, hypocapnia, non-vented mask, end-tidal CO2
Central sleep apnea and Cheyne-Stokes breathing pattern (periodic breathing) are manifestations, in part, of increased respiratory control chemosensitivity during sleep. Complex sleep apnea is characterized by predominantly obstructive sleep apnea on a diagnostic polysomnogram with central sleep apnea or periodic breathing that emerges during positive airway pressure titration.1–3 These patients may have underlying alterations in chemoreflex sensitivity, which only come to prominence when the clinically significant obstructive sleep apnea has been relieved. This concept expands the role of respiratory control dysfunction in clinical practice, previously restricted to those with overt central sleep apnea or Cheyne-Stokes respiration during diagnostic polysomnography.4 This group of disorders might best be considered “chemoreflex modulated sleep apnea.” An incomplete polysomnographic and clinical response to conventional positive airway pressure may be the hallmark of strong chemoreflex modulation of sleep apnea.2,5
Recent attempts to treat these chemoreflex-modulated sleep apnea syndromes include novel applications of positive airway pressure. For example, adaptive servo-ventilation is a newly approved concept in the therapy for central and complex sleep apnea.6–10 Two devices are already available in clinical practice, using different algorithms to vary pressure support in order to stabilize ventilation, and others are likely to follow.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Ventilatory instability during sleep, including that associated with positive airway pressure titration, is induced or worsened by hypocapnia. Methods that can minimize hypocapnia during pressure titration for sleep apnea have the potential to improve the effective CO2 reserve and improve the quality of sleep and breathing.
Study Impact: The authors describe a simple and practical way to minimize hypocapnia during positive pressure treatment. This method may be of special benefit to those demonstrating central apneas or periodic breathing on diagnostic polysomnograms, or those who have emergence of these same phenomena during positive pressure treatment. Improved clinical outcomes seem possible in a subset of positive pressure treatment failures.
We hypothesized that manipulation of arterial carbon dioxide levels might provide an alternative treatment strategy. Specifically, keeping PCO2 just over the apnea threshold would be predicted to buffer chemoreflex influences and make the disease more purely obstructive in physiology, thus remaining responsive to positive airway pressure treatment. Central apneas and periodic breathing can be generated when the arterial PCO2 level falls below that required to stimulate respiration, a set-point that is unmasked during sleep.11 Preventing hypocapnia is a powerful stabilizing influence on sleep respiratory control.12 Hypocapnia is critical in the genesis of altitude-induced periodic breathing,13 idiopathic central sleep apnea syndrome,4 heart failure-related periodic breathing and Cheyne-Stokes respiration,4,14 and stroke-related mixed forms of sleep apnea.15 CO2 can be introduced into the circuit, using dead space,16–18 bleed-in, or by a more precisely controlled flow-independent method that is currently investigational.12
There are, however, several problems with the use of dead space alone: (1) Pure central sleep apnea is rare, and even in these instances, collapse of the upper airway can be demonstrated.19 (2) “Mixed” patterns of disease are typical in clinical practice20 and require simultaneous upper airway support.5 (3) Tidal volume increases are necessary to effectively use the volumes of dead space that appear effective (500-600 mL).17 Consequences of this include respiratory discomfort, increased work of breathing, and the need to inhale the equivalent of 3% to 4% CO2.
We hypothesized that adding dead space to positive airway pressure therapy would be effective in the treatment of central apneas and periodic breathing that emerged or persisted on positive airway pressure therapy, and that sub-tidal volume dead space would be adequate when the upper airway was also supported.21 As dead space is a volume ventilation concept, and our technique was meant to complement positive pressure application, the term enhanced expiratory rebreathing space or EERS was coined. We present a retrospective analysis of our experience with this technique for improved management of positive pressure treatment-associated ventilatory instability.
METHODS
Patient Selection
All patients diagnosed with central sleep apnea, periodic breathing, or complex sleep apnea (obstructive sleep apnea on the diagnostic sleep study with emergence of central sleep apnea or persistent periodic breathing with CPAP application), and referred to the sleep laboratory for EERS titration from 1/1/04 through 7/1/06 were selected for analysis. All subjects reported in this study were patients treated in the Multidisciplinary Sleep Disorders Center at the Beth Israel Deaconess Medical Center, Boston, Massachusetts. For this retrospective review, there were 204 patients who were diagnosed and treated during this time frame. IRB approval was obtained for a retrospective review of the clinical records.
The clinical criteria for evaluation of EERS were the following: (1) Sleep apnea with a respiratory disturbance index (RDI [(apneas + hypopneas, the latter including events characterized by flow limitation only, irrespective of oxygen desaturation]) ≥ 30 and clinical symptoms (e.g., sleepiness, fatigue, and unrefreshing sleep). (2) Suggestive evidence of respiratory control dysfunction as evidenced by one or more of the following: central apnea index ≥ 5 /h; sustained periods (≥ 3 min) of periodic breathing, including Cheyne-Stokes respiration; or polysomnographic failure of standard continuous positive airway pressure titration, with induction of new and persistent central apneas or periodic breathing in the setting of positive airway pressure treatment required to relieve obstruction.
Polysomnography
Standard attended polysomnography was performed. Nasal pressure and thermistors were used for diagnostic airflow assessments, with mask pressure and pneumotachograph signals for titration studies. Sleep scoring used the standard system, while respiratory event scoring tabulated an apnea-hypopnea index (AHI, apneas + hypopneas associated with 4% oxyhemoglobin desaturation/h of sleep) and an RDI (apneas + hypopneas + respiratory event related arousals/h of sleep). Apneas were scored when both flow signal amplitudes dropped to ≤ 10 % of a stable baseline. Hypopneas were scored when a clearly discernible reduction of the flow signal, including flow-limitation only on the nasal pressure signal, was terminated by an abrupt recovery and associated with a 4% desaturation.22 Respiratory event related arousals were scored when clearly discernible reduction of the flow signal or flow-limitation was seen and terminated by abrupt recovery associated with EEG arousal in the absence of 4% desaturation. Sleep stages used standard scoring approaches to generate measures of REM and NREM sleep. To assess effects of EERS on autonomic and respiratory systems, stable state (10 min free of sleep apnea) respiratory rate and heart rates were tabulated in REM and NREM sleep in a subset (144/204) of EERS studies. During record review, comments about leak were specifically noted when available.
