Abstract
Object
Although neuron transplantation to repair the nervous system has shown promise in animal models, there are few practical sources of viable neurons for clinical application and insufficient approaches to bridge extensive nerve damage in patients. Therefore, the authors sought a clinically relevant source of neurons that could be engineered into transplantable nervous tissue constructs. The authors chose to evaluate human dorsal root ganglion (DRG) neurons due to their robustness in culture.
Methods
Cervical DRGs were harvested from 16 live patients following elective ganglionectomies, and thoracic DRGs were harvested from 4 organ donor patients. Following harvest, the DRGs were digested in a dispase–collagenase treatment to dissociate neurons for culture. In addition, dissociated human DRG neurons were placed in a specially designed axon expansion chamber that induces continuous mechanical tension on axon fascicles spanning 2 populations of neurons originally plated ~ 100 μm apart.
Results
The adult human DRG neurons, positively identified by neuronal markers, survived at least 3 months in culture while maintaining the ability to generate action potentials. Stretch-growth of axon fascicles in the expansion chamber occurred at the rate of 1 mm/day to a length of 1 cm, creating the first engineered living human nervous tissue constructs.
Conclusions
These data demonstrate the promise of adult human DRG neurons as an alternative transplant material due to their availability, viability, and capacity to be engineered. Also, these data show the feasibility of harvesting DRGs from living patients as a source of neurons for autologous transplant as well as from organ donors to serve as an allograft source of neurons.
Keywords: axon elongation, axon stretch growth, nervous tissue construct, peripheral nerve injury repair, spinal cord injury repair, tissue engineering
Due to the limited regeneration of both the central and peripheral nervous systems following injury or disease,8,13,19,20 many cell-based therapies have been vigorously pursued to restore damaged nervous tissue. In particular, major emphasis has been placed on transplantation of neuronal stem cells and neuronal cell lines. These efforts, however, have been hampered by poor long-term survival of transplanted neurons derived from these cells, limited differentiation into neuronal phenotypes following transplantation, and the risk of tumor formation from non-neuronal cells.1,2,12 Although an alternative strategy is to transplant neurons harvested from animals into humans, there is a high risk of rejection or transmission of pathogens.7,14,16 Nonetheless, there remains another source of transplantable neurons that has been generally overlooked. Neurons from DRGs have been isolated from both fetal and adult animals and appear to survive well in culture and following transplantation.4,6,10,19,25 In addition, several groups have successfully cultured DRG neurons from adult human organ donors, human fetuses, and recently from patients surgically treated with ganglionectomies to evaluate human DRG neuron physiology.3,11,23,24
Dorsal root ganglia also appear to be an ideal source of neurons to engineer transplantable living nervous tissue constructs. We have recently shown that bundles of axons spanning 2 populations of rat DRG neuronal cell bodies readily tolerate extreme axon stretch-growth, creating living nerve tracts in vitro.17-19 This is accomplished by applying continuous mechanical tension on the integrated axon fascicles, inducing enormous growth of the central cylinder of the stretched axons. Beginning at only ~ 100 μm in length, these DRG axon fascicles containing up to 106 axons can extend up to at least 10 cm at a remarkable rate of 1 cm/day.18,19 Accordingly, these nervous tissue constructs can be rapidly grown to lengths suitable to bridge even extensive damage in the nervous system. Indeed, we have recently found long-term survival and integration of transplanted nervous tissue constructs derived from rat DRG neurons, which spanned 1-cm long lateral hemisection lesions of the rat spinal cord10 and 1.2-cm peripheral nerve lesions in the rat.9
For clinical application, it is notable that an expedient supply of DRG neurons could come from the patients themselves to form autologous grafts or from organ donors for allografts. In the present study, research was performed on isolated human DRG neurons in culture derived from cervical and thoracic ganglionectomies from live patients as well as organ donors. The long-term in vitro survival of these harvested neurons was examined, based on their morphological characteristics and ability to generate action potentials. Moreover, the potential of adult human DRG neurons to be engineered into transplantable nervous tissue constructs was examined.
