Abstract
Sphingosine-1-phosphate and its receptors have emerged as important modulators of the immune response. The sphingosine-1-phosphate prodrug 2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol (FTY720) can alleviate experimental allergic airway inflammation. Nevertheless, the role of individual sphingosine-1-phosphate receptors in the regulation of allergic airway inflammation remains undefined. Using a newly characterized potent and selective sphingosine-1-phosphate receptor 1 (S1P1) agonist with physical properties allowing airway delivery, we studied the contribution of S1P1 signaling to eosinophilic airway inflammation induced in ovalbumin-immunized mice by airway challenges with ovalbumin. Airway delivery of receptor-nonselective sphingosine-1-phosphate prodrug significantly inhibits the sequential accumulation of antigen-presenting dendritic cells and CD4+ T cells in draining lymph nodes. This in turn suppressed by >80% the accumulation of CD4+ T cells and eosinophils in the airways. Systemic delivery of sphingosine-1-phosphate prodrug or of an S1P1-specific agonist at doses sufficient to induce lymphopenia did not inhibit eosinophil accumulation in the airways. In contrast, local airway delivery of S1P1-specific agonist inhibited airways release of endogenous CCL5 and CCL17 chemokines, and significantly suppressed accumulation of activated T cells and eosinophils in the lungs. Specific S1P1 agonism in lungs contributes significantly to anti-inflammatory activities of sphingosine-1-phosphate therapeutics by suppressing chemokine release in the airways, and may be of clinical relevance.
Introduction
Sphingosine-1-phosphate (S1P) is produced by phosphorylation of sphingosine by sphingosine kinases (SK1 and SK2). Along with its five high affinity G-protein-coupled receptors (S1P1–S1P5), S1P has emerged as significant modulator of processes underlying pathogenesis of asthma. For instance, SK1-derived S1P regulates pro-inflammatory signaling pathways, including activation of nuclear factor-κB (Alvarez et al., 2010). S1P1 regulates endothelial barrier integrity (Sanna et al., 2006); cytokine and adhesion molecule expression (Lien et al., 2006) lymphocyte maturation, differentiation, and trafficking (Sanna et al., 2004; Liu et al., 2009); and mast cell migration (Jolly et al., 2004). S1P2 has been involved in the regulation of mast cell degranulation (Jolly et al., 2004) and tissue remodeling (Skoura et al., 2007), whereas S1P3 was shown to modulate dendritic cell trafficking (Niessen et al., 2008). In addition, SK1 and SK2 (Liu et al., 2000), as well as receptors S1P1 to S1P4 are expressed in the lung tissue (Gräler et al., 1998; Zhang et al., 1999). It is noteworthy that S1P levels are increased in the airways of patients with asthma (Ammit et al., 2001) but it remains unclear whether this increase is deleterious or protective.
The role of S1P and its receptors in the pathogenesis of allergic airway inflammation remains controversial. For instance, the S1P prodrug 2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol (FTY720) alleviates the salient features of allergic airway inflammation (AAI), including accumulation of eosinophils and T cells in the airways and development of bronchial hyper-responsiveness (Sawicka et al., 2003; Idzko et al., 2006). In contrast, administration of exogenous S1P exacerbates bronchial hyper-responsiveness (Roviezzo et al., 2004) by favoring mast cell and eosinophil accumulation in the lung, whereas reduction of S1P levels using sphingosine kinases inhibitors leads to alleviation of AAI (Nishiuma et al., 2008).
Nonselective S1P receptor agonists, with significant additional off-target activities, limit mechanistic understanding of the system. Indeed, sphingosine analogs such as FTY720 or (R)-2-amino-4-(4-heptyloxyphenyl)-2-methylbutanol (AAL-R) interact with or inhibit multiple proteins, including sphingosine kinases, S1P lyase, cytosolic phospholipase A2, lipid phosphatases, and, after phosphorylation, four of the five S1P receptors (S1P1, S1P3, S1P4, and S1P5) (for review, see Marsolais and Rosen, 2009). Likewise, modulation of AAI by genetic or chemical inhibition of sphingosine kinase activities can result either from the build up of upstream metabolites in the metabolic pathway leading to S1P generation (Billich et al., 2003; Petrache et al., 2005) or, potentially, from decreased stimulation of any of the 5 S1P receptors. Thus, current genetic systems and receptor-subtype nonselective chemical probes are limited in the analysis of the complex sphingosine-S1P-S1P receptor signaling system in vivo.
S1P1 agonism shows therapeutic potential for alleviation of pulmonary immunopathology because it regulates T-cell trafficking (Brinkmann et al., 2004; Sanna et al., 2004), barrier integrity (Marsolais and Rosen, 2009), and cytokine release (Lien et al., 2006). Genetic deletion of S1P1 did not clarify the role of this receptor in the regulation of pulmonary immune response because it is embryonic lethal. Moreover, existing S1P1 agonists such as 5-[4-phenyl-5-(trifluoromethyl)thiophen-2-yl]-3-[3-(trifluoromethyl)phenyl]1,2,4-oxadiazole (SEW2871) or 3-(((2-(2-(trifluoromethyl)-[1,1'-biphenyl]-4-yl)benzo[b]thiophen-5-yl)methyl)amino)propanoic acid (AUY954) (Pan et al., 2006) had poor water solubility, which limited their use in the airways. Indeed, these agonists need to be dissolved in common solvents including DMSO or methanol, which are incompatible with lung delivery (Massion et al., 1996). Usage of receptor-specific agonists with physical properties allowing airway delivery is therefore a preferred experimental strategy to dissect site- and subtype-specific receptor contributions in the pathogenesis of respiratory diseases. Here we show that airway delivery of a water-soluble S1P1-selective agonist dampens recruitment of activated CD4+ cells and inhibits eosinophilic airway inflammation by regulating the release of chemokines in the airways.
Materials and Methods
Mice.
Male C57BL/6j and BALB/c mice were maintained in a closed breeding facility at The Scripps Research Institute. The handling of all mice conformed to the requirements of the National Institutes of Health and The Scripps Research Institute animal research committee.
Allergic Airway Inflammation Model.
