Abstract
BACKGROUND
Oncogenic fusion genes consisting of EML4 and anaplastic lymphoma kinase (ALK) are present in a subgroup of non–small-cell lung cancers, representing 2 to 7% of such tumors. We explored the therapeutic efficacy of inhibiting ALK in such tumors in an early-phase clinical trial of crizotinib (PF-02341066), an orally available small-molecule inhibitor of the ALK tyrosine kinase.
METHODS
After screening tumor samples from approximately 1500 patients with non–small-cell lung cancer for the presence of ALK rearrangements, we identified 82 patients with advanced ALK-positive disease who were eligible for the clinical trial. Most of the patients had received previous treatment. These patients were enrolled in an expanded cohort study instituted after phase 1 dose escalation had established a recommended crizotinib dose of 250 mg twice daily in 28-day cycles. Patients were assessed for adverse events and response to therapy.
RESULTS
Patients with ALK rearrangements tended to be younger than those without the rearrangements, and most of the patients had little or no exposure to tobacco and had adenocarcinomas. At a mean treatment duration of 6.4 months, the overall response rate was 57% (47 of 82 patients, with 46 confirmed partial responses and 1 confirmed complete response); 27 patients (33%) had stable disease. A total of 63 of 82 patients (77%) were continuing to receive crizotinib at the time of data cutoff, and the estimated probability of 6-month progression-free survival was 72%, with no median for the study reached. The drug resulted in grade 1 or 2 (mild) gastrointestinal side effects.
CONCLUSIONS
The inhibition of ALK in lung tumors with the ALK rearrangement resulted in tumor shrinkage or stable disease in most patients.
Specific genetic lesions that drive the proliferation of cancer cells render some cancers sensitive to therapeutic inhibitors targeting the mutated pathway. For example, inhibitors of the epidermal growth factor receptor (EGFR) have produced consistent responses in a subgroup of patients with non–small-cell lung cancer with activating EGFR mutations.1–3 Such findings suggest that patients’ outcomes may be optimized by testing tumors for specific mutated pathways and directing therapies against those mutant pathways.
Activating mutations or translocations of the anaplastic lymphoma kinase gene (ALK) have been identified in several types of cancer, including anaplastic large-cell lymphoma,4 neuroblastoma,5,6 inflammatory myofibroblastic tumor,7 and non–small-cell lung cancer.8 In non–small-cell lung cancer, EML4-ALK is an aberrant fusion gene that encodes a cytoplasmic chimeric protein with constitutive kinase activity. Multiple distinct EML4-ALK chimeric variants have been identified, representing breakpoints within various EML4 exons, all of which are transforming in vitro9,10 (Fig. 1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). EML4-ALK is uncommon, occurring in 2 to 7% of all non–small-cell lung cancers, and is more prevalent in patients who have never smoked or who have a history of light smoking and in patients with adenocarcinomas.8,10–14 Other, rarer fusion partners for ALK (e.g., KIF5B and TFG) have also been reported in non–small-cell lung cancers.15 Thus, ALK rearrangements may define a molecular subgroup of these tumors that is susceptible to targeted kinase inhibition.
In preclinical analyses of more than 600 cell lines derived from human cancers, an investigational selective ALK inhibitor specifically reduced the proliferation of cells carrying genetic alterations in ALK, supporting the role of ALK in malignant proliferation and as a valid drug target.16 Crizotinib (PF-02341066, Pfizer) is an oral ATP-competitive selective inhibitor of the ALK and MET tyrosine kinases that inhibits tyrosine phosphorylation of activated ALK at nanomolar concentrations.16,17 We conducted an open-label, multi-center, two-part phase 1 trial of crizotinib to evaluate the adverse-event profile and efficacy in an expanded cohort of patients with lung cancers carrying ALK rearrangements.
METHODS
STUDY DESIGN
We administered oral crizotinib on a continuous daily schedule in patients with advanced non–small-cell lung cancer. One cycle was defined as 28 days. The first part of the trial enrolled patients with any solid tumor refractory to standard therapy in a dose-escalation study to determine toxic effects and the maximum tolerated dose (i.e., the highest dose that would not cause unacceptable side effects). Details of dose escalation, toxic effects, and pharmacokinetics have been reported previously.18 The study was conducted in accordance with the protocol, which is available at NEJM.org. On the basis of evidence of activity in two patients with non–small-cell lung cancer with ALK rearrangement who were treated during the dose-escalation period, we enrolled an expanded cohort of patients who had non–small-cell lung cancer with prospectively identified ALK rearrangement.
The protocol was approved by the investigational review board at each study center, and all patients provided written informed consent. The study was designed jointly by representatives of Pfizer and the investigators. Data were collected from the academic sites and analyzed by representatives of Pfizer. The primary data were made available to the investigators, who vouch for the results after independent review and analyses. The first draft of the manuscript was written by the corresponding author, with review and revision by the other coauthors.
