Abstract
Study Objectives:
To evaluate the effectiveness of ramelteon, a melatonin receptor agonist, for the treatment of insomnia in older adults starting auto-titrating positive airway pressure (APAP) therapy for sleep apnea.
Methods:
A parallel group, randomized, double-blind, placebo-controlled pilot effectiveness clinical trial. The study enrolled 21 research study participants who were ≥ 60 years old and had obstructive sleep apnea, defined by an apnea-hypopnea index (AHI) ≥ 5 events/h, with complaints of insomnia. The primary outcome measure was change in sleep onset latency determined from polysomnography at 4 weeks. Research study participants, all of whom were starting on APAP, were randomized to ramelteon 8 mg (n = 8) or placebo (n = 13).
Results:
Ramelteon treatment was associated with a statistically significant difference in sleep onset latency (SOL) as measured by polysomnography of 28.5 min (± 16.2 min) compared to placebo (95% C.I. 8.5 min to 48.6 min, effect size 1.35, p = 0.008). This was due to a 10.7 (± 17.0) min SOL reduction in the ramelteon arm and a 17.8 (± 23.5) min SOL increase in the placebo arm. No change was noted in subjective sleep onset latency (−1.3 min, ± 19.3 min, 95% C.I.: −21.4 min to 18.7 min). No statistically significant changes were noted in the AHI, sleep efficiency (polysomnography and self-report), APAP adherence, Pittsburgh Sleep Quality Index global score, or Epworth Sleepiness Scale score when comparing ramelteon vs. placebo. Four adverse events occurred in the ramelteon arm and 2 in the placebo arm; none were considered to be related to treatment.
Conclusions:
Ramelteon was effective in improving objective, but not subjective, sleep onset latency even in older adults who were starting APAP therapy for sleep apnea. Further research is warranted in examining the role of ramelteon in the care of older adults with insomnia symptoms and sleep apnea.
Citation:
Gooneratne NS; Gehrman P; Gurubhagavatula I; Al-Shehabi E; Marie E; Schwab R. Effectiveness of ramelteon for insomnia symptoms in older adults with obstructive sleep apnea: a randomized placebo-controlled pilot study. J Clin Sleep Med 2010;6(6):572-580.
Keywords: Insomnia, sleep apnea, APAP, ramelteon, aged, polysomnography
Older adults have prevalence rates of many sleep disorders that exceed that of younger populations. Sleep related breathing disorder, defined as an apnea-hypopnea index (AHI) ≥ 15 events/h, for example, was noted in approximately 20% of adults over the age of 65 in the Sleep Heart Health Study cohort.1 Insomnia symptoms are also very prevalent, and may occur in up to 15% to 30% of older adults.2 One area of particular concern that has been relatively understudied, however, is that of coexistent insomnia and sleep apnea in the elderly. This comorbid state may be especially common in older adults given the high prevalence rates of both conditions3: Approximately 29% to 61% of older adults with insomnia complaints have coexisting sleep apnea.4–8
The combination of sleep apnea and insomnia symptoms can create difficult clinical management challenges. Sedative-hypnotics may conceivably worsen sleep apnea,9,10 but this has not been a consistent finding.11–13 In addition, older subjects are more likely to have adverse effects from traditional benzodiazepine sedative-hypnotics.14 On the other hand, adherence with sleep apnea treatment may be lower in patients with untreated insomnia symptoms.15,16
BRIEF SUMMARY
Current Knowledge/Study Rationale: Patients with sleep apnea syndrome may have insomnia symptoms which can cause significant discomfort and influence treatment adherence. Little is known about potential pharmacotherapy options to address these insomnia symptoms.
Study Impact: In this pilot study, relative to placebo, ramelteon was associated with a statistically significant improvement in objective sleep onset latency, but no change in subjective sleep onset latency or adherence to auto-titrating positive airway pressure (APAP) therapy. Ramelteon did not have any significant adverse effects or influence the apnea-hypopnea index while on APAP therapy.
A limited number of studies have examined treatment options for patients with sleep apnea and insomnia symptoms. Cognitive-behavioral therapy may reduce insomnia symptoms in middle-aged adults with sleep apnea.17,18 Berry et al. noted that zolpidem reduced sleep latency relative to placebo for patients using continuous positive airway pressure (CPAP) in a single-night dosing paradigm.11 In addition, treatment of sleep apnea itself may improve insomnia symptoms when patients are compliant with CPAP therapy.18,19
Ramelteon (TAK-375), a melatonin receptor agonist, has been found to have minimal effects on sleep apnea severity.20 Thus, it may be an ideal choice for the treatment of insomnia symptoms in sleep apnea patients. Our specific study hypothesis was that ramelteon would improve sleep latency in older adults with insomnia and sleep apnea. In order to maximize the clinical relevance of the study, we conducted a pragmatic effectiveness trial in older adults newly diagnosed with sleep apnea, all of whom were starting auto-titrating positive airway pressure (APAP) therapy for their sleep apnea. We used a placebo-controlled, parallel-arm, randomized, double-blind clinical trial design with intent-to-treat analysis to test our hypothesis.
METHODS
Participants
Research study participants were recruited from the greater Philadelphia metropolitan area using media ads (radio, print), community flyers, presentations at senior centers, and announcements placed in primary care medical clinics. All study data was collected at the patients' homes, or at the Clinical and Translational Research Center of the University of Pennsylvania Clinical and Translational Science Award (CTSA). Overnight polysomnography studies were performed at the Clinical Research Center for Sleep of the Center for Sleep and Respiratory Neurobiology, also affiliated with the CTSA. Study participants were compensated financially for their participation in the research study protocol.
Inclusion criteria were age greater than 60 years, a diagnosis of sleep apnea syndrome with a minimum AHI of 5 events/h, and insomnia symptoms. Sleep apnea syndrome was defined by the criteria proposed by the International Classification of Sleep Disorders, 2nd edition.21 These included the presence of an AHI ≥ 5 events/h and symptoms of sleep apnea such as insomnia. A hypopnea was defined as a ≥ 10-sec episode with (1) > 50% decrease in airflow amplitude from baseline of any valid respiratory signal (flow or effort belts); (2) < 50% decrease in airflow amplitude preceding > 3% oxyhemoglobin desaturation; or (3) < 50% decrease in airflow amplitude preceding an arousal.22 An obstructive apnea was defined as > 90% decrease from baseline in amplitude of airflow lasting ≥ 10 sec. A central apnea was a ≥ 10-sec period with no fluctuations in airflow or rib cage and abdominal movement channels.
