Abstract
A significant link was previously established between benzodiazepine withdrawal anxiety and a progressive increase in α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) potentiation in hippocampal CA1 neurons from rats withdrawn up to 2 days from 1-week oral administration of the benzodiazepine flurazepam (FZP). Despite AMPAR current potentiation, withdrawal anxiety was masked by a 2-fold reduction in CA1 neuron N-methyl-d-aspartate receptor (NMDAR) currents since preinjection of an NMDA antagonist restored NMDAR currents and unmasked anxiety in 2-day FZP-withdrawn rats. In the current study, GluN subunit levels in postsynaptic density (PSD)-enriched subfractions of CA1 minislices were compared with GluN2B-mediated whole-cell currents evoked in CA1 neurons in hippocampal slices from 1- and 2-day FZP-withdrawn rats. GluN1 and GluN2B, although not the phosphoSer1303-GluN2B ratio or GluN2A subunit levels, were decreased in PSD subfractions from 2-day, but not 1-day, FZP-withdrawn rats. Consistent with immunoblot analyses, GluN2B-mediated NMDAR currents evoked in slices from 2-day FZP-withdrawn rats were decreased in the absence, but not the presence, of the GluN2B subunit-selective antagonist ifenprodil. In contrast, ifenprodil-sensitive NMDAR currents were unchanged in slices from 1-day withdrawn rats. Because AMPA (1 μM) preincubation of slices from 1-day FZP-withdrawn rats induced depression of GluN2B subunit-mediated currents, depression of NMDAR currents was probably secondary to AMPAR potentiation. CA1 neuron NMDAR currents were depressed ∼50% after 2-day withdrawal and offset potentiation of AMPAR-mediated currents, leaving total charge transfer unchanged between groups. Collectively, these findings suggest that a reduction of GluN2B-containing NMDAR may serve as a homeostatic feedback mechanism to modulate glutamatergic synaptic strength during FZP withdrawal to alleviate benzodiazepine withdrawal symptoms.
Introduction
The dynamic regulation of postsynaptic excitatory α-amino-3-hydroxy-5-methyl-4-isozaxolepropionic acid receptors (AMPARs) and N-methyl-d-aspartate receptors (NMDARs) plays a central role in both the stability and experiential modification of synaptic connections in numerous brain regions and contributes prominently to activity-dependent synaptic remodeling (Derkach et al., 2007). As with classic models of activity-dependent plasticity, glutamatergic synaptic plasticity is also associated with dependence on and addiction to a variety of drugs of abuse, including benzodiazepines (Kauer and Malenka, 2007; Tan et al., 2010).
Although benzodiazepines have their direct actions as selective modulators of GABA type A receptors (Wafford, 2005), interactions among inhibitory and excitatory networks in specific brain areas such as the hippocampus and ventral tegmental area provide a basis for the regulation of excitatory receptor systems central to behaviors associated with dependence and addiction (Van Sickle et al., 2004; Xiang and Tietz, 2007; Tan et al., 2010). Glutamate receptor regulation was implicated in mediating manifestations of physical dependence (Dunworth and Stephens, 1998), including anxiety observed in rodents after withdrawal from various benzodiazepines (Izzo et al., 2001; Van Sickle et al., 2004; Katsura et al., 2007; Xiang and Tietz, 2007). We reported previously that anxiety was observed in an elevated plus maze 1 day after withdrawal from 1-week oral administration of flurazepam (FZP) and was closely linked to AMPAR current potentiation in CA1 neurons (Van Sickle et al., 2004; Xiang and Tietz, 2007), a key waypoint in the neural anxiety circuit (McNaughton and Gray, 2000; Shen et al., 2007). Although AMPAR potentiation was further enhanced within 2 days, withdrawal anxiety was not observed when NMDAR currents were reduced by 50% in the same rats (Van Sickle et al., 2004). Systemic preinjection of an AMPA antagonist prevented anxiety expression in 1-day FZP-withdrawn rats, whereas preinjection of an NMDA antagonist alone prevented the reduction in NMDAR currents and “unmasked” anxiety after 2 days when AMPAR potentiation persisted (Xiang and Tietz, 2007).
We have previously shown that the molecular mechanisms associated with benzodiazepine-induced withdrawal anxiety involves membrane insertion of GluA1 homomeric receptors into CA1 neuron asymmetric synapses, increased AMPAR current amplitude followed by Ca2+/calmodulin-dependent protein kinase type II (CaMKII)-mediated Ser831 GluA1 phosphorylation, and enhanced AMPAR single-channel conductance (Song et al., 2007; Das et al., 2008; Shen et al., 2009, 2010), in common with AMPAR potentiation during activity-dependent plasticity (Derkach et al., 2007). However, Ca2+ influx in benzodiazepine-withdrawn rats was mediated via L-type voltage-gated calcium channels (Xiang et al., 2008) and CaMKII was not autophosphorylated (Shen et al., 2010). Thus, although we identified numerous mechanistic similarities, we identified some important differences between drug-induced glutamatergic plasticity and the postsynaptic signaling mechanisms associated with e.g., hippocampal long-term potentiation (LTP).
Distinct from LTP, but consistent with models of emotional learning such as fear conditioning (Zinebi et al., 2003), NMDAR function was reduced by half 2 days after FZP withdrawal and returned to basal levels within 4 days (Van Sickle et al., 2002, 2004). The signaling mechanisms underlying benzodiazepine-induced delayed down-regulation of NMDAR currents are unknown. NMDARs are linked to a group of scaffold and adaptor proteins associated with various kinases and phosphatases to form a large macromolecular signaling complex (Lau and Zukin 2007). Their high calcium (Ca2+) permeability makes NMDAR a hub of Ca2+-mediated signal transduction and, upon activation, Ca2+-activated signaling molecules exert numerous effects on synaptic transmission and plasticity (Xia and Storm 2005). Ca2+ also transiently mediates NMDAR inactivation during sustained neuronal activity (Medina et al., 1999). Although NMDARs seem quite stable in the postsynaptic density (PSD) (Ehlers, 2003), synaptic complexes are extremely mobile structures that rapidly switch receptor subunits and associated scaffold proteins to dynamically influence their signaling properties (Newpher and Ehlers, 2008). NMDAR function is subject to activity- and experience-dependent regulation by lateral movement and endocytosis, which affects synaptic retention of NMDARs and in turn their function (Lau and Zukin 2007). Using immunoblot analysis of PSD-enriched fractions from CA1 minislices combined with whole-cell electrophysiological techniques in CA1 neurons in hippocampal slices, the goal of the present study was to evaluate possible mechanisms underlying the temporal pattern of the reduction in NMDA current in hippocampus during FZP withdrawal and its possible physiological significance.
Materials and Methods
Animals.
