Abstract
The mechanism by which the inhaled anesthetic isoflurane produces amnesia and immobility is not understood. Isoflurane modulates GABAA receptors (GABAA-Rs) in a manner that makes them plausible targets. We asked whether GABAA-R α2 subunits contribute to a site of anesthetic action in vivo. Previous studies demonstrated that Ser270 in the second transmembrane domain is involved in the modulation of GABAA-Rs by volatile anesthetics and alcohol, either as a binding site or a critical allosteric residue. We engineered GABAA-Rs with two mutations in the α2 subunit, changing Ser270 to His and Leu277 to Ala. Recombinant receptors with these mutations demonstrated normal affinity for GABA, but substantially reduced responses to isoflurane. We then produced mutant (knockin) mice in which this mutated subunit replaced the wild-type α2 subunit. The adult mutant mice were overtly normal, although there was evidence of enhanced neonatal mortality and fear conditioning. Electrophysiological recordings from dentate granule neurons in brain slices confirmed the decreased actions of isoflurane on mutant receptors contributing to inhibitory synaptic currents. The loss of righting reflex EC50 for isoflurane did not differ between genotypes, but time to regain the righting reflex was increased in N2 generation knockins. This effect was not observed at the N4 generation. Isoflurane produced immobility (as measured by tail clamp) and amnesia (as measured by fear conditioning) in both wild-type and mutant mice, and potencies (EC50) did not differ between the strains for these actions of isoflurane. Thus, immobility or amnesia does not require isoflurane potentiation of the α2 subunit.
Introduction
The advent of general anesthesia in the mid-19th century was one of the most important developments in the history of medicine. Despite more than 160 years of clinical use, the mechanisms of action of inhaled anesthetics remained largely a mystery until the end of the 20th century (Franks and Lieb, 1994). Although such anesthetics are some of the most widely used drugs in clinical practice, no presently available inhaled anesthetic is ideal. These drugs have a low therapeutic index and exhibit adverse side effects. Knowledge of the mechanism of action might enable the design, synthesis, and testing of improved anesthetics and the prevention of adverse events such as intraoperative awareness and postoperative cognitive dysfunction.
Accumulating evidence suggests that inhaled anesthetics act by modulating various ligand- and/or voltage-gated ion channels, rather than through nonspecific perturbation of membrane lipids as suggested previously by the Meyer-Overton hypothesis (Franks and Lieb, 1994; Franks, 2008). Among the plausible targets, GABAA receptors (GABAA-Rs) have been strongly implicated in anesthetic action because of their leading role in mediating both synaptic and tonic inhibition in the central nervous system. The function of synaptic and extrasynaptic GABAA-Rs is enhanced by inhaled anesthetics at clinically relevant concentrations (Jones et al., 1992). In contrast, GABAA-Rs are insensitive to structurally related compounds that are not anesthetic (nonimmobilizers) (Mihic et al., 1994). Potentiation of GABAA-R function in vitro parallels in vivo anesthetic potency for the inhaled agents (Zimmerman et al., 1994). These receptors are now accepted as the primary targets and mediators of the central nervous system depressant actions of the intravenous anesthetics propofol and etomidate (Jurd et al., 2003; Reynolds et al., 2003).
Pentameric GABAA-Rs are encoded by 19 different GABAA-R subunit genes (Simon et al., 2004). Most native GABAA-Rs are composed of two α, two β, and a γ or δ subunit (McKernan and Whiting, 1996). Our understanding of the roles of various receptor isoforms and specific subunits in the behavioral effects of anesthetics is limited.
One experimental strategy for defining the contribution of individual receptor subunits to drug action is to create and characterize point mutated gene knockin mice that harbor mutations that render individual subunits insensitive to the drug of interest but otherwise do not affect receptor function. This knockin strategy clarified the role of individual GABAA-R subunits in whole animal responses to benzodiazepines (Rudolph et al., 1999; McKernan et al., 2000) and intravenous anesthetics (Jurd et al., 2003; Reynolds et al., 2003).
We used this same genetic strategy to dissect inhaled anesthetic action. We initially identified a key amino acid (serine at position 270; Ser270) in GABAA-R α subunits that, when mutated to histidine (His), abolished sensitivity to isoflurane (Mihic et al., 1997). However, knockin mice with this single S270H mutation in the α1 subunit displayed behavioral impairments because of an increased sensitivity to GABA (Homanics et al., 2005). This increased GABA sensitivity could be corrected by a second mutation (Leu277 to alanine; L277A), which when combined with S270H, resulted in mutant receptors with isoflurane insensitivity and near normal GABA responses (Borghese et al., 2006). GABAA-R α1 subunit knockin mice with these two mutations showed reduced GABAergic cellular responses to isoflurane and reduced sensitivity to isoflurane-induced loss of righting reflex (LORR), but showed normal immobility and amnestic responses to the drug (Borghese et al., 2006; Sonner et al., 2007).
In this article, we present results of analogous studies of the same mutations in the GABAA-R α2 subunit. Approximately 26% of GABAA-Rs in brain contain the α2 subunit (McKernan and Whiting, 1996), and α2-containing receptors are particularly abundant in cerebral cortex, hippocampus, striatum, olfactory bulb, and the dorsal horn of the spinal cord (Fritschy and Mohler, 1995; Bohlhalter et al., 1996). We report on the characterization of recombinant receptors with the two mutations. We also report that α2 knockin mice are overtly normal and have normal isoflurane-induced loss of righting reflex, immobility, and amnesia, but recover more slowly from isoflurane-induced loss of righting reflex.
Materials and Methods
Electrophysiology in Xenopus Oocytes
The materials and methods were detailed in Borghese et al., (2006). In brief, Xenopus laevis oocytes were manually isolated, treated with collagenase, and placed in sterile modified Barth's solution [88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 10 mM HEPES, 0.82 mM MgSO4, 0.33 mM Ca(NO3)2, and 0.91 mM CaCl2, adjusted to pH 7.5], supplemented with 10,000 units penicillin, 50 mg of gentamicin, 90 mg of theophylline, and 220 mg of sodium pyruvate per liter (incubation medium). Oocytes were then injected into the nucleus with cDNA encoding GABAA subunits (α/β/γ 1:1:3 in ng/oocyte). The cDNAs were human α2 (wild type and mutated), rat β2, and human γ2S in vector pCIS2 and human β3 in vector pcDNA1AMP. The injected oocytes were kept at 13°C in incubation medium.
Recordings were carried out 1 to 3 days after injection. The oocytes were placed in a rectangular chamber and continuously perfused with ND96 buffer at room temperature. The perfusion buffer composition was 96 mM NaCl, 1 mM CaCl2, 2 mM KCl, 1 mM MgCl2, and 5 mM HEPES, pH 7.5. The whole-cell voltage clamp at −70 mV was achieved through two glass electrodes filled with 3 M KCl, using an oocyte clamp.
All drugs were applied by bath perfusion. All solutions were prepared the day of the experiment. The concentration response curves were obtained with increasing concentrations of GABA, applied for 20 to 30 s at intervals ranging from 5 to 15 min. From these concentration response curves, the concentration evoking a half-maximal response (EC50) was calculated, along with the Hill coefficient. To study the Zn2+ (1 and 10 μM), etomidate (1 μM), isoflurane (0.3 mM), and flunitrazepam (1 μM) modulation of GABA currents, the GABA concentration equivalent to EC5–10 was determined after 1 mM GABA gave the maximal current, and then the modulator was coapplied with EC5–10 GABA. For zinc, isoflurane, and flunitrazepam, the coapplication was preceded by 1-min application of the modulator alone. Percentage change was then calculated as the percentage change from the control response to EC5–10 GABA in the presence of modulator. To observe the direct effect of etomidate on GABAA-R function, 10 μM etomidate was applied for 1 min. All experiments shown include data obtained from oocytes taken from at least two different frogs.
Nonlinear regression analysis was performed with Prism (GraphPad Software Inc., San Diego, CA). Pooled data are represented as mean ± S.E.M. Statistical significance was determined by Student's t test.
