Abstract
Objective
To determine what variables separate community-dwelling elders from assisted-living dwelling elders.
Design
Cross-sectional
Setting
Community and assisted living facilities in Connecticut
Participants
114 individuals (77 community-dwelling, 37 assisted living)
Assessments
Nutritional survey, 6 minute walk, Mini-Mental Status Exam (MMSE), Center of Epidemiologic Studies (CES)-Depression Scale, 25-OH vitamin D
Results
At baseline, assisted-living dwelling elders appeared to have lower serum 25-OH vitamin D levels, lower MMSE scores, higher CES-depression scale scores, and walked shorter distances in the six minute walk. Serum 25-OH vitamin D levels and six minute walk were significantly different between the two groups using logistic regression analysis. As serum 25-OH vitamin D levels increased, the probability of an elder living in an ALF decreased, and as distance walked during the six minute walk increased, the probability of an elder living in an ALF decreased.
Conclusions
Elders living in assisted living facilities had significantly lower 25-OH vitamin D levels and walked shorter distances during the six minute walk. These variables can be used to predict the probability of an elder living in an assisted living facility. The lack of effect of nutrition suggests that the role of vitamin D in this setting is in physical function.
Introduction
The last US Census in 2000 counted 35 million people 65 years of age and older, a 12 % increase from 1990. Of those 35 million people, 1.5 million live in skilled nursing facilities (SNF) [1]. While the Census did not count the elders living in assisted living facilities (ALF), most long-term care is now provided outside SNFs [2], with an estimated 1.15 million people living in ALFs as of 1998 [3]. This number continues to grow as elders view assisted living as the preferred alternative to entering SNFs due to the lower cost, higher level of independence and the more “home-like” environment [4].
Assisted living encompasses several residential settings but generally includes support available 24 hours a day, facilitation of aging in place, and services and activities to promote independence and maintain dignity, autonomy, and privacy through a homelike environment [5–7]. This population is of interest because, compared to their community-dwelling cohort, ALF-dwelling elders are in general more physically impaired and vulnerable and exhibit a more depressive affect [8, 9]. There is also some evidence of higher rates of mortality in ALF dwelling elders [10]. In a two year comparison of functioning between ALF-dwelling and community-dwelling elders, Fonda et al. (2002) found that 10.9% fewer of the ALF residents had stable high functioning (independent in instrumental activities of daily living and activities of daily living) and 14% more moved into more care intensive institutions at the end of 2 years. Decline in function, discharge to SNFs and death accounted for approximately half of the ALF residents’ outcomes during the 2 year study [9]. ALFs can be considered a transitional setting to SNFs as many residents worsen and develop new morbidities, necessitating transfer to nursing homes (NH). In a one year longitudinal study of the outcomes of ALF residents, Zimmerman et al. (2005) reported that the annual rate of NH transfer was 21.3 per 100 residents. Probability of hospitalization over 100 days per 100 residents was 12.7 [11]. In addition, another study reported an average length of stay at an ALF of 3 years with reasons for leaving including moving to NH (33–36%) and hospital stays (11–18%) [8].
The purpose of this paper is to determine what variables separate ALF-dwelling elders from community dwelling elders and if in turn, those variables can be used to predict the probability of an elder living in an ALF. As it seems that ALF-dwelling elders are more physically impaired to begin with and are at higher risk of requiring more intensive care providing facilities in the future, by identifying factors that predisposed an elder to requiring relocation to an ALF, preventive measures may be put into place.
The variables that will be used are those related to frailty characteristics. Frailty has been defined as a biologic syndrome of increased vulnerability to stressors resulting from aging-associated declines in function and reserve across multiple physiologic systems and ultimately compromising the ability to maintain a stable homeostasis. Given this definition, several markers of frailty have been established: low strength, low energy, slowed motor performance, low physical activity, and/or unintentional weight loss [12]. Frailty is often a risk factor in elders entering ALFs and ALF-dwelling elders are, in general, frailer than community-dwelling elders [13–15]. In addition, factors that often plays a part in residents’ discharge from ALFs to SNFs, such as worsening morbidities, cognitive decline, and requiring more nursing assistance and help with ADLs [12, 16], are associated with increased frailty.
