Abstract
Study Objectives:
Planned naps can improve performance when the habitual or nocturnal sleep schedule is disrupted. It may be difficult, however, to achieve sleep during a nap, particularly during the circadian peak in alertness in the early evening. Prior studies with the melatonin agonist, ramelteon, reported that this hypnotic does not impair neurobehavioral performance. We tested whether ramelteon could improve nap efficiency in the early evening and subsequent performance during a simulated 8-h night shift.
Methods:
10 healthy volunteers aged 19-31 years participated in an inpatient randomized, double-blind, placebo-controlled crossover study. Ramelteon 8 mg or placebo was administered 30 min prior to a 2-h nap opportunity commencing 13 h after each individual's habitual morning wake time.
Results:
Ramelteon did not significantly affect sleep efficiency during the nap prior to the night shift. Following the nap, ramelteon was associated with significantly worse neurobehavioral performance on assessments immediately following the nap and during the simulated night shift.
Conclusions:
Although ramelteon did not significantly affect sleep during the nap, it was associated with significant impairments in neurobehavioral performance for up to 12 h after administration. High homeostatic sleep pressure combined with the circadian performance nadir may increase the vulnerability to hypnotic-induced neurobehavioral impairments. The findings do not support the use of ramelteon prior to an evening prophylactic nap, as there may be residual effects that last for several hours. Furthermore, this study highlights the pitfalls of applying side-effect profiles obtained in one context to another.
Citation:
Cohen DA; Wang W; Klerman EB; Rajaratnam SMW. Ramelteon prior to a short evening nap impairs neurobehavioral performance for up to 12 hours after awakening. J Clin Sleep Med 2010;6(6):565-571.
Keywords: Ramelteon, shift work, sleep deprivation, circadian
Traveling on a long-haul flight, tending to family emergencies or caregiver responsibilities, and rotating shift work schedules are all examples of situations in which the habitual nocturnal sleep episode may be disrupted while an individual may still be required to subsequently remain awake and alert. When possible, a planned nap can minimize the detrimental impact of the impending bout of sustained wakefulness on alertness and performance.1 It may be difficult, however, to achieve sleep outside of the habitual sleep period, particularly during the afternoon and early evening when the circadian system promotes wakefulness.2 Theoretically, a hypnotic medication that does not impair neurobehavioral performance could be an effective strategy to assist with planned naps and improve waking functioning.
Ramelteon is a selective MT1/MT2 melatonin receptor agonist3 approved by the Food and Drug Administration for the treatment of insomnia. Preclinical animal studies of ramelteon in rats, cats, and mice demonstrated that doses up to 30 mg/kg did not impair subsequent performance on tasks of learning, memory, and motor control.4–6 In phase-advance models of transient insomnia in humans and in patients with chronic insomnia, ramelteon has been reported to lack significant adverse neurobehavioral effects on performance on measures such as the digit symbol substitution task (DSST) and delayed recall.7–11 Although these studies generally assessed the effect of ramelteon on next-day neurobehavioral performance following an 8-h nocturnal sleep period, two studies showed that ramelteon at doses of 4-160 mg did not impair measures such as DSST, word recall, balance, or hand-eye coordination during the waking day after morning administration.3,12 Additionally, in a recent crossover study in which elderly participants aged 65 or older received ramelteon, zolpidem, or placebo 30 min prior to sleep and were awakened 2 h after dosing, ramelteon did not impair middle-of-the-night balance or memory, whereas zolpidem impaired these measures.13 Therefore, given its apparent neurobehavioral safety profile when tested under a variety of circumstances, we hypothesized that ramelteon could improve sleep efficiency outside of the habitual sleep episode and subsequently improve waking performance.
BRIEF SUMMARY
Current Knowledge/Study Rationale: A nap is often recommended before working a night shift to improve alertness and performance, but naps in the evening may be difficult to achieve secondary to circadian alerting mechanisms. Modulation of the melatonin receptor system can improve sleep in the early evening, perhaps by attenuating circadian arousal mechanisms, and may potentially improve performance during the following night shift.
Study Impact: While prior studies suggested that ramelteon lacks cognitive side effects, we demonstrate neurobehavioral impairments for up to 12 hours after administration. We caution that conditions of high homeostatic sleep pressure coinciding with the circadian performance nadir during a night shift may increase the sensitivity for residual hypnotic-induced deficits on subsequent waking performance.