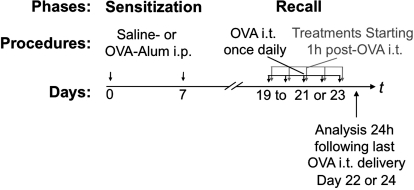
Male mice (6–8 weeks old) were immunized intraperitoneally on day 0 and 7 with 10 μg of ovalbumin (OVA) (grade V; Sigma-Aldrich, St. Louis, MO) in 0.1 ml of aluminum hydroxide gel (Sigma) or 0.1 ml of aluminum hydroxide gel with saline for control. Recall response was induced on days 19 to 21 or 23 with intratracheal delivery, once daily, of PBS containing 0.1% OVA (grade V; Sigma) (Fig. 1).
Fig. 1.
Experimental model. Mice were sensitized on days 0 and 7 by intraperitoneal (i.p.) injections of OVA coupled to the adjuvant aluminum hydroxide (Alum). Recall phase consisted of intratracheal (i.t.) delivery of OVA, once daily, on consecutive days, starting on day 19. OVA/OVA mice developed allergic airway inflammation. When required, sham procedure was performed and consisted of injecting mice with a mixture of saline and aluminum hydroxide before OVA intratracheal challenges (saline/OVA mice). Depending on the experiment, three to five intratracheal OVA challenges were performed, and mice were euthanized 24 h after the last intratracheal OVA delivery (on days 22 and 24, respectively) for analyses. AUY954 or AAL-R was administered once daily starting 1 h after intratracheal OVA delivery. To account for the short in vivo half-life of CYM-5442, this compound was delivered 1 and 13 h after each intratracheal delivery of OVA.
Compounds.
AUY954 [synthesized according to published methods (Pan et al., 2006)] was dissolved in polyethylene glycol 300 and 5% dextrose, whereas AAL-R and the tartrate salt of 2-(4-(5-(3,4-diethoxyphenyl)-1,2,4-oxadiazol-3-yl)-2,3-dihydro-1H-inden-1-yl amino)ethanol (CYM-5442) were dissolved in water. AUY954 was administered by gavage, whereas AAL-R and CYM-5442 were either delivered intraperitoneally or intratracheally (local treatment). Mice were anesthetized with isoflurane for intratracheal delivery of compounds.
Flow Cytometric Analyses.
Single-cell suspensions were obtained from bronchoalveolar lavage fluid (BALF), whole lungs, or lymph nodes as described previously (Marsolais et al., 2008). The frequencies of dendritic cells, T cells, and eosinophils were assessed by flow cytometry. To do so, single-cell suspensions were processed for surface staining with fluorochrome-labeled antibodies raised against murine CD3e, CD4, CD8a, CD11b, CD11c, CD45.2, CD44, CD62L, GR-1, F4/80, and CCR-3 (BD Biosciences Pharmingen, San Diego, CA, and eBioscience, San Diego, CA). Total number of viable cells was evaluated by the trypan-blue exclusion method and multiplied by frequencies obtained by flow cytometry to compute the absolute number of specific cell subsets.
Cytokine Quantification.
Bronchoalveolar lavage was performed with 1 ml of PBS. Samples were kept on ice, and cell-free supernatant was harvested after centrifugation at 4°C. A cocktail of protease inhibitors (Complete; Roche Diagnostics) was added to the samples, which were stored at −20°C until multiplex enzyme-linked immunosorbent assay analysis (Quansys Biosciences, Logan, UT) (Marsolais et al., 2009).
In Vivo Uptake of OVA-Alexa Fluor 647.
Mice were administered by gavage with either 3 mg/kg AUY954 or vehicle (polyethylene glycol 300, 5% dextrose). Three hours later, 50 μl of PBS or 100 μg of OVA-Alexa Fluor 647 (Invitrogen) in 50 μl of PBS was administered intratracheally under isoflurane anesthesia. Twenty-four hours after injection, Alexa Fluor 647+ CD11C+ dendritic cells (DCs) were quantified in lungs and mediastinal lymph nodes.
Bone Marrow-Derived DCs In Vivo Migration Assay.
DCs were derived from bone marrow cells as described previously (Lutz et al., 1999). Cultured DCs were incubated with DMSO or 1 μM AUY954. Thirty minutes later, 100 μg/ml OVA was added into the DCs culture and incubated overnight. DCs were then washed and labeled with carboxyl-fluorescein diacetate, succinimidyl ester (CFSE) (Invitrogen). CFSE- labeled DCs (2 × 106) were transferred intratracheally into congenic mice. The number of CFSE+ CD11c+ cells was quantified in lungs and mediastinal LNs 48 h after DC transfer.
Statistical Analysis.
Bars represent means of a group of mice ± S.E. of the mean. Averages between groups were compared using one-way analysis of variance (with Tukey-Kramer post hoc test) or with two-sided unpaired Student's t test. When appropriate, logarithmic transformation was applied on the data to insure stability of variance between groups. For analyses of variance, labeling with a same alphabetic letter represents absence of differences between groups. Asterisk (*) was used to mark significant differences between two groups when T tests were performed. The results were considered significant with p-values ≤0.05. Three to six mice were used in each individual group included in an experiment; a minimum of two experiments was performed for each set of data presented in figures. Data were analyzed using the statistical package STATA (ver. 10.10; StataCorp LP, College Station, TX).
Results
Nonselective S1P Receptor Agonist Prodrug Alleviates AAI.
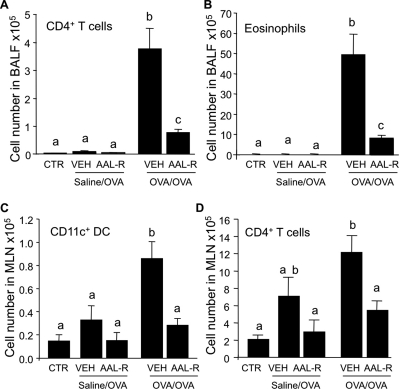
In a first series of experiments, we determined how local intratracheal delivery of the S1P receptor agonist prodrug AAL-R modulated the immune response during the recall response of AAI. As in nonimmunized mice (CTR), mice immunized with saline and challenged with OVA (saline/OVA mice; Fig. 1) did not display salient features of AAI (i.e., massive accumulation of T cells and eosinophils in BALF) (Fig. 2, A and B). As expected, significant accumulation of DCs and T cells in draining mediastinal lymph nodes (Fig. 2, C and D), as well as infiltration of CD4+ T cells and eosinophils into the airways (Fig. 2, A and B), was observed in mice sensitized and then challenged with OVA (OVA/OVA mice).
