SUMMARY
Inflammatory myofibroblastic tumor (IMT) is a distinctive mesenchymal neoplasm characterized by a spindle-cell proliferation with an inflammatory infiltrate. Approximately half of IMTs carry rearrangements of the anaplastic lymphoma kinase (ALK) locus on chromosome 2p23, causing aberrant ALK expression. We report a sustained partial response to the ALK inhibitor crizotinib (PF-02341066, Pfizer) in a patient with ALK-translocated IMT, as compared with no observed activity in another patient without the ALK translocation. These results support the dependence of ALK-rearranged tumors on ALK-mediated signaling and suggest a therapeutic strategy for genomically identified patients with the aggressive form of this soft-tissue tumor.
Inflammatory myofibroblastic tumors (IMTS) occur primarily during the first two decades of life and typically arise in the lung, retroperitoneum, or abdominopelvic region.1,2 Abdominal tumors may be multifocal. Lesional cells are predominantly myofibroblasts in a myxoid to collagenous stroma admixed with inflammatory cells.2,3 Local recurrence may occur after initial surgery, with a low risk of distant metastases,1,2 so that IMTs are considered to be soft-tissue tumors of intermediate biologic potential, with a small fraction behaving aggressively.4
Rearrangements involving the ALK locus on chromosome 2p23 have been documented in approximately 50% of IMTs.5,6 ALK aneuploidy has also been described, with a gain in copy number without rearrangement.5 Among cancers with rearrangements, several fusion partners have been identified that serve to constitutively activate ALK.7–10 ALK expression reliably correlates with ALK rearrangement.11 Distant metastases occur primarily in ALK-negative IMTs, but local recurrence occurs regardless of ALK expression.5,12
Several ALK fusion proteins, including TPM3-ALK found in IMT, induce transformation in cell lines and animal models,13 a finding that suggests that ALK rearrangement may define a subgroup of IMTs that is sensitive to targeted kinase inhibition. We therefore enrolled two patients with IMT in a dose-escalation phase 1 trial of crizotinib, an orally bioavailable ATP-competitive inhibitor of the ALK and MET tyrosine kinases.14,15
CASE REPORTS
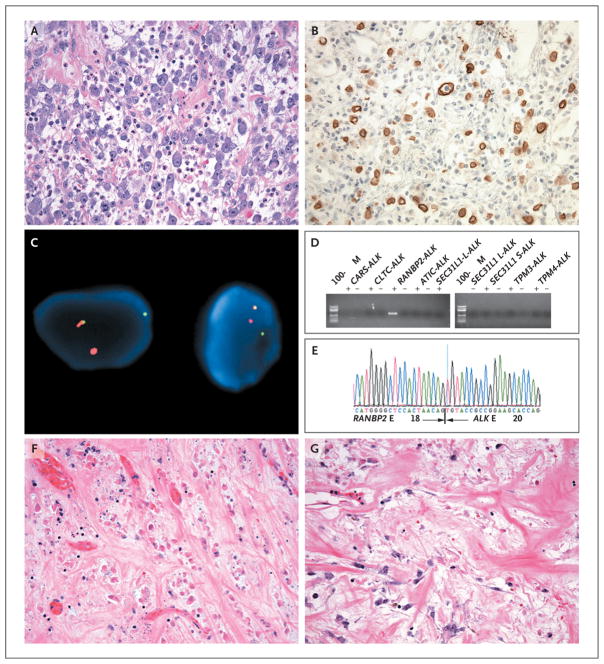
Patient 1 was a 44-year-old man who had been well until May 2007, when he reported having early satiety and abdominal pain. Computed tomography (CT) of the abdomen and pelvis revealed ascites, a mass in the right upper quadrant, and omental caking. The results of esophagogastroduodenoscopy and colonoscopy were unremarkable. The patient then underwent paracentesis. Combined 18F-fluorodeoxyglucose positron-emission tomography and CT (FDG-PET–CT) revealed hypermetabolic masses in the abdomen and pelvis. In June 2007, he underwent exploratory laparotomy, which showed massive omental caking with discrete, round, gelatinous, grape-size tumor nodules and extensive peritoneal disease. Maximal tumor debulking was performed along with catheter placement to facilitate administration of a hyperthermic peritoneal perfusion of cisplatin, doxorubicin, and mitomycin C. The tumor was composed of predominantly epithelioid cells with large vesicular nuclei, prominent nucleoli, and amphophilic cytoplasm, embedded in a myxoid stroma with prominent neutrophils (Fig. 1A). On immunohistochemical analysis, tumor cells were positive for desmin, a finding that is consistent with a myofibroblastic origin, and ALK (Fig. 1B) and were negative for SMA, cytokeratin, and myogenin. Fluorescence in situ hybridization (FISH) with the use of break-apart probes showed ALK rearrangement (Fig. 1C). The nuclear membrane pattern of ALK staining suggested the RANBP2 fusion partner, which encodes a nuclear pore protein.16 This rearrangement was confirmed by reverse-transcriptase–polymerase-chain-reaction (RT-PCR) and sequencing assays (Fig. 1D and 1E).
