Abstract
Biologic tumor necrosis factor inhibitors (TNFIs) include TNF decoy receptors (TNFRs). TNFα plays a pathologic role in both acute and chronic brain disease. However, biologic TNFIs cannot be developed as brain therapeutics because these large molecule drugs do not cross the blood-brain barrier (BBB). To enable penetration of the brain via receptor-mediated transport, the human TNFR type II was re-engineered as an IgG fusion protein, where the IgG part is a chimeric monoclonal antibody (MAb) against the mouse transferrin receptor (TfR), and this fusion protein is designated cTfRMAb-TNFR. The cTfRMAb part of the fusion protein acts as a molecular Trojan horse to ferry the TNFR across the BBB via transport on the endogenous BBB TfR. cTfRMAb-TNFR was expressed by stably transfected Chinese hamster ovary cells and purified by affinity chromatography to homogeneity on electrophoretic gels. The fusion protein reacted with antibodies to both mouse IgG and the human TNFR and bound TNFα with high affinity (Kd = 96 ± 34 pM). cTfRMAb-TNFR was rapidly transported into mouse brain in vivo after intravenous administration, and the brain uptake of the fusion protein was 2.8 ± 0.5% of injected dose per gram of brain, which is >45-fold higher than the brain uptake of an IgG that does not recognize the mouse TfR. This new IgG-TNFR fusion protein can be tested in mouse models of brain diseases in which TNFα plays a pathologic role.
Introduction
Tumor necrosis factor alpha (TNFα) plays a pathologic role in both acute brain disorders, including stroke (Nawashiro et al., 1997), traumatic brain injury (Knoblach et al., 1999), and spinal cord injury (Marchand et al., 2009), as well as chronic brain conditions, such as Parkinson's disease (PD) (McCoy et al., 2006) or Alzheimer's disease (AD) (McAlpine et al., 2009). TNFα action in brain can be suppressed by TNF inhibitors (TNFIs), and the most potent TNFIs are biologic drugs, including the extracellular domain of the type II TNF receptor (TNFR-II ECD) (Tansey and Szymkowski, 2009). Etanercept is a fusion protein of the TNFR-II ECD and the Fc region of human IgG1 and a leading biologic TNFI used to suppress inflammation in peripheral human diseases (Tansey and Szymkowski, 2009). Etanercept and other biologic TNFIs cannot be developed as drugs for disorders of the central nervous system (CNS), because these large molecules do not cross the blood-brain barrier (BBB) (Boado et al., 2010a).
Biologic drugs such as etanercept can be re-engineered to enable brain penetration by fusion of the biologic drug to a BBB molecular Trojan horse (MTH). The latter is a peptide or peptidomimetic monoclonal antibody (MAb) that binds an endogenous peptide receptor on the BBB. This binding triggers receptor-mediated transport of the MAb across the BBB, which enables the biologic drug that is fused to the MTH to penetrate the brain (Pardridge, 2010a). The most potent BBB MTH is a MAb against the human insulin receptor (HIR). A fusion protein of engineered MAb against the HIR (HIRMAb) and the TNFR-II ECD was engineered (Hui et al., 2009), and this protein, designated HIRMAb-TNFR, was shown to rapidly penetrate the BBB in vivo after intravenous (IV) injection in the rhesus monkey (Boado et al., 2010a). The HIRMAb part of the fusion protein cross-reacts with the insulin receptor of Old World primates, such as the rhesus monkey, but does not cross-react with the insulin receptor of lower animals, including rodents (Pardridge et al., 1995). Therefore, HIRMAb-TNFR cannot be tested in rat or mouse models of brain disease. A surrogate BBB MTH, which is a chimeric MAb against the mouse transferrin receptor (TfR), has been engineered, and this antibody is designated cTfRMAb (Boado et al., 2009). Biologic drugs, such as single-chain Fv antibodies, or neurotrophins, such as glial-derived neurotrophic factors, have been fused to the cTfRMAb, and these fusion proteins rapidly penetrate the mouse brain in vivo with a brain uptake of 3 to 4% of injected dose per gram of brain after IV administration (Boado et al., 2010b; Zhou et al., 2010). To develop a brain-penetrating biologic TNFI that can be tested in mouse models of CNS disease, in this study, we engineered and expressed a new IgG-TNFR fusion protein. The amino terminus of the human TNFR-II ECD was fused to the carboxyl terminus of the heavy chain (HC) of the cTfRMAb, and this new fusion protein is designated cTfRMAb-TNFR. The structure of cTfRMAb-TNFR is shown in Fig. 1. This study describes the isolation of a stably transfected line of Chinese hamster ovary (CHO) cells expressing cTfRMAb-TNFR, as well as the purification and biochemical properties of the fusion protein. This study then describes the in vivo pharmacokinetics and brain uptake of cTfRMAb-TNFR after IV administration in the adult mouse.
