Abstract
Objectives:
The video-polysomnographic criteria of REM sleep behavior disorder (RBD) have not been well described. We evaluated the between-night reproducibility of phasic and tonic enhanced muscle activity during REM sleep as well as the associated behaviors and vocalizations of the patients.
Methods:
Fifteen patients with clinical RBD underwent two consecutive video-polysomnographies. The amount of excessive phasic and tonic chin muscle activity during REM sleep was measured in 15 patients in 3-sec mini-epochs. The time spent with motor (minor, major, complex, and scenic) or vocal (sounds, mumblings, and comprehensible speeches) events was measured in 7 patients during REM sleep.
Results:
There was a good between-night agreement for tonic (Spearman rho = 0.55, p = 0.03; Kendall tau = 0.48, p = 0.01) but not for phasic (rho = 0.47, p = 0.1; tau = 0.31, p = 0.1) excessive chin muscle activity. On the video and audio recordings, the minor RBD behaviors tended to occur more frequently during the second night than the first, whereas the patients spoke longer during the first than the second night.
Conclusion:
The excessive tonic activity during REM sleep is a reliable marker of RBD. It could represent the extent of dysfunction in the permissive atonia systems. In contrast, the more variable phasic activity and motor/vocal events could be more dependent on dream content (executive systems).
Citation:
Cygan F; Oudiette D; Leclair-Visonneau L; Leu-Semenescu S; Arnulf I. Night-to-night variability of muscle tone, movements, and vocalizations in patients with REM sleep behavior disorder. J Clin Sleep Med 2010;6(6):551-555.
Keywords: REM sleep behavior disorders, phasic chin muscle, tonic chin muscle, reproducibility, between-night variability, vocalization, motor behaviors
REM sleep behavior disorder (RBD) is a recently described parasomnia characterized by violent, or potentially violent, movements during REM sleep, mostly corresponding to enacted dreams. During sleep monitoring, there is a partial or total loss of normal muscle atonia during REM sleep.1 REM sleep behavior disorder predominantly affects elderly subjects without any other disease (idiopathic RBD) or suffering from various neurological and neurodegenerative diseases, mainly synucleinopathies.2 Because the RBD symptoms may indicate a future neurodegenerative disease such as Parkinson disease, dementia with Lewy bodies, and multiple system atrophy,3 there is a need for accurate diagnostic criteria for the disorder. In this regard, the recent diagnostic consensus indicates that a history of sleep related injurious, potentially injurious, or disruptive behaviors should be associated with the presence of REM sleep without atonia.1 The latter is defined in practice as excessive sustained or intermittent elevation of chin electromyographic (EMG) tone or excessive phasic chin or limb EMG twitching.1 However, the methods for scoring enhanced muscle tone and the cut-off for abnormal muscle tone were not defined in this classification. More recently, the measure of tonic muscle activity in REM sleep was defined as the sum of REM sleep epochs with ≥ 50% of the duration of the epoch having a chin EMG amplitude greater than the minimum amplitude in NREM sleep.4 The enhanced phasic chin muscle activity in REM sleep was defined as the percentage of 30-sec epochs containing ≥ five 3-sec mini-epochs with bursts of transient muscle activity. A transient muscle activity was an enhancement of EMG signal ≥ 4 times as high in amplitude as the background EMG activity, lasting 0.1 to 5 sec. When contrasting muscle activity during REM sleep in 33 patients with Parkinson disease (with and without RBD) and 16 healthy controls, it was suggested that 20% of REM sleep without atonia be defined as abnormal.5 When combining the tonic and phasic muscle activity, and using mini-epochs of 3 sec instead of 2 sec in 17 patients, Consens et al. found that a cut-off of 10% is more sensitive to diagnose RBD.6 Because a recent study showed that the amount of tonic muscle activity during REM sleep predicts the future development of Parkinson disease in idiopathic RBD,7 it is important to determine if one night is sufficient for the diagnosis, or if the sleep test should be repeated to enhance the accuracy of RBD diagnosis. The night-to-night variability in tonic and phasic muscle tone is also an important factor to take into account when calculating the power and size of drug or interventions against RBD. When studying 2 consecutive nights, the tonic, phasic, and combined muscle activity measured on Night 1 closely correlated with the same measures on Night 2 in 17 patients at risk for RBD and 6 controls.6 However, an agreement (rather than a correlation) between diagnosis on both nights may be more appropriate to evaluate if one measure is sufficient or not. In a previous study using 55 patients with RBD, a threshold ≥ 10% enhanced tonic (or phasic plus tonic) muscle submental activity yielded a positive diagnosis of RBD in 80% of patients during the first night.8 This percentage was increased to 95% when combining the polysomnography results with the videos. We aimed to replicate these results in our series of patients with clinically suspected RBD, and to measure night-to-night variability in motor and vocal behaviors.