Knockin Mouse Production and Molecular Characterization
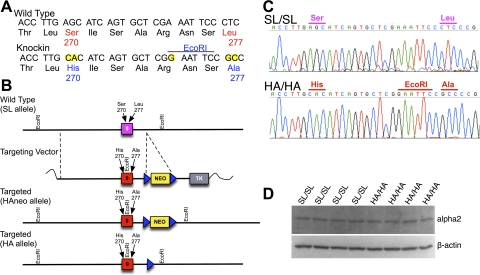
The targeting construct shown in Fig. 2B was designed to modify the α2 locus. The targeting vector was created with mouse genomic DNA (Strain 129 × 1). A 6.2-kb PstI fragment containing exon 9 was isolated from a bacterial artificial chromosome and subcloned into pZErO-2 (Invitrogen, Carlsbad, CA). A 3.05-kb NdeI fragment containing exon 9 was further subcloned and subsequently mutated by using a QuikChange XL site-directed mutagenesis kit (Stratagene, La Jolla, CA) to change the Ser270 codon (AGC) to His (CAC), the Leu277 codon (CTC) to Ala (GCC), and a silent single-base pair (A to G) substitution 7 bp upstream of Leu277 to create an EcoRI site. [Note: amino acid numbering is based on the human α1 GABAA-R protein sequence (Ensembl protein ID ENSP00000377517) for comparison.] A BamHI site ∼340 bp downstream of exon 9 was destroyed and converted to a SmaI, and a blunt-end fragment containing a PGK-neomycin resistance gene (Neo) flanked 5′ and 3′ by Frt sites was inserted. The modified NdeI fragment was returned to the parent vector, and a PGK-thymidine kinase cassette was cloned downstream of the 3′ arm of homology. The resulting targeting construct was linerized by using NotI and electroporated into R1 embryonic stem cells (Nagy et al., 1993) using conditions described previously (Homanics et al., 1997). G418 and gancyclovir-resistant embryonic stem cell clones were screened by Southern blot analysis using EcoRV and hybridization with a 3′ external probe. Targeted clones were further analyzed with multiple enzyme/probes to verify the integrity of the modified locus. Details of embryonic stem cell Southern blot analysis (and mouse genotyping) are available in Supplemental Fig. 1. Correctly targeted embryonic stem cell clones (∼4% of clones screened) were microinjected in C57BL/6J blastocysts, and male chimeras were subsequently bred to female C57BL/6J mice to produce the F1 generation. The resultant α2 colony was derived from embryonic stem cell clone 160-I4. Heterozygous mice were subsequently bred to FLPe mice (Rodriguez et al., 2000) on a C57BL/6J background to remove the Neo cassette. The FLPe transgene eventually was eliminated from the pedigree by further breeding. For some experiments, the knockin mutation was further backcrossed to C57BL/6J for an additional two generations (i.e., resulting in the N4 generation). For all experiments, heterozygous mice for the knockin allele were interbred to obtain wild-type controls (SL/SL), heterozygotes (SL/HA), or knockin (HA/HA) littermates. Mice were housed in a controlled environment with lights on at 7:00 AM and off at 7:00 PM. Mice had unlimited access to water and rodent chow. Institutional Animal Care and Use Committees approved all experiments.
Fig. 2.
Production of α2 knockin mice by gene targeting. A, partial DNA sequence of wild-type and mutant α2 genes. Note that the nucleotides highlighted in yellow in the knockin sequence denote the bases changed to alter the codons at amino acid positions 270 and 277. An additional nucleotide was altered to introduce an EcoRI restriction endonuclease recognition site that did not change the codon at position 274. B, gene-targeting strategy used to modify the α2 locus in embryonic stem cells. Exon 9 corresponds to nucleotides 859 to 1059 (note: nucleotide 1 corresponds to the start of translation) of the mouse α2 cDNA (Ensembl Transcript ID: ENSMUST00000000572). C, DNA sequence analysis of whole brain α2 reverse transcription-PCR products from SL/SL (top) or HA/HA (bottom) mice. This analysis demonstrates the presence of the introduced mutations in the α2 gene product in the brain of knockin mice. D, Western blot analysis of α2 GABAA-R subunits (top) or β-actin (bottom) in cortex. Shown is a representative immunoblot. After normalization to β-actin, the amount of α2 did not differ with respect to genotype (n = 4/genotype).
For analysis of α2 mRNA, whole-brain total RNA was extracted by using TRIzol (Invitrogen), and cDNA was prepared by using the Superscript First Strand Kit (Invitrogen) and oligo(dT) as recommended by the manufacturer. α2 cDNA was selectively amplified by using PCR Supermix (Invitrogen) with an exon 8 primer (5′-TGGCTGAACAGAGAATCGGTG-3′) and an exon 10 primer (5′-ATGGACTGACCCCTAATACAGGC-3′). PCR cycles consisted of 95°C for 1 min, followed by 35 cycles of 95°C for 20 s, 60°C for 30 s, and 72°C for 60 s. DNA sequence analysis of cDNA from exon 8 to exon 10 from SL/SL and HA/HA mice were compared.
For analysis of α2 protein levels in mice from the N3 generation, Western blot analysis was performed as described previously (Borghese et al., 2006). In brief, cerebral cortices were rapidly dissected out, frozen on dry ice, and stored at −80°C until further use. P2 membrane fractions were isolated, and 25 μg of protein per sample was denatured and subjected to SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA). Membranes were probed with an α2 GABAA-R subunit-specific antibody (Novus Biologicals Inc., Littleton, CO) followed by horseradish peroxidase-conjugated goat anti-rabbit (Novus Biologicals Inc.) and visualized by enhanced chemiluminescence (Western Lightning; PerkinElmer Life and Analytical Sciences, Waltham, MA). Membranes were reprobed with β-actin as a loading control (Abcam Inc., Cambridge, MA). Multiple exposures were used to ensure that the proteins fell within the linear range. Band intensities were quantified by densitometry, and genotypes were compared with Student's t test.
Electrophysiological Recordings in Brain Slices
Postnatal days 28 to 40, α2 SL/SL and HA/HA mice of the N4 generation were anesthetized with isoflurane, and coronal slices were prepared. Whole-cell patch-clamp recordings on dentate gyrus granule cells were performed under voltage-clamp using an Axopatch 200A (Axon Instruments, Union City, CA) amplifier at room temperature. This cell type was selected because the hippocampus is potentially involved in the amnestic effects of anesthetics and these cells robustly express the α2 subunit. Intracellular solution for voltage-clamp recordings contained 130 mM Cs-methanesulfonate, 8.3 mM Na-methanesulfonate, 1.7 mM NaCl, 1 mM CaCl2, 10 mM EGTA, 2 mM ATP-Mg2+, 0.3 mM GTP-Na+, and 10 mM HEPES, pH adjusted to 7.2 with CsOH. Access resistance was monitored by using a 5-mV test pulse throughout the recording period; cells were included for analysis only if the series resistance was less than 25 MOhm and the change of resistance was less than 25% during the experiment. During recordings, GABAA-R-mediated miniature inhibitory postsynaptic currents (mIPSCs) were recorded at 0 mV and isolated pharmacologically by bath application of the ionotropic excitatory amino acid receptor blocker kynurenic acid (5 mM) and tetrodotoxin (0.5 μM). Both drugs were obtained from Sigma-Aldrich (St. Louis, MO) and dissolved in artificial cerebrospinal fluid, which contained 124 mM NaCl, 2.5 mM KCl, 2 mM MgSO4, 2 mM CaCl2, 26 mM NaHCO3, 1.25 mM NaH2PO4, and 10 mM glucose. Isoflurane was obtained from Abbott Laboratories (Abbott Park, IL). Isoflurane solutions were prepared by injection of liquid anesthetic with a gas-tight syringe (Hamilton Co., Reno, NV) into intravenous solution bags containing artificial cerebrospinal fluid solution and were used within 2 h (Krasowski and Harrison, 2000).