Figure 1. Histologic, Immunohistochemical, and Molecular Analyses of IMT Samples from Patient 1.
A sample of the inflammatory myofibroblastic tumor (IMT) obtained on biopsy shows epithelioid cells containing vesicular nuclei, prominent nucleoli, and amphophilic cytoplasm embedded in a myxoid stroma containing prominent neutrophils (Panel A, hematoxylin and eosin). Immunohistochemical analysis for ALK shows positive staining in tumor cells, with a nuclear membrane pattern (Panel B). Dual-color fluorescence in situ hybridization (FISH) shows rearrangement of centromeric (green) and telomeric (orange) probes flanking the ALK locus at 2p23 (Panel C). Gel electrophoresis of polymerase-chain-reaction (PCR) products after reverse-transcriptase PCR (RT-PCR) is shown for primers directed at known ALK translocation partners in IMT, including CARS, CLTC, RANBP2, ATIC, SEC31L1 (which generates both long-form [L] and short-form [S] fusion transcripts), TPM3, and TPM44,9 (Panel D). Only RANBP2-ALK primers produce an amplification product in the presence of (but not in the absence of) reverse transcriptase. Sequencing of the PCR product confirmed that RANBP2 exon 18 is fused in frame with ALK exon 20 (Panel E). Sections from progressing tumor masses in the liver (Panel F) and perirectal region (Panel G), which were resected after approximately 8 months of crizotinib administration, show histologic heterogeneity, with cellular areas similar in appearance to the initial biopsy sample but also revealing extensive areas suggestive of a treatment effect, with foci of tumor-cell necrosis in the liver sample and marked stromal hyalinization in the perirectal sample (hematoxylin and eosin in Panels F and G). Methods for immunostaining, FISH, and RT-PCR are described in the Supplementary Appendix, available with the full text of this article at NEJM.org.
The patient received doxorubicin and ifosfamide from August through November 2007, followed by maintenance therapy with imatinib until February 2008, when follow-up CT revealed asymptomatic, multifocal, recurrent peritoneal nodules. After meeting eligibility criteria and providing written informed consent, the patient began receiving crizotinib on March 25, 2008, at a dose of 200 mg twice daily. On May 21, 2008, and June 19, 2008, CT scanning showed reductions of 40% and 53%, respectively, in the sum of unidimensional measurements of target lesions, which was classified as a partial response, according to the Response Evaluation Criteria in Solid Tumors (RECIST)17 (Fig. 2). The maximal response was achieved in October 2008. At that time, despite the continued partial response of multiple mesenteric and peritoneal lesions, growth of three lesions (hepatic, peripancreatic, and perirectal masses) was noted.
Figure 2. CT Scans Showing the Response to Crizotinib in Patient 1.