PATIENTS
We evaluated 82 patients with ALK-rearranged advanced non–small-cell lung cancer who were enrolled through February 10, 2010. These patients underwent a baseline tumor assessment, received a dose of crizotinib on the first day of cycle 1, and underwent at least one post-baseline tumor assessment or discontinued the study before the first protocol-planned tumor assessment. We present safety and response data that were available in the database as of April 7, 2010. Key eligibility requirements included ALK positivity on fluorescence in situ hybridization (FISH), measurable disease, adequate bone marrow and organ function, and resolution of all previous treatment-related toxic effects to grade 1 or less. Also required was an Eastern Cooperative Oncology Group performance status19 between 0 (fully active and able to carry out all predisease performance without restriction) and 2 (ambulatory and capable of all self care but unable to carry out any work activities; up and about more than 50% of waking hours). There was no limit on the number of previous treatment regimens for any patient.
MOLECULAR SCREENING AND ANALYSES
Unstained slides from formalin-fixed, paraffin-embedded (FFPE) tumor samples were analyzed prospectively by means of FISH with the use of an ALK break-apart (or split-signal) probe.20 Samples were deemed to be FISH-positive if more than 15% of scored tumor cells had split ALK 5′ and 3′ probe signals or had isolated 3′ signals. Reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assays for specific EML4-ALK fusions and immunohistochemical analyses for ALK protein were performed retrospectively on a subgroup of FFPE tumor samples (for details, see the Supplementary Appendix). A total of 70 of 82 samples (85%) were confirmed to be FISH-positive by a central laboratory under standards and conditions certified according to the Clinical Laboratory Improvement Amendments.
TREATMENT
We escalated the doses of crizotinib from 50 mg once daily to 300 mg twice daily, using a standard dose-escalation design. Doses of 200 mg or more twice daily resulted in a mean plasma trough concentration of more than 120 ng per milliliter, the preclinically predicted effective concentration. During dose escalation, one patient with non–small-cell lung cancer with ALK rearrangement entered the cohort receiving 300 mg twice daily, and one entered a de-escalated cohort receiving 200 mg twice daily. Dose-limiting fatigue in the cohort receiving 300 mg twice daily led to the establishment of a regimen of 250 mg twice daily as the maximum tolerated dose. The expanded cohort with FISH-positive results for ALK rearrangement received 250 mg twice daily. Patients continued to receive therapy as long as they did not have progressive disease or intolerable side effects.
STUDY ASSESSMENTS
Patients were evaluated for safety at least once every 2 weeks for the first two cycles and then at least every 4 weeks thereafter. Radiologic assessments were performed at baseline and generally after every two cycles of treatment with the use of Response Evaluation Criteria in Solid Tumors (RECIST), version 1.0 (for details, see the Supplementary Appendix). Patients who had a partial response were scheduled to undergo confirmatory scans at least 4 weeks after the initial partial response. A window for radiologic assessment that varied by 1 week was allowed. However, three patients underwent computed tomographic (CT) scanning 6 weeks after treatment initiation and were classified as having stable disease. These three patients were not included in the calculation of the disease-control rate (for patients with a complete or partial response or stable disease) at 8 weeks. At the discretion of the investigator, 18F-fluorodeoxyglucose–positron-emission tomography (18FDG-PET), 18F-fluoro-3′-deoxy-3′-L-fluorothymidine-PET (18FLT-PET), or both were performed. Graded adverse events were summarized and reported according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf).
STATISTICAL ANALYSIS
We calculated progression-free survival from the date of the first administration of crizotinib until the date of objective disease progression or death from any cause. We used the Kaplan–Meier method to estimate progression-free survival. All confidence intervals that are reported are two-sided. All analyses were performed with the use of SAS statistical software, version 9.2 (SAS Institute).
RESULTS
CLINICOPATHOLOGICAL CHARACTERISTICS
Two patients with non–small-cell lung cancer with FISH-positive results for ALK rearrangement who were treated with crizotinib during dose escalation had dramatic improvement in symptoms, prompting large-scale prospective screening for non–small-cell lung cancers with ALK rearrangement and enrollment into an expanded molecular cohort. From August 2008 through February 2010, the study centers screened approximately 1500 patients with non–small-cell lung cancers for ALK rearrangement with the use of FISH (Fig. 1A). The protocol placed no restrictions on the subtypes of non–small-cell lung cancer that were submitted for screening. However, as the clinicopathological features associated with ALK positivity emerged, tumor samples that were screened were increasingly adenocarcinomas.
Figure 1. Diagnosis of an EML4-ALK–Positive Non–Small-Cell Lung Cancer in a Single Representative Patient.