EERS Technology
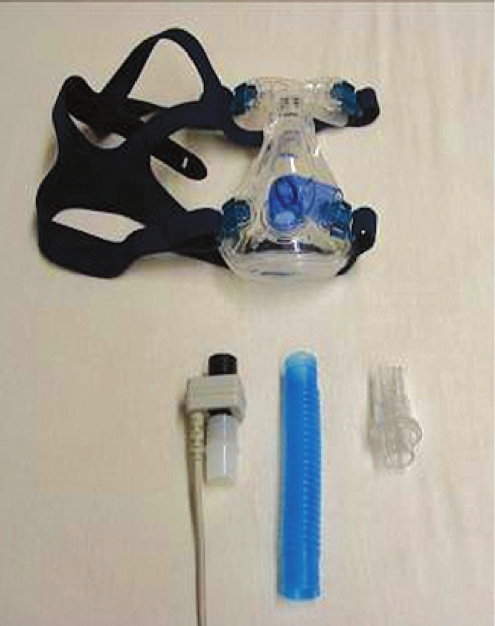
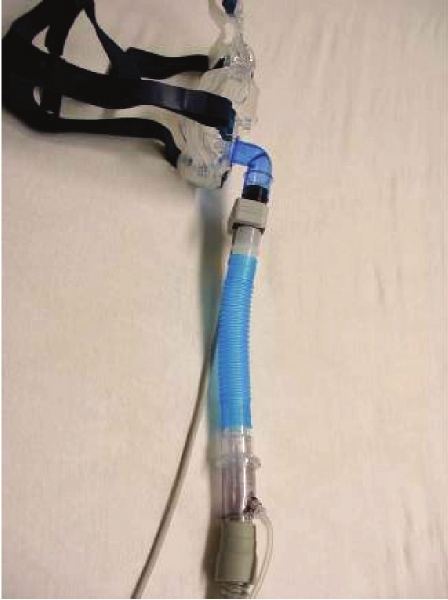
The laboratory requirement for evaluation of EERS is relatively straightforward. The critical components for laboratory use are a non-vented nasal or oronasal mask, a mainstream ETCO2 sensor (e.g., Capnogard, Phillips Respironics), the required volume of standard tubing, an exhalation valve (e.g., Whisper Swivel, Phillips Respironics), followed by the rest of the conventional set-up (Figures 1, 2). While transcutaneous CO2 may usefully complement monitoring, it is not critical and is limited by slow response times relative to end-tidal recordings. Real-time integration into the polysomnographic screen of the end-tidal recordings is useful but not critical: the ETCO2 monitor can be taken to the patient's bedside as needed (Figure 3).
Figure 1.
Enhanced expiratory rebreathing space components
The essential components are available and FDA approved; the assembly and use is off-label. A non-vented mask such as the ResMed Mirage non-vented (top) or a mask that can be made non-vented (such as the Breeze, Activa, Quattro, Liberty, Swift) is required. Other essential components are (from left to right) mainstream CO2 sensing, segments of 22 mm tubing that is the re-breathing space, and the Whisper Swivel valve (Respironics). Use of mainstream CO2 sensing has the additional advantage of providing functional leak information.
Figure 2.
Enhanced expiratory rebreathing space assembled
The fully assembled EERS system consists of, in series, a non-vented interface, CO2 sensing (during laboratory use only), the rebreathing space, exhalation valve, and then the conventional positive pressure circuit. To add additional rebreathing space, our technicians are able to manually add 6-inch segments (approximately 50 mL) in less than 3 seconds, without disturbing sleep. Such changes occur infrequently during a titration study, as the typical range tested is 100-150 mL (2-3 segments). One method of choosing the initial rebreathing volume is described in Table 1.
Figure 3.
Enhanced expiratory rebreathing space montage
The mainstream ETCO2 signal (arrow) provides information about leak (loss of plateau), and maximum/ minimum CO2. Note that during inspiration the ETCO2 usually drops to low/ atmospheric levels as the EERS volume is less than tidal (e.g., 100 mL EERS vs. 500 mL tidal volume), and pressurized fresh incoming air washes out the rebreathing space.
EERS Application
Normal sleep results in a 2-8 mm Hg increase in end-tidal CO2.11 With the EERS technique, the target was to keep the ETCO2 at the lowest possible to enable respiratory stability; hypercapnia was not desired or achieved. CO2 biocalibration was performed before sleep onset. The ETCO2 with steady state relaxed breathing for 30 sec with a non-vented mask alone, and 50, 100, and 150 mL added dead space, without positive airway pressure, were noted, and helped to determine the starting EERS volume (Table 1). The use of pressure and EERS was carefully balanced during pressure titration to obtain optimal synergism (Table 2). The technique was highly leak intolerant, as leaks normally acceptable (20-30 L/ min) resulted in loss of breathing stability if the leak washed out the rebreathing space. The range of EERS evaluated was 50-200 mL. Those subjects with a resting ETCO2 ≥ 50 mm Hg or with a resting ETCO2 between 45 and 50 mm Hg but an increase to ≥ 50 mm Hg with 50 mL EERS were studied with a NV mask alone (to track CO2 during sleep), and are excluded from this analysis.
Table 1.
Determination of starting EERS volume
| Starting Dead Space condition | Baseline wake ETCO2* | Suggested starting EERS |
|---|---|---|
| ETCO2 with NV mask only* and ≥ 45 mm Hg with 150 mL EERS | ≤ 40 | 100 mL |
| ETCO2 with NV mask only* and ≥ 45 mm Hg with 100 mL EERS | ≤ 40 | 50 mL |
| ETCO2 with NV mask only* and ≥ 45 mm Hg with 50 mL EERS | ≤ 40 | NV mask only |
| ETCO2 with NV mask only* and ≥ 50 mm Hg with 50 mL EERS | 40-45 | NV mask only |
| ETCO2 with NV mask only* and ≤ 50 mm Hg with 50 mL EERS | 40-45 | NV mask only or 50 mL |
| ETCO2 with NV mask only* and ≤ 50 mm Hg with 100 mL EERS | 40-45 | 50 mL |
| ETCO2 with NV mask only* and ≤ 50 mm Hg with 150 mL EERS | 40-45 | 100 mL |
| ETCO2 with NV mask only* | ≥ 45 | Physician approval |
EERS refers to enhanced expiratory rebreathing space; ET, end-tidal; NV, non-vented. The starting dead space condition is with the subject resting comfortably sitting, using a NV mask, and adding 50 mL segment increments of dead space (without any positive airway pressure). This is a “CO2 biocalibration” procedure.
Table 2.