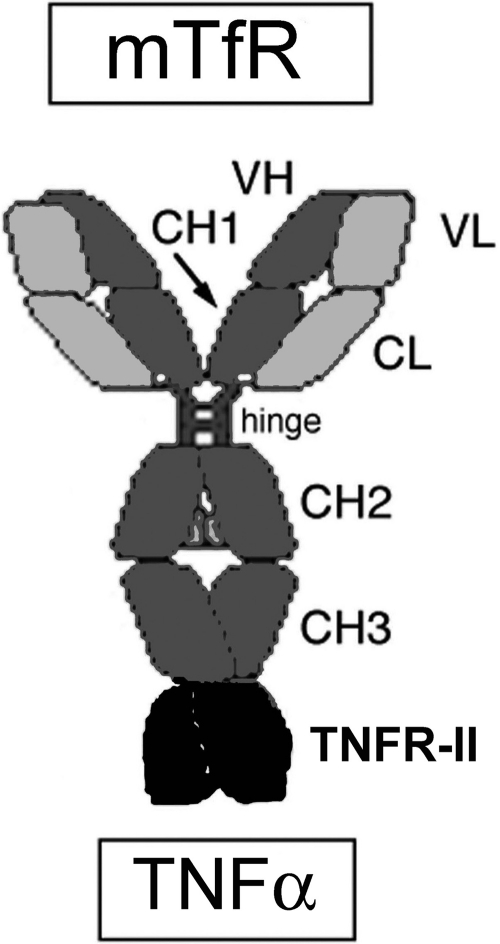
Fig. 1.
cTfRMAb-TNFR is comprised of two heavy chains and two light chains. The heavy chain is formed by fusion of the variable region of the heavy chain (VH) of the rat 8D3 MAb against the mouse transferrin receptor (mTfR) to the amino terminus of mouse IgG1 constant (C) region as well as fusion of human TNFR-II ECD to the carboxyl terminus of the heavy chain C region. The light chain is formed by fusion of the variable region of the light chain (VL) of the rat 8D3 MAb to the mouse κ light chain C region (CL). The heavy chain C region is comprised of four domains: CH1, hinge, CH2, and CH3.
Materials and Methods
Production of CHO Line.
A tandem vector (TV) plasmid DNA encoding the cTfRMAb heavy chain-TNFR fusion protein, the cTfRMAb light chain (LC), and the murine dihydrofolate reductase was engineered similar to a TV described previously (Boado et al., 2010b). The cDNA encoding the 235-amino acid (AA) human mature TNFR-II ECD was amplified by polymerase chain reaction as described previously (Hui et al., 2009) and subcloned at the 3′ end of the cTfRMAb HC to form the pcTfRMAb-TNFR tandem vector. The structure of the TV has been described previously (Boado et al., 2010b). The sequence of the TV was confirmed by bidirectional DNA sequencing performed at Eurofins MWG Operon (Huntsville, AL) using custom sequencing oligodeoxynucleotides synthesized at Midland Certified Reagent (Midland, TX). The TV was linearized and CHO cells were electroporated, followed by selection in hypoxanthine-thymine-deficient medium and amplification with graded increases of up to 80 nM in methotrexate in serum-free medium. High-producing clones were identified by measurement of medium concentrations of mouse IgG by enzyme-linked immunosorbent assay (ELISA). The CHO line was stable through multiple generations and produced medium IgG levels of 5 to 10 mg/l in shake flasks at a cell density of 1 to 2 million cells/ml.
Protein Purification.
The CHO cells were propagated in 1-liter bottles, until 2.4 liters of conditioned serum-free medium was collected. The medium was ultrafiltered with a 0.2-μm Sartopore 2 sterile-filter unit (Sartorius Stedim Biotech, Goettingen, Germany) and applied to a 25-ml column of Protein G Sepharose 4 Fast Flow (GE Healthcare, Chalfont St. Giles, UK) equilibrated in 25 mM Tris/25 mM NaCl/5 mM EDTA, pH 7.1. The column was washed with 25 mM Tris/1 M NaCl/5 mM EDTA, pH 7.1, and the fusion protein was eluted with 0.1 M glycine, pH 2.8. The acid eluate was pooled and neutralized to pH 6.5 with 1 M Tris base and concentrated with an Ultra-15 microconcentrator (Millipore Corporation, Billerica, MA) then sterile-filtered and stored at 4°C or at −70°C.
SDS-Polyacrylamide Gel Electrophoresis and Western Blotting.
The homogeneity of cTfRMAb-TNFR was evaluated with SDS-polyacrylamide gel electrophoresis (PAGE) under reducing and nonreducing conditions using 12 and 7.5% Tris-HCl gels (Bio-Rad Laboratories, Hercules, CA), respectively. Western blot analysis was performed with a goat anti-mouse IgG (H+L) antibody (Bethyl Laboratories, Montgomery, TX) for the mouse IgG Western blot and with a goat anti-human TNFR-II antibody (R&D Systems, Minneapolis, MN) for the TNFR Western blot. The immunoreactivity of cTfRMAb-TNFR was compared with that for the cTfRMAb described previously (Boado et al., 2009), and the 20-kDa human TNFR-II ECD (amino acids Pro24 to Thr206) was expressed in Escherichia coli (R&D Systems).
Amino-Terminal Amino Acid Sequence Analysis.
After SDS-PAGE under reducing conditions, the purified cTfRMAb-TNFR was electroblotted onto a 0.45-μm polyvinylidene fluoride filter (Immobilon-P Transfer Membrane; Millipore Corporation), followed by staining with Coomassie Blue. Edman sequence analysis of the heavy chain was performed through seven cycles at the Protein/Peptide Micro Analytical Laboratory at the California Institute of Technology (Pasadena, CA). The amino acid sequence Glu-Val-Gln-Leu-Val-Glu-Ser was a 100% match with the amino acid sequence deduced from nucleotide sequence analysis of the heavy chain of cTfRMAb (Boado et al., 2009).
TNFα Binding ELISA.