Materials and Methods
Tissue Harvesting
Two different avenues were used to acquire human DRGs, both fully approved by the institutional review board at the University of Pennsylvania. For the first source of DRG neurons, a protocol was developed to grow DRG neurons that were harvested from 16 patients undergoing bilateral C-2 ganglionectomy for treatment of intractable occipital neuralgia. Immediately after removal of the DRGs during the elective surgery, the tissue was placed in cold Hibernate A medium (Brain Bits) and stored on ice in preparation for further processing in a cell culture environment.
The second source of DRG neurons was acquired from organ donors through the Gift of Life program, the Philadelphia region’s organ and tissue transplant network. The donors were , < 60 years old who met brain death criteria and in whom the results of serological testing were negative for HIV. In close cooperation with the Gift of Life program, DRG isolation was added to the consent form for all organ donors in the region. After the respective organ transplant surgeons harvested the major organs, the DRGs were harvested through the same anterior incision used for organ harvesting. To avoid repositioning the body during organ harvest, an anterior 2-level corpectomy was performed at the midthoracic level to expose the spinal cord. The DRGs were identified at their entrance to the dorsolateral spinal cord and incised with a blade. The average total length of time it took for harvesting 4 DRGs (2 at each level) was 20 minutes. Following the harvesting, the entire thoracic incision was closed in anatomical layers. Sterility was maintained throughout the entire procedure. All DRG harvestings were performed within 2 hours after aortic clamping. Immediately after harvesting, the DRG tissue was placed in cold Hibernate A solution and stored on ice for further processing.
Human DRG Cell Culture
For these procedures, we modified protocols kindly communicated to us by Drs. Patrick Wood and Thomas Baumann, respectively from the Miami Project and Oregon Health and Sciences University. Under a dissection microscope, the ganglia were cleaned of excess fat, connective tissue, and nerve roots. The ganglia were then sliced into small pieces. The pieces were digested in an enzyme cocktail of 0.25% Collagenase P (Roche Diagnostics) and 0.1% Dispase I (Roche Diagnostics) and incubated at 37°C for 18 hours. Following digestion, the cells were washed free of the enzyme solution with Hanks balanced salt solution. The DRG neurons were separated from myelin debris and nonneuronal cells using a density gradient of 5% and 10% bovine serum albumin (Sigma).
After purification, the dissociated cells were plated onto tissue culture dishes, were treated with 10 μg/ml poly-L-lysine for 2 hours, and were then removed and allowed to dry for 1 hour before being rinsed 3 times with sterile water. Type 1 rat-tail collagen (Becton Dickinson) as supplied was spread over the surface (10–20 μl/cm2) and polymerized by exposure to ammonia vapors for 2 minutes. The collagen was then allowed to dry completely in the cell culture hood before the cells were plated. Cells were maintained in Neurobasal-A medium (Invitrogen) supplemented with B-27 Supplement (Invitrogen), 1% penicillin/streptomycin (Invitrogen), 0.4 mM L-glutamine (Sigma), 2.5 g/L glucose (Sigma), 10 ng/ml 2.5S nerve growth factor (Becton Dickinson), and 1% fetal bovine serum (HyClone). Cultures were treated with the mitotic inhibitors (MI#1, 5 μM cytosine β-D-arabinofuranoside, 20 μM 5-fluoro-2’-deoxyuridine and 20 μM uridine [Sigma]) on the day of plating. After 2 days, the medium was exchanged including the mitotic inhibitors (MI#2, 20 μM 5-fluoro-2’-deoxyuridine [Sigma] and 20 μM uridine [Sigma]) for 3 days. Thereafter the media were changed every 2 to 3 days and the mitotic inhibitors MI#2 were applied once a week.
Electrophysiological Measurements
For a limited number of DRG neurons we examined their ability to generate action potentials to corroborate visual assessment of long-term survival. Action potentials were stimulated and recorded using the whole cell patch clamp technique at room temperature using electrodes (4–8MX, P-30, Sutter Instrument Co.), Axopatch 1D amplifier, Digidata data acquisition system, and pClamp 8 software (Axon Instruments). To minimize instances of inadequate space clamp, neurons were only included in the analysis if 80% series resistance compensation was achieved, and recordings where discarded if resistance increased > 20% during the experiment.
