Abstract
A fundamental role for protein-protein interactions in the organization of signal transduction pathways is evident. Anchoring, scaffolding and adapter proteins function to enhance the precision and directionality of these signaling events by bringing enzymes together. The cAMP signaling pathway is organized by A-kinase Anchoring Proteins (AKAPs). This family of proteins assembles enzyme complexes containing the cAMP dependent protein kinase (PKA), phosphoprotein phosphatases, phosphodiesterases (PDEs) and other signaling effectors to optimize cellular responses to cAMP and other second messengers. Selected AKAP signaling complexes will be highlighted in this review.
Keywords: cAMP, AKAP, enzyme complexes, signal transduction
Introduction
Knowing how signal transduction cascades are effectively organized inside cells is key to understanding how cells communicate. Insight into how this is achieved has been forthcoming from research on anchoring and scaffolding proteins [1]. A number of protein kinases with broad substrate specificities associate with proteins that target them to precise sites inside the cell. Signaling events that are initiated by the second messenger cAMP involve the activation of discrete pools of anchored Protein Kinase A (PKA) [1]. The tetrameric PKA holoenzyme is composed of two regulatory R subunits and two catalytic C subunits. Multiple genes encode the PKA subunits. Accordingly, the differential expression of the RIα, RIβ, RIIα, RIIβ, Cα and Cβ genes can generate a range of holoenzyme combinations with slightly different physiochemical properties [2]. PKA type II holoenzymes (RIIα2C2, RIIβ2C2) turn on with an activation constant (Kact) of 200–400 nM cAMP whereas PKA type I holoenzymes (RIα2C2, RIβ2C2) are triggered with lower concentrations of the second messenger (50–100 nM [3]). One clear distinction between these two isoenzymes is their preference for interaction with A-kinase Anchoring Proteins (AKAPs) [4]. A majority of AKAPs associate with PKA type II, however dual specificity AKAPs have been identified [5]. Much less is known about PKA type I-selective anchoring proteins. PKA type II, hereafter referred to as simply PKA, binds via an RII dimer interacting with a 14–18 residue amphipathic helix within the AKAP [6]. Crystallographic analysis of this complex revealed that this interaction requires the formation of a groove on one face of a four-helix bundle formed between RII protomers [7,8]. Biochemical characterization of this complex has led to the generation of several valuable tools for determining the biological significance of these complexes. These include membrane permeant peptides that bind RII with high affinity and therefore can be used to disrupt AKAP/PKA interactions inside cells [9,10]. This review will focus on some of the recent work elucidating the functions of selected AKAPs. Three anchoring proteins (AKAP150, mAKAP and AKAP-Lbc) and their interacting partners will be discussed in detail (Table 1 and acronyms and abreviations section).
Table 1.
| AKAP79/150 | mAKAP | AKAP-Lbc | |
|---|---|---|---|
| Interaction partners: signaling proteins, receptors and ion channels | PKA, PKC, PP2B, MAGUKs (SAP97, PSD- 95), AC5, AMPA receptor, NMDA receptor, KCNQ2 channel, M1 muscarinic receptor, β-adrenergic receptor, L-type calcium channel, aquaporin channel | PKA, PDE4D3, Epac1, ERK5, HIF-1α, Siah2, PHD, pVHL | PKA, PKC, PKD, Rho, 14-3-3 |
| Subcellular targeting | Membranes | Perinuclear membrane | Cytosol |
AKAP79/150 signaling complexes
To date, AKAP150 (the murine homolog of human AKAP79) remains the best-understood anchoring protein. In hippocampal neurons AKAP150 positions PKA, PP2B and PKC at membranes proximal to AMPA type glutamate receptors through it’s binding with SAP97 [11–13]. This complex permits the robust phosphorylation of AMPA receptors by PKA at key residues that enhance the flow of ions through the channel [11–13]. This effect is counter balanced by AKAP150-targeting of the calcium/calmodulin dependent protein phosphatase PP2B [14]. In the absence of PKA binding, PP2B dephosphorylates these ligand gated ion-channels resulting in decreased conductance [14]. The anchored PKC is inactive in this complex. However, AKAP150-anchored PKC plays an important role in another context. In superior cervical ganglion (SCG) neurons, AKAP150 coordinates suppression of current through M-type channels in response to muscarinic receptors [15–17]. M channels allow the passage of potassium ions through the plasma membrane, and suppression of the current results in enhanced neuronal excitability. AKAP150 modulates the M channel by positioning PKC close to critical residues necessary for the passage of ions through the channel and silencing of AKAP150 reduces the M-current suppression by muscarinic agonists. The anchored PKA and PP2B remain inactive in this context [15–17]. The importance of AKAP150-coordinated signaling inside neurons is supported by evidence that mice lacking AKAP150 exhibit deficiencies in muscarinic suppression of M currents, motor coordination, memory retention, and resistance to pilocarpine-induced seizures [18].