The following criteria were used for insomnia symptoms and were adapted from the Diagnostic and Statistical Manual-IV: (1) complaint of difficulty initiating or maintaining sleep ≥ 1 month; and (2) the sleep disturbance causes clinically significant distress or impairment.23 Potential subjects with insomnia due to caffeine use, inadequate sleep hygiene (TV disturbing sleep, etc.), major depression, or anxiety disorder were not eligible, as sedative-hypnotics are generally not indicated as initial therapy in these cases. Insomnia assessment was obtained during a medical history/exam by a board-certified sleep disorders medicine physician (performed by N.S.G.).
Exclusion criteria were as follows: (1) active use of sedative-hypnotics; (2) restless legs syndrome (RLS), because difficulty initiating sleep (insomnia) can be one of the hallmarks of RLS21; (3) periodic limb movement disorder (PLMD), as defined by a periodic limb movement with arousal index > 5 on polysomnography21; (4) alcohol abuse identified by the CAGE/Quantity Frequency Index, with subjects who were CAGE-positive and had consumed > 10 drinks during the week being excluded24; (5) cognitive impairment (dementia), as cognitive impairment would impair their ability to comprehend the study protocol and provide informed consent. The Clock Drawing Test was used as a rapid initial screen for cognitive impairment. If abnormal, potential subjects underwent a Mini-Mental Status Exam (MMSE), a widely used assessment for cognitive impairment.25 Subjects with scores < 24 were excluded from the study; (6) liver abnormalities, as defined by liver function test results more than twice the normal range; (7) prior history of CPAP use; (8) active use of fluvoxamine, which may interact with ramelteon; and (9) presence of severe emphysema (COPD).
Experimental Design
This pilot research study protocol utilized a parallel arm, randomized, placebo-controlled, double-blind, effectiveness trial design. Research study participants were randomized to either ramelteon 8 mg or identical-appearing placebo treatment for 4 weeks. Randomization was performed by the Investigational Drug Service of the University of Pennsylvania CTSA using a computer-generated central system and opaque, sequentially numbered sealed envelopes.
The research study protocol was approved by the University of Pennsylvania Institutional Review Board, underwent audits by an independent Data and Safety Monitoring Board, was submitted to the FDA (IND #75102), and was also audited yearly by the Office of Human Research at the University of Pennsylvania. All research study participants provided informed consent.
The research study protocol followed standard clinical practices for the treatment of sleep apnea: it included a patient education session, sleep physician history/exams (NSG), and a research study participant outpatient follow-up visit within 2 weeks of starting APAP.
Intervention
Study participants were randomized to receive oral ramelteon 8 mg or placebo daily, 30 min before their habitual bedtime. The placebo tablet was identical in appearance to the ramelteon tablet. All study research medications (ramelteon and placebo tablets) were provided by Takeda Pharmaceuticals North America, Inc. Study participants took ramelteon or placebo for 30 days, starting the day after their baseline (pre-study drug polysomnography) and continuing up to and including the night of their post-treatment polysomnography. A study drug log and pill count was used to confirm adherence to the study drug regimen.
Study Procedure
Sleep apnea education/counseling
All study participants, whether they were randomized to ramelteon or placebo, received APAP therapy for their sleep apnea using an autotitrating unit. Prior to starting APAP, they received a 45-min counseling session about sleep apnea that consisted of the following: (1) review of the etiology of sleep apnea, including a discussion of anatomical risk factors for sleep apnea and a sample patient video; (2) discussion of consequences of sleep apnea; (3) explanation of the use and benefits of APAP; (4) overview of the procedure for receiving and caring for their APAP equipment; and (5) a detailed review of their screening sleep study findings with a sleep disorders physician (N.S.G.).
Baseline measurements
Research study participants then completed the baseline questionnaires, and underwent a physician general history/physical and sleep history (N.S.G.). The sleep history included questions about their average sleep onset latency, total sleep time, and other sleep parameters during the prior week. They also had a baseline (pre-study drug treatment) polysomnography during which they had their first night of APAP. The purpose of this baseline polysomnography was to determine sleep metrics (sleep latency, sleep duration, etc.) and AHI while on APAP, but prior to randomization to the study intervention (ramelteon or placebo). The polysomnography used a 16-channel system, which included electroencephalogram, electrooculogram, electrocardiogram, snoring, chin and limb electromyogram, chest and abdominal respiratory belts, finger oximetry, and airflow monitoring with nasal and oral thermistors. Sleep records were manually scored in 30-sec epochs according to standard criteria.22,26 The APAP unit was a ResMed S8 AutoSet Vantage (ResMed Inc., Poway, CA). Study participants were then sent home with the APAP unit and the study medication (ramelteon or placebo). The APAP unit remained in the auto-titrating mode to help maximize the research study participants' tolerance of APAP.27 In cases where the APAP unit was not able to adequately treat the study participant's sleep apnea, they were then asked to return for a manual CPAP titration to determine the optimal CPAP setting. These study participants were then placed on that optimal CPAP setting instead of the auto-titration mode for the 4-week duration of the study.
Interval visit
After 2 weeks, research study participants returned for an interval visit during which they completed the study questionnaires, had their pill count assessed, had a physician history and physical examination (N.S.G.), and were screened for adverse events. Blood specimens were drawn for routine laboratory studies.
Post-treatment measurements
After 4 weeks of therapy, research study participants completed the study questionnaires, underwent a repeat physician history/physical, provided a blood specimen for routine labs, and had a final polysomnography. This polysomnography was performed while they were on study drug (ramelteon or placebo) and APAP.
Follow-up visit
Research study participants were evaluated again 4 weeks after the post-treatment visit and the cessation of study medication (a total of 8 weeks after randomization to study medication).