Fig. 2.
Local activation of S1P receptors efficiently inhibits eosinophilic airway inflammation. A–D, C57BL/6J mice were either left intact (CTR), submitted to the sham procedure (saline/OVA) or to the procedure inducing allergic airway inflammation (OVA/OVA). saline/OVA, or OVA/OVA mice were treated intratracheal with 50 μl of water (VEH) or AAL-R (0.1 mg/kg) 1 h after each daily intratracheal delivery of OVA, during a 3-day recall response. Mice were euthanized 24 h after the last intratracheal delivery of OVA. A, the accumulation of CD4+ T cells (A) and eosinophils (B) was inhibited in the BALF by the AAL-R treatment, compared with VEH, in the OVA/OVA mice. In addition, the number of CD11c+ DCs (C) and of CD4+ T cells (D) were significantly decreased in mediastinal lymph nodes (MLN) by AAL-R treatment compared with VEH in OVA/OVA mice. n = 3–5 mice per group; bars represent means ± S.E.M. *, significantly different from VEH, p < 0.05.
Local intratracheal delivery of 0.1 mg/kg AAL-R during the recall phase of AAI inhibited the accumulation of CD11c+ DCs in the draining mediastinal lymph nodes (Fig. 2C) of OVA/OVA mice, leading to impaired expansion of CD4+ T cells in mediastinal lymph nodes (Fig. 2D) and to >70% inhibition of CD4+ T cell accumulation in the BALF (Fig. 2A). Moreover, the accumulation of eosinophils in BALF was reduced by 80% compared with vehicle (VEH)-treated OVA/OVA mice, 24 h after the last OVA intratracheal challenge (Fig. 2B). These results confirm alteration of DC trafficking from lungs to lymph nodes as a primary immunosuppressive mechanism of airway-delivered sphingosine analogs (Idzko et al., 2006; Marsolais et al., 2008; Marsolais et al., 2009).
Contrasting the Effects of Systemic Delivery of Prodrug or Selective S1P1 Agonist on AAI.
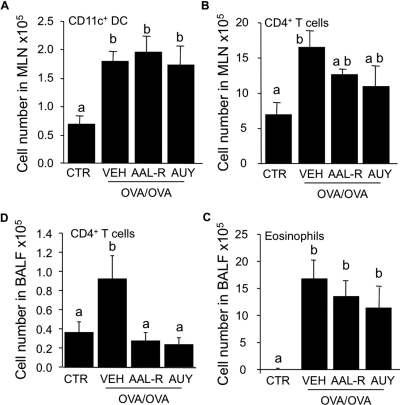
To quantify the contribution of systemic effects of S1P receptor activation in the recall phase of AAI, mice were gavaged with either AAL-R or with the S1P1-selective agonist AUY954 (Pan et al., 2006) at doses sufficient to induce sustained lymphopenia (Pan et al., 2006; Marsolais et al., 2008; data not shown). Systemic delivery of either AAL-R or AUY954 failed to significantly inhibit accumulation of CD11c+ DCs in mediastinal lymph nodes of OVA/OVA mice 24 h after the last OVA challenge compared with OVA/OVA mice treated with VEH (Fig. 3A). As expected by lymphocyte sequestration from peripheral blood, systemic delivery of these drugs mildly altered CD4+ T cell content of mediastinal lymph nodes (Fig. 3B), reduced the number of transiting CD4+ T cells in the lungs (Fig. 3C), but failed to significantly alter the accumulation of eosinophils in BALF (Fig. 3D). Systemic delivery of S1P prodrug increased the number of total T cells in nondraining inguinal popliteal lymph nodes (not shown) through sequestration. Thus, systemic delivery of either S1P prodrug or S1P1-specific agonist after allergen challenge, at doses sufficient to induce lymphopenia, fails to replicate the effects of airway-delivered agonist, and uncouples pulmonary effects from the alteration of trafficking of naive lymphocytes.
Fig. 3.
Systemic delivery of AAL-R or AUY954 is impotent for control of allergic airway inflammation. BALB/c mice were left intact (CTR), or submitted to the procedure inducing allergic airway inflammation (OVA/OVA). OVA/OVA mice were administered by gavage with water (VEH), AAL-R (0.1 mg/kg), or AUY954 (AUY; 3 mg/kg) 3 h before each daily intratracheal instillation of OVA, during a 5-day recall response. Mice were euthanized 24 h after the last intratracheal delivery of OVA. Systemic delivery of AAL-R or AUY954 did not significantly inhibit of the accumulation of CD11c+ cells (A) or CD4+ T cells (B) in the draining mediastinal lymph nodes, compared with VEH. AAL-R and AUY954 did not strongly reduce the accumulation of T cells (C) or eosinophils (D) in the BALF. n = 4–6 mice per group; bars represent means ± S.E.M. *, significantly different from VEH, p < 0.05.
S1P1 Receptor Activation Does Not Interfere with Antigen Transport from Lungs to Mediastinal Lymph Nodes.