All experiments were performed at room temperature. External bathing solution (consisting of 115 mM NaCl, 5.6 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 11 mM glucose, 1 mM NaH2PO4, and 25 mM NaHCO3) was continuously perfused. Internal pipette solution consisted of 5 mM NaCl, 155 mM KCl, 1 mM MgCl2, 3 mM EGTA, and 10 mM HEPES, adjusted to pH 7.3. Action potentials were measured and analyzed using pClamp software (Axon Instruments). Under current-clamp conditions, each patched cell was injected with 2 nA of current > 2 msec to stimulate an action potential, while the change in membrane potential was recorded.
Immunocytochemical Studies
Dorsal root ganglion cultures were fixed using 4% paraformaldehyde in 1M PBS for 60 minutes at room temperature. Following fixation, the cells were rinsed 3 times with 1M PBS and blocked with 4% normal goat serum in 0.1% Triton-X PBS for 30 minutes at room temperature. They were then incubated with antibodies to the DRG marker CGRP (Chemicon) and several neurofilament markers: SMI-31 (specific for phosphorylated neurofilament-H, Sternberger Monoclonal, Inc.); RMO-254 (specific for phosphorylated neurofilament-M, a generous gift from V. M.-Y. Lee); and neurofilament-200 (Clone N52, Sigma). Each primary antibody was labeled with goat anti–mouse or goat anti–rabbit immunoglobulin G conjugated to Alexa 488 (Molecular Probes) for 60 minutes at room temperature. Fluorescence microscopy was performed on a Nikon TE300 inverted microscope with a Cooke Sensicam digital camera.
Mechanical Axon Elongation
Dorsal root ganglion axons were stretch-grown within a custom-designed axon expansion chamber, which served as tissue culture support and housing. The chamber consists of a sealed enclosure with a gas exchange port, a removable axon stretching frame, and connecting rods to apply displacements.18,22 The entire apparatus was sterilized prior to plating by a regular water-steam autoclave (Tuttnauer 2450M). This device arranges 2 adjoining Aclar membranes on which neural cells are cultured. Axons, growing in culture, readily bridged the interface between the 2 adjoining substrates and integrated with neurons on either side, spanning an ~ 100-μm gap. These bridged axons were then stretch-grown by displacing the 2 integrated populations of neurons apart in a stepwise fashion, using an automated microstepper motor and controller system (Servo Systems) according to an optimized stretch-growth paradigm previously described in detail18,19 and summarized below.
Elongator Aclar substrates were coated with Type I collagen prior to DRG plating. Aclar was washed with laboratory soap, treated in 1M NaOH for 24 hours, and then sterilized in 100% ethanol for 10 minutes. Aclar substrates were attached to the stretching frame using medical-grade room-temperature vulcanizing silicone (NuSil) and allowed a minimum of 2 days to cure. Culture surfaces were treated with 10 μg/ml poly-L-lysine for 2 hours, then removed, and allowed to dry for 1 hour before being rinsed 3 times with sterile water. Type 1 rat-tail collagen (Becton Dickinson) as supplied was spread over the surface (10–20 μl/cm2) and polymerized by exposure to ammonia vapors for 2 minutes. The collagen was then allowed to dry completely in the cell-culture hood before the cells were plated. Dissociated DRG neurons were plated on each of the 2 adjacent substrates within the elongation chamber and allowed 14 days for axons to develop in culture and span the dividing region between the 2 membranes. Axon stretch-growth was initiated at a rate of 0.25 mm/ day and gradually escalated to a rate of 1 mm/day to reach a total length of 1 cm.
Results
Dorsal root ganglia were obtained from 18 individuals, including 16 live patients (6 men and 10 women) who underwent elective C-2 ganglionectomy and 4 organ donors (all male) in whom thoracic ganglionectomy was performed after death. All elective surgeries were performed in adults (mean age 42.6 years, range 26–57 years). The organ donors included 3 adults and 1 child (mean age 23.8 years, range 2–52 years). The overall mean age for both groups was 38.4 years (range 2–57 years). The male/female sex ratio was 1:1 for the combined group.