We hypothesize that a combination of variables, including physical function, cognition, mood, and nutritional factors will separate ALF-dwelling and community-dwelling elders and can be used to predict elders at risk of entering assisted living.
Methods
Secondary data analysis was performed on a data set obtained for a previous study [17]. One hundred fourteen individuals were evaluated in the study; 77 individuals from the community and 37 from assisted living environments. Assisted living was defined as congregate housing with meals and housekeeping provided and with nurse and home-health care availability. Inclusion criteria were living in assisted living or being age- and gender-matched control living in the community. There were no exclusion criteria for this study.
Physical function measures were collected based on the frailty phenotype described by Fried et al. [12] including reported weights loss of ≥ 10 pounds in the preceding year, grip strength measured by hand-held Jamar dynamometer, sense of exhaustion as evaluated by two question from the Center of Epidemiologic Studies-Depression Scale [18], walking speeding by an 8-foot walk, and level of physical activity reported in kcals/week using the Physical Activity Scale in the Elderly [19]. Individuals were reported as frail if they met criteria for 3 or more of the 5 characteristics. The six minute walk was performed as described by Guyatt et al [20]. Depression was assessed using the Center for Epidemiologic Studies-Depression (CESD) Scale [18] and cognitive status using the Folstein Mini-mental Status Exam (MMSE) [21]. Vitamin D and fat consumption were estimated using a standardized food log.
Serum 25-hydroxyvitamin D level was also measured. Serum was divided into 0.5 ml aliquots and stored at −70°C. 25-hydroxyvitamin D levels were measured by enzyme immunoassay (Immunodiagnostic Systems Inc., Fountain Hills, AZ) with an intra-assay CV of less than 6.6%.
Statistical Analysis
Summary statistics for baseline characteristics of ALF and community-dwelling samples were calculated using means (± standard deviations) or percentages (with counts), as appropriate. Differences between group proportions were evaluated with either ANOVA or chi-square tests. Continuous variables were checked for normality.
Total frailty score was calculated for each group by summing the number of qualifying frailty criteria. Frailty status was represented by classifying subjects into one of three categories: non-frail (Frailty score of 0), intermediate frail (Frailty score 1 or 2), or frail (frailty score 3–5). We then compared the proportion of frail (frailty score 3–5) to nonfrail/intermediate (frailty score 0–2) subjects.
Logistic regression was performed to identify variables that predict the likelihood of living in ALF or the community. The dependent variable was living situation. The model included age, gender, BMI, CESD score, MMSE score, serum 25-hydroxyvitamin D, daily intake of fat calories and 6-minute walk results. Odds ratios and 95% confidence intervals indicated the effect of each predictor and whether it met statistical significance. Logistic functions were calculated and graphed to depict the probability of living in an assisted living facility, based on significant predictors from the logistic regression model. Analyses were performed using SPSS (version 16.0.1, SPSS Inc., Chicago, Illinois).
Results
The subject characteristics are summarized in Table 1. Both groups were similar in average age, gender (predominantly female), BMI and intake of calories from fat. The assisted living participants appeared to have significantly higher depression scores, lower serum 25 OH vitamin D levels, lower MMSE scores and walked shorter distances in the six minute walk (Table 1).
Table 1.
Characteristics of subjects from community and assisted living facilities
| Community Living (77) | Assisted Living (37) | P Value | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age | 82.4 ± 4.6 | 83.3 ± 5.6 | 0.44 |
| Female | 56 (73%) | 25 (68%) | 0.57 |
| Body Mass Index (Kg/m2) | 24.5 ± 3.3 | 25.3 ± 3.5 | 0.29 |
| CES-depression score | 5.9 ± 6.2 | 9.5 ± 9.6 | 0.02 |
| MiniMental score | 28.3 ± 1.9 | 27.2 ± 2.7 | 0.009 |
| 25 OH Vitamin D (nmol/L) | 113.1 ± 40.1 | 81.8 ± 37.0 | <0.001 |
| Fat Calories | 506 ± 203 | 502 ± 260 | 0.94 |
| Six Minute Walk (feet) | 1026 ± 339 | 723 ± 370 | <0.001 |
| Frailty Criteria Total | 1.8 ± 0.9 | 2.4 ± 0.9 | 0.002 |
| Frail % (N) | 21% (16) | 46% (17) | 0.006 |
p<0.05 using chi square tests or analysis of variance
Logistic regression was used to test several factors for the likelihood of living in an assisted-living facility (Table 1). Serum 25-OH vitamin D levels and distance walked during the six minute walk were significantly associated with living situation (Table 2). Odds ratios revealed that decreases in serum 25-OH vitamin D and distance walked significantly increased the likelihood of being in an assisted living facility. Age, gender, BMI, CESD scores, MMSE scores, and calories from fat were not associated with ALF-dwelling or community-dwelling. Frailty was not entered into the model.
