Abstract
A 28-year-old woman was evaluated for 4 months of excessive daytime sleepiness (EDS), after an overnight polysomnogram (PSG) revealed neither sleep disordered breathing nor a sleep related movement disorder. A full sleep evaluation revealed the presence of heavy daytime napping and pervasive fatigue. Epworth Sleepiness Scale (ESS) Score was 10/24. No features characteristic for depression or narcolepsy were present. Chronic pain in the low back and thighs, as well as chronic daily headaches were identified as potential sleep-disrupting forces. Risk factors for hypovitaminosis D included limited natural sun exposure, dark skin tone, and obesity. A 25-hydroxyvitamin D level was low, at 5.9 ng/mL. Vitamin D supplementation was initiated at a dose of 50,000 IU once weekly, and EDS improved within 2 weeks. One week later, a PSG with next-day multiple sleep latency testing (MSLT) failed to show significant pathology. At follow-up, she reported resolution of thigh pain and headaches, with a significant improvement in her low back pain syndrome. EDS had resolved, and her ESS score was 1/24. Follow-up 25-hydroxyvitamin D level was normal at 39 ng/mL. Mechanisms for her clinical improvement could include enhanced sleep quality due to resolution of hypovitaminisos D-associated noninflammatory myopathy, or a possible immunomodulatory effect of vitamin D decreasing central nervous system (CNS) homeostatic sleep pressure via its effects on tumor necrosis factor-alpha (TNF-α) and/or prostaglandin D2. More research is needed to determine if patients presenting with EDS should be more broadly screened for vitamin D deficiency.
Citation:
McCarty DE. Resolution of hypersomnia following identification and treatment of vitamin D deficiency. J Clin Sleep Med 2010;6(6):605-608.
Keywords: Hypersomnia, vitamin D, hypovitaminosis D, osteomalacic myopathy, chronic pain
REPORT OF CASE
A 28-year-old African American female presented to an academic sleep disorders center for further evaluation of approximately 4 months of EDS symptoms. Her symptoms began gradually, insidiously worsening to the point that she began having functional difficulties staying awake to engage in social responsibilities. She kept a standard bedtime between 21:00 and 22:00 and reported that she fell asleep easily and within minutes. She subjectively felt as though the quality of her sleep was good, stating she did not believe she had frequent nocturnal awakenings. She awakened to start her day at 07:30, would assist her children in getting ready for school, and then would routinely return to bed at 08:00 and would often sleep until noon. She would then rise to perform house-related chores and assist her children with returning home from school, often going back to bed for another nap from 16:00–19:00. In total, the patient stated that she was obtaining ≥ 14 h of sleep per day, as a result of her daytime sleepiness. Her ESS score was 10/24, indicating a pathological degree of daytime sleepiness. Her primary care physician requested a sleep study to evaluate for obstructive sleep apnea (OSA).
An overnight PSG using a digital acquisition system (Alice 5, Philips-Respironics) was performed. The study was scored according to rules described in the American Academy of Sleep Medicine (AASM) scoring manual,1 and raw PSG data were reviewed by a physician board certified in Sleep Medicine. Hypopnea rule 4(A)—requiring 4% oxygen desaturation, along with a 30% drop in nasal pressure transducer airflow tracing—was used during the scoring of this PSG. Results from this study showed reasonably good sleep efficiency, with no evidence of sleep disordered breathing or sleep related movement disorder (Figure 1).
Figure 1.
Results of original diagnostic polysomnography
A primary hypersomnia syndrome was clinically suspected, and the patient was scheduled for a complete Sleep Medicine evaluation. This consultation occurred approximately 3 weeks after the original PSG was performed. At this visit, she continued to endorse a functionally limiting, pervasive sense of fatigue and “fighting sleep” whenever she was inactive. She denied cataplexy, sleep related hallucinations, and sleep paralysis. She denied emotional depression or anxiety. She endorsed a significant musculoskeletal pain syndrome involving her low back and thighs, which she rated as 6 out of 10 in severity, and stated she believed that this could be negatively affecting her sleep quality. She also endorsed frequent retro-orbital headaches, which were poorly responsive to medications.
