Abstract
Pyrethroid insecticides bind to voltage-gated sodium channels (VGSCs) and modify their gating kinetics, thereby disrupting neuronal function. Pyrethroids have also been reported to alter the function of other channel types, including activation of voltage-gated calcium channels. Therefore, the present study compared the ability of 11 structurally diverse pyrethroids to evoke Ca2+ influx in primary cultures of mouse neocortical neurons. Nine pyrethroids (tefluthrin, deltamethrin, λ-cyhalothrin, β-cyfluthrin, esfenvalerate, S-bioallethrin, fenpropathrin, cypermethrin, and bifenthrin) produced concentration-dependent elevations in intracellular calcium concentration ([Ca2+]i) in neocortical neurons. Permethrin and resmethrin were without effect on [Ca2+]i. These pyrethroids displayed a range of efficacies on Ca2+ influx; however, the EC50 values for active pyrethroids all were within one order of magnitude. Tetrodotoxin blocked increases in [Ca2+]i caused by all nine active pyrethroids, indicating that the effects depended on VGSC activation. The pathways for deltamethrin- and tefluthrin-induced Ca2+ influx include N-methyl-d-aspartic acid receptors, L-type Ca2+ channels, and reverse mode of operation of the Na+/Ca2+ exchanger inasmuch as antagonists of these sites blocked deltamethrin-induced Ca2+ influx. These data demonstrate that pyrethroids stimulate Ca2+ entry into neurons subsequent to their actions on VGSCs.
Introduction
Pyrethroids are synthetic insecticidal compounds that resemble pyrethrins, natural toxins present in the flowers of Chrysanthemum sp. Pyrethroids account for approximately one-fourth of the insecticide market worldwide (Casida and Quistad, 1998), and evidence indicates that use of these compounds is increasing (Amweg et al., 2005; Spurlock and Lee, 2008). This usage results in an increased potential for human exposure, including exposures to pregnant women, infants, and children (Whyatt et al., 2002; Morgan et al., 2007). Based on both their chemical structures and biological effects of high-dose acute exposure, pyrethroids are classified into two major groups: type I pyrethroids lack a cyano group at the α carbon of the 3-phenoxybenzyl alcohol moiety and produce a poisoning syndrome consisting of hyperexcitation, tremors, and convulsions (T syndrome), whereas type II pyrethroids have a cyano group at the α carbon of the 3-phenoxybenzyl alcohol moiety and produce a syndrome consisting of hypersensitivity, choreoathetosis, salivation, and seizures (CS syndrome). There are a few pyrethroids that produce mixed signs, including both tremors and salivation, and these are classified accordingly as type I/II (Verschoyle and Barnes, 1972; Barnes and Verschoyle, 1974; Verschoyle and Aldridge, 1980).
Voltage-gated sodium channels (VGSCs) are responsible for the rapid influx of sodium that underlies the rising phase of the action potential in most electrically excitable cells, including neurons. VGSCs represent the molecular target for a broad range of potent neurotoxins that bind specifically to at least six distinct receptor sites (sites 1–6) on the sodium channel α-subunit and affect the permeability and gating of the channel (Catterall et al., 2007). Pyrethroids bind to a site on the sodium channel α-subunit that is distinct from sites 1 to 6 (Catterall et al., 2007) and affect nervous system function by altering their normal gating kinetics (Narahashi, 1996; Soderlund et al., 2002). As such, VGSCs are the primary molecular targets for pyrethroid's insecticidal activity and their acute neurotoxicity in vertebrates (Soderlund et al., 2002).