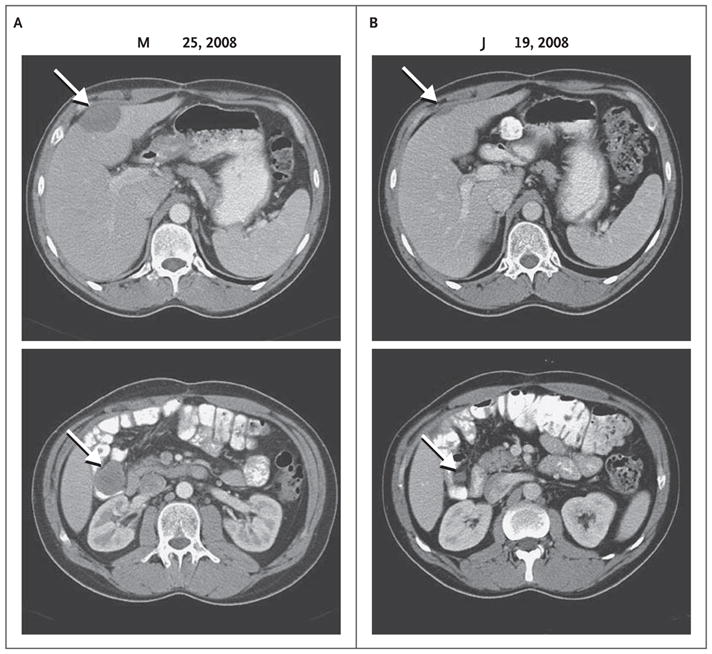
The baseline abdominal CT scan shows a hepatic mass measuring 4.8 by 3.3 cm (top) and one of several mesenteric masses measuring 3.8 by 3.3 cm (bottom) (Panel A, arrows). After 13 weeks of treatment with crizotinib, the hepatic and mesenteric masses measured 2.3 by 0.8 cm and 1.3 by 1.2 cm, respectively (Panel B, arrows). In October 2008, these masses measured 3.6 by 2.2 cm and 0.5 by 0.5 cm, respectively (not shown), indicating that the hepatic mass had regrown, despite a continued response, according to the Response Evaluation Criteria in Solid Tumors.
In December 2008, further growth of these masses occurred, and the patient subsequently underwent exploratory laparotomy to resect the growing lesions with maximal debulking (Fig. 1F and G). After he had recovered from the operation and with approval from the sponsor and the institutional review board, treatment with crizotinib was restarted at a dose of 250 mg twice daily, which had been defined as the maximum tolerated dose.15 As of September 2010, he remained in complete radiographic remission. While receiving crizotinib, the patient has had edema in the legs and feet, intermittent joint aches, hypocalcemia, hypophosphatemia, leukopenia, and anemia, all of grade 1 severity, according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf). He has not reported fatigue (the known dose-limiting toxic effect) and has not had aminotransferase elevations, a finding that has complicated the treatment course in a small percentage of patients.15
Patient 2 was a 21-year-old man who presented with vomiting and new-onset jaundice in November 2007. CT scanning revealed a calcified mass near the greater curvature of the stomach in the region of the pancreatic head. Endoscopic retrograde cholangiopancreatography was performed, and samples that were obtained on biopsy were not diagnostic. In December 2007, increasing abdominal pain led to exploratory laparotomy for debulking, partial gastrectomy, partial right colectomy, cholecystectomy, and splenectomy and placement of internal and external biliary draining devices.