Data were analyzed as described previously (Jia et al., 2005); in brief, off-line analysis was performed by using MiniAnalysis 5.5 (Synaptosoft, Decatur, GA), SigmaPlot 6.0 (SPSS Inc., Chicago, IL), and Excel 2000 (Microsoft, Redmond, WA). IPSCs were detected and analyzed by using MiniAnalysis. Unless otherwise indicated, averaged data are expressed as mean ± S.E.M. Statistical significance was assessed by using Student's t test or one-way analysis of variance (ANOVA) with a Dunnett test, and p < 0.05 was considered statistically significant.
Animal Behavioral Responses
Gross Behavioral Response to Anesthetics.
Adult (6–9 weeks old) mice of the N2 generation were placed in a sealed acrylic chamber and anesthetized with 0, 0.4, or 1.2% isoflurane or halothane (Halocarbon Laboratories, River Edge, NJ) in oxygen. Chamber temperature was maintained at 35 ± 0.2°C. Anesthetic concentrations were monitored by using a Datex Capnomac Ultima device (Datex-Ohmeda, Helsinki, Finland), and oxygen was delivered at a rate of 1.5 L/min. Mice were equilibrated at each concentration of anesthetic for at least 15 min. Mice were observed for overt behavioral responses (e.g., sedation, motor activity) while anesthetized and after termination of anesthetic exposure.
Recovery from Anesthesia.
Adult mice (∼10–15 weeks old) of the N2 or N4 generations were anesthetized with 1.5% isoflurane or 1.5% halothane as described above for 60 min. Approximately 30 min into the exposure period, the mice were placed in a supine position. Delivery of anesthesia to the chamber was terminated, anesthetic was removed from the chamber with an exhaust fan, and the air was replaced with 100% O2 at 5 L/min. The time from termination of anesthesia until each mouse spontaneously rolled over was recorded. Differences in recovery time between genotypes were compared by Student's t test.
Loss of Righting Reflex.
Adult (∼8–13 weeks old) male and female mice of the N2 generation were tested for LORR to inhalational anesthetics as described previously (Homanics et al., 1997). In brief, mice were placed into individual wire mesh cages and placed onto a rotating carousel within a sealed acrylic chamber and anesthetized with either isoflurane or halothane. Mice were equilibrated with concentrations ranging from 0.4 to 0.8% inhalational anesthetic for 15 min, and then LORR was assessed. Scoring was done by using a quantal method. The carousel rotated a total of five times at a speed of 4 rpm. Animals that passively rolled over twice while the carousel was rotating were given a positive score. After scoring at each concentration, mice recovered in anesthetic-free air for 20 min before the procedure was repeated at a different concentration. The dose response for each anesthetic was analyzed by using the Z statistic (Waud, 1972; Alifimoff et al., 1987).
Minimum Alveolar Concentration.
Mice (age ∼8–13 weeks) of the N2 generation were placed into an acrylic chamber and exposed to isoflurane or halothane as described previously (Homanics et al., 1997). In brief, mice were equilibrated with a given concentration of anesthetic for 20 min, after which the base of the tail was pinched with a hemostat. Scoring was done by using a quantal method. Any purposeful movement was considered a positive response. After scoring, mice recovered in anesthetic-free air for 20 min before the procedure was repeated at a different concentration. Data were analyzed as described above for LORR.
Fear Conditioning.
Fear conditioning was used to assess mice (age 10–12 weeks) of the N4 generation for isoflurane-induced amnesia. An average of eight mice of each genotype were assessed at each isoflurane concentration. Concentrations of 0.0, 0.2, 0.4, and 0.8% were tested. Before fear conditioning, mice were placed in a chamber (28 cm long × 12.5 cm wide × 17.5 cm high) equilibrated to the desired isoflurane concentration. A Gow-Mac gas chromatograph (Gow-Mac Instrument Corp., Bridgewater, NJ) equipped with a flame ionization detector and an infrared monitor (Instrumentarium Corp., Helsinki, Finland) were used to measure concentrations of isoflurane throughout the training procedures.
After 30 min of equilibration, the mice were quickly transferred to the training chambers, which contained the same isoflurane concentration as in the equilibration chamber. The chambers (32 cm long × 25 cm wide × 25 cm high) were constructed of clear acrylic. The grid floor used to deliver shock was composed of 36 stainless-steel bars, each 2.5 mm in diameter, spaced 6 mm apart. These floors were connected to a shock delivery system (MED Associates, St. Albans, VT). The chambers were wiped down with a pine-scented cleaner (5% pine-scented disinfectant; Midland, Inc., Sweetwater, TN) before and after each session. In the room in which training took place, the overhead fluorescent bulbs were left on and a ventilation fan provided background noise (65 db). The appearance, odor, and texture of the chambers and room comprised the training context.
After 3 min in the chambers, the mice received three tone (2000 Hz, 90 db)–shock (1 mA, 2 s) pairings, separated by 1 min. During training, freezing was scored only in animals receiving 0% isoflurane, because freezing may be visually difficult to distinguish from sedation in mice treated with isoflurane, thus confounding scoring procedures. Freezing, the absence of all movement except that necessary for respiration, is an innate defensive response in rodents and is a reliable measure of learned fear (Fanselow, 1994). Each animal's behavior was scored every 8 s during the observation period, and a percentage was calculated by dividing the number of freezing observations a mouse had by the total number possible during the observation period.
Over the next 2 days, mice were tested for fear to the training context and fear to tone. The testing order was counterbalanced between isoflurane concentration and genotype. For the context test, each mouse was placed back in the chamber in which it was trained for 8 min (in the absence of shock). For the tone test, groups of mice were transported in separate plastic pots (14 cm high × 15.5 cm diameter) to a different context in a different room. The test chambers were triangular in shape with an acrylic floor (28 cm long × 25 cm wide) and two acrylic sidewalls (28 cm long × 22 cm wide) at a 45° angle. The chambers were equipped with a speaker and were wiped down with 1% acetic acid (Fisher Scientific, Pittsburgh, PA) before and after each session. The room, lit by a single red 30-W bulb, seemed dark to the mice. No background noise was present during this test. Mice were given a 3-min exploratory period, then six 30-s tones (2000 Hz, 90 db) were presented, separated by 60 s. As with the context test, no shocks were administered during the tone test. Animals were removed from the chamber after an additional 30 s. Freezing was scored during both tests.
Split plot ANOVA was used to analyze freezing scores during training, and t tests were used to analyze context and tone freezing scores at 0% isoflurane. Nonlinear regression was used to calculate EC50 values and the maximum value of the dose-response curve for context and tone freezing scores. Equation 1 was used in the regression, with n = the Hill coefficient, and A = the maximal value.