All animal procedures were performed in accordance with protocols approved by the University of Toledo Health Science Campus Animal Care and Use Committee and were consistent with the National Institutes of Health animal care and use policy. Male Sprague-Dawley rats (Harlan, Indianapolis IN), postnatal days 36 to 42 when euthanized, were first adapted to 0.02% saccharin vehicle for 2 days, then offered FZP in vehicle for 1 week (100 mg/kg × 3 days; 150 mg/kg × 4 days) as their sole source of drinking water. Daily water consumption was monitored to adjust the drug concentration to offer the desired dose. Rats that did not reach a weekly average of 120 mg/kg/day were excluded. FZP treatment was followed by 1 or 2 days of drug withdrawal when saccharin water was offered. Matched control rats received saccharin vehicle in parallel throughout the study.
The oral bioavailability of FZP is such that this dosing regimen results in therapeutic brain concentrations of FZP in the 1.2-μM range (equivalent to ∼0.6 μM diazepam) similar to that achieved with intraperitoneal injections, without the side effects related to repeated injections (Gonsalves and Gallager, 1985). The time course of the appearance and disappearance of anxiety is also related to the treatment drug, its half-life, and the duration and route of treatment. Izzo et al. (2001) reported increased AMPAR GluA1 subunit expression in the hippocampus and frontal and occipital cortex beginning 4 days after withdrawal from 2-week diazepam injection, which was also associated with increased anxiety-like behavior in an elevated plus maze. Likewise, we have repeatedly shown that the aforementioned oral FZP dosing regimen reliably induces anxiety-like behavior in the elevated plus maze upon drug withdrawal and that anxiety was positively correlated with a progressive increase in CA1 neurons AMPAR current amplitude and modulated by the regulation of NMDAR function in neurons from the same rats (Van Sickle et al., 2004; Xiang and Tietz, 2007).
PSD-Enriched Subcellular Fractionation.
PSD-enriched subcellular fractions of CA1 minislices were prepared at 0 to 4°C as described previously (Song et al., 2007). In brief, rats were decapitated and the brains were removed to bubbled ice-cold artificial cerebrospinal fluid (ACSF). Hippocampi were rapidly isolated on ice and pooled from three matched pairs of control or FZP-withdrawn rats (n = 12–21 rats/experimental group), and the CA1 region was homogenized and triturated with a 30-gauge needle in ice-cold buffer containing 10 mM Tris 10, pH 7.4, 320 mM sucrose, 1 mM EDTA, 1 mM EGTA, 5 mM NaF, 1 mM Na orthovanadate, 0.5 mM okadaic acid, 1 μM cyclosporin A, and 1% protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Homogenates were centrifuged at 960g for 10 min to remove large debris. The supernatant (S1) was centrifuged at 10,000g for 30 min to obtain the crude membrane pellet (P2) and the cytosolic fraction (S2). The PSD-enriched fraction was obtained by incubating P2 pellets in Triton-homogenate buffer on ice for 20 min and centrifugation at 32,000g for 1 h. Final pellets were sonicated in resuspension buffer (10 mM Tris 10, pH 8, 1 mM EDTA 1, and 0.1% SDS). Protein concentrations were determined with a BCA protein assay kit (Pierce Chemical, Rockford, IL).
Immunoblotting.
Subfractionated protein (15 μg per well) was mixed with sample buffer (Bio-Rad Laboratories, Hercules, CA) plus 5% β-mercaptoethanol and running buffer (25 mM Tris base, 200 mM glycine, 0.1% SDS), then loaded on a 10% polyacrylamide gel. Protein was wet-transferred to a nitrocellulose membrane. Primary antibodies were incubated with membranes overnight at 4°C. The antibody signal was detected with horseradish peroxidase-conjugated secondary antibodies (1:10,000, Jackson ImmunoResearch Laboratories Inc., West Grove, PA), followed by enhanced chemiluminescence (Denville Scientific, Metuchen, NJ). Primary antibodies used included: anti-GluN1 (1:2000; BD Biosciences, San Jose, CA), anti-GluN2A (1:5000; Millipore Corporation, Billerica, MA), anti-GluN2B (1 μg/ml; Zymed Laboratories, South San Francisco, CA), anti-phosphoSer1303-GluN2B (1 μg/ml; Millipore Corporation), anti-actin (1:50,000; Millipore Corporation), and anti-glyceraldehyde-3-phosphate dehydrogenase (1:20,000; Abcam Inc., Cambridge, MA). Images of immunoblots were scanned and quantified with ImageJ (National Institutes of Health, Bethesda, MD). NMDAR-integrated density signals were normalized to the corresponding loading control. The GluN2B subunit phosphorylation ratio was defined as the phosphoSer1303-GluN2B signal density divided by the GluN2B signal density.
Hippocampal Slice Preparation.
After decapitation, the hippocampus was rapidly dissected, and transverse dorsal hippocampal slices (400 μm) were cut on a vibratome in ice-cold, pregassed low-calcium ACSF containing 120 mM NaCl, 2.5 mM KCl, 0.5 mM CaCl2, 7.0 mM MgCl2, 1.2 mM NaH2PO4, 2 mM NaHCO3, 20 mM d-glucose, and 1.3 mM ascorbate, pH 7.4. The CA3 region was dissected from slices to prevent the spread of epileptiform activity. Slices were maintained at room temperature for 15 min in gassed low-calcium ACSF, then transferred to normal ACSF containing 119 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, 1.3 mM MgSO4, 1.25 mM NaH2PO4, 26 mM NaHCO3, and 10 mM d-glucose, pH 7.4 for >2 h recovery in ACSF before electrophysiological recording. During recording, slices were submerged and perfused at a rate of 2.5 ml/min with gassed ACSF at room temperature.
Electrophysiological Recording.
Blind whole-cell patch-clamp recordings were made from CA1 pyramidal neurons using borosilicate micropipettes (3–7 MΩ; WPI, Sarasota, FL). The pipette solution contained 132.5 mM Cs methanesulfonate, 17.5 mM CsCl, 10 mM HEPES, 0.2 mM EGTA, 8 mM NaCl, 2 mM Mg-ATP, 0.3 mM Na3-GTP, and 2 mM lidocaine N-ethyl bromide (QX-314), pH 7.2. Signals were amplified with an Axoclamp2A amplifier coupled to a four-pole Bessel filter/amplifier (1 kHz, 100×; Dagan Corp., Minneapolis, MN) and digitized online (20 kHz; Digidata 1200A; Molecular Devices, Sunnyvale, CA) then stored on disks for later analysis. Miniature NMDAR currents are not reliably measureable beyond postnatal day 15; therefore, NMDAR-mediated EPSCs were evoked in FZP-withdrawn neurons as described previously (Van Sickle et al., 2004). Hippocampal slices were continuously perfused with oxygenated ACSF and visualized on an upright Carl Zeiss Inc. (Thornwood, NY) Axioskop. A bipolar stimulating electrode made from a gold-coated tungsten fiber was placed in the center of stratum radiatum, 50 to 100 μm from the recording electrode. Voltage-gated channels were inactivated by adjusting the holding potential to VH = +10 mV in 10-mV increments. Stimulus intensity was determined by inducing half-maximal eEPSCs at VH = −20 mV in the presence of 10 μM DNQX and 50 μM picrotoxin in ACSF. EPSCs evoked using the half-maximal stimulus intensity were recorded under voltage clamp (VH = +40 mV) with the same antagonists. Ten-minute perfusion of the GluN2B-selective antagonist ifenprodil (1 μM) was used to block GluN2B subunit-containing NMDARs.