Study Assessments
Polysomnography
The primary study outcome measurement was the sleep onset latency derived from polysomnography. The sleep onset latency was defined as latency to stage 1 (N1) sleep. Baseline polysomnography was performed during the research study participants' first night of APAP, prior to starting research study drug treatment (ramelteon or placebo). The post-treatment polysomnography was performed one month later while on both the APAP and research study drug. Additional measures derived from the polysomnography included the sleep efficiency (time in bed divided by time asleep), the AHI, and the periodic limb movement index.21
Sleep
The Pittsburgh Sleep Quality Index (PSQI), which assesses sleep quality and is responsive to changes in insomnia,28,29 was used to provide a global measure of overall sleep quality. The Insomnia Severity Index (ISI) was used to assess the level of insomnia symptoms.30 Study participants were also asked to complete a 7-day at-home sleep diary; however, sleep diaries were properly completed by less than half of all study participants in both arms. We determined that inclusion of the sleep diary data was not justified because of this low response rate and self-report data was available from the PSQI.
Daytime functioning
The Epworth Sleepiness Scale (ESS), an 8-item self-administered scale that is used to measure daytime sleep propensity/sleepiness in a variety of standardized daily situations,31 provided information regarding level of sleepiness in common situations that are relevant to daily life.32 The Functional Outcomes of Sleepiness Scale (FOSQ), a 30-item scale that measures the effects of daytime sleepiness on a broad range of daily activities,33 provided a functional assessment of sleepiness. The RAND Medical Outcomes Study Short Form (SF-36) was used as a quality of life assessment tool because it is sensitive to changes in health status and has demonstrated validity among elderly subjects.34,35 The Cumulative Illness Rating Scale (CIRS) is a reliable, standardized instrument for rating of health status which was used to identify severity of comorbid illness in 13 different organ systems as well as provide a global measure of comorbidity.36 It was administered by a physician (N.S.G.) during the baseline assessment.
Statistical Analysis
Summary statistics including means, standard deviations, and ranges were used to describe changes from baseline for all primary and secondary outcome variables within each of the intervention groups. Intent-to-treat comparisons were used to preclude the possibility of bias due to selectively excluding subjects from the analyzed study groups. All randomized subjects, regardless of APAP or study drug compliance, were included in the primary effectiveness assessment of the changes in sleep efficiency. This technique allows for a more accurate reflection of treatment effects as it factors in compliance (effectiveness study). It also enhances the clinical relevance of the study findings.
Study comparisons between treatment arms were performed using generalized linear modeling (PROC GLM) due to the unbalanced study arms that resulted from random allocation to the study arms. All statistical analysis was performed using SAS v9.1 (SAS Institute, Cary, NC). Effect size estimates were derived using the Centre for Evaluation and Monitoring (Durham University) Effect Size Calculator.37
RESULTS
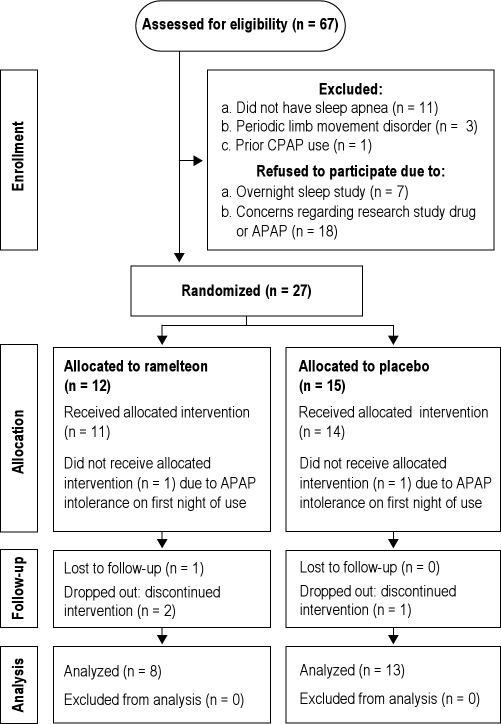
A total of 89 potential study participants attended informed consent sessions. Of these, 86 (96.6%) consented to participate in the research study and underwent eligibility assessment and screening. Complete screening data was gathered on 67 (75.3%) potential participants: Four were unreachable after the initial consent session, and 15 dropped out of the study before completing the screening process. Figure 1 contains a flow diagram of study recruitment, enrollment and analysis according to CONSORT guidelines.38 Five subjects did not tolerate CPAP after randomization and thus did not complete the on-treatment sleep study: Two subjects dropped out immediately after the pre-treatment sleep study (when they wore APAP for the first night, one subject in each arm—see “Allocation” section in Figure 1), and 3 later (identified in the “Follow-up” section in Figure 1 as “dropped-out: discontinued intervention”, 2 in the ramelteon arm and 1 in the placebo arm). Two subjects were also lost to follow-up and did not provide a reason (one in each arm). Table 1 provides demographic characteristics of all consented study participants and of participants in each study arm. The mean AHI prior to treatment (before starting APAP and the study drug intervention) was 35.8 (± 14.0) events/h for the ramelteon arm and 28.2 (± 25.8) events/h for the placebo arm (p = 0.6).
Figure 1.
CONSORT study diagram
Table 1.