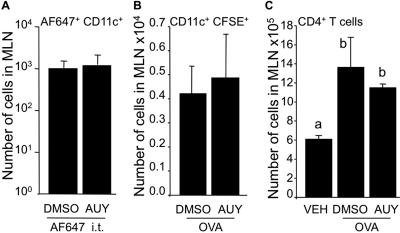
Given that the number of CD4+ T cells was reduced in BALF by local intratracheal S1P prodrug treatment, we tested the hypothesis that S1P1 activation short-circuited DC trafficking from lungs to mediastinal lymph nodes, as previously shown with the broad S1P receptor agonist prodrugs (Fig. 2) (Idzko et al., 2006; Marsolais et al., 2009). Systemic activation of S1P1 can increase the number of DCs in the blood (Lan et al., 2005). However, whether this effect is mediated through direct activation of S1P1 on DCs remains unknown. In agreement with results shown in Fig. 3A, oral delivery of AUY954 did not alter the movement of CD11c+ carrying Alexa Fluor 647-coupled OVA from lungs to mediastinal lymph nodes (Fig. 4A). In addition, in vitro treatment of CFSE-stained OVA-treated BMDCs with micromolar concentration of AUY954 did not impair the ability of DCs to migrate to the mediastinal lymph nodes (Fig. 4B), which resulted in a normal T cell response in the BALF (Fig. 4C). In addition, S1P3-null DCs pulsed with OVA-induced AAI (not shown). Together, these results suggest that the mechanism of S1P1-mediated inhibition of T cell accumulation in BALF during AAI is independent of both migration and antigen presentation by DC.
Fig. 4.
Neither local nor systemic S1P1-specific activation impair dendritic cell movement from lungs to mediastinal lymph nodes. A, mice were administered by gavage with AUY954 (3 mg/kg), then Alexa Fluor 647-OVA (AF647) (50 μg) was administered intratracheally 24 hours later, mice were euthanized and CD11c+ AF647+ cells were detected by flow cytometry in the mediastinal lymph nodes. B and C, BMDCs were incubated for 18 h in the presence of OVA (0.2 mg/ml) in DC medium without FBS containing either 0.1% DMSO or AUY954 (1 μM). BMDCs were then stained with CFSE and 50 μl of a 1.5 × 106 cells/ml suspension was administered intratracheal to intact mice. Mice were euthanized 48 h later. No CFSE signal could be detected in mediastinal lymph nodes (MLN) of in PBS-treated DCs (not shown). A, systemic AUY954 treatment did not affect movement of DCs from lungs to mediastinal lymph nodes. The number of CFSE+ CD11c+ DCs in the mediastinal lymph nodes was not affected by incubation with AUY954, compared with DMSO treatment (B), nor was the total number of CD4+ T cells (C). n = 3 mice per group; bars represent means ± S.E.M. *, significantly different, p < 0.05.
S1P1 Activation in Lungs Inhibits CD4+ T Cell and Eosinophilic Responses.
Because S1P1 activation did not alter DC function in the airways, we tested whether this receptor was not essential for local inhibition of AAI. Selective airway modulation of S1P1 was made possible by the recent development of a water-soluble tartaric salt of the S1P1-selective receptor agonist [CYM5442 (Gonzalez-Cabrera et al., 2008)]. We were surprised to find that daily local intratracheal delivery of CYM-5442 during the recall response of AAI strongly inhibited the T cell and eosinophilic responses in OVA/OVA mice compared with the VEH group (Fig. 5, A and B). Local intratracheal treatment with CYM-5442 inhibited the accumulation of effector/memory CD44hi CD4+ T cells subsets (Fig. 5C) in the BALF, compared with the VEH group. On the other hand, intraperitoneal delivery of CYM-5442 was inefficient at inhibiting the accumulation of eosinophils, total T cells, and effector/memory T cells in BALF (Fig. 5) compared with the local intratracheal delivery route. Along with the DC data presented in Fig. 4, these data (Fig. 5) support a mechanism of AAI inhibition by local activation of S1P1 that is independent of the chain of events leading to efficient clonal T cell expansion.
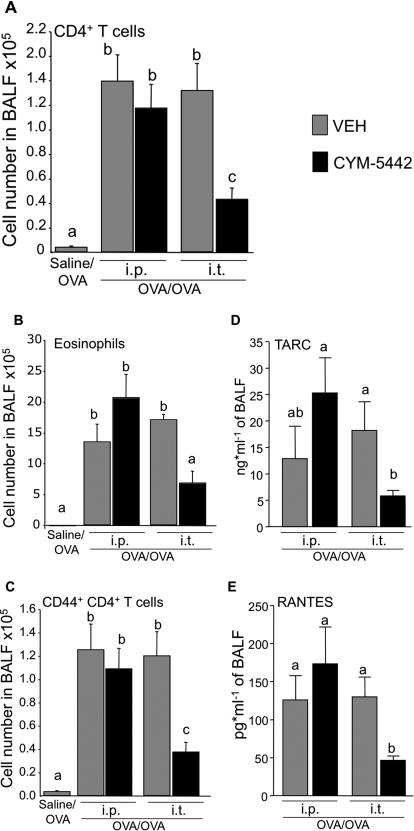
Fig. 5.
Local intratracheal (i.t.) delivery of CYM-5442 inhibits the development of allergic airway inflammation and chemokine release in the airways. BALB/c mice were submitted to the sham procedure (saline/OVA) or to the procedure inducing allergic airway inflammation (OVA/OVA). OVA/OVA mice were treated intratracheally or intraperitoneally (i.p.) with water (VEH) or CYM-5442 (2 mg/kg) 1 and 13 h after the daily intratracheal delivery of OVA during a 5-day recall response. Mice were euthanized 24 h after the last intratracheal delivery of OVA. The number of total CD4+ T cells (A), eosinophils (B), and effector/memory CD44+ CD4+ T cells (C) were quantified in BALF. Intratracheal delivery of CYM5442 is efficient at inhibiting CD44+ CD4+ T cells and eosinophils in BALF compared with intraperitoneal delivery. TARC (D) and RANTES (E) levels were also quantified in BALF. RANTES was not consistently detected in saline/OVA mice (<12 pg/ml, when detected), whereas basal levels of TARC were 148 ± 55 pg/ml (not shown). Compared with VEH treatments, intratracheal but not intraperitoneal delivery of CYM5442 strongly inhibited the release of these two cytokines in the BALF. n = 3–5 mice per group, experiment was repeated twice; bars represent means ± S.E.M. *, significantly different from VEH, p < 0.05.
Local Intratracheal Delivery of S1P1 Receptor Agonist Suppresses Recruitment Signals for Activated T Cells and Eosinophils in the Airways.