To evaluate the combined effect of AMPAR current potentiation and the down-regulation of NMDAR function in the same neurons, AMPAR- and NMDAR-mediated evoked EPSCs were recorded at a stimulus intensity to induce half-maximal response in the absence of Mg2+ (119 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, 1.25 mM NaH2PO4, 26 mM NaHCO3, 10 mM d-glucose, pH 7.4). Because currents at positive holding potentials are less stable, mixed AMPAR- and NMDAR-mediated currents were first recorded at VH = −40 mV, in the presence of picrotoxin (50 μM) only. The AMPAR-mediated component was recorded after 10-min application of APV (50 μM) and confirmed by further application of DNQX (10 μM). NMDAR-mediated currents were calculated by measuring the difference between currents with and without APV. The area under each separate and combined current trace was defined as the charge transfer.
Data Analysis.
Data are expressed as mean ± S.E.M. For each subunit, analysis of immunoblot signal density between experimental groups was by Student's t test. Statistical differences in CA1 neuron glutamatergic currents between experimental groups were assessed by one-way analysis of variance followed by post hoc analysis using Bonferroni's multiple comparison test and plotted using Prism software (GraphPad Software Inc., San Diego, CA). A p value < 0.05 was considered statistically significant.
Results
GluN1 and GluN2B Expression Patterns in PSD-Enriched Subfractions during FZP Withdrawal.
In the absence of a change in glutamate release (Shen et al., 2010), decreased NMDAR-mediated eEPSC amplitude (Van Sickle et al., 2002, 2004) was indicative of changes in postsynaptic NMDAR function. Therefore, NMDAR subunit expression levels were hypothesized to parallel modulation of NMDAR function across the drug-withdrawal period. PSD-enriched subcellular fractions were collected from CA1 minislices derived from 1- and 2-day FZP-withdrawn rats (Song et al., 2007; Shen et al., 2010) and probed for the expression of NMDAR subunits. Because most NMDARs are GluN1/GluN2 assemblies (Lau and Zukin 2007), GluN2C subunits are restricted largely to cerebellum, and GluN2D subunits are more prominently expressed early in development (Wenthold et al., 2003), only GluN1, GluN2A. and GluN2B subunits were investigated. The expression levels of the phosphoSer1303-GluN2B subunit, a substrate of CaMKII, and its ratio to total GluN2B subunit levels were also probed to evaluate whether CaMKII-mediated phosphorylation of GluN2B subunits might play a role in NMDAR regulation during benzodiazepine withdrawal.
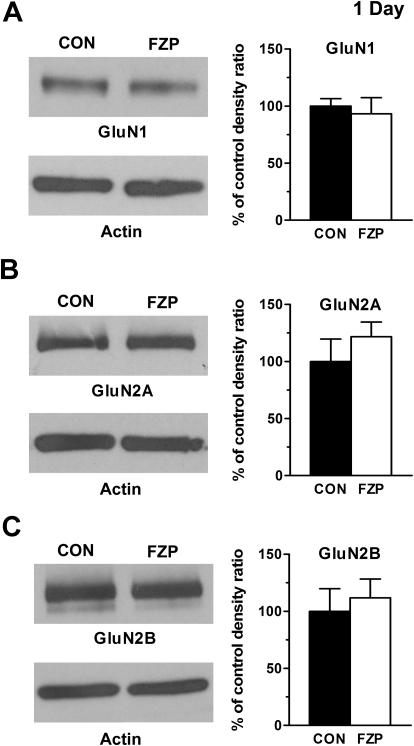
In 1-day FZP-withdrawn rats, no differences were found in the levels of any of the three GluN subunits (Fig. 1): GluN1 (CON: 100 ± 6.6% versus FZP: 93.4 ± 14.0%, n = 7), GluN2A (CON: 100 ± 19.7% versus FZP: 121.7 ± 12.9%, n = 7), or GluN2B (CON: 100 ± 19.9% versus FZP: 111.8 ± 16.6%, n = 7) consistent with earlier findings of a lack of modulation in NMDAR currents at this withdrawal time point (Van Sickle et al., 2004). Furthermore, neither the amount of phosphoSer1303-GluN2B (CON: 100 ± 11.9% versus FZP: 100.2 ± 15.8%, n = 7) nor the phospho ratio to total GluN2B (CON: 100 ± 5.9% versus FZP: 82.6 ± 10.9%, n = 4) was modified at this time point.
Fig. 1.
NMDAR subunit expression levels in PSD-enriched subfractions were unchanged in CA1 minislices from 1-day FZP-withdrawn rats. Left, representative blots of GluN1 (A), GluN2A (B), and GluN2B (C) subunit protein levels are shown. Right, quantitative analyses of integral density signal are shown in the histograms. No significance differences were found among any subunit levels between experimental groups.
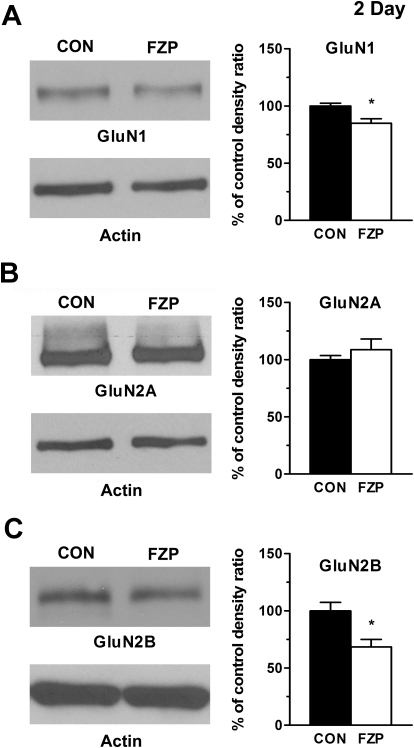
The decrease in NMDAR subunit expression was also consistent with earlier in situ findings in 2-day FZP-withdrawn rats (Van Sickle et al., 2002). A significant decrease in the levels of expression of GluN1 (Fig. 2) (CON: 100 ± 2.4% versus FZP: 85.0 ± 3.9%, n = 4) and GluN2B subunits (CON: 100 ± 7.3% versus FZP: 68.5 ± 6.6%, n = 4) was detected, whereas GluN2A subunit levels were unchanged (CON: 100 ± 3.6% versus FZP: 108.7 ± 9.3%, n = 4). Because GluN2A and GluN2B are the two major GluN2 subunits expressed in the hippocampus, the lack of change in the levels of GluN2A expression excluded the possibility of either a compensatory increase or a parallel decrease in GluN2A subunit levels. The findings imply that the depression of NMDAR currents is mediated largely by GluN2B-containing receptors, probably GluN1/GluN2B heterodimers. Although the absolute amount of phosphoSer1303-GluN2B decreased (CON: 100 ± 5.3% versus FZP: 75.6 ± 7.6%, n = 4), the ratio of phospho to total GluN2B subunit levels was unchanged (CON: 100 ± 2.3% versus FZP: 111.7 ± 9.3%, n = 4), suggesting that CaMKII-mediated phosphorylation of synaptic GluN2B subunits may not be increased despite the increased CaMKII levels in PSD subfractions of FZP-withdrawn rats (Shen et al., 2010).