Titration guidelines for EERS
| Choose starting EERS using guidelines from Table 1. |
| Titrate positive airway pressure to obtain best response in REM and NREM sleep. |
| Add EERS is 50 mL increments if periodic breathing or central apneas persist. |
| If ETCO2 increases ≥ 5 mm from wake baseline, remove 50 mL of the added rebreathing space. |
| If stable sleep and breathing for more than 20-30 minutes, reduce EERS by 50 mL. |
| If patients complain of difficulty exhalation, consider use of one of the commercial expiratory pressure relief modes. |
| Add 2-4 L O2 if central apnea and periodic breathing persists despite EERS that increases ETCO2 ≥ 5 mm Hg from wake baseline. |
| If new cardiac tachyarrhythmia, headache, or subjective dyspnea is experience, discontinue EERS and inform M.D. |
EERS, enhanced expiratory rebreathing space; ETCO2, end-tidal CO2
The usual pressure titration protocol of the sleep laboratory, which was similar, but not identical, to that recently endorsed by the American Academy of Sleep Medicine,23 was followed. EERS was added if central apneas or periodic breathing (without or without flow-limitation) were observed, using the guidelines in Table 2.
Supplemental Oxygen
Supplemental oxygen was added to the positive airway pressure circuit proximal to the rebreathing space, and not the mask side port (to prevent washout of rebreathing space). Oxygen was used if one or more of the following conditions occurred: (1) baseline oxygen saturation ≤ 90%while awake; (2) sleep oxygen saturation ≤ 90% after optimal positive pressure and ERRS; (3) persistent periodic breathing despite use of recommended strategies for EERS, regardless of the oxygen saturation. Typically, oxygen was added during the second half of the titration. After adequate control of disordered breathing, oxygen was down titrated and discontinued if possible.
Arterial Blood Gas Analysis
Arterial blood gasses were performed on all subjects at the start of EERS evaluations (8 subjects), but then discontinued, as added value to clinical management was not found. Blood gas analysis was then restricted to those with unexpectedly high (≥ 45 mm Hg, 6 subjects) ETCO2 during the polysomnogram.
Clinical Therapy and Follow-Up
The home therapeutic set-up was identical to the laboratory, other than the mainstream CO2 monitor. The clinical records were reviewed to assess tolerance, a new diagnosis of hypertension, outcomes based on use, and perceived benefit. Objective compliance with treatment was documented, obtained with inbuilt meters of the positive pressure devices, and represented the average of a month within 3 months after the final prescription. Clinical outcome categories were as follows: 1: “Using nightly,” full subjective clinical recovery (refreshing sleep, no problematic daytime sleepiness or fatigue, no prominent midafternoon dip in alertness); 2: “Using nightly,” good benefit, but not fully resolved (persistent daytime fatigue, sleepiness, or mild afternoon dip in alertness); 3: “Using most nights,” small benefit (better than no treatment); 4: Intermittent use, unable to judge benefit; 5: Abandoned therapy; 6: Not enough follow-up data (did not return to clinic after set up with treatment); 7: Chose not to use positive airway pressure therapy.
Statistical Methods
Summary measures are means and standard deviations. Comparison of polysomnographic characteristics across conditions used analysis of variance for normally distributed data (e.g., stage 1, sleep efficiency) and the nonparametric Kruskal-Wallis test for data not normally distributed (e.g., AHI, RDI). The comparison time-points were baseline study, conventional positive airway pressure titration, and EERS assessment. Logistic regression was used to assess clinical success or not (clinical outcomes category less than 3 or higher) predictive capability of polysomnographic measures (sleep stages, sleep efficiency, AHI, RDI, minimum nocturnal oxygen desaturation), presence of uncontrollable leak, or presence of fragmented sleep despite positive pressure and EERS use. All subjects had complete diagnostic and EERS titration polysomnogram data. Twenty-three subjects did not have useable conventional positive pressure titration data because of immediate induction of central apneas that prevented sleep—these individuals were immediately converted to an EERS titration. STATA 9.2 SE (StataCorp LP, Texas, USA) was used for analysis.
RESULTS
Clinical Characteristics
These are summarized in Table 3. There was a significant male predominance (89%), and BMI was moderately elevated (29.1 ± 6.1 kg/m2). Ten subjects had wake ETCO2 levels ≥ 40 mm Hg, and 4 had levels ≥ 45 mm Hg. Although pre-treatment hypertension was present in the majority of patients (68.6%), congestive heart failure was present in a minority of patients (10.8%). In addition, the concomitant use of stimulants and antidepressant medications was prevalent in this population (50% and 59.8%, respectively). The majority of subjects, 182/204 (89.2%), had abandoned treatment because of intolerance or lack of perceived benefit prior to EERS titration. Arterial blood gas analysis (14 subjects) revealed the following: pH: 7.4 ± 0.1, PaO2: 92.4 ± 3.6 mm Hg, PaCO2: 39.1 ± 3.2 mm Hg, HCO3−: 22.2 ± 2.2 mEq/Liter.
Table 3.
Demographic and clinical characteristics (n = 204)
| Characteristic | Mean (%) |
|---|---|
| Age-y | 51.2 ± 14.2 |
| Age-range (y) | 21 − 82 |
| BMI (kg/m2) | 29.1 ± 6.1 |
| Male sex | 182 (89.2) |
| Wake ETCO2 mm Hg | 38.1 ± 3.1 |
| Stimulant use | 102 (50) |
| Antidepressants | 122 (59.8) |
| Opiates | 11 (5.4%) |
| Restless legs | 12 (5.9) |
| Delayed sleep phase syndrome | 24 (7.4) |
| Shift work | 4 (2) |
| Congestive heart failure | 22 (10.8) |
| Hypertension | 140 (68.6) |
| Diabetes mellitus | 38 (18.6) |
| CVA | 7 (3.4) |
| Epilepsy | 3 (1.5) |
| Atrial fibrillation | 20 (9.8) |
| Attention deficit disorder | 22 (11.8) |
| Renal transplant | 1 (0.5) |
| Hydrocephalus | 1 (0.5) |
| Syringomyelia | 1 (0.5) |
| Neuroborreliosis | 1 (0.5) |
| Klinefelter syndrome | 1 (0.5) |
Polysomnography and Positive Pressure Titration
The data reflect full-night diagnostic sleep studies or diagnostic components of a “split-night” study. Similarly, “standard titration” reflects the treatment part of a split-night study or a full night (152/204) of positive pressure titration. The “EERS” titration data is the whole night; thus, some underestimation of treatment effects is possible.
As a group, the patients showed the following baseline polysomnographic characteristics (Table 4): (1) high RDI; (2) severe sleep fragmentation; (3) NREM dominance of disease; and (4) relatively modest oxygen desaturation. Standard titration with continuous or bilevel positive airway pressure, often supplemented by adding oxygen into the circuit, was characterized by an inability to obtain an adequate therapeutic setting (Table 4). A particularly problematic feature was the inability to control obstruction without inducing central apneas or worsening periodic breathing. The patients were nearly always responsive to conventional positive airway pressure treatment during REM sleep.
Table 4.