In addition to their actions on VGSCs, pyrethroids have been reported to interact with other ion channel types in a variety of in vitro systems, including altering the function of voltage-gated calcium channels (VGCCs) (Soderlund et al., 2002; Clark and Symington, 2007; Neal et al., 2010). However, the extent to which actions on VGCCs contribute to pyrethroid neurotoxicity remains to be clarified (Shafer and Meyer, 2004). For example, electrophysiological assessments have reported either inhibition (allethrin; Hildebrand et al., 2004) or facilitation (deltamethrin; Symington et al., 2008) of current through calcium channels expressed in HEK cells and oocytes, respectively. Furthermore, deltamethrin-induced increases in [Ca2+]i and glutamate release measured in synaptosomal preparations (Symington et al., 2005) were not replicated in intact neurons in vitro (Meyer and Shafer, 2006) and may depend on phosphorylation status of the channel (Symington et al., 2007). These uncertainties regarding pyrethroid actions on VGCC function confound a better understanding of how effects on VGCCs may contribute to pyrethroid acute neurotoxicity (Shafer and Meyer, 2004). It has been proposed that, in addition to actions on VGSC, pyrethroid effects on VGCC may constitute a separate key initiating event in pyrethroid neurotoxicity (Soderlund et al., 2002; Breckenridge et al., 2009). Thus, the extent to which actions of pyrethroids on VGCC observed in non-neuronal/nonmammalian or subcellular fractions can be replicated in intact neurons will inform the robustness of proposed modes of action involving direct effects on VGCC. To date, studies of the actions of pyrethroids on calcium channel function in intact neurons are lacking, particularly those in which actions on voltage-gated sodium channel function are also available. Consequently, the calcium-responsive fluorescent dye fluo-3 was used in the present studies to compare the effects of 11 structurally diverse pyrethroids on [Ca2+]i in primary cultures of neocortical neurons. The use of intact neurons allows for measurement of pyrethroid effects on VGCC function in a manner that preserves intracellular modulation of ion channel function such as by phosphorylation. In addition, the use of primary cultures of mammalian neurons provides a model system that is more relevant to mammalian neurotoxicity than clonal cell lines or mammalian or nonmammalian expression systems (e.g., HEK cells or oocytes, respectively), because the native complement of ion channels and other proteins will be present in this system.
Materials and Methods
Materials.
Trypsin, penicillin, streptomycin, heat-inactivated fetal bovine serum, horse serum, and soybean trypsin inhibitor were obtained from Atlanta Biologicals (Norcross, GA). Minimum essential medium, deoxyribonuclease (DNase), poly-l-lysine, cytosine arabinoside, veratridine, (5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate (MK-801), nifedipine, 2-aminoethoxydiphenylborane (2-APB), and thapsigargin were from Sigma-Aldrich (St. Louis, MO). The fluorescent dye fluo-3, pluronic acid F-127, Neurobasal medium, and B-27 were obtained from Invitrogen (Carlsbad, CA). 2-[2-[4-(4-Nitrobenzyloxy)phenyl]ethyl]isothiourea mesylate (KBR-7943) and 1-[6-[[(17b)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione (U73122) were from Tocris Bioscience (Ellisville, MO). The pyrethroids used in this study are the same compounds used by Wolansky et al. (2006), and information on the source and isomer composition are provided therein.
The compounds used were (purity): deltamethrin (98.9%), β-cyfluthrin (99.2%), cypermethrin (88%), permethrin (96%), bifenthrin (95%), esfenvalerate (98.6%), λ-cyhalothrin (87.7%), tefluthrin (92.6%), fenpropathrin (91.8%), resmethrin (92.3%), and S-bioallethrin (95.6%). These are the same compounds as used by Wolansky et al. (2006).
Compound Preparation.
Each pyrethroid was dissolved in dimethyl sulfoxide (DMSO) at a stock concentration of 100 mM. An aliquot of stock solution of each pyrethroid was then diluted using DMSO to give a series of secondary stock solutions of 30, 10, 3, 1, 0.3, 0.1, and 0.03 mM. These secondary stock solutions were kept at −20°C in the dark. Before each experiment an equal volume of pluronic acid F-127 (20% in DMSO) was then mixed with the secondary stock solution and diluted 250-fold by using Locke's buffer (8.6 mM HEPES, 5.6 mM KCl, 154 mM NaCl, 5.6 mM glucose, 1.0 mM MgCl2, 2.3 mM CaCl2, 0.0001 mM glycine, pH 7.4). This dilution in Locke's buffer resulted in solutions of pyrethroid that were four times the desired final concentrations in the assay. The final concentration of DMSO was 0.2% for all chemicals, and this was without effect on [Ca2+]i measurements.