AKAP150 has also been identified in association with the L-type calcium channel subunit Cav1.2 in the brain where a complex that includes β2-adrenergic receptor (β2- AR), Cav1.2, G-proteins, adenylyl cyclase (AC), PKA and PP2A plays an essential role in the modulation of Ca2+ signaling downstream of β2-AR stimulation [19,20]. Here the AKAP150-associated PKA is believed to phosphorylate Ser 1928 on the central pore forming subunit Cav1.2 in response to beta-adrenergic stimulation and disruption of AKAP150 prevents this activation step [21]. Likewise in the heart, PKA anchoring to a similar complex plays an essential role in increasing cardiac rate and output in response to β2-AR stimulation. This physiological response requires modulation of L-type calcium channels and Ser 1928 on cardiac α1 subunits has also been identified as the key PKA phosphorylation site [22]. Interestingly in another cellular context, AKAP150-mediated targeting of the kinase PKC to L-type calcium channels in arterial myocytes is necessary for stuttering persistent calcium sparklets and the regulation of myogenic tone and blood pressure [23,24]. Stuttering persistent calcium sparklets produced by the long openings and reopenings of L-type Ca2+ channels lead to increased calcium influx and vascular tone, and are regulated through the AKAP150-anchored PKC. Collectively these studies highlight the role that cellular context and the differential assembly of specific AKAP150-enzyme complexes plays in influencing the diversity of AKAP signaling events.
The mAKAP complex
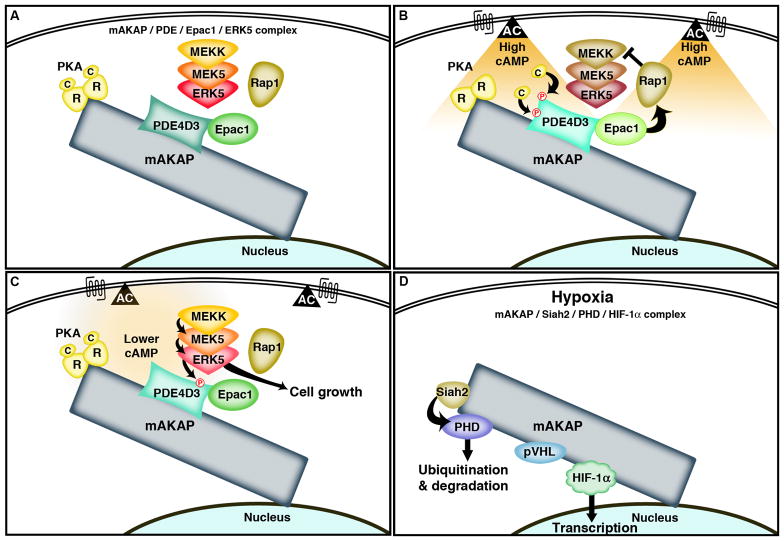
In the heart, the muscle selective anchoring protein mAKAP organizes different combinations of proteins to control diverse aspects of cardiomyocyte physiology that occur close to the nuclear membrane. Although initially described as an anchoring protein for PKA, mAKAP also interacts with the phosphodiesterase PDE4D3, the guanine nucleotide exchange factor Epac1 and the protein kinase ERK5 [25,26]. This provides a locus for the control of cAMP and mitogenic signaling events (Figure 1A-1C). As local cAMP levels increase the mAKAP-associated PKA is activated to phosphorylate PDE4D3 to enhance cAMP metabolism [27]. This mAKAP-PKA-PDE configuration forms a classic enzyme feedback loop as anchored PKA activity eventually leads to the termination of cAMP signals. Interestingly, the same AKAP complex contributes to cAMP mediated regulation of an anchored ERK5 mitogenic signaling pathway. This is achieved through mobilization of an mAKAP-associated pool of cAMP-dependent Epac1, which activates the small G-protein Rap1. Active Rap1 can in turn repress the ERK5 activity associated with the mAKAP-signaling network [26].
Fig. 1. mAKAP signaling complexes.