The binding of cTfRMAb-TNFR to human TNFα was determined by ELISA. The capture reagent was human TNFα from PeproTech (Rocky Hill, NJ). The negative control was mouse IgG1κ from Sigma-Aldrich (St. Louis, MO). The TNFα was dissolved in 0.1 M NaHCO3, pH 9.0, and plated overnight at 4°C in 100 μl/well (0.2 μg/well). After washing with 0.01 M Tris/0.15 M NaCl, pH 7.4 [Tris-buffered saline (TBS)], the wells were blocked with 1% bovine serum albumin in TBS for 30 min. A volume of 100 μl/well of cTfRMAb-TNFR or mouse IgG1κ was plated for 60 min at room temperature. After washing with TBS plus 0.05% Tween 20, a goat anti-mouse κ light chain antibody-alkaline phosphatase conjugate (Bethyl Laboratories) was incubated (0.2 μg/well) for 60 min. After washing with TBS plus 0.05% Tween 20, para-nitrophenylphosphate for color development and chromagen detection at 405 nm was performed with an ELISA plate reader after termination of the reaction with 1.2 M NaOH.
TNFα Radioreceptor Assay.
The saturable binding of human TNFα to cTfRMAb-TNFR was determined with a radioreceptor assay as described previously (Hui et al., 2009). A goat anti-mouse IgG1 Fc antibody (Bethyl Laboratories) was plated in 96-well plates (0.2 μg/well) with an overnight incubation in 0.1 M NaHCO3, pH 8.3, followed by washing, and blocking with 1% bovine serum albumin in 0.01 M Na2HPO4/0.15 M NaCl, pH 7.4 [phosphate-buffered saline (PBS)]. cTfRMAb-TNFR was plated (100 ng/well), followed by a 1-h incubation at room temperature. The wells were then washed with PBS, followed by the addition of 100 μl/well of a comixture of 125I-human TNFα (PerkinElmer Life and Analytical Sciences, Waltham, MA) (specific activity, 91 μCi/μg) at a concentration of 0.01 μCi/well (0.1 μCi/ml; 1.1 ng/ml; 60 pM) and various concentrations of unlabeled human TNFα, followed by a 3-h incubation at room temperature. The wells were emptied by aspiration and washed with ice-cold PBS, and 250 μl/well of 1 N NaOH was added, followed by heating at 60°C for 30 min. Radioactivity was counted in Ultima Gold (PerkinElmer Life and Analytical Sciences) in a Tri-Carb 2100TR liquid scintillation counter (PerkinElmer Life and Analytical Sciences), and the fractional binding per well was computed. The half-saturation constant, Kd, of TNFα binding to cTfRMAb-TNFR was determined by nonlinear regression analysis using BMDP2007e software (Statistical Solutions, Cork, Ireland) after fitting binding data to the following equation: Bound = [(Bmax)(C)]/(Kd + C), where Bmax is the maximal binding and C is the concentration of TNFα. The radiochemical purity of the 125I-TNFα was tested with precipitation with 10% ice-cold trichloroacetic acid (TCA), which was >99%.
Radiolabeling of Fusion Protein.
cTfRMAb-TNFR, which was injected into mice for a pharmacokinetics analysis, was radiolabeled with [3H]-N-succinimidyl propionate from American Radiolabeled Chemicals (St. Louis, MO). The labeled protein was purified with a 1 × 28 cm Sephadex G-25 gel filtration column with an elution buffer of 0.01 M sodium acetate/0.14 M NaCl, pH 5.5. cTfRMAb-TNFR was labeled to a specific activity of 0.8 μCi/μg and a TCA precipitability of 99%.
Pharmacokinetics and Brain Uptake in the Mouse.
Adult male C57BL/6J mice, weighing 25 g, were obtained from The Jackson Laboratory (Bar Harbor, ME). All procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) as adopted and promulgated by the National Institutes of Health. Mice in groups of four were anesthetized with intraperitoneal (IP) ketamine (100 mg/kg) and xylazine (10 mg/kg) and received either IV (in the tail vein) or IP injections with 0.1 ml (7 μCi) of [3H]-cTfRMAb-TNFR. The injection dose (ID) of cTfRMAb-TNFR in each mouse was 350 μg/kg. Because the fusion protein is 26% TNFR by amino acid content (as detailed under Results), the ID of TNFR is 90 μg/kg. An aliquot (50 μl) of venous blood was collected at 0.25, 2, 5, 15, 30, and 60 min after injection by sampling the orbital vein. The blood was centrifuged for collection of plasma, which was analyzed for radioactivity. At 60 min after injection, each mouse was euthanized and the cerebral hemisphere was removed and weighed. One hemisphere was used for total radioactivity after solubilization in Soluene-350 (PerkinElmer Life and Analytical Sciences), and one hemisphere was homogenized for capillary depletion analysis. Plasma and tissue samples were analyzed for 3H radioactivity with Opti-Fluor O (PerkinElmer Life and Analytical Sciences) and a Tri-Carb 2100TR liquid scintillation counter (PerkinElmer Life and Analytical Sciences). Brain uptake data were expressed as volume of distribution (VD), which is the ratio of the 60-min organ radioactivity [disintegrations per minute (DPM) per gram] divided by the 60-min plasma radioactivity (DPM/μl), or as percent ID per gram of tissue. The plasma radioactivity that was precipitable with ice-cold 10% TCA was determined at each time point.
The plasma radioactivity (disintegrations per minute per milliliter) was converted to percent ID per milliliter, which was fit to a biexponential equation, %ID/ml = A1e−k1t + A2e−k2t.
The intercepts (A1, A2) and the slopes (k1, k2) were used to compute the pharmacokinetics parameters, including the median residence time, the central volume of distribution, the steady-state volume of distribution, the area under the plasma concentration curve (AUC), and the systemic clearance. Nonlinear regression analysis used the AR subroutine of the BMDP Statistical Software. Data were weighted by 1/(%ID/ml)2.