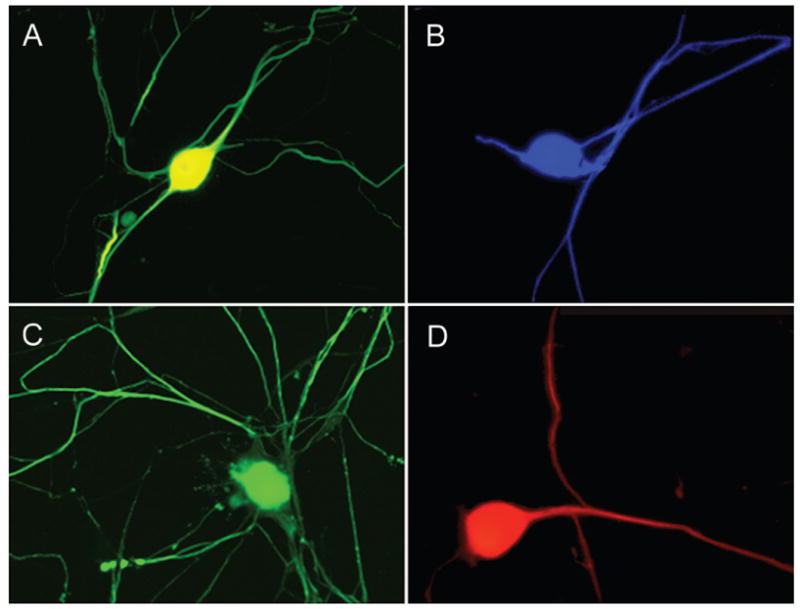
The purified human DRG neurons harvested from both live patients and organ donors tolerated culture well in either the elongation apparatus or in collagen-coated 35-mm-diameter dishes. Within 7 days following plating, an extensive network of axons had developed to interconnect neural cell bodies. Neurons cultured in 35-mm-diameter dishes consistently survived for more than 3 months, identified by both neuronal morphology and immunoreactivity to various neuron markers (Fig. 1). Notably, the robust axon integration and viability of these neurons even after 3 months in culture suggest that much longer survival could be achieved, but this survival was dependent on mitotic inhibitors in the culture media to prevent nonneuronal cell proliferation.
Fig. 1.
Fluorescence micrographs demonstrating robust survival of adult human DRG neurons with elaborate axonal networks that could be maintained in culture for at least 3 months. The human DRG neurons were identified by immunoreactivity to antibodies specific for DRG neurons: A, CGRP or neurofilament proteins; B, RMO-254; C, SMI-31; and D, neurofilament-200. Each neuron cell body is ~ 50 μm in diameter.
To support the visual identification of long-term neuronal survival in culture, nonstretched neuronal cultures grown from 2 of the ganglionectomy harvests were examined for their ability to generate action potentials. Five neurons each from the 2 harvest sources that had been maintained in culture for > 1 month underwent whole cell patch clamping. The median resting potential of the patched cells was found to be −43 mV. Cells were switched to current-clamp mode and stimulated with a 2-nA current injection. All of the neurons examined were found to be able to generate action potentials.
Overall, robust survival of isolated human DRG from organ donors as well as living patients was observed in terms of long-term survival and maintenance of normal geometry. Likewise, the age of the organ donor or patient did not appear to have an effect on DRG neuron viability in culture, including neurons isolated from the only child in the study and from the oldest adult.
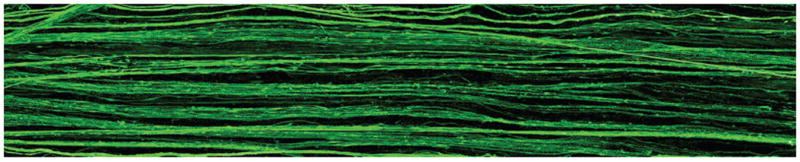
It was also found that axon stretch-growth could be induced in cultured human DRG neurons. Axon fascicles spanning 2 populations of DRG neurons were placed under an elongation paradigm of continuous mechanical tension. Our first successful elongation was performed at a rate of 0.5 mm/day over a period of 6 days and achieved a length of ~ 2.0 mm. Additional experimentation showed that the axon stretch-growth rate could be successfully escalated to a rate of 1.0 mm/day, which could be maintained until the axons had grown to at least a length of 1 cm. Large fascicles 1 cm long spanning 2 populations could be easily visualized by the unaided eye, and these fascicles were identified as axons by fluorescent immunoreactivity to antibodies targeting neurofilament proteins (Fig. 2).
Fig. 2.