Research study sample characteristics
| Parameter | Consented (n = 67) | Ramelteon (n = 8) | Placebo (n = 13) |
|---|---|---|---|
| Age, y; mean (SD) | 74.0 (5.6) | 73.6 (5.6) | 70.6 (3.5) |
| BMI, kg/m2; mean (SD) | 27.2 (5.2) | 25.7 (4.9) | 28.7 (5.8) |
| Gender, female (percent) | 37 (55.2) | 3 (37.5) | 5 (38.5) |
| Race (percent) | |||
| White | 48 (71.6) | 5 (62.5) | 10 (76.9) |
| Black | 18 (26.9) | 3 (37.5) | 3 (23.1) |
| Multiracial | 1 (1.5) | 0 | 0 |
| Refused | 0 | 0 | 0 |
| Ethnicity (percent) | |||
| Hispanic or Latino | 1 (1.5) | 0 | 1 (7.7) |
| Not Hispanic or Latino | 41 (61.2) | 4 (50.0) | 9 (69.2) |
| Refused | 25 (37.3) | 4 (50.0) | 3 (23.1) |
| Marital status (percent) | |||
| Married | 28 (41.8) | 5 (62.5) | 8 (61.5) |
| Single | 12 (18.0) | 1 (12.5) | 1 (7.7) |
| Separated/divorced | 8 (11.9) | 0 | 1 (7.7) |
| Widow | 18 (26.9) | 2 (25.0) | 2 (15.4) |
| Refused | 1 (1.5) | 0 | 1 (7.7) |
| Education (percent) | |||
| Junior high | 1 (1.5) | 0 | 0 |
| High school | 14 (20.9) | 3 (37.5) | 3 (23.1) |
| 2 y college | 15 (22.4) | 1 (12.5) | 2 (15.4) |
| 4 y college | 14 (20.9) | 2 (25.0) | 3 (23.1) |
| Graduate school | 19 (28.4) | 1 (12.5) | 4 (30.8) |
| Refused | 4 (6.0) | 1 (12.5) | 1 (7.7) |
| Current work (percent) | |||
| Full time | 5 (7.5) | 2 (25.0) | 1 (7.7) |
| Part time | 6 (9.0) | 0 | 2 (15.4) |
| Homemaker | 4 (6.0) | 1 (12.5) | 1 (7.7) |
| Unemployed, looking | 3 (4.5) | 0 | 1 (7.7) |
| Retired | 43 (64.2) | 4 (50.0) | 6 (46.2) |
| Unable to work | 3 (4.5) | 1 (12.5) | 1 (7.7) |
| Refused | 3 (4.5) | 0 | 1 (7.7) |
| Living situation (percent) | |||
| At home alone | 30 (44.8) | 3 (37.5) | 3 (23.1) |
| At home with spouse/children | 33 (49.3) | 5 (62.5) | 8 (61.5) |
| Assisted living/retirement community | 1 (1.5) | 0 | 0 |
| Refused | 3 (4.5) | 0 | 2 (15.4) |
The primary study outcome measure for the planned (a priori) analysis was objective sleep onset latency derived from polysomnography (Table 2). There was a statistically significant difference of 28.5 min (± 16.2 min, 95% C.I. 8.5 to 48.6 min, p = 0.008) in research study participants treated with ramelteon relative to placebo when comparing the baseline and post-treatment polysomnography. This was due to 2 factors: (1) a 10.7 (± 17.0) min decrease in the sleep onset latency in the ramelteon arm, and (2) a 17.8 (± 23.5) min increase in the sleep onset latency in the placebo arm. Additional planned analysis included the self-report sleep onset latency: no significant difference was noted in this measure between the 2 study arms (−1.3 min, ± 19.3 min, 95% C.I. −21.4 to 18.7 min, p = 0.9). Furthermore, neither objective nor subjective sleep efficiency differed significantly between study arms.
Table 2.
Polysomnography and self-report findings for key study outcomes
| Parameter | Ramelteon (SD), n = 8 |
Placebo (SD), n = 13 |
Mean Difference (SD, 95% CI | p-value | Effect size95% CI | ||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||||
| Polysomnography | |||||||
| Sleep latency (minutes) | 20.4 (23.2) | 9.7 (10.3) | 16.6 (17.5) | 34.4 (30.7) | 28.5 (16.2, 8.5 to 48.6) | 0.008 | 1.35 (0.32, 2.25)1 |
| Sleep efficiency (percent) | 72.5 (24.5) | 78.6 (10.5) | 70.3 (17.6) | 72.8 (16.9) | −3.6 (22.5, −24.7 to 17.6) | 0.7 | −0.16 (−1.04, 0.73)1 |
| AHI (events/h) | 13.9 (7.5) | 16.0 (11.6) | 10.9 (12.9) | 13.5 (12.1) | 0.5 (12.9, −11.6 to 12.6) | 0.9 | 0.04 (−0.84, 0.92)1 |
| Self-report | |||||||
| Sleep latency (minutes) | 35.2 (43.9) | 31.5 (24.0) | 23.8 (15.0) | 18.8 (11.0) | −1.3 (19.3, −21.4 to 18.7) | 0.9 | −0.07 (-0.94, 0.82)2 |
| Sleep efficiency (percent) | 72.5 (22.6) | 68.5 (24.1) | 71.6 (17.0) | 76.8 (14.9) | 9.2 (19.6, −9.6 to 27.9) | 0.3 | 0.5 (−0.44, 1.35)2 |
The Mean Difference column compares the pre-study drug to post-study drug change between the ramelteon and placebo arms. It is the difference between the change score for the ramelteon arm and the placebo arm. Mean Difference = ramelteon change (i.e., pre-ramelteon – post-ramelteon) – placebo change (i.e., pre-placebo – post-placebo). Effect size interpretation: The direction of the effect size estimate can represent an improvement or worsening in the ramelteon arm relative to the placebo arm depending upon the nature of the variable. To facilitate interpretation, a (1) indicates improvement in the ramelteon arm relative to the placebo arm, and a (2) indicates improvement in the placebo arm relative to the ramelteon arm.
Global perception of sleep quality was measured using the Pittsburgh Sleep Quality Index (PSQI). Insomnia severity was assessed using the Insomnia Severity Index (ISI). Both measures were unchanged between the study arms (Table 3). Sleep apnea severity was measured by the AHI derived both from polysomnography and the internal monitor on the APAP unit. Both measures showed no statistically significant difference between the study arms: (1) during the sleep study, the AHI (events/h) while wearing APAP for the ramelteon arm increased by 2.1 events/h versus the placebo arm increase of 2.6 events/h (p = 0.9) (Table 2); (2) the internal monitor on the APAP unit (collected only on days 22-29, thus there is no pre-treatment value for comparison) was 7.9 events/h (SD 7.6) for the ramelteon arm and 10.9 events/h (SD 13.1) for the placebo arm, with a difference of −3.0 events/h (SD 11.1, 95% C.I.: 15.0 to 9.0), and an effect size of −0.28 (95% C.I.: −1.25, 0.74), p = 0.6.
Table 3.