The organ clearance (microliters per minute per gram), also called the BBB permeability-surface area (PS) product, is computed from the terminal brain uptake (percent ID per gram) and the 60-min plasma AUC (percent ID minutes per milliliter) as follows: PS product = [(%ID/g)/AUC] × 1000.
Capillary Depletion Method.
One cerebral hemisphere from each mouse was processed for the capillary depletion method described previously (Triguero et al., 1990), which separates the brain into three fractions as follows: the total brain homogenate, the microvascular capillary pellet, and the postvascular supernatant. The VD was determined for each of the three fractions from the ratio of total 3H radioactivity in the fraction, DPM/g brain, divided by the 3H radioactivity in the 60-min terminal plasma, DPM/μl. A high VD in the postvascular supernatant, compared to the VD in the capillary pellet, is evidence for transport of the protein through the BBB into brain parenchyma.
Results
A tandem vector was engineered; this vector contained the expression cassettes for the heavy chain fusion gene, the light gene, and the dihydrofolate reductase gene on a single plasmid DNA. DNA sequence analysis showed that the three expression cassettes spanned 9337 nucleotides. The light chain was comprised of 234 AAs, which included a 20-AA signal peptide, a 108-AA variable region of the light chain of cTfRMAb, and a 106-AA mouse κ light chain constant region, which is 100% identical to AAs 113 to 218 of BAA06141. The predicted molecular mass of the light chain is 23,554 Da, with a predicted isoelectric point of 5.73. The fusion protein of the cTfRMAb heavy chain and the TNFR was comprised of 698 AAs, which included a 19-AA signal peptide. The predicted molecular mass of the heavy chain, without glycosylation, is 74,073 Da, with a predicted pI of 6.34. The domains of the fusion heavy chain include a 118-AA variable region of the heavy chain of cTfRMAb, a 324-AA mouse IgG1 constant region, which is 100% identical to AAs 140 to 463 of BAC44885, a 2-AA linker (Ser-Ser), and the 235-AA human TNFR-II ECD, which is 100% identical to AAs 23 to 257 of NP_001057 (GenBank accession number). The HC contains three N-linked glycosylation sites: one site in the constant region of the IgG heavy chain and two sites in the TNFR domain. The predicted molecular mass, without glycosylation, of the heterotetrameric cTfRMAb-TNFR is 195,200 Da, with an isoelectric point of 6.23.
The CHO-derived cTfRMAb-TNFR was homogeneous on both nonreducing (Fig. 2B) and reducing (Fig. 2A) SDS-PAGE. The size of the HC of cTfRMAb-TNFR on the reducing gel (85 kDa) was greater than the size of the HC of cTfRMAb (55 kDa), owing to fusion of the 30-kDa TNFR (Fig. 2A). cTfRMAb-TNFR migrated at approximately 230 kDa on the nonreducing gel (Fig. 2B, lane 2). Western blot analysis with a primary antibody against mouse IgG detected both the HC and the LC of cTfRMAb (Fig. 3A, lane 2) and cTfRMAb-TNFR (Fig. 3A, lane 3) but not the recombinant TNFR-II ECD (Fig. 3A, lane 1). Western blot analysis with a primary antibody against human TNFR-II reacted with the HC of cTfRMAb-TNFR (Fig. 3B, lane 3) and TNFR-II ECD (Fig. 3B, lane 1) but did not react with HC of cTfRMAb (Fig. 3B, lane 2).
Fig. 2.
Results of reducing (A) and nonreducing (B) SDS-PAGE of cTfRMAb (lanes 1) and cTfRMAb-TNFR (lanes 2).
Fig. 3.
Results of Western blotting with a primary antibody against mouse IgG (A) or human TNFR-II (B). A, the anti-mouse antibody reacts with the HC and LC of both cTfRMAb (lane 2) and cTfRMAb-TNFR (lane 3), but not with the TNFR-II ECD (lane 1). B, the anti-TNFR antibody reacts only with the HC of cTfRMAb-TNFR (lane 3), and the TNFR-II ECD (lane 1), but does not react with cTfRMAb (lane 2).
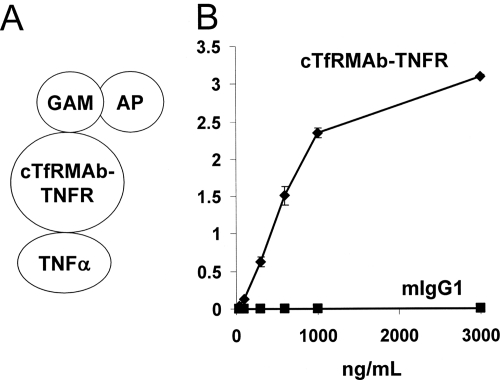
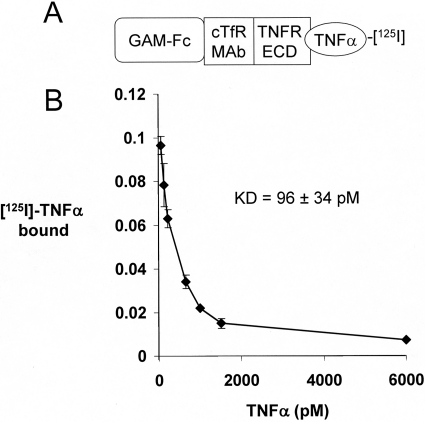
The design of the TNFα ELISA is shown in Fig. 4A. cTfRMAb-TNFR bound to the plated TNFα, whereas there is no binding by the mouse IgG1 control (Fig. 4B). The design of the TNFα radioreceptor assay is shown in Fig. 5A. TNFα bound to cTfRMAb-TNFR with high affinity characterized by a Kd of 96 ± 34 pM (Fig. 5B).
