Abstract
Copper transporter 2 (CTR2) is one of the four copper transporters in mammalian cells that influence the cellular pharmacology of cisplatin and carboplatin. CTR2 was knocked down using a short hairpin RNA interference. Robust expression of CTR2 was observed in parental tumors grown in vivo, whereas no staining was found in the tumors formed from cells in which CTR2 had been knocked down. Knockdown of CTR2 reduced growth rate by 5.8-fold, increased the frequency of apoptotic cells, and decreased the vascular density, but it did not change copper content. Knockdown of CTR2 increased the tumor accumulation of cis-diamminedichloroplatinum(II) [cisplatin (cDDP)] by 9.1-fold and greatly increased its therapeutic efficacy. Because altered endocytosis has been implicated in cDDP resistance, uptake of dextran was used to quantify the rate of macropinocytosis. Knockdown of CTR2 increased dextran uptake 2.5-fold without reducing exocytosis. Inhibition of macropinocytosis with either amiloride or wortmannin blocked the increase in macropinocytosis mediated by CTR2 knockdown. Stimulation of macropinocytosis by platelet-derived growth factor coordinately increased dextran and cDDP uptake. Knockdown of CTR2 was associated with activation of the Rac1 and cdc42 GTPases that control macropinocytosis but not activation of the phosphoinositide-3 kinase pathway. We conclude that CTR2 is required for optimal tumor growth and that it is an unusually strong regulator of cisplatin accumulation and cytotoxicity. CTR2 regulates the transport of cDDP in part through control of the rate of macropinocytosis via activation of Rac1 and cdc42. Selective knockdown of CTR2 in tumors offers a strategy for enhancing the efficacy of cDDP.
Introduction
The platinum-based chemotherapeutic agents cisplatin (cDDP), carboplatin, and oxaliplatin are widely used for the treatment of many types of cancer (Rabik and Dolan, 2007). Unfortunately, patients commonly develop resistance during sequential cycles of treatment with all of these agents. Changes in drug influx and efflux, deficiencies in the mismatch repair pathway, and down-regulation of the apoptotic cascade have all been linked to the mechanism of resistance (Chu, 1994; Crul et al., 1997; Manic et al., 2003; Sedletska et al., 2005; Kuo et al., 2007). The platinum-based drugs are believed to act through the formation of adducts on the purine bases of nuclear DNA (Johnson et al., 1997; Sedletska et al., 2005; Zorbas and Keppler, 2005); however, it is still unclear how these drugs traffic through the cell to reach the nucleus. Tumors that acquire resistance accumulate less drug than those that remain sensitive (Metcalfe et al., 1986; Waud, 1987; Teicher et al., 1991; Kelland et al., 1992; Twentyman et al., 1992; Gately and Howell, 1993; Oldenburg et al., 1994; Song et al., 2004).
There is now strong evidence that the transporters and chaperones that manage copper homeostasis also transport the platinum-containing drugs (Katano et al., 2002; Safaei et al., 2004b; Safaei and Howell, 2005). Specifically, the copper efflux transporters ATP7A and ATP7B are involved in the sequestration and export of the platinum-containing drugs, whereas the major copper influx transporter CTR1 controls their uptake (Safaei and Howell, 2005; Holzer et al., 2006; Larson et al., 2009). In addition, the copper chaperone ATOX1 modulates sensitivity to cDDP by a less well characterized mechanism (Safaei et al., 2009).
In addition to the high-affinity copper influx transporter CTR1, mammalian cells express a second structurally related transporter, copper transporter 2 (CTR2), whose function is less well defined. CTR2 is found predominantly in association with vacuolar membranes in yeast (Portnoy et al., 2001; Rees et al., 2004) and to be localized to late endosomes and lysosomes in mammalian cells (van den Berghe et al., 2007; Bertinato et al., 2008). However, when expressed at the cell surface, CTR2 is capable of mediating copper uptake (Bertinato et al., 2008). Although CTR1 functions to influx both copper and cDDP into the cell, a recent study from this laboratory demonstrated that CTR2 has the opposite effect and serves to limit the accumulation of cDDP in cell line models (Blair et al., 2009). Cells in which the expression of CTR2 was knocked down [CTR2(kd)] accumulated 2- to 3-fold more cDDP than wild-type parental cells; the finding that there was no effect of knocking down CTR2 on cDPP efflux suggested that the enhanced uptake was a result of increased influx (Blair et al., 2009). In addition, these CTR2 knockdown cells exhibited a greatly increased sensitivity to cDDP and carboplatin compared with wild-type cells (Blair et al., 2009). The effect of the loss of CTR2 on both the uptake and sensitivity to cDDP was shown to be independent of CTR1 expression (Blair et al., 2009). The expression of CTR2 has been shown recently to be regulated by the copper level in the cell (Blair et al., 2010). In copper-starved cells, CTR2 is degraded, whereas increased copper causes up-regulation. Further confirmation of the importance of CTR2 as a regulator of the cellular pharmacology and cytotoxicity of cDDP was provided by the fact that down-regulation of CTR2 expression by copper starvation increased cDDP uptake and cytotoxicity, whereas up-regulation by exposure to excess copper has the opposite effect (Blair et al., 2010).
One potential means by which CTR2 may limit cDDP accumulation and cytotoxicity is through the regulation of intracellular sequestration. In yeast, CTR2 is expressed in the vacuolar membrane (Bellemare et al., 2002; Rees et al., 2004), and in mammalian cells, it is found in the mammalian equivalent of the yeast vacuole, which consists of the late endosomal and lysosomal compartments (Rees et al., 2004; van den Berghe et al., 2007; Bertinato et al., 2008). Available evidence suggests that CTR2 functions primarily to efflux copper from these structures under conditions of low environmental copper (van den Berghe et al., 2007). If CTR2 mobilizes cDDP in a similar manner, then the loss of CTR2 would be expected to lead to an increased amount of drug trapped in vesicles and an increase in total cellular cDDP. However, previous studies have provided evidence against this hypothesis (Blair et al., 2009). Knockdown of CTR2 had no effect on the absolute amount of cDDP that accumulated in intracellular vesicles (Blair et al., 2009). In addition, knockdown of CTR2 enhanced the accumulation of cDDP within 5 min, suggesting that CTR2 restrains initial influx, perhaps through regulation of the uptake at the plasma membrane or of trafficking to intracellular sites (Blair et al., 2009). Knockdown of CTR2 also had no effect on cDDP efflux, suggesting that the major role of CTR2 is to mediate cDDP influx rather than to restrain efflux.
Previous studies have reported a link between endocytosis and cDDP sensitivity (Safaei et al., 2004a; Shen et al., 2004a,b). The GTPases Rac1, RhoA, and cdc42, which control clathrin-independent endocytosis, are down-regulated in several cDDP-resistant cell lines (Shen et al., 2004b), as are cytoskeletal proteins necessary for endocytosis (Shen et al., 2004a). Furthermore CTR1, which also regulates cellular accumulation of cDDP, has been closely linked to macropinocytosis; CTR1 degradation triggered by exposure to copper or to cDDP occurs by this process (Holzer and Howell, 2006). In the current study, we sought to determine whether CTR2 is an important determinant of the responsiveness to cDDP in vivo and further define the mechanism by which CTR2 controls the cellular pharmacology of cDDP. We examined the effect of knocking down the expression of CTR2 in malignant mouse embryo fibroblasts on the accumulation of cDDP and the ability of cDDP to slow tumor growth, and we examined the effect on macropinocytosis. We report here that tumors derived from cells in which CTR2 had been knocked down grew more slowly that those derived from wild-type cells and that this was associated with an increased frequency of apoptotic cells and decreased vascular density. However, the tumors in which CTR2 was knocked down accumulated much more platinum after injection of cDDP and exhibited a much greater response to treatment. We found that CTR2 regulates the rate of macropinocytosis through effects on endocytotic factors Rac1 and cdc42. These observations suggest that selective inhibition of CTR2 expression or function may be a useful strategy for enhancing the effectiveness of cDDP chemotherapy.