We tested whether the impairment of effector/memory T cell and eosinophil accumulation in lungs was due to the alteration of recruitment signals after local activation of S1P1. To do so, the concentrations of thymus- and activation-regulated chemokine (TARC; CCL17) and regulated upon activation, normal T-cell expressed, and secreted (RANTES; CCL5) were determined in BALF 24 hours after the last intratracheal delivery of OVA in OVA/OVA mice treated intratracheally or intraperitoneally with CYM-5442 or vehicle. Although low in BALF of saline/OVA mice (not shown), concentrations of TARC (Fig. 5D) and RANTES (Fig. 5E) were 15 and 175 pg/ml, respectively, in OVA/OVA mice. Intratracheal delivery of CYM-5442 strongly suppressed the concentrations of TARC and RANTES by approximatively 75% in the BALF. As predicted by experiments with systemic agonist, intraperitoneal delivery of CYM-5442 did not decrease TARC or RANTES levels compared with the intraperitoneal vehicle treatment. Thus, secreted recruitment signals are significantly modulated by local intratracheal delivery of an S1P1-specific agonist.
Discussion
Airway delivery of S1P prodrugs interferes with pulmonary immune response to alleviate immunopathologic conditions (Idzko et al., 2006; Marsolais et al., 2008), but attribution of function to specific S1P receptors has remained undefined. Using a specific S1P1 agonist with physical properties allowing airway delivery, we showed that S1P1 pharmacological activation in the airways contributes to alleviate AAI. Potent local anti-inflammatory activity of S1P1 agonist cannot be explained only by alterations of antigen presentation by DCs or by the induction of peripheral blood lymphopenia. Current data suggest that S1P1-induced inhibition of chemokine production and release in the airways may be an essential component of inflammation suppression by S1P prodrugs.
Specific S1P1 activation inhibits AAI. A number of in vitro studies suggest anti-inflammatory properties of S1P1 in macrophages (Hughes et al., 2008) and endothelial cells (Bolick et al., 2005). In vivo, nonselective S1P agonist prodrugs, such as FTY720, inhibit inflammation in models of experimental autoimmune encephalopathy (Papadopoulos et al., 2010), AAI (Idzko et al., 2006), and pulmonary viral infections (Marsolais et al., 2008; Marsolais et al., 2009), but contribution of single S1P receptors for alleviation of pulmonary immune response has remained elusive. It is noteworthy that delivery of a nonphosphorylatable sphingosine analog does not inhibit T cell accumulation in lungs of mice infected with influenza virus, strongly supporting the idea that S1P receptors are required for immunomodulation in the airways (Marsolais et al., 2008). Moreover, S1P1 mRNA is expressed in the pulmonary tissue (Zhang et al., 1999). In accordance with the literature, we show, for the first time, that significant anti-inflammatory effects can be achieved with local intratracheal delivery of a selective S1P1 receptor agonist.
Because local S1P1 activation in the airways quantitatively and qualitatively inhibits pulmonary inflammation similar to S1P prodrugs (Fig. 2) (Idzko et al., 2006; Marsolais et al., 2008), we dissected the cellular events in AAI that could be shared by both S1P1-selective agonists and nonselective S1P prodrugs (Fig. 6). In contrast with AAL-R or FTY720 (Czeloth et al., 2005; Idzko et al., 2006; Marsolais et al., 2009), S1P1-specific chemical probes do not interfere with early steps of antigen presentation. Although systemic delivery of an S1P1-specific agonist modestly alters DC trafficking in mice (Lan et al., 2005), and DC functions are modulated by the S1P3 receptor (Maeda et al., 2007; Niessen et al., 2008), no in vivo evidence supports a role for S1P1 located on DCs to modulate their functions. Accordingly, local intratracheal delivery of CYM-5442 during the early phase after influenza virus infection does not interfere with clonal expansion of T cells (Marsolais et al., 2008), and direct activation of S1P1 on DCs does not alter the movement of antigen-loaded DCs from lungs to lymph nodes (Fig. 4). This is consistent with in vitro data showing no effect of the S1P1 agonist SEW2871 on DC migration and endocytosis (Maeda et al., 2007). Thus, our in vivo results show that S1P prodrug-mediated alteration of antigen presentation by DC is independent of S1P1 (Figs. 3–5) and that inhibition of AAI by S1P1 relies on modulation of other mechanisms.
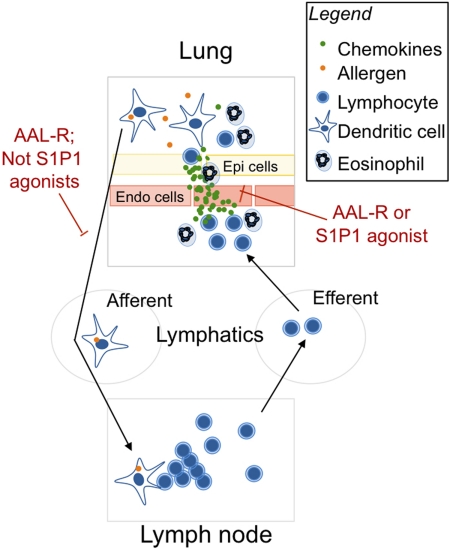
Fig. 6.
Chemical modulation of S1P1 interrupts AAI by inhibiting the release of chemokines in the airways. In sensitized mice, airway allergen exposure triggers rapid release of chemokines and proinflammatory cytokines by several cell subtypes in the airways including mast cells, macrophages, dendritic cells, epithelial cells, and endothelial cells. Antigen is taken up by dendritic cells that migrate toward draining lymph nodes where they present antigen to lymphocytes. There, lymphocytes undergo rapid expansion, egress from lymph nodes to efferent lymphatic vessels, and then reach blood circulation. Because chemokines are released in the lung, activated T cells and eosinophils accumulate in the airways, where they synergize with cells already in that location to further recruit leukocytes. Eosinophils will release a plethora of factors inducing tissue remodeling thus contributing to the development of airway hyper-responsiveness. Current and documented experiments (Idzko et al., 2006; Marsolais et al., 2008; Marsolais et al., 2009) support the idea that intratracheal delivery of nonselective S1P prodrugs interferes with antigen presentation process to inhibit pulmonary immune response. Here we show that selective S1P1 activation in the airways interferes with the development of AAI by inhibiting the release of chemokines in the airways but does not affect steps of antigen presentation.