Polysomnographic characteristics of 204 patients
| Measure | Diagnostic | Standard titration | EERS titration | p |
|---|---|---|---|---|
| Sleep efficiency (%) | 71.3 ± 18.2 | 66.9 ± 21.5 | 75.4 ± 14.9 | < 0.001 |
| TST | 222.4 ± 126.6 | 219.8 ± 105.2 | 308.5 ± 87.5 | < 0.001 |
| Stage 1 (% TST) | 21.6 ± 18 | 24 ± 19 | 20.3 ± 12.7 | 0.09 |
| Stage 2 (% TST) | 60.9 ± 16.8 | 56.7 ± 16.6 | 58.8 ± 13.1 | 0.05 |
| Stage 3 (% TST) | 5.8 ± 7.4 | 4.6 ± 6.5 | 5.5 ± 6.1 | 0.05* |
| Stage 4 (% TST) | 2.2 ± 6.3 | 1.2 ± 4.2 | 1.7 ± 4.2 | 0.10* |
| REM sleep (% TST) | 9.8 ± 9 | 13 ± 10.1 | 14 ± 9.5 | < 0.001 |
| AHI (/ h of sleep) | 36 ± 36.8 | 25.4 ± 59 | 4.1 ± 5.8 | < 0.001* |
| RDI (/ h of sleep) | 69.8 ± 32.8 | 59.4 ± 33.9 | 30.7 ± 19.7 | < 0.001* |
| CAI (/ h of sleep) | 3.8 ± 8.2 | 8.9 ± 11.1 | 1.5 ± 2.8 | < 0.001* |
| Min O2 | 88.7 ± 8.1 | 88.5 ± 4.8 | 92.7 ± 4.5 | < 0.001 |
| PLM index | 6.1 ± 15.7 | 2 ± 4.8 | 15.2 ± 19.2 | < 0.001* |
TST refers to total sleep time; AHI, apnea-hypopnea index; RDI, respiratory disturbance index; CAI, central apnea index; PLM, periodic limb movement.
Analysis of variance was used to estimate significance between groups; metrics designated with a * were not normally distributed and were analyzed using the Kruskal-Wallis nonparametric test.
EERS Titration
Four subjects had a resting ETCO2 ≥ 45 mm Hg. EERS titration was not performed in those with a wake resting ETCO2 ≥ 50 mm Hg. Minimization of hypocapnia allowed an improvement in control, as summarized in Table 4. The mean wake, minimum at point of control (with at least a non-vented mask and 50 mL EERS), and the maximum for the study ETCO2 was 38.1 ± 3.1, 38.6 ± 2.8, and 42.1 ± 3 mm Hg, respectively. The volume of EERS that provided the optimal benefit was 100-150 mL in most instances (non-vented mask only = 9 (4.4%), 50 mL = 31 (15.2%), 100 mL = 89 (43.6%), 150 mL = 63 (30.9%), and 200 mL = 12 (5.9%).
Patients with chemoreflex-dependent sleep apnea patients tend to have the majority of the respiratory abnormality during NREM sleep. The residual sleep fragmentation and uncontrolled respiratory events on conventional positive pressure titration was high. The vast majority of respiratory events were not central apneas, but obstructive hypopneas, often oscillating with a periodicity reminiscent of typical periodic breathing. Use of EERS markedly reduced the residual disease.
The “Sleep Effect”
During the course of data tabulation some subjects were noted to demonstrate disproportionate fragmentation of sleep, not dependent on any of the following: (1) poor sleep hygiene or abnormal circadian phase; (2) presence of untreated depression, anxiety, or pain; (3) withdrawal of sedative medications or alcohol abuse; (4) poor control of sleep disordered breathing during consolidated periods of sleep; (5) mask leak effects or primary subjective pressure intolerance. This subgroup was characterized by poor sleep efficiency (typically < 70%), stage 1 sleep ≥ 10%, and prolonged sleep-wake transitions. It was unlikely that this was a first night effect, as these patients had multiple prior polysomnograms, and uniformly admitted to a similar pattern at home. The polysomnographic features of this subgroup are contrasted with the rest in Table 5.
Table 5.
Primary sleep fragmentation (“sleep effect”)
| Measure | “Regular” group (111, 54.4%) | “Sleep effect” group (93, 46.6%) | p |
|---|---|---|---|
| Sleep efficiency (%) | 83.1 ± 9.5 | 66.3 ± 15 | < 0.001 |
| Total sleep time (minutes) | 346.5 ± 65.3 | 263.1 ± 89.2 | < 0.001 |
| Stage 1 (% TST) | 13.1 ± 5 | 28.8 ± 13.7 | < 0.001 |
| Stage 3 (% TST) | 6.9 ± 5.8 | 3.9 ± 6 | 0.001* |
| Stage 4 (% TST) | 2.3 ± 5.2 | 0.9 ± 2.4 | 0.02* |
| REM sleep (% TST) | 16.2 ± 9.3 | 11.3 ± 9.1 | < 0.001 |
| AASM arousal index | 20.2 ± 11.4 | 33 ± 16.6 | < 0.001 |
| AHI (/ h of sleep) | 2.4 ± 3.2 | 6.1 ± 7.6 | < 0.001* |
| RDI (/ h of sleep) | 20.6 ± 11.5 | 35 ± 16.1 | < 0.001* |
| CAI (/ h of sleep) | 1.1 ± 1.9 | 2 ± 3.5 | 0.05* |
| Min O2 (any stage) | 94.6 ± 3.8 | 94.5 ± 3.2 | 0.9 |
| Min O2 NREM sleep | 93.5 ± 4.5 | 92.5 ± 5.8 | 0.3 |
| PLM index (/ h of sleep) | 15.1 ± 18.5 | 15.4 ± 20.2 | 0.9* |
t-test other than metrics marked *, which used the Wilcoxon rank-sum test as the data did not have a normal distribution.
The “Leak Effect”
Presence and severity of leak as determined by reviewing the technical notes and sleep study reports. “No leak” was a consistent seal, with total leak ≤ 30 L/ min for the whole night and a “plateau” morphology of the ETCO2 signal. “Controllable leak” was noted if mask changes were required during the study for the explicit purpose of leak control and obtaining the ETCO2 plateau, and that total leak was ≤ 40 L/ min. In those with “uncontrollable leak,” a consistent ETCO2 plateau was not obtained despite multiple mask switches, and leak rates ≤ 40 L/ min could not be obtained. All patients had leaks in the range that could have been acceptable for conventional titration.
Optimizing leak was a particular challenge: in 76.4% of titrations, an adequate seal was obtained; it was intermittently excessive but controllable in 12.3%, and of a severity that did not allow optimal titration in 11.3%. That this difference was clinically significant is demonstrated by the polysomnographic outcomes of these 3 categories (Table 6).
