Introduction
The heart is a sophisticated organ that continuously pumps blood to ensure that oxygen and nutrients reach the brain, other organs, and peripheral tissue. The right side of the heart pumps blood to the lungs, where oxygen is taken up and carbon dioxide is removed. The left side of the heart then pumps blood to the rest of the body, where oxygen and nutrients are delivered to tissues. This cycle is sustained by the repeated contraction of cardiomyocytes, specialized muscle cells that are designed to contract and rapidly respond to physiological stimuli.
Normally, contraction of atrial and ventricular myocytes is activated by action potentials (AP) that originate in the sinoatrial (SA) node in the wall of the right atrium; often referred to as the heart's pacemaker. Coupling of this electrical signal to contraction (EC coupling) in cardiac myocytes initiates with Ca2+ influx through L-type Ca2+ channels, which activates intracellular Ca2+ release via ryanodine receptors located in the sarcoplasmic reticulum (SR), and thus induce a global increase in [Ca2+]i that triggers contraction1, 2. The rate at which the SA node myocytes fire action potentials is modulated by two opposing nervous systems. The sympathetic nervous system utilizes the catecholamine hormones noradrenaline and adrenaline to increase the force and rate of atrial and ventricular contraction. In contrast, the parasympathetic nervous system releases the neurotransmitter acetylcholine to reduce action potentials from the SA node. Together these neural systems ensure that cardiac output is matched to the physiological needs of the organism, a process that is often termed “stimulus-response coupling”.
Although numerous signal transduction cascades are operational in cardiomyocytes, G-protein coupled receptor (GPCR) signaling pathways play a prominent role. Agonists such as noradrenaline, adrenaline, angiotensin II and endothelin-1 promote the interaction of their respective receptor with a G-protein heterotrimer comprising α, β, and γ subunits 3. This transient interaction promotes exchange of GTP for GDP on the Gα subunit and thereby activates or inhibits effector molecules and ion channels. Effector molecules, which include enzymes such as adenylate adenylyl cyclases and phospholipases, regulate the production of second messengers 3.
Second messengers are small molecules that are mobilized or generated in response to extracellular stimuli. In the heart, Ca2+ and cAMP are the second messengers most frequently used by cardiac signaling pathways. They act at defined intracellular sites to initiate signaling events 4 that control excitability, contraction, and gene expression. Occupancy of ß-adrenergic receptors engages the cAMP-signaling pathway to activate protein kinases, guanine nucleotide exchange factors and ion channels 5, 6. Combinations of these cAMP responsive enzymes modulate cardiac contraction force (Inotropy), heart rate (chronotropy), and muscle relaxation (lusitropy) 7. Not surprisingly, there is a significant degree of crosstalk between the Ca2+ and cAMP signaling pathways. This is achieved in part because Ca2+ and cAMP responsive proteins are often brought together in multiprotein complexes 8. The goal of this review is to highlight recent progress on our understanding of how anchored signaling complexes influence cardiomyocyte physiology. We will focus on cardiomyocyte A-Kinase Anchoring Proteins (AKAPs), a family of scaffolding proteins that compartmentalize cAMP and Ca2+ responsive enzymes in proximity to preferred substrates such as ion channels, contractile proteins, Ca2+ pumps and the transcriptional machinery.
A-Kinase Anchoring proteins
Seminal experiments in the 1950's demonstrated that hormone mediated stimulation of cAMP synthesis by different agonists induced distinctive physiological outputs, even within the same tissue 9. Thirty years later it was shown that adrenergic stimulation selectively activated a pool of the cAMP dependent protein kinase (PKA) associated with the particulate fraction of cardiomyocytes, whereas prostoglandin E1 stimulation activated a cytosolic pool of PKA in the same cells to induce different physiological effects 10. As more investigators pondered this concept, it became clear that cAMP was not uniformly distributed throughout the cell 11-14. This led to the hypothesis that the opposing actions of adenylyl cyclases (ACs) and phosphodiesterases (PDEs) generate intracellular gradients and compartmentalized pools of cAMP 15, 16. More recently a genetically encoded fluorescence based biosensor was successfully used to measure microdomains of cAMP along sarcomeric Z lines in cardiomyocytes in response to adrenergic stimulation 17.
Although it was accepted that cAMP levels were unevenly distributed within the cell, it remained unclear exactly how PKA was retained within different subcellular compartments 18. Soon a variety of protein-protein interaction screens identified a set of molecules that interact with the R subunits of the PKA holoenzyme (a tetramer consisting of an regulatory (R) subunit dimer and two catalytic (C) subunits) 19, 20. These proteins were named A-Kinase Anchoring Proteins in recognition of their ability to anchor PKA at defined subcellular locations 21. A principle function of AKAPs is to position PKA and other cAMP responsive enzymes in proximity to their substrates 6, 22, 23. However, AKAPs also serve to cluster regulatory enzymes with the cAMP synthesis machinery 24, 25. For example, interactions between AKAP79/150 and the adenylyl cyclase isoforms ACV or ACVI facilitate the preferential PKA phosphorylation of the enzyme to inhibit cAMP synthesis. Such a PKA-AKAP79/150-ACV complex forms a negative feedback loop that generates pulses of cAMP production 24. This configuration may be particularly relevant in the pacemaker cells since studies in ACV knockout mice suggest that this adenylyl cyclase isoform participates in sympathetic and parasympathetic regulation of cardiac contractility 26, 27.
Functional analysis of AKAP action often takes advantage of reagents that can displace PKA from anchoring proteins. Detailed biochemical analyses have demonstrated that R subunit dimerization is necessary for PKA interaction with AKAPs and that each anchoring protein contains a reciprocal binding sequence of 14-18 amino acids that form an amphipathic helix 28, 29. One of these proteins, initially called Ht31, but now known as AKAP-Lbc, contained an 18 amino acid sequence that can be used as a peptide disruptor of RII-AKAP interactions inside cells 19. The utility of Ht31 peptide as a universal PKA anchoring disruptor was initially demonstrated upon perfusion into cultured hippocampal neurons to disrupt the location of PKA in relation to a key substrate, the AMPA type glutamate receptor 30. Later it was shown that perfusion of Ht31 peptide into cardiomyocytes uncoupled cAMP dependent regulation of the L-type calcium channel in 31. More recently adenoviral gene transfer of Ht31 analogs into rat hearts has suggested that anchored pools of PKA contribute to ß-adrenergic stimulation of cardiomyocyte contractility 32, 33. Mislocalization of PKA alters the phosphorylation of key proteins that participate in this process such as L-type Ca channels, the ryanodine receptors, phospholamban and myofibullar tropinin I 34, 35. Calcium imaging and echocardiogram analyses conclude that disruption of PKA anchoring (1) affects cardiac contractility as measured by changes in the rate of calcium transients and (2) increases the left ventricular ejection fraction and stroke volume 33. These studies provide a compelling rationale for more mechanistic studies to assess the contribution of individual AKAP complexes in the regulation of different aspects of cardiac function under normal and pathophysiological conditions.