Her medical history was remarkable for sickle cell disease, with occasional transfusion requirements (the most recent of which was 2 years prior to her initial polysomnogram), morbid obesity, and chronic low back pain. She reported taking daily oral hydroxyurea and folate supplements but no other regular medications. She admitted to occasionally using hydrocodone/acetaminophen for her headache syndrome, with only marginal benefit. She reported no association between the use of this medication and her EDS symptoms, stating that she felt pervasively sleepy whether she used this medication or not. She denied use of alcohol, recreational drugs, and tobacco products. She slept alone, and denied any problems relating to bed comfort or noise disturbances during the sleeping time frame.
Physical examination revealed a pleasant obese African American woman in no distress. Vital signs were within normal limits. Her body mass index was 40. Examination of the oropharynx revealed a Mallampati I posterior inlet, with 1+ tonsils. A grade 2/6 systolic ejection murmur was present. Her affect was euthymic and showed full range. Her neurologic examination was nonfocal. Aside from marked conjunctival pallor, her skin examination was normal.
Laboratory data obtained earlier that month revealed anemia—consistent with her known history of sickle cell disease—with a hemoglobin of 8.1 g/dL, a value no different from those obtained prior to onset of symptoms. Thyroid stimulating hormone (TSH) was normal at 0.7 μIU/mL. A metabolic panel, including liver function tests and renal indices, was normal.
The patient was tentatively diagnosed with idiopathic CNS hypersomnia with long sleep time, and a repeat PSG with next-day MSLT protocol was planned. The presence of musculoskeletal pain associated with risk factors for vitamin D deficiency prompted further testing (see later). A 25-hydroxyvitamin D level was obtained, returning quite low at 5.9 ng/mL (normal range 32-100 ng/mL). Vitamin D supplementation at a dose of 50,000 international units (IU) once weekly was initiated the following week.
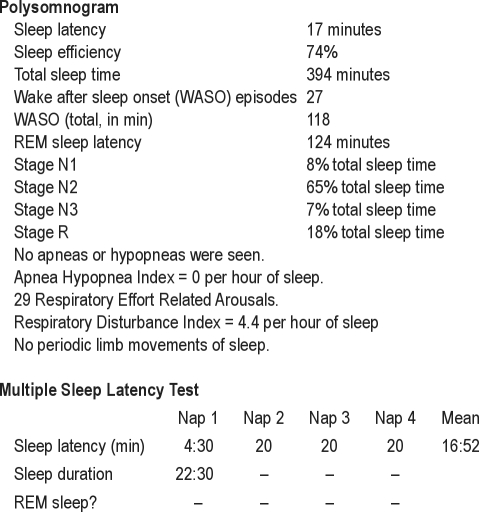
A repeat PSG with next-day MSLT was performed 3 weeks after vitamin D supplementation was initiated. This study showed a few respiratory effort related arousals, but no frank apneas or hypopneas, and fell short of diagnostic criteria for obstructive sleep apnea (Figure 2). Compared with her first PSG, the follow-up study showed decreases in sleep continuity, sleep efficiency, and percentage of sleep time spent in stage N3. The MSLT performed the following day revealed sleep in only the first of 4 naps, with a mean sleep latency calculated at 16:52. No sleep-onset REM sleep periods were seen.
Figure 2.
Polysomnography and next-day MSLT results
At a follow-up clinic visit to discuss results, she admitted that she began feeling better approximately 2 weeks after initiating vitamin D supplementation, and that her EDS symptoms had completely resolved by the time she returned for her repeat PSG and MSLT. She stated that she continued to have mild low back pain, but it no longer seemed to affect the quality of her sleep. The pain in her thighs and daytime headache syndrome had completely resolved. She denied functional limitations due to fatigue, and denied daytime napping. Her ESS score was 1/24. She estimated a total sleep time of approximately 9-10 hours per night. She stated that—aside from initiating vitamin D supplementation—no other circumstances in her life had changed since her initial evaluation: there had been no interval changes in her other medications, diet, social activities, caffeine use, exercise patterns, or work. A 25-hydroxyvitamin D level obtained at her follow-up visit was found to be within normal limits at 39 ng/mL. A hemoglobin level obtained the following day was essentially unchanged from previous levels, at 8.2 g/dL.