Subjective sleep quality and daytime outcomes
| Parameter | Ramelteon (SD), n = 8 |
Placebo (SD), n = 13 |
Mean Difference (SD, 95% CI | p-value | Effect size95% CI | ||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||||
| PSQI | 10.0 (3.9) | 10.9 (3.6) | 9.5 (4.1) | 9.2 (2.5) | −1.11 (3.41, −4.32 to 2.10) | 0.5 | −0.34 (−1.20, 0.57)2 |
| ISI | 13.3 (5.9) | 12.0 (5.5) | 13.6 (6.8) | 11.5 (5.4) | −0.9 (6.5, -7.0 to 5.2) | 0.8 | −0.14 (−1.01, 0.75)2 |
| FOSQ | 18.4 (1.2) | 18.8 (0.9) | 17.6 (1.8) | 18.1 (1.7) | 0.32 (1.33, −1.36 to 1.99) | 0.68 | 0.24 (−0.90, 1.34)1 |
| ESS | 9.8 (3.8) | 6.6 (3.6) | 10.4 (7.0) | 6.8 (4.5) | 0.1 (4.3, −4.9 to 5.1) | 0.97 | 0.03 (−1.10, 1.13)2 |
| SF-36 PCS** | 48.7 (13.5) | 47.6 (13.5) | 45.9 (11.4) | 46.3 (11.7) | 3.1 (3.1, −1.2 to 7.4) | 0.14 | 1.01 (−0.24, 2.11)2 |
| SF-36 MCS** | 53.6 (7.2) | 52.0 (11.2) | 52.6 (6.7) | 55.2 (7.8) | 5.1 (10.6, −9.5 to 19.6) | 0.45 | 0.46 (−0.70, 1.56)2 |
Sleep quality and insomnia measures: PSQI-Pittsburgh Sleep Quality Index, higher scores indicate worse sleep quality. ISI-Insomnia Severity Scale, higher scores indicate worse sleep quality. Daytime functional outcomes and quality of life measures: FOSQ-Functional Outcomes of Sleepiness Questionnaire, higher scores indicate increased levels of daytime functioning. ESS-Epworth Sleepiness Scale, higher scores indicate more daytime sleepiness. SF-36 PCS/MCS-Short-Form 36 Physical Component Score and Mental Component Scores, higher scores indicate improved quality of life. Effect size interpretation: The direction of the effect size estimate can represent an improvement or worsening in the ramelteon arm relative to the placebo arm depending upon the nature of the variable.
To facilitate interpretation, a (1) indicates improvement in the ramelteon arm relative to the placebo arm, and a (2) indicates improvement in the placebo arm relative to the ramelteon arm.
The SF-36 was completed by 7 subjects on ramelteon and 11 subjects on placebo
Daytime consequences of sleep apnea and insomnia treatment were also measured using both disease-specific measures (Functional Outcomes of Sleepiness Questionnaire and Epworth Sleepiness Scale) and a global quality of life measure (Short-Form 36). None of these measures showed statistically significant differences between the ramelteon and placebo groups (Table 3). APAP adherence was measured as the number of minutes of APAP use per night and did not differ significantly between the ramelteon and placebo groups (159.1 ± 117.0 min vs 226.9 ± 180.8 min, p = 0.4). APAP adherence (≥ 4 h of use for ≥ 4 nights per week) was 47.1% and was not influenced by study drug treatment. When including subjects who dropped out due to APAP intolerance (Figure 1), APAP adherence was 38.0%.
A total of 4 adverse events occurred in the ramelteon arm and 2 in the placebo arm. For ramelteon, the adverse events were as follows: gastrointestinal-diarrhea (1); dermatologic-skin ulcer (1); pulmonary-paranasal reaction (sinusitis) (1); and musculoskeletal-fracture after being hit by a bicyclist (1). For placebo, the adverse events were: pain-abdominal (1); and gastrointestinal-nausea (1). All adverse events were thought to be unrelated to study drug treatments, and none were serious adverse events.
DISCUSSION
This pilot study examined pharmacologic treatment options for insomnia symptoms in older adult patients starting CPAP therapy for sleep apnea syndrome. The effectiveness of ramelteon for improving sleep onset latency in older adults starting APAP was tested using a randomized, double-blind, placebo-controlled, parallel arm study design. We observed that in older adults initiating APAP therapy for their sleep apnea, ramelteon was effective in reducing polysomnography-measured sleep onset latency by 10.7 (± 17.0) min, which was a 28.5 min (± 16.2 min, p = 0.008) difference relative to placebo. Other polysomnography parameters did not change. Subjective measures, including sleep onset latency, sleep quality, sleepiness, and daytime functioning, were unchanged between the two arms. No adverse events were attributed to ramelteon in these older adult research participants, and AHI did not increase with the use of ramelteon.
Developing treatment options for patients presenting with coexistent sleep apnea and insomnia is important because this clinical scenario affects many older adults. Indeed, the combination of insomnia symptoms and sleep apnea may be more common in older adults than in younger patients. There are several potential reasons for this. First, even among healthy adults, advancing age is associated with reductions in sleep efficiency,39 usually due to increased wakefulness after sleep onset.40 While this may or may not be considered clinical insomnia, it may increase the potential risk that an older adult may develop clinically significant insomnia. Older adults also experience a reduced arousal threshold to auditory stimuli,41 circadian phase changes with aging,42 and comorbid medical conditions.43,44 The Sleep Heart Health Study also showed that the prevalence rate of sleep related breathing disorders, defined as an AHI ≥ 15 events/h, is approximately 20% in older subjects and is higher than that seen in younger age groups from this cohort.1 For these reasons, it is more likely that an older adult may present to a clinician with both sleep apnea and insomnia complaints. Using current US Census data and the above prevalence rate for sleep related breathing disorder (AHI > 15 events/h), along with an estimated prevalence of 29% for insomnia symptoms in patients with sleep related breathing disorder,45 it is estimated that nearly two million older adults may have sleep related breathing disorder and insomnia symptoms. However, it is worth noting that not all studies show a higher prevalence rate of clinically significant insomnia with age,2 and others have suggested that while sleep related breathing disorders may increase with age, sleep apnea syndrome itself may not.46
While it can be argued that the prevalence rate of coexistent sleep apnea and insomnia increases with age simply due to the rising prevalence rates of each condition independent of one another, sleep apnea itself may have insomnia as one of its symptom presentations.7,16 However, this has not been noted in all studies.45,47 It has also been suggested that patients with coexistent sleep apnea and insomnia are at greater risk for daytime consequences from their sleep disorders.15,45
Irrespective of the prevalence rate, the clinical management of older adult patients with coexistent insomnia and sleep apnea can be challenging for many reasons. In theory, the presence of insomnia may undermine adherence with sleep apnea therapy, since oral appliances and APAP may be associated with some level of physical discomfort that could worsen insomnia.16 Treatment with sedative-hypnotics may worsen sleep apnea in some cases because of reduced upper airway muscle tone or because of an increased arousal threshold that may lead to more prolonged apneas with associated oxyhemoglobin desaturations.9,10 Older adults, in particular, are especially susceptible to the risks of polypharmacy, which can result in falls.14 Thus, a clinician may be reluctant to start an older adult patient with sleep apnea and insomnia on sedative-hypnotics without some assurance that the patient will use their sleep apnea treatment regularly.
