Abstract
Objective:
To further explore the effects of sodium oxybate (SXB) administration on nocturnal sleep in narcolepsy patients during a double-blind, placebo-controlled, parallel group study conducted with 228 adult patients with narcolepsy/cataplexy in the United States, Canada, and Europe.
Method:
Patients were withdrawn from antidepressants and sedative/hypnotics, and then randomized to receive 4.5, 6, or 9 g SXB or placebo nightly for 8 weeks. Patients receiving 6 and 9 g/night doses were titrated to their final dose in weekly 1.5 g increments, while patients receiving placebo were randomized to undergo a similar mock dose titration. The use of stimulant therapy continued unchanged. Changes in sleep architecture were measured using centrally scored nocturnal polysomnograms. Daily diaries were used to record changes in narcolepsy symptoms and adverse events.
Results:
Following 8 weeks of SXB treatment, study patients demonstrated significant dose-related increases in the duration of stage 3 and 4 sleep, reaching a median increase of 52.5 minutes in patients receiving 9 g nightly. Compared to placebo-treated patients, delta power was significantly increased in all dose groups. Stage 1 sleep and the frequency of nocturnal awakenings were each significantly decreased at the 6 and 9 g/night doses. The changes in nocturnal sleep coincided with significant decreases in the severity and frequency of narcolepsy symptoms.
Conclusions:
The nightly administration of SXB to narcolepsy patients significantly impacts measures of slow wave sleep, wake after sleep onset, awakenings, total sleep time, and stage 1 sleep in a dose-related manner. The frequency and severity of narcolepsy symptoms decreased with treatment.
Citation:
Black J; Pardi D; Hornfeldt CS; Inhaber N. The nightly use of sodium oxybate is associated with a reduction in nocturnal sleep disruption: a double-blind, placebo-controlled study in patients with narcolepsy. J Clin Sleep Med 2010;6(6):596-602.
Keywords: Narcolepsy, polysomnography, sodium oxybate, sleep architecture, delta power
The efficacy of nightly administered sodium oxybate (SXB) for the treatment of cataplexy and excessive daytime sleepiness (EDS) in patients with narcolepsy has been well established.1–5 While the mechanism whereby SXB diminishes the diurnal symptoms of narcolepsy is unknown, it has been observed that SXB also has pharmacodynamic effects on nocturnal sleep, which is frequently disrupted in patients with narcolepsy.6 Of particular interest, SXB has been shown to consistently increase the duration of stage 3 and 4 (slow wave or delta) sleep.7–10
An initial 10-week pilot study in 21 patients with narcolepsy tested the hypothesis that nightly SXB administration produces dose-related changes in sleep architecture. After withdrawing patients from antidepressants and sedative-hypnotics, the administration of SXB decreased nightly awakenings and increased the duration of sleep stages 3 and 4. In addition, delta power was significantly increased at all doses tested. The duration of REM sleep increased initially, and then decreased modestly over the duration of the 10-week trial.11 As the patients in this preliminary study were titrated from 4.5 to 9 g nightly, it was not possible to determine whether the effects of SXB were dependent upon the dose used, the 10-week duration of therapy, or a combination of both.
BRIEF SUMMARY
Current Knowledge/Study Rationale: The efficacy of sodium oxybate (SXB) for the treatment of cataplexy and excessive daytime sleepiness in patients with narcolepsy has been previously demonstrated in randomized controlled trials and may be due, in part, to SXB-related improvement in disrupted nocturnal sleep. The present study is the first, large, randomized, controlled, parallel group trial in patients with narcolepsy examining the impact of nightly administration of SXB on sleep architecture and narcolepsy symptoms.
Study Impact: The nocturnal administration of SXB to narcolepsy results in significant dose-related changes in sleep architecture resulting in decreased sleep disruption and increased slow wave sleep. These findings are consistent with improvement in measures of sleep continuity and suggest SXB may promote some amelioration of the sleep fragmentation that is common in narcolepsy.
The following double-blind, placebo-controlled, parallel group study, designed to assess the efficacy of SXB for the treatment of excessive daytime sleepiness in narcolepsy, permitted further examination of the effects of nightly SXB administration on sleep architecture. Specifically, changes in nocturnal polysomnography (PSG) parameters were measured, providing additional information on the effects of SXB on nocturnal sleep. Other measures of efficacy have been published elsewhere.1–5,12
METHODS
Subjects
Patients included in the trial were ≥ 16 years of age and met the following criteria: diagnosis of narcolepsy based on an overnight PSG and multiple sleep latency test (MSLT)13, and current symptoms of narcolepsy, including excessive daytime sleepiness, cataplexy, and recurrent sleep attacks for > 3 months (all patients met current ICSD-2 criteria for narcolepsy with cataplexy). Additional criteria included: willingness to forgo operating a car or heavy machinery if indicated by the investigator; and willingness to complete the entire trial as described in the protocol by signing an informed consent. Women of child-bearing potential agreed to use a medically accepted method of birth control, unless surgically sterile or 2 years post-menopausal.