A) mAKAP assembles a cAMP-responsive complex of signaling enzymes at the perinuclear membrane in the heart. PKA, PDE4D3 Epac1 and ERK5 are brought together with other associated enzymes to control different aspects of cardiomyocyte physiology. B) When intracellular cAMP levels are elevated the mAKAP-associated PKA phosphorylates the PDE4D3 in the complex at two sites, leading to increased metabolism of cAMP by the phosphodiesterase. Likewise, cAMP activation of Epac1 in the complex activates Rap1 to inhibit ERK5 signaling. C) As cAMP levels fall, the Epac1-mediated inhibition of ERK signaling is lost and mitogenic signaling favors cell growth. D) mAKAP assembles an oxygen sensitive signaling pathway that includes the ubiquitin E3 ligase Siah2, prolyl hydroxylase, von Hippel-Lindau protein and the transcription factor HIF-1α. Under normoxic conditions, HIF-1α is continually degraded however, when oxygen levels fall, the mAKAP associated PHD is degraded and HIF-1α accumulates and translocates into the nucleus.
So why are so many enzymes brought together by mAKAP at the same point in the cell. One explanation is that these multienzyme complexes create a situation where subtle changes in the concentration of cAMP can have profound effects on the cellular processes that are active. As cAMP levels rise, anchored PKA works to deplete the second messenger by activating a local pool of PDE4D (Figure 1B). Yet when cAMP levels fall, Epac1 mediated inhibition of the ERK5 cascade is lost (Figure 1C). The concomitant de-repression of ERK5 turns on mitogenic signals that favor cell growth (Figure 1C). Thus these mAKAP complexes exemplify how distinct enzyme cascades constrained within the same macromolecular complex can respond and contribute to the ebb and flow of cAMP.
Recently, it has been discovered that mAKAP organizes additional and diverse signaling proteins [28]. This includes enzymes that coordinate the oxygen-dependent control of the transcription factor HIF-1α (Figure 1D). Under normoxic conditions, HIF-1α protein levels are kept low by the action of prolyl hydroxylases (PHD’s), a family of oxygen-sensitive dioxygenases [28]. Hydroxylated proline residues in HIF-1α constitute a binding site for the von Hippel-Lindau protein (pVHL), which is part of a multiprotein complex that ubiquitinates HIF-1α resulting in degradation by the proteasome. Under hypoxic conditions, HIF-1α protein levels rise as a result of two factors: 1) The enzymatic activity of the PHDs is reduced in the absence of oxygen and 2), the ubiquitin E3 ligase, seven in absentia homolog 2 (Siah2) ubiquitinates selected PHDs. Together these processes terminate the destruction of HIF-1α. The consequence of bringing these enzymes in proximity to their substrates was illustrated in cells lacking mAKAP. Gene silencing of mAKAP blunted hypoxia induced HIF-1α-dependent gene transcription [28]. Delocalizing mAKAP from perinuclear membranes using a peptide corresponding to the perinuclear targeting domain of mAKAP reduced movement of HIF-1α into the nucleus and HIF-1α-dependent gene transcription [28]. Thus, mAKAP participates in response to oxygen tension by facilitating the proteasomal degradation or stabilization of the transcription factor HIF-1α.
AKAP-Lbc signaling complex
AKAP-Lbc is another multivalent anchoring protein that organizes PKA and PKC in a manner that favors activation of protein kinase D (PKD) [29,30]. An added feature of AKAP-Lbc is that it functions as a guanine nucleotide exchange factor (GEF) for Rho, a small GTP binding protein, thereby creating a point of convergence between the cAMP and Rho signaling pathways [31]. This anchored signaling complex interfaces with the cytoskeleton as AKAP-Lbc has the capacity to remodel actin upon activation of Rho [32,33]. Termination of AKAP-Lbc’s Rho GEF activity involves homo-oligomerization of the anchoring protein and PKA mediated recruitment of 14-3-3 [34].
In the heart, chronic activation of PKD is associated with hypertrophy. In support of this notion AKAP-Lbc expression is increased approximately 50% in hypertrophic cardiomyocytes, [36]. Reciprocal experiments demonstrated that cardiomyocytes lacking AKAP-Lbc are resistant to phenylephrine induced hypertrophy [36]. Several lines of inquiry have implicated AKAP-Lbc as a co-factor in the mobilization of the fetal gene response that is emblematic of pathological cardiomyocyte hypertrophy [35]. A key event in this process is the PKD phosphorylation and subsequent nuclear export of class II histone deacetylases (HDACs) [36]. Using a combination of live cell imaging and gene silencing approaches it was shown that depletion of AKAP-Lbc suppressed the nuclear export of HDAC5 and repressed transcription of the ANF gene, a marker for pathological cardiac hypertrophy [35]. These data provided some of the initial evidence that altered expression of AKAPs can influence the control of pathophysiological processes.