Table 2.
Logistic regression model evaluating predictors of assisted living
| Variables | Unstandardized Beta coefficients | Odds ratio | 95% CI | P value |
|---|---|---|---|---|
| Age | −0.011 | 0.989 | 0.90 to 1.08 | 0.80 |
| Gender (Males=1) | −0.325 | 0.723 | 0.23 to 2.33 | 0.59 |
| Body Mass Index (Kg/m2) | −0.039 | 0.962 | 0.82 to 1.13 | 0.64 |
| CES-depression score | 0.033 | 1.033 | 0.97 to 1.11 | 0.35 |
| MiniMental score | −0.132 | 0.877 | 0.70 to 1.10 | 0.26 |
| 25 OH Vitamin D (nmol/L) | −0.018 | 0.982 | 0.97 to 0.997 | 0.018 |
| Fat Calories | 0.000 | 1.0 | 0.997 to 1.00 | 0.81 |
| Six Minute Walk (feet) | −0.002 | 0.998 | 0.997 to 1.00 | 0.038 |
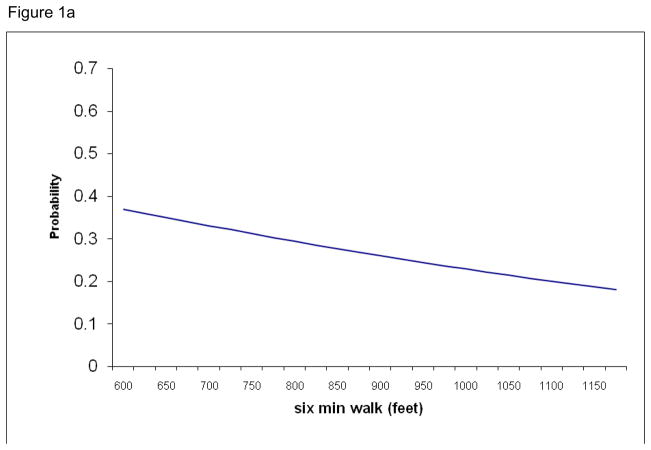
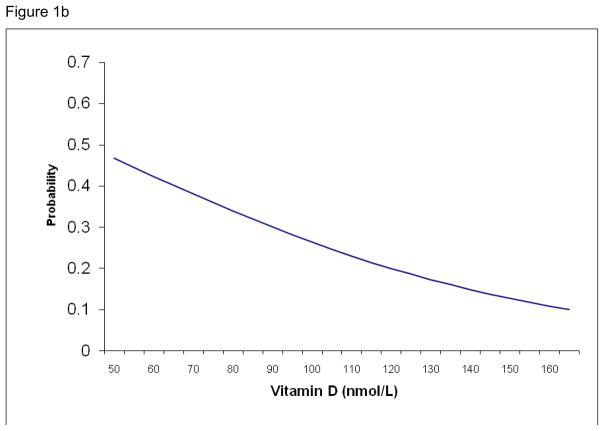
Using the results of the logistic regression, a logistic function was calculated for the two significant variables, six minute walk and serum 25-OH vitamin D. The probability of individuals living in an assisted living facility based on those variables was fitted to the logistic curves (Figures 1a and b). Figure 1a demonstrates the increased probability of an individual living in assisted living facility as they cover less distance during the six minute walk. For example, an individual who walked about 1100 feet in six minutes had a 20% chance of living in an ALF while someone who walked 650 feet in six minutes has an almost 35% probability of living in an ALF. Figure 1b shows that as serum 25-OH vitamin D levels decrease, the probability of individual living in ALF increases. The similar association is seen with serum 25-OH vitamin D: an individual with a vitamin D level of 53 nmol/L (approx. 21 ng/dL) has a 45% probability of living in an ALF while a two-fold increase in vitamin D level halves that probability.