Panel A shows the results of a break-apart fluorescence in situ hybridization assay of tumor cells from a patient with rearrangement of the gene encoding anaplastic lymphoma kinase (ALK). The green probe hybridizes to the region immediately 5′ to ALK, and the red probe to the 3′ region. The separation of red and green probe signals (arrows) indicates a chromosomal rearrangement involving ALK. Close apposition of red and green probe signals indicates an intact wild-type copy of ALK. The probe that was used was the Vysis LSI ALK Dual Color, Break Apart Rearrangement Probe (Abbott Molecular). Panel B shows a light micrograph of the same tumor, revealing adenocarcinoma (hematoxylin and eosin). Panel C shows a representative sequence electropherogram of a reverse-transcriptase–polymerase-chain-reaction assay of EML4-ALK. The sequence of a junction between EML4 exon 6b and ALK exon 20 is shown. Panel D shows immunohistochemical analysis of ALK protein expression in tumor cells (brown) but not in adjacent normal bronchial epithelium (diaminobenzidine).
The characteristics of 82 patients who had tumors with FISH-positive ALK rearrangement are summarized in Table 1. Patients tended to be younger than the average age of patients with lung cancer, with a history of never having smoked or of former light smoking (≤10 pack-years). However, five patients had a history of more than 10 pack-years, including three who had smoked for at least 35 pack-years. Tumors were found to be predominantly adenocarcinomas on histologic analyses (Fig. 1B, and Table 1 in the Supplementary Appendix), with signet-ring cells frequently seen.20 Information on previous therapy was available for 81 patients, with 76 patients (94%) having received at least one previous therapy and 5 receiving first-line therapy with crizotinib (Table 1, and Table 2 in the Supplementary Appendix).
Table 1.
Demographic and Clinicopathological Characteristics of the 82 Patients.
| Characteristic | Value |
|---|---|
| Male sex — no. (%) | 43 (52) |
| Age — yr | |
| Mean | 51 |
| Range | 25–78 |
| Race — no. (%)* | |
| White | 46 (56) |
| Asian | 29 (35) |
| Other | 7 (9) |
| ECOG performance status — no. (%)† | |
| 0 | 24 (29) |
| 1 | 44 (54) |
| 2 | 13 (16) |
| 3 | 1 (1) |
| No. of previous therapies — no. (%) | |
| 0 | 5 (6) |
| 1 | 27 (33) |
| 2 | 15 (18) |
| ≥3 | 34 (41) |
| Not reported | 1 (1) |
| Histologic analysis — no. (%) | |
| Adenocarcinoma | 79 (96) |
| Squamous-cell carcinoma | 1 (1) |
| Other | 2 (2) |
| Smoking history — no. (%)‡ | |
| Never | 62 (76) |
| ≤10 pack-yr | 15 (18) |
| >10 pack-yr | 5 (6) |
Race was self-reported.
The Eastern Cooperative Oncology Group (ECOG) performance score ranges from 0 to 5, with higher scores indicating a greater degree of disability. One patient with an ECOG performance score of 2 at enrollment had a score of 3 by the start of treatment.
Data on patients’ smoking history are based on correspondence with investigators.
RESPONSE TO ALK INHIBITION
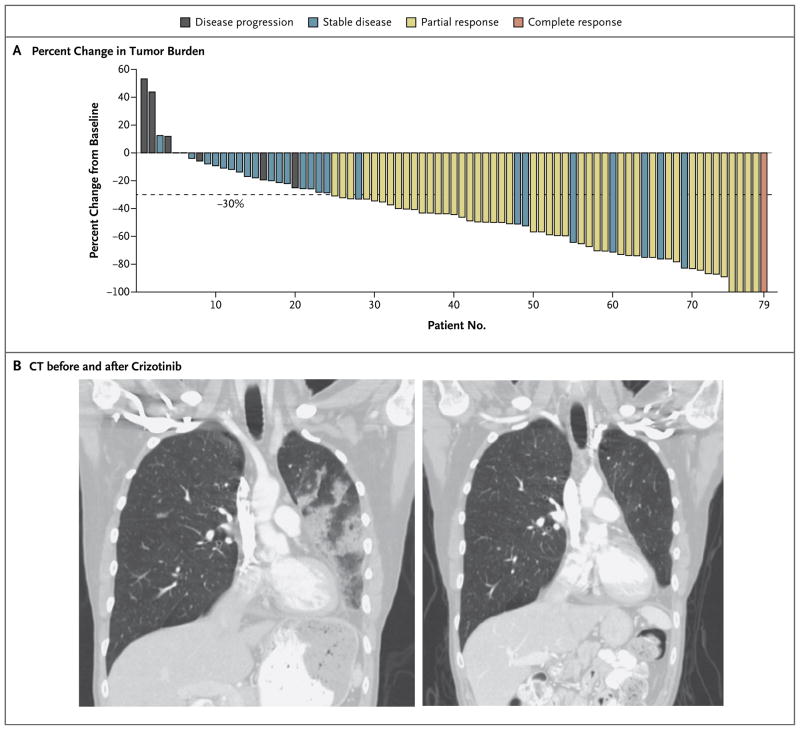
We evaluated 82 patients with FISH-positive ALK rearrangements, including 2 who were treated during dose escalation, for response and safety. Of these patients, 46 from study centers in the United States, Australia, and Korea met RECIST criteria for a confirmed partial response, and 1 met the criteria for a complete response, for an overall response rate of 57% (95% confidence interval [CI], 46 to 68). An additional group of 27 patients (33%) met criteria for stable disease, including 5 who had an unconfirmed partial response but who were classified as having stable disease because confirmatory CT results were not available by the cutoff date (Fig. 2A). The disease-control rate at 8 weeks was 87% (71 of 82 patients). A subgroup of unselected patients was also evaluated by 18FDG-PET, and representative radiographic images of responses to therapy are shown in Figure 2B (and Fig. 2 in the Supplementary Appendix). Exploratory 18FLT-PET scans were performed after one cycle of treatment in several unselected patients, with results supporting the clinical impression that responses to ALK inhibition can be rapid (Fig. 3 in the Supplementary Appendix).