We used a simulated night-shift model to mimic a real-world situation in which individuals may use a hypnotic prior to a nap with the goal of improving nap sleep efficiency and enhancing subsequent alertness and performance. This proof-of-concept study was conducted using a randomized, double-blind, placebo controlled, crossover inpatient study design.
METHODS
Participants
Ten healthy volunteers aged 19-31 years (mean age 24.6 years, 6 female, 4 male) completed the study. These participants were medically healthy as determined by history, physical examination, electrocardiography, blood chemistry, and hematology. Their self-reported habitual sleep onset occurred between 21:00 and 02:00 (mean 23:12), and habitual sleep duration was reported to be 7-9 h. The study protocol was approved by the Partners Human Research Committee.
Protocol
Each participant completed 2 inpatient laboratory visits of approximately 28 h in duration, separated by approximately 4 weeks to minimize potential differences in phase of the menstrual cycle on the crossover visit, and to ensure complete recovery from sleep deprivation. Eligible participants maintained a self-selected, fixed 8-h sleep: 16-h wake schedule for approximately 3 weeks prior to each inpatient admission, which was verified by sleep logs and time-stamped voicemail messages left before going to bed and upon waking. Furthermore, wrist actigraphy (Philips Respironics, Murrysville, PA) was recorded for at least one week prior to each visit. All inpatient events were timed relative to each participant's habitual sleep and wake times. Beginning 3 weeks prior to each inpatient admission, participants refrained from caffeine, alcohol, medications (except oral contraceptives), supplements, and illicit drugs. Urine toxicology screen and serum pregnancy testing (for female participants) were performed at the time of each admission.
The inpatient study was conducted in the Brigham and Women's Hospital inpatient Center for Clinical Investigation. The same experimental suite was used for all participants to minimize variability within and across participants. The suite was free of time cues, including no phone, internet access, or television. Ambient light, measured from a point in the center of the suite in 4 directions of gaze, ranged from 53-127 lux (mean 88 lux). When measured in the direction of gaze when seated at the desk, ambient light averaged 89 lux. An anteroom leading to the suite minimized the transmission of external noise during sleep and neurobehavioral testing.
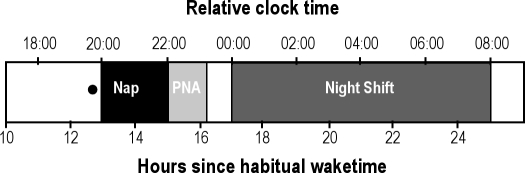
Ramelteon (8 mg) or placebo was administered double-blind during the inpatient study (Figure 1). Treatment was administered 30 min prior to a 2-h sleep opportunity, which was approximately 12.5 h after each participant's habitual morning wake time. Sleep propensity is usually low at this time, which is termed the “forbidden zone” for sleep onset or the “wake maintenance zone,” secondary to circadian alerting mechanisms.14,15 Immediately after the nap, the head of the bed was raised to 45 degrees, and participants performed a computerized battery of neurobehavioral tests every 10 min for 71 min for post-nap performance assessments. They ate dinner, and the simulated night shift started 2 h after the end of the nap. During the simulated night shift, participants remained seated at a desk except during scheduled 5-min breaks every hour. A 30-min neurobehavioral test battery was administered each hour to assess performance during the simulated night shift. A small snack was given during the middle of the night shift. A technician remained in the room to ensure that participants did not fall asleep during the simulated night shift. Following the night shift, participants ate breakfast and were provided an 8-h recovery sleep opportunity prior to being discharged.
Figure 1.
Schematic representation of inpatient protocol
Relative clock time (h) represents clock time for a participant whose habitual wake time is 07:00h. Actual times were adjusted for each participant depending on their habitual wake time. PNA is post-nap assessments. The black circle represents the time of double-blind treatment administration.
Neurobehavioral measures
Sleep
Sleep during the 2-h nap was assessed by polysomnography (Vitaport digital sleep recorder, TEMEC Instruments B.V. Kerkrade, The Netherlands; sampling rate 256 Hz, montage: C3, C4, O1, O2 referenced to contralateral mastoid A1, A2). Sleep data were visually scored16 to determine sleep stage distributions.