The following criteria were used to exclude patients from the trial: use of SXB or investigational drug therapy within 30 days of trial entry; sleep apnea or any other cause of daytime sleepiness; use of hypnotics, anxiolytics, or any other sedating medications; any unstable disease that might place the patient at risk during the study or might compromise the study objectives; history of a substance abuse disorder; serum creatinine > 2.0 mg/dL, liver function tests more than twice the normal upper limit, serum bilirubin > 1.5 times the normal upper limit, or an ECG demonstrating clinically significant arrhythmias; history of myocardial infarction within 6 months; an occupation requiring changing shifts or routine night shifts; or history of seizure disorder, head trauma, or invasive intracranial surgery. Patients were also excluded from the study if the initial PSG study revealed the presence of moderate to severe sleep apnea syndrome, defined as an apnea index of > 10/h, or apnea/hypopnea index > 15/h, or any sleep disorder except narcolepsy.
Dosing and Administration of Study Drug
Trial medication consisted of a concentrated oral solution containing 500 mg/mL SXB; placebo consisted of a sodium citrate solution that was equimolar to the study drug with respect to sodium. Previous taste tests confirmed the placebo is indistinguishable from SXB solution (Jazz Pharmaceuticals, Inc., data on file). Study drug or placebo was administered in 2 equally divided doses each night. Patients participating in the trial were instructed to take the second dose of SXB 2.5 to 4 h following the first dose, when dosing the medication at home. However, during the in-lab PSG nights, the total PSG-recording duration was set at exactly 8 h and the 8-h night was split into 2 consecutive 4-h periods. SXB was dosed at the beginning of each 4-h period.
Overall, 78% of patients were taking CNS stimulants for the treatment of EDS; the dosage of these medications was held constant throughout the trial. A post hoc analysis revealed the use of stimulant medications was uniformly distributed across placebo and active drug groups (range 74.6% to 83.6%) (Jazz Pharmaceuticals, Inc., data on file). Patients were cautioned against the use of alcoholic beverages and potentially sedating medications such as opiate analgesics or skeletal muscle relaxants at any time during the trial and were required to discuss the use of all medicines with a study investigator.
Study Design
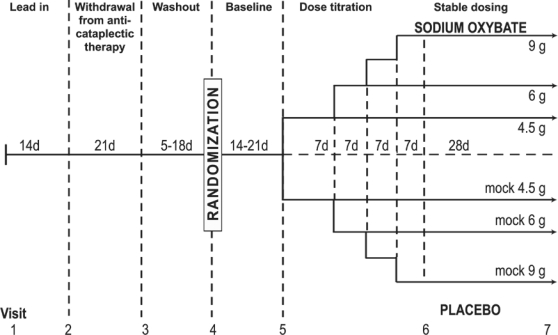
The study design, including visit number and frequency, is illustrated in Figure 1. Following clinic Visit 1, patients recorded narcolepsy symptoms and adverse events associated with current narcolepsy treatments in daily diaries during a 14-day lead-in period. Following clinic Visit 2, patients were gradually tapered from antidepressants or any other medication used for the treatment of cataplexy during a 21-day withdrawal period. This was followed by a washout period lasting 5 days or 5 times the half-life of the discontinued drug, whichever was longer, but not exceeding 18 days. Withdrawal from fluoxetine was initiated at clinic Visit 1 due to its very long half-life. If withdrawal from an antidepressant was not required, patients entered a mock 5-day washout period.
Figure 1.
Baseline cataplexy frequency was recorded during the 2-week lead-in phase. Antidepressant medications used for the treatment of cataplexy were withdrawn over a 21-day period following Visit 2 with the exception of fluoxetine, which was withdrawn beginning at Visit 1. The washout period lasted 5 days or 5 times the half-life of the withdrawn medication, whichever was longer, but did not exceed 18 days. Following randomization, patients were started on placebo in single-blind fashion and recorded baseline cataplexy occurrences over a 14-day period; however, this was extended to 21 days if the investigator felt cataplexy had not yet stabilized. Patients then received study medication or placebo and were titrated to their final dose, as shown. Polysomnography, the maintenance of wakefulness test, and assessment of narcolepsy symptoms were performed at Visits 2, 5, 6, and 7.
Following the washout period, patients entered a 14-day baseline period and received placebo in single-blind fashion. During this phase of the trial, patients were acclimated to the use of daily diaries, and baseline assessments of narcolepsy symptoms were recorded. The 14-day baseline period was extended to 21 days if, in the opinion of the investigator, the frequency of cataplexy attacks had not stabilized. To remain eligible for the double-blind phase of the trial, each patient was required to record a minimum average of 8 cataplexy attacks per week during the baseline period. Patients were kept unaware of this requirement. To be classified as cataplexy for this trial, the event must have had sudden onset, been localized to a specific muscle group(s) or part of the body in a bilateral manner, and occurred while the patient was lucid (i.e., not experiencing a sleep attack or microsleep).
The dose-titration phase (Visit 5) was conducted in randomized, double-blind fashion as follows:
Week 1: One-fourth of the study patients received placebo, while three-fourths received 4.5 g SXB nightly.
Week 2: One-third of patients receiving SXB remained at the 4.5 g/night dose, while two-thirds increased their dose to 6 g nightly; two-thirds of placebo patients increased the volume of their placebo dose by an equivalent amount to match the 6 g/night dose.
Week 3: One-half of patients taking SXB at the 6 g/night dose remained at this dose, while the remaining half increased their dose to 7.5 g nightly; one-half of the placebo patients taking the mock 6 g/night dose continued taking the same volume, while the remaining half increased their volume of their placebo dose to match the 7.5 g/night dose.