Figure 1.
Figure 1a. Probability estimates for residency in an assisted-living facility by six minute walk
Figure 1b. Probability estimates for residing in an assisted living facility for every 5 nmol/L increase in serum 25-OH vitamin D
Discussion
Lower serum 25-OH vitamin D and six minute walk distance were significant predictors of living situation between community-dwelling and ALF-dwelling elders. These results were not surprising. Previous studies have shown that ALF-residents tend to be more physically impaired and vulnerable [8, 9, 11]. Thus, the fact that the six minute walk separates the two populations of community-dwelling and ALF-dwelling elders can be expected. Lower extremity function in elders over age 70 has been found to be predictive of subsequent disability [22, 23], and a study of assisted living residents testing grip strength, walking speed, chair rise and balance found better performance on those tests was associated with reduced risk of nursing home placement, fracture and decline over one year [24]. In addition, frail women are at increased risk of falls and fractures [25] and ALF-dwelling elders have increased risk factors for falls and fractures compared to community dwelling elders [17, 25].
Results relating to the serum 25-OH vitamin D levels warrant further examination. It is unclear whether the effect of vitamin D levels can be attributed to nutritional influences or to a physical function role. Additional data (not shown) demonstrated that the amount of daily protein consumed was not significant in separating the two populations, neither was calories consumed from fat, or BMI. This suggests that nutrition alone does not explain how vitamin D levels significantly affect an individual’s likelihood of living in an ALF.
The importance of vitamin D, in combination with calcium, in bone health has been well established. Multiple meta-analyses have shown a positive effect of vitamin D on fracture and fall incidence, with vitamin D supplementation reducing the risk of falls by more than 20% [26, 27]. Furthermore, another meta-analysis reported that serum 25-hydroxyvitamin D levels should exceed 74 nmol/L to prevent fractures in the elderly with optimal fracture prevention appearing with levels of up to 100 nmol/L [28]. A recent trial of in nursing homes, bread was fortified with 5000 IU of vitamin D3 to achieve serum 25-OH vitamin D levels of greater than 75 nmol/L and was found to increase bone density over 12 months [29].
Vitamin D’s effect is not only on the skeleton itself but also plays a role in extraskeletal health as well. Vitamin D deficiency has been associated with sarcopenia, weakness and gait instability [30–32] with supplementation leading to improvement in balance and physical performance [33, 34]. Furthermore, in a study performed in men and women 60 years and older, higher serum 25-OH vitamin D levels were associated with faster walking speeds and the ability to rise out of a chair faster [35]. Vitamin D supplementation has been associated with lower incidence of rheumatoid arthritis, lower risk of developing multiple sclerosis; and with deficiency associated with increased risk for type 1 and type 2 diabetes mellitus, metabolic syndrome, heart failure and sudden cardiac death [36–40]. It seems that vitamin D can affect an elder’s probability of living in an ALF in several ways, with an effect on physical functioning at the forefront.
Several other variables were tested that were not significant in separating the two populations. By direct comparison, MMSE and CES-depression scores were significantly different between the two groups. However, these factors did not significantly contribute to the logistic regression analysis. These tests were considered because depressed mood has been found to be more prevalent in the assisted-living population of elders compared to community dwelling elders [41] and has been associated with increased risk of strength decline in older men, especially in combination with low body weight [42]. Depressed elders are also at increased risk for death, impairment in ADLs and IADLs, and cognitive impairment [43, 44]. Cognitive impairment has been associated with increased mortality, unintended weight loss, functional disability, and increased frailty [45–48].
In addition to measures of cognitive function and mood, nutritional assessments were initially included. Poor appetite, weight loss and subsequent sarcopenia and visceral protein depletion has been suggested as contributors to frailty [49], and frail elders have demonstrated lower levels of body fat with corresponding lower leptin levels [50]. If the effects of nutritional deficiency were contributory, they must have been an indirect effect that affected the six minute walk.