Materials and Methods
Drugs and Reagents.
Platinol AQ was a gift from Bristol-Myers Squibb Co. (Stamford, CT); it contains cDDP at a concentration of 3.33 mM in 0.9% NaCl. The anti-CTR2 antibody was a gift from Dr. Jessie Bertinato (Health Canada, Ottawa, ON, Canada). Transferrin-Alexa Fluor 546 and 70-kDa dextran-Texas red were purchased from Invitrogen (Carlsbad, CA). Amiloride and wortmannin were purchased from Tocris Bioscience (Ellisville, MO). Bradford reagent was purchased from Bio-Rad Laboratories (Hercules, CA). Sulforhodamine B was obtained from Sigma-Aldrich (St. Louis, MO), and 0.4% sulforhodamine B (w/v) was solubilized in 1% (v/v) acetic acid solution.
Cell Types.
Parental mouse embryonic fibroblasts in which both copies of CTR1 had been somatically knocked out [CTR1(−/−)] were a gift from Dr. Dennis Thiele (Lee et al., 2002). The CTR2(kd) sublines were constructed by infecting the CTR1(−/−) cells with lentivirus expressing an shRNAi targeting mouse CTR2 mRNA purchased from Sigma-Aldrich as described previously (Blair et al., 2009).
Western Blotting.
Cell lysates were prepared, and subsequent Western blots were conducted as described previously (Blair et al., 2009). The primary antibodies used for Western blot analysis were anti-CTR2 (provided by Dr. Jesse Bertinato), anti-CTR2 (Novus Biologicals, Littleton, CO), anti-tubulin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and anti-Rac1, anti-cdc42, anti-Akt, and anti pAkt S473 (Cell Signaling Technology, Danvers, MA).
Immunohistochemistry and Chemiluminescent Immunoblotting.
Upon harvesting, tumors were either imbedded in paraffin or frozen in Tissue-Tek O.C.T. compound (Sakura Finetech USA, Torrance, CA) as described previously (Holzer et al., 2006). Sections were stained according to the protocol outlined in the Catalyzed Signal Amplification System (Dako North America, Inc., Carpinteria, CA). Endogenous biotin was blocked by first overlaying the slides with 0.1% avidin for 15 min and washing three times with 0.1% Triton-X in PBS. Slides were then overlaid with 0.01% biotin for 15 min followed by another three washes with 0.1% Triton-X in PBS. Nonspecific protein binding was blocked by immersion of the slides in 1% bovine serum albumin in PBS for 20 min. Slides were incubated with anti-hCTR2 antibody at a dilution of 1:200 in 1% bovine serum albumin in PBS overnight at 4°C. As a negative control, parallel sections were incubated with nonimmune rabbit IgG1 sera (prediluted; Dako North America). Slides were also stained with anti-Ki67 to determine the fraction of S-phase nuclei and anti-CD31 antibody (BD Pharmingen, San Diego, CA) to quantify vascular density and apoptotic nuclei were detect by TUNEL assay (Latimer et al., 2009).
Quantification of Vascular Density.
The mean vascular density (vessels per square millimeter) for each tumor was calculated as described previously (Lucidarme et al., 2006). Five light microscope pictures at 40× magnification were taken at different locations in each tumor sample. The total count of CD31-stained vessels was divided by the area of the five fields to obtain the mean vascular density. Eight to 10 tumors of each cell type were scored by two blinded observers; the values reported are the mean of the ratio for the two types of tumors.
Measurement of Drug Accumulation in Tumors.
One hour after intraperitoneal injection of 10 mg/kg cDDP, tumors were harvested and digested in 70% nitric acid overnight, diluted to a final 5% nitric acid (0.1% Triton X-100, 1.4% nitric acid, 1 ppb indium in double-distilled H2O). Platinum concentration was measured using an Element 2 ICP-OES (PerkinElmer Life an Analytical Sciences, Waltham, MA) located at the Analytical Facility at the Scripps Institute of Oceanography (University of California, San Diego, CA). As a method of normalization, total sulfur was also measured by ICP-OES in each sample. Copper levels were measured using the same instrument. All data presented are the means of 40 samples of each type of tumor.
Determination of Drug Sensitivity In Vivo.
All animal experiments were carried out in accordance with guidelines of the University of California San Diego Institutional Animal Care and Use Committee. To grow the various types of cells as xenografts, 3 × 106 cells in 100 μl were inoculated at 4 subcutaneous sites into female nu/nu mice; 19 mice were included in the cDDP-treated group, and 18 in the untreated group. Cell types were randomized between shoulder and hip, left and right, ensuring that there were always tumors of the same type on left and right. Tumors were allowed to grow until they were ∼2 mm in diameter, at which point each mouse received either a single dose of cDDP 10 mg/kg by intraperitoneal injection or vehicle only. Tumor size was monitored three times per week for 7 weeks. Tumor volume was estimated using the equation (length × width2)/2.
Tyrosinase Activity Assay.
CTR1(+/+), CTR1(+/+) CTR2(kd), CTR1(−/−), and CTR1(−/−) CTR2(kd) cells were plated in 96-well tissue culture dishes (∼20,000 cells per well, 15 wells per cell type) and 24 h later were treated l-DOPA-containing assay buffer [10 mM l-DOPA and 500 mM phosphate buffer, pH 6.8]. After a 72-h exposure, the absorbance of each well at 400 nm was recorded using a Versamax Tunable Microplate Reader (Molecular Devices, Sunnyvale, CA). All experiments were repeated at least three times using three cultures for each drug concentration, and the data are presented as the percentage of tyrosinase activity relative to wild-type control cells. Parallel plates were stained using 100 μl of 0.4% sulforhodamine B in 1% acetic acid at room temperature for 15 min. After washing, the absorbance of each well at 515 nm was recorded using a Versamax Tunable Microplate Reader (Molecular Devices) as a control for cell density.
Measurement of Macropinocytosis by Dextran Uptake.
CTR1(+/+), CTR1(+/+) CTR2(kd), CTR1(−/−), and CTR1(−/−) CTR2(kd) cells were plated in 96-well tissue culture dishes (∼2–5 × 104 cells per well, 15 wells per cell type) and 24 h later were treated with transferrin-Alexa Fluor 546 and 70-kDa dextran-Texas red for 30 min. The wells were immediately washed with ice-cold PBS three times and fixed with 3.7% formalin in PBS for 30 min followed by three 10-min washes with PBS. Fluorescence was measured using an Infinite M-200 microplate reader (Tecan US, Inc., San Jose, CA). The cells were then stained using 100 μl of 0.4% sulforhodamine B in 1% acetic acid at room temperature for 15 min. After washing, the absorbance of each well at 515 nm was recorded using a Versamax Tunable Microplate Reader (Molecular Devices) as a control for cell density. All experiments were repeated at least three times using three cultures for each drug concentration. To access the effect of copper starvation on macropinocytosis, CTR1(+/+) and CTR1(−/−) cells were pretreated with 100 μM BCS for 1 h or left untreated. Cells were then washed with 37°C PBS three times, and uptake of Texas-red dextran was measured.
Measurement of Dextran Exocytosis.
CTR1(+/+), CTR1(+/+) CTR2(kd), CTR1(−/−), and CTR1(−/−) CTR2(kd) cells were plated in 96-well tissue culture dishes (∼2–5 × 104 cells per well, 15 wells per cell type), and 24 h later, cells were treated with transferrin-Alexa Fluor 546 and 70-kDa dextran-Texas red for 30 min. Cells were immediately washed with 37°C PBS three times and placed in normal media. At 0, 1, 2, 4, and 10 min after removal of dextran, plates were fixed with 3.7% formalin in PBS for 30 min followed by three 10-min washes with PBS. Fluorescence and absorbance were then measured. All conditions were repeated at least three times using three cultures for each drug concentration. Retention of Texas-red dextran was assayed as a marker for rate of dextran exocytosis.