Post-nap Assessments
The post-nap assessment battery consisted of: Visual analog scale (VAS alert)–a non-numeric scale of increasing alertness scored from 0-100; Karolinska Sleepiness Scale (KSS)–a numeric scale of increasing sleepiness from 1-917; Digit symbol substitution test (DSST)–a cognitive throughput task consisting of matching symbols to a number key; Karolinska Drowsiness Test (KDT)18 to obtain artifact-free EEG recordings, while participants stare at a central target for 3 minutes. These waking EEG data (Fz, Cz, Pz, Oz referenced against linked mastoids [A1-A2]) were subjected to spectral analysis in 2-sec epochs by applying a 10% cosine window, resulting in power spectra with a 0.5 Hz frequency resolution. The primary outcome was power density in the 5.5-9 Hz theta-low frequency alpha (TLFA) range: higher values indicate greater degrees of drowsiness. The total time for each post-nap assessment battery was approximately 8 minutes.
Simulated Night Shift Performance
The neurobehavioral battery during the simulated night shift included: Psychomotor Vigilance Task (PVT)–a 10-min visuo-motor test of sustained attention measured by median reaction time (RT) and the number of lapses (responses with RT > 0.5 seconds); VAS alert–administered at the beginning and end of the battery to determine the change (delta) in subjective alertness related to performing cognitively demanding tasks; KSS– administered at the beginning and end of the battery to determine the change (delta) in sleepiness related to cognitively demanding tasks; DSST; Addition task (ADD)–a cognitive throughput task consisting of adding 2-digit numbers; Flanker–a 7-min task that measures the RT cost of inhibiting distracting stimuli19; Probed Recall Memory (PRM)–a free recall and recognition task for 6 word pairs; and KDT.
Statistical Analysis
Primary endpoints were specified a priori for sleep, post-nap assessments, and simulated night shift performance. For each of the 3 sets of primary endpoints,α = 0.05 was used to determine statistical significance and Bonferroni correction was applied when multiple measures were included for each set of endpoints. The primary sleep measure was sleep efficiency (total sleep time/2 h x 100); the study was powered to detect a 25% difference in sleep efficiency. All tests in the post-nap assessment battery were included as primary endpoints, and the correctedα* wasα/4 = 0.0125. PVT median RT and lapses were the primary night shift battery endpoints, and the correctedα* wasα/2 = 0.025. Mixed-effects models were used to determine interactions between time since awakening (post-nap assessments) or time across the night shift and the effect of drug condition.
RESULTS
Sleep