AKAPs signal cardiac contractility
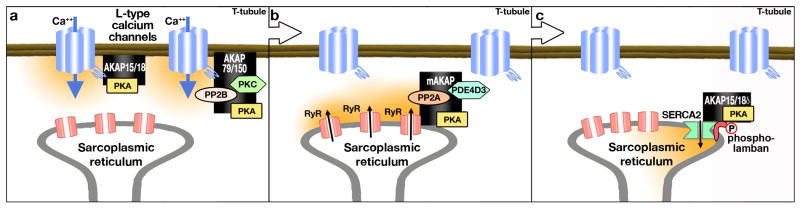
As noted above, EC coupling is the process whereby an action potential triggers a myocyte to contract. This involves a transient raise in intracellular calcium that drives contraction [reviewed by Bers 2008 2]. From a signaling perspective, EC coupling can be considered to occur in three phases (Figure 1 a-c). Phase 1 is initiated by brief openings of voltage-gated L-type Ca2+ channels. Small amounts of Ca2+ enter specialized regions of the ventricular myocyte where the junctional SR is close to the sarcolemma 36-38. In phase 2, this localized Ca2+ influx triggers the synchronous activation of multiple ryanodine-sensitive Ca2+ channels (RyRs) in the SR to produce a global Ca2+ transient 39 40. The concomitant activation of Ca2+ responsive contractile proteins such as cardiac troponin C initiates contraction. Phase 3, requires termination of SR Ca2+ release and the transport of Ca2+ back into the SR through the ATP dependent Ca2+ pump SERCA2 (sarcoplasmic/endoplasmic reticulum Ca2+ pump 2), which decreases [Ca2+]i and begins myocyte relaxation. Recent evidence suggests that distinct AKAP complexes contribute to each phase of EC coupling by optimizing the phosphorylation of ion channels and contractile proteins.
Figure 1. Schematic diagram of the role of AKAPs in Excitation-Contraction coupling in cardiomyocytes.
a) Phase 1 of EC coupling: AKAP79/150 and the short forms of AKAP15/18 (α and β) maintain signaling complexes tethered to L-type calcium channels to enable calcium influx. b) Phase 2 of EC coupling: Ryanodine receptors at the sarcoplasmic reticulum are bound to the mAKAP signaling complex as it responds to the increased cytosolic Ca++. mAKAP clusters PKA, PDE4D3 and PP2A to regulate the phosphorylation status of the Ryr and the resultant Ca++ release from the SR. c) Phase 3 of EC coupling: Active transport of calcium back into the SR via SERCA2 is regulated by phospholamban. The AKAP15/18δ long isoform brings PKA into complex with phospholamban and SERC2 where it can augment the phosphorylation of phospholamban and the reuptake of calcium into the SR.
The junction between the sarcolemma and the SR is decorated with local Ca2+ signaling complexes that contain between 10-25 L-type Ca2+ channels and about 100-200 RyRs2. ß-andrenergic agonists stimulate PKA to increase the amplitude of L-type Ca2+ currents to favor Ca2+ influx. The AKAP79/150 family of anchoring proteins (human AKAP79, bovine AKAP75 and murine AKAP150) facilitates this process by directing the phosphorylation L-type Ca2+ channel subunits 41, 42 (Figure 1a). Biochemical and electrophysiological studies initially showed that expression of recombinant AKAP79 enhanced the cAMP dependent stimulation of L-type Cav1.2 currents 43. AKAP79/150 also associates with the calcium calmodulin dependent phosphatase PP2B and a variety of calcium phospholipid dependent protein kinase (PKC) isoforms 44-46. These additional AKAP79/150 binding partners are important in other aspects of cardiac myocyte function 47.
Recent studies suggest that AKAP79/150 has multiple regulatory roles in the cardiovascular system. For example, AKAP79/150 associated pools of PKCα have been implicated in the induction of persistent Ca2+ sparklets; local Ca2+ signals produced by recurrent openings of L-type Ca2+ channels in arterial myocytes that enhances vascular tone 48(Figure 1a). Arterial myocytes isolated from AKAP150 knockout mice do not generate persistent Ca2+ sparklets. Furthermore, AKAP150-/- mice are naturally hypotensive and do not develop angiotensin II-induced hypertension 48. Collectively, these results suggest that local modulation of L-type Ca2+ channels by AKAP150-targeted pools of PKA, PKCα, and PP2B may control different aspects of Ca2+ influx that contribute to cardiac EC coupling, vascular tone, and changes in blood pressure.
Another AKAP also modulates cardiac L-type Ca2+ channels. Alternate splicing of the AKAP15/18 gene yields several isoforms that reside in distinct subcellular locations 49. The α & ß isoforms of AKAP15/18 are found at the plasma membrane in complex with L-type Ca2+ channels 50, 51. Lipid modification of three residues in the extreme amino terminus of the anchoring protein are required for association with the inner face of the plasma membrane whereas a leucine zipper like motif in the carboxyl terminus interacts with the cytoplasmic domain of the L-type Ca2+ channel 50, 52. This arrangement ensures that an anchored pool of PKA is optimally positioned and available to phosphorylate the channel (Figure 1a). Experimental support for this notion has been provided by electrophysiological analysis showing that expression of a targeting-defective mutant of AKAP15/18α lacking the sites of lipid modification reduces the rate of cAMP dependent stimulation of L-type Ca2+ currents 50. Complementary studies have shown that peptide-mediated disruption of the AKAP15/18α-channel interaction with a leucine zipper peptide mimetic has similar effects on myocyte L-type Ca2+ currents 52 (Figure 1a). A leucine-like-zipper motif has also been identified in AKAP79/150 53 (Figure 1a). Thus, it would appear that cardiac myocytes utilize AKAP15/18α or AKAP79/150 to modulate the entry of extracellular Ca2+ through L-type Ca channels 54, 55. Although both anchoring proteins seem equally adept in coordinating cAMP signaling events that initiate EC coupling, by virtue of their ability to anchor PKA it may be worthy to note that AKAP79/150 has the capacity to recruit additional binding partners such as PKC's and the phosphatase PP2B into its channel associated complex 56, 57. In addition, PP2B may also interact directly with cardiac L-type Ca2+ channels as phosphatase binding sites have been mapped to the cytoplasmic regions of the α1 1.2 subunit of the channel 58. The formation of these more sophisticated multiprotein signaling complexes that are mediated by AKAP79/150 may permit the more precise transmission of second messenger signals to this Ca2+ channel. Another advantage of the formation of more sophisticated AKAP signaling complexes in proximity to Ca2+ channels may permit the integration of cAMP and Ca/phospholipid signals at this intracellular locus.