A non-pharmacologic treatment option that may be attempted is cognitive-behavioral therapy (CBT). Research studies using CBT for insomnia in young/middle-aged patients with sleep apnea have found beneficial results on sleep parameters.17,18 However, not all patients are amenable to using CBT for their insomnia, and a paucity of CBT practitioners continues to limit access to this service.
Sedatives that act via non-GABA pathways, such as ramelteon, a melatonin (MT) analogue that is a selective MT(1) and MT(2)-receptor agonist, may represent an alternative treatment option. Ramelteon does not increase sleep apnea severity in mild-moderate cases of sleep apnea.20 In addition, ramelteon has been well-tolerated in older adults,48,49 with relatively low risk of psychomotor impairments that may result in falls, a major concern with traditional benzodiazepine and non-benzodiazepine agents. While ramelteon has been found to improve objective sleep onset latency in insomnia patients, no research studies have been conducted to date to determine if this treatment benefit would also occur in older adult patients on CPAP therapy, a group for whom improvements in sleep onset latency may be attenuated by the physical discomfort of the CPAP therapy itself.
Our data showed that ramelteon was associated with a statistically significant difference of 28.5 minutes in sleep onset latency compared to placebo treatment. The effect size of this difference was large and clinically significant. While this improvement is potentially beneficial, there are several important caveats to consider. First, research study participants in the placebo arm had an overall worsening of their sleep onset latency of 17.8 minutes during the 4-week period of the study trial, and this, when compared to the 10.7-min improvement in the ramelteon arm, resulted in the 28.5-min difference between the two arms. Thus, the change associated with ramelteon was the result of both a benefit from ramelteon and a further deterioration in sleep onset latency in research study participants on placebo while both groups were habituating to APAP. It is possible that patients started on APAP therapy without any pharmacotherapy sleep aids had an overall worsening over time in their sleep latency due to discomfort from their APAP therapy. Second, while we noted a statistically significant difference in sleep onset latency as determined by polysomnography, there was no subjective difference in sleep onset latency, other subjective sleep parameters, or subjective measures of daytime functioning. It has been postulated that ramelteon, due to its non-GABA mechanism, is not associated with as prominent a perception of sedation/sleepiness as traditional sedative-hypnotics despite having objective benefits.
Other findings from this study include the observation that ramelteon was well-tolerated with no significant increase in study drug adverse effects relative to placebo. In addition, there was no increase in the AHI in patients on ramelteon. Of note, all study participants were on APAP, and this may have mitigated any potential worsening of the AHI. However, other research has shown that ramelteon had little effect on the underlying AHI in patients with mild to moderate sleep apnea.20
In general, we noted that APAP adherence was only approximately 50% in our study sample of older adults with insomnia symptoms and sleep apnea, and many potential study participants dropped out of the study during the initial baseline autotitrating CPAP polysomnogram because of CPAP intolerance, suggesting that actual CPAP adherence rates may have been closer to 40%. It is also interesting to compare this study with the findings of Lettieri and colleagues in their study of eszopiclone to improve adherence with CPAP therapy.50 They evaluated newly diagnosed sleep apnea patients aged 18-65 years, most of whom presumably presented with sleepiness complaints and did not identify subpopulations with insomnia, while our study participants were over age 65, were recruited with insomnia complaints, and then were screened for sleep apnea. Their study had a higher overall adherence rate (61.7%). This adherence rate may be attributed in part to the fact that our research study participants presented with primary complaints of insomnia, thus they found the prospect of using a CPAP unit disruptive of their already impaired sleep. While the education session emphasized the concept that treatment of sleep apnea may improve their insomnia symptoms, many study participants may have remained skeptical of this physiological link. The shorter duration of action of ramelteon and its primary effects on sleep onset latency may also be a factor; further research examining sleep maintenance effects of longer study treatment periods may be useful to assess if improvements in sleep latency ultimately lead to decreases in conditioned nocturnal arousal that may result in reduced wakefulness after sleep onset.
Our study has several limitations that warrant discussion. First, we elected to use APAP units which may not necessarily be used in a clinical practice setting. We chose to use these units in order to maximize adherence through their theoretical benefit of increased patient comfort. Second, our polysomnography paradigm called for a single night of overnight polysomnography as opposed to a dual-night approach, with one night being used for adaptation and the other for data analysis. We chose to use a single-night approach to minimize research study participant burden and maintain a high enrollment rate, thereby minimizing the risk of undermining study generalizability by self-selecting for a more enthusiastic and compliant cohort willing to undergo four polysomnography tests. It is possible that a single-night approach may have resulted in higher than average levels of sleep onset latency because of reduced opportunities for adaptation to polysomnography. Of note, though, the research study participants had previously undergone a diagnostic polysomnogram, thus proving an opportunity for some degree of habituation to the polysomnography experience. Third, while we attempted to model our study intervention on a typical clinical pattern, i.e., review of the patient's sleep study, an education session, and follow-up within a few weeks of starting APAP to address any concerns or issues, we understand that actual clinical practice may differ and result in different compliance rates and treatment benefits. This is a common problem in randomized controlled trials where the emphasis on subject retention results in high levels of subject contact/monitoring that may not be feasible in all clinical environments. Fourth, two subjects had very low sleep onset latency (one subject in the ramelteon arm and one in the placebo arm): they fell asleep during the PSG set-up process, thus creating an effective sleep onset latency of zero on two sleep studies in total (one for each subject). It is possible that this led to a slight underreporting of the treatment difference in sleep onset latency due to a floor effect (i.e., sleep onset latency cannot be improved beyond zero), and thus the actual sleep onset latency benefit of ramelteon treatment may be higher than we reported. Since we conducted an intent-to-treat analysis as per CONSORT guidelines, we are obligated to report all PSG results and not exclude these two subjects. Fifth, our study had unequal randomized allocation to the two study arms. Most likely this was a result of the small sample size. Sixth, our analysis methodology did not adjust for APAP adherence effects on sleep onset latency and other parameters. Again, this is because we used an intent-to-treat analysis, which requires that all randomized study participants with available data be included in the analysis without exclusion of certain subgroups. A study that excluded non-adherent PAP users would, in essence, be an efficacy study and thus less clinically relevant than the current effectiveness design we used. Furthermore, this type of analysis would represent a post hoc subgroup analysis that was not initially included in our study analysis plan. Future research specifically addressing this question may be warranted. Of note, all study participants used APAP during the baseline polysomnography night and the post-treatment polysomnography night, thus the improvement in the ramelteon arm in terms of sleep onset latency could not be attributed to non-use of APAP during the post-treatment polysomnography. Seventh, we had a low rate of completion of our at-home sleep diary data and relied on the PSQI self-report sleep times to provide these estimates. We are unable to explain the low rate of completion of the sleep diary except to note that anecdotally, completion of at-home self-report measures is often problematic. And finally, our definition of sleep onset latency was the onset of stage 1 sleep. Stage 1 sleep is scored less reliably than other sleep stages and this could contribute to measurement error, which is of some concern with a small sample size. To address this, we re-examined sleep onset latency using the first epoch of stage 2 sleep and noted findings that were consistent with our prior analysis.