Table 6.
Leak effects on laboratory EERS effectiveness
| Measure | No leak (156, 76.4 %) | Controllable leak (25, 12.3 %) | Uncontrollable leak (23, 11.3 %) | p |
|---|---|---|---|---|
| Sleep efficiency (%) | 75.9 ± 15.1 | 76.9 ± 11.6 | 70.3 ± 15.8 | 0.2 |
| Total sleep time (minutes) | 309.7 ± 88.4 | 320.7 ± 67.7 | 286.5 ± 99.7 | 0.4 |
| Stage 1 (% TST) | 18.6 ± 11.5 | 22 ± 10.2 | 29.7 ± 17.6 | < 0.001 |
| Stage 3 (% TST) | 5.4 ± 6 | 5.5 ± 6.2 | 6.4 ± 6.9 | 0.6* |
| Stage 4 (% TST) | 1.7 ± 4.6 | 1.9 ± 3.9 | 0.9 ± 1.8 | 0.4* |
| REM sleep (% TST) | 15.2 ± 9.6 | 12.4 ± 8.6 | 7.1 ± 5.5 | < 0.001 |
| AASM arousal index | 23.1 ± 13.4 | 30.2 ± 12.5 | 45.2 ± 18.5 | < 0.001 |
| AHI (/ h of sleep) | 3.3 ± 5 | 4.3 ± 4.9 | 9 ± 9.2 | < 0.001* |
| RDI (/ h of sleep) | 23 ± 13.5 | 34 ± 10.3 | 46.4 ± 15.8 | < 0.001* |
| CAI (/ h of sleep) | 1.1 ± 2.3 | 2.1 ± 3.1 | 2.8 ± 4.1 | 0.004* |
| Min O2 (any stage) | 94.4 ± 3.7 | 96.1 ± 2.9 | 93.9 ± 2.6 | 0.10 |
| Min O2 NREM sleep | 93.3 ± 5.1 | 92.4 ± 7.3 | 92.3 ± 3.7 | 0.6 |
| PLM index (/ h of sleep) | 12.8 ± 16.7 | 26.1 ± 28.9 | 17.8 ± 16.1 | 0.003* |
Analysis of variance was used to estimate significance between groups; metrics designated with a * were not normally distributed and were analyzed using the Kruskal-Wallis nonparametric test.
Oxygen Use
Supplemental oxygen during CPAP was evaluated in 154/ 204 (75.5%) patients, and was considered useful in improving respiratory control stability by the interpreting physician in 62/ 154 instances (40.3% of those evaluated). Oxygen was prescribed for home use in 46/204 (22.6%) overall. The use of supplemental oxygen was guided by the instruction to the sleep technologist “add O2 if periodic breathing and central apneas persist after 100 mL EERS.”
Outcomes
Tolerance
No patient complained of a new throbbing headache on awakening that could suggest a response to hypercapnia. Headache attributable to the tightness of the mask straps was noted in 11/204 (5.4%). No patient complained of palpitations or dyspnea.
Technical/mask
The paucity of available non-vented masks posed a major practical difficulty in fitting. The interfaces used in the laboratory were the non-vented ResMed Mirage (83.5%), a nasal pillow with taped orifice (16%), and non-vented Hans Rudolph (0.5%).
Polysomnographic
When leak and sleep fragmentation effects were absent, control of sleep disordered breathing was excellent (Figures 4, 5). In the 88 subjects who were able to achieve this target, the following were the whole night polysomnographic outcomes: sleep efficiency: 83.1% ± 9.6%, total sleep time (TST) 346.7 ± 66.1 min, NREM stage 1: 12.9% ± 5.1% TST, stage 3: 6.3% ± 5.7% TST, stage 4: 2.2% ± 5.4% TST, REM sleep: 16.9% ± 9.1% TST, American Academy of Sleep Medicine arousal index: 9.2 ± 4.6/ h sleep, AHI: 1.9 ± 2.1/ h of sleep, RDI: 18.4 ± 10.5/ h of sleep, central apnea index: 0.8 ± 1.8/ h of sleep, minimal nocturnal O2 desaturation: 94.1% ± 2.9%, periodic limb movement index: 13.4 ± 17.8/ h of sleep. In the 94 subjects for whom a separate central apnea index was available during the conventional CPAP titration study, the index was 8.9 ± 11.1/ h of sleep, and 48/94 (51%) demonstrated a central apnea index ≥ 5/ h of sleep. The central apnea index was ≤ 5 in 188/204 (92.2%) subjects on the EERS titration night, while the apnea hypopnea index was ≤ 5 in 156/204 (76.5%).
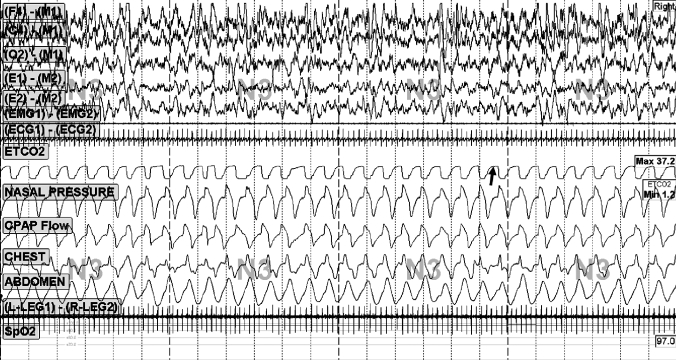
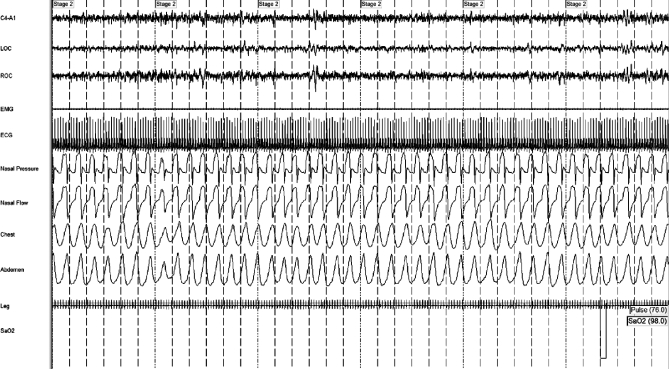
Figure 4.
Uncontrolled sleep apnea without rebreathing
This patient was a 56-year old male. Note persistent central and “mixed” events despite bilevel ventilation, back-up rate, and supplemental oxygen. “Nasal pressure” is mask pressure measures by connecting a pressure transducer to the mask side port. “Nasal flow” is obtained as an output from the positive pressure therapy machine—the unusual appearance of this trace may reflect desynchrony between the patient and the ventilator, as it was not seen when rebreathing space was used.