Inhibition of Endocytosis.
Cells were treated with 5 mM amiloride, 100 nM wortmannin, or control medium for 30 min and then washed with 37°C PBS three times. The media were then removed, and the cells were exposed to 500 μl of OptiMEM medium (Invitrogen) containing 30 μM cDDP at 37°C for either 0 or 15 min, after which the drug-containing medium was removed, and the plates were washed three times with ice-cold PBS and were processed for platinum measurement.
Measurement of Whole-Cell Drug Accumulation after PDGF Stimulation.
Cells were grown to 90% confluence in T-150 tissue culture flasks. Cells were then harvested using trypsin, and 7.5 × 105 cells were placed into each well of six-well tissue culture plates and allowed to grow overnight in 2.5 ml of reduced serum media at 37°C in 5% CO2. The next day, medium was removed by aspiration, and the cells were treated with either 5 ng/ml PDGF-BB or control media for 4 h. The media were then removed, and the cells were exposed to 500 μl of cDDP-containing OptiMEM medium (Invitrogen) at 37°C for either 0 or 60 min. The drug-containing medium was removed, and the plates were washed three times with ice-cold PBS and then placed on ice. In the case of the time 0 samples, the drug-containing medium was aspirated within 15 s of the start of drug exposure. Concentrated (50–70%) nitric acid (215 μl) was added to each well, and the plate was incubated overnight at room temperature. The following day, the acid was moved into Omni-vials (Wheaton Science Products, Millville, NJ) and incubated at room temperature overnight to thoroughly dissolve all cellular debris. The samples were then diluted with 3 ml of buffer (0.1% Triton X-100, 1.4% nitric acid, and 1 ppb In in double-distilled H2O). Platinum concentration was measured using a PerkinElmer Element 2 ICP-MS located at the Analytical Facility at the Scripps Institute of Oceanography. As a method of normalization, total sulfur was measured using a PerkinElmer ICP-OES also located at the Scripps Institute of Oceanography. Samples that were prepared previously for the ICP-MS were then introduced into the ICP-OES, in which the total micrograms of sulfur was measured. All data presented are the means of at least three independent experiments each performed with six wells per concentration tested.
Western Blot Analysis of Activated Rac1 and cdc42.
Cells grown in serum-free media for 24 h to ∼80% confluence were washed three times with PBS and immediately lysed in 1 ml of lysis buffer (50 mM Tris, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% Triton X-100, and protease inhibitor). The lysate was then incubated with 10 μg of PAK-1 PBD agarose (Millipore Corporation, Billerica, MA) for 1 h at 4°C. Beads were isolated and washed three times with lysis buffer and then resuspended in Laemmli buffer and analyzed by Western blot using antibodies specific for total and phosphorylated forms of Rac1, cdc2, and Akt.
Statistical Analysis.
All data shown were derived from at least three independent experiments, each of which included triplicate cultures for each data point. Values are presented as mean ± S.E.M. Statistical comparisons were performed using a two-tailed Student's t test with the assumption of unequal variance. A total of 23 mice were used for tumor experiments (12 CDDP-treated, 11 untreated), each with 4 tumors distributed as described above.
Results
Effect of CTR2 Knockdown on Tumor Growth Rate.
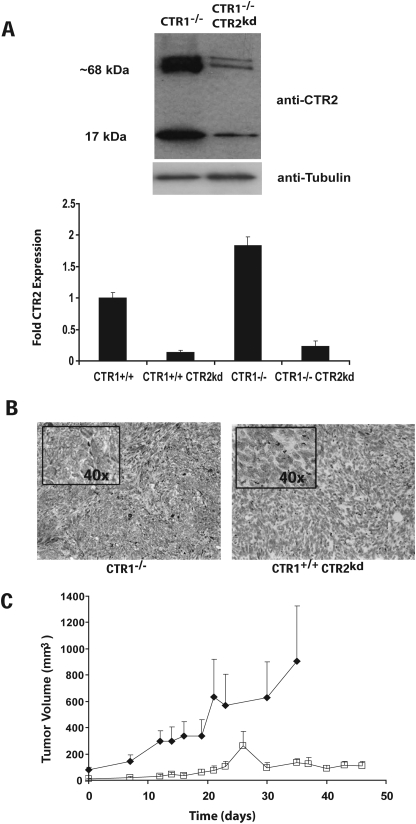
To determine the dependence of tumor growth on CTR2 in vivo, we used a malignant mouse embryo fibroblast cell line, in which both alleles of CTR1 had already been deleted. The expression of CTR2 in these CTR1(−/−) cells was constitutively knocked down using a lentiviral vector expressing an shRNAi directed to the CTR2 mRNA (Blair et al., 2009). Figure 1A shows a Western blot analysis that documents that the level of expression of CTR2 protein in the cell line before tumor inoculation was reduced by 87.1 ± 4.6 (S.E.M.) % below that in the parental CTR1(−/−) cells. Both the parental CTR1(−/−) and CTR1(−/−) CTR2(kd) cells were inoculated subcutaneously into nu/nu mice, and both types of cells formed tumors with equal frequency. Immunohistochemical analysis of sections from these tumors demonstrated robust expression of CTR2 in the CTR1(−/−) tumors, but no detectable CTR2 expression in the CTR1(−/−) CTR2(kd) tumors (Fig. 1B). As shown in Fig. 1C, CTR1(−/−) tumors grew 5.8-fold more rapidly than CTR1(−/−) CTR2(kd) tumors.
Fig. 1.
Expression of CTR2 and growth rate of CTR1(−/−) and CTR2(kd) tumors. A, Western blot analysis of CTR2 levels in CTR1(+/+), CTR1(−/−), CTR(+/+) CTR2(kd), and CTR(−/−) CTR2(kd) cells. B, immunohistochemical staining of CTR1(−/−) and CTR(−/−) CTR2(kd) tumors for expression of CTR2 (brown). C, tumor volume as a function of time; ■, CTR1(−/−) tumors; □, CTR1(−/−) CTR2(kd) tumors. Vertical bars, S.E.M.
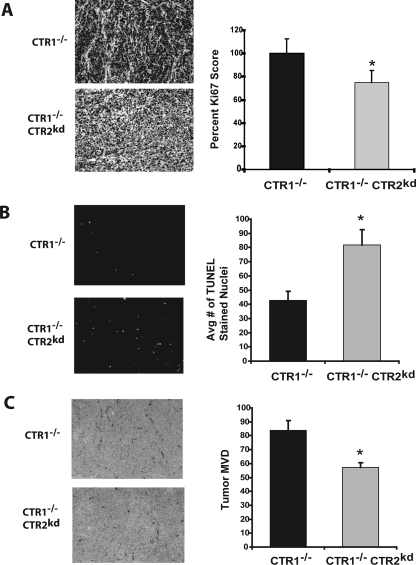
Effect of CTR2 on Proliferation and Apoptosis In Vivo.
To examine the basis for the difference in growth rate, CTR1(−/−) and CTR1(−/−) CTR2(kd) tumors were harvested and fixed in formalin. Ki67 is an antigen expressed in the S-phase of the cell cycle that is widely used to quantify the fraction of proliferating cells in tumors (Iatropoulos and Williams, 1996). The tumors were stained with an antibody to Ki67 to determine the effect of knocking down CTR2 on proliferation rate. CTR2(kd) tumors contained 24.3 ± 10.3% (p < 0.02) fewer Ki67-positive cells than CTR1(−/−) tumors (Fig. 2A). The tumors were sectioned, and the frequency of apoptotic cells were measured by TUNEL assay, as shown in Fig. 2B. The average number of TUNEL-positive nuclei per high-power field was determined for each tumor type. CTR1(−/−) tumors had an average of 42.8 ± 6.2 TUNEL-positive nuclei per high-power field. In contrast, CTR1(−/−) CTR2(kd) tumors had an average of 81.8 ± 10.8 TUNEL-positive nuclei per high-power field. Thus, the frequency of apoptotic cells in the CTR1(−/−) CTR2(kd) tumors was 1.9-fold higher than in the CTR1(−/−) tumors, suggesting that the death rate of tumor cells was increased by a large amount when CTR2 was knocked down.