Ramelteon was associated with a 5.7% increase in sleep efficiency, yielding approximately 7 more minutes of sleep during the nap. This result was not statistically significant (p = 0.1725, see Table 1).
Table 1.
Nap sleep measures
| Measure | Placebo Mean ± SD | Ramelteon Mean ± SD | Effect Size | p value |
|---|---|---|---|---|
| Sleep efficiency % | 83.3 ± 14.3 | 89.1 ± 9.8 | 0.1725 | |
| Sleep latency (min) | 20.3 ± 17.5 | 13.6 ± 11.9 | 0.1971 | |
| Total sleep time (min) | 100.3 ± 17.5 | 106.8 ± 12.1 | 0.2016 | |
| Stage 1 (%) | 5.4 ± 2.0 | 6.8 ± 5.9 | 0.3549 | |
| Stage 2 (%) | 36.8 ± 12.8 | 47.1 ± 15.9 | 0.0502 | |
| Stage 3&4 (slow wave sleep)(%) | 34.1 ± 6.0 | 30.4 ± 16.5 | 0.3759 | |
| Stage REM (%) | 7.0 ± 9.7 | 4.9 ± 7.9 | 0.2276 | |
| Wake (%) | 16.6 ± 14.3 | 10.9 ± 9.8 | 0.1747 | |
| Awakenings | 3.6 ± 3.0 | 10.9 ± 9.8 | 1.01 | 0.0193*** |
Secondary endpoints uncorrected α*** = p < 0.05
Post-nap Assessments
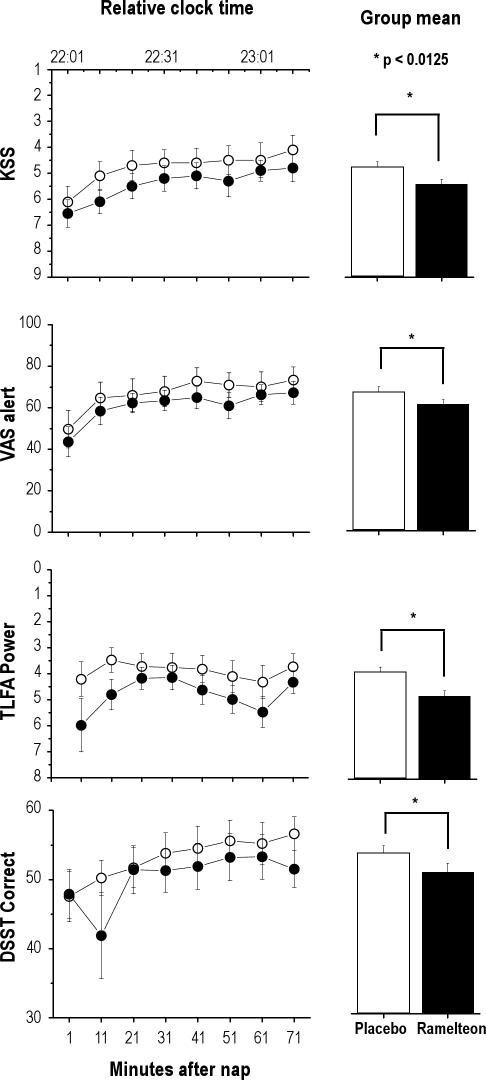
Ramelteon was associated with small but significant worsening of all post-nap measures, corrected for multiple comparisons (Table 2, Figure 2) atα* = 0.0125 level. Performance generally improved across the duration of post-nap testing, but there was no statistically significant interaction between time and drug condition.
Table 2.
Post-nap assessments
| Measure | Placebo Mean ± SD | Ramelteon Mean ± SD | Effect Size | p value |
|---|---|---|---|---|
| VAS alert | 67.1 ± 22.8 | 61.1 ± 18.6 | 0.29 | 0.0013* |
| KSS | 4.8 ± 1.8 | 5.4 ± 1.6 | 0.35 | 0.0003* |
| DSST correct | 53.3 ± 9.3 | 50.4 ± 9.3 | 0.31 | 0.0068* |
| KDT - TLFA | 4.0 ± 5.6 | 4.9 ± 4.5 | 0.18 | < 0.0001* |
Primary endpoints corrected α:* = p < 0.0125
VAS, visual analog scale alertness; KSS, Karolinska Sleepiness Scale; DSST, digit symbol substitution task; KDT, Karolinska Drowsiness Test; TLFA, theta low frequency alpha (5.5-9 Hz) power
Figure 2.
Post-nap assessments
Performance within the post-nap assessments is shown as a function of minutes after waking and the relative clock time for a participant with a habitual sleep period of 23:00 to 07:00. Higher values on the Y-axis of all graphs indicates improved performance.
Night Shift Performance
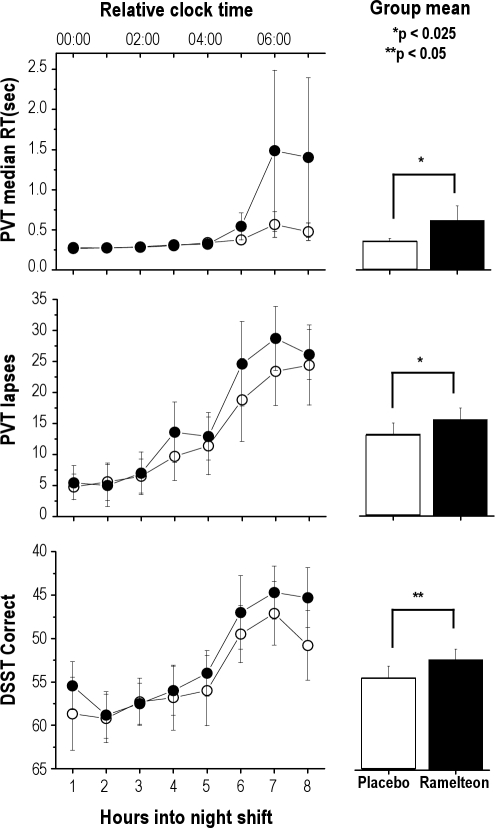
The primary measures, PVT median RT and lapses were significantly better with placebo compared to ramelteon, corrected for multiple comparisons. Secondary measures, including the DSST, the PRM, the decrease in alertness (VAS delta score) and increase in sleepiness (KSS delta score) from beginning to end of each performance battery were worse in the ramelteon condition (all p-values < 0.05 uncorrected). On all other secondary neurobehavioral measures, there was a trend for worsened performance in the ramelteon condition (Table 3, Figure 3).