Results
Pharmacology of Recombinant Receptors in Xenopus Oocytes
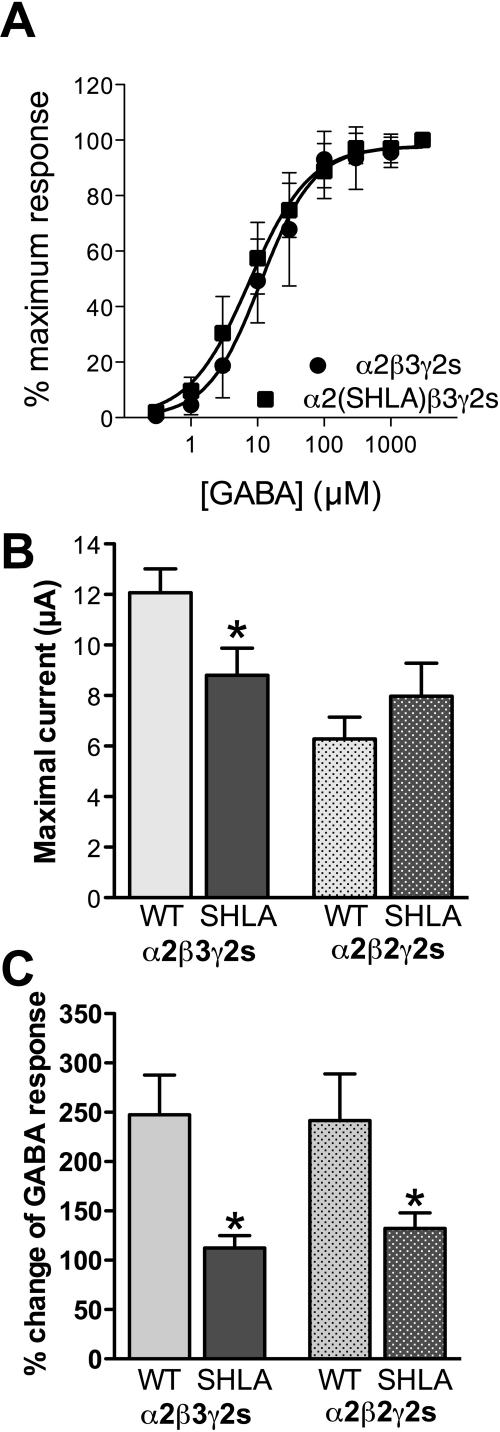
Recombinant GABAA-Rs containing either WT or mutant (SHLA) α2 subunits in combination with either β2γ2 or β3γ2 subunits were assessed. The GABA concentration response curves were similar for control and mutant α2 subunits coexpressed with β3γ2s subunits (Fig. 1A); the GABA EC50 values [WT, 11.0 μM (95% confidence interval = 8.44 to 14.87); SHLA, 7.6 μM (95% confidence interval = 5.82 to 9.84)] and the Hill coefficients (WT, 1.08 ± 0.14, SHLA, 0.96 ± 0.10) were not different. However, the maximal currents of mutant α2β3γ2s receptors were decreased 3 days after injection compared with WT receptors (p < 0.05; Fig. 1B). This difference was not observed for α2 coexpressed with β2γ2s (Fig. 1B). Potentiation by isoflurane (0.3 mM) of GABA responses was decreased ∼60% in mutant α2β3γ2s and α2β2γ2s compared with WT receptors (p < 0.05; Fig. 1C). GABA responses were inhibited similarly for WT and mutant receptors by 1 or 10 μM Zn2+ (Table 1). GABA responses were potentiated equally for the WT and mutant receptors by a benzodiazepine, flunitrazepam (1 μM), and by the intravenous anesthetic etomidate (1 μM). At higher concentrations, etomidate can act as a channel activator in the absence of GABA. Therefore, we tested the direct effects of 10 μM etomidate on receptor responses. There were no differences between control and mutant receptors.
Fig. 1.
GABA responses and their modulation in α2 wild-type and mutant (SHLA) subunits coexpressed with β2/3γ2s subunits in Xenopus oocytes. A, GABA concentration response curves (n = 7–8). B, maximal GABA-induced current 3 days after injection (n = 4–13). C, isoflurane (0.3 mM) modulation of EC5–10 GABA (n = 4). *, p < 0.05, t test.
TABLE 1.
Drug effects on α2β3γ2s wild-type and SHLA mutant receptors
Data are expressed as mean ± S.E.M. Numbers in parentheses are numbers of oocytes tested.
| Drug | Change of EC5–10 GABA Response |
Maximal GABA Response |
||
|---|---|---|---|---|
| α2β3γ2s | α2(SHLA)β3γ2s | α2β3γ2s | α2(SHLA)β3γ2s | |
| % | ||||
| Coapplied with EC5–10 GABA | ||||
| 1 μM Zn2+ | −12.2 ± 1.5 (4) | −9.2 ± 0.8 (4) | ||
| 10 μM Zn2+ | −49.1 ± 1.6 (8) | −44.4 ± 1.6 (8) | ||
| 1 μM flunitrazepam | 234 ± 22 (8) | 200 ± 42 (7) | ||
| 1 μM etomidate | 355 ± 83 (4) | 316 ± 29 (4) | ||
| Applied alone | ||||
| 10 μM etomidate | 3.9 ± 1.5 (4) | 3.9 ± 0.8 (4) | ||
Production and Characterization of α2 Knockin Mice
The α2 locus in embryonic stem cells was modified by using the targeting strategy outlined in Fig. 2. Correctly targeted embryonic stem cells were used to create chimeric mice, and the mutant locus was transferred through the germ line to generate heterozygous offspring. The neomycin-selectable marker flanked by FRT sites was deleted by site-specific recombination. Homozygous wild-type (SL/SL) and knockin (HA/HA) mice were produced from heterozygous (SL/HA) mating pairs for subsequent analysis.
GABAA α2 subunit mRNA was reverse-transcribed and amplified by PCR, and the resulting α2 cDNA was analyzed by sequence analysis. The sequence of the mRNA encoded by exon 9 from SL/SL mice was identical to that reported previously (Ensembl Transcript ID: ENSMUST00000000572). Analysis of HA/HA mice verified that only the intended mutations were introduced (Fig. 2C). Western blot analysis revealed that the amount of the α2 GABAA-R subunit did not differ between SL/SL and HA/HA mice in cortex (100 ± 9% versus 109 ± 12%, respectively; Fig. 2D) or hippocampus (100 ± 17% versus 94 ± 15%, respectively; data not shown).
We did observe an altered Mendelian distribution of genotypes of offspring at weaning from SL/HA breeding pairs; HA/HA mice were underrepresented. The genotype distribution of SL/SL, SL/HA, and HA/HA mice was 373:655:156. This differed from the 1:2:1 distribution expected (p < 0.0001; χ2 analysis). However, genotype analysis of a small number of fetuses collected during late gestation revealed that 11 of 27 fetuses were HA/HA. Thus, HA/HA animals were not underrepresented before birth. Those HA/HA mice that survived were normal in appearance and weight (e.g., at 10 weeks: SL/SL = 27.7 ± 0.6 g versus HA/HA = 27.8 ± 0.5 g), did not display any gross abnormality in overt behavior, and were indistinguishable from littermates in the home cage environment. Both male and female HA/HA mice were able to mate and reproduce. In addition, HA/HA females were able to feed and care for their offspring.
Slice Electrophysiology
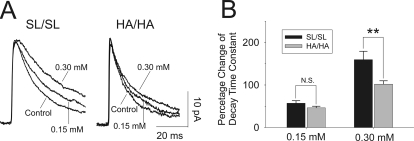
mIPSCs were recorded from hippocampal dentate granule cells (Fig. 3). The amplitude (SL/SL = 21.2 ± 4.5 pA, n = 13 versus HA/HA = 20.4 ± 2.0 pA, n = 14) and frequency of mIPSCs (SL/SL = 4.3 ± 2.4 Hz, n = 13 versus HA/HA = 3.9 ± 3.0 Hz, n = 14) did not differ significantly between genotypes. The average decay time constant of mIPSCs from HA/HA mice (24.1 ± 2.4 ms, n = 14) was reduced (p < 0.01) compared with SL/SL controls (34.6 ± 2.4 ms, n = 13).
Fig. 3.
Whole-cell patch clamp recordings from hippocampal dentate gyrus granule cells. A, the left traces illustrate the averaged mIPSCs (from 100 single events) recorded from a granule neuron from a SL/SL mouse in the absence or presence of 0.15 and 0.30 mM isoflurane. The right traces show the averaged mIPSCs from an α2 ΗΑ/ΗΑ animal. Under control conditions, the decay time constant of mIPSCs recorded from HA/HA mice was reduced compared with SL/SL (P < 0.01). B, histogram summarizing the effects of isoflurane on decay time constants. Isoflurane dose-dependently increased the decay time constants in both genotypes, but the increase in HA/HA mice was reduced compared with SL/SL mice in response to 0.30 mM isoflurane. **, p < 0.01.