Fig. 2.
Decreased GluN1 and GluN2B subunit expression levels in PSD-enriched subfractions were unchanged in CA1 minislices from 2-day FZP-withdrawn rats. Left, representative blots of (A) GluN1, (B) GluN2A, and (C) GluN2B (subunit protein levels are shown. Right, quantitative analyses of integral signal density are shown in the histograms. GluN1 (CON, 100 ± 2.4% versus FZP, 85 ± 3.9%, p < 0.05) and GluN2B (CON, 100 ± 7.3% versus FZP, 69 ± 6.6%, p < 0.05) subunit levels showed significant decreases in PSD-enriched subfractions from 2-day FZP-withdrawn rats (* p < 0.05), without a change in phosphoSer1303-GluN2B ratio (CON, 100 ± 6.1% versus FZP 133 ± 22.1%, p > 0.05). GluN2A subunit levels were unchanged (CON, 100 ± 3.6% versus FZP, 109 ± 9.3%, p = 0.42).
Depression of NMDAR Currents Is Mediated by GluN2B-Containing Receptors in 2-Day FZP-Withdrawn Rats.
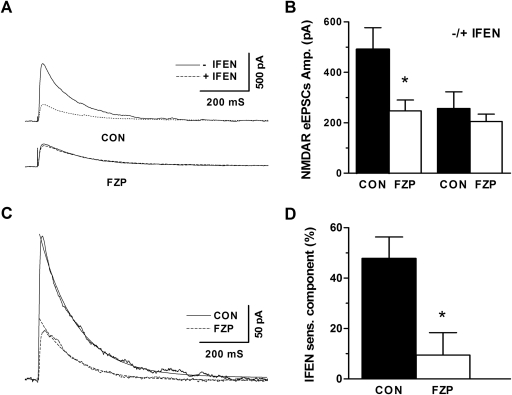
The GluN2B-selective antagonist ifenprodil was used to evaluate the contribution of GluN2B-containing receptors to the depression of isolated CA1 neuron NMDAR currents. Representative traces of NMDAR-mediated eEPSCs are shown in the absence or presence of ifenprodil (Fig. 3A). Similar to earlier studies (Van Sickle et al., 2002, 2004), the peak amplitude of NMDAR-mediated eEPSCs was significantly reduced by nearly 50% (Fig. 3B; CON: 491 ± 87 pA; FZP: 247 ± 43 pA, p < 0.05). Bath application (10 min) of 1 μM ifenprodil eliminated the difference between NMDAR-mediated peak eEPSC amplitude (Fig. 3B; CON: 257 ± 65 pA versus FZP: 205 ± 29 pA, p > 0.05), supporting the hypothesis that GluN2B subunit-containing NMDAR mediated the current depression. The GluN2B-mediated current from both control and FZP-withdrawn rats could be fit with a single exponential decay function and confirmed a decreased in current amplitude, yet similar kinetic properties (Fig. 3C; CON: tau = 0.15 s versus FZP: tau = 0.14 s, p > 0.05). The ifenprodil-sensitive eEPSC component, which represents the percentage of GluN2B-containing NMDAR, was calculated as the difference between the percentage of amplitude change before and after ifenprodil application. A significant decrease in the ifenprodil-sensitive component was also observed in CA1 neurons from 2-day FZP-withdrawn rats (Fig. 3D; CON: 47.9 ± 8.5% versus FZP: 9.5 ± 8.9%, p < 0.05).
Fig. 3.
Reduction in GluN2B-mediated eEPSCs in CA1 neurons from 2-day FZP-withdrawn rats. A, representative traces recorded before (solid line) and after (dotted line) application of 1 μM ifenprodil, a GluN2B subunit-selective antagonist. B, the amplitude of NMDAR-mediated evoked EPSCs was decreased in CA1 neurons from 2-day FZP-withdrawn rats, an effect abolished by ifenprodil. C, a single-exponential decay fit of mean ifenprodil-sensitive current revealed a similar decay time constant (CON: τ = 0.15 s versus FZP: τ = 0.14 s). *, p < 0.05. D, the amplitude of the ifenprodil-sensitive component was significantly decreased in neurons from 2-day FZP-withdrawn rats, whereas the non-GLUN2B component was not different between experimental groups.
NMDAR Current Depression Observed after AMPAR Overactivation.
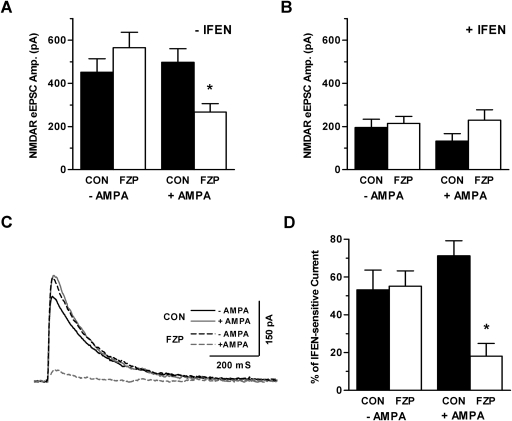
Although NMDAR currents were not down-regulated after 1 day of drug withdrawal, AMPAR currents were already potentiated and linked to withdrawal anxiety at that time point (Song et al., 2007; Xiang and Tietz, 2007). Systemic AMPAR antagonist injection eliminated both AMPAR current enhancement and NMDAR current reduction (Van Sickle et al., 2004; Xiang and Tietz 2007). Therefore, the depression of GluN2B-containing NMDAR was hypothesized to be triggered by the more sizeable AMPAR potentiation observed after 2 days. As predicted, one-way analysis of variance (F value = 4.029, p = 0.015) showed that in vitro activation of partially amplified AMPAR after 1 μM AMPA preincubation of slices derived from 1-day FZP-withdrawn rats decreased NMDAR-mediated eEPSC amplitude in comparison with slices from matched control rats (p < 0.05) or 1-day FZP-withdrawn rats (p < 0.01) (Fig. 4A) not preincubated with AMPA. Similar to NMDAR depression in slices from 2-day withdrawn rats, GluN2B subunit-containing NMDAR played a major role because ifenprodil also blocked the NMDAR depression induced by AMPA preincubation in 1-day FZP-withdrawn rats (F value = 1.318, p = 0.286) (Fig. 4B). The decreased mean GluN2B-mediated current occurred only in slices from 1-day FZP-withdrawn preincubated with AMPA (Fig. 4C). Likewise, a significant decrease in the percentage ifenprodil-sensitive current was observed in hippocampal slices from 1-day FZP-withdrawn rats after AMPA preincubation (F value = 6.79, p = 0.001), in comparison with matched control rats (p < 0.001) and 1-day FZP-withdrawn rats without AMPAR activation (p < 0.001) (Fig. 4D). The decrease in the percentage ifenprodil current was blocked (FZP: +AMPA = 55.2 ± 8.1%; FZP: −AMPA = 18.2 ± 6.6%; FZP + AMPA + DNQX = 46%) in one cell coincubated with AMPA and 10 μM DNQX. No significant effect of AMPA incubation was detectable in slices from control rats.