Limitations of this study included a small study population. Further, results were a cross-section and thus comments about causal relationships cannot be made. Further studies to confirm the relationship among vitamin D, six minute walk and living situation are warranted.
Conclusion
Elders living in assisted living facilities had significantly lower serum 25-OH vitamin D levels and walked shorter distances during the six minute walk compared to their community-dwelling counterparts. The data suggests that as serum 25-OH vitamin D levels decrease and distance walked during the six minute walk decrease, probability of an individual living in an ALF increases. While the lack of effect of nutrition suggests that the role of vitamin D is through physical function, further research is warranted to examine the relationship among vitamin D, physical function and living situation.
Acknowledgments
This work has been supported by a grant from the Society for Clinical Densitometry and the General Clinical Research Center (MO1-RR06192).
Footnotes
All authors give final approval to the manuscript submitted. We certify that no affiliations with or financial involvement with any organization with a financial interest are present.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hetzel L, Smith A. The 65 years and Over Population: 2000 October 2001. US Census Bureau; 2000. p. 8. [Google Scholar]
- 2.Bishop CE. Where are the missing elders? The decline in nursing home use, 1985 and 1995. Health Aff (Millwood) 1999 Jul–Aug;18(4):146–155. doi: 10.1377/hlthaff.18.4.146. [DOI] [PubMed] [Google Scholar]
- 3.American_Health_Care_Association; NCfA. Facts and trends: The assisted living sourcebook In: N.C.f.A. Living . 1998 Washington, DC: 1998. [Google Scholar]
- 4.Kane RA. Long-term care and a good quality of life: bringing them closer together. Gerontologist. 2001 Jun;41(3):293–304. doi: 10.1093/geront/41.3.293. [DOI] [PubMed] [Google Scholar]
- 5.Hawes CaP, Charles, Rose, Miriam, Holan, Scott, Sherman, Michael A National Survey of Assisted Living Facilities. The Gerontologist. 2003;43(6):8. doi: 10.1093/geront/43.6.875. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerman S, Sloane PD. Definition and classification of assisted living. Gerontologist. 2007;47 Spec(3):33–39. doi: 10.1093/geront/47.supplement_1.33. [DOI] [PubMed] [Google Scholar]
- 7.Zimmerman S, Gruber-Baldini AL, Sloane PD, et al. Assisted living and nursing homes: apples and oranges? Gerontologist. 2003 Apr;43 Spec(2):107–117. doi: 10.1093/geront/43.suppl_2.107. [DOI] [PubMed] [Google Scholar]
- 8.Golant SM. Do Impaired Older Persons With Health Care Needs Occupy U.S. Assisted Living Facilities? An Analysis of Six National Studies. Journal of Gerontology: Social Sciences. 2004;59B(2):12. doi: 10.1093/geronb/59.2.s68. [DOI] [PubMed] [Google Scholar]
- 9.Fonda SJ, Clipp EC, Maddox GL. Patterns in functioning among residents of an affordable assisted living housing facility. Gerontologist. 2002 Apr;42(2):178–187. doi: 10.1093/geront/42.2.178. [DOI] [PubMed] [Google Scholar]
- 10.Stones MJ, Dornan B, Kozma A. The prediction of mortality in elderly institution residents. J Gerontol. 1989 May;44(3):P72–79. doi: 10.1093/geronj/44.3.p72. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerman S, Sloane PD, Eckert JK, et al. How good is assisted living? Findings and implications from an outcomes study. J Gerontol B Psychol Sci Soc Sci. 2005 Jul;60(4):S195–204. doi: 10.1093/geronb/60.4.s195. [DOI] [PubMed] [Google Scholar]
- 12.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 13.Winograd CH. Targeting strategies: an overview of criteria and outcomes. J Am Geriatr Soc. 1991 Sep;39(9 Pt 2):25S–35S. doi: 10.1111/j.1532-5415.1991.tb05930.x. [DOI] [PubMed] [Google Scholar]
- 14.Winograd CH, Gerety MB, Chung M, Goldstein MK, Dominguez F, Jr, Vallone R, Winograd CH, et al. Screening for frailty: criteria and predictors of outcomes. J Am Geriatr Soc. 1991 Aug;39(8):778–784. doi: 10.1111/j.1532-5415.1991.tb02700.x. [DOI] [PubMed] [Google Scholar]