Table 3.
Simulated night shift assessments
| Measure | Placebo Mean ± SD | Ramelteon Mean ± SD | Effect Size | p value |
|---|---|---|---|---|
| PVT median RT (sec) | 0.362 ± 0.25 | 0.662 ± 0.16 | 0.22 | 0.0193** |
| PVT lapses | 13.2 ± 16 | 15.5 ± 16.3 | 0.14 | 0.0001** |
| VAS alert (delta) | 12.0 ± 12.7 | 16.9 ± 13.7 | 0.37 | 0.0143*** |
| ADD correct | 42.9 ± 15.7 | 42.6 ± 13.8 | 0.7104 | |
| DSST correct | 54.4 ± 11.6 | 52.3 ± 10.6 | 0.19 | 0.0341*** |
| KSS (delta) | 1.1 ± 1.2 | 1.5 ± 1.2 | 0.33 | 0.0091*** |
| Flanker (msec) | 29.6 ± 114 | 46.8 ± 97.3 | 0.2995 | |
| PRM - free recall | 4.2 ± 1.8 | 4 ± 1.8 | 0.2707 | |
| PRM - recognition | 5.6 ± 0.87 | 5.3 ± 1.0 | 0.32 | 0.0432*** |
| KDT - TLFA | 1.7 ± 1.6 | 1.8 ± 1.7 | 0.5050 |
Primary endpoints correctedα:** = p < 0.025;
Secondary endpoints uncorrectedα*** = p < 0.05
PVT, Psychomotor Vigilance Task; RT, reaction time; VAS, visual analog scale alertness; ADD, addition task; DSST, digit symbol substitution task; KSS, Karolinska Sleepiness Scale; PRM, Probe Recall Memory; KDT, Karolinska Drowsiness Test; TLFA, theta low frequency alpha (5.5-9 Hz) power
Figure 3.
Simulated night shift assessments
Performance within each hour of the simulated night shift is shown. Higher values on the Y-axis of all graphs indicate worsened performance.
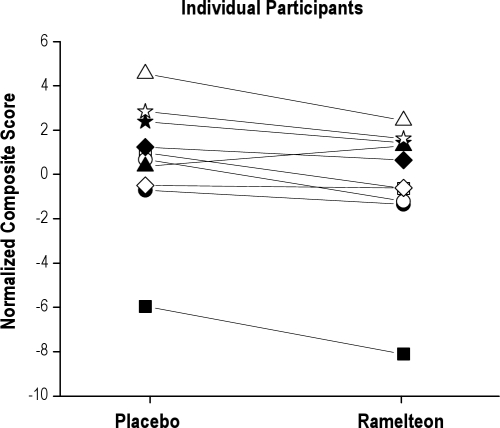
For each performance test (PVT, DSST, ADD, PRM, Flanker), individual performance scores were normalized as the deviation from the group mean relative to the standard deviation for that test. The composite score for each individual's night shift was calculated by adding all the normalized test scores together. Although the magnitude of impairment on any individual measure was small (effect sizes 0.2-0.4,20 Table 1), the composite score reflecting the overall performance during the entire night shift was worse in 9 of the 10 individuals (Figure 4).
Figure 4.
Individual performance composite scores during the simulated night shift
Individual composite scores reflecting overall performance during the simulated night shift are shown as a function of drug condition. Nine of the 10 individuals performed worse after receiving ramelteon prior to the prophylactic nap; the one individual who performed better after receiving ramelteon is represented by a black triangle.
Adverse Events
One participant who received ramelteon during the first admission developed significant nausea and small amounts of emesis toward the end of the night shift, leading to his withdrawal of consent. This participant was replaced in the randomization scheme. One participant who received placebo during the second admission reported mild nausea toward the end of the night shift but felt well enough to continue participation.
DISCUSSION
Ramelteon did not significantly increase sleep efficiency during the two-hour prophylactic nap, but post-nap assessments revealed worse performance on all measures compared to placebo immediately after the nap. In addition, ramelteon significantly worsened performance on the PVT, the primary night shift neurobehavioral measure. On the secondary neurobehavioral measures, there was either a significant worsening or trend for worse performance in the ramelteon condition compared to placebo. The composite performance score, reflecting overall performance during the entire night shift, was worse in 9 of the 10 individuals after ramelteon (Figure 4). These findings therefore do not support the use of ramelteon prior to an early evening nap to improve subsequent waking performance.