In conclusion, this randomized, double-blind, placebo-controlled pilot trial examined the effectiveness of ramelteon to improve sleep parameters in older study participants starting APAP therapy. We noted that ramelteon resulted in a statistically significant difference in polysomnography-determined sleep onset latency relative to placebo of 28.5 minutes, a difference that was consistent with a large effect size and considered clinically meaningful. However, there was no change in subjective sleep onset latency, sleep quality, adherence, or daytime functioning parameters. These results suggest that ramelteon may improve sleep onset latency in this population, despite the disruptive effects of initiating APAP therapy. Future research in this area should examine additional ways to maximize compliance with APAP therapy in older adults, and identify subgroups of patients that are most likely to demonstrate benefit from specific interventions. This will provide clinicians with additional options to improve APAP tolerance and thereby reduce morbidity and mortality from untreated sleep apnea and insomnia.
DISCLOSURE STATEMENT
This study was supported by an investigator-initiated pilot grant from Takeda Pharmaceuticals North America, Inc. Takeda Pharmaceuticals had no role in the study design, research study participant enrollment, data collection, data storage, data interpretation, and manuscript preparation. They had no role in the decision to submit the manuscript for publication, nor did they edit the final manuscript. The authors have indicated no other financial conflicts of interest.
ACKNOWLEDGMENTS
The investigators would like to thank Ella Yost and Adrienne Juarascio for their assistance with this project. We would also like to thank Judi Miller of Allcare Medical, Inc., for assisting with the set-up of APAP adherence monitoring.
REFERENCES
- 1.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 2.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 3.Beneto A, Gomez-Siurana E, Rubio-Sanchez P. Comorbidity between sleep apnea and insomnia. Sleep Med Rev. 2009 doi: 10.1016/j.smrv.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Johansson P, Alehagen U, Svanborg E, Dahlstrom U, Brostrom A. Sleep disordered breathing in an elderly community-living population: Relationship to cardiac function, insomnia symptoms and daytime sleepiness. Sleep Med. 2009;10:1005–11. doi: 10.1016/j.sleep.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Lichstein KL, Riedel BW, Lester KW, Aguillard RN. Occult sleep apnea in a recruited sample of older adults with insomnia. J Consult Clin Psychol. 1999;67:405–10. doi: 10.1037//0022-006x.67.3.405. [DOI] [PubMed] [Google Scholar]
- 6.Krakow B, Melendrez D, Ferreira E, et al. Prevalence of insomnia symptoms in patients with sleep-disordered breathing. Chest. 2001;120:1923–9. doi: 10.1378/chest.120.6.1923. [DOI] [PubMed] [Google Scholar]
- 7.Chung KF. Relationships between insomnia and sleep-disordered breathing. Chest. 2003;123:310–1. doi: 10.1378/chest.123.1.310. author reply 311-3. [DOI] [PubMed] [Google Scholar]
- 8.Chung KF. Insomnia subtypes and their relationships to daytime sleepiness in patients with obstructive sleep apnea. Respiration. 2005;72:460–5. doi: 10.1159/000087668. [DOI] [PubMed] [Google Scholar]
- 9.Mendelson WB, Garnett D, Gillin JC. Flurazepam-induced sleep apnea syndrome in a patient with insomnia and mild sleep-related respiratory changes. J Nerv Ment Dis. 1981;169:261–4. doi: 10.1097/00005053-198104000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Berry RB, Kouchi K, Bower J, Prosise G, Light RW. Triazolam in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:450–4. doi: 10.1164/ajrccm.151.2.7842205. [DOI] [PubMed] [Google Scholar]
- 11.Berry RB, Patel PB. Effect of zolpidem on the efficacy of continuous positive airway pressure as treatment for obstructive sleep apnea. Sleep. 2006;29:1052–6. doi: 10.1093/sleep/29.8.1052. [DOI] [PubMed] [Google Scholar]
- 12.Sateia MJ, Hauri P, Kripke D, Roehrs T. Clinical safety of flurazepam and midazolam during 14-day use in chronic insomniacs. J Clin Psychopharmacol. 1990;10:28S–31S. [PubMed] [Google Scholar]
- 13.Camacho ME, Morin CM. The effect of temazepam on respiration in elderly insomniacs with mild sleep apnea. Sleep. 1995;18:644–5. doi: 10.1093/sleep/18.8.644. [DOI] [PubMed] [Google Scholar]
- 14.Neutel CI, Perry S, Maxwell C. Medication use and risk of falls. Pharmacoepidemiol Drug Saf. 2002;11:97–104. doi: 10.1002/pds.686. [DOI] [PubMed] [Google Scholar]
- 15.Smith S, Sullivan K, Hopkins W, Douglas J. Frequency of insomnia report in patients with obstructive sleep apnoea hypopnea syndrome (OSAHS) Sleep Med. 2004;5:449–56. doi: 10.1016/j.sleep.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Cherniack NS. Sleep apnea and insomnia: sleep apnea plus or sleep apnea minus. Respiration. 2005;72:458–9. doi: 10.1159/000087667. [DOI] [PubMed] [Google Scholar]
- 17.Guilleminault C, Davis K, Huynh NT. Prospective randomized study of patients with insomnia and mild sleep disordered breathing. Sleep. 2008;31:1527–33. doi: 10.1093/sleep/31.11.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krakow B, Melendrez D, Lee SA, Warner TD, Clark JO, Sklar D. Refractory insomnia and sleep-disordered breathing: a pilot study. Sleep Breath. 2004;8:15–29. doi: 10.1007/s11325-004-0015-5. [DOI] [PubMed] [Google Scholar]
- 19.Krakow B, Melendrez D, Sisley B, et al. Nasal dilator strip therapy for chronic sleep-maintenance insomnia and symptoms of sleep-disordered breathing: a randomized controlled trial. Sleep Breath. 2006;10:16–28. doi: 10.1007/s11325-005-0037-7. [DOI] [PubMed] [Google Scholar]