Week 4: All patients taking the 7.5 g/night SXB dose increased their dose to 9 g nightly, while the placebo patients taking the mock 7.5 g/night dose increased the volume of their dose by an equivalent amount.
Each subject returned to the clinic at Visit 6 when study measures for efficacy were conducted and safety assessments were made. Patients then continued at their assigned dose for the remaining 28 days of the study before returning for the final efficacy and safety assessments at Visit 7. PSG and MWT were performed at Visits 2, 5, 6, and 7; and awakenings, defined as the average number of awakenings during the night per week based on diary data from the 2 weeks immediately preceding the visit, were recorded.
Polysomnographic Recordings
The PSG data were obtained as described elsewhere11 using previously described techniques.14 Briefly, data from electroencephalographic (EEG) and other parameters, including eye movements (EOG), submentalis muscle tone (chin-EMG), electrocardiogram (ECG), and right and left anterior tibialis muscle activity (leg-EMG) as well as nasal air flow, thoracic and abdominal effort, and oxygen saturation, were digitally recorded and manually scored by trained registered polysomnographers in a blind manner using a validated software program.
The following variables were measured during each half of the night, corresponding with the first and second doses of SXB, and were subsequently added together for the entire night: sleep latency; total sleep time (TST); wake after sleep onset (WASO); duration of stages 1, 2, 3 and 4, and REM sleep; sleep stage shifts per hour; and nocturnal awakenings. REM density was defined as the percent of 2-sec REM epochs containing one or more rapid eye movements; REM sleep latency was examined for the first half of the night only; delta power was defined as the accumulated EEG signal power for all frequencies between and including 0.5 Hz and 4 Hz.
Data Analysis
All computations were performed using the Statistical Analysis System (SAS; SAS Institute Inc., Cary, NC) and were performed on an intent-to-treat basis using patients who received trial drug and completed at least one post-treatment evaluation visit. All analyses were based on the change from baseline to endpoint. Baseline was defined as the PSG measures at Visit 5. Endpoint was defined as the PSG measures at Visit 7, or in the absence of Visit 7 data, Visit 6. The test for a normal distribution of data was performed using the Wilks-Shapiro test, and homogeneity of the variability was examined graphically. If the data were found to be normal and homogeneous in distribution, an analysis of variance (ANOVA) model was used to analyze the data. Factors in the ANOVA model included treatment group, trial site, and the interaction between treatment and trial site. If the interaction was not found to be statistically significant (p ≥ 0.10) the term was dropped from the model. If a significant interaction effect was found, the nature of the interactions and the impact on the study conclusion was assessed. If a significant difference between treatment groups was found, each of the treatment groups was compared to placebo using the Dunnett test. If the data were not normally distributed, nonparametric tests were used. The Kruskal-Wallis test was used to assess the differences between treatment groups in the change from baseline. The Wilcoxon signed rank test was used to assess the change from baseline within treatment groups, and the Mann-Whitney test was used for pairwise comparisons of active treatment versus placebo. Interpretation and generalization of the study results in this multi-center study were assessed by examining the treatment by trial site interactions. In the data analysis involving ANOVA models, treatment by trial site interaction was tested and if the interaction was significant, the impact on the study results was evaluated. This interaction was modeled as a fixed effect, although modeling as a random effect may further increase the ability to generalize these results. The dose-response relationship in the PSG measures was assessed by testing the slope of simple linear regression model of individual PSG measure and dose, using data from the 3 active treatment groups. Statistical significance was accepted if the adjusted p-value was < 0.05. The significance of the mean change from baseline for each treatment group was determined using a paired t-test.
Ethics
This trial was conducted at 42 sites between November 2000 and March 2004 in the United States, Canada, United Kingdom, Germany, France, Switzerland, Netherlands, and the Czech Republic, and was approved by the institutional review board/ethics committee of each participating trial center. Written informed consent was obtained from each patient prior to initiation of the study. This study was conducted in accordance with the ethical principles delineated in the Helsinki Declaration of 1975, as revised in 1997.
RESULTS
Of 401 patients who signed informed consent and entered the screening phase, 353 were enrolled, 285 were randomized to treatment, 246 received at least one dose of study drug, and 228 entered the double-blind phase of the trial. The intent-to-treat (ITT) population included these 228 patients who received at least one dose of study drug and had baseline PSG efficacy data and week 4 (Visit 6) and/or week 8 (Visit 7) PSG data. Of the patients randomized to treatment (285), 78% were taking stimulants, 14.7% (42/285) were taking tricyclic antidepressants (TCAs), and 14% (40/285) were taking serotonin selective reuptake inhibitors (SSRIs).
Of the ITT population, 149 (65.4%) were female and 79 (34.6%) were male. The average age was 40.5 years (range 16–75); the average height was 168.1 cm (range 139.7–202.0); the average weight was 85.7 kg (range 46.3–170.6). A total of 196 patients were Caucasian, 25 were of African descent, 2 were Asian, 2 were Hispanic, and 2 were of other ethnic origins. An analysis across all treatment groups indicated that patients were evenly distributed with respect to the above demographic parameters. The study was completed by 206 patients and polysomnographic data were available at weeks 4 and 8 for 191 and 193 patients, respectively. The duration of REM sleep and NREM sleep were found to be normally distributed and were analyzed with ANOVA models. For the other variables, nonparametric methods were used.