Fig. 2.
Immunohistochemical characterization of proliferation, apoptosis, and vessel density in CTR1(−/−) and CTR(−/−) CTR2(kd) tumors. A, Ki67 staining for proliferation; numerical quantification of Ki67-positive cells per five high-power fields. B, TUNEL staining for apoptotic nuclei; numerical quantification of TUNEL-positive nuclei per five high-power fields. C, immunohistochemical staining for CD31; numerical quantification of vessel density. Vertical bars, ± S.E.M. *, p < 0.02.
Effect of CTR2 on Vessel Density In Vivo.
Copper is essential for angiogenesis, and adequate vascularization is required for tumor growth. To determine whether knockdown of CTR2 altered the extent of angiogenesis in tumors, subcutaneously implanted CTR1(−/−) and CTR1(−/−) CTR2(kd) tumors were harvested and frozen in O.C.T. compound. Tumors were sectioned and stained with an antibody to the endothelial cell marker CD31, which stains tumor capillaries. Figure 2C shows a reduced density of CD31-expressing cells in the CTR1(−/−) CTR2(kd) tumors. The mean number of vessels per square mm was 83.7 ± 7.0 in the CTR1(−/−) tumors but was reduced to 57.3 ± 3.5 in the CTR1(−/−) CTR2(kd) tumors (Fig. 3B). Thus, the vessel density was 1.5-fold higher in the CTR1(−/−) tumors (p = 0.00003), indicating that CTR2 has a substantial effect on tumor vessel formation.
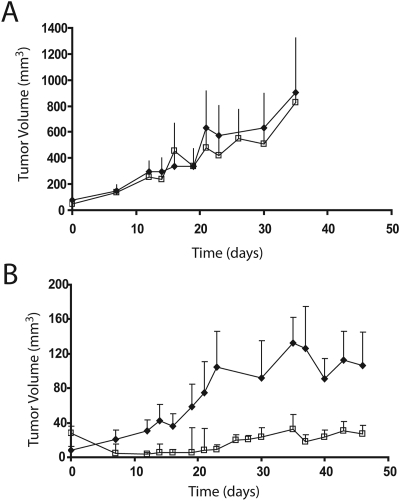
Fig. 3.
Effect of knocking down CTR2 on responsiveness to cDDP in vivo. Tumor volume as a function of time with (□) or without (♦) intraperitoneal injection of 10 mg/kg cDDP. A, CTR1(−/−) tumors; B, CTR1(−/−) CTR2(kd) tumors. Vertical bars, ± S.E.M.
Effect of CTR2 on Copper Content In Vitro and In Vivo.
The exact role of CTR2 in copper homeostasis remains poorly defined. Knockdown of CTR2 was found to increase the steady-state level of copper in the CTR1(−/−) CTR2(kd) cells when grown in vitro in the absence of any added copper. The level in CTR1(+/+) cells was 1.10 ± 0.02 ng of copper/μg of sulfur when grown in standard tissue culture medium. The level in the CTR1(−/−) cells did not significantly differ being 0.90 ± 0.10 ng of copper/μg of sulfur. Knockdown of CTR2 in the CTR1(−/−) cells increased the steady-state copper level by 2.1-fold to 1.89 ± 0.01 ng of copper/μg of sulfur. To determine whether similar differences were observed when the CTR1(−/−) and CTR1(−/−) CTR2(kd) cells were grown in vivo, untreated CTR1(−/−) and CTR1(−/−) CTR2(kd) subcutaneous tumors were harvested and dissolved in nitric acid, and the copper levels were assayed by ICP-MS. There was no significant difference in steady-state copper levels, which were 552.2 ± 18.3 ng copper/mg of tumor in the CTR1(−/−) tumors and 535.9 ± 36.0 ng copper/mg of tumor in the CTR1(−/−) CTR2(kd) tumors (p = 0.7). Thus, despite the clear effect of knocking down CTR2 on cellular copper levels when grown in vitro, when grown in vivo, the knockdown of CTR2 did not alter copper levels when measured in the mixture of all cell types in the tumor.
Tyrosinase activity was measured as an additional assay of the affect of CTR2 loss on the ability of copper to reach downstream targets. The tyrosinase activity in the CTR1(−/−) cells was 56.6 ± 7.7% (p < 0.0001) and in the CTR1(−/−) CTR2(kd) cells was 58.2 ± 4.9% (p < 0.0002) of that in the CTR1(+/+) cells. Thus, loss of CTR1 reduced the amount of copper reaching tyrosinase. In contrast, knockdown of CTR2 did not significantly affect tyrosinase activity in either the CTR1(+/+) or CTR1(−/−) background. The tyrosinase activity in the CTR1(+/+) CTR2(kd) cells was 113.5 ± 12.7% of that in the CTR1(+/+) cells (p = 0.15), and that in the CTR1(−/−) CTR2(kd) cells was 97.2 ± 6.0% (p = 0.48) of that in the CTR1(−/−) cells. Thus, despite similar whole-cell copper levels, the loss of CTR1 limited the availability of intracellular copper, at least as measured by tyrosinase activity, whereas knockdown of CTR2 did not.
Effect of CTR2 Knockdown on cDDP Accumulation In Vivo.
nu/nu mice with subcutaneous CTR1(−/−) and CTR1(−/−) CTR2(kd) tumors were injected intraperitoneally with 10 mg/kg cDDP, and 1 h later, the mice were sacrificed and tumors harvested. The platinum level in each tumor was determined by ICP-OES. The average platinum level in the CTR1(−/−) tumors was 2.26 ± 0.36 ng platinum/mg of tumor. The average platinum level in the CTR1(−/−) CTR2(kd) tumors was 20.62 ± 3.53 ng platinum/mg of tumor. Thus, the CTR1(−/−) CTR2(kd) tumors accumulated 9.1-fold more platinum at 1 h after injection of cDDP than the CTR1(−/−) tumors (p = 0.006). This is a very large difference in platinum accumulation compared with the 3.5-fold difference in uptake observed for these cells when grown in vitro (Blair et al., 2009) and what is generally observed in cDDP-sensitive and cDDP-resistant cell lines.
Effect of CTR2 Knockdown on Responsiveness to cDDP In Vivo.
As shown in Fig. 3A, a single intraperitoneal injection of the maximum tolerated dose of cDDP (10 mg/kg) produced little slowing of the growth of CTR1(−/−) tumors relative to the growth rate of the untreated control tumors (p = 0.75). However, the same dose of cDDP clearly slowed the growth of CTR1(−/−) CTR2(kd) tumors (p < 0.0009) (Fig. 3B). The average volume of the cDDP-treated CTR1(−/−) CTR2(kd) tumors was 74% smaller than the untreated CTR1(−/−) CTR2(kd) tumors by week 7. Many of these tumors shrank in size and remained smaller than they were before treatment. Six of the 16 tumors became undetectable and never regrew during the period of observation. The remaining tumors either stopped growing or grew at a much slower rate than the CTR1(−/−) tumors. Thus, consistent with the effect of CTR2 on cDDP accumulation, CTR2 is a major determinant of therapeutic efficacy of cDDP in vivo.
Loss of CTR2 Increases the Rate of Endocytosis.