Fig. 4.
A, format of ELISA used to measure binding of cTfRMAb-TNFR to human TNFα. Human TNFα is the capture agent and a conjugate of a goat anti-mouse (GAM) IgG, and alkaline phosphatase (AP) is the detector reagent. B, cTfRMAb-TNFR binds to TNFα, whereas there is no binding to TNFα by mouse IgG1 (mIgG1).
Fig. 5.
A, outline of radioreceptor assay binding of TNFα to cTfRMAb-TNFR. A goat anti-mouse (GAM) IgG1 Fc was plated, which bound the Fc region of cTfRMAb-TNFR. The TNFR extracellular domain (ECD) region of the fusion protein then bound the 125I-TNFα, which was displaced by the addition of unlabeled TNFα. B, the saturable binding was analyzed by a nonlinear regression analysis to yield the concentration, Kd, that gave 50% inhibition of TNFα binding to cTfRMAb-TNFR.
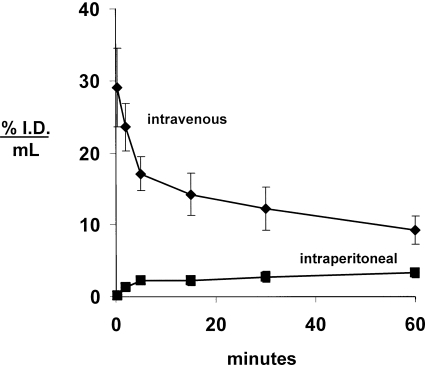
cTfRMAb-TNFR was radiolabeled with the 3H-N-succinimidyl propionate reagent (as detailed under Material and Methods) and injected into adult male C57BL/6J mice via either IV or IP administration. The clearance of [3H]-cTfRMAb-TNFR from plasma after IV administration and the appearance of plasma radioactivity after IP administration of the fusion protein are plotted in Fig. 6. For the intravenous injection studies, the plasma radioactivity decay curve was fit to a biexponential equation to yield the pharmacokinetics parameters shown in Table 1. [3H]-cTfRMAb-TNFR was metabolically stable after IV administration, because the plasma radioactivity that was precipitable by TCA was 99 ± 1, 96 ± 1, 92 ± 3, 88 ± 3, 86 ± 3, and 84 ± 4% at 0.25, 2, 5, 15, 30, and 60 min, respectively, after intravenous injection. After IP injection, the concentration of cTfRMAb-TNFR in blood increased with time (Fig. 6). Based on the trapezoid rule, the plasma AUC of cTfRMAb-TNFR at 60 min after IP injection is 158% ID · min/ml, which is 20% of the plasma AUC after intravenous injection at the 60-min time point (Table 1). The plasma AUC after IP injection may approach the AUC after intravenous injection at later time points.
Fig. 6.
Plasma concentration, expressed as percent ID per milliliter, of the [3H]-cTfRMAb-TNFR after intravenous or intraperitoneal injection in the mouse. Data are means ± S.E. (n = 4 mice/point).
TABLE 1.
Pharmacokinetic parameters of cTfRMAb-TNFR in the mouse
The injection dose was 350 μg/kg, and the body weight of the mice was 0.028 kg. The parameters were determined from the plasma profile shown in Fig. 6 for IV administration.
| Parameter | Unit | cTfRMAb-TNFR |
|---|---|---|
| A1 | %ID/ml | 14.8 ± 1.3 |
| A2 | %ID/ml | 16.1 ± 0.7 |
| k1 | min−1 | 0.38 ± 0.06 |
| k2 | min−1 | 0.0094 ± 0.0011 |
| MRT | min | 105 ± 12 |
| Vc | ml/kg | 130 ± 5 |
| Vss | ml/kg | 238 ± 10 |
| AUC (60 min) | %ID · min/ml | 778 ± 13 |
| AUCss | %ID · min/ml | 1761 ± 144 |
| CL | ml/(min · kg) | 2.3 ± 0.2 |
MRT, mean residence time; Vc, plasma volume; Vss, steady-state volume of distribution; AUC (60 min), AUC first 60 min; AUCss, steady-state AUC; CL, clearance from plasma.
The capillary depletion analysis showed that the VD of cTfRMAb-TNFR in the brain homogenate was high, 431 ± 39 μl/g (Table 2). The VD of the fusion protein in the postvascular supernatant, 326 ± 19 μl/g (Table 2), is 76% of the homogenate VD, indicating that the majority of cTfRMAb-TNFR had completed transcytosis through the BBB by 60 min after intravenous injection.
TABLE 2.
Capillary depletion analysis for brain uptake of cTfRMAb-TNFR fusion protein
Mean ± S.E. (n = 4 mice). The fusion protein was administered by IV injection, and brain measurements were made 60 min after injection.
| Fraction | VD |
|---|---|
| μl/g | |
| Brain homogenate | 431 ± 39 |
| Postvascular supernatant | 326 ± 19 |
| Vascular pellet | 155 ± 18 |
The uptake of [3H]-cTfRMAb-TNFR by brain was determined at 60 min after intravenous injection and was 2.8 ± 0.5% ID/g brain (Table 3). cTfRMAb-TNFR was also taken up by spinal cord, and the uptake was 2.6 ± 0.3, 3.1 ± 0.4, and 3.6 ± 0.3% ID/g in cervical, thoracic, and lumbar spinal cord, respectively. The BBB PS product of cTfRMAb-TNFR in brain, which is equal to the ratio of the 60-min percent ID per gram (Table 3) and the 60-min plasma AUC (Table 1), is 3.6 ± 0.6 μl · min/g. The concentration of cTfRMAb-TNFR in brain at 60 min after intravenous injection is 250 ± 45 ng/g (Table 3). Because the TNFR content of the fusion protein is 26% by amino acid content, the brain concentration of TNFR at 60 min after intravenous injection of the fusion protein is 65 ± 12 ng/g (Table 3).