The impairment associated with ramelteon persisted on some measures for up to 12 h after drug administration (Figure 3). Although ramelteon has a half-life of 1-2 h, the active monohydroxylated metabolite, M II, has a half-life of 4-5 h and may contribute to the clinical effect.3 A direct hypnotic effect of the medication on performance would be expected to be maximal shortly after drug administration, with a decline in the impairment as the medication is metabolized.
While additional mechanisms may be involved, the principal mechanism by which melatonin is thought to promote sleep is through inhibition of neuronal activity in the hypothalamic suprachiasmatic nuclei (SCN),21 the site of the dominant circadian pacemaker in mammals. This inhibitory effect appears to be MT1 receptor-mediated22 and is evident when SCN neuronal activity is high during the late subjective day.21 Assuming that ramelteon promotes sleep by the same mechanism, one would expect that in humans the largest effects on sleepiness and reduced performance would occur during the late evening.
Contrary to what we predict from the pharmacokinetics and hypothesized sleep promoting mechanisms of action of ramelteon, there was a trend for worsening in the ramelteon condition in the second half of the simulated night shift rather than the first (Figure 3). The second half of the night shift corresponds to the circadian nadir in performance (approximately 03:00–07:00 during normal circadian alignment).2,23 In addition, this worsening in performance parallels the time course of increasing homeostatic sleep pressure as the participants had only a 2-hour sleep opportunity in the last 24 hours by that time in the protocol. Based on the known non-linear interactions between homeostatic sleep pressure and circadian phase on performance23–25 one interpretation of our results is that high homeostatic sleep pressure coinciding with the circadian nadir in performance increases the sensitivity of medication-induced neurobehavioral deficits. In other words, while the active metabolite is still in the systemic circulation, there is an intensification of the potential performance deficit under these physiological conditions that already predispose to poor performance.26 In contrast, during the waking day after a normal night of sleep, conditions in which homeostatic and circadian interactions promote maximal alertness, previous studies failed to identify significant performance deficits.3,12 Therefore, it remains to be tested whether ramelteon could have a beneficial effect on sleep and subsequent waking performance when administered before an early afternoon nap in order to extend wakefulness several hours later than the habitual bedtime, but prior to the circadian performance nadir.
This study has practical implications for the almost 15% of the full-time workforce that are involved in shift work.27 The transition to the first of a series of night shift, as modeled in this study, may be a particularly vulnerable time since extended wakefulness for almost 24 hours may coincide with the circadian nadir in alertness and performance.28 The worsening of performance on all measures of the post-nap assessments could interfere with safety sensitive activities following a truncated sleep episode. In addition, the overall increased probability of an error across a variety of tasks during an 8-hour night shift can have important real-world safety consequences depending on the nature of the operations.
Potential limitations of the study should be noted. Ramelteon improved sleep latency and total sleep time by approximately 7 minutes, but this finding was not statistically significant, possibly due to the sample size. The nonsignificant increase in sleep efficiency induced by ramelteon may also be partially explained by a higher than expected sleep efficiency in the placebo condition during a nap placed within the circadian wake maintenance zone. In a forced desynchrony protocol in which sleep and wake episodes were scheduled to a 28-hour cycle so that sleep episodes occurred at all phases of the endogenous circadian cycle, sleep latency was increased and total sleep time decreased during times corresponding to the circadian wake maintenance zone.2 Since circadian phase assessments were not performed, it is possible that, in the present study, the nap did not coincide with the predicted circadian peak in alertness. However, the goal of this study was to mimic a real-world situation, and clinicians who treat actual workers with shift work disorder rarely have precise circadian phase information. Alternatively, participants may have had a preexisting sleep debt that led to an increase in sleep efficiency in the placebo condition despite the adverse circadian timing of the nap. Conceivably, individuals without any degree of chronic sleep loss prior to the protocol could have achieved relatively better sleep with ramelteon and an improvement in performance during the night shift compared to placebo conditions rather than the observed worsening. However, the rigor in which we tried to ensure that participants had 8 hours time in bed for 3 weeks prior to each inpatient admission rarely occurs in a clinical population, and individuals with disrupted sleep schedules such as shift workers are likely to have a history of chronic sleep loss at least to the same degree or greater than these carefully monitored healthy volunteers.26 It is possible that individuals who are prone to insomnia or complain of difficulty napping outside of their habitual sleep hours would have had a relatively greater effect on sleep efficiency and subsequent improvement in waking performance with ramelteon. However, all performance measures trended in the same direction with relative impairment in the ramelteon condition, and 9 of 10 subjects in this counterbalanced crossover design did worse on the overall composite shift score in the ramelteon condition. These observations suggest that the pharmacological effect of the medication, particularly during the circadian nadir at high homeostatic pressure, increases the vulnerability to impairment. It would be challenging for clinicians to be able to weigh these direct pharmacodynamic effects against the subjective degree of insomnia complaints of an individual when recommending this strategy as a potential countermeasure. Finally, it is not known whether the observed pharmacodynamic responses to ramelteon under these physiological conditions would have been different in an elderly population. The findings of Zammit et al.13 suggest that ramelteon is less likely than zolpidem to impair middle-of-the-night balance, but whether activities that require full alertness, such as driving, are safe under these circumstances remains to be tested.