Effect of Sodium Oxybate on Nocturnal Polysomnography Variables
Sleep Latency and Total Sleep Time
Sleep latency was not significantly different among the treatment groups after the initial dose or after the 2nd dose (data not shown). TST was increased after 8 weeks of treatment, reaching significance at the 9 g/night dose (Table 2), but no change was seen at 4 weeks (Table 1). At 8 weeks, there was a significant relationship between dose and increased TST (p = 0.0127)
Table 1.
Changes in polysomnographic parameters following 4 weeks of treatment*†(change from baseline; median)
| Placebo N = 48 | SXB 4.5 g N = 59 | SXB 6 g N = 49 | SXB 9 g N = 35 | |
|---|---|---|---|---|
| Total Sleep Time (min) | ||||
| 1st Half | 1.50 | 1.75 | −0.25 | −1.50 |
| 2nd Half | 0.00 | 0.00 | 9.25 | 9.00 |
| TOTAL | 1.75 | 1.00 | 9.25 | 7.00 |
| — | NS | NS | NS | |
| Total NREM Sleep (min) | ||||
| 1st Half | 9.25 | 6.75 | 0.50 | 8.50 |
| 2nd Half | −0.50 | 10.00 | 15.50 | 38.50 |
| TOTAL | 4.25 | 16.25 | 13.00 | 41.50 |
| — | NS | NS | p = 0.001 | |
| Total REM Sleep (min) | ||||
| 1st Half | −5.25 | −0.25 | −3.50 | −9.50 |
| 2nd Half | −1.75 | 1.00 | −5.50 | −19.00 |
| TOTAL | −8.50 | −0.50 | −8.00 | −22.00 |
| — | NS | NS | p = 0.010 | |
| REM Sleep Latency (min) | ||||
| −0.75 | −3.25 | −9.00 | 1.00 | |
| — | NS | NS | NS | |
| Stage 1 Sleep (min) | ||||
| 1st Half | −1.50 | −6.75 | −6.00 | −9.00 |
| 2nd Half | 0.00 | −7.50 | −13.00 | −12.50 |
| TOTAL | −0.50 | −11.75 | −17.00 | −22.50 |
| — | p = 0.002 | p = 0.002 | p < 0.001 | |
| Stage 2 Sleep (min) | ||||
| 1st Half | 0.00 | 9.00 | −1.00 | 3.00 |
| 2nd Half | 1.50 | 11.75 | 7.00 | 6.00 |
| TOTAL | −0.25 | 11.00 | 4.25 | 11.00 |
| — | NS | NS | NS | |
| Stage 3 and 4 Sleep (min) | ||||
| 1st Half | 0.00 | 1.00 | 9.75 | 27.50 |
| 2nd Half | 0.00 | 0.50 | 5.75 | 34.00 |
| TOTAL | 0.00 | 3.25 | 19.25 | 71.00 |
| — | NS | p = 0.002 | p < 0.001 | |
| Wake After Sleep Onset (min) | ||||
| 1st Half | −2.50 | −3.75 | −2.50 | −0.50 |
| 2nd Half | 1.25 | 2.25 | −8.50 | −8.00 |
| TOTAL | −3.75 | −0.25 | −13.75 | −7.50 |
| — | NS | NS | NS | |
| Sleep Stage Shifts Per Hour | ||||
| 1st Half | 0.07 | −1.32 | 1.59 | −4.08 |
| 2nd Half | −3.13 | −1.67 | −2.64 | −1.05 |
| AVERAGE | −0.59 | −0.55 | −0.20 | −1.86 |
| — | NS | NS | NS | |
| Delta Power (microvolts^2/Hz) | ||||
| 1st Half | −3720.20 | 5320.09 | 14868.03 | 26498.77 |
| 2nd Half | 1142.55 | 7889.88 | 17058.46 | 28035.86 |
| AVERAGE | −782.46 | 4842.03 | 14812.20 | 29629.76 |
| — | p = 0.026 | p < 0.001 | p < 0.001 | |
| Nocturnal Awakenings | ||||
| 1st Half | 0.00 | −3.50 | −3.00 | −4.00 |
| 2nd Half | −1.50 | −6.00 | −6.00 | −9.00 |
| TOTAL | −1.00 | −7.50 | −10.50 | −15.00 |
| — | p = 0.011 | p = 0.011 | p = 0.015 |
Statistical significance was established compared to placebo. NS = not significant.
Expressed as medians following transformation of non-normal data.
Awakenings and Wake after Sleep Onset
The number of nocturnal awakenings was significantly decreased in all dose groups at 4 weeks (Table 1) and in the 6 g/night and 9 g/night groups at 8 weeks (Table 2). There was a significant relationship between increased dose and decreased number of awakenings at 8 weeks (p = 0.0444). WASO was significantly decreased in the 9 g/night group at 8 weeks (Table 2). There was a significant dose relationship for the decrease in WASO at 8 weeks as well (p = 0.0075). Sleep stage shifts per hour was not different between the treatment groups at either time point.
Table 2.