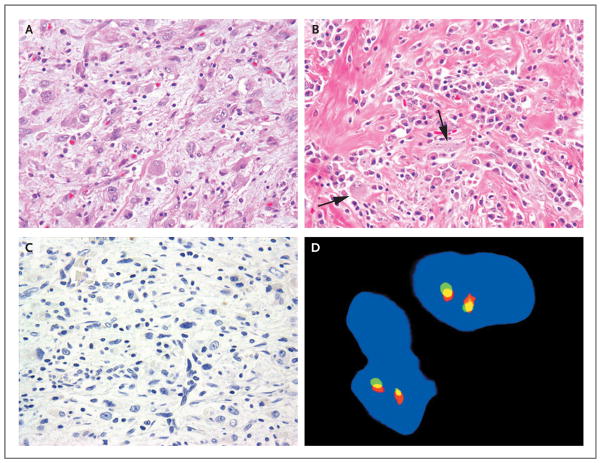
The tumor showed a multinodular growth pattern involving the gastric and colonic wall. Morphologically, the tumor had a heterogeneous appearance, ranging from hypercellular areas arranged in intersecting fascicles and associated with an abundant lymphoplasmacytic inflammatory infiltrate (Fig. 3A and 3B) to hypocellular and sclerotic regions with dystrophic calcification. The tumor cells ranged from slender, fusiform cells with fibrillary cytoplasm and open chromatin, reminiscent of myofibroblasts, to epithelioid cells with abundant eosinophilic cytoplasm and macronucleoli, resembling ganglion cells. A diagnosis of IMT was made on the basis of the histologic analysis. The tumor cells were focally positive for muscle-specific actin (HHF35), with a characteristic tram-track pattern of staining, a finding that is consistent with myofibroblastic differentiation. All other markers were negative, including CD117, CD34, desmin, SMA, AE1:AE3, S-100, CD21, CD35, clusterin, and CD68. The cellular morphologic features, prominent inflammatory infiltrate, and negativity for these markers ruled out entities that mimic IMT, such as spindle-cell melanoma, sarcomatoid carcinoma, gastrointestinal stromal tumor, follicular or interdigitating dendritic-cell sarcoma, inflammatory leiomyosarcoma, and desmoid fibromatosis. ALK immunohistochemical analysis was also negative, and FISH studies did not reveal a rearranged ALK gene (Fig. 3C and 3D).
Figure 3. Histologic and Immunohistochemical Analyses of IMT Samples from Patient 2.
A sample of the inflammatory myofibroblastic tumor (IMT) obtained on biopsy shows ganglion-like, plump epithelioid cells with abundant eosinophilic cytoplasm and nuclei with open chromatic and prominent nucleoli; scattered small lymphocytes are in the background (Panel A, hematoxylin and eosin). A different area of the tumor shows a prominent inflammatory component that is composed primarily of plasma cells, dense fibrous stroma, and scattered plump spindle and ganglion-like cells (Panel B, arrows; hematoxylin and eosin). Results of immunostaining for ALK are negative (Panel C), and FISH analysis shows no ALK rearrangement (Panel D).
After the patient had recovered from surgery, in February 2008, treatment with prednisone and ibuprofen was started.18 He had increasing abdominal pain, and progressive disease was documented on imaging. In July 2008, after meeting eligibility criteria and providing written informed consent, the patient began to receive crizotinib at a dose of 250 mg twice daily. In August 2008, crizotinib therapy was interrupted because of an increased level of total bilirubin. Repeat imaging showed worsening of abdominal disease, and the patient was removed from the trial.
DISCUSSION
Patient 1 had advanced IMT with ALK rearrangement and had only a short disease-free interval after initial surgery with aggressive treatment, consisting of hyperthermic peritoneal chemotherapy perfusion, followed by adjuvant systemic doxorubicin–ifosfamide and empirical imatinib. This patient’s disease showed a rapid and substantial partial response to crizotinib that lasted at least 6 months, despite an extensive tumor burden. This response suggests a primary role of aberrantly expressed and constitutively activated ALK in the growth and maintenance of the patient’s tumor. Consistent with this biologic hypothesis is the finding that the tumor in Patient 2, which lacked ALK rearrangement, had no response to crizotinib. Together, these cases support the dependence on ALK-mediated signal transduction in a subgroup of IMTs.
