Abstract
Congenital central hypoventilation syndrome (CCHS) is an uncommon disorder characterized by the absence of adequate autonomic control of respiration, which results in alveolar hypoventilation and decreased sensitivity to hypercarbia and hypoxemia, especially during sleep.1 Patients with CCHS need lifelong ventilatory support. The treatment options for CCHS include intermittent positive pressure ventilation administered via tracheostomy, noninvasive positive pressure ventilation, negative-pressure ventilation by body chamber or cuirass, and phrenic nerve pacing.2 However, it may be necessary to alter the mode of ventilation according to age, psychosocial reasons, complications of therapy, and emergence of new modes of ventilation.3 We present a case of a 16-year-old girl with CCHS who was mechanically ventilated via tracheostomy for 16 years and was successfully transitioned to a new modality of noninvasive ventilation (average volume-assured pressure support [AVAPS]) that automatically adjusts the pressure support level in order to provide a consistent tidal volume.
Citation:
Vagiakis E; Koutsourelakis I; Perraki E; Roussos C; Mastora Z; Zakynthinos S; Kotanidou A. Average volume-assured pressure support in a 16-year-old girl with central congenital hypoventilation syndrome. J Clin Sleep Med 2010;6(6):609-612.
Keywords: Central congenital hypoventilation syndrome, tracheostomy, respiratory management
Congenital central hypoventilation syndrome (CCHS) is an uncommon disorder characterized by the absence of adequate autonomic control of respiration, which results in alveolar hypoventilation and decreased sensitivity to hypercarbia and hypoxemia, especially during sleep.1 Patients with CCHS need lifelong ventilatory support. The treatment options for CCHS include intermittent positive pressure ventilation administered via tracheostomy, noninvasive positive pressure ventilation, negative-pressure ventilation by body chamber or cuirass, and phrenic nerve pacing.2 However, it may be necessary to alter the mode of ventilation according to age, psychosocial reasons, complications of therapy, and emergence of new modes of ventilation.3 We present a case of a 16-year-old girl with CCHS who was mechanically ventilated via tracheostomy for 16 years and was successfully transitioned to a new modality of noninvasive ventilation (average volume-assured pressure support [AVAPS]) that automatically adjusts the pressure support level in order to provide a consistent tidal volume.
REPORT OF CASE
The 16-year-old girl was born at term with a birth weight of 2.950 kg to healthy parents after an uneventful pregnancy and delivery. Ten hours following an uncomplicated elective cesarean section, she was transferred to the neonatal intensive care unit, where she was intubated and mechanically ventilated because of severe cyanosis. The infant could not be weaned successfully from mechanical ventilation because of frequent apneas and absence of effective spontaneous respiration during sleep. A tracheostomy was performed at the age of 3 months. A series of studies including bronchoscopic examination, muscle biopsy, electromyography (leg muscles), muscle enzyme levels, brainstem magnetic resonance imaging, showed normal findings. She was discharged home with nocturnal volume-cycled mechanical ventilation via tracheostomy (Puritan Bennett LP10) at the age of 6 years after overcoming several respiratory tract infections. For the following 10 years she consistently used the ventilator during sleep without serious complications.
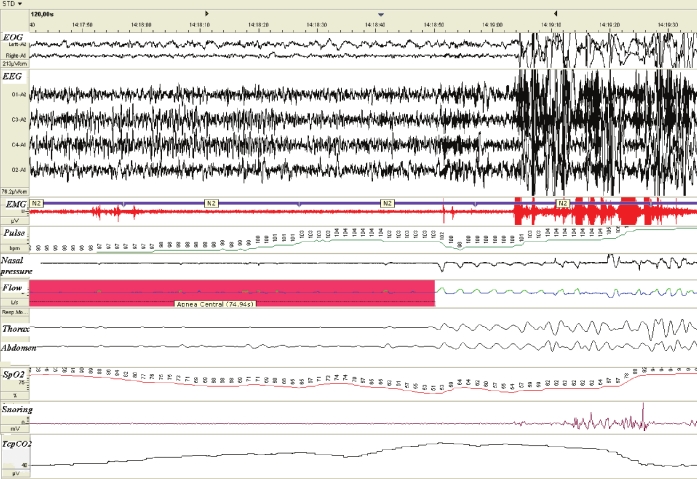
At the age of 16 years, her parents expressed their will for tracheostomy removal in an effort to alleviate the associated psychosocial problems. Initial clinical assessment did not reveal any autonomic nervous system dysfunction such as body temperature dysregulation, esophageal dysphagia, pupillary abnormalities, constipation, or heart rate variability. Respiration was adequate while awake. Arterial blood gases were as follows: pH was 7.41; PaO2 was 102 mm Hg, PaCO2 was 35 mm Hg, and HCO3− concentration was 22 mEq/L. Subsequently, diagnostic polysomnography (EMBLA S7000, Medcare Flaga, Iceland) with concomitant measurement of transcutaneous partial pressure of CO2 (Tina TCM2; Radiometer; Copenhagen; Denmark) was performed for several consecutive nights. No study lasted more than one hour because the lack of ventilatory response to hypoxemia and hypercarbia led to such a significant increase in partial pressure of CO2 and decrease in oxygen saturation made it necessary to awaken her so that she could resume breathing. Oxyhemoglobin saturation decreased to a minimum of 51%, and transcutaneous partial pressure of CO2 increased to a maximum of 70 mm Hg (Figure 1).