S1P1 activation in the lungs inhibits the release of chemokines. S1P1 can alleviate the inflammatory response in vitro (Hughes et al., 2008) and in vivo (Lien et al., 2006). However, limited information is available regarding its mechanisms of action. The release of CCR4 ligands TARC and RANTES is decreased in the lungs by S1P1-specific agonist. It is noteworthy that recruitment of Th-2 polarized CD4+ T cells is mediated through ligands of CCR4, because this chemokine receptor is specifically expressed on Th-2 polarized T cells (as opposed to naive memory Th-0 or Th-1 polarized T cells) that mainly express CXCR4 or CXCR3 (Sallusto et al., 1998). It is noteworthy that TARC induces mouse eosinophil chemotaxis, which would also explain the inhibition of eosinophil infiltration by local intratracheal delivery of the S1P1 agonist (Borchers et al., 2002). A significant component of the immunosuppressive mechanism of local S1P1 activation is therefore through suppression of chemokine release within the pulmonary tissue.
Lymphocyte sequestration in secondary lymphoid organs partially explains the anti-inflammatory efficacy of pharmacological S1P1 activation. Indeed, systemic delivery of AAL-R (0.1 mg/kg) or the S1P1-specific agonists AUY954 (3 mg/kg) or CYM-5442 (2 mg/kg), which were all documented to induce significant lymphopenia at the doses used herein (Pan et al., 2006; Gonzalez-Cabrera et al., 2008; Marsolais et al., 2008), did not significantly inhibit eosinophil accumulation in lung during the recall phase of AAI (Figs. 3 and 5), compared with airway treatment (Figs. 2 and 5). In the current study, compounds were administered 1 h after allergen exposure, which allows early chemotactic factor release to occur. Our results contrast with those of others (Sawicka et al., 2003; Blé et al., 2009) who showed in similar AAI models that systemic delivery of FTY720 or S1P1-specific agonist inhibits T cell and eosinophil accumulation in the airways. This discrepancy is probably explained by the timing of treatment. It is noteworthy that Sawicka et al. (2003) and Blé et al. (2009) have administered mice with FTY720 before allergen exposure, thus sequestering T cells in nondraining lymph nodes, preventing their chemokine-driven recruitment in the airways. Moreover, FTY720 or AUY954-induced enhancement of barrier integrity before allergen exposure might also have inhibited inflammation in their systems (Blé et al., 2009). Thus, in our study, chemical probe delivery after allergen exposure revealed a critical role for S1P1 in the control of ongoing AAI by blunting chemokine release and recruitment of T cells and eosinophils.
In the past, systemic delivery of S1P prodrugs or specific S1P1 agonists had been shown to induce immunosuppression by sequestering lymphocytes in lymph nodes and Peyer's patches, preventing T and B cells from reaching the primary site of insult (Mandala et al., 2002; Sanna et al., 2004). On this basis, FTY720 was tested for efficacy in models of solid grafts only to undergo rejection with massive accumulation of myeloid leukocytes (Sis et al., 2008). This is similar to what we observed with systemic delivery of S1P prodrugs or S1P1-specific agonists; systemic doses sufficient to induce profound lymphopenia did not inhibit myeloid leukocyte infiltration. In more recent studies, airway delivery of S1P prodrugs has proven efficient at alleviating myeloid and lymphoid leukocyte accumulation in lungs of mice infected with an influenza virus (Marsolais and Rosen, 2009; Marsolais et al., 2009), or undergoing AAI (Idzko et al., 2006). The current experiments define specific molecular events in local S1P1 modulation of inflammatory cell recruitment. Systemic dosage of S1P1 agonists (Sanna et al., 2004; Pan et al., 2006) has been titrated for induction and maintenance of lymphopenia. However, accumulating evidence supports differential distribution of S1P receptor probes in various tissues (Meno-Tetang et al., 2006; Gonzalez-Cabrera et al., 2008), supporting potential distributional contributions to tissue-dependent modulatory activities.
In this study, we used a water-soluble tartaric salt of CYM-5442 to determine whether local activation of S1P1 in the airways could contribute to inhibit AAI. It is noteworthy that the half-life of CYM-5442 in the lungs after local intratracheal delivery is the same as that measured by systemic delivery (Gonzalez-Cabrera et al., 2008). CYM-5442 does not preferentially accumulate in the lungs like other S1P receptor chemical probes (Meno-Tetang et al., 2006), and local effects can thus be dissociated from systemic effects using different routes of delivery. Moreover, in contrast to S1P, CYM-5442 induces significant S1P1 ubiquitination (Gonzalez-Cabrera et al., 2008), which is consistent with receptor internalization and prolonged intracellular signaling (Mullershausen et al., 2009). Although in vivo selectivity is often hard to assess, CYM-5442 has high S1P1 selectivity upon S1P2–S1P5, without antagonistic activities (at 10 μM) on S1P1–S1P5 (Gonzalez-Cabrera et al., 2008; M.-T. Schaeffer and S. Brown, unpublished observations). In addition, in vivo selectivity is supported by the ability of CYM-5442 to induce lymphopenia (S1P1-specific effect), which can be competed using S1P1-specific antagonist 3-amino-4-(3-hexylphenylamino)-4-oxobutylphosphonic acid (W146) (Gonzalez-Cabrera et al., 2008). Considering that CYM-5442-induced lymphopenia is competed in vivo with a selective antagonist in a dose-range similar to that used in the current study, we expect off-target effects of the chemical probe to be minimal in the current setting.
Here, we show a new mechanism of inflammatory modulation by S1P prodrugs that involves S1P1 activation leading to suppression of cytokine production in the lung. Further characterization of S1P1 cellular distribution in the pulmonary environment and the molecular basis of modulation of allergic inflammation could ultimately lead to specific new therapies for pulmonary immunopathology.
Acknowledgments
We acknowledge the technical assistance of the personnel from The Scripps Research Institute Flow Cytometry Core Facility and of Serge Simard (Biostatistician, Centre de Recherche de l'Institut Universitaire de Cardiologie et de Pneumologie de Québec).