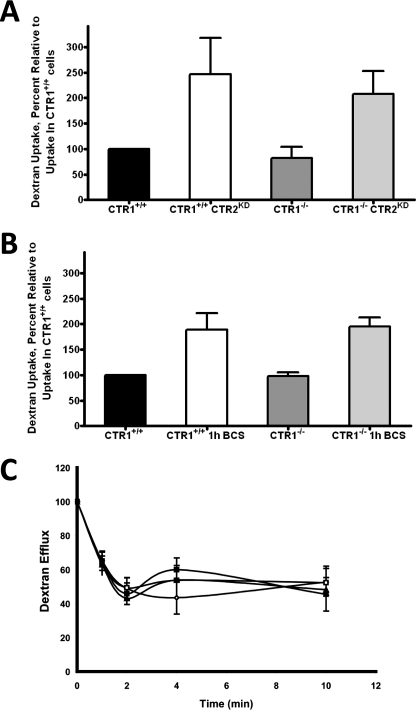
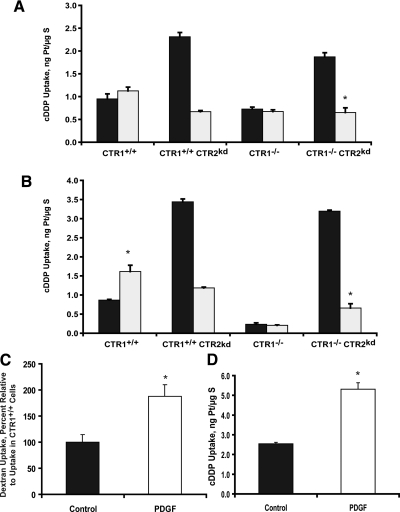
Changes in sensitivity to cDDP has been linked to perturbations in the rate of endocytosis in the past, and CTR1 is cleared from the plasma membrane by macropinocytosis. The accumulation of Texas red-labeled dextran is a well validated measure of the rate of macropinocytosis (Chauhan et al., 2003; Holzer and Howell, 2006). The rate of macropinocytosis in the CTR1(+/+), CTR1(+/+) CTR2(kd), CTR1(−/−), and CTR1(−/−) CTR2(kd) cells was assessed by exposing them to the labeled dextran for 30 min. As shown in Fig. 4A, loss of CTR1 did not significantly change the rate of macropinocytosis, as measured by the uptake of dextran; there was no difference in its accumulation in CTR1(−/−) and CTR1(+/+) cells (p = 0.45). In contrast, loss of CTR2 significantly increased the uptake of dextran. The CTR1(+/+) CTR2(kd) cells took up 2.5-fold more dextran over a 30-min period than the CTR1(+/+) cells (p = 0.01). This increase in macropinocytosis in CTR2(kd) cells occurred independently of CTR1 status. CTR1(−/−) CTR2(kd) cells accumulated 2.1-fold more dextran over a 30-min period than CTR1(−/−) cells (p = 0.049).
Fig. 4.
Effect of CTR2 on whole cell accumulation and efflux of Texas red- labeled dextran. A, dextran accumulation CTR1(+/+), CTR1(+/+) CTR2(kd), CTR1(−/−) and CTR1(−/−) CTR2(kd) cells. B, dextran accumulation in cells after a 1 h exposure to 100 μM BCS. C, dextran content as a function of efflux time in ♢, CTR1(+/+); □, CTR1(+/+) CTR2(kd); ▴, CTR1(−/−); and ■, CTR1(−/−) CTR2(kd) cells. Vertical bars, ± S.E.M. *p < 0.04.
CTR2 Degradation Due to Copper Starvation Induces Endocytosis.
Copper depletion by exposure to the high-affinity copper chelator BCS results in a rapid and near total degradation of CTR2 in these mouse embryo fibroblasts (Blair et al., 2010). To strengthen the link between CTR2 and macropinocytosis, cells were exposed to BCS for 1 h to reduce CTR2 levels by ∼90 to 95%. As shown in Fig. 4B, BCS-induced down-regulation of CTR2 expression enhanced dextran uptake by 1.9-fold in both the CTR1(+/+) cells (p = 0.039) and CTR1(−/−) cells (p = 0.021). Thus, macropinocytosis was enhanced when CTR2 expression was reduced by either of two independent means.
Enhanced Dextran Accumulation Is Not Due to Changes in Exocytotic Rate.
Enhanced accumulation of dextran may be due to either increased macropinocytosis or reduced exocytosis. Exocytosis of Texas-red dextran was measured in the CTR1(+/+), CTR1(+/+) CTR2(kd), CTR1(−/−), and CTR1(−/−) CTR2(kd) cells. As shown in Fig. 4C, the majority (∼55%) of Texas-red dextran exited the cells in the first 2 min, and there was no significant difference in the rate of Texas-red dextran exocytosis in CTR1(+/+), CTR1(+/+) CTR2(kd), CTR1(−/−), and CTR1(−/−) CTR2(kd) cells during either the initial or late phases of efflux.
Inhibition of Macropinocytosis Blocks CTR2-Dependent cDDP Accumulation.
As described in previous studies, knockdown of CTR2 mediated by either shRNAi or copper starvation substantially increases cDDP uptake in vitro (Blair et al., 2009) and in vivo. To determine whether this increase is due to enhanced macropinocytosis, cDDP accumulation was measured in the CTR1(+/+), CTR1(+/+) CTR2(kd), CTR1(−/−), and CTR1(−/−) CTR2(kd) cells with and without inhibition of macropinocytosis by pretreatment with either 5 mM amiloride or 100 nM wortmannin for 30 min. Both of these drugs have been widely used to inhibit macropinocytosis, although neither is entirely specific for this process (Ivanov, 2008). As shown in Fig. 5A, knockdown of CTR2 in untreated cells caused a 2.8-fold increase in cDDP accumulation in the CTR1(+/+) cells (p = 0.01) and a 2.7-fold increase in the CTR1(−/−) cells (p = 0.03). Treatment with amiloride did not significantly change cDDP uptake in either the CTR1(+/+) or CTR1(−/−) cells, but it completely blocked the increased cDDP uptake in the CTR2(kd) cells. Compared with untreated cells, amiloride pretreatment produced a 71.0% (p = 0.001) and 65.3% (p = 0.002) decrease in cDDP accumulation in CTR1(+/+) CTR2(kd) and CTR1(−/−) CTR2(kd) cells, respectively. As shown in Fig. 5B, CTR1(+/+) cells pretreated with wortmannin accumulated 1.8-fold (p = 0.02) more cDDP than untreated CTR1(+/+) cells; wortmannin had no effect on the accumulation of cDDP in CTR1(−/−) cells, indicating that this effect was specific to CTR1-expressing cells. This effect may be related to the ability of wortmannin to interfere with the clearance of CTR1 from the plasma membrane in a manner similar to the effect of proteosomal inhibition (Jandial et al., 2009). As observed with amiloride, wortmannin completely blocked the increased accumulation of cDDP in untreated CTR1(+/+) CTR2(kd) and CTR1(−/−) CTR2(kd) cells, producing 65.7 (p = 0.001) and 79.4% (p = 0.0001) decreases in cDDP accumulation, respectively. These results provide further evidence that the enhanced cDDP uptake that accompanies knockdown of CTR2 is mediated by an increased rate of macropinocytosis.
Fig. 5.
Effect of amiloride, wortmannin, and PDGF on the CTR2 regulation of cDDP accumulation. A, accumulation of platinum after a 30-min exposure to cDDP without (■) or with (□) inhibition of macropinocytosis by amiloride. B, accumulation of platinum after a 1-h exposure to cDDP without (■) or with (□) inhibition of macropinocytosis by wortmannin. C, 30-min dextran accumulation with and without PDGF pretreatment. D, effect of PDGF pretreatment on uptake of cDDP after an exposure to 30 μM cDDP for 1 h. Vertical bars, S.E.M. *, p < 0.001.
Induction of Macropinocytosis by PDGF Increases cDDP Accumulation.