Representative fluorescence micrograph of the center region of a 1-cm long living nervous tissue construct engineered from harvested adult human DRG neurons. To create this construct, 2 populations of the neurons were plated on adjacent membranes in an axon elongation apparatus. Axons grew out across the ~ 100-μm gap between the 2 membranes to integrate with the neuron population on the other side. Subsequently, continuous tension was placed on the bridging axons at an escalating rate, by mechanically separating the 2 membranes. This process induced axon “stretch-growth” of up to 1 mm/day and promoted fasciculation of the axons into large bundles reaching 1 cm in length. The stretch-grown axon bundles are demonstrated by immunoreactivity to an antibody specific for phosphorylated neurofilament protein (SMI-31).
Discussion
The results of this study demonstrate that adult human DRG neurons can be isolated from patients undergoing elective ganglionectomies and from organ donors and can be successfully maintained in tissue culture. Under optimized culturing conditions, we found that these neurons could survive in culture for at least 3 months, supporting previous observations of long-term survival.11,14,23,24 Moreover, we have demonstrated that harvested DRG neurons can be engineered to create the first living human nervous tissue constructs using a newly identified process of axon growth. To form the constructs, axon tracts spanning 2 populations of DRG neuron bodies were stretch-grown from the initial length of ~ 100 μm and extended 10 mm in length, while maintaining normal morphological characteristics. Due to their long-term viability in culture and capacity to be engineered, harvested DRGs appear to be an expedient source of neurons for transplantation and potential repair of nervous system damage in humans.
The culturing characteristics and potential therapeutic value of DRG neurons have been evaluated in animal models. Neonatal rat DRG cells have been cultured and studied for their physiological and pharmacological properties,6,15 while adult mouse DRG neurons have been maintained in vitro for up to 5 months.21 In addition, adult rat DRG neurons transplanted into central nervous system white matter have been shown to survive and regenerate their axons5 and can extend axons into cografts of peripheral nerve in the spinal cord.25
Previous studies of DRG neurons harvested from humans have focused primarily on evaluating survival in culture and physiological function. In one study,24 adult human DRG neurons were used to characterize a γ-aminobutyric acid receptor and were maintained for 5–7 days in vitro. Kim and colleagues11 maintained dissociated neurons from human superior cervical ganglia in vitro for > 2 months and demonstrated that action potentials could be recorded extracellularly from these neurons. Likewise, Sosa et al.23 were able to isolate DRG neurons from human organ donors and maintain them for 2.5 months and record action potentials after 8 weeks in culture. Finally, Baumann and colleagues3 performed extensive electrophysiological examination of DRG neurons in relation to neuropathic pain with survival up to 90 days in culture. Adapting culturing techniques used previously for the present study, it was also demonstrated that DRG neurons harvested from live adult patients as well as from organ donors can be isolated and maintained in long-term culture. Specifically, it was found that these human neurons could survive for at least 3 months in the culture environment, while maintaining a morphologically normal appearance as well as generating action potentials.
A major goal of neuronal transplantation in humans is to restore function by bridging extensive damage in the nervous system. Using a new tissue-engineering technique, we have created transplantable human nervous tissue constructs composed of adult DRG neurons. The geometry of these constructs consists of long axon tracts spanning 2 populations of neurons. Ideally, following transplantation, these constructs would serve as living “jumper cables,” forming a functional relay between viable host tissues at each end of a lesion. Alternatively, the constructs may act as living conduits, facilitating outgrowth and guidance of host axons across damaged tissue to synapse with neurons or reinnervate tissue on the other side. Furthermore, human nervous tissue constructs may more broadly serve as prototypes to study complex neurological problems.
Using rat models, we have recently found very promising evidence of the potential of engineered nervous tissue constructs to repair the nervous system. Transplanted 1-cm-long constructs derived from fetal rat DRGs were found to survive at least 1 month and integrate with host tissue at both ends of a 1-cm lateral hemisection spinal cord lesion in the adult rat.10 Similar constructs used to span a 1.3-cm excised portion of a rat sciatic nerve were found to survive for at least 4 months following transplantation while promoting both physiological and motor recovery. Here, host axons were actually found intertwined with graft axons.10 These recent data support the feasibility of using nervous tissue constructs for clinical application.