Changes in polysomnographic parameters following 8 weeks of treatment*†(change from baseline; median)
| Placebo N = 50 | SXB 4.5 g N = 59 | SXB 6 g N = 49 | SXB 9 g N = 35 | |
|---|---|---|---|---|
| Total Sleep Time (min) | ||||
| 1st Half | 0.50 | −0.50 | 2.00 | 3.00 |
| 2nd Half | −1.75 | 5.00 | 4.50 | 10.50 |
| TOTAL | 0.25 | 0.00 | 13.00 | 18.00 |
| — | NS | NS | p = 0.049 | |
| Total NREM Sleep (min) | ||||
| 1st Half | −2.75 | −1.50 | 4.00 | 15.50 |
| 2nd Half | 1.75 | 15.00 | 17.00 | 40.50 |
| TOTAL | 2.00 | 10.00 | 24.00 | 51.50 |
| — | NS | p = 0.010 | p < 0.001 | |
| Total REM Sleep (min) | ||||
| 1st Half | −0.75 | −1.00 | −3.50 | −5.50 |
| 2nd Half | 2.00 | −5.00 | −4.50 | −17.00 |
| TOTAL | −1.00 | −6.00 | −7.00 | −22.00 |
| — | NS | NS | p = 0.026 | |
| REM Sleep Latency (min) | ||||
| 0.00 | −1.00 | −1.50 | 6.00 | |
| — | p = 0.521 | p = 0.203 | p = 0.547 | |
| Stage 1 Sleep (min) | ||||
| 1st Half | −2.75 | −3.00 | −5.00 | −7.50 |
| 2nd Half | 1.25 | −1.50 | −9.50 | −14.00 |
| TOTAL | −2.25 | −9.50 | −13.50 | −22.50 |
| — | p = 0.086 | p < 0.001 | p < 0.001 | |
| Stage 2 Sleep (min) | ||||
| 1st Half | 1.50 | −1.00 | −5.00 | −4.50 |
| 2nd Half | 0.25 | 12.00 | 13.50 | 24.00 |
| TOTAL | 3.50 | 9.50 | 13.00 | 31.50 |
| — | NS | NS | NS | |
| Stage 3 and 4 Sleep (min) | ||||
| 1st Half | −0.25 | 1.00 | 10.00 | 20.50 |
| 2nd Half | 0.00 | 0.50 | 4.50 | 26.50 |
| TOTAL | 0.00 | 3.00 | 21.00 | 52.50 |
| — | p = | p < 0.001 | p < 0.001 | |
| Wake After Sleep Onset (min) | ||||
| 1st Half | −0.50 | 0.25 | −0.25 | −1.00 |
| 2nd Half | −2.00 | −1.50 | −2.75 | −9.50 |
| TOTAL | 2.00 | −5.75 | −3.75 | −22.00 |
| — | NS | NS | p = 0.052 | |
| Sleep Stage Shifts Per Hour | ||||
| 1st Half | −1.82 | −1.51 | −1.18 | −1.91 |
| 2nd Half | −0.88 | −0.45 | −0.85 | 0.00 |
| AVERAGE | −1.03 | −1.36 | −1.11 | −0.68 |
| — | NS | NS | NS | |
| Delta Power (microvolts^2/Hz) | ||||
| 1st Half | 85.42 | 8413.11 | 14624.92 | 25164.43 |
| 2nd Half | −3538.83 | 7725.27 | 19726.61 | 32951.43 |
| AVERAGE | −1102.98 | 7166.20 | 14736.32 | 28796.65 |
| — | p = 0.006 | p < 0.001 | p < 0.001 | |
| Nocturnal Awakenings | ||||
| 1st Half | −1.50 | −2.00 | −3.00 | −3.00 |
| 2nd Half | −0.00 | −3.00 | −5.00 | −9.00 |
| TOTAL | −0.50 | −5.00 | −8.00 | −12.00 |
| — | NS | p = 0.005 | p = 0.009 |
Statistical significance was established compared to placebo. NS = not significant.
Expressed as medians following transformation of non-normal data.
Stage 1 and 2 Sleep
The duration of Stage 1 sleep was significantly decreased in all SXB treatment groups at 4 weeks (Table 1) and in the 6 g/night and 9 g/night groups at 8 weeks of treatment (Table 2). There was a significant dose relationship for the decrease in stage 1 sleep (p = 0.0355). The duration of stage 2 sleep was not significantly different among the treatment groups at either 4 or 8 weeks.
Stage 3 and 4 Sleep
The duration of stage 3 and 4 sleep was significantly increased for the 6 g/night and 9 g/night groups at 4 weeks (Table 1) and for all 3 SXB treatment groups at 8 weeks (Table 2). As shown in Tables 1 and 2, substantial increases occurred during both halves of the night. This increase in stage 3 and 4 sleep was significantly dose-related at both 4 weeks (p < 0.0001) and 8 weeks (p < 0.0001).
Delta Power
Median delta power was significantly increased in all SXB treatment groups at both 4 weeks (Table 1) and 8 weeks (Table 2). These increases were proportionately greater during the second half of the night (Tables 1 and 2). A significant dose relationship was not observed (p = 0.1353 at 4 weeks and p = 0.1323 at 8 weeks) due to high variability.