The second phase of EC coupling begins when openings of L-type Ca2+ channels cause a local increase of [Ca2+]i from 100 nM to about 10-20 μM that activates nearby RyR to release Ca2+ from the SR (Figure 1b). Planar bilayer work suggests that phosphorylation of the RyR by PKA increases the sensitivity of the channel to Ca2+ and accelerates the kinetics of adaptation 59. In the intact cell, PKA is recruited to the RyR complex by the muscle selective anchoring protein (mAKAP) 60-63. mAKAP is a multivalent anchoring protein that brings PKA and the cAMP metabolizing enzyme phosphodiesterase PDE4D3 to the RyR 64-66. The clustering of a cAMP metabolizing enzyme with PKA limits the availability of cAMP to ensure that kinase activation is transient 64 (Figure 1b). Furthermore, the phosphorylation state of the RyR is constantly monitored by the mAKAP associated phosphatase PP2A 60. Loss of anchored PDE4D3 and hyperactivation of the anchored PKA, may correlate with the onset of “leaky” channels found in certain models for exercise induced cardiac arrhythmias and heart failure 67-69. However an equally plausible explanation is that compensatory changes in Ca content of the SR make the RyR leaky during arrythmias. These latter events could be critical in determining whether abnormal Ca waves and sparks are propagated 70.
The third phase of EC coupling, involves active transport of Ca2+ back into the lumen of the sarcoplasmic reticulum to intiate myocyte relaxation (Figure 1c). The SERCA2 pump and its modulator protein phospholamban control this process 71-73. Monomeric phospholamban is a 52 amino acid protein that represses SERCA2. However, ß-adrenergic stimulation favors PKA phosphorylation of phospholamban. This promotes its pentamerization and the de-repression of SERCA2 72, 74, 75. Precise regulation of this system is achieved in part because all of the components are organized into supramolecular complex consisting of the SERCA2, phospholamban, PKA and a high molecular weight isoform of AKAP15/18 76, 77. Elegant biochemical and imaging studies show that disruption of PKA interaction with the AKAP15/18δ isoform interferes with the phosphorylation of phospholamban (Figure 1c). This prevents the release of phospholamban from SERCA2 to blunt Ca2+ re-uptake and myocyte relaxation 76. Reversible control of the cAMP response may be provided by an anchored pool of type 4 phosphodiesterase which is believed to associate with AKAP15/18δ 78, 79.
AKAP signaling under pathophysiolgical conditions
Adverse genetic profiles and unfavorable environmental factors contribute to the etiology of many cardiac disorders. Since AKAPs orchestrate signaling events that modulate cardiac contractility, it seems reasonable that defects in anchoring protein genes or pathophysiological changes in AKAP signaling complexes may underlie certain heart diseases. Several recent reports now support this notion.
Sudden death due to arrhythmia kills over 400,000 people a year in the USA and many more through out the world. As a result there is a concerted international effort to identify genes that regulate heart rhythm. Several independent findings point toward a role for a dual function anchoring proteins (d-AKAP-2) in heart rhythm control. A genetic screen of single-nucleotide polymorphisms (SNPs) in DNA pools of 6500 age-stratified healthy, European, Asian and American individuals detected an amino acid change from Ile to Val at position 646 in d-AKAP-2 (AKAP10) 80. Individuals harboring the d-AKAP-2 646Val variant often exhibited a decrease in the length of the PR interval on electrocardiograms (the time from the onset of atrial depolarization to the beginning of ventricular depolarization). In vitro biochemical analyses of the d-AKAP-2 646Val variant suggested that this amino acid substitution in the PKA anchoring domain of the protein enhanced its affinity for the type 1 PKA holoenzyme three fold 81. Although a mechanistic connection between these two observations remains unclear, it is worthy to note d-AKAP-2 mutant mice expressing a truncated form of d-AKAP2 which are unable to anchor PKA develop cardiac arrhythmias and die prematurely 82. Furthermore cultured myocytes isolated from these mice were reported to display an increased contractile response to cholinergic signals 82.
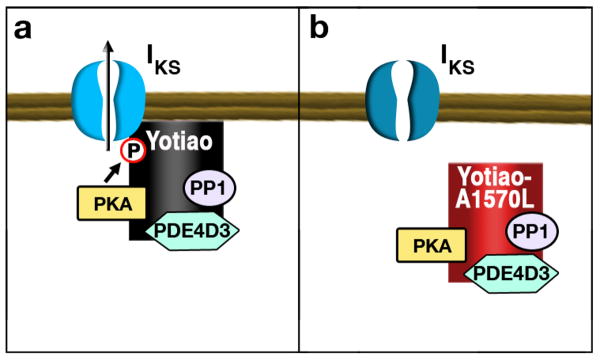
Long QT syndrome is a rare congenital heart condition that can result in sudden death as a consequence of exercise or excitement induced arrhythmia. This condition occurs because of the delayed repolarization of myocyte membranes. This prolongs the duration of the ventricular action potential, and thus is diagnosed as a lengthening of the QT interval. Myocyte repolarization requires the movement of potassium ions out of the cell. Consequently, the most common type of long QT syndrome (LQT1) is associated with mutations in KCNQ1 subunit of a potassium channel 83. The anchoring protein Yotiao associates with a variety of ion channels, including the KCNQ1 subunit responsible for IKS currents that shape cardiac action potential duration in response to ß-adrenergic agonists 84. The primary function of Yotiao is to facilitate phosphorylation of Ser27 on KCNQ1 to modulate ion channel activity, however anchored PKA also phosphorylated serine 43 on Yotiao to enhance cAMP-dependent activation of IKS 85, 86. Inherited mutations in another region of Yotiao (S1570L) that reduce the interaction between KCNQ1 suppress cAMP-induced phosphorylation of the IKS channel. The functional consequence of the S1570L mutation in mice is delayed repolarization of the ventricular action potential, a recognized symptom of LQT1 87 (Figure 2a). Taken together these findings suggest that spatial organization of the cAMP signaling enzymes by Yotiao is important to orchestrate ion channel activity during myocyte repolarization 88. More recent evidence suggests that the phosphodiesterase PDE4D3, a negative regulator of anchored PKA, is also present in the Yotiao signaling complex (Figure 2a). Electrophysiological studies have shown that pharmacological inhibition of anchored PDE activity enhances cAMP dependent stimulation of IKS 89. In this context the recruitment of a PDE4D3 constrains the local availability cAMP to more precisely regulate the anchored pool of PKA.
Figure 2. Schematic diagram of Yotiao and Iκs currents.
a) In myocytes Yotiao associates with the KCNQ1 subunit of the Iκs potassium channel. Anchored PKA phosphorylation of Yotiao enhances activation of the channel. b) The S1570L Yotiao mutant has reduced interaction with KCNQ1 and delayed repolarization of ventricular action potential.
Another means to vary the modulation of signaling scaffolds may be to induce time dependent changes in the composition or amount of AKAP complexes. There have been recent reports suggesting that temporal changes in AKAP complexes can happen; either in response to acute physiochemical changes in the environment or more gradually as a cellular adaptation to stress. For example, activation of ß-adrenergic receptors during periods of cardiac stress initially improves cardiac output by increasing heart rate and contractility. However, chronic mobilization of the same signaling pathway ultimately harms the heart 90.