Neocortical Neuron Culture.
All animal use protocols were approved by the Creighton University Institutional Animal Care and Use Committee. Primary cultures of neocortical neurons were obtained from embryonic day 16 Swiss-Webster mice as described by Cao et al. (2007). In brief, pregnant mice were euthanized by CO2 asphyxiation, embryos were removed from the dam, and their neocortices were collected. Isolated neocortices were then removed of their meninges, minced by trituration using a Pasteur pipette, and treated with trypsin for 25 min at 37°C. The cells were then dissociated by two successive trituration and sedimentation steps in soybean trypsin inhibitor and DNase-containing isolation buffer, centrifuged, and resuspended in Eagle's minimal essential medium with Earle's salt and supplemented with 2 mM l-glutamine, 10% fetal bovine serum, 10% horse serum, 100 I.U./ml penicillin, and 0.10 mg/ml streptomycin, pH 7.4. Cells were plated onto poly-l-lysine-coated 96-well (9 mm) clear-bottomed black plates at a density of 1.5 × 105 cells/well (∼500,000 cells/cm2). Plates were incubated at 37°C with 5% CO2 and 95% humidity. Cytosine arabinoside (10 μM) was added to the culture medium on day 2 after plating to prevent proliferation of non-neuronal cells. The culture medium was changed on days 5 and 7 by using a serum-free growth medium containing Neurobasal medium supplemented with B-27, 100 units/ml penicillin, 0.10 mg/ml streptomycin, and 0.2 mM l-glutamine. Neocortical cultures were used in experiments between 8 and 10 days in vitro (DIV).
Measurement of Intracellular Calcium Concentration.
The culture medium from neocortical neurons in 96-well clear-bottomed black plates was removed and replaced with dye loading buffer (100 μl/well) containing 4 μM fluo-3 and 0.04% Pluronic F-127 in Locke's buffer. After 1-h incubation in dye loading buffer, cells were washed five times with Locke's buffer using an automated cell washer (BioTek Instruments, Winooski, VT), leaving a final volume of 150 μl in each well. The plate was then transferred to a fluorescence laser plate reader (Molecular Devices, Sunnyvale, CA) chamber. Cells were excited at 488 nm, and Ca2+-bound fluo-3 emission in the 500- to 600-nm range was recorded. Fluorescence readings were taken once every 3 s for 2 min to establish the baseline, and then 50 μl of test compound solution (4×) was added to each well from the compound plate, yielding a final volume of 200 μl/well. The cells were exposed to test compounds for another 13 min. Background fluorescence was automatically subtracted from all fluo-3 fluorescence measurements.
Data Analysis.
Time-response and concentration-response relationships were generated by using Prism software (GraphPad Software, Inc., San Diego, CA). The potency and efficacy values for pyrethroid-induced calcium influx were determined by nonlinear regression analysis. A three-parameter logistic equation was used to fit the concentration-response data: Y = bottom + (top − bottom)/(1 + 10(LogEC50−X)). In this equation, X is the logarithm of the agonist concentration and Y is the response. The variable bottom is the Y value at the bottom plateau; top is the Y value at the top plateau, and LogEC50 is the logarithm of the EC50 value (50% effective concentration). Statistical significance was determined by an ANOVA and, where appropriate, a Dunnett's multiple comparison test was performed to compare responses of vehicle and pyrethroid-induced calcium influx values.
Results
Pyrethroids Produce Ca2+ Influx in Neocortical Neurons.