Figure 2. Response to ALK Inhibition.
Panel A shows the best response of patients with ALK-positive tumors who were treated with crizotinib, as compared with pretreatment baseline. Numbers along the x axis indicate arbitrarily assigned subject numbers from 1 to 79. The bars indicate the percent change in tumor burden from baseline. Three study patients are not included in this plot: one patient was clinically assessed as having had a partial response, although the response was primarily in areas of nonmeasurable disease, so the patient was classified as having stable disease; two patients with abrupt clinical deterioration could not be assessed. Four patients had complete resolution of their target lesions but were classified as having had a partial response on the basis of stability in nontarget lesions. Eight patients had tumor shrinkage of more than 30% but were classified as having stable disease either because confirmatory scans were not available by the data-cutoff point (for five patients) or early restaging was performed at 6 weeks after crizotinib initiation (for three patients). The dashed line indicates a tumor reduction of 30% from baseline, the minimal percent decrease that constitutes a partial response, according to Response Evaluation Criteria in Solid Tumors. Panel B shows the results of CT with coronal reconstruction in a representative patient at baseline (left) and after two cycles of therapy (right). This patient had undergone previous left lower lobectomy.
Of the 82 patients, 6 (7%) had disease progression at the time of their first restaging scans. Two patients (Patients 1 and 2) had an increase of more than 20% in disease burden after two cycles of therapy (Fig. 2A). Three patients (Patients 4, 8, and 20) had stable disease in target lesions but concurrently had development of new lesions, so these patients were categorized as having progressive disease. One patient (Patient 16) had tumor regression of 25% on the basis of early CT scanning during cycle 1 but subsequently had an increase in tumor burden of more than 20% at the time of protocol-specified restaging. Two patients had abrupt clinical deterioration, which was probably caused by acute complications of disease (thrombosis and acute pulmonary hemorrhage), and could not be radiographically evaluated for response.
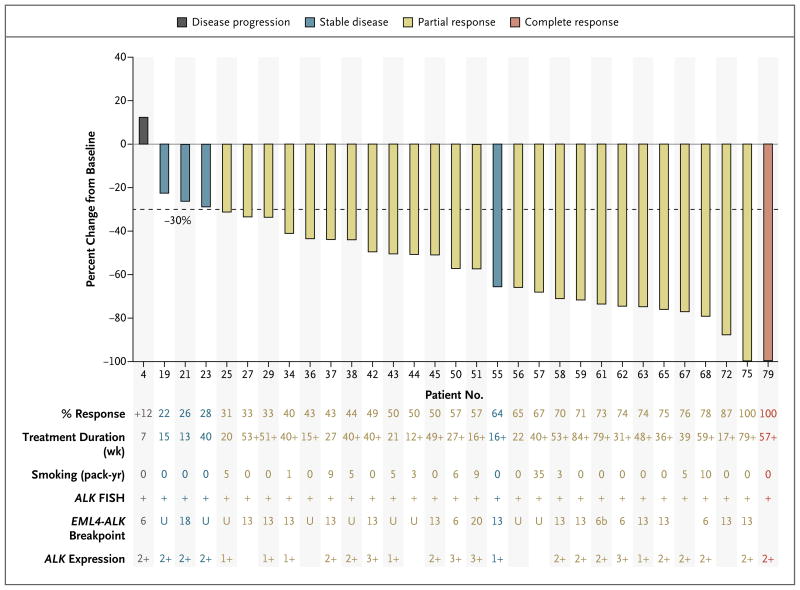
MOLECULAR ANALYSIS OF EML4–ALK BREAKPOINTS
The break-apart FISH assay detects disruption of the ALK locus but does not confirm EML4 as the partner fusion gene. Therefore, RT-PCR was used to analyze exon breakpoints in 31 patients with FISH-positive ALK rearrangements who had sufficient amounts of FFPE tumor material (Fig. 1C and 3). The most frequently identified genotype was exon 13 of EML4 fused to exon 20 of ALK (in 13 of 29 patients). EML4 exon 6, alternative exon 6b, exon 18, and exon 20 breakpoints were also observed (in 7 of 29 patients). An EML4-ALK fusion transcript could not be confirmed on RT-PCR in 9 patients, and the analysis failed in 2 additional patients. RT-PCR assays were unable to detect all known ALK rearrangements. Thus, the presence of RT-PCR–negative results suggests that either alternative EML4 exons were involved or that EML4 was not the ALK fusion partner in these patients. In the 29 analyzed samples, data were insufficient to correlate the presence of the EML4-ALK breakpoint with either smoking history or response rate (Fig. 3). Immunohistochemical analysis of ALK in FFPE tumor sections with an anti-ALK rabbit monoclonal antibody21 revealed positive ALK protein expression in all 25 samples that had sufficient tissue available (Fig. 1D and 3). FISH-negative samples and normal lung tissues did not express ALK protein (data not shown).