DISCUSSION
Vitamin D refers to a group of fat-soluble secosteroid hormones, and is typically ingested in dietary sources (dairy products and fatty fish), or is manufactured in the skin after exposure to UVB light. Vitamin D deficiency/insufficiency is increasingly recognized as a global epidemic, estimated to affect over a billion persons worldwide.2 Though vitamin D deficiency is commonly understood to be disproportionately represented in underserved populations,3 patients residing in northern latitudes,4 individuals with darker skin tones,2,5,6 the elderly,7 the obese,8 and pregnant or lactating women,9 it is also commonly found in children10,11 and is surprisingly common in areas with a high degree of year-round sunshine.12,13 It has been postulated that increasing urbanization of the population as well as an increased awareness of potential dangers of sun exposure with subsequent reliance on sun-blocking skin products are both factors underlying the seeming increase in prevalence.
Over the past decade, scientific understanding of the role of vitamin D has expanded greatly, beyond its classically described effects on gut and bone,2 with an explosion of new associations tied to its deficiency. Vitamin D and its analogues appear to have potent immunomodulatory activities, and deficiency of vitamin D has been linked to multiple pulmonary disorders, including worsening lung function in patients with chronic obstructive pulmonary disease,14 reactivation of tuberculosis,15 and increased incidence of childhood asthma and wheezing.16–18 Vitamin D appears to be necessary for optimum functioning of skeletal muscle as well, with deficiency associated with increased risk for falls19 and disability20 among elderly patients, as well as chronic pain,21 low back pain,22 and cases of overt painful myopathy.23–25 In one study of patients admitted to a Minneapolis hospital, 93% of those complaining of nonspecific musculoskeletal pain were found to have significant vitamin D deficiency.26 Recent publications point to a potential link between vitamin D deficiency and the metabolic syndrome,27 type 2 diabetes,28 incident hypertension,29 as well as a possible association with cancers of the breast, colon, and prostate.30 Vitamin D deficiency may lead to problems with higher cognitive functioning as well, with associations found with poor stress resilience,31 depression,32 and cognitive decline.33 Finally, some evidence supports a link between vitamin D deficiency and all-cause mortality.34
The mechanism for the improvement in this patient's hypersomnia syndrome after identification and remediation of vitamin D deficiency is not known. A simple explanation is that she experienced a significant improvement in her musculoskeletal pain syndrome—which was identified as a sleep-disrupting force on her initial interview—thus leading to improved quality of sleep and decreased daytime consequences. Corroborating this theory is the patient's own description of an improvement in her subjective pain syndrome, along with subjectively more restorative sleep. If this were the sole explanation, however, one would expect that her post-replacement PSG would show an improvement in sleep continuity, as reflected by a decrease in episodes and duration of wake after sleep onset. In reality, the opposite was seen.
Another possible explanation is the notion that vitamin D may play some yet unidentified role in the regulation of homeostatic sleep drive via CNS inflammatory signaling. Slow wave sleep is widely considered to be a marker for homeostatic sleep drive. In this patient, stage N3 sleep showed an interval decrease following vitamin D replacement, suggesting a reduction in homeostatic sleep pressure following this intervention. Recent research on vitamin D lends support to this theory. Petersen and Heffernan found an inverse correlation between serum 25-hydroxyvitamin D and TNF-α levels, suggesting a mechanism by which vitamin D may influence the presentation of inflammatory diseases.35 Though data are mixed, some research has demonstrated that TNF-α is mechanistically implicated in the sleepiness associated with OSA. In a study of sleepy adults with OSA, Vgontzas and colleagues found that administration of the TNF-α antagonist etanercept markedly decreased daytime sleepiness symptoms.36 Recently, Barcelo and colleagues showed that patients with so-called “non-sleepy apnea” had significantly lower levels of lipocalin-type D2 synthase compared with their sleepy counterparts.37 Lipocalin-type D2 synthase is the rate-limiting enzyme responsible for the production of prostaglandin D2, the major prostanoid in the brain, and is a physiologic regulator of sleep. Dovetailing with this is the recent work by Feldman and colleagues showing that vitamin D is a potent biologic regulator of prostaglandin synthesis via inhibition of cyclooxygenase-2.38 Taken together, these data suggest the possibility that vitamin D deficiency could represent a condition which predisposes a patient to the development of pathologic degrees of CNS-induced sleepiness, mediated by components of the inflammatory cascade.