TABLE 3.
cTfRMAb-TNFR brain uptake parameters
Mean ± S.E. (n = 4 mice). The fusion protein was administered by IV injection, and brain measurements were made 60 min after injection.
| Parameter | Value | Units |
|---|---|---|
| Brain uptake | 2.8 ± 0.5 | %ID/g |
| BBB PS product | 3.6 ± 0.6 | μl/(min · g) |
| Injection dose | 350 ± 5 | μg/kg |
| cTfRMAb-TNFR brain concentration | 250 ± 45 | ng/g |
| TNFR brain concentration | 65 ± 12 | ng/g |
Discussion
Biologic TNFIs include TNFR decoy receptors and anti-TNFα monoclonal antibodies, and these biologic drugs are potent anti-inflammatory agents for the treatment of peripheral tissues (Tansey and Szymkowski, 2009). Biologic TNFIs could also prove to be important new treatments for brain disorders (Pardridge, 2010b), because TNFα plays a proinflammatory role in both acute disorders of the brain [such as traumatic brain injury, spinal cord injury, and stroke (Nawashiro et al., 1997; Knoblach et al., 1999; Marchand et al., 2009)] as well as chronic brain diseases [such as Parkinson's disease or Alzheimer's disease (McCoy et al., 2006; McAlpine et al., 2009)]. However, biologic TNFIs cannot be developed for the brain, because these large molecule drugs do not cross the BBB, as recently demonstrated for the TNFR in the primate (Boado et al., 2010a).
The CNS drug development of biologic TNFIs requires in vivo validation in animal models. However, in vivo animal studies cannot go forward until the biologic TNFI is re-engineered to cross the BBB. The purpose of this study was to re-engineer the TNFR as the first biologic TNFI that is brain-penetrating in the mouse. The TNFR was re-engineered as a fusion protein with cTfRMAb, which acts as a molecular Trojan horse to ferry the TNFR across the BBB. This re-engineering of the TNFR was accomplished by fusion of the amino terminus of the human TNFR-II ECD to the carboxyl terminus of the heavy chain of cTfRMAb, as illustrated in Fig. 1. The human TNFR was fused to cTfRMAb rather than the mouse TNFR, because the human TNFR is the receptor that will ultimately be used for human therapeutics and because the human TNFR binds human and murine TNFα with the same high affinity (Scallon et al., 2002). The binding of murine TNFα by the human TNFR is compatible with the high (78%) amino acid identity between the monomeric human TNFα (GenBank accession number NP_000585) and the monomeric mouse TNFα (GenBank accession number NP038721). The fusion of the TNFR to the carboxyl terminus of the IgG heavy chain is a departure from the genetic engineering of all other decoy receptors. The engineering of all prior decoy receptor/Fc fusion proteins involves the fusion of the carboxyl terminus of the decoy receptor to the amino terminus of the human IgG Fc fragment (Peppel et al., 1991; Scallon et al., 1995). The structure shown in Fig. 1 was chosen because the amino terminus of the TNFR receptor is not involved in ligand binding (Banner et al., 1993), and this design leaves free the amino terminus of the receptor-specific MAb. The variable regions of the antibody that bind to the BBB receptor are located in the amino-terminal parts of the IgG chains (Boado et al., 2009), and fusion of proteins to the antibody amino terminus impairs binding of the antibody to the target BBB receptor (Boado and Pardridge, 2010).
The structure of cTfRMAb-TNFR shown in Fig. 1 was validated by DNA sequencing and the deduced amino acid sequence (as detailed under Results). The SDS-PAGE (Fig. 2) and the mouse IgG and human TNFR Western blots (Fig. 3) also verify the structure of the fusion protein. cTfRMAb-TNFR is a bifunctional molecule that binds both TNFα and the murine TfR with high affinity characterized by low-nanomolar binding constants. cTfRMAb-TNFR binds human TNFα in the ELISA (Fig. 4) and in the radioreceptor assay (Fig. 5). The Kd of fusion protein binding to human TNFα is low, 96 ± 34 pM (Fig. 5B), and this high binding will enable sequestration of TNFα in brain at pM concentrations.