Figure 3. Best Response to Crizotinib in 31 Patients with ALK-Positive Tumors, as Correlated with Clinicopathological Characteristics.
Percent tumor response, treatment duration, smoking history, and selected tumor characteristics are listed in the table below the graph, with each table entry corresponding to a patient in the graph above. Patients are listed in order of increasing percentage response to crizotinib, with listed patient numbers corresponding to those in Figure 2A. Smoking history is reported in pack-years. The EML4-ALK genotype is reported as the EML4 exon that is fused to ALK, as assayed by nucleotide sequencing of RT-PCR products. U denotes undetermined for patients for whom RT-PCR assays using primers to ALK exon 20 along with EML4 exons 6, 13, and 18 produced no product. Blank fields indicate that adequate tumor samples were not available for analysis. ALK expression is reported as 0, 1+, 2+, or 3+, per convention for immunohistochemical analysis.
Because crizotinib also inhibits the MET tyrosine kinase, tumors from 33 patients with available tissue were tested for MET amplification. All samples were negative, arguing against crizotinib’s MET inhibitory activity as a determinant of response in these patients. In addition, none of the patients with ALK rearrangement had a concurrent mutation in EGFR (data not shown). This finding was noteworthy, since these patients shared several key clinical features with patients with EGFR mutations (adenocarcinoma histology and nonsmoking history).
SAFETY AND ADVERSE EVENTS
Safety data from 82 patients who were treated primarily with 250 mg twice daily in the expansion cohort are shown in Table 2. Grade 1 nausea and diarrhea were the most commonly reported side effects. Mild visual disturbances were reported by 34 patients (41%). These events were most frequently described as trails of light following objects moving relative to the observer, particularly noticed during changes in ambient lighting from dark to light, often improving with length of time receiving therapy. No abnormalities were found in three of these patients who underwent detailed ophthalmologic examination. Increases in levels of hepatic transaminases were generally grade 1 or 2; however, grade 3 elevations in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were observed in 4 patients (5%) and 5 patients (6%), respectively, usually with onset during cycle 2; 1 patient had a grade 4 elevation in ALT. These increases reversed on cessation of crizotinib, and 4 of 5 patients with elevated transaminase levels were able to resume treatment at a lower dose without recurrence of dose-limiting toxic effects. One patient with grade 3 ALT discontinued crizotinib because of recurrence of grade 3 ALT, despite dose reduction.
Table 2.
Adverse Events in the 82 Patients.*
| Adverse Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total |
|---|---|---|---|---|---|
| no. of patients (%) | |||||
| Any adverse event† | |||||
| Nausea | 43 (52) | 1 (1) | 0 | 0 | 44 (54) |
| Diarrhea | 38 (46) | 1 (1) | 0 | 0 | 39 (48) |
| Vomiting | 35 (43) | 1 (1) | 0 | 0 | 36 (44) |
| Visual disturbance | 34 (41) | 0 | 0 | 0 | 34 (41) |
| Constipation | 18 (22) | 2 (2) | 0 | 0 | 20 (24) |
| Peripheral edema | 13 (16) | 0 | 0 | 0 | 13 (16) |
| Dizziness | 12 (15) | 0 | 0 | 0 | 12 (15) |
| Decreased appetite | 11 (13) | 0 | 0 | 0 | 11 (13) |
| Fatigue | 8 (10) | 0 | 0 | 0 | 8 (10) |
| Grade 3 or 4 adverse events‡ | |||||
| ALT elevation | 4 (5) | 1 (1) | |||
| AST elevation | 5 (6) | 0 | |||
| Lymphopenia | 2 (2) | 0 | |||
| Hypophosphatemia | 1 (1) | 0 | |||
| Neutropenia | 1 (1) | 0 | |||
| Hypoxia | 1 (1) | 0 | |||
| Pneumonitis | 1 (1) | 0 | |||
| Pulmonary embolism | 1 (1) | 0 | |||
ALT denotes alanine aminotransferase, and AST aspartate aminotransferase.