The use of targeted inhibitors to disrupt mutant signaling pathways that cancers require for continued growth has resulted in substantial progress in cancer treatment. Other examples include imatinib inhibition of BCR-ABL in chronic myeloid leukemia19 and of KIT in gastrointestinal stromal tumors20 and the inhibition of epidermal growth factor receptor (EGFR) by tyrosine kinase inhibitors (gefitinib or erlotinib) in non–small-cell lung cancers with activating EGFR mutations.21 Crizotinib has also shown striking clinical activity in non–small-cell lung cancers with EML4-ALK rearrangements.22,23
Several histologic patterns of IMT have been described, including those with compact spindle-cell, myxoid–vascular, and hypocellular fibrous morphologic features.2 However, it is not possible to predict which tumors will have ALK rearrangement or ALK aneuploidy. Rearrangement correlates well with ALK expression and kinase domain activation, both of which probably predict ALK dependence and crizotinib sensitivity. ALK immunoreactivity has not been reliably shown in cases of aneuploidy,5 and the dependence on ALK signaling or propensity to respond to targeted treatment in this subgroup of tumors is unknown.
Although there have been no clear associations among ALK expression, histologic analysis, and disease recurrence, tumors that have RANBP2-ALK fusion, including samples obtained from Patient 1, have common morphologic features and biologic behavior.16,24 Histologically, such tumors are dominated by epithelioid or round cells and have prominent myxoid stroma and a neutrophilic inflammatory infiltrate. In addition, nearly all patients with such tumors have presented with intraabdominal disease, many with multifocal tumors. These patients have had a locally aggressive clinical course, with early recurrence after initial surgery. Although not all cases of IMT with round-cell transformation express RANBP2-ALK, it is possible that specific fusion partners influence both histologic features and prognosis. Crizotinib therapy along with surgery may be useful in cases that are complicated by local recurrences. Similarly, unresectable IMTs may respond to crizotinib, facilitating their complete surgical removal.
Despite the impressive rates of tumor regression and control achieved with the use of targeted tyrosine kinase inhibitors in diseases such as EGFR-mutant non–small-cell lung cancer or KIT-mutant gastrointestinal stromal tumors, resistance develops in most patients within 1 to 2 years. Resistance results from the acquisition or selection of secondary mutations in the targeted kinase, which serve to inhibit drug binding through steric effects or increase affinity for ATP, or involves the activation of alternative tyrosine kinases that the cancer requires.25 Although Patient 1 did not have objective disease progression according to RECIST after 8 months of receiving crizotinib, three masses were growing at that time and were resected. These tumors are under study to determine the mechanism of resistance to crizotinib. Various sites of solid- tumor metastases may behave heterogeneously, and several other IMT lesions were continuing to respond at the time of surgery. We therefore elected to continue crizotinib therapy after surgical tumor resection, and an additional 19 months have passed without evidence of recurrence.
Although the role of crizotinib in this prolonged disease-free interval cannot be conclusively established, our observations suggest a long-term response of certain tumor-cell populations. In addition, Patient 1 continues to have an excellent performance status and only mild side effects, supporting the tolerability of the long-term administration of crizotinib.
Supplementary Material
Acknowledgments
Funded by Pfizer and others; ClinicalTrials.gov number, NCT00585195.
Supported by Pfizer and by grants from the National Institutes of Health (R01-CA136851, to Dr. Jänne; and P01-CA47179 and RC2-CA148260, to Dr. Maki), a Clinical Investigator Team Leadership Supplemental Award from the National Cancer Institute–American Society of Clinical Oncology Cancer Foundation (to Dr. Maki), and a grant from Cycle for Survival (to Dr. Maki).