Perspectives
Considering the spatial and temporal distribution of intracellular signaling molecules is now recognized as an important determinant in the control of cell signaling. A defining characteristic of the AKAP family is the ability to shape the local environment through scaffolding both effectors and signal terminating enzymes. This article has highlighted the advantage of AKAP signaling complexes in the organization of responses to second messengers. The examples we have used illustrate the utility of AKAPs as a family of co-factors that uphold the molecular organization of enzyme cascades and the fidelity of cell signaling events. Delineating these local environments will become increasingly more important to understanding these pathways. Advances in mass spectrometry and the development and utilization of FRET based reporters of kinase activity and second messengers inside living cells will greatly aid these efforts.
Acknowledgments
Thanks to Lorene K. Langeberg for editing the text of this manuscript. National Institutes of Health grant DK54441 and the Leducq Foundation Transatlantic Network support JDS.
Acronyms and Abreviations
- AKAPs
A-kinase anchoring proteins, a family of targeting proteins that optimally position PKA and other signaling enzymes in proximity to activating signals and substrates
- cAMP
The second messenger, cyclic adenosine-3′,5′-monophosphate is cyclic mononucleotide of adenosine that is synthesized from ATP by adenylyl cyclase in response to stimulation of G-protein coupled receptors. cAMP is responsible for the intracellular mediation of various cellular processes
- PKA
The cAMP-dependent protein kinase A, which phosphorylates substrates in response to elevation of cAMP levels
- R subunit
The regulatory subunit of PKA. The type II regulatory subunit exists as a dimer, thus creating a surface for interaction with AKAPs. When cAMP levels are low each R subunit can bind one inactive C subunit
- C subunit
The catalytic subunit of PKA. When cAMP levels rise and bind to R subunits the C subunits are released and are then catalytically active to phosphorylate substrates
- PDE
PDE4D3, cyclic nucleotide phophodiesterase and the 4D3 isoform of phosphodiesterase. This enzyme metabolizes cAMP, reducing the amplitude of cAMP-dependent signaling
- PKC
Protein kinase C phosphorylates substrates in response to increases in Ca2+, diacylglycerol and phospholipid
- PKD
Protein kinase D is another important signaling enzyme that phosphorylates its substrates downstream of other kinases. Phospho-PKD can be translocated to the nucleus
- PP2B
Protein phosphatase 2B, also known as calcineurin and protein phosphatase 3, is a calcium/calmodulin dependent phosphatase that dephosphorylates proteins in regulation of signaling pathways
- SAP97
Synapse-associated protein 97 is a MAGUK-family protein that has a multiple domain structure with various interaction partners and is involved with ion channel clustering at the synapse
- AC
Adenylyl Cyclase catalyzes the conversion of ATP to the second messenger cAMP when the enzyme is activated via G proteins at the plasma membrane
- Epac1
Exchange protein directly activated by cAMP is a guanine nucleotide exchange factor that activates Rap1
- ERK5
Extracellular signal regulated kinase 5 also known as MAPK7. This kinase is a member of the MAP kinase family, is activated by MEK5, and plays a role in cardiovascular development
- Rap1
A small GTP binding protein that has been shown to be a substrate of Epac1
- HIF-1α
Hypoxia-inducible factor 1α is a transcription factor that responds to low oxygen
- PHD
Prolyl hydroxylase is an enzyme that contributes to the regulation of HIF-1α via oxygen-dependent hydroxylation of proline residues
- E3
A ubiquitin ligase that in combination with E2 attaches ubiquitin to a lysine residue on a target protein. This process of ubiquitination is important for various cellular processes including degradation and interaction with other proteins
- Rho
A family of small GTPases that have been shown to regulate cytoskeletal dynamics
- 14-3-3
A family of conserved regulatory proteins that can bind many diverse signaling proteins
- HDAC5
Histone deacetylase 5 is a transcriptional regulator that interacts with the myocyte enhancer factor 2 (MEF2), a transcription factor that regulates embryonic muscle development
- ANF