REM Sleep Latency and Duration
Sodium oxybate administration had no appreciable effect on REM sleep. The duration of REM sleep was significantly decreased in the 9 g/night group at 4 weeks (Table 1) and 8 weeks (Table 2).
Effect of Sodium Oxybate on the Symptoms of Narcolepsy
Data reported elsewhere4,5 indicate that the nightly administration of 4.5, 6, and 9 g/night doses of SXB resulted in significant decreases in median weekly cataplexy attacks. Patients also experienced significant improvements in both subjective and objective measures of excessive daytime sleepiness (Epworth Sleepiness Scale; 40-min maintenance of wakefulness test) and quality of life,12 as well as significant improvements in the clinical investigator-rated evaluation of disease severity.
Safety
Twenty-one patients discontinued the trial due to an adverse event, with most occurring in the 9 g/night dose group; 15 unique events occurred overall with a frequency significantly greater than placebo. Nausea, headache, dizziness, nasopharyngitis, and enuresis occurred with an overall incidence greater than 5%. Of these, only nausea and dizziness reached a level of statistical significance compared with placebo. These appeared to be dose related and occurred in 34/186 (18.3%) and 31/186 (16.7%) of the SXB-treated subjects. There were no deaths.
Six serious adverse events were reported during the study. Three of these occurred in patients receiving placebo. Of the remaining 3 events, an episode of pneumonitis was reported in one patient receiving 6 g of SXB and was reported to be unrelated to drug. Another patient receiving 9 g of SXB suffered a fractured ankle following an accidental fall during the night. In this case, the relationship to study medication was reported as unknown. The third patient receiving 4.5 g of SXB demonstrated abnormal amino alanine transferase (ALT) and aspartate amino transferase (AST) at the conclusion of the trial, which returned to normal approximately 8 months later. This event was reported to be possibly due to study medication. These events are described in greater detail in another report.5
DISCUSSION
One of the earliest reports describing the use of sodium oxybate (also known as γ-hydroxybutyrate sodium; GHB sodium) for the treatment of narcolepsy indicated that doses of 50 mg/kg (approximately 3.75–6.25 g nightly) significantly increased the duration of nocturnal slow wave sleep at the expense of stage 1 sleep and generally improved the continuity of nocturnal sleep.8 These changes in nocturnal sleep coincided with patient reports of improvements in the quality of sleep, diminished daytime drowsiness and decreased cataplexy.15
Subsequent investigations into the use of SXB have yielded similar results on nocturnal sleep in patients with narcolepsy.9–11,16,17 Collectively, these studies demonstrate that the nightly administration of SXB decreases nocturnal awakenings and increases slow wave sleep in a dose-dependent fashion, as well as imparting effects on REM sleep. These studies also demonstrate that the changes in nighttime sleep are accompanied by improvements in other clinical manifestations of narcolepsy, including cataplexy, subjective and objective measures of excessive daytime sleepiness, hypnagogic hallucinations, and sleep paralysis.
The present study is the largest controlled study to compare multiple doses of SXB to placebo performed to date, permitting a more thorough evaluation of impact on nighttime sleep and narcolepsy symptoms following the nightly administration of SXB in patients with narcolepsy. As predicted by a previous open-label pilot study,11 the nocturnal administration of SXB produced significant dose-related increases in both slow wave sleep and total sleep time. Robust increases in stage 3 and 4 sleep and delta power occurred in association with corresponding decreases in stage 1 sleep, REM sleep, and number of nocturnal awakenings, while stage 2 sleep remained unaffected. These findings are consistent with previously published SXB studies.9,10,16,17
Whether the observed impact of SXB on stages 3 and 4 sleep and on delta power represents a true sleep effect, delta-wave effects similar to those seen with CNS anesthetic agents, or an epiphenomenon unrelated to either sleep or anesthesia is unknown. With the data available, it is difficult to evaluate this question as it is currently not possible to investigate this issue meaningfully through characterization of EEG spectral features alone. One may hypothesize that an altered pattern of increased delta power activity may suggest a pharmacological effect that is not representative of sleep. Yet, such a conclusion may be inaccurate as this variant pattern of EEG activity may reflect an alteration of some aspects of sleep but not others, or an impact on all aspects of sleep, but weighted in a fashion distinct from normal physiological sleep. Taken together, the observed impacts of SXB on sleep, coupled with the observed improvements in daytime symptoms, are consistent with the hypothesis that sodium oxybate enhances SWS processes. Further work to better characterize the nature of the increased delta activity may be of interest.
Similar to the initial pilot study,11 changes in sleep architecture following the nightly administration of SXB in the present study coincided with significant improvements in narcolepsy symptoms, including significant dose-related reductions in median number of weekly cataplexy attacks; a significant dose-related improvement in EDS, as measured with the Epworth Sleepiness Scale, the maintenance of wakefulness test, and incidence of inadvertent naps; and significant dose-related improvements in the CGI-c. These results are reported elsewhere.5 Although some of the changes in sleep architecture reported here are significant only at the highest dose, the relationship between sleep architecture and subjective outcomes remains unclear and warrants further study.