CONCLUSIONS
Excessive daytime sleepiness is a symptom which may result from a number of different sources, and a comprehensive approach to each patient is needed.39 To our knowledge, this is the first reported case of clinical excessive daytime sleepiness resolving upon identification and remediation of severe vitamin D deficiency. Further study is needed to elucidate the possible mechanism for this phenomenon, and, importantly, whether more widespread screening for vitamin D deficiency among patients complaining of excessive daytime sleepiness is warranted.
DISCLOSURE STATEMENT
The author has indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The author wishes to thank Dr. Douglas Moul and Dr. Cesar Liendo for participating in helpful discussions regarding this case and during the preparation of this manuscript.
ABBREVIATIONS
- CNS
central nervous system
- EDS
excessive daytime sleepiness
- ESS
Epworth Sleepiness Scale
- MSLT
multiple sleep latency test
- PSG
polysomnogram
- TNF-α
tumor necrosis factor-alpha
- WASO
wake after sleep onset
REFERENCES
- 1.Iber C. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. American Academy of Sleep Medicine. [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Kakarala RR, Chandana SR, Harris SS, Kocharla LP, Dvorin E. Prevalence of vitamin D deficiency in uninsured women. J Gen Intern Med. 2007;22:1180–3. doi: 10.1007/s11606-007-0245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–8. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 5.Matsuoka LY, Wortsman J, Chen TC, Holick MF. Compensation for the interracial variance in the cutaneous synthesis of vitamin D. J Lab Clin Med. 1995;126:452–7. [PubMed] [Google Scholar]
- 6.Matsuoka LY, Wortsman J, Haddad JG, Kolm P, Hollis BW. Racial pigmentation and the cutaneous synthesis of vitamin D. Arch Dermatol. 1991;127:536–8. [PubMed] [Google Scholar]
- 7.Holick MF, Siris ES, Binkley N, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215–24. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 8.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–3. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 9.Lee JM, Smith JR, Philipp BL, Chen TC, Mathieu J, Holick MF. Vitamin D deficiency in a healthy group of mothers and newborn infants. Clin Pediatr (Phila) 2007;46:42–4. doi: 10.1177/0009922806289311. [DOI] [PubMed] [Google Scholar]
- 10.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158:531–7. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan SS, Rosen CJ, Halteman WA, Chen TC, Holick MF. Adolescent girls in Maine are at risk for vitamin D insufficiency. J Am Diet Assoc. 2005;105:971–4. doi: 10.1016/j.jada.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Faiz S, Panunti B, Andrews S. The epidemic of vitamin D deficiency. J La State Med Soc. 2007;159:17–20. quiz, 55. [PubMed] [Google Scholar]
- 13.Zargar AH, Ahmad S, Masoodi SR, et al. Vitamin D status in apparently healthy adults in Kashmir Valley of Indian subcontinent. Postgrad Med J. 2007;83:713–6. doi: 10.1136/pgmj.2007.059113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128:3792–8. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- 15.Sita-Lumsden A, Lapthorn G, Swaminathan R, Milburn HJ. Reactivation of tuberculosis and vitamin D deficiency: the contribution of diet and exposure to sunlight. Thorax. 2007;62:1003–7. doi: 10.1136/thx.2006.070060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85:788–95. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devereux G, Litonjua AA, Turner SW, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. 2007;85:853–9. doi: 10.1093/ajcn/85.3.853. [DOI] [PubMed] [Google Scholar]
- 18.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120:1031–5. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 19.Burleigh E, McColl J, Potter J. Does vitamin D stop inpatients falling? A randomised controlled trial. Age Ageing. 2007;36:507–13. doi: 10.1093/ageing/afm087. [DOI] [PubMed] [Google Scholar]