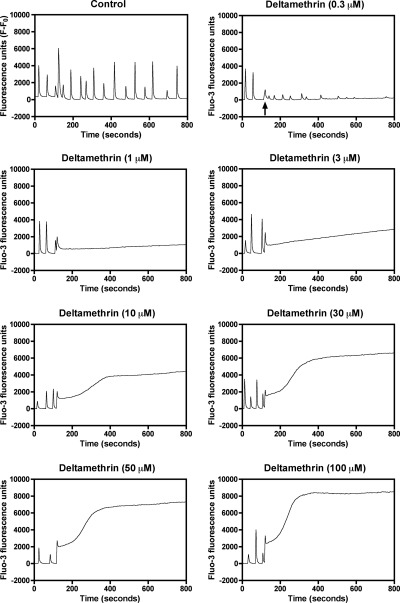
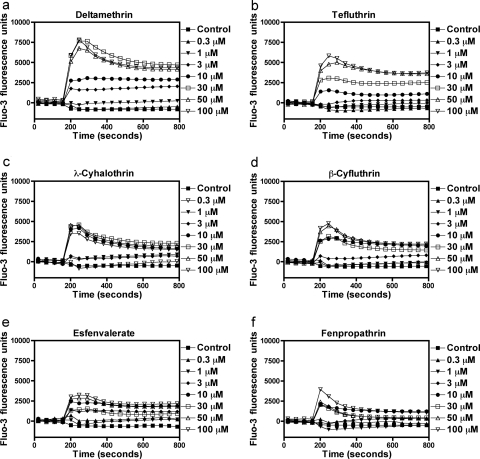
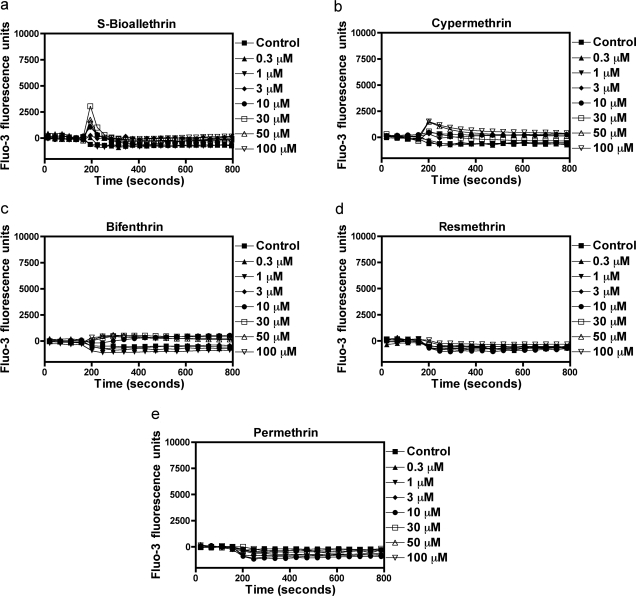
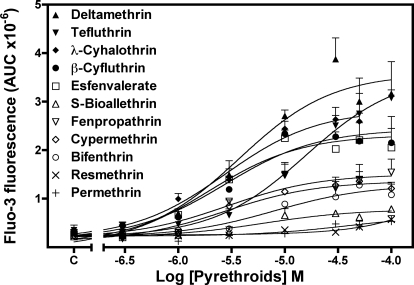
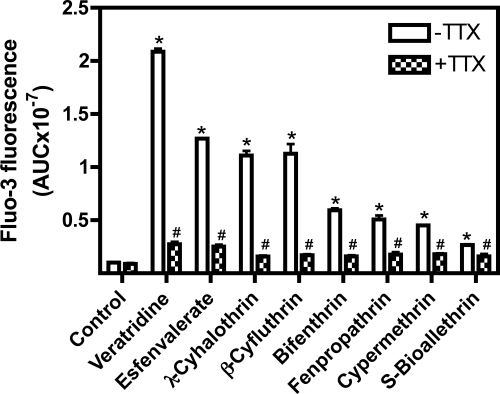
Type II pyrethroids have been reported to increase current through N-type calcium channels expressed in oocytes (Symington and Clark, 2005) and stimulate TTX-insensitive Ca2+ influx into synaptosomes (Symington et al., 2008). By contrast, the neurotoxicity induced by brevetoxins, sodium channel neurotoxin site 5 agonists, is primarily through the indirect activation of NMDA receptors with attendant Ca2+ influx in cerebellar granule cells (Berman and Murray, 2000). Radioligand binding and electrophysiological experiments have revealed that the pyrethoid binding site is intrinsic to the sodium channel α subunit (Smith and Soderlund, 1998; Choi and Soderlund, 2006). Primary cultures of neocortical neurons at DIV 10 to 13 display synchronized spontaneous Ca2+ oscillations that are associated with action potential generation (Fig. 1). The ability of 11 pyrethroids to stimulate Ca2+ influx was examined in these DIV 10 to 13 neocortical neurons. As a positive control for activation of Ca2+ influx secondary to activation of VGSCs, neocortical neurons were treated with veratridine, a sodium channel activator. As illustrated in Fig. 1, deltamethrin produced a robust Ca2+ influx in a concentration-dependent manner (Fig. 1). Similar to deltamethrin, five additional pyrethroids (tefluthrin, λ-cyhalothrin, β-cyfluthrin, esfenvalerate, and fenpropathrin) produced substantial elevations of neuronal [Ca2+]i in neocortical neurons (Fig. 2), as did the VGSC activator veratridine (Supplemental Fig. 1). Three pyrethroids (S-bioallethrin, cypermethrin, and bifenthrin) produced very little in neuronal [Ca2+]i in neocortical neurons (Fig. 3). The Ca2+ influx response to the active pyrethroids was rapid and sustained for at least 5 min with the exception of S-bioallethrin. S-bioallethrin-induced Ca2+ influx recovered to baseline levels within 2 min. Two