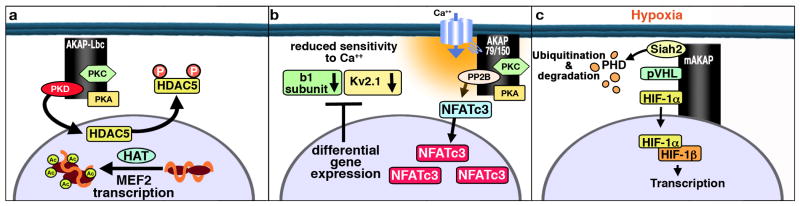
Elevated catecholamines in the heart evoke transcriptional activation of the Myocyte Enhancer Factor (MEF) pathway to induce a cellular response known as pathological myocardial hypertrophy 91, 92. This initiates a developmental gene reprogramming paradigm known as the “fetal gene response” 93, 94. A clearer picture of how individual steps in this hypertrophic signaling pathway are linked is beginning to emerge. The anchoring protein AKAP-Lbc is one of the genes upregulated in hypertrophic myocytes and functions as a scaffolding protein for PKA and PKC to mediate activation of a third enzyme, protein kinase D (PKD1) 95 (Figure 3a). A combination of cellular and live cell imaging approaches support a model where AKAP-Lbc facilitates activation of protein kinase D, which in turn phosphorylates the histone deacetylase HDAC5 to promote its nuclear export. Finally, the concomitant reduction in nuclear histone deacetylase activity favors MEF2 transcription and the onset of cardiac hypertrophy 96. Although most of this study was conducted in neonatal rat cardiomyocytes, further support for this concept was provided by analysis of human heart tissue samples obtained postmortem from individuals exhibiting hypertrophic cardiomyopathy, showing that AKAP-Lbc mRNA increased 2 ± 0.5-fold over normal age-matched patient controls 96. Since myocardial hypertrophy is extremely common, affecting 14%–18% of the general adult population, further investigation of the AKAP-Lbc/PKD/HDAC5 is warranted to establish whether AKAP-Lbc is a valid biomarker for hypertrophic cardiomyopathy.
Figure 3. Schematic diagram of the role of AKAPs in transcriptional regulation in the heart.
a) An upregulation of AKAP-Lbc facilitates PKD activation and phosphorylation of HDAC5, leading to transcriptional activation of the MEF pathway and the onset of hypertrophy. b) Calcium mediated activation of AKAP150-targeted PP2B leads to dephosphorylation of NFATc3 in arterial myocytes. Dephosphorylated NFATc3 accumulates in the nucleus where it leads to decreased gene expression of channel subunits. c) mAKAP organizes ubiquitin E3 ligases that managed the stability of the transcription factor HIF-1α During hypoxia, HIF1α translocated to the nucleus initiates transcription of proangiogenic, metabolic, and antiapoptotic genes that promote cell survival during hypoxia.
AKAP79/150 may also be involved in pathophysiological changes of gene expression that occur in vascular smooth muscle during the development of hypertension 97. In these cells, activation of PKCα induces persistent Ca2+ sparklets, producing an increase in local Ca2+ influx that activates nearby AKAP150-targeted PP2B. Upon activation, PP2B dephosphorylates NFATc3 allowing this transcription factor to translocate into the nucleus of arterial myocytes where it modulates gene expression (Figure 3b). The AKAP79/150 associated phosphatase PP2B and NFATc3 activities are low in arterial smooth muscle under physiological conditions because of low levels of persistent Ca2+ sparklet activity. However, during hypertension, elevated PKCα activity increases persistent Ca2+ sparklet activity thereby increasing PP2B activity and consequently NFATc3 nuclear import rate, leading to high nuclear accumulation of this transcription factor. This, in turn, leads to down-regulation of the b1 subunit of the large conductance, Ca2+-activated K+ channel and the Kv2.1 channel subunit in arterial myocytes 98, 99. When these findings are considered in light of evidence that abnormalities in the AKAP79/150 associated PKC contributes to the onset hypertension it seems reasonable to speculate that an anchored pool of PP2B may work via a distinct mechanisms to counteract this process.
Like all organs, the heart needs a constant supply of oxygen-rich blood. A system of arteries and veins supplies the myocardium with oxygen-rich blood and then returns oxygen-depleted blood to the right atrium. As a result the concentration of cellular oxygen is maintained within a narrow range (termed normoxia). A key cellular response to a state of reduced oxygen tension (hypoxia) involves induction of genes by the transcription factor known as hypoxia-inducible factor 1α (HIF-1α) 100. Under normoxic conditions HIF-1 α is kept low through its ubiquitin mediated proteasomal degradation. The half-life of HIF-1 α under normoxic conditions has been calculated to be around 5 min 101. However, if the oxygen supply suddenly drops, myocytes enter a hypoxic state and the continual destruction of HIF-1α halts instantly (Figure 3c). This allows the protein to form a stable heterodimeric complex with the HIF-1β subunit to initiate transcription of proangiogenic, metabolic, and anti-apoptotic genes to promote cell survival in response to a sudden ischemic event 102-104. Accordingly, the accumulation of HIF-1α is an early marker of myocardial infarction105.
Although the molecular mechanisms underlying the destruction or maintenance of HIF-1α are well-defined 106, the subcellular organization of the factors that regulate these processes has not been investigated. It has recently been shown that mAKAP organizes ubiquitin E3 ligases that manage the stability of HIF-1α and optimally position it close to its site of action inside the nucleus 107. Functional experiments in cardiomyocytes showed that depletion of mAKAP or disruption of its targeting to the perinuclear region altered the stability of HIF-1α and transcriptional activation of genes associated with hypoxia 107. Compartmentalization of oxygen-sensitive signaling components may influence the fidelity and magnitude of the hypoxic response (Figure 3c). These findings infer a link between hypoxia and hypertrophic signaling pathways. Indeed, mAKAP may provide such a link because the abundance of mAKAP is increased in response to hypertrophic stimuli 61. Further support for this notion comes from evidence that mAKAP anchors two signaling enzymes that act sequentially to influence cardiomyocyte hypertrophy and the stability of HIF-1a. One of these, the cAMP-responsive guanine nucleotide exchange factor Epac-1, is the upstream element in a signaling pathway that modulates the activity of extracellular signal–regulated kinase 5 (ERK5), a protein kinase that augments the hypertrophic response and influences the stability of HIF-1α 65, 108, 109. Thus, certain mAKAP complexes may create cellular microenvironments in which cAMP signals can feed into oxygen-responsive transcriptional activation pathways.
Cardiomyocytes are exquisitely adapted to facilitate rapid changes in heart rate in response to sympathetic and parasympathetic nervous impulses. AKAPs are central to this process as they position protein kinases, phosphatases, and phosphodiesterases in proximity to selected substrates. The material discussed in this article draws attention to the importance of anchored enzyme activity in the spatial and temporal synchronization of persistent intracellular process such as cardiac contractility. However, recent evidence suggests that the anchoring proteins themselves may play a more active role in the regulation of cardiac contractility that merely functioning as scaffolding proteins. For example, Yotiao binding to IKS channels is believed to evoke allosteric changes that increase magnitude of IKS currents 88 and interaction with AKAP15/18 δ is believed to enhance the phospholamban rate of multimerization 77. Finally, it may be worthy to note that anchoring proteins such as AKAP-Lbc and mAKAP interface with the protein acetylation and ubiquitination machinery respectively to evoke changes in transcriptional activation pathways 96, 107. These latter observations imply that AKAPs not only contribute to the bi-directional control of second messenger phosphorylation events but also shape broader aspects of cardiovascular physiology and pathophysiology.