Inhibition of macropinocytosis blocked the increased cDDP accumulation produced by knocking down CTR2. To further explore the role of macropinocytosis in the cellular accumulation of cDDP, PDGF was used to up-regulate macropinocytosis in the wild-type CTR1(+/+) cells. As reported previously (Dharmawardhane, 2000), pretreatment with 5 ng/ml PDGF-BB for 4 h increased dextran uptake by 1.8-fold (p < 0.02) (Fig. 5A). As demonstrated in Fig. 5B, CTR1(+/+) cells pretreated with PDGF accumulated 2.1-fold (p < 0.001) more cDDP after a 1-h exposure to 30 μM cDDP, consistent with the conclusion that cDDP can enter cells through the macropinocytotic pathway.
Knockdown of CTR2 Activates Rac1 and cdc42.
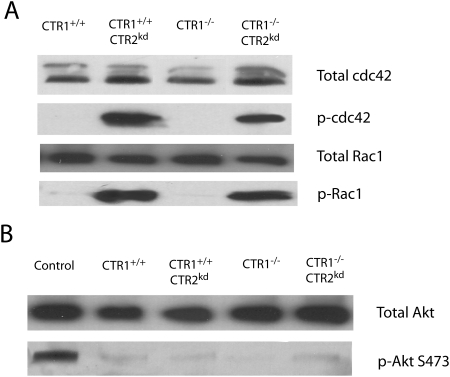
The active form of the Rho GTPases Rac1 and cdc42 are necessary for macropinocytosis. To determine whether CTR2 can regulate the activation of these proteins, the levels of active Rac1 and cdc42 were quantified by Western blot analysis using antibodies to the phosphorylated forms of these proteins in the CTR1(+/+), CTR1(+/+) CTR2(kd), CTR1(−/−), and CTR1(−/−) CTR2(kd) cells. As shown in Fig. 6A, under basal conditions, neither the CTR1(+/+) nor CTR1(−/−) cells contained detectable active Rac1 or cdc42. However, knockdown of CTR2 resulted in a very strong activation of both Rac1 and cdc42 regardless of CTR1 status. The total amount of Rac1 and cdc42 protein was similar in all four cell lines, indicating that loss of CTR2 constitutively activates these GTPases without changing their level.
Fig. 6.
CTR2 activates the GTPases that control macropinocytosis. A, relative levels of total and phosphorylated Rac1 and cdc42 in CTR1(+/+), CTR1(+/+) CTR2(kd), CTR1(−/−), and CTR1(−/−) CTR2(kd) cells. B, relative levels of phosphorylated Akt.
Loss of CTR2 Does Not Affect Akt Activation.
PI3K has been shown to activate Rac1 and cdc42. To determine whether the loss of CTR2 activates Rac1 and cdc42 via the PI3K pathway, the extent of Akt phosphorylation was measured in the CTR1(+/+), CTR1(+/+) CTR2(kd), CTR1(−/−), and CTR1(−/−) CTR2(kd) cells because Akt becomes phosphorylated upon PI3K activation. As shown in Fig. 6B, Western blot analysis using an antibody specific for Akt phosphorylated at Ser473 demonstrated that the loss of either CTR1 or CTR2 did not change Akt phosphorylation, thus suggesting that the loss of CTR2 regulates Rac1 and cdc42 phosphorylation downstream of PI3K.
Discussion
In this study, we sought to investigate the effect of CTR2 on tumor growth rate and cDDP accumulation and responsiveness in vivo, and to further elucidate the mechanism by which CTR2 controls the cellular pharmacology of cDDP. The first finding of importance was that the growth rate of the CTR1(−/−) CTR2(kd) tumors was substantially slower than that of the CTR1(−/−) tumors. The finding of a higher frequency of apoptotic cells, lower proliferation index and a lower density of CD31-positive capillaries in the CTR1(−/−) CTR2(kd) tumors provides a reasonable explanation for why the growth rate was impaired, but how the loss of CTR2 produces these effects remains unknown. There was a 2.1-fold higher level of copper in the CTR1(−/−) CTR2(kd) cells when growth in vitro that was not observed when they were grown in vivo. Because we harvested the whole tumor, which contains mouse-derived endothelial cells, mesenchymal, and inflammatory cells in addition to tumor cells, it is conceivable that the difference in the copper content of the actual tumor cells was missed. However, the fact that there was such a large difference in platinum levels suggests that the extent of contamination of the tumor cells by normal mouse cells was not large, and the fact that knockdown of CTR2 did not impair tyrosinase activity suggests no major limitation of intracellular copper availability. There is a substantial body of data indicating that copper is required for angiogenesis (Finney et al., 2009) but the fact that no differences were detected in steady-state copper levels between the two types of tumors leaves open the possibility that, despite its important role in copper homeostasis, CTR2 modulates the angiogenic response via effects on other pathways. It is of interest that CTR1 has been shown to regulate signaling via the fibroblast growth factor receptor pathway during embryonic development of neurectoderm in Xenopus laevis (Haremaki et al., 2007). Given the structural similarity of CTR1 and CTR2, it is conceivable that CTR2 regulates the synthesis or release of angiogenic factors from tumor cells.
CTR2 is an important determinant of the cytotoxicity of cDDP when cells are grown in tissue culture (Blair et al., 2009); however, results obtained using cultured cells do not always extrapolate to the in vivo setting. Fortunately, the results of the current study show that CTR2 is a major determinant of both cDDP accumulation and its therapeutic effectiveness in vivo and in vitro. Given the fact that CTR2 also regulates sensitivity to carboplatin in vitro, it is likely that CTR2 expression is also important to the tumor pharmacology and responsiveness of this drug in vivo as well. The magnitude of the effect of knocking down CTR2 on cDDP accumulation in vivo (9.1-fold) was quite a bit larger than the difference in uptake observed when the cells were grown in vitro (3.5-fold) and is very large relative to the ∼50% difference in cDDP uptake typically observed in isogenic pairs of cDDP-sensitive and -resistant cell lines (Andrews and Howell, 1990; Muggia and Los, 1993). This suggests that CTR2 is particularly important to cDDP accumulation in the complex in vivo environment in which rates of drug delivery, protein binding, and aquation are different from those obtained when cells are exposed to CDDP in vitro. Although the cellular level of copper does modulate the uptake of cDDP, the similarity of the copper levels in the CTR1(−/−) and CTR1(−/−) CTR2(kd) tumors makes it unlikely that intracellular copper availability alone accounts for the difference in cDDP accumulation.
Prior studies from this laboratory demonstrated that CTR2 has a large effect on the cellular accumulation of cDDP, and the results reported here provide insight into the mechanism of this effect. We have reported previously that the increased cDDP uptake in CTR2(kd) cells is not due to a change in drug efflux or microsomal storage of cDDP (Blair et al., 2009). The results reported here indicate that CTR2 regulates cDDP accumulation at least in part through an effect on macropinocytosis. Knockdown or BCS-induced degradation of CTR2 was accompanied by a large increase in macropinocytosis, and inhibition of macropinocytosis with either amiloride or wortmannin blocked this effect. Stimulation of macropinocytosis with PDGF mimicked the effect of knocking down CTR2 on dextran and cDDP uptake, and knockdown of CTR2 was associated with the activation of two Rho GTPases that regulate various endocytotic pathways. Taken together, these data suggest that the increased uptake of cDDP in CTR2(kd) cells is due to the observed up-regulation of macropinocytosis.
Endocytosis, and specifically macropinocytosis, is a tightly controlled process requiring activation of the GTPases Rac1 and cdc42 (Conner and Schmid, 2003). Once activated, these GTPases stabilize the formation of actin and filamin projections into the extracellular space, allowing the formation of endocytotic vesicles. Previous studies have shown that Rac1, cdc42, actin, and filamin are all down-regulated in many cDDP-resistant cell lines (Shen et al., 2004a,b). Endocytosis itself has also been reported to be reduced in many cDDP-resistant cells (Chauhan et al., 2003; Liang et al., 2006). CTR2(kd) cells were found to have constitutively active Rac1 and cdc42, inferring that this mechanism may underlie up-regulated rate of macropinocytosis in these cells.