Bath application of isoflurane (0.15 and 0.30 mM; 1 minimum alveolar concentration corresponds to 0.31 mM) dose-dependently increased the decay phase of mIPSCs recorded from dentate granule cells in SL/SL mice; 0.15 and 0.30 mM isoflurane increased the decay time constants to 54.5 ± 2.4 ms (n = 10) and 91.7 ± 6.3 ms (n = 13), respectively (Fig. 3A). In HA/HA mice, 0.15 and 0.30 mM isoflurane increased the averaged decay time constants to 30.7 ± 2.7 ms (n = 9) and 48.2 ± 4.8 ms (n = 14), respectively. To compare isoflurane responses between two genotypes, we normalized decay time constants to the control for each genotype (Fig. 3B). At 0.15 mM, isoflurane increased decay time constants similarly in both genotypes [SL/SL = 57.2 ± 6.0% (n = 10) versus HA/HA = 46.4 ± 0.38% (n = 13)]. In contrast, at 0.30 mM isoflurane, the percentage change of the decay time constant was significantly reduced in knockins (101.9 ± 8.0%, n = 13) compared with controls (159.6 ± 19.7%, n = 14; p < 0.01). Isoflurane did not affect frequency or amplitude of mIPSCs in either genotype (data not shown).
Behavioral Responses
Gross Response to Inhaled Anesthetics.
In initial experiments after production of the first HA/HA mice, we qualitatively compared them to SL/SL littermates for gross behavioral responses to isoflurane and halothane (see Supplemental Video). Upon exposure to 0.4% atm isoflurane, HA/HA mice were sedated and displayed less locomotor activity compared with SL/SL control littermates. At 1.2% atm isoflurane, both genotypes were sedated and did not ambulate, i.e., they were immobile. However, upon discontinuation of isoflurane anesthesia, SL/SL mice quickly recovered from anesthesia and moved about the test chamber. In contrast, HA/HA mice remained sedated and immobile for a longer time. In contrast to isoflurane, gross behavioral response to halothane did not differ on this assay (data not shown).
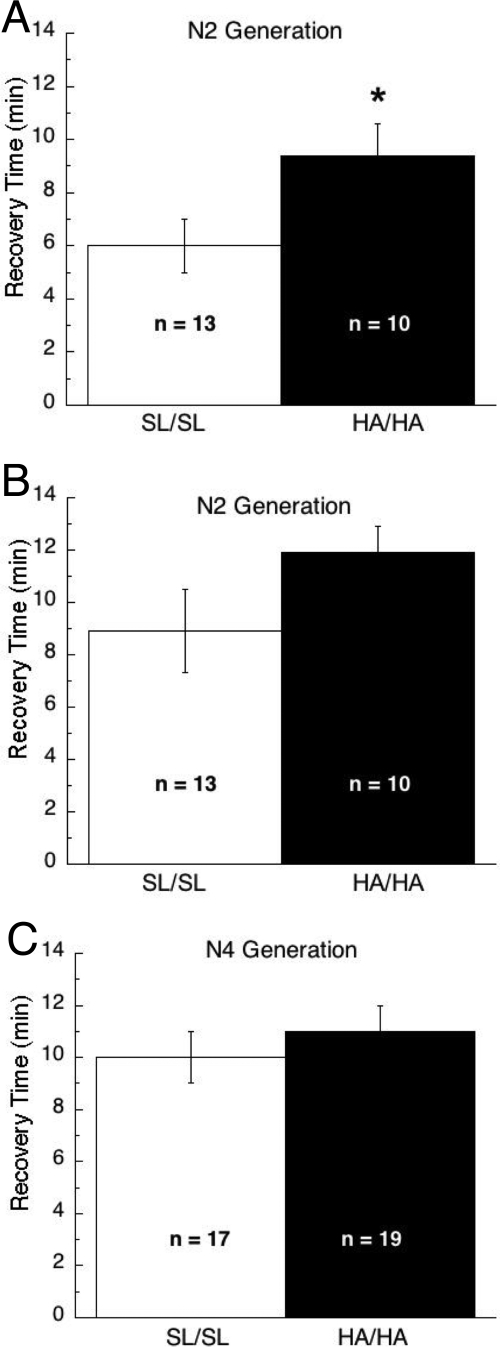
Recovery From Anesthesia.
To quantitatively assess recovery from anesthesia, mice were deeply anesthetized with 1.5% atm isoflurane or halothane. Mice were placed on their backs, anesthesia was discontinued, and the time until the animals regained the righting reflex and spontaneously rolled over was determined. Time to recover from isoflurane anesthesia was significantly longer for HA/HA mice compared with SL/SL mice (p < 0.05; Fig. 4A). In contrast, time to recover from halothane anesthesia did not differ between genotypes (Fig. 4B). To determine the reproducibility of the altered isoflurane phenotype and determine whether the effect persisted on a more uniform inbred genetic background, this same assay was later repeated on mice that had been backcrossed onto the C57BL/6J for two additional generations (i.e., the N4 generation). We were surprised to find that time to recover from isoflurane anesthesia did not differ between genotypes on this genetic background (Fig. 4C).
Fig. 4.
Recovery from isoflurane anesthesia. A, recovery time after exposure to 1.5% atm isoflurane was significantly different between genotypes when studied on mice on a N2 genetic background. B, recovery time after exposure to 1.5% atm halothane did not differ between mice on a N2 genetic background. C, no differences between genotypes were observed on this same assay when mice of the N4 genetic background were tested with 1.5% atm isoflurane. *, p < 0.05.
Loss of Righting Reflex.
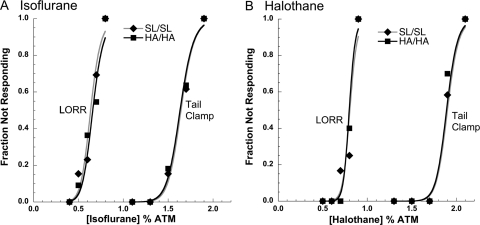
A standard LORR assay was used to compare genotypes on the N2 genetic background for the obtunding effect of isoflurane (Fig. 5A) and halothane (Fig. 5B). For isoflurane, neither the slopes of the concentration response curves (SL/SL: 11.3 ± 2.6 versus HA/HA: 11.2 ± 2.6) or the EC50 values (SL/SL: 0.64 ± 0.02% atm versus HA/HA: 0.66 ± 0.02% atm) differed between genotypes. Likewise, no differences between genotypes were observed for responses to halothane on this same assay; the slopes of the concentration response curves (SL/SL: 20.1 ± 5.4 versus HA/HA: 23.8 ± 7.6) and the EC50 values (SL/SL: 0.81 ± 0.18% atm versus HA/HA: 0.80 ± 0.02% atm) did not differ between genotypes.
Fig. 5.
Concentration response curves for LORR and tail clamp/withdrawal assays in response to isoflurane (A; n = 11–13/genotype) and halothane (B; n = 10–12/genotype). Genotypes did not differ on either assay for either anesthetic.
Minimum Alveolar Concentration.
A tail clamp/withdrawal assay was used to compare genotypes on the N2 genetic background for the effects of isoflurane (Fig. 5A) and halothane (Fig. 5B) on suppression of movement in response to a noxious stimulus. For isoflurane, neither the slopes of the concentration response curves (SL/SL: 22.6 ± 6.1 versus HA/HA: 21.7 ± 6.3) nor the EC50 values (SL/SL: 1.64 ± 0.03% atm versus HA/HA: 1.63 ± 0.04% atm) differed between genotypes. Likewise, no differences between genotypes were observed for responses to halothane on this same assay; the slopes of the concentration response curves (SL/SL: 30.7 ± 9.2 versus HA/HA: 31.1 ± 9.8) and the EC50 values (SL/SL: 1.90 ± 0.03% atm versus HA/HA: 1.89 ± 0.04% atm) did not differ between genotypes.
Fear Conditioning: Training and Testing Without Anesthesia.