BRIEF SUMMARY
Current Knowledge/Study Rationale: It is not yet determined if a single night-time video and sleep monitoring are sufficient for diagnosing REM sleep behavior disorder (RBD). Plus, the night-to-night variability of chin muscle tonic and phasic activity, as well as movements and vocalizations during REM sleep, is unknown.
Study Impact: As the enhanced tonic muscle activity during REM sleep is a more reliable marker of RBD between nights than the phasic activities (chin muscle, movements and speeches), it can be used as a marker of efficacy in future drug trials in RBD. Plus, it shows that one night is enough for diagnosing RBD.
METHODS
Subjects
Eighteen patients with RBD underwent 2 consecutive nights with video- and sleep- polysomnography. Diagnosis of RBD was confirmed by clinical interview plus video-polysomnography and defined as one of the following: a history of sleep related injurious, potentially injurious, or disruptive behavior; the presence of REM sleep without atonia; and the absence of EEG epileptiform activity during REM sleep.1 To be included in the test-retest study, patients had to present ≥ 5 min of REM sleep time during both nights and to have measurable EMG tone for at ≥ 95% of REM sleep time. Eventually, 15/18 patients met these criteria and were included in the study. They had idiopathic RBD (n = 9), idiopathic Parkinson disease (n = 4), and idiopathic narcolepsy (n = 2); 12 of 15 (80%) were men. Patients were 54 to 81 years old, with a mean age of 66.3 ± 10.2 years and a mean body mass index of 26.6 ± 4.5 kg/m2. Prescribed medications included antidepressants (n = 6) and melatonin (n = 3), unchanged during the 2 nights. No patients were taking benzodiazepines. The mean score of the group on the Epworth Sleepiness Scale was 10.0 ± 5.2, and 53.3% of the patients had a score ≥ 10.
Video and sleep monitoring
All patients underwent a video-polysomnography on 2 consecutive nights. The monitoring included the following: Fp1-A2, C3-A2, C3-O1 electroencephalography; electro-oculography (2 channels); levator menti and tibialis anterior muscle electromyography; nasal pressure though a cannula; tracheal sounds through a microphone; thoracic and abdominal belts to assess respiratory efforts; electrocardiography; pulse oximetry; EEG-synchronized infrared video-monitoring; and ambiance microphone.
Scoring
The sleep stages, arousals, alpha rhythm on EEG, respiratory events, and periodic leg movements were scored by visual inspection according to standard criteria,4 with allowance for scoring REM sleep despite persistence of tonic and phasic muscle activity, according to the international criteria. Tonic and phasic muscle activities were quantified during REM sleep using the EMG chin channel.9 The periods of REM sleep with a microarousal or snoring artifact were removed from the analysis. The tonic enhanced muscle activity was scored on 30-sec epochs. The epochs were scored as tonic when the background EMG activity was 2 times greater than in stage N3 during ≥ 50% of the epoch. The phasic enhanced EMG activity was evaluated in 3-sec mini-epochs during REM sleep, and any burst 4 times greater than the epoch background EMG activity, lasting between 0.1 and 5 sec, was counted. To be considered as distinct events, 2 phasic events had to be separated by at least one second. The phasic REM sleep activity (in percent) was the sum of all mini-epochs containing enhanced muscle activity, divided by the REM sleep time. We did not combine the phasic and tonic activities, as they could be superimposed in the same patient, which would lead to a sum > 100%. We measured the proportion of REM sleep time during which movements and behaviors were apparent and also noted the type of behaviors. For this purpose, we scrutinized, second-by-second, the videos obtained during REM sleep for 7 patients with RBD over 2 consecutive nights. When we observed a movement or a vocalization (regardless of type, amplitude, and duration), we put a marker at the start and end of the event and determined the event duration. Motor events were classified according to complexity as previously described.10 The behaviors were considered minor when they were myoclonic or too simple to be noticed by the bed partner (e.g., finger twitch). They were major when their amplitude was large or when more than one limb was moving (e.g., large arm movement or whole body jerk). Complex behaviors were behaviors that involved apparent “acting out” of a dream but were not clear enough to determine the exact meaning of the behavior (e.g., multiple disordered gestures of the superior limbs). Scenic behaviors were behaviors that involved “acting out” the dream and were easily understood by the observer (e.g., mimicking the gesture of smoking a cigarette). The vocalizations were classified according to articulation (sounds, mumblings, or words). The side of the limb movements was noted.