Fig. 4.
AMPA preincubation induced GluN2B-mediated NMDAR down-regulation in slices from 1-day FZP-withdrawn rats. A, incubation (30 min) of hippocampal slices in 1 μM AMPA resulted in decreased NMDAR-mediated eEPSC amplitude in CA1 neurons from 1-day FZP-withdrawn rats, as in 2-day FZP-withdrawn rats, but not in neurons from control rats. B, blockade of GluN2B-containing NMDARs with ifenprodil diminished the effect of AMPA incubation in slices from 1-day FZP-withdrawn rats. C, average traces of ifenprodil-sensitive currents in slices from control − AMPA (solid black line), control + AMPA (solid gray line), 1-day FZP − AMPA (black dotted line), and 1-day FZP + AMPA (gray dotted line) indicated that AMPA incubation promoted a decrease in GluN2B-containing NMDAR currents in 1-day FZP-withdrawn rats. D, the ifenprodil-sensitive component was significantly decreased in slices from 1-day FZP-withdrawn rats preincubated with AMPA.
Depression of NMDAR Currents: A Natural Brake for AMPAR Overexcitation during FZP Withdrawal.
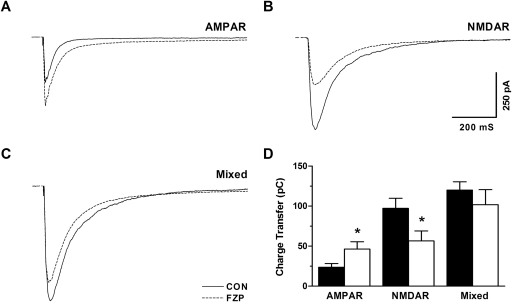
Based on the cumulative findings in our present and earlier work, the depression of GluN2B-mediated receptor function probably is triggered by AMPAR overactivation, which might act as a homeostatic negative feedback mechanism to avert hyperactivity of glutamatergic neurotransmission. To further evaluate this possibility, evoked currents mediated by AMPAR and NMDAR CA1 neuron glutamate receptors were recorded at VH = −40 mV in the presence of a GABA antagonist. To evaluate the contribution of each component to total neuronal output, combined currents were recorded, then AMPAR-mediated currents were recorded in the presence of APV, and the APV-sensitive components were defined as NMDAR-mediated currents by subtraction. As shown in Fig. 5A, charge transfer-mediated AMPAR current was increased, whereas charge transfer mediated by NMDAR current in the same neurons was decreased (Fig. 5B). The lack of difference in the total charge transferred by the mixed current in the same neuron suggested that the decreased NMDAR current could offset the increased AMPAR current, leaving CA1 neuron output unchanged in 2-day FZP-withdrawn rats versus control rats (Fig. 5C). Figure 5D shows the average charge transfer mediated by AMPAR (CON: 23.4 ± 4.7 versus FZP: 46.5 ± 0.0, n = 7, p < 0.05), NMDAR (CON: 97.2 ± 12.7 versus FZP: 56.4 ± 12.7, n = 7, p < 0.05), and mixed currents (CON: 120.0 ± 10.2 versus FZP: 101.7 ± 18.8, n = 7, p = 0.41).
Fig. 5.
Depression of NMDAR current counteracted AMPAR potentiation in CA1 neurons from 2-day FZP-withdrawn rats. A–C, average traces of eEPSCs recorded in ACSF without Mg2+ at VH = −40 mV using a stimulus intensity to elicit a half-maximal response. The charge transfer of mixed current mediated by both AMPAR and NMDAR was not different between neurons from control rats (120.0 ± 10.2 pC, n = 7) and 2-day FZP-withdrawn rats (101.7 ± 18.8 pC, n = 7, p = 0.4099). A, AMPAR-mediated charge transfer, dissected using APV, increased in neurons from 2-day FZP-withdrawn rats (CON, 23.5 ± 4.7 pC versus FZP, 46.5 ± 9.0 pC, n = 7, p = 0.04024). B, in contrast, charge transfer mediated by NMDAR showed a significant decrease in neurons from 2-day FZP-withdrawn rats (CON, 97.2 ± 16.7 pC versus FZP 56.4 ± 12.7 pC, n = 7, p = 0.0419). C, charge transfer mediated by mixed AMPAR and NMDAR currents was unchanged in CA1 neurons from 2-day FZP-withdrawn rats, reflecting the increased AMPAR-mediated and decreased NMDAR-mediated charge transfer. *, p < 0.05.
Discussion
Whole-cell electrophysiological recording of hippocampal slices, coupled with immunoblots of PSD-enriched subfractions from CA1 minislices, revealed that depression of NMDAR currents after 2-day FZP withdrawal was mediated by GluN2B subunit-containing NMDAR. Previous (Xiang and Tietz, 2007) and current findings indicated that NMDAR depression was secondary to AMPAR potentiation and counteracted the overactivation of AMPAR-mediated glutamatergic transmission during FZP withdrawal. These findings may explain why in most cases benzodiazepine withdrawal is of relatively short duration and self-limited with less severe consequences (O'Brien, 2005) in contrast to the life-threatening withdrawal symptoms that may accompany nonselective central nervous system depressants such as ethanol and barbiturates, which may enhance NMDAR function upon drug withdrawal (Short and Tabakoff, 1993; Kash et al., 2009; see also Obara et al., 2009).
Dual Modulation of Ionotropic Glutamate Receptors during FZP Withdrawal.
Ionotropic glutamate receptors have been implicated in neuronal development, synaptic plasticity (Dingledine et al., 1999), excitotoxicity (Auzmendi et al., 2009), and addiction (Lau and Zukin, 2007; Tan et al., 2010). AMPAR and NMDAR, the two major subtypes of ionotropic glutamate receptors in the central nervous system, undergo bidirectional modulation during FZP withdrawal (Van Sickle et al., 2004). Potentiation of AMPAR function in hippocampus was associated with GluA1 homomer incorporation into CA1 pyramidal synapses (Song et al., 2007; Shen et al., 2010) and positively correlated with benzodiazepine withdrawal anxiety (Izzo et al., 2001; Xiang and Tietz, 2007), as well as other animal models of anxiety (Shen et al., 2007). An LTP-like modulation of excitatory responses and AMPAR responses was also found in amygdala neurons of fear-conditioned rats (Zinebi et al., 2001; Iosoardi et al., 2004), another key locus within the neural circuit mediating anxiety (McNaughton and Gray, 2000). On the contrary, maintenance of fear conditioning, in which Pavlovian contingencies induced fear and anxiety, was associated with decreased expression of both GluN2A and GluN2B subunits and proposed to guard against enhanced glutamatergic strength or serve as a mechanism to rapidly reactivate emotional memories through rapid NMDAR recycling (Zinebi et al., 2003).