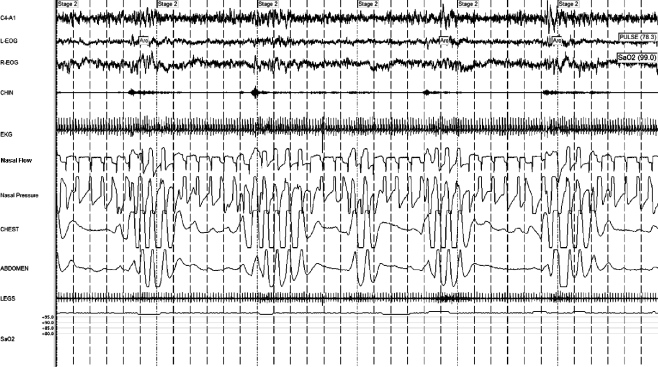
Figure 5.
Controlled sleep apnea with rebreathing
The same patient as in Figure 3, on CPAP with pressure of 12 cm H2O, with 150 mL of enhanced expiratory rebreathing space, with complete resolution of disease. This patient has used rebreathing + CPAP for over 5 years, and has had 3 further positive pressure titration demonstrating the continued requirement for rebreathing for optimal control of mixed obstructive and central sleep apnea.
Respiration and Heart Rate
The respiratory rates (breaths per minute) during NREM and REM sleep were 13.7 ± 2.5 and 15.1 ± 2.7, respectively. The heart rates (beats per minute) during NREM and REM sleep were 63.6 ± 10.6 and 66.3 ± 10.2, respectively.
Clinical
Objective compliance was available in 125 subjects. The mean duration of use was 5.5 ± 1.6 h; 113/125 (90.4) used therapy ≥ 4 h/ night. Clinical outcomes were assessed with a follow-up duration of 569 ± 403 (range: 30-1872) days, and were in categories 1 to 7 as follows (n/204, % of total): 38 (18.6%), 77 (37.7%), 24 (11.8%), 10 (4.9%), 18 (8.8%), 24 (11.8%), and 12 (5.9%). Categories 1 to 3 were considered acceptable clinical outcomes, and were achieved by 139/204 (68.1%).
Prediction of Success
No clinical or individual polysomnographic variable predicted clinical success (category ≤ 3). However, the “sleep non-fragmented” group had an odds ratio of 2.0 (p = 0.02, C.I. = 1.2-3.8) for reaching this threshold (“using, some benefit”). Similarly, those who had no leak during titration had success odds of 2.2 (p = 0.02, C.I. = 1.1-4.3); leak and sleep fragmentation effects were independently associated with adverse long-term outcomes. Fragmentation effects were not dependent on leak effects; in fact, the odds of demonstrating sleep fragmentation as described above was 0.36 (p = 0.003, C.I. = 0.18-0.71) when there was also a problem containing leak (no leak = plateau on the ETCO2 signal).
DISCUSSION
This retrospective analysis of EERS treatment in patients with positive pressure therapy-associated respiratory instability demonstrates the following key points: (1) Addition of low volumes of EERS as an adjunct to conventional mask-based positive pressure provided immediate stabilization benefits during positive airway pressure titration. (2) The therapy was well tolerated physiologically. (3) Mask leak and a subset of patients with sleep fragmentation are important challenges and may limit the clinical response. (4) Home use of EERS resulted in sustained benefits, but partial clinical responses were frequent. This simple approach uses available off-the-shelf components, and has the potential to provide benefit to otherwise difficult to treat sleep apnea patients. Improved control of difficult-to-treat sleep apnea was achieved without inducing hypercapnia. With the use of EERS in conjunction with positive airway pressure therapy, the main result is a reduction in hypocapnia, and the ETCO2 remains close to wake values. Our patients had substantial degrees of obstructive sleep apnea; it is unlikely that dead space alone would have been effective therapy.
There were large differences between the AHI and RDI in our study. The former used a 4% desaturation association, and desaturating hypopneas and apneas were readily eliminated with EERS. The RDI included respiratory effort related arousals that showed features of flow-limitation only. These patients were hard to treat; the residual RDI reflects both the limitations of the technique and the strong propensity for respiratory cycling in those with strong chemoreflex effects on sleep-breathing, yet patients had clear clinical benefits.
This study adds to the growing body of evidence for the importance of respiratory control mechanisms in the pathophysiology of sleep apnea syndromes.24–28 Tolerance of EERS was excellent; only one patient felt “suffocated” and unable to use during the period under consideration, but this was while awake. It is unclear from the chart review if the problem was claustrophobia. No patient had a new diagnosis of hypertension during the follow-up period. No patient complained of morning dyspnea or palpitations. The respiratory and heart rates during stable NREM sleep and REM sleep were in the normal range. An increase in scored periodic limb movements without associated arousals were seen in the EERS group; possibilities include an effect of the small increase in ETCO2 and more precise scoring enabled by improved treatment of sleep apnea.
Use of continuous or bilevel positive airway pressure in those prone to positive pressure therapy-induced respiratory instability can result in significant residual respiratory and sleep quality abnormalities.29,30 A subset of our patients had severe sleep fragmentation during EERS titration that adversely impacted long-term outcomes. Arousals from sleep and repetitive transitions across the sleep-wake interface can increase the severity of sleep apnea.31 Use of benzodiazepine or non-benzodiazepine sedatives can improve central sleep apnea,32 sleep and periodic breathing at altitude,33,34 and sleep during positive pressure titration in the sleep laboratory.35 It is a testable hypothesis that those with severe fragmentation during a titration polysomnogram could benefit from long-term use of hypnotic medications as an adjunct to positive airway pressure therapy.
The majority of our patients had discontinued positive airway pressure therapy before the trial of EERS, following which we were ultimately able to salvage about half of these patents. Our population was highly selected (tertiary care center, the majority had “failed” therapy). It will take prospective evaluations of unselected consecutive sleep apnea patients and accurate phenotype determination to establish if the presence of strong chemoreflex modulation of sleep apnea is an independent risk factor for non-compliance and tolerance problems. An important issue yet to be systematically evaluated is if long term use of EERS is necessary, as it is possible the only role for such approaches may be to improve initial tolerance. Both significant persistence36 and resolution37 of central apneas over time have been reported in complex sleep apnea. One caution with these reports is that central hypopneas were not quantified; if only classic central apneas were scored, or if a 4% oxygen desaturation association was required, they may have underestimated residual chemoreflex-modulated sleep-breathing. Once positive pressure is applied, oxygenation often improves, regardless of the persistence of residual respiratory events.