Figure 1.
Oxyhemoglobin desaturation reaching 51% during sleep with spontaneous breathing
Blood samples were taken from the patient, her siblings, and her parents and sent for analysis to the Molecular Diagnostic Laboratory at Rush University Medical Center (Chicago, IL). DNA sequencing of the girl revealed that she carried a 25-repeat polyalanine expansion mutation in paired-like homeobox (PHOX)2B gene located on chromosome 4p12. DNA analysis of the mother and siblings were normal, whereas father's DNA analysis showed that he carried alleles of 20 and 25 repeats. The mutated 25 repeat allele gave a lighter band than the normal allele suggesting somatic mosaicism.
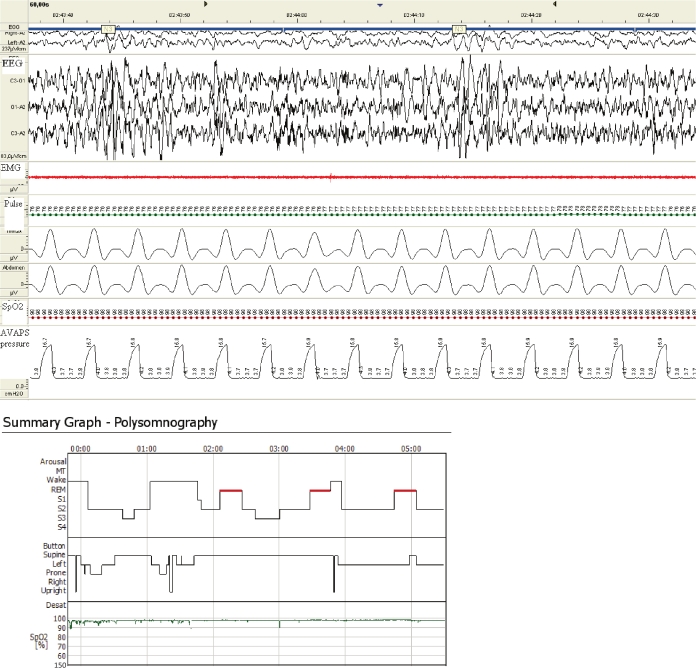
With the tracheostomy capped, a trial of bilevel pressure ventilation-spontaneous/timed with average volume-assured pressure (BiPAP Synchrony & AVAPS support function; Respironics Inc; Murrysville, PA) mode was successfully undertaken. Average volume-assured pressure support (AVAPS), combines both the pressure and the volume characteristics of ventilation and, accordingly, delivers a range of inspiratory pressures to guarantee a prefixed inspiratory tidal volume. During AVAPS titration, the actual inspiratory positive airway pressure (IPAP) level ranged between expiratory positive airway pressure (EPAP) and 19 cm H2O to ensure adequate tidal volume (450 mL) under a constant rate of 16 breaths per minute. EPAP was set at the minimum level (4 cm H2O). Adequate tidal volume was 8 mL per kilogram of predicted body weight (calculated as equal to 45.5+0.91[centimeters of height-152.4]).4 Respiratory rate was chosen according to the setting of the previously used ventilator. Under close supervision, during a one-month period several polysomnographic studies were performed with the ventilator on and the tracheostomy corked to ensure adequate titration of the patient. Indeed, all sleep studies revealed normal sleep architecture with a minimum SpO2 of 96% and a maximum PtcCO2 of 40% (Figure 2). After these results, the tracheostomy was downsized and closed on its own. A residual tracheo-cutaneous fistula required surgical closure 3 months later.
Figure 2.