The concordance of the in vitro and in vivo results with respect to cDDP accumulation and cytotoxicity further validates CTR2 as a target against which one could develop drugs capable of sensitizing tumors to cDDP. Gene silencing via targeted siRNA therapy is being explored in clinical trials (Soutschek et al., 2004; Castanotto and Rossi, 2009; Siomi, 2009), and our success in knocking down CTR2 suggests that it is a good target for this approach. Furthermore, CTR2 is quickly down-regulated upon copper starvation (Blair et al., 2010). Many copper chelating drugs such as d-penicillamine, tetrathiomolybdate, clioquinol, and trientine either have been or are currently being investigated in clinical trials for their ability to slow tumor growth (Yoshii et al., 2001; Pan et al., 2002; Yu et al., 2006; Gupte and Mumper, 2009). If these drugs effectively reduce copper levels in tumor cells, we would predict a concurrent down-regulation of CTR2 and enhanced cDDP uptake and tumor cell kill that would be expected to result in clinical synergy between these agents and the platinum-containing drugs.
Acknowledgments
We thank Dr. Dennis Thiele (Department of Pharmacology and Cancer Biology, Duke University Medical Center, Durham, NC) for kindly providing the CTR1(+/+) and CTR1(−/−) cells used in this study, Dr. Jesse Bertinato (Nutrition Research Division, Health Products and Food Branch, Health Canada, Ottawa, ON, Canada) for the providing the anti-CTR2 antibody, Dr. Alexandra Newton (Department of Pharmacology. University of California at San Diego, La Jolla, CA) for the anti-Akt antibodies, and Dr. Minji Jo (Department of Pathology, University of California at San Diego) for the anti-Rac1 and anti-cdc42 antibodies. Dr. Sandra Schmid provided valuable insight and suggestions as to the understanding of endocytosis.
This work was supported by the National Institutes of Health National Cancer Institute [Grant CA095298]; and the Department of Defense [Grant W81XWH-08-1-0135].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.068411.
- cDDP
- cisplatin [cis-diamminedichloroplatinum(II)]
- BCS
- bathocuproine disulfonate
- CTR1
- copper transporter 1, SLC31A1
- CTR2
- copper transporter 2, SLC31A2
- ICP-MS
- inductively coupled plasma mass spectrometry
- ICP-OES
- inductively coupled plasma optical emission spectroscopy
- PI3K
- phosphoinositide 3-kinase
- PDGF
- platelet-derived growth factor
- shRNAi
- short hairpin RNA interference
- PBS
- phosphate-buffered saline
- TUNEL
- terminal deoxynucleotidyl transferase dUTP nick-end labeling.
Authorship Contributions
Participated in research design: Blair, Safaei, and Howell.
Conducted experiments: Blair, Larson, Adams, Abada, and Pesce.
Performed data analysis: Blair, Larson, and Howell.
Wrote or contributed to the writing of the manuscript: Blair and Howell.
References
- Andrews PA, Howell SB. (1990) Cellular pharmacology of cisplatin: perspectives on mechanisms of acquired resistance. Cancer Cells 2:35–43 [PubMed] [Google Scholar]
- Bellemare DR, Shaner L, Morano KA, Beaudoin J, Langlois R, Labbe S. (2002) Ctr6, a vacuolar membrane copper transporter in Schizosaccharomyces pombe. J Biol Chem 277:46676–46686 [DOI] [PubMed] [Google Scholar]
- Bertinato J, Swist E, Plouffe LJ, Brooks SP, L'abbé MR. (2008) Ctr2 is partially localized to the plasma membrane and stimulates copper uptake in COS-7 cells. Biochem J 409:731–740 [DOI] [PubMed] [Google Scholar]
- Blair BG, Larson CA, Adams PL, Abada PB, Safaei R, Howell SB. (2010) Regulation of copper transporter 2 expression by copper and cisplatin in human ovarian carcinoma cells. Mol Pharmacol 77:912–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair BG, Larson CA, Safaei R, Howell SB. (2009) Copper transporter 2 regulates the cellular accumulation and cytotoxicity of cisplatin and carboplatin. Clin Cancer Res 15:4312–4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanotto D, Rossi JJ. (2009) The promises and pitfalls of RNA-interference-based therapeutics. Nature 457:426–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan SS, Liang XJ, Su AW, Pai-Panandiker A, Shen DW, Hanover JA, Gottesman MM. (2003) Reduced endocytosis and altered lysosome function in cisplatin-resistant cell lines. Br J Cancer 88:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G. (1994) Cellular responses to cisplatin. The roles of DNA-binding proteins and DNA repair. J Biol Chem 269:787–790 [PubMed] [Google Scholar]
- Conner SD, Schmid SL. (2003) Regulated portals of entry into the cell. Nature 422:37–44 [DOI] [PubMed] [Google Scholar]
- Crul M, Schellens JH, Beijnen JH, Maliepaard M. (1997) Cisplatin resistance and DNA repair. Cancer Treat Rev 23:341–366 [DOI] [PubMed] [Google Scholar]
- Dharmawardhane S, Schürmann A, Sells MA, Chernoff J, Schmid SL, Bokoch GM. (2000) Regulation of macropinocytosis by p21-activated kinase-1. Mol Biol Cell 11:3341–3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney L, Vogt S, Fukai T, Glesne D. (2009) Copper and angiogenesis: unravelling a relationship key to cancer progression. Clin Exp Pharmacol Physiol 36:88–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gately DP, Howell SB. (1993) Cellular accumulation of the anticancer agent cisplatin: a review. Br J Cancer 67:1171–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte A, Mumper RJ. (2009) Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat Rev 35:32–46 [DOI] [PubMed] [Google Scholar]
- Haremaki T, Fraser ST, Kuo YM, Baron MH, Weinstein DC. (2007) Vertebrate Ctr1 coordinates morphogenesis and progenitor cell fate and regulates embryonic stem cell differentiation. Proc Natl Acad Sci USA 104:12029–12034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer AK, Howell SB. (2006) The internalization and degradation of human copper transporter 1 following cisplatin exposure. Cancer Res 66:10944–10952 [DOI] [PubMed] [Google Scholar]
- Holzer AK, Manorek GH, Howell SB. (2006) Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol Pharmacol 70:1390–1394 [DOI] [PubMed] [Google Scholar]
- Iatropoulos MJ, Williams GM. (1996) Proliferation markers. Exp Toxicol Pathol 48:175–181 [DOI] [PubMed] [Google Scholar]
- Ivanov AI. (2008) Pharmacological inhibition of endocytic pathways: is it specific enough to be useful? Methods Mol Biol 440:15–33 [DOI] [PubMed] [Google Scholar]
- Jandial DD, Farshchi-Heydari S, Larson CA, Elliott GI, Wrasidlo WJ, Howell SB. (2009) Enhanced delivery of cisplatin to intraperitoneal ovarian carcinomas mediated by the effects of bortezomib on the human copper transporter 1. Clinical Cancer Research 15:553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, Laub PB, Beesley JS, Ozols RF, Hamilton TC. (1997) Increased platinum-DNA damage tolerance is associated with cisplatin resistance and cross-resistance to various chemotherapeutic agents in unrelated human ovarian cancer cell lines. Cancer Res 57:850–856 [PubMed] [Google Scholar]
- Katano K, Kondo A, Safaei R, Holzer A, Samimi G, Mishima M, Kuo YM, Rochdi M, Howell SB. (2002) Acquisition of resistance to cisplatin is accompanied by changes in the cellular pharmacology of copper. Cancer Res 62:6559–6565 [PubMed] [Google Scholar]