Conclusions
The present study demonstrates that adult human DRGs are a practical source of neurons for transplantation, due to their availability, viability, and capacity to be engineered to form tissue constructs. These neurons could be used for immediate transplantation or as an “off-the-shelf” cell therapy either as dissociated cells or engineered constructs. Moreover, it was shown that DRGs can be harvested from living patients as a source of neurons that could be used for autologous transplantation or from organ donors as a source of neurons for allografts. Preclinical studies suggest that dissociated neurons and nervous tissue constructs from DRGs can be used to repair both the central and peripheral nervous systems. Thus, while more popularized sources of neurons for human transplantation remain in development, including neuronal stem cells, human DRG neurons represent an immediately available and promising alternative.
Acknowledgments
We thank the Gift of Life program and the family members of the organ donors for their generous support and selfless sacrifice. Our method of culturing human DRG cells was based on protocols kindly provided to us by Dr. Pat Wood from the Miami Project at the University of Miami and by Dr. Thomas Baumann from the Oregon Health and Sciences University. We also acknowledge the skillful technical assistance of Xiao-Han Chen, Joycelyn Cabuhat, Kevin Browne, and Lesley Cruz, as well as generous help given by Sherman Stein, M.D., and Akira Iwata, M.D., Ph.D.
This study was supported by a Sharpe Foundation grant (D.H.S.); National Institutes of Health (NIH) grants NS048949 (D.H.S.), AG 21527 (D.H.S.), and NS38104 (D.H.S.); an AANS/CNS Codman Award (J.H.H.); and an AANS/CNS David Kline Award (J.H.H.). Dr. Huang is a recipient of an NIH National Research Service Award (NS46170).
Abbreviations used in this paper
- CGRP
calcitonin gene related peptide
- DRG
dorsal root ganglion
- PBS
phosphate-buffered saline
References
- 1.Armstrong RJ, Harrower TP, Hurelbrink CB, McLaughin M, Ratcliffe EL, Tyers P, et al. Porcine neural xenografts in the immunocompetent rat: immune response following grafting of expanded neural precursor cells. Neuroscience. 2001;106:201–216. doi: 10.1016/s0306-4522(01)00273-1. [DOI] [PubMed] [Google Scholar]
- 2.Arnhold S, Klein H, Semkova I, Addicks K, Schraermeyer U. Neurally selected embryonic stem cells induce tumor formation after long-term survival following engraftment into the subretinal space. Invest Ophthalmol Vis Sci. 2004;45:4251–4255. doi: 10.1167/iovs.03-1108. [DOI] [PubMed] [Google Scholar]
- 3.Baumann TK, Chaudhary P, Martenson ME. Background potassium channel block and TRPV1 activation contribute to proton depolarization of sensory neurons from humans with neuropathic pain. Eur J Neurosci. 2004;19:1343–1351. doi: 10.1111/j.1460-9568.2004.03097.x. [DOI] [PubMed] [Google Scholar]
- 4.Caffrey JM, Eng DL, Black JA, Waxman SG, Kocsis JD. Three types of sodium channels in adult rat dorsal root ganglion neurons. Brain Res. 1992;592:283–297. doi: 10.1016/0006-8993(92)91687-a. [DOI] [PubMed] [Google Scholar]
- 5.Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–683. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- 6.Drew LJ, Wood JN, Cesare P. Distinct mechanosensitive properties of capsaicin-sensitive and -insensitive sensory neurons. J Neurosci. 2002;22:RC228. doi: 10.1523/JNEUROSCI.22-12-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dymecki J, Poltorak M, Freed WJ. The degree of genetic disparity between donor and host correlates with survival of intraventricular substantia nigra grafts. Reg Immunol. 1990;3:17–22. [PubMed] [Google Scholar]
- 8.Fournier AE, Strittmatter SM. Repulsive factors and axon regeneration in the CNS. Curr Opin Neurobiol. 2001;11:89–94. doi: 10.1016/s0959-4388(00)00178-1. [DOI] [PubMed] [Google Scholar]