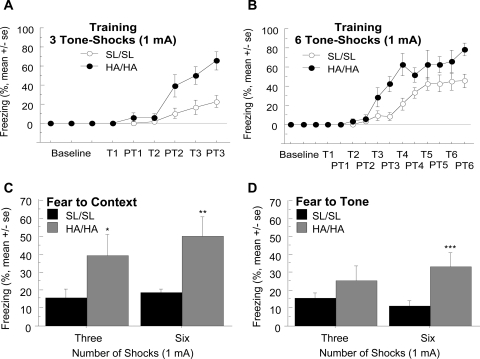
Mice on the N4 genetic background were tested for basal learning and memory by using fear conditioning. HA/HA mice showed enhanced acquisition during training compared with SL/SL control mice (Fig. 6A). This was confirmed by the split plot ANOVA, in which there was a main effect of genotype (F1,90 = 13.14, P < 0.005), a main effect of time (F5,90 = 28.79, P < 0.0001), and an interaction of the two (F5,90 = 6.76, P < 0.0001). Given that differences in freezing at 0% isoflurane during the test would make it difficult to evaluate a shift in EC50 values for isoflurane, we modified the protocol by increasing the number of tone–shock pairings from three to six. This method has worked previously to equate freezing in mutant and control strains, thus allowing for an appropriate assessment of the effect isoflurane on conditional fear (Rau et al., 2009). However, as with the protocol with three tone–shock pairings, HA/HA mice also showed enhanced acquisition during training compared with SL/SL mice (Fig. 6B). Split plot ANOVA indicated a main effect of genotype (F1, 20 = 13.37, P < 0.005), a main effect of time (F11,220 = 30.18, P < 0.0001), and an interaction of the two (F11,220 = 2.52, P < 0.01). HA/HA mice also displayed higher fear to context scores after both the three [t(18) = 4.30, P = 0.05] and six [t test, t(19) = 15.45, P < 0.001] tone–shock training protocols (Fig. 6C). HA/HA mice also had a higher level of fear to tone after the six tone–shock training protocol [t(20) = 10.15, P < 0.005], but not for the three tone–shock training protocol (Fig. 6D).
Fig. 6.
Fear conditioning during training. A and B, HA/HA mice show facilitated acquisition of conditional fear during training to the three tone–1-mA shock pairings (A, p < 0.0001) and the six tone–1-mA shock pairings (B, p < 0.005) compared with SL/SL mice. T refers to tone, and PT refers to posttone. C, HA/HA mice displayed increased performance on the fear to context test compared with SL/SL mice after training with either the three or six tone–shock pairings. D, HA/HA mice displayed increased performance on the fear to tone test compared with SL/SL mice after the six, but not the three, tone–shock pairings. n = 8–14/genotype. *, p < 0.05; **, p < 0.001; ***, p < 0.005.
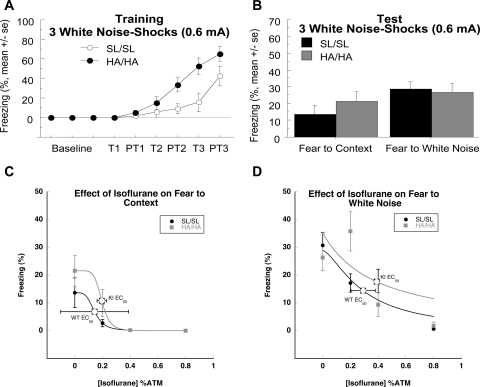
Because HA/HA mice displayed enhanced acquisition of learning and perform better in memory tests compared with SL/SL controls when the task is easier (higher shock intensities), mice were tested at a lower shock intensity of 0.6 mA. Because it is possible that the mice used in this study may have had impaired hearing (Zheng et al., 1999; Maison et al., 2006) instead of giving the mice pure tone as in the previous three and six tone–shock pairings, they were given three white noise (0 Hz, 75–80 db)–shock (0.6 mA) pairings. White noise contains a wide spectrum of tones, so it will be less affected by hearing loss of specific Hz intervals. Again, HA/HA mice displayed enhanced acquisition compared with SL/SL mice (Fig. 7A). Repeated measures showed there was a main effect of genotype (F1,80 = 7.69, P < 0.05), a main effect of time (F5,80 = 27.29, P < 0.0001), and an interaction of the two (F5,80 = 3.32, P < 0.01). However, SL/SL and HA/HA mice did not differ on fear to context and white noise tests (Fig. 7B).
Fig. 7.
Fear conditioning with white noise. A, SL/SL and HA/HA mice were trained by using three white noise (0 Hz, 75–80 db)–shock (0.6 mA) pairings. HA/HA show facilitated acquisition of learning compared with SL/SL mice (p < 0.05). n = 8–10/genotype. B, There was no difference between genotypes during testing, however. C, Effect of isoflurane on fear to context. SL/SL and HA/HA mice were statistically similar at 0% isoflurane during the fear to context test. EC50 values did not differ between genotypes for fear to context. D, SL/SL and HA/HA mice did not differ in fear to white noise at 0% isoflurane. EC50 values did not differ between genotypes for fear to tone. n = 6–10/genotype per concentration.
Isoflurane Effects on Context and Tone.
Because genotypes did not differ on context and white noise tests when trained with three white noise–0.6 mA shock pairings, this protocol was used to test isoflurane at 0.0, 0.2, 0.4, and 0.8% atm. Calculated EC50 values for SL/SL and HA/HA mice did not significantly differ for fear to context; isoflurane EC50 values were 0.14 ± 0.25% atm for SL/SL mice and 0.20 ± 0.03% atm for HA/HA mice (Fig. 7C). There was also no significant difference between the EC50 values calculated for fear to white noise; isoflurane EC50 values were 0.29 ± 0.09% atm for SL/SL mice and 0.39 ± 0.01% atm for HA/HA mice (Fig. 7D).
Discussion
In this article, we report on the characterization of mutant recombinant GABAA-R α2 subunits that are functionally normal with the exception that they show reduced sensitivity to potentiation by isoflurane. We subsequently introduced these mutations into the mouse germ line to produce gene knockin mice. Although approximately half of the knockin mice died prematurely, those that survived seemed overtly normal and were used to investigate the role of the α2 subunit of the GABAA-R in inhaled anesthetic action. In the first generation of knockin mice that were studied, the knockins were found to be paradoxically more sensitive to isoflurane exposure and recovered more slowly from isoflurane anesthesia. However, this altered sensitivity was not observed after backcrossing to C57BL/6J for two additional generations. Other isoflurane-induced behavioral alterations (LORR, tail clamp/withdrawal, and amnesia) were unaltered by the knockin. These results must be interpreted with caution because of the neonatal mortality observed. Because we could only study surviving knockin mice for behavioral responses to isoflurane, it is possible that the phenotype of the survivors may not be representative of those that did not survive. It is conceivable that a unique blend of genetic factors in combination with the knockin mutations may be responsible for animal viability and anesthetic responses in this mouse line.
Our studies of recombinant GABAA-Rs with mutations of Ser270 to His and Leu277 to Ala in the α2 subunit demonstrated normal affinity for GABA, but substantially reduced responses to the volatile anesthetic isoflurane. Previous studies have demonstrated that Ser270 is involved in modulation of GABAA-Rs by volatile anesthetics and alcohol both in vitro and in vivo (Mihic et al., 1997; Borghese et al., 2006). The L277A mutation is important for rescuing the alteration in GABA sensitivity induced by the S270H mutation (Borghese et al., 2006). It is noteworthy that, although not tested here on α2 receptors, incorporation of these mutations selectively reduces sensitivity to isoflurane but not to halothane (Borghese et al., 2006). The action of additional GABAA-R modulators such as Zn2+, etomidate, and flunitrazepam were not altered between control and mutant α2 receptors.