Acknowledgments
The authors wish to thank Lorene K. Langeberg for creating the artwork in this article.
Sources of Funding: JDS is supported in part by NIH grant HL08366 and Leducq transatlantic network 06CVD02 and LFS is supported in part by HL08568.
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kubalova Z, Terentyev D, Viatchenko-Karpinski S, Nishijima Y, Gyorke I, Terentyeva R, da Cunha DN, Sridhar A, Feldman DS, Hamlin RL, Carnes CA, Gyorke S. Abnormal intrastore calcium signaling in chronic heart failure. Proc Natl Acad Sci U S A. 2005;102:14104–14109. doi: 10.1073/pnas.0504298102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 3.Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008;9:60–71. doi: 10.1038/nrm2299. [DOI] [PubMed] [Google Scholar]
- 4.Sutherland EW. Studies on the mechanism of hormone action. Science. 1972;171:401–408. doi: 10.1126/science.177.4047.401. [DOI] [PubMed] [Google Scholar]
- 5.Beavo JA, Brunton LL. Cyclic nucleotide research -- still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- 6.Wong W, Scott JD. AKAP Signalling complexes: Focal points in space and time. Nature Reviews Molecular Cell biology. 2004;52:959–971. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 7.Zaccolo M. cAMP signal transduction in the heart: understanding spatial control for the development of novel therapeutic strategies. Br J Pharmacol. 2009;158:50–60. doi: 10.1111/j.1476-5381.2009.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faux MC, Scott JD. Molecular glue: kinase anchoring and scaffold proteins. Cell. 1996;70:8–12. doi: 10.1016/s0092-8674(00)81075-2. [DOI] [PubMed] [Google Scholar]
- 9.Sutherland EW, Rall TW. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J Biol Chem. 1958;232:1077–1091. [PubMed] [Google Scholar]
- 10.Buxton ILO, Brunton LL. Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. JBC. 1983;258:10233–10239. [PubMed] [Google Scholar]
- 11.Faux MC, Scott JD. More on target with protein phosphorylation: conferring specificity by location. TIBS. 1996;21:312–315. [PubMed] [Google Scholar]
- 12.Jurevicius J, Fischmeister R. cAMP compartmentation is responsible for a local activation of cardiac Ca2+ channels by beta-adrenergic agonists. Proc Natl Acad Sci U S A. 1996;93:295–299. doi: 10.1073/pnas.93.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosey MM, Green RD. Effects of isoproterenol on cyclic AMP and cyclic AMP-dependent protein kinase in developing chick myocardium. Biochim Biophys Acta. 1977;500:152–161. doi: 10.1016/0304-4165(77)90055-1. [DOI] [PubMed] [Google Scholar]
- 14.Puri TS, Gerhardstein BL, Zhao XL, Ladner MB, Hosey MM. Differential effects of subunit interactions on protein kinase A- and C-mediated phosphorylation of L-type calcium channels. Biochemistry. 1997;36:9605–9615. doi: 10.1021/bi970500d. [DOI] [PubMed] [Google Scholar]
- 15.Bacskai BJ, Hochner B, Mahaut-Smith M, Adams SR, Kaang BK, Kandel ER, Tsien RY. Spatially resolved dynamics of cAMP and protein kinase A subunits in aplysia sensory neurons. Science. 1993;260:222–226. doi: 10.1126/science.7682336. [DOI] [PubMed] [Google Scholar]
- 16.Hempel CM, Vincent P, Adams SR, Tsien RY, Selverston AI. Spatio-temporal dynamics of cyclic AMP signals in an intact neural circuitm. Nature. 1996;384:166–169. doi: 10.1038/384166a0. [DOI] [PubMed] [Google Scholar]
- 17.Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- 18.Scott JD. Cyclic nucleotide-dependent protein kinases. Pharmacol Ther. 1991;50:123–145. doi: 10.1016/0163-7258(91)90075-w. [DOI] [PubMed] [Google Scholar]
- 19.Carr DW, Hausken ZE, Fraser ID, Stofko-Hahn RE, Scott JD. Association of the type II cAMP-dependent protein kinase with a human thyroid RII-anchoring protein. Cloning and characterization of the RII-binding domain. J Biol Chem. 1992;267:13376–13382. [PubMed] [Google Scholar]
- 20.Carr DW, Stofko-Hahn RE, Fraser IDC, Cone RD, Scott JD. Localization of the cAMP-dependent protein kinase to the postsynaptic densities by A-kinase anchoring proteins: characterization of AKAP79. J Biol Chem. 1992;24:16816–16823. [PubMed] [Google Scholar]
- 21.Scott JD. Compartmentalized cAMP signalling: a personal perspective. Biochem Soc Trans. 2006;34:465–467. doi: 10.1042/BST0340465. [DOI] [PubMed] [Google Scholar]
- 22.Ruehr ML, Russell MA, Bond M. A-kinase anchoring protein targeting of protein kinase A in the heart. J Mol Cell Cardiol. 2004;37:653–665. doi: 10.1016/j.yjmcc.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Negro A, Dodge-Kafka K, Kapiloff MS. Signalosomes as Therapeutic Targets. Prog Pediatr Cardiol. 2008;25:51–56. doi: 10.1016/j.ppedcard.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauman AL, Soughayer J, Nguyen BT, Willoughby D, Carnegie GK, Wong W, Hoshi N, Langeberg LK, Cooper DM, Dessauer CW, Scott JD. Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Mol Cell. 2006;23:925–931. doi: 10.1016/j.molcel.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piggott LA, Bauman AL, Scott JD, Dessauer CW. The A-kinase anchoring protein Yotiao binds and regulates adenylyl cyclase in brain. Proc Natl Acad Sci U S A. 2008;105:13835–13840. doi: 10.1073/pnas.0712100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadana R, Dessauer CW. Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. Neurosignals. 2009;17:5–22. doi: 10.1159/000166277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okumura S, Kawabe J, Yatani A, Takagi G, Lee MC, Hong C, Liu J, Takagi I, Sadoshima J, Vatner DE, Vatner SF, Ishikawa Y. Type 5 adenylyl cyclase disruption alters not only sympathetic but also parasympathetic and calcium-mediated cardiac regulation. Circ Res. 2003;93:364–371. doi: 10.1161/01.RES.0000086986.35568.63. [DOI] [PubMed] [Google Scholar]
- 28.Scott JD, Stofko RE, McDonald JR, Comer JD, Vitalis EA, Mangeli J. Type II regulatory subunit dimerization determines the subcellular localization of the cAMP-dependent protein kinase. J Biol Chem. 1990;265:21561–21566. [PubMed] [Google Scholar]
- 29.Carr DW, Stofko-Hahn RE, Fraser IDC, Bishop SM, Acott TS, Brennan RG, Scott JD. Interaction of the regulatory subunit (RII) of cAMP-dependent protein kinase with RII-anchoring proteins occurs through an amphipathic helix binding motif. J Biol Chem. 1991;266:14188–14192. [PubMed] [Google Scholar]