These adverse events occurred in at least 10% of the 82 patients. The adverse events that occurred in two patients who received crizotinib during dose escalation are included. The remaining patients started treatment at 250 mg of crizotinib twice daily.
These grade 3 or 4 adverse events were evaluated in 82 patients; laboratory data were not always available for all 82 patients.
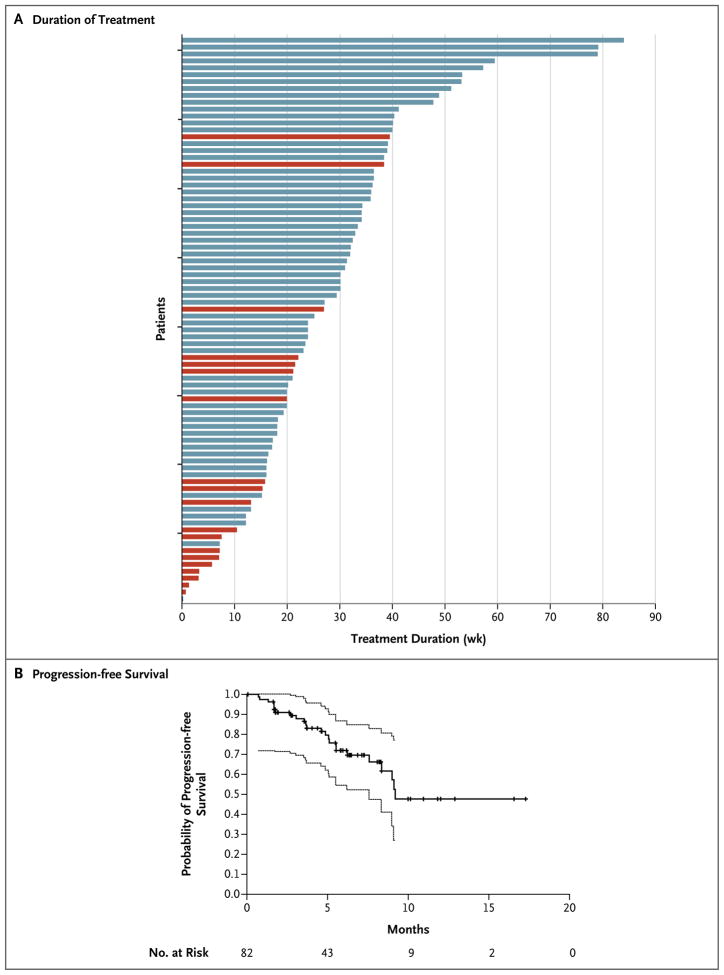
DURATION OF TREATMENT
Of the 82 patients, 63 (77%) continued to receive crizotinib after the data cutoff for this study (Fig. 4A). The mean duration of treatment was 6.4 months, with follow-up ongoing. This trial was not designed to address progression-free survival as an end point; the responses seen in patients with ALK rearrangement were unanticipated at the time the protocol was written, and no statistically predetermined enrollment goal for ALK-positive patients was established. Furthermore, ALK-positive patients receiving crizotinib had a heterogeneous history of previous treatments. On the basis of these limitations, and with a median follow-up for progression-free survival of 6.4 months (95% CI, 5.5 to 7.2), the probability of progression-free survival at 6 months was estimated at 72% (95% CI, 61 to 83) (Fig. 4B).
Figure 4. Duration of Treatment and Estimated Progression-free Survival.
Panel A shows the duration of treatment for the 82 patients, with blue bars indicating patients who were continuing to receive crizotinib by data cutoff. Red bars indicate 19 patients who discontinued treatment (13 because of disease progression, 1 because of crizotinib-related adverse events, 1 because of unrelated adverse events, 2 because of death from unrelated causes, and 2 because of other reasons). Panel B shows a Kaplan–Meier curve of estimated progression-free survival, with the lighter curves above and below the Kaplan–Meier curve representing 95% Hall–Wellner confidence limits.