- Kelland LR, Mistry P, Abel G, Freidlos F, Loh SY, Roberts JJ, Harrap KR. (1992) Establishment and characterization of an in vitro model of acquired resistance to cisplatin in a human testicular nonseminomatous germ cell line. Cancer Res 52:1710–1716 [PubMed] [Google Scholar]
- Kuo MT, Chen HH, Song IS, Savaraj N, Ishikawa T. (2007) The roles of copper transporters in cisplatin resistance. Cancer Metastasis Rev 26:71–83 [DOI] [PubMed] [Google Scholar]
- Larson CA, Blair BG, Safaei R, Howell SB. (2009) The role of the mammalian copper transporter 1 in the cellular accumulation of platinum-based drugs. Mol Pharmacol 75:324–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latimer P, Menchaca M, Snyder RM, Yu W, Gilbert BE, Sanders BG, Kline K. (2009) Aerosol delivery of liposomal formulated paclitaxel and vitamin E analog reduces murine mammary tumor burden and metastases. Exp Biol Med (Maywood) 234:1244–1252 [DOI] [PubMed] [Google Scholar]
- Lee J, Petris MJ, Thiele DJ. (2002) Characterization of mouse embryonic cells deficient in the ctr1 high affinity copper transporter. Identification of a Ctr1-independent copper transport system. J Biol Chem 277:40253–40259 [DOI] [PubMed] [Google Scholar]
- Liang XJ, Mukherjee S, Shen DW, Maxfield FR, Gottesman MM. (2006) Endocytic recycling compartments altered in cisplatin-resistant cancer cells. Cancer Res 66:2346–2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucidarme O, Kono Y, Corbeil J, Choi SH, Golmard JL, Varner J, Mattrey RF. (2006) Angiogenesis: noninvasive quantitative assessment with contrast-enhanced functional US in murine model. Radiology 239:730–739 [DOI] [PubMed] [Google Scholar]
- Manic S, Gatti L, Carenini N, Fumagalli G, Zunino F, Perego P. (2003) Mechanisms controlling sensitivity to platinum complexes: role of p53 and DNA mismatch repair. Curr Cancer Drug Targets 3:21–29 [DOI] [PubMed] [Google Scholar]
- Metcalfe SA, Cain K, Hill BT. (1986) Possible mechanism for differences in sensitivity to cis-platinum in human prostate tumor cell lines. Cancer Lett 31:163–169 [DOI] [PubMed] [Google Scholar]
- Muggia FM, Los G. (1993) Platinum resistance: laboratory findings and clinical implications. Stem Cells 11:182–193 [DOI] [PubMed] [Google Scholar]
- Oldenburg J, Begg AC, van Vugt MJ, Ruevekamp M, Schornagel JH, Pinedo HM, Los G. (1994) Characterization of resistance mechanisms to cis-diamminedichloroplatinum(II) in three sublines of the CC531 colon adenocarcinoma cell line in vitro. Cancer Res 54:487–493 [PubMed] [Google Scholar]
- Pan Q, Kleer CG, van Golen KL, Irani J, Bottema KM, Bias C, De Carvalho M, Mesri EA, Robins DM, Dick RD, et al. (2002) Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res 62:4854–4859 [PubMed] [Google Scholar]
- Portnoy ME, Schmidt PJ, Rogers RS, Culotta VC. (2001) Metal transporters that contribute copper to metallochaperones in Saccharomyces cerevisiae. Mol Genet Genomics 265:873–882 [DOI] [PubMed] [Google Scholar]
- Rabik CA, Dolan ME. (2007) Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev 33:9–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees EM, Lee J, Thiele DJ. (2004) Mobilization of intracellular copper stores by the ctr2 vacuolar copper transporter. J Biol Chem 279:54221–54229 [DOI] [PubMed] [Google Scholar]
- Safaei R, Howell SB. (2005) Copper transporters regulate the cellular pharmacology and sensitivity to Pt drugs. Crit Rev Oncol Hematol 53:13–23 [DOI] [PubMed] [Google Scholar]
- Safaei R, Katano K, Samimi G, Holzer AK, Naerdemann W, Howell SB. (2004a) Contribution of endocytic pathways to the uptake of cisplatin in sensitive and resistant ovarian cancer cells. Proc Am Assoc Cancer Res 45:120 [Google Scholar]
- Safaei R, Katano K, Samimi G, Naerdemann W, Stevenson JL, Rochdi M, Howell SB. (2004b) Cross-resistance to cisplatin in cells with acquired resistance to copper. Cancer Chemother Pharmacol 53:239–246 [DOI] [PubMed] [Google Scholar]
- Safaei R, Maktabi MH, Blair BG, Larson CA, Howell SB. (2009) Effects of the loss of Atox1 on the cellular pharmacology of cisplatin. J Inorg Biochem 103:333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedletska Y, Giraud-Panis MJ, Malinge JM. (2005) Cisplatin is a DNA-damaging antitumour compound triggering multifactorial biochemical responses in cancer cells: importance of apoptotic pathways. Curr Med Chem Anticancer Agents 5:251–265 [DOI] [PubMed] [Google Scholar]
- Shen DW, Liang XJ, Gawinowicz MA, Gottesman MM. (2004a) Identification of cytoskeletal [14C]carboplatin-binding proteins reveals reduced expression and disorganization of actin and filamin in cisplatin-resistant cell lines. Mol Pharmacol 66:789–793 [DOI] [PubMed] [Google Scholar]
- Shen DW, Su A, Liang XJ, Pai-Panandiker A, Gottesman MM. (2004b) Reduced expression of small GTPases and hypermethylation of the folate binding protein gene in cisplatin-resistant cells. Br J Cancer 91:270–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomi MC. (2009) Short interfering RNA-mediated gene silencing; towards successful application in human patients. Adv Drug Deliv Rev 61:668–671 [DOI] [PubMed] [Google Scholar]
- Song IS, Savaraj N, Siddik ZH, Liu P, Wei Y, Wu CJ, Kuo MT. (2004) Role of human copper transporter Ctr1 in the transport of platinum-based antitumor agents in cisplatin-sensitive and cisplatin-resistant cells. Mol Cancer Ther 3:1543–1549 [PubMed] [Google Scholar]
- Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, et al. (2004) Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 432:173–178 [DOI] [PubMed] [Google Scholar]
- Teicher BA, Holden SA, Herman TS, Sotomayor EA, Khandekar V, Rosbe KW, Brann TW, Korbut TT, Frei E., 3rd (1991) Characteristics of five human tumor cell lines and sublines resistant to cis-diamminedichloroplatinum(II). Int J Cancer 47:252–260 [DOI] [PubMed] [Google Scholar]
- Twentyman PR, Wright KA, Mistry P, Kelland LR, Murrer BA. (1992) Sensitivity to novel platinum compounds of panels of human lung cancer cell lines with acquired and inherent resistance to cisplatin. Cancer Res 52:5674–5680 [PubMed] [Google Scholar]
- van den Berghe PV, Folmer DE, Malingré HE, van Beurden E, Klomp AE, van de Sluis B, Merkx M, Berger R, Klomp LW. (2007) Human copper transporter 2 is localized in late endosomes and lysosomes and facilitates cellular copper uptake. Biochem J 407:49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waud WR. (1987) Differential uptake of cis-diamminedichloroplatinum (II) by sensitive and resistant murine L1210 leukemia cells. Cancer Res 47:6549–6555 [PubMed] [Google Scholar]
- Yoshii J, Yoshiji H, Kuriyama S, Ikenaka Y, Noguchi R, Okuda H, Tsujinoue H, Nakatani T, Kishida H, Nakae D, et al. (2001) The copper-chelating agent, trientine, suppresses tumor development and angiogenesis in the murine hepatocellular carcinoma cells. Int J Cancer 94:768–773 [DOI] [PubMed] [Google Scholar]
- Yu Y, Wong J, Lovejoy DB, Kalinowski DS, Richardson DR. (2006) Chelators at the cancer coalface: desferrioxamine to Triapine and beyond. Clin Cancer Res 12:6876–6883 [DOI] [PubMed] [Google Scholar]
- Zorbas H, Keppler BK. (2005) Cisplatin damage: are DNA repair proteins saviors or traitors to the cell? Chembiochem 6:1157–1166 [DOI] [PubMed] [Google Scholar]