- 15.Speechley M, Tinetti M, Tinetti M. Falls and injuries in frail and vigorous community elderly persons. J Am Geriatr Soc. 1991 Jan;39(1):46–52. doi: 10.1111/j.1532-5415.1991.tb05905.x. [DOI] [PubMed] [Google Scholar]
- 16.Aud MA, Rantz MJ. Admissions to skilled nursing facilities from assisted living facilities. J Nurs Care Qual. 2005 Jan–Mar;20(1):16–25. doi: 10.1097/00001786-200501000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Kenny AM, Smith J, Noteroglu E, et al. Osteoporosis risk in frail older adults in assisted living. J Am Geriatr Soc. 2009 Jan;57(1):76–81. doi: 10.1111/j.1532-5415.2008.02072.x. [DOI] [PubMed] [Google Scholar]
- 18.Orme JG, Reis J, Herz EJ. Factorial and discriminant validity of the Center for Epidemiological Studies Depression (CES-D) scale. J Clin Psychol. 1986 Jan;42(1):28–33. doi: 10.1002/1097-4679(198601)42:1<28::aid-jclp2270420104>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 19.Washburn RA, Smith KW, Jette AM, Janney CA, Washburn RA, et al. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993 Feb;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 20.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985 Apr 15;132(8):919–923. [PMC free article] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB, Guralnik JM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995 Mar 2;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000 Apr;55(4):M221–231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giuliani CA, Gruber-Baldini AL, Park NS, et al. Physical performance characteristics of assisted living residents and risk for adverse health outcomes. Gerontologist. 2008 Apr;48(2):203–212. doi: 10.1093/geront/48.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ensrud KE, Ewing SK, Taylor BC, et al. Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2007 Jul;62(7):744–751. doi: 10.1093/gerona/62.7.744. [DOI] [PubMed] [Google Scholar]
- 26.O’Donnell S, Moher D, Thomas K, Hanley DA, Cranney A, et al. Systematic review of the benefits and harms of calcitriol and alfacalcidol for fractures and falls. J Bone Miner Metab. 2008;26(6):531–542. doi: 10.1007/s00774-008-0868-y. [DOI] [PubMed] [Google Scholar]
- 27.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of Vitamin D on falls: a meta-analysis. JAMA. 2004 Apr 28;291(16):1999–2006. doi: 10.1001/jama.291.16.1999. [DOI] [PubMed] [Google Scholar]
- 28.Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B, et al. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005 May 11;293(18):2257–2264. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 29.Mocanu V, Stitt PA, Costan AR, et al. Long-term effects of giving nursing home residents bread fortified with 125 microg (5000 IU) vitamin D(3) per daily serving. Am J Clin Nutr. 2009 Apr;89(4):1132–1137. doi: 10.3945/ajcn.2008.26890. [DOI] [PubMed] [Google Scholar]
- 30.Szulc P, Duboeuf F, Marchand F, Delmas PD, et al. Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: the MINOS study. Am J Clin Nutr. 2004 Aug;80(2):496–503. doi: 10.1093/ajcn/80.2.496. [DOI] [PubMed] [Google Scholar]
- 31.Visser M, Deeg DJ, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003 Dec;88(12):5766–5772. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- 32.Lau EM, Lynn HS, Woo JW, Kwok TC, Melton LJ, 3, Lau EM, et al. Prevalence of and risk factors for sarcopenia in elderly Chinese men and women. J Gerontol A Biol Sci Med Sci. 2005 Feb;60(2):213–216. doi: 10.1093/gerona/60.2.213. [DOI] [PubMed] [Google Scholar]
- 33.Pfeifer M, Begerow B, Minne HW, Abrams C, Nachtigall D, Hansen C, et al. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res. 2000 Jun;15(6):1113–1118. doi: 10.1359/jbmr.2000.15.6.1113. [DOI] [PubMed] [Google Scholar]
- 34.Bischoff HA, Stahelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003 Feb;18(2):343–351. doi: 10.1359/jbmr.2003.18.2.343. [DOI] [PubMed] [Google Scholar]