A shortcoming of the initial pilot study was the lack of a control for the dose and duration of SXB therapy.11 An open-label 12-month extension study of SXB in the treatment of narcolepsy demonstrated sustained improvement in cataplexy and EDS.2 The present study was not designed to detect differences in sleep architecture variables at different time points with steady doses of SXB. These matters may merit further study. Although the greatest percentage of changes occurred within the initial 4-week treatment period, some parameters of sleep were further impacted during the subsequent 4 weeks of stable-dose treatment including total sleep time, total NREM sleep, SWS, and number of awakenings. The further changes from week 4 to week 8 may suggest a time-on-drug effect for these parameters, but this cannot be clarified with these data.
CONCLUSION
The nocturnal administration of SXB to narcolepsy patients in two equally divided doses results in significant dose-related changes in sleep architecture, including an increase in slow wave sleep and TST and a decrease in stage 1 sleep, wake after sleep onset, and number of awakenings. These findings are consistent with improvement in measures of sleep continuity and suggest SXB may promote some amelioration of the sleep fragmentation that is common in narcolepsy. At SXB doses of 4.5 g, 6 g, and 9 g/night, dose-related improvements in cataplexy and overall change in severity of patient's disease state and at doses of 6 g and 9 g/night, decreases in excessive daytime sleepiness were noted as previously reported.4,5
DISCLOSURE STATEMENT
This study was sponsored by Jazz Pharmaceuticals, Inc., Palo Alto, CA and conducted by the members of the Xyrem International Study Group (see Acknowledgments for names of the group). Dr. Black is an employee of Actelion Pharmaceuticals, LTD and has received research support from Cephalon, GlaxoSmithKline, Jazz Pharmaceuticals, Merck, and Takeda, and honoraria from Boehringer-Ingelheim, Takeda, and UCB. Dr. Hornfeldt is a paid consultant to Jazz Pharmaceuticals. Dr. Inhaber and Mr. Pardi are former employees of Jazz Pharmaceuticals and own shares of stock.
ACKNOWLEDGMENTS
This study was sponsored by Jazz Pharmaceuticals, Inc., Palo Alto, CA and conducted by the members of the Xyrem International Study Group who are: Mansoor Ahmed, MD, Cleveland Sleep Center, Middlebrook Heights, OH; Philip Becker, MD, Sleep Medicine Association of Texas, Plano, TX; Michael Biber, MD, Center for Sleep Diagnostics, Newton, MA; Jed Black, MD, Stanford Sleep Disorder Clinic, Stanford, CA; Richard Bogan, MD, Palmetto Baptist Medical Center, Columbia, SC; Andrew Chesson Jr, MD, Louisiana State University Sleep Disorders Center, Shreveport, LA; James Cook, MD, The Center for Sleep & Wake Disorders, Danville, IN; Stephen Duntley, MD, Washington University Medical Center, St. Louis, MO; Helene Emsellem, MD, Center for Sleep & Wake Disorders, Chevy Chase, MD; Milton Erman, MD and Roza Hayduk, MD, Pacific Sleep Medicine Services, La Jolla, CA; Neil Feldman, MD, St. Petersburg Sleep Disorders Center, St. Petersburg, FL; John Fleming, MD, Vancouver Hospital, Vancouver, British Columbia, Canada; Peter Geisler, MD, Psychiatrische Universitäatsklinik, Regensburg, Germany; Martha Hagaman, MD, Children's Sleep Institute, Nashville, TN; Dennis Hill, MD, Central Carolina Neurology & Sleep, Salisbury, NC; William C. Houghton, MD and Carl S. Hornfeldt, PhD, Orphan Medical, Inc., Minnetonka, MN; Aatif M. Husain, MD, Duke Health Center at Morreene Road, Durham, NC; Thomas Kaelin, MD, Lowcountry Lung & Critical Care PA, Charleston, SC; Gert Jan Lammers MD, PhD, Leiden University Medical Center, Leiden, the Netherlands; D. Alan Lankford, PhD, Sleep Disorders Center of Georgia, Atlanta, GA; Judith Leech, MD, The Ottawa Hospital Sleep Centre, Ottawa, Ontario, Canada; Mortimer Mamelak, MD, Brain & Sleep Diagnostic Center, Toronto, Ontario, Canada; Geert Mayer, MD, Hepatic Klinik, Schwalmstadt-Treysa, Germany; Harvey Moldofsky, MD, Center for Sleep and Chronobiology, Toronto, Ontario, Canada; Jacques Montplaisir, MD, Sleep Disorder Centre, Montreal, Quebec, Canada; Rachel Morehouse, MD, Saint John Regional Hospital, Saint John, New Brunswick, Canada; Adam Moscovitch, MD, Canadian Sleep Institute, Calgary, Alberta, Canada; Sonka Nevsimalova, MD, Charles University, Prague, Czech Republic; William Orr, PhD, Lynn Health Science Institute, Oklahoma City, OK; Ralph Pascualy, MD, Swedish Sleep Medicine Institute, Seattle, WA; Vernon Pegram, PhD, Sleep Disorders Center of Alabama, Birmingham, AL; Thomas Perkins, MD, PhD, Raleigh Neurology Associates PA, Raleigh, NC; Jayant Phadke, MD, St. Vincent Hospital, Worcester, MA; Ruzica Ristanovic, MD, Evanston Hospital Sleep Disorders Center, Evanston, IL; John Shneerson, MD, Papworth Hospital, Cambridge, England; James Stevens, MD, Fort Wayne, IN; Todd Swick, MD, The Houston Sleep Center, Houston, TX; Joyce Walsleben, PhD, Bellevue Hospital Sleep/Wake Center, New York, NY; Timothy Walter, MD, Grove City Sleep Diagnostic Center, Grove City, OH; J. Catesby Ware, PhD, Sleep Disorders Center, Norfolk, VA; Patrick Whitten, MD, Peoria, IL; David Winslow, MD, Sleep Medicine Specialists, Louisville, KY. The authors wish to thank the Stanford Sleep Disorders Clinic (Oscar Carillo) for assistance in data collection and data analysis. The authors also acknowledge Howard Liang, MS, and Chinglin Lai, PhD, (Jazz Pharmaceuticals, Inc.) in performing statistical analyses and Teresa Steininger, PhD, and Chinglin Lai, PhD, (Jazz Pharmaceuticals, Inc.) for editorial assistance and helpful suggestions.