The mechanism of EERS likely involves capturing some CO2 during the expiratory part of the respiratory cycle within the rebreathing space, as the sub-tidal volume EERS and the continuous inflow of air resulted in the ETCO2 reaching the baseline room air values immediately at the end of expiration (Figure 3). The baseline of the ETCO2 signal typically registers 0 to 0.5 mm Hg. We hypothesize that during the switch from expiration to inspiration, a small volume of CO2 is trapped and inhaled, resulting in a transient increase in inspired CO2 that ultimately reaches the peripheral and central chemoreceptors. As the beneficial effects occur within 20-30 seconds (as do loss of these effects with increased mask leak), the carotid chemoreceptors38,39 may mediate most of the benefits of EERS. There appears to be a plateau in the beneficial effect of EERS, as increasing the rebreathing volume to greater than 150 mL rarely provided an incremental benefit.
Limitations of this study are important. We did not directly evaluate hypoxic and hypercapnic ventilatory responses—our assumption of the importance of the respiratory chemoreflexes in these patients is indirect. The retrospective approach does not provide information on the efficacy of EERS in relation to other approaches, including supplemental oxygen,40 CO2,41 and acetazolamide.42 However, dead space alone may impair sleep quality, and cause a decrease in total sleep and an increase in arousals, possibly due to hypercapnia (5.6 ± 0.5 mm Hg rise from restful wake).41 The use of supplemental oxygen, which has been shown to be useful for central sleep apnea and to improve stabilization of respiratory control in obstructive sleep apnea patients with high loop gain,43 was not performed systematically here during the EERS titration. Although lack of a continuous positive pressure-responsive obstructive sleep apnea control group limits interpretation, the use of methods to minimize hypocapnia12,21 offers powerful synergistic stabilizing effects and could be a logical adjunct to conventional positive pressure therapy in appropriately selected patients. This study does not explicitly address who would need long-term rebreathing therapy, and for what duration; it is possible that with time some patients will lose positive pressure therapy-respiratory instability.
There are certain subgroups of patients for whom this approach may not be feasible or safe. For example, obese individuals may have hypoventilation, especially during REM sleep, which may be associated with unacceptable degrees of hypercapnia. Claustrophobic patients will not likely tolerate the tight fit of the mask required. There are additional costs associated with EERS treatment, including equipment, disposables, technician training, patient education, and physician supervision effort.
In summary, EERS offers an immediate, simple, and practical option as salvage therapy for patients with respiratory instability associated with or induced by positive airway pressure therapy, without induction of hypercapnia.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Thomas is assigned a patent to treat central/complex sleep apnea with CO2 using a flow-compensated device call the Positive Airway Pressure Gas Modulator. This device is licensed by Beth Israel Deaconess Medical Center to Embla. Mr. Daly has conducted research into leak-proof patient interfaces that would improve performance of EERS. In addition, he has designed approaches to evaluate inherently low-gain breathing circuits with and without adaptive electronics.
ACKNOWLEDGMENTS
Dr. Ramesh Donepudi assisted in data cleaning. The homecare companies affiliated with the Beth Israel Deaconess Medical Center (North Atlantic Medical, Reliable Respiratory, and Total Sleep Therapy) enabled practical home use of EERS.
Performance site: Beth Israel Deaconess Medical Center
Financial support: The Periodic Breathing Foundation
REFERENCES
- 1.Morgenthaler TI, Kagramanov V, Hanak V, Decker PA. Complex sleep apnea syndrome: is it a unique clinical syndrome? Sleep. 2006;29:1203–9. doi: 10.1093/sleep/29.9.1203. [DOI] [PubMed] [Google Scholar]
- 2.Pusalavidyasagar SS, Olson EJ, Gay PC, Morgenthaler TI. Treatment of complex sleep apnea syndrome: a retrospective comparative review. Sleep Med. 2006;7:474–9. doi: 10.1016/j.sleep.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Gilmartin GS, Daly RW, Thomas RJ. Recognition and management of complex sleep-disordered breathing. Curr Opin Pulm Med. 2005;11:485–93. doi: 10.1097/01.mcp.0000183061.98665.b0. [DOI] [PubMed] [Google Scholar]
- 4.Badr MS. Central sleep apnea. Prim Care. 2005;32:361–74. doi: 10.1016/j.pop.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Thomas RJ, Terzano MG, Parrino L, Weiss JW. Obstructive sleep-disordered breathing with a dominant cyclic alternating pattern--a recognizable polysomnographic variant with practical clinical implications. Sleep. 2004;27:229–34. doi: 10.1093/sleep/27.2.229. [DOI] [PubMed] [Google Scholar]
- 6.Pepin JL, Chouri-Pontarollo N, Tamisier R, Levy P. Cheyne-Stokes respiration with central sleep apnoea in chronic heart failure: proposals for a diagnostic and therapeutic strategy. Sleep Med Rev. 2006;10:33–47. doi: 10.1016/j.smrv.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Szollosi I, O'Driscoll DM, Dayer MJ, Coats AJ, Morrell MJ, Simonds AK. Adaptive servo-ventilation and deadspace: effects on central sleep apnoea. J Sleep Res. 2006;15:199–205. doi: 10.1111/j.1365-2869.2006.00515.x. [DOI] [PubMed] [Google Scholar]
- 8.Teschler H, Dohring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164:614–9. doi: 10.1164/ajrccm.164.4.9908114. [DOI] [PubMed] [Google Scholar]
- 9.Arzt M, Wensel R, Montalvan S, et al. Effects of dynamic bilevel positive airway pressure support on central sleep apnea in men with heart failure. Chest. 2008;134:61–6. doi: 10.1378/chest.07-1620. [DOI] [PubMed] [Google Scholar]
- 10.Randerath WJ, Galetke W, Kenter M, Richter K, Schafer T. Combined adaptive servo-ventilation and automatic positive airway pressure (anticyclic modulated ventilation) in co-existing obstructive and central sleep apnea syndrome and periodic breathing. Sleep Med. 2009;10:898–903. doi: 10.1016/j.sleep.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Dempsey JA, Smith CA, Przybylowski T, et al. The ventilatory responsiveness to CO(2) below eupnoea as a determinant of ventilatory stability in sleep. J Physiol. 2004;560:1–11. doi: 10.1113/jphysiol.2004.072371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas RJ, Daly RW, Weiss JW. Low-concentration carbon dioxide is an effective adjunct to positive airway pressure in the treatment of refractory mixed central and obstructive sleep-disordered breathing. Sleep. 2005;28:69–77. doi: 10.1093/sleep/28.1.69. [DOI] [PubMed] [Google Scholar]