- 20.Kryger M, Wang-Weigand S, Roth T. Safety of ramelteon in individuals with mild to moderate obstructive sleep apnea. Sleep Breath. 2007;11:159–64. doi: 10.1007/s11325-006-0096-4. [DOI] [PubMed] [Google Scholar]
- 21.American Academy of Sleep Medicine. International classification of sleep disorders, 2nd ed.: Diagnostic and coding manual. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 22.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 23.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4th ed. Washington DC: American Psychiatric Association; 2000. [Google Scholar]
- 24.Buchsbaum DG, Buchanan RG, Welsh J, Centor RM, Schnoll SH. Screening for drinking disorders in the elderly using the CAGE questionnaire. J Am Geriatr Soc. 1992;40:662–5. doi: 10.1111/j.1532-5415.1992.tb01956.x. [DOI] [PubMed] [Google Scholar]
- 25.Folstein M, Folstein S, McHugh P. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Rechtschaffen A, Kales A. A manual of standardization terminology: techniques and scoring system for sleep stages of human subjects. Los Angeles: Brain Information Services/Brain Research Institute, University of California at Los Angeles; 1968. [Google Scholar]
- 27.Konermann M, Sanner BM, Vyleta M, et al. Use of conventional and self-adjusting nasal continuous positive airway pressure for treatment of severe obstructive sleep apnea syndrome: a comparative study. Chest. 1998;113:714–8. doi: 10.1378/chest.113.3.714. [DOI] [PubMed] [Google Scholar]
- 28.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 29.Dolberg OT, Hirschmann S, Grunhaus L. Melatonin for the treatment of sleep disturbances in major depressive disorder. Am J Psychiatry. 1998;155:1119–21. doi: 10.1176/ajp.155.8.1119. [DOI] [PubMed] [Google Scholar]
- 30.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 31.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 32.Chervin R, Aldrich M, Pickett R, Guilleminault C. Comparison of the results of the Epworth Sleepiness Scale and the Multiple Sleep Latency Test. J. Psychosomatic Res. 1997;42:145–55. doi: 10.1016/s0022-3999(96)00239-5. [DOI] [PubMed] [Google Scholar]
- 33.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- 34.Ware J, Sherborne C. The MOS-36 item short form health survey (SF-36): I. conceptual framework and item selection. Med Care. 1992;30:473–481. [PubMed] [Google Scholar]
- 35.Lyons RA, Perry HM, Littlepage BN. Evidence for the validity of the Short-form 36 Questionnaire (SF-36) in an elderly population. Age Ageing. 1994;23:182–4. doi: 10.1093/ageing/23.3.182. [DOI] [PubMed] [Google Scholar]
- 36.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–48. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 37.Effect Size Calculator [Computer program]. Centre for Evaluation and Monitoring, Durham University. 2006. Available at: http://www.cemcentre.org/renderpage.asp?linkID=30325017.
- 38.Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285:1987–91. doi: 10.1001/jama.285.15.1987. [DOI] [PubMed] [Google Scholar]
- 39.Vgontzas AN, Zoumakis M, Bixler EO, et al. Impaired nighttime sleep in healthy old versus young adults is associated with elevated plasma interleukin-6 and cortisol levels: physiologic and therapeutic implications. J Clin Endocrinol Metab. 2003;88:2087–95. doi: 10.1210/jc.2002-021176. [DOI] [PubMed] [Google Scholar]
- 40.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 41.Bonnet MH. The effect of sleep fragmentation on sleep and performance in younger and older subjects. Neurobiol Aging. 1989;10:21–5. doi: 10.1016/s0197-4580(89)80006-5. [DOI] [PubMed] [Google Scholar]
- 42.Youngstedt SD, Kripke DF, Elliott JA, Klauber MR. Circadian abnormalities in older adults. J Pineal Res. 2001;31:264–72. doi: 10.1034/j.1600-079x.2001.310311.x. [DOI] [PubMed] [Google Scholar]
- 43.Katz DA, McHorney CA. Clinical correlates of insomnia in patients with chronic illness. Arch Intern Med. 1998;158:1099–107. doi: 10.1001/archinte.158.10.1099. [DOI] [PubMed] [Google Scholar]
- 44.Avidan AY. Sleep in the geriatric patient population. Semin Neurol. 2005;25:52–63. doi: 10.1055/s-2005-867076. [DOI] [PubMed] [Google Scholar]
- 45.Gooneratne NS, Gehrman PR, Nkwuo JE, et al. Consequences of comorbid insomnia symptoms and sleep-related breathing disorder in elderly subjects. Arch Intern Med. 2006;166:1732–8. doi: 10.1001/archinte.166.16.1732. [DOI] [PubMed] [Google Scholar]
- 46.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–8. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 47.Unruh ML, Redline S, An MW, et al. Subjective and objective sleep quality and aging in the Sleep Heart Health Study. J Am Geriatr Soc. 2008;56:1218–1227. doi: 10.1111/j.1532-5415.2008.01755.x. [DOI] [PubMed] [Google Scholar]
- 48.Mini LJ, Wang-Weigand S, Zhang J. Self-reported efficacy and tolerability of ramelteon 8 mg in older adults experiencing severe sleep-onset difficulty. Am J Geriatr Pharmacother. 2007;5:177–84. doi: 10.1016/j.amjopharm.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Reynoldson JN, Elliott E, Sr, Nelson LA. Ramelteon: a novel approach in the treatment of insomnia. Ann Pharmacother. 2008;42:1262–71. doi: 10.1345/aph.1K676. [DOI] [PubMed] [Google Scholar]
- 50.Lettieri CJ, Shah AA, Holley AB, Kelly WF, Chang AS, Roop SA. Effects of a short course of eszopiclone on continuous positive airway pressure adherence: a randomized trial. Ann Intern Med. 2009;151:696–702. doi: 10.7326/0003-4819-151-10-200911170-00006. [DOI] [PubMed] [Google Scholar]