Atrial natriuretic factor (peptide) is released by cardiac myocytes in response to stress, stretching or injury. This is a marker for cardiac pathophysiology
- FRET
Forster (or fluorescence) resonance energy transfer or FRET refers to the direct transfer of energy from a donor fluorophore in its excited state to an acceptor fluorophore. This technique allows detection of protein-protein interactions that are less than 50 angstroms apart
References
- 1.Scott JD, Pawson T. Cell signaling in space and time: where proteins come together and when they’re apart. Science. 2009;326:1220–4. doi: 10.1126/science.1175668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skalhegg BS, Tasken K. Specificity in the cAMP/PKA signaling pathway. Differential expression, regulation, and subcellular localization of subunits of PKA. Front Biosci. 2000;5:D678–93. doi: 10.2741/skalhegg. [DOI] [PubMed] [Google Scholar]
- 3.Dostmann WR, Taylor SS. Identifying the molecular switches that determine whether (Rp)-cAMPS functions as an antagonist or an agonist in the activation of cAMP-dependent protein kinase I. Biochemistry. 1991;30:8710–6. doi: 10.1021/bi00099a032. [DOI] [PubMed] [Google Scholar]
- 4.Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol. 2004;5:959–70. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, et al. Cloning and mitochondrial localization of full-length D-AKAP2, a protein kinase A anchoring protein. Proc Natl Acad Sci USA. 2001;98:3220–5. doi: 10.1073/pnas.051633398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr DW, Stofko-Hahn RE, Fraser ID, Bishop SM, Acott TS, Brennan RG, Scott JD. Interaction of the regulatory subunit (RII) of cAMP-dependent protein kinase with RII-anchoring proteins occurs through an amphipathic helix binding motif. J Biol Chem. 1991;266:14188–92. [PubMed] [Google Scholar]
- 7.Gold MG, et al. Molecular basis of AKAP specificity for PKA regulatory subunits. Mol Cell. 2006;24:383–95. doi: 10.1016/j.molcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Kinderman FS, et al. A dynamic mechanism for AKAP binding to RII isoforms of cAMP-dependent protein kinase. Mol Cell. 2006;24:397–408. doi: 10.1016/j.molcel.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alto NM, Soderling SH, Hoshi N, Langeberg LK, Fayos R, Jennings PA, Scott JD. Bioinformatic design of A-kinase anchoring protein-in silico: A potent and selective peptide antagonist of type II protein kinase A anchoring. Proc Natl Acad Sci USA. 2003;100:4445–50. doi: 10.1073/pnas.0330734100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlson CR, Lygren B, Berge T, Hoshi N, Wong W, Tasken K, Scott JD. Delineation of Type I Protein Kinase A-selective Signaling Events Using an RI Anchoring Disruptor. J Biol Chem. 2006;281:21535–45. doi: 10.1074/jbc.M603223200. [DOI] [PubMed] [Google Scholar]
- 11.Coghlan VM, Hausken ZE, Scott JD. Subcellular targeting of kinases and phosphatases by association with bifunctional anchoring proteins. Biochem Soc Trans. 1995;23:591–596. doi: 10.1042/bst0230592. [DOI] [PubMed] [Google Scholar]
- 12.Klauck TM, Faux MC, Labudda K, Langeberg LK, Jaken S, Scott JD. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science. 1996;271:1589–1592. doi: 10.1126/science.271.5255.1589. [DOI] [PubMed] [Google Scholar]
- 13.Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD. Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron. 2000;27:107–19. doi: 10.1016/s0896-6273(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 14.Tavalin SJ, Colledge M, Hell JW, Langeberg LK, Huganir RL, Scott JD. Regulation of GluR1 by the A-kinase anchoring protein 79 (AKAP79) signaling complex shares properties with long-term depression. J Neurosci. 2002;22:3044–51. doi: 10.1523/JNEUROSCI.22-08-03044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoshi N, Langeberg LK, Scott JD. Distinct enzyme combinations in AKAP signalling complexes permit functional diversity. Nat Cell Biol. 2005;7:1066–73. doi: 10.1038/ncb1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoshi N, et al. AKAP150 signaling complex promotes suppression of the M-current by muscarinic agonists. Nat Neurosci. 2003;6:564–571. doi: 10.1038/nn1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoshi N, Langeberg LK, Gould CM, Newton AC, Scott JD. Interaction with AKAP79 modifies the cellular pharmacology of PKC. Mol Cell. 37:541–50. doi: 10.1016/j.molcel.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tunquist BJ, Hoshi N, Guire ES, Zhang F, Mullendorff K, Langeberg LK, Raber J, Scott JD. Loss of AKAP150 perturbs distinct neuronal processes in mice. Proc Natl Acad Sci U S A. 2008;105:12557–62. doi: 10.1073/pnas.0805922105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliveria SF, Dell’Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 2007;55:261–75. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davare MA, et al. A beta2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science. 2001;293:98–101. doi: 10.1126/science.293.5527.98. [DOI] [PubMed] [Google Scholar]