In conclusion, ramelteon administration intended to improve sleep efficiency during a pre-night shift nap was associated with potentially clinically significant impairments during post-nap assessments and night shift performance. These results are contrary to previous reports that indicate no significant neurobehavioral impairment associated with ramelteon. The likelihood of detecting medication-induced neurobehavioral impairments reflects multiple factors, including: the processing demands of the task, ambient conditions, pharmacokinetic factors, population differences in drug metabolism, and individual vulnerability to receptor-mediated effects. Conditions of high homeostatic sleep pressure and adverse circadian phase in particular may be physiological conditions that increase the likelihood of hypnotic-induced neurobehavioral impairments. Our results suggest caution in using hypnotics for an evening prophylactic nap before sustained wakefulness across the night. It is possible that a compound (and its active metabolites) with a shorter half-life prior to a prophylactic nap may prove to be a reasonable countermeasure for individuals with disrupted sleep schedules, but this hypothesis remains to be tested.
DISCLOSURE STATEMENT
This study was funded by Takeda Pharmaceuticals North America, grant #05-016R (Investigator Initiated). Dr. Cohen was project leader for this study. Dr. Klerman has received research support from Vanda, Respironics, and Sepracor and has consulted for Sanofi-Aventis. Dr. Rajaratnam has received research support from Takeda, Vanda, ResMed Foundation, Respironics Sleep and Respiratory Research Foundation, Philips Lighting and Cephalon and has consulted for Vanda.
ACKNOWLEDGMENTS
Work was performed at the Center for Clinical Investigation, Brigham and Women's Hospital, Boston, MA 02115
REFERENCES
- 1.Dinges DF, Orne MT, Whitehouse WG, Orne EC. Temporal placement of a nap for alertness: contributions of circadian phase and prior wakefulness. Sleep. 1987;10:313–29. [PubMed] [Google Scholar]
- 2.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–38. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karim A, Tolbert D, Cao C. Disposition kinetics and tolerance of escalating single doses of ramelteon, a high-affinity MT1 and MT2 melatonin receptor agonist indicated for treatment of insomnia. J Clin Pharmacol. 2006;46:140–8. doi: 10.1177/0091270005283461. [DOI] [PubMed] [Google Scholar]
- 4.Hirai K, Kita M, Ohta H, et al. Ramelteon (TAK-375) accelerates reentrainment of circadian rhythm after a phase advance of the light-dark cycle in rats. J Biol Rhythms. 2005;20:27–37. doi: 10.1177/0748730404269890. [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto M. Effect of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist, on motor performance in mice. Neurosci Lett. 2006;402:201–4. doi: 10.1016/j.neulet.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 6.Miyamoto M, Nishikawa H, Doken Y, Hirai K, Uchikawa O, Ohkawa S. The sleep-promoting action of ramelteon (TAK-375) in freely moving cats. Sleep. 2004;27:1319–25. doi: 10.1093/sleep/27.7.1319. [DOI] [PubMed] [Google Scholar]
- 7.Roth T, Seiden D, Wang-Weigand S, Zhang J. A 2-night, 3-period, crossover study of ramelteon's efficacy and safety in older adults with chronic insomnia. Curr Med Res Opin. 2007;23:1005–14. doi: 10.1185/030079907x178874. [DOI] [PubMed] [Google Scholar]
- 8.Roth T, Stubbs C, Walsh JK. Ramelteon (TAK-375), a selective MT1/MT2-receptor agonist, reduces latency to persistent sleep in a model of transient insomnia related to a novel sleep environment. Sleep. 2005;28:303–7. [PubMed] [Google Scholar]