GluN2B Subunit-Containing NMDAR: Mediators of NMDAR Depression.
Using both biochemical (Fig. 2) and electrophysiological methods (Fig. 3), our findings revealed that both GluN2B-containing NMDAR function and synaptic expression were down-regulated after 2-day FZP withdrawal. The difference between control and 2-day withdrawn rats was precluded by ifenprodil application, which implied that down-regulation of GluN2B-containing NMDARs was the major mediator of NMDAR current depression. These results were further supported by our earlier in situ study carried out at the same time point after drug withdrawal (Van Sickle et al., 2002). The involvement of the GluN2B subunit in fear conditioning and anxiety was supported by either application of ifenprodil (Rodrigues et al., 2001) or overexpression of the GluN2B subunit C terminus (Zhang et al., 2008), which disrupted acquisition of fear conditioning (LeDoux, 2003). The smaller reduction in GluN1 subunit expression level herein probably was related to its obligatory role in the formation of all functional receptors (Cull-Candy et al., 2001). Therefore, the removal of functional heteromeric GluN1/GluN2B receptors might serve as a molecular basis for the decreased NMDAR eEPSC amplitude during FZP withdrawal at CA1 neuron synapses, a finding extended by ultrastructural analyses (Das et al., 2010). Unlike at early developmental stages in which the GluN2B subunits are replaced by GluN2A subunits (Cull-Candy et al., 2001), no change in GluN2A subunit expression was observed during FZP withdrawal.
Possible Mechanism for GluN2B Removal.
Of the two major GluN2 subunit isoforms expressed in forebrain, GluN2B subunits are more mobile compared with GluN2A (Groc et al., 2006) and are more likely to be internalized (Lavezzari et al., 2004). Studies also have revealed that GluN2B homodimers, unlike GluN2A homodimers, predominantly sort to late endosomes and tended to traffic to recycling endosomes (Tang et al., 2010). These properties render GluN2B subunits better candidates for regulated removal from the synapse. The removal of NMDAR from synapses requires several steps: 1) disruption of the interaction with scaffold proteins; 2) lateral movement; and 3) clathrin-mediated internalization from extrasynaptic sites (Groc et al., 2009). In fact, calmodulin can competitively displace α-actinin-NMDAR binding, whereas α-actinin can bridge NMDAR binding with actin filaments (Wyszynski et al., 1997) and facilitate NMDAR clustering in dendritic spines (Rao et al., 1998). So, increased postsynaptic Ca2+/calmodulin can undermine the stability of NMDAR and may serve as an underlying mechanism for removal of GluN2B-containing NMDAR from synapses during FZP withdrawal.
Several indirect lines of evidence suggest that Ca2+ influx may be elevated in CA1 neurons from FZP-withdrawn rats. First, electrophysiological evidence suggested that incorporation of Ca2+-permeable homomeric GluA1 AMPARs was responsible for the progressive potentiation of AMPAR currents at CA1 neuron synapses (Song et al., 2007; Shen et al., 2010). These findings were further supported by the increased synaptic expression of GluA1, but not GluR2, subunits measured using electron microscopic techniques (Das et al., 2008). After 1-day withdrawal, newly inserted AMPARs might also turn silent synapses into active synapses, resulting in further Ca2+ accumulation. These possibilities were supported by the findings that either systemic injection of AMPAR or NMDAR antagonists can block NMDAR down-regulation in 2-day FZP-withdrawn rats (Van Sickle et al., 2004; Xiang and Tietz, 2007). The hypothesis that activation of Ca2+-permeable GluA1 homomers may contribute to NMDAR down-regulation was evaluated by activation of already potentiated CA1 neuron AMPARs from 1-day FZP-withdrawn rats (Fig. 4). Preincubation (0.5 h) of hippocampal slices with 1 μM AMPA induced significant reduction in NMDA currents (Fig. 4), at a time when decreases in NMDA eEPSCs were previously undetectable (Van Sickle et al., 2004). These findings implied that additional Ca2+ influx through activation of newly inserted Ca2+-permeable GluA1 homomeric AMPARs might play an important role to induce NMDAR down-regulation. It is noteworthy that voltage-gated Ca2+ channel activation might also play a central role in Ca2+ accumulation and thus regulation of CA1 neuron NMDAR function in FZP-withdrawn rats because a 2-fold increase in voltage-gated Ca2+ channel-mediated current density emerged upon withdrawal from 1-week FZP treatment and lasted up to 2 days (Xiang et al., 2008).
Physiological Implication of NMDAR Down-Regulation.
NMDARs composed of GluN2A or GluN2B subunits have high channel conductance states and are sensitive to Mg2+ blockade (Cull-Candy et al., 2001), but GluN2B-containing NMDARs have a much longer deactivation time compared with GluN2A (Cull-Candy et al., 2001), which means that upon activation GluN2B-containing NMDARs can introduce a greater amount of intracellular Ca2+ during synaptic transmission. In fact, GluN1/GluN2B heterodimers carried more charge for a single synaptic event than GluN1/GluN2A heterodimers (Erreger et al., 2005), and Ca2+ imaging studies implied that GluN2B-containing NMDAR introduced more Ca2+ influx per unit current (Sobczyk et al., 2005). Thus, removal of GluN2B-containing NMDAR may significantly reduce Ca2+ influx and its downstream cascades. Reduced Ca2+ influx might affect the unique dynamic interaction between GluN2B subunits and autophosphorylated CaMKII. Residues surrounding the GluN2B Ser1303 phosphorylation site target CaMKII translocation, although Thr286 autophosphorylation is not required for CaMKII-mediated GluN2B phosphorylation (Strack et al., 2000). The latter phosphorylation might not only promote desensitization of GluN1/GluN2B channels (Sessoms-Sikes et al., 2005), but also promote the slow dissociation of activated CaMKII from the phosphorylated GluN2B subunit (Strack et al., 2000). The latter negative feedback regulation of GluN1/GluN2B channel function was Ca2+-dependent because 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid abolished desensitization (Sessoms-Sikes et al., 2005). This might explain why the phosphorylation ratio of phosphoSer1303-GluN2B was unchanged despite increased CA1 neuron CaMKII levels, consistent with the absence of an increase in autophosphorylated CaMKII (Shen et al., 2010). Loss of the GluN2B anchor point could then serve as one mechanism for later CaMKII removal.