- 30.Rosenmund C, Carr DW, Bergeson SE, Nilaver G, Scott JD, Westbrook GL. Anchoring of protein kinase A is required for modulation of AMPA/kainate receptors on hippocampal neurons. Nature. 1994;368:853–856. doi: 10.1038/368853a0. [DOI] [PubMed] [Google Scholar]
- 31.Florio Va, Sonnenburg WK, Johnson R, Kwak KS, Jensen GS, Walsh KA, Beavo JA. Phosphorylation of the 61-kDa calmodulin-stimulated cyclic nucleotide phosphodiesterase at serine 120 reduces its affinity for calmodulin. Biochem. 1994;33:8948–8954. doi: 10.1021/bi00196a012. [DOI] [PubMed] [Google Scholar]
- 32.Mauban JR, O'Donnell M, Warrier S, Manni S, Bond M. AKAP-scaffolding proteins and regulation of cardiac physiology. Physiology (Bethesda) 2009;24:78–87. doi: 10.1152/physiol.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McConnell BK, Popovic Z, Mal N, Lee K, Bautista J, Forudi F, Schwartzman R, Jin JP, Penn M, Bond M. Disruption of protein kinase A interaction with A-kinase-anchoring proteins in the heart in vivo: effects on cardiac contractility, protein kinase A phosphorylation, and troponin I proteolysis. J Biol Chem. 2009;284:1583–1592. doi: 10.1074/jbc.M806321200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manni S, Mauban JH, Ward CW, Bond M. Phosphorylation of the cAMP-dependent protein kinase (PKA) regulatory subunit modulates PKA-AKAP interaction, substrate phosphorylation, and calcium signaling in cardiac cells. J Biol Chem. 2008;283:24145–24154. doi: 10.1074/jbc.M802278200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fink MA, Zakhary DR, Mackey JA, Desnoyer RW, Apperson-Hansen C, Damron DS, Bond M. AKAP-mediated targeting of protein kinase a regulates contractility in cardiac myocytes. Circ Res. 2001;88:291–297. doi: 10.1161/01.res.88.3.291. [DOI] [PubMed] [Google Scholar]
- 36.Niggli E, Lederer WJ. Voltage-independent calcium release in heart muscle. Science. 1990;250:565–568. doi: 10.1126/science.2173135. [DOI] [PubMed] [Google Scholar]
- 37.Klein MG, Cheng H, Santana LF, Jiang YH, Lederer WJ, Schneider MF. Two mechanisms of quantized calcium release in skeletal muscle. Nature. 1996;379:455–458. doi: 10.1038/379455a0. [DOI] [PubMed] [Google Scholar]
- 38.Navedo MF, Amberg GC, Votaw VS, Santana LF. Constitutively active L-type Ca2+ channels. Proc Natl Acad Sci U S A. 2005;102(31):11112–11117. doi: 10.1073/pnas.0500360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983;245:C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- 40.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 41.Catterall WA. Excitation-contraction coupling in vertebrate skeletal muscle: a tale of two calcium channels. Cell. 1991;64:871–874. doi: 10.1016/0092-8674(91)90309-m. [DOI] [PubMed] [Google Scholar]
- 42.Dodge K, Scott JD. AKAP79 and the evolution of the AKAP model. FEBS Lett. 2000;476:58–61. doi: 10.1016/s0014-5793(00)01671-9. [DOI] [PubMed] [Google Scholar]
- 43.Gao T, Yatani A, Dell'Acqua ML, Sako H, Green SA, Dascal N, Scott JD, Hosey MM. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997;19:185–196. doi: 10.1016/s0896-6273(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 44.Coghlan VM, Perrino BA, Howard M, Langeberg LK, Hicks JB, Gallatin WM, Scott JD. Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science. 1995;267:108–112. doi: 10.1126/science.7528941. [DOI] [PubMed] [Google Scholar]
- 45.Klauck TM, Faux MC, Labudda K, Langeberg LK, Jaken S, Scott JD. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science. 1996;271:1589–1592. doi: 10.1126/science.271.5255.1589. [DOI] [PubMed] [Google Scholar]
- 46.Hoshi N, Langeberg LK, Scott JD. Distinct enzyme combinations in AKAP signalling complexes permit functional diversity. Nat Cell Biol. 2005;7(11):1066–1073. doi: 10.1038/ncb1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santana LF, Chase EG, Votaw VS, Nelson MT, Greven R. Functional coupling of calcineurin and protein kinase A in mouse ventricular myocytes. J Physiol. 2002;544:57–69. doi: 10.1113/jphysiol.2002.020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Navedo MF, Nieves-Cintron M, Amberg GC, Yuan C, Votaw VS, Lederer WJ, McKnight GS, Santana LF. AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension. Circ Res. 2008;102:e1–e11. doi: 10.1161/CIRCRESAHA.107.167809. [DOI] [PubMed] [Google Scholar]
- 49.Trotter KW, Fraser ID, Scott GK, Stutts MJ, Scott JD, Milgram SL. Alternative splicing regulates the subcellular localization of A-kinase anchoring protein 18 isoforms. J Cell Biol. 1999;147:1481–1492. doi: 10.1083/jcb.147.7.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fraser ID, Tavalin SJ, Lester LB, Langeberg LK, Westphal AM, Dean RA, Marrion NV, Scott JD. A novel lipid-anchored A-kinase Anchoring Protein facilitates cAMP- responsive membrane events. Embo J. 1998;17:2261–2272. doi: 10.1093/emboj/17.8.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gray PC, Tibbs VC, Catterall WA, Murphy BJ. Identification of a 15-kDa cAMP-dependent protein kinase-anchoring protein associated with skeletal muscle L-type calcium channels. J Biol Chem. 1997;272:6297–6302. doi: 10.1074/jbc.272.10.6297. [DOI] [PubMed] [Google Scholar]
- 52.Hulme JT, Ahn M, Hauschka SD, Scheuer T, Catterall WA. A novel leucine zipper targets AKAP15 and cyclic AMP-dependent protein kinase to the C terminus of the skeletal muscle Ca2+ channel and modulates its function. J Biol Chem. 2002;277:4079–4087. doi: 10.1074/jbc.M109814200. [DOI] [PubMed] [Google Scholar]
- 53.Oliveria SF, Dell'Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 2007;55:261–275. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gray PC, Scott JD, Catterall WA. Regulation of ion channels by cAMP-dependent protein kinase and A-kinase anchoring proteins. Curr Opin Neurobiol. 1998;8:330–334. doi: 10.1016/s0959-4388(98)80057-3. [DOI] [PubMed] [Google Scholar]
- 55.Shcherbakova OG, Hurt CM, Xiang Y, Dell'Acqua ML, Zhang Q, Tsien RW, Kobilka BK. Organization of beta-adrenoceptor signaling compartments by sympathetic innervation of cardiac myocytes. J Cell Biol. 2007;176:521–533. doi: 10.1083/jcb.200604167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fraser ID, Scott JD. Modulation of ion channels: a “current” view of AKAPs. Neuron. 1999;23:423–426. doi: 10.1016/s0896-6273(00)80795-3. [DOI] [PubMed] [Google Scholar]