This work was supported in part by the National Institutes of Health National Institute of Allergy and Infectious Diseases [Grants AI05509, AI074564]; the National Institutes of Health National Institute on Mental Health [Grant MH074404]; by a grant from Kyorin Pharmaceuticals [SFP-1799]; by Le Fonds de la Recherche en Santé du Québec (FRSQ); the Respiratory Health Network of FRSQ; and the La Chaire de Pneumologie de la Fondation J-D Bégin de l'Université Laval et de la Fondation de l'Institut Universitaire de Cardiologie et de Pneumologie de Québec.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.066811.
- S1P
- sphingosine-1-phosphate
- S1P1–S1P5
- sphingosine-1-phosphate receptors 1 to 5
- SK
- sphingosine kinase
- FTY720
- 2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol
- AAI
- allergic airway inflammation
- AAL-R
- (R)-2-amino-4-(4-heptyloxyphenyl)-2-methylbutanol
- OVA
- ovalbumin
- CYM-5442
- 2-(4-(5-(3,4-diethoxyphenyl)-1,2,4-oxadiazol-3-yl)-2,3-dihydro-1H-inden-1-yl amino)ethanol
- BALF
- bronchoalveolar lavage fluid
- PBS
- phosphate-buffered saline
- DC
- dendritic cell
- DMSO
- dimethyl sulfoxide
- CFSE
- carboxyl-fluorescein diacetate, succinimidyl ester
- CTR
- control
- OVA/OVA mice
- mice sensitized and then challenged with OVA
- VEH
- vehicle
- TARC
- thymus- and activation-regulated chemokine
- RANTES
- regulated upon activation, normal T-cell expressed, and secreted
- CXCR
- chemokine CXC motif receptor
- CCR
- chemokine receptor
- W146
- 3-amino-4-(3-hexylphenylamino)-4-oxobutylphosphonic acid
- SEW2871
- 5-[4-phenyl-5-(trifluoromethyl)thiophen-2-yl]-3-[3-(trifluoromethyl)phenyl]1,2,4-oxadiazole
- AUY954
- 3-(((2-(2-(trifluoromethyl)-[1,1'-biphenyl]-4-yl)benzo[b]thiophen-5-yl)methyl)amino)propanoic acid.
Authorship Contributions
Participated in research design: Marsolais, Yagi, and Rosen.
Conducted experiments: Marsolais, Yagi, Kago, and Leaf.
Performed data analysis: Marsolais and Yagi.
Wrote or contributed to the writing of the manuscript: Marsolais, Yagi, and Rosen.
References
- Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, Maceyka M, Jiang H, Luo C, Kordula T, et al. (2010) Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 465:1084–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammit AJ, Hastie AT, Edsall LC, Hoffman RK, Amrani Y, Krymskaya VP, Kane SA, Peters SP, Penn RB, Spiegel S, et al. (2001) Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. FASEB J 15:1212–1214 [DOI] [PubMed] [Google Scholar]
- Billich A, Bornancin F, Dévay P, Mechtcheriakova D, Urtz N, Baumruker T. (2003) Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem 278:47408–47415 [DOI] [PubMed] [Google Scholar]
- Blé FX, Cannet C, Zurbruegg S, Gérard C, Frossard N, Beckmann N, Trifilieff A. (2009) Activation of the lung S1P(1) receptor reduces allergen-induced plasma leakage in mice. Br J Pharmacol 158:1295–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolick DT, Srinivasan S, Kim KW, Hatley ME, Clemens JJ, Whetzel A, Ferger N, Macdonald TL, Davis MD, Tsao PS, et al. (2005) Sphingosine-1-phosphate prevents tumor necrosis factor-{alpha}-mediated monocyte adhesion to aortic endothelium in mice. Arterioscler Thromb Vasc Biol 25:976–981 [DOI] [PubMed] [Google Scholar]
- Borchers MT, Ansay T, DeSalle R, Daugherty BL, Shen H, Metzger M, Lee NA, Lee JJ. (2002) In vitro assessment of chemokine receptor-ligand interactions mediating mouse eosinophil migration. J Leukoc Biol 71:1033–1041 [PubMed] [Google Scholar]
- Brinkmann V, Cyster JG, Hla T. (2004) FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant 4:1019–1025 [DOI] [PubMed] [Google Scholar]
- Czeloth N, Bernhardt G, Hofmann F, Genth H, Förster R. (2005) Sphingosine-1-phosphate mediates migration of mature dendritic cells. J Immunol 175:2960–2967 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cabrera PJ, Jo E, Sanna MG, Brown S, Leaf N, Marsolais D, Schaeffer MT, Chapman J, Cameron M, Guerrero M, et al. (2008) Full pharmacological efficacy of a novel S1P1 agonist that does not require S1P-like headgroup interactions. Mol Pharmacol 74:1308–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräler MH, Bernhardt G, Lipp M. (1998) EDG6, a novel G-protein-coupled receptor related to receptors for bioactive lysophospholipids, is specifically expressed in lymphoid tissue. Genomics 53:164–169 [DOI] [PubMed] [Google Scholar]
- Hughes JE, Srinivasan S, Lynch KR, Proia RL, Ferdek P, Hedrick CC. (2008) Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ Res 102:950–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idzko M, Hammad H, van Nimwegen M, Kool M, Müller T, Soullié T, Willart MA, Hijdra D, Hoogsteden HC, Lambrecht BN. (2006) Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function. J Clin Invest 116:2935–2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, Milstien S, Spiegel S. (2004) Transactivation of sphingosine-1-phosphate receptors by FcepsilonRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med 199:959–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan YY, De Creus A, Colvin BL, Abe M, Brinkmann V, Coates PT, Thomson AW. (2005) The sphingosine-1-phosphate receptor agonist FTY720 modulates dendritic cell trafficking in vivo. Am J Transplant 5:2649–2659 [DOI] [PubMed] [Google Scholar]
- Lien YH, Yong KC, Cho C, Igarashi S, Lai LW. (2006) S1P(1)-selective agonist, SEW2871, ameliorates ischemic acute renal failure. Kidney Int 69:1601–1608 [DOI] [PubMed] [Google Scholar]