Statistics
As no measures were normally distributed, we compared the measures by Wilcoxon signed rank (with correction for multiple testing) and tested their agreement between nights by using the Kendall tau coefficient and the Spearman rho. Data are shown as mean ± standard deviation or frequencies (percentage). Between-nights change is calculated as the measure during the second night minus the measure during the first night (in absolute value), divided by the measure during the first night. It is expressed as a percentage.
RESULTS
Night-to-Night Variability of Sleep Measures
As shown in Table 1, the patients had a higher percentage of N3 sleep during the second night than the first. The sleep duration, efficiency, sleep onset, and REM sleep latencies, as well as N1, N2, and REM sleep percentages were not different between nights. Over the whole group, REM sleep time was 77.9 ± 27.6 min (range 6-118 min) during Night 1 and 77.4 ± 40.8 min (range 18-144 min) during Night 2, which was a nonsignificant change.
Table 1.
Sleep measures during two consecutive nights in 15 patients with RBD
| Night 1 | Night 2 | Individual night to night change | p | |
|---|---|---|---|---|
| Number of patients | 15 | 15 | ||
| Total sleep time, min | 403.9 ± 83.1 | 385.4 ± 131.2 | 19 ± 21% | 0.8 |
| Total sleep period, min | 542.2 ± 110.8 | 529.00 ± 110.8 | 23 ± 16% | 0.8 |
| Sleep efficiency, % | 75.4 ± 12.9 | 69.1 ± 23.9 | 18.8 ± 18.7% | 0.07 |
| Latency to | ||||
| Sleep onset, min | 46.0 ± 40.3 | 36.1 ± 26.4 | 55.3 ± 54.6% | 0.4 |
| REM sleep, min | 143.1 ± 137.3 | 131.7 ± 150.1 | 51 ± 64% | 0.2 |
| Sleep stages, % of total sleep time | ||||
| N1 | 7.7 ± 5.1 | 7.1 ± 5.5 | 64 ± 70% | 0.4 |
| N2 | 48.1 ± 7.6 | 47.4 ± 9.1 | 11 ± 8% | 0.9 |
| N3 | 22.7 ± 6.1 | 26.99 ± 7.93 | 26 ± 23% | 0.03* |
| REM sleep | 21.1 ± 7.7 | 18.5 ± 8.9 | 28 ± 23% | 0.2 |
| REM sleep time, min | 77.9 ± 27.6 | 77.4 ± 40.8 | 184 ± 586% | 0.7 |
| Quantification of tonic and phasic muscle activity | ||||
| REM sleep with tonic activity, % | 45.9 ± 28.4 | 42.6 ± 26.1 | 46 ± 44% | 0.7 |
| REM sleep with phasic activity, % | 20.9 ± 5.2 | 23.6 ± 5.9 | 28 ± 31% | 0.1 |
Night-to-Night Variability of Phasic and Tonic Muscle Activity during REM Sleep
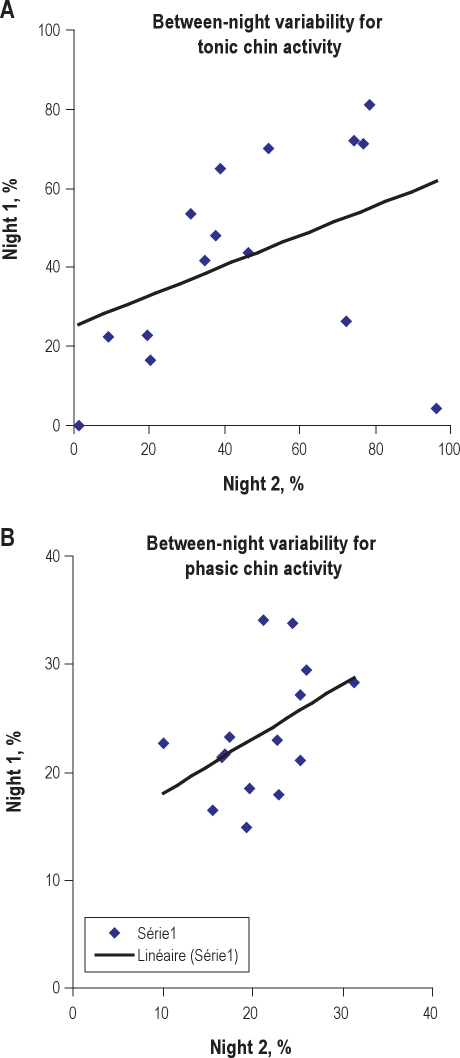
There was no difference between Night 1 and Night 2 in terms of percentages of tonic and phasic muscle activity during REM sleep. There was a 46% between-night change in tonic enhanced muscle activity and a 28% change in phasic muscle activity. Figure 1A and Figure 1B illustrate the between-night correlations for tonic and phasic muscle activity.