The finding that these mutations markedly reduced isoflurane sensitivity of α2 receptors but minimally affected other receptor responses, including response to the endogenous ligand GABA, prompted their introduction into the mouse germ line. We subsequently generated a knockin mouse line harboring these mutations to allow for in vivo analysis of α2-containing GABAA-Rs. We observed that the number of HA/HA mice at weaning from heterozygous matings was only ∼50% of that expected, suggesting lethality before weaning. A genotype analysis of several litters collected before birth did not reveal a deficiency of HA/HA embryos. This suggests that the HA/HA pups that died did so between birth and weaning. We do not know why those animals died. HA/HA knockin mice that survived were overtly normal and indistinguishable from controls. The amount of α2 subunit protein in cortex and hippocampus from HA/HA mice did not differ from controls.
In electrophysiological recordings from dentate gyrus granule cells, although most parameters measured were normal, mIPSCs decayed more quickly in cells from HA/HA mice compared with SL/SL mice, as evidenced by the decreased decay time constant. A similar result was observed in knockin mice with the same mutations in the α1 subunit of the GABAA-R (Sonner et al., 2007). In response to isoflurane, although the decay phase of mIPSCs was prolonged in both genotypes, this prolongation was substantially decreased in HA/HA mice compared with SL/SL mice, at the higher concentration of isoflurane tested, 0.30 mM. This result indicates that GABAA-R isoforms containing α2 subunits normally contribute to the mIPSC envelope in dentate granule cells and to prolongation of IPSCs by isoflurane. It is not surprising that anesthetic potentiation of mIPSC is not completely ablated, because these cells also express other α subunits (Sperk et al., 1997), notably α1 and α4, which retain their anesthetic sensitivity in the knockin animals. In addition, the knockin mutations do not totally remove isoflurane sensitivity (Fig. 1).
Our studies of the behavioral effects of isoflurane suggest that α2-containing GABAA-Rs do not play a key role in mediating several primary clinically relevant behavioral responses, namely obtunding effects (assayed by LORR), immobility (assayed by tail clamp/withdrawal), or amnesia (assayed by impairment of fear conditioning). Although immobility results from anesthetic action in the spinal cord (Antognini and Schwartz, 1993; Rampil et al., 1993), and α2 is highly expressed in the dorsal horn of the spinal cord (Bohlhalter et al., 1996), this negative result is consistent with the conclusions of previous studies with GABAA-R mutant mice and pharmacologic perturbations that GABAA-Rs have at most a limited role in mediating inhaled anesthetic-induced immobility (Eger et al., 2008). Furthermore, immobility is caused by actions of inhaled anesthetics on the ventral horn of the spinal cord (Barter et al., 2008; Jinks et al., 2008).
In contrast to the negative results observed on the LORR, tail clamp, and fear conditioning assays in response to isoflurane, we made the unexpected observation that HA/HA mice were more sensitive to isoflurane as evidenced by locomotor activity (see Supplemental Video) and a recovery from anesthesia assay. This is surprising because we hypothesized that if α2 receptors were important for an isoflurane-induced behavioral response, then mice with isoflurane-insensitive α2 receptors would be less (not more) sensitive to isoflurane. Such a result is not unprecedented, however. As we reported previously (Werner et al., 2006), this same mutation in the α1 subunit eliminated potentiation by ethanol at the receptor level, but at the behavioral level we observed that sensitivity to ethanol was decreased, unchanged, or actually increased depending on the behavioral endpoint tested. It is conceivable that these paradoxical effects reveal a circuit level phenomenon about the location of GABAA-Rs that are important for drug effects. For example, if α2 receptors on inhibitory interneurons are normally potentiated by isoflurane, this could serve to limit inhibitory output from those cells. In contrast, impairment of isoflurane potentiation at that same location by our knockin mutations would remove this limitation on inhibitory output, i.e., result in disinhibition. It is also possible that unexpected effects of the mutant and compensatory changes could account for the unexpected phenotypes. It is important to note that this paradoxical increase in isoflurane sensitivity on these particular endpoints was observed only in the first generation studied. Backcrossing to C57BL/6J for two additional generations eliminated the genotypic difference. This observation, together with the low number of homozygous mutant mice in the litters, suggests that the mutant subunit is not completely normal in function. At birth, the α2 is the major α subunit in brain, and only after birth does α1 emerge as the main GABAA-R subunit (Fritschy et al., 1994). Thus, newborns rely on α2 for GABAergic function and would be particularly sensitive to changes in function of this subunit.
Another indication that the knockin mutations are not completely “silent” is the finding that in studies of learning and memory in the absence of anesthesia we observed that HA/HA mice displayed increased learning and memory during training and testing compared with SL/SL controls. This is somewhat surprising, given that functional studies of recombinant and native mutant receptors in the current study showed near normal GABA responses and the levels of the mutant α2 subunit were not different from wild type. However, the introduced mutations produced some subtle changes in GABAA-R function. We observed some decrease of maximal current in recombinant receptors and HA/HA mice had faster mIPSCs. This could result in decreased inhibition and may thereby facilitate enhanced learning. This behavioral effect is consistent with other findings indicating that altering GABAA-R subunits facilitates acquisition of learning. For example, knockout of the α1 subunit produces enhanced fear to tone (Sonner et al., 2005; Wiltgen et al., 2009). Knockout of the α4 subunit generally facilitates acquisition of fear conditioning (Rau et al., 2009; Moore et al., 2010), whereas knockout of the α5 or delta subunit results in facilitated trace fear conditioning (Crestani et al., 2002; Wiltgen et al., 2005). Although the exact nature of the facilitation depends on the subunit and its anatomical distribution (Wiltgen et al., 2009), the phenotype found is typically a facilitation, not depression, of fear conditioning. Thus, although the α2 subunit may not play a role in mediating isoflurane-induced amnesia, it does play a role in both hippocampal-dependent and independent learning.
In summary, we engineered a mutant α2 GABAA-R subunit with substantially reduced response to the inhaled anesthetic isoflurane in Xenopus oocytes and in hippocampal slice recordings from gene-targeted mice. This allowed a critical test of the role of this subunit in isoflurane anesthesia. Behavioral analysis of gene knockin mice demonstrated that isoflurane potentiation of the α2 subunit is not required for immobility or amnesia, the major clinically relevant behavioral effects associated with isoflurane anesthesia.
Supplementary Material
Acknowledgments
We thank Carolyn Ferguson for expert technical assistance.
This work was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grants AA10422, AA16046, AA06399] and the National Institutes of Health National Institute of General Medical Sciences [Grant GM47818].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.170431.
 The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- GABAA-R
- GABAA receptor
- LORR
- loss of righting reflex
- mIPSC
- miniature inhibitory postsynaptic current
- ANOVA
- analysis of variance
- WT
- wild type
- PCR
- polymerase chain reaction.
Authorship Contributions
Participated in research design: Homanics, Harris, Fanselow, Eger, Sonner, Harrison, and Jia.
Conducted experiments: Werner, Iyer, Homanics, Borghese, McCracken, Rau, Eger, Swihart, Oh, and Jia.
Contributed new reagents or analytic tools: Homanics.
Performed data analysis: Werner, Iyer, Homanics, Borghese, McCracken, Rau, and Jia.
Wrote or contributed to the writing of the manuscript: Werner, Iyer, Homanics, Harris, Borghese, McCracken, Rau, Fanselow, Eger, Sonner, Harrison, and Jia.