- Liu G, Burns S, Huang G, Boyd K, Proia RL, Flavell RA, Chi H. (2009) The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat Immunol 10:769–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Sugiura M, Nava VE, Edsall LC, Kono K, Poulton S, Milstien S, Kohama T, Spiegel S. (2000) Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol Chem 275:19513–19520 [DOI] [PubMed] [Google Scholar]
- Lutz MB, Kukutsch N, Ogilvie AL, Rössner S, Koch F, Romani N, Schuler G. (1999) An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods 223:77–92 [DOI] [PubMed] [Google Scholar]
- Maeda Y, Matsuyuki H, Shimano K, Kataoka H, Sugahara K, Chiba K. (2007) Migration of CD4 T cells and dendritic cells toward sphingosine 1-phosphate (S1P) is mediated by different receptor subtypes: S1P regulates the functions of murine mature dendritic cells via S1P receptor type 3. J Immunol 178:3437–3446 [DOI] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, et al. (2002) Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296:346–349 [DOI] [PubMed] [Google Scholar]
- Marsolais D, Hahm B, Edelmann KH, Walsh KB, Guerrero M, Hatta Y, Kawaoka Y, Roberts E, Oldstone MB, Rosen H. (2008) Local not systemic modulation of dendritic cell S1P receptors in lung blunts virus-specific immune responses to influenza. Mol Pharmacol 74:896–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsolais D, Hahm B, Walsh KB, Edelmann KH, McGavern D, Hatta Y, Kawaoka Y, Rosen H, Oldstone MB. (2009) A critical role for the sphingosine analog AAL-R in dampening the cytokine response during influenza virus infection. Proc Natl Acad Sci USA 106:1560–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsolais D, Rosen H. (2009) Chemical modulators of sphingosine-1-phosphate receptors as barrier-oriented therapeutic molecules. Nat Rev Drug Discov 8:297–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massion PP, Lindén A, Inoue H, Mathy M, Grattan KM, Nadel JA. (1996) Dimethyl sulfoxide decreases interleukin-8-mediated neutrophil recruitment in the airways. Am J Physiol 271:L838–L843 [DOI] [PubMed] [Google Scholar]
- Meno-Tetang GM, Li H, Mis S, Pyszczynski N, Heining P, Lowe P, Jusko WJ. (2006) Physiologically based pharmacokinetic modeling of FTY720 (2-amino-2[2-(-4-octylphenyl)ethyl]propane-1,3-diol hydrochloride) in rats after oral and intravenous doses. Drug Metab Dispos 34:1480–1487 [DOI] [PubMed] [Google Scholar]
- Mullershausen F, Zecri F, Cetin C, Billich A, Guerini D, Seuwen K. (2009) Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat Chem Biol 5:428–434 [DOI] [PubMed] [Google Scholar]
- Niessen F, Schaffner F, Furlan-Freguia C, Pawlinski R, Bhattacharjee G, Chun J, Derian CK, Andrade-Gordon P, Rosen H, Ruf W. (2008) Dendritic cell PAR1–S1P3 signalling couples coagulation and inflammation. Nature 452:654–658 [DOI] [PubMed] [Google Scholar]
- Nishiuma T, Nishimura Y, Okada T, Kuramoto E, Kotani Y, Jahangeer S, Nakamura S. (2008) Inhalation of sphingosine kinase inhibitor attenuates airway inflammation in asthmatic mouse model. Am J Physiol Lung Cell Mol Physiol 294:L1085–L1093 [DOI] [PubMed] [Google Scholar]
- Pan S, Mi Y, Pally C, Beerli C, Chen A, Guerini D, Hinterding K, Nuesslein-Hildesheim B, Tuntland T, Lefebvre S, et al. (2006) A monoselective sphingosine-1-phosphate receptor-1 agonist prevents allograft rejection in a stringent rat heart transplantation model. Chem Biol 13:1227–1234 [DOI] [PubMed] [Google Scholar]
- Papadopoulos D, Rundle J, Patel R, Marshall I, Stretton J, Eaton R, Richardson JC, Gonzalez MI, Philpott KL, Reynolds R. (2010) FTY720 ameliorates MOG-induced experimental autoimmune encephalomyelitis by suppressing both cellular and humoral immune responses. J Neurosci Res 88:346–359 [DOI] [PubMed] [Google Scholar]
- Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. (2005) Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med 11:491–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roviezzo F, Del Galdo F, Abbate G, Bucci M, D'Agostino B, Antunes E, De Dominicis G, Parente L, Rossi F, Cirino G, et al. (2004) Human eosinophil chemotaxis and selective in vivo recruitment by sphingosine 1-phosphate. Proc Natl Acad Sci USA 101:11170–11175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. (1998) Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med 187:875–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, Webb B, Lefebvre S, Chun J, Gray N, et al. (2004) Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem 279:13839–13848 [DOI] [PubMed] [Google Scholar]
- Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, Wei SH, Parker I, Jo E, Cheng WC, et al. (2006) Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol 2:434–441 [DOI] [PubMed] [Google Scholar]
- Sawicka E, Zuany-Amorim C, Manlius C, Trifilieff A, Brinkmann V, Kemeny DM, Walker C. (2003) Inhibition of Th1- and Th2-mediated airway inflammation by the sphingosine 1-phosphate receptor agonist FTY720. J Immunol 171:6206–6214 [DOI] [PubMed] [Google Scholar]
- Sis B, Grynoch R, Murray AG, Campbell P, Solez K. (2008) Antibody-mediated rejection with a striking interstitial monocyte/macrophage infiltration in a renal allograft under FTY720 treatment. Am J Kidney Dis 51:127–130 [DOI] [PubMed] [Google Scholar]
- Skoura A, Sanchez T, Claffey K, Mandala SM, Proia RL, Hla T. (2007) Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J Clin Invest 117:2506–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Contos JJ, Weiner JA, Fukushima N, Chun J. (1999) Comparative analysis of three murine G-protein coupled receptors activated by sphingosine-1-phosphate. Gene 227:89–99 [DOI] [PubMed] [Google Scholar]