Figure 1.
Tonic (A) and phasic (B) enhanced muscle activity, as a percent of REM sleep time, during Night 1 (x axis) and Night 2 (y axis)
The correlation lines are indicated in Figure 1A (rho = 0.41, p = 0.1) and 1B (rho = 0.45, p = 0.09).
As indicated in Table 2, the Kendall tau coefficient was significant (meaning that there was a good between-night agreement) for tonic but not for phasic activity. Similarly, the Spearman rho coefficient was significant (p = 0.03) for tonic but not (p = 0.1) for phasic muscle activity during REM sleep.
Table 2.
Between-night concordance for RBD-related measures, as a percent of REM sleep time
| Kendall tau coefficient | Spearman rho coefficient | |
|---|---|---|
| REM sleep without atonia, % (n = 15) | ||
| Phasic | 0.31 | 0.47 |
| Tonic | 0.48* | 0.55* |
| Duration of behaviors, % (n = 7) | ||
| All | 0.24 | 0.21 |
| Minor | 0.43 | 0.50 |
| Major | 0.33 | 0.46 |
| Complex | 0.39 | 0.50 |
| Scenic | −0.28 | −0.38 |
| Duration of vocalizations, % (n = 7) | ||
| All | 0.05 | 0.28 |
| Mumbling | 0.29 | 0.57 |
| Sounds | −0.24 | −0.43 |
| Comprehensible speech | 0.09 | 0.05 |
p < 0.05
Night-to-Night Variability in RBD-Associated Behaviors and Vocalizations
In the video and audio recordings (Table 3), the RBD-associated behaviors represented 8.1% ± 6% of REM sleep time during the first night (range: 2% to 19%; 1.4-11.6 min), and increased (p = 0.4) to 8.7% ± 4.9% the second night (range: 3% to 16%; 1.3-21.0 min), but this change was not significant. Only 0.9% of the time was spent in scenic behaviors, whereas complex and major behaviors were more frequent. There were no between-night differences in the time and proportion of these behaviors. The vocalizations during RBD contained mainly comprehensible words and sentences, followed by mumbling and sounds. There were no difference in the frequency of these vocalizations between the first and the second night, except that patients tended to speak (with a comprehensible speech) more during the first than the second night. There was a weak, but not significant, correlation between nights for the duration of all types of behaviors, sounds, and mumbling. The time spent speaking did not, however, correlate between nights (rho = 0.09, p = 0.1). Most limb movements were bilateral, and the left and right limbs were used as frequently, with no difference between nights.
Table 3.