Oxygen saturation consistently above 96% during AVAPS ventilation
DISCUSSION
It is well known that the major problem in the respiratory management of CCHS is the choice of lifelong ventilatory support. Among the factors that determine the choice are efficacy, practicality, psychosocial acceptance, complications, and cost. The existing options for ventilatory support include intermittent positive pressure ventilation via tracheostomy, phrenic nerve pacing, noninvasive positive pressure ventilation, and negative pressure ventilation by body chamber or cuirass. Although mechanical ventilation is facilitated via tracheostomy and is regarded as the standard method of respiratory support for CCHS, it is not ideal. Tracheostomy in children is intuitively associated with impaired speech and language development and with frequent infections of the lower airway tract.5,6
Previous reports have already described the successful use of bilevel PAP for ventilatory support of children with CCHS.7 Transition from mechanical ventilation via tracheostomy to bilevel PAP during childhood has also been reported in children older than 7 years old.7 The case we describe is the first in which a patient with CCHS was successfully transitioned to a new mode of noninvasive ventilation after using mechanical ventilation via tracheostomy for 16 years. AVAPS has been recently introduced as a new additional mode for a bilevel pressure ventilation device that automatically adjusts the pressure support level to provide a consistent tidal volume. Studies on its physiologic and clinical effects are few.8,9 In particular, AVAPS ventilation has been showed to be more efficient in decreasing PtcCO2 than bilevel pressure ventilation in patients with obesity hypoventilation syndrome.8 Furthermore, in patients with chronic respiratory insufficiency AVAPS offered greater minute ventilation in comparison with noninvasive ventilation with pressure support therapy.9
The impetus to use noninvasive techniques for the respiratory management is multifactorial, including psychosocial reasons associated with tracheostomy. Additionally, the availability of noninvasive positive pressure ventilation machines, which provide flow-triggered breaths with automatic breath-by-breath compensation for airleaks, and the advent of soft self-molding or cushioned nasal masks, all represent a practical and reliable alternative to invasive mechanical ventilation. This equipment is generally more transportable than a mechanical ventilator, and may be used easily away from home or during a journey when the child falls asleep. Lastly, the possible requirement for less attendant care with noninvasive techniques is financially attractive.
The disease-defining gene mutation was identified in the DNA analysis of the patient. Most expansion mutations occur de novo in CCHS probands, and rarely are inherited as an autosomal dominant trait. Somatic mosaicism for the expansion mutation in an unaffected parent of a CCHS child is seen in about 10% of cases.
We conclude that nasally applied AVAPS mode ventilation may be a reliable alternative to mechanical ventilation via tracheostomy in the management of CCHS.
DISCLOSURE STATEMENT
The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The present work was funded by the Thorax Foundation.
REFERENCES
- 1.BWeese-Mayer DE, Berry-Kravis EM, et al. Idiopathic congenital central hypoventilation syndrome: analysis of genes pertinent to early autonomic nervous system embryologic development and identification of mutations in PHOX2B. Am J Med Genet A. 2003;123:267–78. doi: 10.1002/ajmg.a.20527. [DOI] [PubMed] [Google Scholar]
- 2.BVilla M, Dotta A, Castello D, et al. Bi-level positive airway pressure (BiPAP) ventilation in an infant with central hypoventilation syndrome. Pediatr Pulmonol. 1997;24:66–9. doi: 10.1002/(sici)1099-0496(199707)24:1<66::aid-ppul12>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Berry-Kravis E, Zhou L, Rand C, Weese-Mayer D. Congenital central hypoventilation syndrome. Am J Respir Crit Care Med. 2006;174:1139–44. doi: 10.1164/rccm.200602-305OC. [DOI] [PubMed] [Google Scholar]
- 4.The acute respiratory distress syndrome network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 5.Hill BP, Singer LT. Speech and language development after infant tracheostomy. J Speech Hear Disord. 1990;55:15–20. doi: 10.1044/jshd.5501.15. [DOI] [PubMed] [Google Scholar]
- 6.Morar P, Singh V, Makura Z, et al. Oropharyngeal carriage and lower airway colonization/infection in 45 tracheotomised children. Thorax. 2002;57:1015–20. doi: 10.1136/thorax.57.12.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tibballs J, Henning R. Noninvasive ventilatory strategies in the management of a newborn infant and three children with congenital central hypoventilation syndrome. Pediatr Pulmonol. 2003;36:544–8. doi: 10.1002/ppul.10392. [DOI] [PubMed] [Google Scholar]
- 8.Storre JH, Seuthe B, Fiechter R. Average volume-assured pressure support in obesity hypoventilation: a randomized controlled trial. Chest. 2006;130:815–21. doi: 10.1378/chest.130.3.815. [DOI] [PubMed] [Google Scholar]
- 9.Ambrogio C, Lowman X, Kuo M, Malo J, Prasad A, Parthasarathy S. Sleep and non-invasive ventilation in patients with chronic respiratory insufficiency. Intensive Care Med. 2009;35:306–13. doi: 10.1007/s00134-008-1276-4. [DOI] [PubMed] [Google Scholar]