DISCUSSION
Our results show that a targeted inhibitor of ALK is effective against advanced non–small-cell lung cancers carrying activated ALK kinase. In 82 patients, the majority of whom had received multiple previous therapies, we observed an overall response rate of 57% (confirmed partial and complete responses) and a rate of stable disease of 33% (stable disease plus unconfirmed partial responses). The response rate is impressive, as compared with the approximate 10% response rate in such cancers that were treated with second-line chemotherapy.22 The rate and speed of clinical response were similar to those shown by EGFR tyrosine kinase inhibitors in EGFR-mutant non–small-cell lung cancers,23–26 which suggests that ALK-positive tumors constitute a second genetically defined subgroup of oncogene-driven lung cancer that is highly susceptible to targeted therapy. It is notable that patients who were treated with therapeutic doses of crizotinib and whose tumors did not have known ALK abnormalities (including two patients with non–small-cell lung cancer who were treated during dose escalation) did not have a response to treatment, which was consistent with preclinical data16 (data not shown).
The benefit that we observed from crizotinib therapy in ALK-positive patients is promising, with an estimated 6-month probability of progression-free survival of 72%; follow-up is ongoing. Since more than 90% of the patients in our study received crizotinib as at least a second-line therapy, our findings compare favorably with those in a meta-analysis involving patients with such tumors who were treated with second-line multiagent chemotherapy, which showed a median duration of progression-free survival of 14 weeks and a 6-month rate of progression-free survival of 27.2%.25,27 Therefore, targeted inhibition of ALK in genotyped patients may lead to improved survival, as compared with conventional chemotherapy. Because our discovery of crizotinib’s activity in this population occurred so proximal to the identification of EML4-ALK,8 it is difficult to determine the outcome of patients with ALK-positive disease after standard chemotherapy in the absence of ALK inhibition. However, preliminary data from a small number of patients who were retrospectively identified as having the ALK rearrangement suggest that ALK-positive tumors that are treated with platinum-based chemotherapy have a response that is similar to that of such tumors without ALK or EGFR mutations.28
Crucial to the identification of a rare genetic subgroup was our ability to molecularly prescreen large numbers of non–small-cell lung cancers in real time and incorporate an expanded genotype-driven study into this phase 1 trial. The ALK rearrangement occurred in about 5% of patients who underwent screening. FISH positivity for ALK rearrangement was required for entry into the expanded cohort. However, the findings that patients with the ALK rearrangement had strong aberrant expression of the ALK protein and that many patients had positive results for the presence of EML4-ALK on RT-PCR assay raise the possibility that other diagnostic approaches could be used to identify ALK-rearranged tumors.
The use of prospective tumor genotyping has the potential to streamline drug development. Approximately 2 years after the initial report describing EML4-ALK rearrangement,8 we first reported that ALK inhibition shrank tumors in a targeted population of patients with non–small-cell lung cancers.18 Only 3 years after the initiation of the phase 1 trial, a phase 3 registration trial of crizotinib in ALK-positive patients started enrollment. In contrast, it took approximately 10 years to advance from the initially unsuccessful trials of EGFR inhibitors in nongenotyped patients to a randomized phase 3 trial that unequivocally showed the effectiveness of the EGFR inhibitor gefitinib, as compared with first-line chemotherapy, in EGFR-mutant tumors.25
Prospective genotyping may also more effectively direct patients to the trials that are most likely to provide benefit. In the randomized phase 3 trial of gefitinib, the selection of patients on the basis of clinicopathological characteristics (e.g., adenocarcinoma histology and absent or light tobacco exposure) was successful in enriching the group for tumors with EGFR mutations. However, these characteristics alone were inadequate to predict which patients would have a response to gefitinib.25 Among patients with a history of no smoking or light smoking who have adenocarcinoma, the genetic drivers of tumorigenesis include EGFR in 50% and ALK in a separate 20%,25,28 indicating that definitive molecular genetic categorization is critical to guide the selection of the appropriate targeted therapy. This concept is further supported by the finding that several patients in our trial had a heavy smoking history and yet had ALK-rearranged tumors that responded to crizotinib.
In conclusion, we have shown the importance and feasibility of prospective genotyping in an early clinical trial and have found that non–small-cell lung cancers with ALK rearrangement are highly sensitive to ALK kinase inhibition.
Supplementary Material
Acknowledgments
Funded by Pfizer and others; ClinicalTrials.gov number, NCT00585195.
Supported by Pfizer; by grants from the Massachusetts General Hospital (MGH) Cancer Center, the Aid for Cancer Research Foundation, and from an anonymous donor (to MGH); grants from the National Cancer Institute (CA090578, to MGH, Dana–Farber Cancer Institute [DFCI], and Beth Israel Deaconess Medical Center [BIDMC]) and the National Institutes of Health (R01CA136851, to DFCI); a Career Development Award from the American Society of Clinical Oncology (ASCO) Cancer Foundation (to BIDMC); Clinical Investigator Team Leadership Awards from the NCI–ASCO Cancer Foundation (CA47179, to Memorial Sloan-Kettering Cancer Center; and CA06516, to DFCI); a John and Carol Barry Award (to Dr. Kwak); and grants from the National Cancer Institute (CA58187 and CA46934, to the University of Colorado Cancer Center).