Numerous reports from our lab (Zeng and Tietz, 1999) and others (Izzo et al., 2001; Isoardi et al., 2004) have shown that neuronal hyperexcitability in specific brain areas may involve a complex interplay among decreased GABAA receptor-mediated inhibition and increased AMPAR-mediated excitation across the benzodiazepine withdrawal period. As reported previously, CA1 neuron hyperexcitability and AMPAR potentiation in 1-day FZP-withdrawn rats closely paralleled withdrawal anxiety (Van Sickle et al., 2004; Xiang et al., 2007). Here, we show that down-regulation of GluN2B-containing NMDARs in 2-day FZP-withdrawn rats counteracted the hyperactivity mediated by AMPAR potentiation measured as a reduction in the total charge transferred during glutamatergic transmission (Fig. 5), previously shown to lead to the disappearance of withdrawal anxiety (Van Sickle et al., 2004). Thus the depression of NMDAR function during FZP withdrawal may act as a compensatory negative feedback mechanism to protect the neuron from AMPAR-mediated overactivation as in amygdala neurons during fear conditioning (Zinebi et al., 2001, 2003), striatal neurons after chronic amphetamine exposure (Mao et al., 2009), or spinal cord nociceptive synapses during chronic peripheral inflammation (Vikman et al., 2008). The reduction of GluN2B subunit-mediated NMDAR currents would serve as a homeostatic mechanism to offset neuronal hyperactivity generated by the progressive enhancement of AMPAR current and thus may serve as a physiological brake to down-regulate neuronal output, limiting withdrawal symptoms such as anxiety.
Acknowledgments
We thank Brian Behrle, Krista Pettee, and Margarete Otting for expert technical assistance and Drs. Paromita Das and Jun Song and Mr. Damien Earl for helpful discussions. Flurazepam was supplied by the National Institute of Drug Abuse Drug Supply Program (Bethesda, MD).
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant RO1-DA018342] (to E.I.T.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.174235.
- AMPAR
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- NMDAR
- N-methyl-d-aspartate receptor
- ACSF
- artificial cerebrospinal fluid
- APV
- (2R)-amino-5-phosphonovaleric acid
- CaMKII
- Ca2+/calmodulin-dependent protein kinase type II
- CON
- control
- DNQX
- 6,7-dinitroquinoxaline-2,3-dione
- eEPSC
- evoked excitatory postsynaptic current
- FZP
- flurazepam
- LTP
- long-term potentiation
- PSD
- postsynaptic density
- QX-314
- lidocaine N-ethyl bromide
- VH
- holding potential.
Authorship Contributions
Participated in research design: Shen and Tietz.
Conducted experiments: Shen.
Performed data analysis: Shen and Tietz.
Wrote or contributed to the writing of the manuscript: Shen and Tietz.
References
- Auzmendi J, González N, Girardi E. (2009) The NMDAR subunit NR2B expression is modified in hippocampus after repetitive seizures. Neurochem Res 34:819–826 [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. (2001) NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol 11:327–335 [DOI] [PubMed] [Google Scholar]
- Das P, Lilly SM, Zerda R, Gunning WT, 3rd, Alvarez FJ, Tietz EI. (2008) Increased AMPA receptor GluR1 subunit incorporation in rat hippocampal CA1 synapses during benzodiazepine withdrawal. J Comp Neurol 511:832–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Zerda R, Alvarez FJ, Tietz EI. (2010) Immunogold electron microscopic evidence of differential regulation of GluN1, GluN2A and GluN2B, NMDA-type glutamate receptor subunits in rat hippocampal CA1 synapses during benzodiazepine withdrawal. J Comp Neurol 518:4311–4328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR. (2007) Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci 8:101–113 [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. (1999) The glutamate receptor ion channels. Pharmacol Rev 51:7–61 [PubMed] [Google Scholar]
- Dunworth SJ, Stephens DN. (1998) Sensitisation to repeated withdrawal, in mice treated chronically with diazepam, is blocked by an NMDA receptor antagonist. Psychopharmacology 136:308–310 [DOI] [PubMed] [Google Scholar]
- Ehlers MD. (2003) Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci 6:231–242 [DOI] [PubMed] [Google Scholar]
- Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF. (2005) Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signaling profiles. J Physiol 563:345–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalves SF, Gallager DW. (1985) Spontaneous and RO 15-1788-induced reversal of subsensitivity to GABA following chronic benzodiazepines. Eur J Pharmacol 110:163–170 [DOI] [PubMed] [Google Scholar]
- Groc L, Bard L, Choquet D. (2009) Surface trafficking of N-methyl-d-aspartate receptors: physiological and pathological perspectives. Neuroscience 158:4–18 [DOI] [PubMed] [Google Scholar]
- Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, Choquet D. (2006) NMDA receptor surface mobility depends on NR2A–2B subunits. Proc Natl Acad Sci USA 103:18769–18774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoardi NA, Martijena ID, Carrer HF, Molina VA. (2004) Increased fear learning coincides with neuronal dysinhibition and facilitated LTP in the basolateral amygdale following benzodiazepine withdrawal in rats. Neuropsychopharmacology 29:1852–1864 [DOI] [PubMed] [Google Scholar]
- Izzo E, Auta J, Impagnatiello F, Pesold C, Guidotti A, Costa E. (2001) Glutamic acid decarboxylase and glutamate receptor changes during tolerance and dependence to benzodiazepines. Proc Natl Acad Sci USA 98:3483–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Baucum AJ, 2nd, Conrad KL, Colbran RJ, Winder DG. (2009) Alcohol exposure alters NMDAR function in the bed nucleus of the stria terminalis. Neuropsychopharmacology 34:2420–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsura M, Shibasaki M, Kurokawa K, Tsujimura A, Ohkuma S. (2007) Up-regulation of L-type high voltage-gated calcium channel subunits by sustained exposure to 1,4- and 1,5-benzodiazepines in cerebrocortical neurons. J Neurochem 103:2518–2528 [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. (2007) Synaptic plasticity and addiction. Nat Rev Neurosci 8:844–858 [DOI] [PubMed] [Google Scholar]
- Lau CG, Zukin RS. (2007) NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci 8:413–426 [DOI] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Dewey CM, Roche KW. (2004) Subunit-specific regulation of NMDA receptor endocytosis. J Neurosci 24:6383–6391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. (2003) The emotional brain, fear, and the amygdale. Cell Mol Neurobiol 23:727–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LM, Wang W, Chu XP, Zhang GC, Liu XY, Yang YJ, Haines M, Papasian CJ, Fibuch EE, Buch S, et al. (2009) Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nat Neurosci 12:602–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton N, Gray JA. (2000) Anxiolytic action on the behavioural inhibition system implies multiple types of arousal contribute to anxiety. J Affect Disord 61:161–176 [DOI] [PubMed] [Google Scholar]