Comparison of REM sleep behaviors in 7 patients between Night 1 and Night 2
| Night 1 | Night 2 | Individual night-to-night change | p value | |
|---|---|---|---|---|
| Number of patients | 7 | 7 | ||
| REM sleep duration, min | 74.6 ± 18.6 | 81.3 ± 57.2 | 42 ± 29% | 0.7 |
| Duration of behavior, % of total REM sleep time | ||||
| All | 8.1 ± 6.0 | 8.7 ± 4.9 | 97 ± 183% | 0.65 |
| Minor | 3.6 ± 3.4 | 4.6 ± 2.8 | 169 ± 202% | 0.06 |
| Major | 2.1 ± 1.8 | 2.1 ± 2.4 | 119 ± 189% | 0.7 |
| Complex | 1.5 ± 1.1 | 1.7 ± 1.8 | 88 ± 76% | 0.7 |
| Scenic | 0.9 ± 1.3 | 0.4 ± 0.5 | 43 ± 53% | 0.4 |
| Duration of vocal type behavior, % of total REM sleep time | ||||
| All | 3.6 ± 0.03 | 3.0 ± 0.03 | 173 ± 296% | 0.3 |
| Sound | 0.9 ± 0.02 | 0.6 ± 0.01 | 140 ± 296% | 0.3 |
| Mumbling | 1.2 ± 0.01 | 1.5 ± 0.02 | 160 ± 222% | 0.4 |
| Comprehensible speech | 1.5 ± 0.01 | 0.8 ± 0.01 | 914 ± 2226% | 0.06 |
| Behavior laterality, % of total behaviors | ||||
| Both | 52.5 ± 0.2 | 50.0 ± 0.3 | 19 ± 21% | 0.7 |
| Left | 21.6 ± 0.1 | 26.5 ± 0.2 | 56 ± 79% | 0.7 |
| Right | 26.0 ± 0.1 | 23.5 ± 0.2 | 41 ± 36% | 0.7 |
DISCUSSION
In 15 patients with RBD, the rate of tonic enhanced muscle activity during REM sleep remained stable between the first and second night, with a correlation of 0.48. However, the percent of phasic enhanced muscle activity and the time spent with abnormal behaviors and vocalizations during REM sleep was not reproducible between nights. In particular, the minor RBD behaviors tended to be more frequent during the second night, whereas the patients spoke longer during the first night.
The night-to-night changes in total sleep time and REM sleep percentage were small and not significant in our sample, as shown in a previous study of patients with RBD.8 These results suggest that the “first-night effect” is not a problem in patients with RBD, except for the percent of N3 sleep. This effect is believed to result from sleeping for the first time with electrodes in an unfamiliar environment. It is characterized by a decreased quality and quantity of first-night sleep as indicated by more stage 1 and intra-sleep wake, paralleled by reduced sleep efficiency and longer sleep onset latency.11 Although there are numerous reports that suggest that sleeping at home or in a hotel room (rather than in the sleep laboratory) minimizes the first-night effect,12 this option of home recording cannot be offered to patients with suspicion of RBD, as there is yet no easy means of simultaneously monitoring the behavior with an infrared video and the electrophysiological measures at home. The time and percentage of REM sleep is one of the key measures, as far as the diagnosis of RBD is concerned. Here, the REM sleep time and percentage were similar between nights, provided that a minimum of 5 min of REM sleep had been observed during both nights.
There was a moderate change and good agreement in the percentage of enhanced tonic chin muscle activity during REM sleep between the 2 nights. This result is important because tonic muscle activity during REM sleep is a widely used measure, especially in patients with idiopathic RBD, as it can predict the later development of Parkinson disease.7 We found a mean 44% enhanced chin tonic muscle activity during REM sleep in our series, which is close to percentages reported in other RBD groups in Western countries,3,6,7,13 but is double that reported in an Asian series.8 Because the methods for measuring muscle activities are identical, these differences suggest that Asian patients have decreased muscle activity during RBD, or that patients with less severe RBD were included. The stability and reproducibility of tonic REM sleep muscle activity suggests that it is a state marker for RBD. It could possibly represent the extent of the damage (neuronal loss or dysfunction) to the REM sleep atonia system.