REFERENCES
- 1.U.S. Xyrem Multicenter Study Group. A randomized, double blind, placebo controlled multicenter trial comparing the effects of three doses of orally administered sodium oxybate with placebo for the treatment of narcolepsy. Sleep. 2002;25:42–9. [PubMed] [Google Scholar]
- 2.U.S. Xyrem Multicenter Study Group. A 12-month, open-label, multicenter extension trial of orally administered sodium oxybate for the treatment of narcolepsy. Sleep. 2003;26:31–5. [PubMed] [Google Scholar]
- 3.U.S. Xyrem Multicenter Study Group. Sodium oxybate demonstrates long-term efficacy for the treatment of cataplexy in patients with narcolepsy. Sleep Med. 2004;5:119–123. doi: 10.1016/j.sleep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Xyrem International Study Group. A double-blind, placebo-controlled, study demonstrates sodium oxybate is effective for the treatment of excessive daytime sleepiness in narcolepsy. J Clin Sleep Med. 2005;1:289–95. [PubMed] [Google Scholar]
- 5.Xyrem International Study Group. Further evidence supporting the use of sodium oxybate for the treatment of cataplexy: a double-blind, placebo-controlled study in 228 patients. Sleep Med. 2005;6:415–21. doi: 10.1016/j.sleep.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Overeem S, Mignot E, van Dijk JG, Lammers GJ. Narcolepsy: Clinical features, new pathophysiologic insights, and future perspectives. J Clin Neurophysiol. 2001;18:78–105. doi: 10.1097/00004691-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Mamelak M, Escriu JM, Stokan O. The effects of gamma-hydroxybutyrate on sleep. Biol Psychiatry. 1977;12:273–88. [PubMed] [Google Scholar]
- 8.Broughton R, Mamelak M. Effects of nocturnal gamma-hydroxybutyrate on sleep waking patterns in narcolepsy-cataplexy. Can J Neurol Sci. 1980;7:23–30. [PubMed] [Google Scholar]
- 9.Scrima L, Hartman PG, Johnson FH, Thomas EE, Hiller FC. The effects of γ-hydroxybutyrate on the sleep of narcolepsy patients: A double blind study. Sleep. 1990;13:479–90. doi: 10.1093/sleep/13.6.479. [DOI] [PubMed] [Google Scholar]
- 10.Lammers GJ, Arends J, Declerck AC, Ferrari MN, Schouwink G, Troost J. Gamma-hydroxybutyrate and narcolepsy: a double blind placebo controlled study. Sleep. 1993;16:216–20. doi: 10.1093/sleep/16.3.216. [DOI] [PubMed] [Google Scholar]
- 11.Mamelak M, Black J, Montplaisir J, Ristanovic R. A dose response study on the effects of sodium oxybate on sleep architecture and daytime alertness in narcolepsy. Sleep. 2004;27:1327–34. doi: 10.1093/sleep/27.7.1327. [DOI] [PubMed] [Google Scholar]
- 12.Weaver TE, Cuellar N. A randomized trial evaluating the effectiveness of sodium oxybate therapy on quality of life in narcolepsy. Sleep. 2006;29:1189–94. doi: 10.1093/sleep/29.9.1189. [DOI] [PubMed] [Google Scholar]
- 13.American Academy of Sleep Medicine. International classification of sleep disorders: diagnostic and coding manual. 2nd. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 14.Rechtschaffen A, Kales A, editors. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Public Health Service, US Government Printing Office; 1968. [Google Scholar]
- 15.Broughton R, Mamelak M. The treatment of narcolepsy-cataplexy with nocturnal gamma-hydroxybutyrate. Can J Neurol Sci. 1979;6:1–6. doi: 10.1017/s0317167100119304. [DOI] [PubMed] [Google Scholar]
- 16.Scharf MB, Brown D, Woods M, Brown L, Hirschowitz J. The effects and effectiveness of gamma-hydroxybutyrate in patients with narcolepsy. J Clin Psychiatry. 1985;46:222–5. [PubMed] [Google Scholar]
- 17.Lapierre O, Montplaisir J, Lamarre M, Bedard MA. The effect of gamma-hydroxybutyrate on nocturnal and diurnal sleep of normal subjects: further considerations on REM sleep. Sleep. 1990;13:24–30. doi: 10.1093/sleep/13.1.24. [DOI] [PubMed] [Google Scholar]