- 13.Weil JV. Sleep at high altitude. High Alt Med Biol. 2004;5:180–9. doi: 10.1089/1527029041352162. [DOI] [PubMed] [Google Scholar]
- 14.Naughton M, Benard D, Tam A, Rutherford R, Bradley TD. Role of hyperventilation in the pathogenesis of central sleep apneas in patients with congestive heart failure. Am Rev Respir Dis. 1993;148:330–8. doi: 10.1164/ajrccm/148.2.330. [DOI] [PubMed] [Google Scholar]
- 15.Nopmaneejumruslers C, Kaneko Y, Hajek V, Zivanovic V, Bradley TD. Cheyne-Stokes respiration in stroke: relationship to hypocapnia and occult cardiac dysfunction. Am J Respir Crit Care Med. 2005;171:1048–52. doi: 10.1164/rccm.200411-1591OC. [DOI] [PubMed] [Google Scholar]
- 16.Parthasarathy S, Tobin MJ. Effect of ventilator mode on sleep quality in critically ill patients. Am J Respir Crit Care Med. 2002;166:1423–9. doi: 10.1164/rccm.200209-999OC. [DOI] [PubMed] [Google Scholar]
- 17.Xie A, Rankin F, Rutherford R, Bradley TD. Effects of inhaled CO2 and added dead space on idiopathic central sleep apnea. J Appl Physiol. 1997;82:918–26. doi: 10.1152/jappl.1997.82.3.918. [DOI] [PubMed] [Google Scholar]
- 18.Khayat RN, Xie A, Patel AK, Kaminski A, Skatrud JB. Cardiorespiratory effects of added dead space in patients with heart failure and central sleep apnea. Chest. 2003;123:1551–60. doi: 10.1378/chest.123.5.1551. [DOI] [PubMed] [Google Scholar]
- 19.Badr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol. 1995;78:1806–15. doi: 10.1152/jappl.1995.78.5.1806. [DOI] [PubMed] [Google Scholar]
- 20.Eckert DJ, Malhotra A, Jordan AS. Mechanisms of apnea. Prog Cardiovasc Dis. 2009;51:313–23. doi: 10.1016/j.pcad.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas RJ. Effect of added dead space to positive airway pressure for treatment of complex sleep-disordered breathing. Sleep Med. 2005;6:177–8. doi: 10.1016/j.sleep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 22.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 23.Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4:157–71. [PMC free article] [PubMed] [Google Scholar]
- 24.Hudgel DW, Gordon EA, Thanakitcharu S, Bruce EN. Instability of ventilatory control in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1998;158:1142–9. doi: 10.1164/ajrccm.158.4.9712105. [DOI] [PubMed] [Google Scholar]
- 25.Meza S, Mendez M, Ostrowski M, Younes M. Susceptibility to periodic breathing with assisted ventilation during sleep in normal subjects. J Appl Physiol. 1998;85:1929–40. doi: 10.1152/jappl.1998.85.5.1929. [DOI] [PubMed] [Google Scholar]
- 26.Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med. 2003;168:645–58. doi: 10.1164/rccm.200302-201OC. [DOI] [PubMed] [Google Scholar]
- 27.Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1181–90. doi: 10.1164/ajrccm.163.5.2007013. [DOI] [PubMed] [Google Scholar]
- 28.Wellman A, Jordan AS, Malhotra A, et al. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:1225–32. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–33. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 30.Johnson KG, Johnson DC. Bilevel positive airway pressure worsens central apneas during sleep. Chest. 2005;128:2141–50. doi: 10.1378/chest.128.4.2141. [DOI] [PubMed] [Google Scholar]
- 31.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–33. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- 32.Quadri S, Drake C, Hudgel DW. Improvement of idiopathic central sleep apnea with zolpidem. J Clin Sleep Med. 2009;5:122–9. [PMC free article] [PubMed] [Google Scholar]
- 33.Nickol AH, Leverment J, Richards P, et al. Temazepam at high altitude reduces periodic breathing without impairing next-day performance: a randomized cross-over double-blind study. J Sleep Res. 2006;15:445–54. doi: 10.1111/j.1365-2869.2006.00558.x. [DOI] [PubMed] [Google Scholar]
- 34.Beaumont M, Batejat D, Pierard C, et al. Zaleplon and zolpidem objectively alleviate sleep disturbances in mountaineers at a 3,613 meter altitude. Sleep. 2007;30:1527–33. doi: 10.1093/sleep/30.11.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lettieri CJ, Quast TN, Eliasson AH, Andrada T. Eszopiclone improves overnight polysomnography and continuous positive airway pressure titration: a prospective, randomized, placebo-controlled trial. Sleep. 2008;31:1310–6. [PMC free article] [PubMed] [Google Scholar]
- 36.Kuzniar TJ, Pusalavidyasagar S, Gay PC, Morgenthaler TI. Natural course of complex sleep apnea--a retrospective study. Sleep Breath. 2008;12:135–9. doi: 10.1007/s11325-007-0140-z. [DOI] [PubMed] [Google Scholar]
- 37.Dernaika T, Tawk M, Nazir S, Younis W, Kinasewitz GT. The significance and outcome of continuous positive airway pressure-related central sleep apnea during split-night sleep studies. Chest. 2007;132:81–7. doi: 10.1378/chest.06-2562. [DOI] [PubMed] [Google Scholar]
- 38.Nakayama H, Smith CA, Rodman JR, Skatrud JB, Dempsey JA. Carotid body denervation eliminates apnea in response to transient hypocapnia. J Appl Physiol. 2003;94:155–64. doi: 10.1152/japplphysiol.00722.2002. [DOI] [PubMed] [Google Scholar]
- 39.Smith CA, Nakayama H, Dempsey JA. The essential role of carotid body chemoreceptors in sleep apnea. Can J Physiol Pharmacol. 2003;81:774–9. doi: 10.1139/y03-056. [DOI] [PubMed] [Google Scholar]
- 40.Javaheri S, Ahmed M, Parker TJ, Brown CR. Effects of nasal O2 on sleep-related disordered breathing in ambulatory patients with stable heart failure. Sleep. 1999;22:1101–6. doi: 10.1093/sleep/22.8.1101. [DOI] [PubMed] [Google Scholar]
- 41.Szollosi I, Jones M, Morrell MJ, Helfet K, Coats AJ, Simonds AK. Effect of CO2 inhalation on central sleep apnea and arousals from sleep. Respiration. 2004;71:493–8. doi: 10.1159/000080634. [DOI] [PubMed] [Google Scholar]
- 42.Javaheri S. Acetazolamide improves central sleep apnea in heart failure: a double-blind, prospective study. Am J Respir Crit Care Med. 2006;173:234–7. doi: 10.1164/rccm.200507-1035OC. [DOI] [PubMed] [Google Scholar]
- 43.Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respir Physiol Neurobiol. 2008;162:144–51. doi: 10.1016/j.resp.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]