We thank the patients who participated in this study.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 2.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 3.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–11. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kutok JL, Aster JC. Molecular biology of anaplastic lymphoma kinase-positive anaplastic large-cell lymphoma. J Clin Oncol. 2002;20:3691–702. doi: 10.1200/JCO.2002.12.033. [DOI] [PubMed] [Google Scholar]
- 5.George RE, Sanda T, Hanna M, et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature. 2008;455:975–8. doi: 10.1038/nature07397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mossé YP, Laudenslager M, Longo L, et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008;455:930–5. doi: 10.1038/nature07261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pulford K, Lamant L, Espinos E, et al. The emerging normal and disease-related roles of anaplastic lymphoma kinase. Cell Mol Life Sci. 2004;61:2939–53. doi: 10.1007/s00018-004-4275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 9.Choi YL, Takeuchi K, Soda M, et al. Identification of novel isoforms of the EML4-ALK transforming gene in non-small cell lung cancer. Cancer Res. 2008;68:4971–6. doi: 10.1158/0008-5472.CAN-07-6158. [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi K, Choi YL, Soda M, et al. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin Cancer Res. 2008;14:6618–24. doi: 10.1158/1078-0432.CCR-08-1018. [DOI] [PubMed] [Google Scholar]
- 11.Koivunen JP, Mermel C, Zejnullahu K, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14:4275–83. doi: 10.1158/1078-0432.CCR-08-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mano H. Non-solid oncogenes in solid tumors: EML4-ALK fusion genes in lung cancer. Cancer Sci. 2008;99:2349–55. doi: 10.1111/j.1349-7006.2008.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perner S, Wagner PL, Demichelis F, et al. EML4-ALK fusion lung cancer: a rare acquired event. Neoplasia. 2008;10:298–302. doi: 10.1593/neo.07878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong DW, Leung EL, So KK, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009;115:1723–33. doi: 10.1002/cncr.24181. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi K, Choi YL, Togashi Y, et al. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin Cancer Res. 2009;15:3143–9. doi: 10.1158/1078-0432.CCR-08-3248. [DOI] [PubMed] [Google Scholar]
- 16.McDermott U, Iafrate AJ, Gray NS, et al. Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Res. 2008;68:3389–95. doi: 10.1158/0008-5472.CAN-07-6186. [DOI] [PubMed] [Google Scholar]
- 17.Christensen JG, Zou HY, Arango ME, et al. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer Ther. 2007;6:3314–22. doi: 10.1158/1535-7163.MCT-07-0365. [DOI] [PubMed] [Google Scholar]
- 18.Kwak EL, Camidge DR, Clark J, et al. Clinical activity observed in a phase I dose escalation trial of an oral c-MET and ALK inhibitor, PF-02341066. J Clin Oncol. 2009;27(Suppl):148s. [Google Scholar]
- 19.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 20.Rodig SJ, Mino-Kenudson M, Dacic S, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res. 2009;15:5216–23. doi: 10.1158/1078-0432.CCR-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mino-Kenudson M, Chirieac LR, Law K, et al. A novel, highly sensitive antibody allows for the routine detection of ALK-rearranged lung adenocarcinomas by standard immunohistochemistry. Clin Cancer Res. 2010;16:1561–71. doi: 10.1158/1078-0432.CCR-09-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gridelli C, Ardizzoni A, Ciardiello F, et al. Second-line treatment of advanced non-small cell lung cancer. J Thorac Oncol. 2008;3:430–40. doi: 10.1097/JTO.0b013e318168c815. [DOI] [PubMed] [Google Scholar]
- 23.Asahina H, Yamazaki K, Kinoshita I, et al. A phase II trial of gefitinib as first-line therapy for advanced non-small cell lung cancer with epidermal growth factor receptor mutations. Br J Cancer. 2006;95:998–1004. doi: 10.1038/sj.bjc.6603393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inoue A, Suzuki T, Fukuhara T, et al. Prospective phase II study of gefitinib for chemotherapy-naive patients with advanced non-small-cell lung cancer with epidermal growth factor receptor gene mutations. J Clin Oncol. 2006;24:3340–6. doi: 10.1200/JCO.2005.05.4692. [DOI] [PubMed] [Google Scholar]
- 25.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 26.Sequist LV, Martins RG, Spigel D, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol. 2008;26:2442–9. doi: 10.1200/JCO.2007.14.8494. [DOI] [PubMed] [Google Scholar]
- 27.Di Maio M, Chiodini P, Georgoulias V, et al. Meta-analysis of single-agent chemotherapy compared with combination chemotherapy as second-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:1836–43. doi: 10.1200/JCO.2008.17.5844. [DOI] [PubMed] [Google Scholar]
- 28.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27:4247–53. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.