In contrast to the tonic muscle activity, which had a good (0.48-0.55) night-to-night agreement, the phasic chin muscle activity changed significantly (with a mean 28% change) between the first and the second night. This result is different from a previous study,8 which reported nonsignificant differences for phasic muscle activity between Night 1 and Night 2 in 50 patients with RBD. The mean phasic activity in the RBD group was, however, lower (9% vs. 22%) than in our sample, and the agreement between phasic measures was not tested. The frequency and complexity of behaviors and vocalizations also varied between nights in our sample, with more time spent with minor behaviors during the second night than the first, and longer speeches during the first night than the second. The between-night reproducibility of behaviors and speeches has not been tested in other series, to the best of our knowledge. Altogether, these results suggest that the brain systems generating these phasic activities (chin muscle phasic activity, limb movements, and vocalizations) during REM sleep are more variable than the atonia system. This finding may suggest that the atonia system is permissive and damaged in RBD, whereas the phasic system is executive and dysfunctional, rather than damaged. Alternatively, the phasic activity during REM sleep may depend on the nature and between-night variability of the dreaming process, if one assumes that most dreams are translated into visible behaviors. It is notable that REM sleep dream content is highly variable among subjects and between nights.14
There are several limitations in this study, including a small sample size (n = 15), especially for behavioral aspects (n = 7), but these time-consuming, detailed measures of movements and vocalizations have previously yielded important and reproducible results in samples as small as 5 patients.9,10 In addition, our sample includes patients with various diseases, including idiopathic RBD and RBD associated with Parkinson disease and narcolepsy, some of them taking antidepressants. It is a realistic, clinically based sample. The sample size is, however, too small to allow measuring between-night variability in homogenous diseases.
In conclusion, enhanced tonic chin muscle activity seems to be a reliable measure for RBD diagnosis, whereas enhanced phasic chin muscle activity as well as motor and vocal behaviors during REM sleep are more variable between nights.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Arnulf has received research support from Actelion and has participated in speaking engagements for UCB. Dr. Leu-Semenescu has received research support from Bioprojet.
ACKNOWLEDGMENTS
The study was financed by a grant from the Federation pour la Recherche sur le Cerveau (2007).
REFERENCES
- 1.American Academy of Sleep Medicine. The international classification of sleep disorders, revised: diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 2.Boeve BF, Silber MH, Saper CB, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130:2770–88. doi: 10.1093/brain/awm056. [DOI] [PubMed] [Google Scholar]
- 3.Iranzo A, Molinuevo JL, Santamaria J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 2006;5:572–7. doi: 10.1016/S1474-4422(06)70476-8. [DOI] [PubMed] [Google Scholar]
- 4.Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 5.Gagnon JF, Bedard MA, Fantini ML, et al. REM sleep behavior disorder and REM sleep without atonia in Parkinson's disease. Neurology. 2002;59:585–9. doi: 10.1212/wnl.59.4.585. [DOI] [PubMed] [Google Scholar]
- 6.Consens FB, Chervin RD, Koeppe RA, et al. Validation of a polysomnographic score for REM sleep behavior disorder. Sleep. 2005;28:993–7. doi: 10.1093/sleep/28.8.993. [DOI] [PubMed] [Google Scholar]
- 7.Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology. 2009;72:1296–300. doi: 10.1212/01.wnl.0000340980.19702.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Lam SP, Ho CK, et al. Diagnosis of REM sleep behavior disorder by video-polysomnographic study: is one night enough? Sleep. 2008;31:1179–85. [PMC free article] [PubMed] [Google Scholar]
- 9.Lapierre O, Montplaisir J. Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology. 1992;42:1371–4. doi: 10.1212/wnl.42.7.1371. [DOI] [PubMed] [Google Scholar]
- 10.Frauscher B, Gschliesser V, Brandauer E, et al. Video analysis of motor events in REM sleep behavior disorder. Mov Disord. 2007;22:1464–70. doi: 10.1002/mds.21561. [DOI] [PubMed] [Google Scholar]
- 11.Curcio G, Ferrara M, Piergianni A, Fratello F, De Gennaro L. Paradoxes of the first-night effect: a quantitative analysis of antero-posterior EEG topography. Clin Neurophysiol. 2004;115:1178–88. doi: 10.1016/j.clinph.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Sharpley AL, Solomon RA, Cowen PJ. Evaluation of first night effect using ambulatory monitoring and automatic sleep stage analysis. Sleep. 1988;11:273–6. doi: 10.1093/sleep/11.3.273. [DOI] [PubMed] [Google Scholar]
- 13.Frauscher B, Iranzo A, Hogl B, et al. Quantification of electromyographic activity during REM sleep in multiple muscles in REM sleep behavior disorder. Sleep. 2008;31:724–31. doi: 10.1093/sleep/31.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domhoff B. The content of dreams: methodological and theorical implications. In: Kryger M, Dement WC, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia: Elsevier Saunders; 2005. p. 1517. [Google Scholar]