cTfRMAb binds the mouse TfR with a Kd of 2.6 ± 0.3 nM (Boado et al., 2009), and fusion of therapeutics to cTfRMAb does not impair fusion protein binding to the murine TfR (Boado et al., 2010b; Zhou et al., 2010). Evidence for the high-affinity binding of cTfRMAb-TNFR to the TfR on the mouse BBB is the high brain uptake of cTfRMAb-TNFR, 2.8 ± 0.5% ID/g brain (Table 3). In contrast, the brain uptake of a MAb that does not recognize the mouse TfR is 47-fold lower, 0.06 ± 0.01% ID/g brain (Lee et al., 2000). The binding of cTfRMAb-TNFR to the BBB TfR triggers transcytosis through the BBB and penetration of the brain parenchyma. This activity is demonstrated with the capillary depletion method (Table 2). The VD of the fusion protein in the postvascular supernatant, 326 ± 19 μl/g, is manyfold above the brain blood volume in the mouse, 10 ± 1 μl/g (Lee et al., 2000), and is 76% of the fusion protein VD in the brain homogenate (Table 2). Measurement of the specific activity of capillary-specific enzymes, such as γ-glutamyl transpeptidase and alkaline phosphatase, shows that the microvasculature of brain is 95% removed from the postvascular supernatant with the capillary depletion method (Triguero et al., 1990). Therefore, the high VD in the postvascular supernatant represents fusion protein penetration into the brain parenchyma. The findings with the capillary depletion method have been verified with emulsion autoradiography of brain, which show rapid penetration of brain parenchyma after fusion protein targeting of the BBB receptor (Boado and Pardridge, 2009). The neuroprotection in experimental PD observed after IV administration of cTfRMAb-glial-derived neurotrophic factor is pharmacologic evidence for fusion protein penetration of brain parenchyma from blood (Fu et al., 2010).
The concentration of the TNFR in mouse brain after the intravenous injection of 350 μg/kg is 65 ± 12 ng/g (Table 3), which is equivalent to a brain TNFR concentration of 3.7 nM, which is high compared to the concentration of TNFα in brain. Under normal conditions, the brain TNFα concentration is 20 to 100 pg/g or 1 to 5 pM (Zhao et al., 2007; Liu et al., 2009). In experimental stroke, the brain TNFα concentration increases to approximately 0.5 nM (Shohami et al., 1994). Therefore, a relatively low dose of fusion protein, 350 μg/kg, is sufficient to sequester even the very high concentrations of TNFα that occur immediately after stroke. This dose of IgG-TNFR fusion protein is small compared to the dose of TNFR that is required to induce immune suppression in peripheral tissues (Boado et al., 2010a). Therefore, chronic conditions such as AD or PD could be treated with brain-penetrating biologic TNFIs such as IgG-TNFR fusion proteins. Several lines of investigation confirm the primary role played by TNFα in the pathogenesis of both PD (Ferger et al., 2004; McCoy et al., 2006) and AD (Janelsins et al., 2008; McAlpine et al., 2009).
In summary, this study describes the genetic engineering, CHO cell expression, purification, and biochemical analysis of a new IgG-TNFR fusion protein that is specific for the mouse. cTfRMAb-TNFR is the first biologic TNFI that is capable of penetration of the BBB after peripheral administration in the mouse. Future investigations of murine models of acute and chronic brain disease may evaluate the efficacy of brain-penetrating biologic TNFIs such as cTfRMAb-TNFR.
Acknowledgments
Winnie Tai and Phuong Tram provided technical assistance.
This work was supported by the National Institutes of Health National Institute on Aging [Grant R01-AG032244].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.110.036012.
- TNFα
- tumor necrosis factor alpha
- PD
- Parkinson's disease
- AD
- Alzheimer's disease
- TNFI
- TNF inhibitor
- TNFR-II ECD
- extracellular domain of the type II TNF receptor
- CNS
- central nervous system
- BBB
- blood-brain barrier
- MTH
- molecular Trojan horse
- MAb
- monoclonal antibody
- HIR
- human insulin receptor
- IV
- intravenous
- TfR
- transferrin receptor
- CHO
- Chinese hamster ovary
- TV
- tandem vector
- LC
- light chain
- AA
- amino acid
- HC
- heavy chain
- ELISA
- enzyme-linked immunosorbent assay
- PAGE
- polyacrylamide gel electrophoresis
- TBS
- Tris-buffered saline
- PBS
- phosphate-buffered saline
- TCA
- trichloroacetic acid
- IP
- intraperitoneal
- ID
- injection dose
- VD
- volume of distribution
- DPM
- disintegrations per minute
- AUC
- area under the plasma concentration curve
- PS
- permeability-surface area
- C
- constant
- BAA06141
- mouse immunoglobulin gamma-1 light chain
- BAC44885
- mouse immunoglobulin gamma-1 heavy chain.
Authorship Contributions
Participated in research design: Boado and Pardridge.
Conducted experiments: Zhou, Boado, Hui, and Lu.
Contributed new reagents or analytic tools: Boado.
Performed data analysis: Zhou, Boado, Hui, Lu, and Pardridge.
Wrote or contributed to writing of the manuscript: Zhou, Boado, Hui, Lu, and Pardridge.