- 35.Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am J Clin Nutr. 2004 Sep;80(3):752–758. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 36.Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG, Merlino LA, et al. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women’s Health Study. Arthritis Rheum. 2004 Jan;50(1):72–77. doi: 10.1002/art.11434. [DOI] [PubMed] [Google Scholar]
- 37.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A, Munger KL, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006 Dec 20;296(23):2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 38.Mathieu C, Gysemans C, Giulietti A, Bouillon R, et al. Vitamin D and diabetes. Diabetologia. 2005 Jul;48(7):1247–1257. doi: 10.1007/s00125-005-1802-7. [DOI] [PubMed] [Google Scholar]
- 39.Ford ES, Ajani UA, McGuire LC, Liu S, Ford ES, et al. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care. 2005 May;28(5):1228–1230. doi: 10.2337/diacare.28.5.1228. [DOI] [PubMed] [Google Scholar]
- 40.Pilz S, Marz W, Wellnitz B, et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab. 2008 Oct;93(10):3927–3935. doi: 10.1210/jc.2008-0784. [DOI] [PubMed] [Google Scholar]
- 41.Grayson P, Lubin B, Van Whitlock R. Comparison of depression in the community-dwelling and assisted-living elderly. J Clin Psychol. 1995 Jan;51(1):18–21. doi: 10.1002/1097-4679(199501)51:1<18::aid-jclp2270510104>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 42.Rantanen T, Penninx BW, Masaki K, Lintunen T, Foley D, Guralnik JM, et al. Depressed mood and body mass index as predictors of muscle strength decline in old men. J Am Geriatr Soc. 2000 Jun;48(6):613–617. doi: 10.1111/j.1532-5415.2000.tb04717.x. [DOI] [PubMed] [Google Scholar]
- 43.Gallo JJ, Rabins PV, Lyketsos CG, Tien AY, Anthony JC, Gallo JJ, et al. Depression without sadness: functional outcomes of nondysphoric depression in later life. J Am Geriatr Soc. 1997 May;45(5):570–578. doi: 10.1111/j.1532-5415.1997.tb03089.x. [DOI] [PubMed] [Google Scholar]
- 44.Penninx BW, Leveille S, Ferrucci L, van Eijk JT, Guralnik JM, Penninx BW, et al. Exploring the effect of depression on physical disability: longitudinal evidence from the established populations for epidemiologic studies of the elderly. Am J Public Health. 1999 Sep;89(9):1346–1352. doi: 10.2105/ajph.89.9.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riccio D, Solinas A, Astara G, Mantovani G, et al. Comprehensive geriatric assessment in female elderly patients with Alzheimer disease and other types of dementia. Arch Gerontol Geriatr. 2007;44(Suppl 1):343–353. doi: 10.1016/j.archger.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 46.van Dijk PT, van de Sande HJ, Dippel DW, Habbema JD, van Dijk PT, et al. The nature of excess mortality in nursing home patients with dementia. J Gerontol. 1992 Mar;47(2):M28–34. doi: 10.1093/geronj/47.2.m28. [DOI] [PubMed] [Google Scholar]
- 47.Landi F, Onder G, Cattel C, et al. Functional status and clinical correlates in cognitively impaired community-living older people. J Geriatr Psychiatry Neurol Spring. 2001;14(1):21–27. doi: 10.1177/089198870101400106. [DOI] [PubMed] [Google Scholar]
- 48.Samper-Ternent R, Al Snih S, Raji MA, Markides KS, Ottenbacher KJ, et al. Relationship between frailty and cognitive decline in older Mexican Americans. J Am Geriatr Soc. 2008 Oct;56(10):1845–1852. doi: 10.1111/j.1532-5415.2008.01947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vanitallie TB. Frailty in the elderly: contributions of sarcopenia and visceral protein depletion. Metabolism. 2003 Oct;52(10 Suppl 2):22–26. doi: 10.1016/s0026-0495(03)00297-x. [DOI] [PubMed] [Google Scholar]
- 50.Hubbard RE, O’Mahony MS, Calver BL, Woodhouse KW, Hubbard RE, et al. Nutrition, inflammation, and leptin levels in aging and frailty. J Am Geriatr Soc. 2008 Feb;56(2):279–284. doi: 10.1111/j.1532-5415.2007.01548.x. [DOI] [PubMed] [Google Scholar]