- 9.Zammit G, Erman M, Wang-Weigand S, Sainati S, Zhang J, Roth T. Evaluation of the efficacy and safety of ramelteon in subjects with chronic insomnia. J Clin Sleep Med. 2007;3:495–504. [PMC free article] [PubMed] [Google Scholar]
- 10.Zammit G, Schwartz H, Roth T, Wang-Weigand S, Sainati S, Zhang J. The effects of ramelteon in a first-night model of transient insomnia. Sleep Med. 2009;10:55–9. doi: 10.1016/j.sleep.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Erman M, Seiden D, Zammit G, Sainati S, Zhang J. An efficacy, safety, and dose-response study of Ramelteon in patients with chronic primary insomnia. Sleep Med. 2006;7:17–24. doi: 10.1016/j.sleep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Johnson MW, Suess PE, Griffiths RR. Ramelteon: a novel hypnotic lacking abuse liability and sedative adverse effects. Arch Gen Psychiatry. 2006;63:1149–57. doi: 10.1001/archpsyc.63.10.1149. [DOI] [PubMed] [Google Scholar]
- 13.Zammit G, Wang-Weigand S, Rosenthal M, Peng X. Effect of ramelteon on middle-of-the-night balance in older adults with chronic insomnia. J Clin Sleep Med. 2009;5:34–40. [PMC free article] [PubMed] [Google Scholar]
- 14.Lavie P. Ultrashort sleep-waking schedule. III. ‘Gates' and ‘forbidden zones' for sleep. Electroencephalogr Clin Neurophysiol. 1986;63:414–25. doi: 10.1016/0013-4694(86)90123-9. [DOI] [PubMed] [Google Scholar]
- 15.Strogatz SH, Kronauer RE, Czeisler CA. Circadian pacemaker interferes with sleep onset at specific times each day: role in insomnia. Am J Physiol. 1987;253:R172–8. doi: 10.1152/ajpregu.1987.253.1.R172. [DOI] [PubMed] [Google Scholar]
- 16.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington, DC: US Government Printing Office; 1968. [Google Scholar]
- 17.Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 18.Gillberg M, Kecklund G, Akerstedt T. Relations between performance and subjective ratings of sleepiness during a night awake. Sleep. 1994;17:236–41. doi: 10.1093/sleep/17.3.236. [DOI] [PubMed] [Google Scholar]
- 19.Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16:143–9. [Google Scholar]
- 20.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 21.Shibata S, Cassone VM, Moore RY. Effects of melatonin on neuronal activity in the rat suprachiasmatic nucleus in vitro. Neurosci Lett. 1989;97:140–4. doi: 10.1016/0304-3940(89)90153-5. [DOI] [PubMed] [Google Scholar]
- 22.Liu C, Weaver DR, Jin X, et al. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997;19:91–102. doi: 10.1016/s0896-6273(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 23.Cohen DA, Wang W, Wyatt JK, et al. Uncovering residual effects of chronic sleep loss on human performance. Sci Transl Med. 2010;2:14ra3. doi: 10.1126/scitranslmed.3000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1:112–7. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 25.Wyatt JK, Cajochen C, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Low-dose repeated caffeine administration for circadian-phase-dependent performance degradation during extended wakefulness. Sleep. 2004;27:374–81. doi: 10.1093/sleep/27.3.374. [DOI] [PubMed] [Google Scholar]
- 26.Barger LK, Lockley SW, Rajaratnam SM, Landrigan CP. Neurobehavioral, health, and safety consequences associated with shift work in safety-sensitive professions. Curr Neurol Neurosci Rep. 2009;9:155–64. doi: 10.1007/s11910-009-0024-7. [DOI] [PubMed] [Google Scholar]
- 27.Workers on flexible and shift schedules in 2004 summary. 2005. cited; Available from: http://www.bls.gov/news.release/flex.nr0.htm.
- 28.Santhi N, Horowitz TS, Duffy JF, Czeisler CA. Acute sleep deprivation and circadian misalignment associated with transition onto the first night of work impairs visual selective attention. PLoS One. 2007;2:e1233. doi: 10.1371/journal.pone.0001233. [DOI] [PMC free article] [PubMed] [Google Scholar]