- 9.Groff RF, Huang JH, Zhang J, Pfister BJ, Zager EL, Smith DH. Surrogate nervous tissue survives and integrates in host nerve. J Neurotrauma. 2005;22:1192. Abstract. [Google Scholar]
- 10.Iwata A, Browne KD, Pfister BJ, Gruner JA, Smith DH. Long-term survival and outgrowth of mechanically engineered nervous tissue constructs implanted into spinal cord lesions. Tissue Eng. 2006;12:101–110. doi: 10.1089/ten.2006.12.101. [DOI] [PubMed] [Google Scholar]
- 11.Kim SU, Warren KG, Kalia M. Tissue culture of adult human neurons. Neurosci Lett. 1979;11:137–141. doi: 10.1016/0304-3940(79)90116-2. [DOI] [PubMed] [Google Scholar]
- 12.Li P, Tessler A, Han SS, Fischer I, Rao MS, Selzer ME. Fate of immortalized human neuronal progenitor cells transplanted in rat spinal cord. Arch Neurol. 2005;62:223–229. doi: 10.1001/archneur.62.2.223. [DOI] [PubMed] [Google Scholar]
- 13.Liu BP, Fournier A, GrandPré T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- 14.Mason DW, Charlton HM, Jones AJ, Lavy CB, Puklavec M, Simmonds SJ. The fate of allogeneic and xenogeneic neuronal tissue transplanted into the third ventricle of rodents. Neuroscience. 1986;19:685–694. doi: 10.1016/0306-4522(86)90292-7. [DOI] [PubMed] [Google Scholar]
- 15.McCarter GC, Reichling DB, Levine JD. Mechanical transduction by rat dorsal root ganglion neurons in vitro. Neurosci Lett. 1999;273:179–182. doi: 10.1016/s0304-3940(99)00665-5. [DOI] [PubMed] [Google Scholar]
- 16.Ostenfeld T, Caldwell MA, Prowse KR, Linskens MH, Jauniaux E, Svendsen CN. Human neural precursor cells express low levels of telomerase in vitro and show diminishing cell proliferation with extensive axonal outgrowth following transplantation. Exp Neurol. 2000;164:215–226. doi: 10.1006/exnr.2000.7427. [DOI] [PubMed] [Google Scholar]
- 17.Pfister BJ, Bonislawski DP, Smith DH, Cohen AS. Rapid stretch-growth of axons induces an increase in Na and K channel density. FEBS Lett. 2006;580:3525–3531. doi: 10.1016/j.febslet.2006.05.030. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfister BJ, Iwata A, Meaney DF, Smith DH. Extreme stretch growth of integrated axons. J Neurosci. 2004;24:7978–7983. doi: 10.1523/JNEUROSCI.1974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfister BJ, Iwata A, Taylor AG, Wolf JA, Meaney DF, Smith DH. Development of transplantable nervous tissue constructs comprised of stretch grown axons. J Neurosci Methods. 2006;153:95–103. doi: 10.1016/j.jneumeth.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Qiu J, Cai D, Filbin MT. Glial inhibition of nerve regeneration in the mature mammalian CNS. Glia. 2000;29:166–174. [PubMed] [Google Scholar]
- 21.Scott BS. Adult mouse dorsal root ganglia neurons in cell culture. J Neurobiol. 1977;8:417–427. doi: 10.1002/neu.480080503. [DOI] [PubMed] [Google Scholar]
- 22.Smith DH, Wolf JA, Meaney DF. A new strategy to produce sustained growth of central nervous system axons: continuous mechanical tension. Tissue Eng. 2001;7:131–139. doi: 10.1089/107632701300062714. [DOI] [PubMed] [Google Scholar]
- 23.Sosa IJ, Reyes O, Inserni J, Kuffler DP. Isolation and long-term survival of adult human sensory neurons in vitro. Neurosurgery. 1998;42:681–686. doi: 10.1097/00006123-199803000-00054. [DOI] [PubMed] [Google Scholar]
- 24.Valeyev AY, Hackman JC, Wood PM, Davidoff RA. Pharmacologically novel GABA receptor in human dorsal root ganglion neurons. J Neurophysiol. 1996;76:3555–3558. doi: 10.1152/jn.1996.76.5.3555. [DOI] [PubMed] [Google Scholar]
- 25.Ye JH, Rhrich F, Baillet-Derbin C, Horvat JC. Co-transplantation of fetal or adult dorsal root ganglia and of autologous peripheral nerve segments to the adult rat spinal cord: extensive reinnervation of the grafted nerves by the transplanted DRG cells. Dev Neurosci. 1992;14:123–129. doi: 10.1159/000111656. [DOI] [PubMed] [Google Scholar]