We thank Paul Meyers, M.D., Monica Bertagnolli, M.D., Andrew Wolanski, N.P., Kristen Johnson, R.N., and Linda Ahn, N.P., who participated in the care of the patients described in this report; and Gina-Lee Emory, Dongpo Cai, Lindsey Burgess, and Trina Watson for their assistance with the clinical trial.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Meis JM, Enzinger FM. Inflammatory fibrosarcoma of the mesentery and retro-peritoneum: a tumor closely simulating inflammatory pseudotumor. Am J Surg Pathol. 1991;15:1146–56. doi: 10.1097/00000478-199112000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor): a clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859–72. doi: 10.1097/00000478-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Pettinato G, Manivel JC, De Rosa N, Dehner LP. Inflammatory myofibroblastic tumor (plasma cell granuloma): clinicopathologic study of 20 cases with immunohistochemical and ultrastructural observations. Am J Clin Pathol. 1990;94:538–46. doi: 10.1093/ajcp/94.5.538. [DOI] [PubMed] [Google Scholar]
- 4.Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol. 2008;61:428–37. doi: 10.1136/jcp.2007.049387. [DOI] [PubMed] [Google Scholar]
- 5.Coffin CM, Patel A, Perkins S, Elenitoba-Johnson KS, Perlman E, Griffin CA. ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol. 2001;14:569–76. doi: 10.1038/modpathol.3880352. [DOI] [PubMed] [Google Scholar]
- 6.Griffin CA, Hawkins AL, Dvorak C, Henkle C, Ellingham T, Perlman EJ. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res. 1999;59:2776–80. [PubMed] [Google Scholar]
- 7.Lawrence B, Perez-Atayde A, Hibbard MK, et al. TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am J Pathol. 2000;157:377–84. doi: 10.1016/S0002-9440(10)64550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ladanyi M. Aberrant ALK tyrosine kinase signaling: different cellular lineages, common oncogenic mechanisms. Am J Pathol. 2000;157:341–5. doi: 10.1016/S0002-9440(10)64545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webb TR, Slavish J, George RE, et al. Anaplastic lymphoma kinase: role in cancer pathogenesis and small-molecule inhibitor development for therapy. Expert Rev Anticancer Ther. 2009;9:331–56. doi: 10.1586/14737140.9.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmer RH, Vernersson E, Grabbe C, Hallberg B. Anaplastic lymphoma kinase: signalling in development and disease. Biochem J. 2009;420:345–61. doi: 10.1042/BJ20090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook JR, Dehner LP, Collins MH, et al. Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor: a comparative immunohistochemical study. Am J Surg Pathol. 2001;25:1364–71. doi: 10.1097/00000478-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007;31:509–20. doi: 10.1097/01.pas.0000213393.57322.c7. [DOI] [PubMed] [Google Scholar]
- 13.Giuriato S, Faumont N, Bousquet E, et al. Development of a conditional bioluminescent transplant model for TPM3-ALK-induced tumorigenesis as a tool to validate ALK-dependent cancer targeted therapy. Cancer Biol Ther. 2007;6:1318–23. doi: 10.4161/cbt.6.8.4508. [DOI] [PubMed] [Google Scholar]
- 14.Christensen JG, Zou HY, Arango ME, et al. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer Ther. 2007;6:3314–22. doi: 10.1158/1535-7163.MCT-07-0365. [DOI] [PubMed] [Google Scholar]
- 15.Kwak EL, Camidge DR, Clark J, et al. Clinical activity observed in a phase I dose escalation trial of an oral c-Met and ALK inhibitor, PF-02341066. J Clin Oncol. 2009;27(Suppl):148s. [Google Scholar]
- 16.Ma Z, Hill DA, Collins MH, et al. Fusion of ALK to the Ran-binding protein 2 (RANBP2) gene in inflammatory myofibroblastic tumor. Genes Chromosomes Cancer. 2003;37:98–105. doi: 10.1002/gcc.10177. [DOI] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Berger A, Kim C, Hagstrom N, Ferrer F. Successful preoperative treatment of pediatric bladder inflammatory myofibroblastic tumor with anti-inflammatory therapy. Urology. 2007;70(2):372.e13–372.e15. doi: 10.1016/j.urology.2007.04.047. [DOI] [PubMed] [Google Scholar]
- 19.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–17. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 20.Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342–9. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 21.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 22.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 23.Kwak EL, Bang Y-J, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non–small-cell lung cancer. N Engl J Med. 2010;363:1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hornick JL, Wang WL, Roy A, et al. Round cell inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK: an aggressive intra-abdominal variant. Mod Pathol. 2010;23(Suppl):21A. doi: 10.1097/PAS.0b013e318200cfd5. [DOI] [PubMed] [Google Scholar]
- 25.Engelman JA, Jänne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008;14:2895–9. doi: 10.1158/1078-0432.CCR-07-2248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.