- 20.Wicherts IS, van Schoor NM, Boeke AJ, et al. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92:2058–65. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 21.Turner MK, Hooten WM, Schmidt JE, Kerkvliet JL, Townsend CO, Bruce BK. Prevalence and clinical correlates of vitamin D inadequacy among patients with chronic pain. Pain Med. 2008;9:979–84. doi: 10.1111/j.1526-4637.2008.00415.x. [DOI] [PubMed] [Google Scholar]
- 22.Lotfi A, Abdel-Nasser AM, Hamdy A, Omran AA, El-Rehany MA. Hypovitaminosis D in female patients with chronic low back pain. Clin Rheumatol. 2007;26:1895–901. doi: 10.1007/s10067-007-0603-4. [DOI] [PubMed] [Google Scholar]
- 23.Boltan DD, Lachar W, Khetan A, Bouffard JP, Roberts WC. Fatal and widespread skeletal myopathy confirmed morphologically years after initiation of simvastatin therapy. Am J Cardiol. 2007;99:1171–6. doi: 10.1016/j.amjcard.2006.11.071. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein MR. Myopathy, statins, and vitamin D deficiency. Am J Cardiol. 2007;100:1328. doi: 10.1016/j.amjcard.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 25.Prabhala A, Garg R, Dandona P. Severe myopathy associated with vitamin D deficiency in western New York. Arch Intern Med. 2000;160:1199–203. doi: 10.1001/archinte.160.8.1199. [DOI] [PubMed] [Google Scholar]
- 26.Plotnikoff GA, Quigley JM. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc. 2003;78:1463–70. doi: 10.4065/78.12.1463. [DOI] [PubMed] [Google Scholar]
- 27.Botella-Carretero JI, Alvarez-Blasco F, Villafruela JJ, Balsa JA, Vazquez C, Escobar-Morreale HF. Vitamin D deficiency is associated with the metabolic syndrome in morbid obesity. Clin Nutr. 2007;26:573–80. doi: 10.1016/j.clnu.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Mattila C, Knekt P, Mannisto S, et al. Serum 25-hydroxyvitamin D concentration and subsequent risk of type 2 diabetes. Diabetes Care. 2007;30:2569–70. doi: 10.2337/dc07-0292. [DOI] [PubMed] [Google Scholar]
- 29.Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–9. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 30.Garland CF, Garland FC, Gorham ED, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96:252–61. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bracha HS, Ralston TC, Matsukawa JM, Williams AE, Bernstein DM. Diminished stress resilience in institutionalized elderly patients: is hypovitaminosis D a factor? Am J Geriatr Psychiatry. 2004;12:544–5. doi: 10.1176/appi.ajgp.12.5.544. [DOI] [PubMed] [Google Scholar]
- 32.Berk M, Sanders KM, Pasco JA, et al. Vitamin D deficiency may play a role in depression. Med Hypotheses. 2007;69:1316–9. doi: 10.1016/j.mehy.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. 2007;460:202–5. doi: 10.1016/j.abb.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 34.Giovannucci E. Can vitamin D reduce total mortality? Arch Intern Med. 2007;167:1709–10. doi: 10.1001/archinte.167.16.1709. [DOI] [PubMed] [Google Scholar]
- 35.Peterson CA, Heffernan ME. Serum tumor necrosis factor-alpha concentrations are negatively correlated with serum 25(OH)D concentrations in healthy women. J Inflamm (Lond) 2008;5:10. doi: 10.1186/1476-9255-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vgontzas AN, Zoumakis E, Lin HM, Bixler EO, Trakada G, Chrousos GP. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-alpha antagonist. J Clin Endocrinol Metab. 2004;89:4409–13. doi: 10.1210/jc.2003-031929. [DOI] [PubMed] [Google Scholar]
- 37.Barcelo A, de la Pena M, Barbe F, Pierola J, Bosch M, Agusti AG. Prostaglandin D synthase (beta trace) levels in sleep apnea patients with and without sleepiness. Sleep Med. 2007;8:509–11. doi: 10.1016/j.sleep.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Feldman D, Krishnan A, Moreno J, Swami S, Peehl DM, Srinivas S. Vitamin D inhibition of the prostaglandin pathway as therapy for prostate cancer. Nutr Rev. 2007;65:S113–5. doi: 10.1111/j.1753-4887.2007.tb00335.x. [DOI] [PubMed] [Google Scholar]
- 39.McCarty DE. Beyond Ockham's razor: redefining problem-solving in clinical sleep medicine using a “five-finger” approach. J Clin Sleep Med. 2010;6:292–6. [PMC free article] [PubMed] [Google Scholar]