pyrethroids, resmethrin and permethrin, were without effect on Ca2+ influx (Fig. 3). The concentration-response (area under the curve) relationships fit by nonlinear regression analysis are depicted in Fig. 4. The EC50 values for pyrethroid-induced Ca2+ influx were within one order of magnitude ranging from 1.93 (esfenvalerate) to 15.7 μM (tefluthrin). Pyrethroid-induced Ca2+ influx also displayed a range of efficacies. The potencies (EC50 values) and efficacies of these nine active pyrethroids and veratridine on Ca2+ influx are summarized in Table 1. All of the cyano-containing compounds were slightly more potent than veratridine, although their efficacies were lower. The noncyano pyrethroids all were less potent than veratridine. The rank order of efficacy was veratridine > tefluthrin = deltamethrin > λ-cyhalothrin > β-cyfluthtin > esfenvalerate > fenpropathrin > cypermethrin > bifenthrin > S-bioallethrin.
Fig. 1.
Representative time-response relationships for deltamethrin-induced elevation of neuronal [Ca2+]i after exposure to different concentrations of deltamethrin. The fluo-3 fluorescence raw data depicted used a sampling frequency of every 4 s. This experiment was repeated three times with similar results.
Fig. 2.
Time-response relationships for the ability of active pyrethroids to stimulate neuronal [Ca2+]i. The active pyrethroids were deltamethrin (a), tefluthrin (b), λ-cyhalothrin (c), β-cyfluthrin (d), esfenvalerate (e), and fenpropathrin (f). This experiment was repeated three times with similar results. For clarity, the fluorescence signals over 45-s epochs were averaged.
Fig. 3.
Time-response relationships for minimally active or inactive pyrethroids on neuronal [Ca2+]i. These pyrethroids were S-bioallethrin (a), cypermethrin (b), bifenthrin (c), resmethrin (d), and permethrin (e). These representative data for pyrethroid-induced Ca2+ influx were simultaneously recorded in neocortical neurons cultured in one 96-well plate using a fluorescence laser plate reader. This experiment was repeated three times with similar results.