- Medina I, Leinekugel X, Ben-Ari Y. (1999) Calcium-dependent inactivation of the monosynaptic NMDA EPSCs in rat hippocampal neurons in culture. Eur J Neurosci 11:2422–2430 [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. (2008) Glutamate receptor dynamics in dendritic microdomains. Neuron 58:472–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara I, Bell RL, Goulding SP, Reyes CM, Larson LA, Ary AW, Truitt WA, Szumlinski KK. (2009) Differential effects of chronic ethanol consumption and withdrawal on homer/glutamate receptor expression in subregions of the accumbens and amygdala of P rats. Alcohol Clin Exp Res 33:1924–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP. (2005) Benzodiazepine use, abuse, and dependence. J Clin Psychiatry 66(Suppl 2):28–33 [PubMed] [Google Scholar]
- Rao A, Kim E, Sheng M, Craig AM. (1998) Heterogeneity in the molecular composition of excitatory postsynaptic sites during development of hippocampal neurons in culture. J Neurosci 18:1217–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. (2001) Intra-amygdala blockade of the NR2B subunit of the NMDA receptor disrupts the acquisition but not the expression of fear conditioning. J Neurosci 21:6889–6896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessoms-Sikes S, Honse Y, Lovinger DM, Colbran RJ. (2005) CaMKIIα enhances the desensitization of NR2B-containing NMDA receptors by an autophosphorylation-dependent mechanism. Mol Cell Neurosci 29:139–147 [DOI] [PubMed] [Google Scholar]
- Shen G, Mohamed MS, Das P, Tietz EI. (2009) Positive allosteric activation of GABAA receptors bi-directionally modulates hippocampal glutamate plasticity and behavior. Biochem Soc Trans 37:1394–1398 [DOI] [PubMed] [Google Scholar]
- Shen G, Van Sickle BJ, Tietz EI. (2010) Calcium/calmodulin-dependent protein kinase II mediates hippocampal AMPAR plasticity during BZ withdrawal. Neuropsychopharmacology 35:1897–1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, Smith SS. (2007) Reversal of neurosteroid effects at α4β2δ GABAA receptors triggers anxiety at puberty. Nat Neurosci 10:469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KR, Tabakoff B. (1993) Chronic barbiturate treatment increases NMDA receptors but decreases kainate receptors in mouse cortex. Eur J Pharmacol 230:111–114 [DOI] [PubMed] [Google Scholar]
- Sobczyk A, Scheuss V, Svoboda K. (2005) NMDA receptor subunit-dependent [Ca2+] signaling in individual hippocampal dendritic spines. J Neurosci 25:6037–6046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Shen G, Greenfield LJ, Jr, Tietz EI. (2007) Benzodiazepine withdrawal-induced glutamatergic plasticity involves up-regulation of GluR1-containing α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors in hippocampal CA1 neurons. J Pharmacol Exp Ther 322:569–581 [DOI] [PubMed] [Google Scholar]
- Strack S, McNeill RB, Colbran RJ. (2000) Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-d-aspartate receptor. J Biol Chem 275:23798–23806 [DOI] [PubMed] [Google Scholar]
- Tan KR, Brown M, Labouèbe G, Yvon C, Creton C, Fritschy JM, Rudolph U, Lüscher C. (2010) Neural bases for addictive properties of benzodiazepines. Nature 463:769–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang TT, Badger JD, 2nd, Roche PA, Roche KW. (2010) Novel approach to probe subunit-specific contributions to N-methyl-d-aspartate (NMDA) receptor trafficking reveals a dominant role for NR2B in receptor recycling. J Biol Chem 285:20975–20981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle BJ, Cox AS, Schak K, Greenfield LJ, Jr, Tietz EI. (2002) Chronic benzodiazepine administration alters hippocampal CA1 neuron excitability: NMDA receptor function and expression(1). Neuropharmacology 43:595–606 [DOI] [PubMed] [Google Scholar]
- Van Sickle BJ, Xiang K, Tietz EI. (2004) Transient plasticity of hippocampal CA1 neuron glutamate receptors contributes to benzodiazepine withdrawal-anxiety. Neuropsychopharmacology 29:1994–2006 [DOI] [PubMed] [Google Scholar]
- Vikman KS, Rycroft BK, Christie MJ. (2008) Switch to Ca2+-permeable AMPA and reduced NR2B NMDA receptor-mediated neurotransmission at dorsal horn nociceptive synapses during inflammatory pain in the rat. J Physiol 586:515–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA. (2005) GABAA receptor subtypes: any clues to the mechanism of benzodiazepine dependence? Curr Opin Pharmacol 5:47–52 [DOI] [PubMed] [Google Scholar]
- Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. (2003) Trafficking of NMDA receptors. Annu Rev Pharmacol Toxicol 43:335–358 [DOI] [PubMed] [Google Scholar]
- Wyszynski M, Lin J, Rao A, Nigh E, Beggs AH, Craig AM, Sheng M. (1997) Competitive binding of α-actinin and calmodulin to the NMDA receptor. Nature 385:439–442 [DOI] [PubMed] [Google Scholar]
- Xia Z, Storm DR. (2005) The role of calmodulin as a signal integrator for synaptic plasticity. Nat Rev Neurosci 6:267–276 [DOI] [PubMed] [Google Scholar]
- Xiang K, Tietz EI. (2007) Benzodiazepine-induced hippocampal CA1 neuron α-amino-3-hydroxy-5-methylisoxasole-4-propionic acid (AMPA) receptor plasticity linked to severity of withdrawal anxiety: differential role of voltage-gated calcium channels and N-methyl-d-aspartic acid receptors. Behav Pharmacol 18:447–460 [DOI] [PubMed] [Google Scholar]
- Xiang K, Earl DE, Davis KM, Giovannucci DR, Greenfield LJ, Jr, Tietz EI. (2008) Chronic benzodiazepine administration potentiates high voltage-activated calcium currents in hippocampal CA1 neurons. J Pharmacol Exp Ther 327:872–883 [DOI] [PubMed] [Google Scholar]
- Zeng XJ, Tietz EI. (1999) Benzodiazepine tolerance at GABAergic synapses on hippocampal CA1 pyramidal cells. Synapse 31:263–277 [DOI] [PubMed] [Google Scholar]
- Zhang XH, Liu F, Chen Q, Zhang CL, Zhuo M, Xiong ZQ, Li BM. (2008) Conditioning-strength dependent involvement of NMDA NR2B subtype receptor in the basolateral nucleus of amygdala in acquisition of auditory fear memory. Neuropharmacology 55:238–246 [DOI] [PubMed] [Google Scholar]
- Zinebi F, Russell RT, McKernan M, Shinnick-Gallagher P. (2001) Comparison of paired-pulse facilitation of AMPA and NMDA synaptic currents in the lateral amygdala. Synapse 42:115–127 [DOI] [PubMed] [Google Scholar]
- Zinebi F, Xie J, Liu J, Russell RT, Gallagher JP, McKernan MG, Shinnick-Gallagher P. (2003) NMDA currents and receptor protein are down-regulated in the amygdala during maintenance of fear memory. J Neurosci 23:10283–10291 [DOI] [PMC free article] [PubMed] [Google Scholar]