References
- Banner DW, D'Arcy A, Janes W, Gentz R, Schoenfeld HJ, Broger C, Loetscher H, Lesslauer W. (1993) Crystal structure of the soluble human 55 kd TNF receptor-human TNF beta complex: implications for TNF receptor activation. Cell 73:431–445 [DOI] [PubMed] [Google Scholar]
- Boado RJ, Pardridge WM. (2009) Comparison of blood-brain barrier transport of glial-derived neurotrophic factor (GDNF) and an IgG-GDNF fusion protein in the rhesus monkey. Drug Metab Dispos 37:2299–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ, Zhang Y, Wang Y, Pardridge WM. (2009) Engineering and expression of a chimeric transferrin receptor monoclonal antibody for blood-brain barrier delivery in the mouse. Biotechnol Bioeng 102:1251–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ, Pardridge WM. (2010) Genetic engineering of IgG-glucuronidase fusion proteins. J Drug Target 18:205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ, Hui EK, Lu JZ, Zhou QH, Pardridge WM. (2010a) Selective targeting of a TNFR decoy receptor pharmaceutical to the primate brain as a receptor-specific IgG fusion protein. J Biotechnol 146:84–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ, Zhou QH, Lu JZ, Hui EK, Pardridge WM. (2010b) Pharmacokinetics and brain uptake of a genetically engineered bifunctional fusion antibody targeting the mouse transferrin receptor. Mol Pharm 7:237–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferger B, Leng A, Mura A, Hengerer B, Feldon J. (2004) Genetic ablation of tumor necrosis factor-alpha (TNF-alpha) and pharmacological inhibition of TNF-synthesis attenuates MPTP toxicity in mouse striatum. J Neurochem 89:822–833 [DOI] [PubMed] [Google Scholar]
- Fu A, Zhou QH, Hui EK, Lu JZ, Boado RJ, Pardridge WM. (2010) Intravenous treatment of experimental Parkinson's disease in the mouse with an IgG-GDNF fusion protein that penetrates the blood-brain barrier. Brain Res 1352:208–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui EK, Boado RJ, Pardridge WM. (2009) Tumor necrosis factor receptor-IgG fusion protein for targeted drug delivery across the human blood-brain barrier. Mol Pharm 6:1536–1543 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Janelsins MC, Mastrangelo MA, Park KM, Sudol KL, Narrow WC, Oddo S, LaFerla FM, Callahan LM, Federoff HJ, Bowers WJ. (2008) Chronic neuron-specific tumor necrosis factor-alpha expression enhances the local inflammatory environment ultimately leading to neuronal death in 3xTg-AD mice. Am J Pathol 173:1768–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblach SM, Fan L, Faden AI. (1999) Early neuronal expression of tumor necrosis factor-alpha after experimental brain injury contributes to neurological impairment. J Neuroimmunol 95:115–125 [DOI] [PubMed] [Google Scholar]
- Lee HJ, Engelhardt B, Lesley J, Bickel U, Pardridge WM. (2000) Targeting rat anti-mouse transferrin receptor monoclonal antibodies through blood-brain barrier in mouse. J Pharmacol Exp Ther 292:1048–1052 [PubMed] [Google Scholar]
- Liu N, Chen R, Du H, Wang J, Zhang Y, Wen J. (2009) Expression of IL-10 and TNF-alpha in rats with cerebral infarction after transplantation with mesenchymal stem cells. Cell Mol Immunol 6:207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand F, Tsantoulas C, Singh D, Grist J, Clark AK, Bradbury EJ, McMahon SB. (2009) Effects of Etanercept and Minocycline in a rat model of spinal cord injury. Eur J Pain 13:673–681 [DOI] [PubMed] [Google Scholar]
- McAlpine FE, Lee JK, Harms AS, Ruhn KA, Blurton-Jones M, Hong J, Das P, Golde TE, LaFerla FM, Oddo S, et al. (2009) Inhibition of soluble TNF signaling in a mouse model of Alzheimer's disease prevents pre-plaque amyloid-associated neuropathology. Neurobiol Dis 34:163–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MK, Martinez TN, Ruhn KA, Szymkowski DE, Smith CG, Botterman BR, Tansey KE, Tansey MG. (2006) Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson's disease. J Neurosci 26:9365–9375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawashiro H, Martin D, Hallenbeck JM. (1997) Neuroprotective effects of TNF binding protein in focal cerebral ischemia. Brain Res 778:265–271 [DOI] [PubMed] [Google Scholar]
- Pardridge WM. (2010a) Biopharmaceutical drug targeting to the brain. J Drug Target 18:157–167 [DOI] [PubMed] [Google Scholar]
- Pardridge WM. (2010b) Biologic TNFα-inhibitors that cross the human blood-brain barrier. Bioengineered Bugs 1:233–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM, Kang YS, Buciak JL, Yang J. (1995) Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transcytosis through the blood-brain barrier in vivo in the primate. Pharm Res 12:807–816 [DOI] [PubMed] [Google Scholar]
- Peppel K, Crawford D, Beutler B. (1991) A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity. J Exp Med 174:1483–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallon BJ, Trinh H, Nedelman M, Brennan FM, Feldmann M, Ghrayeb J. (1995) Functional comparisons of different tumour necrosis factor receptor/IgG fusion proteins. Cytokine 7:759–770 [DOI] [PubMed] [Google Scholar]
- Scallon B, Cai A, Solowski N, Rosenberg A, Song XY, Shealy D, Wagner C. (2002) Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther 301:418–426 [DOI] [PubMed] [Google Scholar]
- Shohami E, Novikov M, Bass R, Yamin A, Gallily R. (1994) Closed head injury triggers early production of TNF alpha and IL-6 by brain tissue. J Cereb Blood Flow Metab 14:615–619 [DOI] [PubMed] [Google Scholar]
- Tansey MG, Szymkowski DE. (2009) The TNF superfamily in 2009: new pathways, new indications, and new drugs. Drug Discov Today 14:1082–1088 [DOI] [PubMed] [Google Scholar]
- Triguero D, Buciak J, Pardridge WM. (1990) Capillary depletion method for quantification of blood-brain barrier transport of circulating peptides and plasma proteins. J Neurochem 54:1882–1888 [DOI] [PubMed] [Google Scholar]
- Zhao C, Ling Z, Newman MB, Bhatia A, Carvey PM. (2007) TNF-alpha knockout and minocycline treatment attenuates blood-brain barrier leakage in MPTP-treated mice. Neurobiol Dis 26:36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QH, Boado RJ, Lu JZ, Hui EK, Pardridge WM. (2010) Monoclonal antibody-glial-derived neurotrophic factor fusion protein penetrates the blood-brain barrier in the mouse. Drug Metab Dispos 38:566–572 [DOI] [PMC free article] [PubMed] [Google Scholar]