References
- Alifimoff JK, Firestone LL, Miller KW. (1987) Anesthetic potencies of secondary alcohol enantiomers. Anesthesiology 66:55–59 [DOI] [PubMed] [Google Scholar]
- Antognini JF, Schwartz K. (1993) Exaggerated anesthetic requirements in the preferentially anesthetized brain. Anesthesiology 79:1244–1249 [DOI] [PubMed] [Google Scholar]
- Barter LS, Mark LO, Jinks SL, Carstens EE, Antognini JF. (2008) Immobilizing doses of halothane, isoflurane or propofol, do not preferentially depress noxious heat-evoked responses of rat lumbar dorsal horn neurons with ascending projections. Anesth Analg 106:985–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlhalter S, Weinmann O, Mohler H, Fritschy JM. (1996) Laminar compartmentalization of GABAA-receptor subtypes in the spinal cord: an immunohistochemical study. J Neurosci 16:283–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese CM, Werner DF, Topf N, Baron NV, Henderson LA, Boehm SL, 2nd, Blednov YA, Saad A, Dai S, Pearce RA, et al. (2006) An isoflurane- and alcohol-insensitive mutant GABAA receptor α1 subunit with near normal apparent affinity for GABA: characterization in heterologous systems and production of knock-in mice. J Pharmacol Exp Ther 319:208–218 [DOI] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Blüthmann H, Möhler H, Rudolph U. (2002) Trace fear conditioning involves hippocampal α5 GABA(A) receptors. Proc Natl Acad Sci USA 99:8980–8985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger EI, 2nd, Raines DE, Shafer SL, Hemmings HC, Jr, Sonner JM. (2008) Is a new paradigm needed to explain how inhaled anesthetics produce immobility? Anesth Analg 107:832–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow M. (1994) Neural organization of the defensive behavior system responsible for fear. Psychon Bull Rev 1:429–438 [DOI] [PubMed] [Google Scholar]
- Franks NP. (2008) General anesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci 9:370–386 [DOI] [PubMed] [Google Scholar]
- Franks NP, Lieb WR. (1994) Molecular and cellular mechanisms of general anesthesia. Nature 367:607–614 [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. (1995) GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol 359:154–194 [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Paysan J, Enna A, Mohler H. (1994) Switch in the expression of rat GABAA-receptor subtypes during postnatal development: an immunohistochemical study. J Neurosci 14:5302–5324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homanics GE, Elsen FP, Ying SW, Jenkins A, Ferguson C, Sloat B, Yuditskaya S, Goldstein PA, Kralic JE, Morrow AL, et al. (2005) A gain-of-function mutation in the GABA receptor produces synaptic and behavioral abnormalities in the mouse. Genes Brain Behav 4:10–19 [DOI] [PubMed] [Google Scholar]
- Homanics GE, Ferguson C, Quinlan JJ, Daggett J, Snyder K, Lagenaur C, Mi ZP, Wang XH, Grayson DR, Firestone LL. (1997) Gene knockout of the α6 subunit of the γ-aminobutyric acid type A receptor: Lack of effect on responses to ethanol, pentobarbital, and general anesthetics. Mol Pharmacol 51:588- 596 [DOI] [PubMed] [Google Scholar]
- Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA. (2005) An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol 94:4491–4501 [DOI] [PubMed] [Google Scholar]
- Jinks SL, Bravo M, Hayes SG. (2008) Volatile anesthetic effects on midbrain-elicited locomotion suggest that the locomotor network in the ventral spinal cord is the primary site for immobility. Anesthesiology 108:1016–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MV, Brooks PA, Harrison NL. (1992) Enhancement of γ-aminobutyric acid-activated Cl− currents in cultured rat hippocampal neurones by three volatile anesthetics. J Physiol 449:279–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, et al. (2003) General anesthetic actions in vivo strongly attenuated by a point mutation in the GABAA receptor β3 subunit. FASEB J 17:250–252 [DOI] [PubMed] [Google Scholar]
- Krasowski MD, Harrison NL. (2000) The actions of ether, alcohol and alkane general anesthetics on GABAA and glycine receptors and the effects of TM2 and TM3 mutations. Br J Pharmacol 129:731–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Rosahl TW, Homanics GE, Liberman MC. (2006) Functional role of the cochlea's GABAergic innervation: phenotypic analysis of mice lacking GABA(A) receptor subunits α1, α2,α5, α6, β2, β3, or δ. J Neurosci 26:10315–10326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. (1996) Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci 19:139–143 [DOI] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, et al. (2000) Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nat Neurosci 3:587–592 [DOI] [PubMed] [Google Scholar]
- Mihic SJ, McQuilkin SJ, Eger EI, 2nd, Ionescu P, Harris RA. (1994) Potentiation of γ-aminobutyric acid type A receptor-mediated chloride currents by novel halogenated compounds correlates with their abilities to induce general anesthesia. Mol Pharmacol 46:851–857 [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, et al. (1997) Sites of alcohol and volatile anesthetic action on GABAA and glycine receptors. Nature 389:385–389 [DOI] [PubMed] [Google Scholar]
- Moore MD, Cushman J, Chandra D, Homanics GE, Olsen RW, Fanselow MS. (2010) Trace and contextual fear conditioning is enhanced in mice lacking the α4 subunit of the GABA(A) receptor. Neurobiol Learn Mem 93:383–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. (1993) Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci USA 90:8424–8428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampil IJ, Mason P, Singh H. (1993) Anesthetic potency (MAC) is independent of forebrain structures in the rat. Anesthesiology 78:707–712 [DOI] [PubMed] [Google Scholar]
- Rau V, Iyer SV, Oh I, Chandra D, Harrison N, Eger EI, 2nd, Fanselow MS, Homanics GE, Sonner JM. (2009) Gamma-aminobutyric acid type A receptor α4 subunit knockout mice are resistant to the amnestic effect of isoflurane. Anesth Analg 109:1816–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds DS, Rosahl TW, Cirone J, O'Meara GF, Haythornthwaite A, Newman RJ, Myers J, Sur C, Howell O, Rutter AR, et al. (2003) Sedation and anesthesia mediated by distinct GABAA receptor isoforms. J Neurosci 23:8608–8617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM. (2000) High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet 25:139–140 [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brünig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Möhler H. (1999) Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature 401:796–800 [DOI] [PubMed] [Google Scholar]
- Simon J, Wakimoto H, Fujita N, Lalande M, Barnard EA. (2004) Analysis of the set of GABAA and glycine receptor genes in the human genome. J Biol Chem 279:41422–41435 [DOI] [PubMed] [Google Scholar]
- Sonner JM, Cascio M, Xing Y, Fanselow MS, Kralic JE, Morrow AL, Korpi ER, Hardy S, Sloat B, Eger EI, 2nd, et al. (2005) α1 subunit-containing GABA type A receptors in forebrain contribute to the effect of inhaled anesthetics on conditioned fear. Mol Pharmacol 68:61–68 [DOI] [PubMed] [Google Scholar]
- Sonner JM, Werner DF, Elsen FP, Xing Y, Liao M, Harris RA, Harrison NL, Fanselow MS, Eger EI, 2nd, Homanics GE. (2007) Effect of isoflurane and other potent inhaled anesthetics on minimum alveolar concentration, learning, and the righting reflex in mice engineered to express α1 γ-aminobutyric acid type A receptors unresponsive to isoflurane. Anesthesiology 106:107–113 [DOI] [PubMed] [Google Scholar]
- Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. (1997) GABAA receptor subunits in the rat hippocampus I: immunocytochemical distribution of 13 subunits. Neuroscience 80:987–1000 [DOI] [PubMed] [Google Scholar]
- Waud DR. (1972) On biological assays involving quantal responses. J Pharmacol Exp Ther 183:577–607 [PubMed] [Google Scholar]
- Werner DF, Blednov YA, Ariwodola OJ, Silberman Y, Logan E, Berry RB, Borghese CM, Matthews DB, Weiner JL, Harrison NL, et al. (2006) Knockin mice harboring ethanol insensitive α1-containing GABAA receptors display selective alterations in behavioral responses to ethanol. J Pharmacol Exp Ther 319:219–227 [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Godsil BP, Peng Z, Saab F, June HL, Linn ML, Cook JM, Houser CR, O'Dell TJ, Homanics GE, et al. (2009) The α1 subunit of the GABAA receptor modulates fear learning and plasticity in the lateral amygdala. Front Behav Neurosci 3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Ferguson C, Homanics GE, Fanselow MS. (2005) Trace fear conditioning is enhanced in mice lacking the delta subunit of the GABAa receptor. Learn Mem 12:327–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. (1999) Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res 130:94–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman SA, Jones MV, Harrison NL. (1994) Potentiation of γ-aminobutyric acid A receptor Cl− current correlates with in vivo anesthetic potency. J Pharmacol Exp Ther 270:987–991 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.