- 21.Gao T, Yatani A, Dell’Acqua ML, Sako H, Green SA, Dascal N, Scott JD, Hosey MM. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997;19:185–196. doi: 10.1016/s0896-6273(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 22.De Jongh KS, Murphy BJ, Colvin AA, Hell JW, Takahashi M, Catterall WA. Specific phosphorylation of a site in the full-length form of the alpha 1 subunit of the cardiac L-type calcium channel by adenosine 3′,5′-cyclic monophosphate-dependent protein kinase. Biochemistry. 1996;35:10392–402. doi: 10.1021/bi953023c. [DOI] [PubMed] [Google Scholar]
- 23.Navedo MF, Amberg GC, Votaw VS, Santana LF. Constitutively active L-type Ca2+ channels. Proc Natl Acad Sci U S A. 2005;102:11112–7. doi: 10.1073/pnas.0500360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navedo MF, Nieves-Cintron M, Amberg GC, Yuan C, Votaw VS, Lederer WJ, McKnight GS, Santana LF. AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension. Circ Res. 2008;102:e1–e11. doi: 10.1161/CIRCRESAHA.107.167809. [DOI] [PubMed] [Google Scholar]
- 25.Dodge-Kafka KL, Langeberg L, Scott JD. Compartmentation of cyclic nucleotide signaling in the heart: the role of A-kinase anchoring proteins. Circ Res. 2006;98:993–1001. doi: 10.1161/01.RES.0000218273.91741.30. [DOI] [PubMed] [Google Scholar]
- 26.Dodge-Kafka KL, Soughayer J, Pare GC, Carlisle Michel JJ, Langeberg LK, Kapiloff MS, Scott JD. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature. 2005;437:574–8. doi: 10.1038/nature03966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlisle Michel JJ, Dodge KL, Wong W, Mayer NC, Langeberg LK, Scott JD. PKA phosphorylation of PDE4D3 facilitates recruitment of the mAKAP signaling complex. Biochem J. 2004;381:587–92. doi: 10.1042/BJ20040846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong W, Goehring AS, Kapiloff MS, Langeberg LK, Scott JD. mAKAP compartmentalizes oxygen-dependent control of HIF-1alpha. Sci Signal. 2008;1:ra18. doi: 10.1126/scisignal.2000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diviani D. Modulation of cardiac function by A-kinase anchoring proteins. Curr Opin Pharmacol. 2007;8:166–173. doi: 10.1016/j.coph.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Carnegie GK, Smith FD, McConnachie G, Langeberg LK, Scott JD. AKAP-Lbc nucleates a protein kinase D activation scaffold. Mol Cell. 2004;15:889–99. doi: 10.1016/j.molcel.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Diviani D, Soderling J, Scott JD. AKAP-Lbc anchors protein kinase A and nucleates Galpha 12-selective Rho-mediated stress fiber formation. J Biol Chem. 2001;276:44247–57. doi: 10.1074/jbc.M106629200. [DOI] [PubMed] [Google Scholar]
- 32.Diviani D, Abuin L, Cotecchia S, Pansier L. Anchoring of both PKA and 14–3-3 inhibits the Rho-GEF activity of the AKAP-Lbc signaling complex. EMBO J. 2004;23:2811–20. doi: 10.1038/sj.emboj.7600287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin J, et al. Proteomic, functional, and domain-based analysis of in vivo 14–3-3 binding proteins involved in cytoskeletal regulation and cellular organization. Curr Biol. 2004;14:1436–50. doi: 10.1016/j.cub.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 34.Baisamy L, Jurisch N, Diviani D. Leucine zipper-mediated homo-oligomerization regulates the Rho-GEF activity of AKAP-Lbc. J Biol Chem. 2005;280:15405–12. doi: 10.1074/jbc.M414440200. [DOI] [PubMed] [Google Scholar]
- 35.Carnegie GK, et al. AKAP-Lbc mobilizes a cardiac hypertrophy signaling pathway. Mol Cell. 2008;32:169–79. doi: 10.1016/j.molcel.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vega RB, Harrison BC, Meadows E, Roberts CR, Papst PJ, Olson EN, McKinsey TA. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol. 2004;24:8374–85. doi: 10.1128/MCB.24.19.8374-8385.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]