- 57.Smith FD, Langeberg LK, Scott JD. The where's and when's of kinase anchoring. Trends Biochem Sci. 2006;31:316–323. doi: 10.1016/j.tibs.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 58.Tandan S, Wang Y, Wang TT, Jiang N, Hall DD, Hell JW, Luo X, Rothermel BA, Hill JA. Physical and functional interaction between calcineurin and the cardiac L-type Ca2+ channel. Circ Res. 2009;105:51–60. doi: 10.1161/CIRCRESAHA.109.199828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valdivia HH, Kaplan JH, Ellis-Davies GC, Lederer WJ. Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation. Science. 1995;267:1997–2000. doi: 10.1126/science.7701323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kapiloff MS, Jackson N, Airhart N. mAKAP and the ryanodine receptor are part of a multi-component signaling complex on the cardiomyocyte nuclear envelope. J Cell Sci. 2001;114:3167–3176. doi: 10.1242/jcs.114.17.3167. [DOI] [PubMed] [Google Scholar]
- 61.Kapiloff MS, Schillace RV, Westphal AM, Scott JD. mAKAP: an A-kinase anchoring protein targeted to the nuclear membrane of differentiated myocytes. J Cell Sci. 1999;112:2725–2736. doi: 10.1242/jcs.112.16.2725. [DOI] [PubMed] [Google Scholar]
- 62.Yang J, Drazba JA, Ferguson DG, Bond M. A-kinase anchoring protein 100 (AKAP100) is localized in multiple subcellular compartments in the adult rat heart. J Cell Biol. 1998;142:511–522. doi: 10.1083/jcb.142.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruehr ML, Russell MA, Ferguson DG, Bhat M, Ma J, Damron DS, Scott JD, Bond M. Targeting of protein kinase A by muscle A kinase-anchoring protein (mAKAP) regulates phosphorylation and function of the skeletal muscle ryanodine receptor. J Biol Chem. 2003;278:24831–24836. doi: 10.1074/jbc.M213279200. [DOI] [PubMed] [Google Scholar]
- 64.Dodge KL, Khouangsathiene S, Kapiloff MS, Mouton R, Hill EV, Houslay MD, Langeberg LK, Scott JD. mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. EMBO J. 2001;20:1921–1930. doi: 10.1093/emboj/20.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dodge-Kafka KL, Soughayer J, Pare GC, Carlisle Michel JJ, Langeberg LK, Kapiloff MS, Scott JD. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature. 2005;437:574–578. doi: 10.1038/nature03966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bauman AL, Michel JJ, Henson E, Dodge-Kafka KL, Kapiloff MS. The mAKAP signalosome and cardiac myocyte hypertrophy. IUBMB Life. 2007;59:163–169. doi: 10.1080/15216540701358593. [DOI] [PubMed] [Google Scholar]
- 67.Lehnart SE, Marks AR. Phosphodiesterase 4D and heart failure: a cautionary tale. Expert Opin Ther Targets. 2006;10:677–688. doi: 10.1517/14728222.10.5.677. [DOI] [PubMed] [Google Scholar]
- 68.Marx SO, Reiken S, Hisamatsu Y, Gaburjakova M, Gaburjakova J, Yang YM, Rosemblit N, Marks AR. Phosphorylation-dependent regulation of ryanodine receptors: a novel role for leucine/isoleucine zippers. J Cell Biol. 2001;153:699–708. doi: 10.1083/jcb.153.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lehnart SE, Wehrens XH, Reiken S, Warrier S, Belevych AE, Harvey RD, Richter W, Jin SL, Conti M, Marks AR. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell. 2005;123:25–35. doi: 10.1016/j.cell.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eisner DA, Kashimura T, O'Neill SC, Venetucci LA, Trafford AW. What role does modulation of the ryanodine receptor play in cardiac inotropy and arrhythmogenesis? J Mol Cell Cardiol. 2009;46:474–481. doi: 10.1016/j.yjmcc.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 71.Schmitt JP, Kamisago M, Asahi M, Li GH, Ahmad F, Mende U, Kranias EG, MacLennan DH, Seidman JG, Seidman CE. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science. 2003;299:1410–1413. doi: 10.1126/science.1081578. [DOI] [PubMed] [Google Scholar]
- 72.MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 73.Katz AM. Discovery of phospholamban. A personal history. Ann N Y Acad Sci. 1998;853:9–19. doi: 10.1111/j.1749-6632.1998.tb08252.x. [DOI] [PubMed] [Google Scholar]
- 74.Robia SL, Flohr NC, Thomas DD. Phospholamban pentamer quaternary conformation determined by in-gel fluorescence anisotropy. Biochemistry. 2005;44:4302–4311. doi: 10.1021/bi0478446. [DOI] [PubMed] [Google Scholar]
- 75.Kelly EM, Hou Z, Bossuyt J, Bers DM, Robia SL. Phospholamban oligomerization, quaternary structure, and sarco(endo)plasmic reticulum calcium ATPase binding measured by fluorescence resonance energy transfer in living cells. J Biol Chem. 2008;283:12202–12211. doi: 10.1074/jbc.M707590200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lygren B, Carlson CR, Santamaria K, Lissandron V, McSorley T, Litzenberg J, Lorenz D, Wiesner B, Rosenthal W, Zaccolo M, Tasken K, Klussmann E. AKAP complex regulates Ca2+ re-uptake into heart sarcoplasmic reticulum. EMBO Rep. 2007;8:1061–1067. doi: 10.1038/sj.embor.7401081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lygren B, Tasken K. The potential use of AKAP18delta as a drug target in heart failure patients. Expert Opin Biol Ther. 2008;8:1099–1108. doi: 10.1517/14712598.8.8.1099. [DOI] [PubMed] [Google Scholar]
- 78.Stefan E, Wiesner B, Baillie GS, Mollajew R, Henn V, Lorenz D, Furkert J, Santamaria K, Nedvetsky P, Hundsrucker C, Beyermann M, Krause E, Pohl P, Gall I, MacIntyre AN, Bachmann S, Houslay MD, Rosenthal W, Klussmann E. Compartmentalization of cAMP-dependent signaling by phosphodiesterase-4D is involved in the regulation of vasopressin-mediated water reabsorption in renal principal cells. J Am Soc Nephrol. 2007;18:199–212. doi: 10.1681/ASN.2006020132. [DOI] [PubMed] [Google Scholar]
- 79.Lygren B, Tasken K. Compartmentalized cAMP signalling is important in the regulation of Ca(2+) cycling in the heart. Biochem Soc Trans. 2006;34:489–491. doi: 10.1042/BST0340489. [DOI] [PubMed] [Google Scholar]
- 80.Kammerer S, Burns-Hamuro LL, Ma Y, Hamon SC, Canaves JM, Shi MM, Nelson MR, Sing CF, Cantor CR, Taylor SS, Braun A. Amino acid variant in the kinase binding domain of dual-specific A kinase-anchoring protein 2: a disease susceptibility polymorphism. Proc Natl Acad Sci U S A. 2003;100:4066–4071. doi: 10.1073/pnas.2628028100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burns-Hamuro LL, Ma Y, Kammerer S, Reineke U, Self C, Cook C, Olson GL, Cantor CR, Braun A, Taylor SS. Designing isoform-specific peptide disruptors of protein kinase A localization. Proc Natl Acad Sci U S A. 2003;100:4072–4077. doi: 10.1073/pnas.2628038100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tingley WG, Pawlikowska L, Zaroff JG, Kim T, Nguyen T, Young SG, Vranizan K, Kwok PY, Whooley MA, Conklin BR. Gene-trapped mouse embryonic stem cell-derived cardiac myocytes and human genetics implicate AKAP10 in heart rhythm regulation. Proc Natl Acad Sci U S A. 2007;104:8461–8466. doi: 10.1073/pnas.0610393104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kass RS, Moss AJ. Long QT syndrome: novel insights into the mechanisms of cardiac arrhythmias. J Clin Invest. 2003;112:810–815. doi: 10.1172/JCI19844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Westphal RS, Tavalin SJ, Lin JW, Alto NM, Fraser ID, Langeberg LK, Sheng M, Scott JD. Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science. 1999;285:93–96. doi: 10.1126/science.285.5424.93. [DOI] [PubMed] [Google Scholar]
- 85.Marx SO, Kurokawa J, Reiken S, Motoike H, D'Armiento J, Marks AR, Kass RS. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002;295:496–499. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- 86.Chen L, Kurokawa J, Kass RS. Phosphorylation of the A-kinase-anchoring protein Yotiao contributes to protein kinase A regulation of a heart potassium channel. J Biol Chem. 2005;280:31347–31352. doi: 10.1074/jbc.M505191200. [DOI] [PubMed] [Google Scholar]
- 87.Kurokawa J, Motoike HK, Rao J, Kass RS. Regulatory actions of the A-kinase anchoring protein Yotiao on a heart potassium channel downstream of PKA phosphorylation. Proc Natl Acad Sci U S A. 2004;101:16374–16378. doi: 10.1073/pnas.0405583101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen L, Marquardt ML, Tester DJ, Sampson KJ, Ackerman MJ, Kass RS. Mutation of an A-kinase-anchoring protein causes long-QT syndrome. Proc Natl Acad Sci U S A. 2007;104:20990–20995. doi: 10.1073/pnas.0710527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Terrenoire C, Houslay MD, Baillie GS, Kass RS. The cardiac IKs potassium channel macromolecular complex includes the phosphodiesterase PDE4D3. J Biol Chem. 2009;284:9140–9146. doi: 10.1074/jbc.M805366200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Perrino C, Rockman HA. Reversal of cardiac remodeling by modulation of adrenergic receptors: a new frontier in heart failure. Curr Opin Cardiol. 2007;22:443–449. doi: 10.1097/HCO.0b013e3282294d72. [DOI] [PubMed] [Google Scholar]
- 91.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 92.Olson EN. A decade of discoveries in cardiac biology. Nat Med. 2004;10:467–474. doi: 10.1038/nm0504-467. [DOI] [PubMed] [Google Scholar]
- 93.Molkentin JD, Dorn IG., 2nd Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu Rev Physiol. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- 94.McKinsey TA, Olson EN. Toward transcriptional therapies for the failing heart: chemical screens to modulate genes. J Clin Invest. 2005;115:538–546. doi: 10.1172/JCI24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Carnegie GK, Smith FD, McConnachie G, Langeberg LK, Scott JD. AKAP-Lbc nucleates a protein kinase D activation scaffold. Mol Cell. 2004;15:889–899. doi: 10.1016/j.molcel.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 96.Carnegie GK, Soughayer J, Smith FD, Pedroja BS, Zhang F, Diviani D, Bristow MR, Kunkel MT, Newton AC, Langeberg LK, Scott JD. AKAP-Lbc mobilizes a cardiac hypertrophy signaling pathway. Mol Cell. 2008;32:169–179. doi: 10.1016/j.molcel.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nieves-Cintron M, Amberg GC, Navedo MF, Molkentin JD, Santana LF. The control of Ca2+ influx and NFATc3 signaling in arterial smooth muscle during hypertension. Proc Natl Acad Sci U S A. 2008;105:15623–15628. doi: 10.1073/pnas.0808759105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nieves-Cintron M, Amberg GC, Nichols CB, Molkentin JD, Santana LF. Activation of NFATc3 down-regulates the beta1 subunit of large conductance, calcium-activated K+ channels in arterial smooth muscle and contributes to hypertension. J Biol Chem. 2007;282:3231–3240. doi: 10.1074/jbc.M608822200. [DOI] [PubMed] [Google Scholar]
- 99.Amberg GC, Navedo MF, Nieves-Cintron M, Molkentin JD, Santana LF. Calcium sparklets regulate local and global calcium in murine arterial smooth muscle. J Physiol. 2007;579:187–201. doi: 10.1113/jphysiol.2006.124420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001;13:167–171. doi: 10.1016/s0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 101.Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med. 2004;36:1–12. doi: 10.1038/emm.2004.1. [DOI] [PubMed] [Google Scholar]
- 102.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 103.Wenger RH, Gassmann M. Oxygen(es) and the hypoxia-inducible factor-1. Biol Chem. 1997;378:609–616. [PubMed] [Google Scholar]
- 104.Koh MY, Spivak-Kroizman TR, Powis G. HIF-1 regulation: not so easy come, easy go. Trends Biochem Sci. 2008;33:526–534. doi: 10.1016/j.tibs.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 105.Lee SH, Wolf PL, Escudero R, Deutsch R, Jamieson SW, Thistlethwaite PA. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N Engl J Med. 2000;342:626–633. doi: 10.1056/NEJM200003023420904. [DOI] [PubMed] [Google Scholar]
- 106.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wong W, Goehring AS, Kapiloff MS, Langeberg LK, Scott JD. mAKAP compartmentalizes oxygen-dependent control of HIF-1alpha. Sci Signal. 2008;1:ra18. doi: 10.1126/scisignal.2000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nicol RL, Frey N, Pearson G, Cobb M, Richardson J, Olson EN. Activated MEK5 induces serial assembly of sarcomeres and eccentric cardiac hypertrophy. Embo J. 2001;20:2757–2767. doi: 10.1093/emboj/20.11.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pi X, Garin G, Xie L, Zheng Q, Wei H, Abe J, Yan C, Berk BC. BMK1/ERK5 is a novel regulator of angiogenesis by destabilizing hypoxia inducible factor 1alpha. Circ Res. 2005;96:1145–1151. doi: 10.1161/01.RES.0000168802.43528.e1. [DOI] [PubMed] [Google Scholar]