Fig. 4.
Nonlinear regression analysis of the [Ca2+]i response of area under curve (AUC) as a function of the concentration of pyrethroids (deltamenthrin, tefluthrin, λ-cyhalothrin, β-cyfluthrin, esfenvalerate, S-bioallethrin, fenpropathrin, cypermethrin, bifenthrin, resmethrin, and permethrin).
TABLE 1.
Potencies and efficacies for veratridine and pyrethroid-induced Ca2+ influx
| Compounds | EC50 (95% CI) | Efficacy |
|---|---|---|
| μM | ||
| Veratridine | 4.76 (2.36–9.61) | 1 |
| Tefluthrin | 15.7 (7.97–31.1) | 0.48 |
| Deltamethrin | 3.75 (1.70–9.99) | 0.48 |
| λ-Cyhalothrin | 2.76 (1.39–5.46) | 0.38 |
| β-Cyfluthrin | 2.76 (1.36–5.59) | 0.33 |
| Esfenvalerate | 1.93 (0.95–3.92) | 0.31 |
| Fenpropathrin | 2.89 (0.70–11.9) | 0.20 |
| Cypermethrin | 2.73 (1.21–6.15) | 0.18 |
| Bifenthrin | 7.95 (1.86–33.9) | 0.17 |
| S-Bioallethrin | 6.34 (1.53–26.3) | 0.11 |
| Resmethrin | N.A. | N.A. |
| Permethrin | N.A. | N.A. |
N.A., no activity.
Pyrethroid-Induced Ca2+ Influx in Neocortical Neurons Is TTX Sensitive.
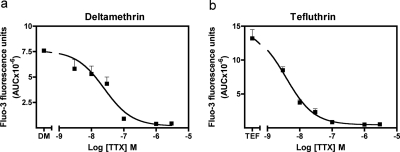
To determine whether pyrethroid-induced Ca2+ influx in neocortical neurons was secondary to activation of VGSC, or triggered by direct actions on voltage-gated calcium channels, we first performed a titration with TTX in the presence of a fixed concentration (30 μM) of the type I pyrethroid, tefluthrin, or a type II pyrethroid, deltamehtirn. As depicted in Fig. 5a, titration with TTX produced a concentration-dependent reduction in the deltamethrin-induced integrated fluo-3 response with an IC50 value of 24.5 nM [15.5–38.8, 95% confidence interval (95% CI)]. Likewise, TTX concentration dependently inhibited tefluthrin (30 μM)-induced Ca2+ influx with an IC50 value of 3.75 nM (2.68–5.24, 95% CI) (Fig. 5b). These data indicate that TTX has nanomolar potency in blocking both type I and II pyrethroid-induced calcium influx. As shown in Fig. 6, TTX (1 μM) abrogated Ca2+ influx stimulated by all of the other active pyrethroids (30 μM). These data demonstrate that pyrethroid-induced Ca2+ influx is secondary to the activation of VGSC. TTX also eliminated veratridine-induced Ca2+ influx in neocortical neurons (Fig. 6).
Fig. 5.
Effects of TTX on the elevation of [Ca2+]i induced by 30 μM deltamenthrin (a) or tefluthrin (b). TTX concentration dependently inhibited deltamethrin- or tefluthrin-induced Ca2+ influx. These experiments were repeated twice in quadruplicate with similar results.
Fig. 6.
Effects of TTX on the elevation of [Ca2+]i induced by 30 μM veratridine, esfenvalerate, λ-cyhalothrin, β-cyfluthrin, bifenthrin, fenpropathrin, S-bioallethrin, or cypermethrin. These experiments were performed twice in triplicate with similar results. *, p < 0.01, pyrethroid (veratridine) versus control; #, p < 0.01, pyrethroid (veratridine) + TTX versus pyrethroid (veratridine).
Pharmacological Evaluation of Deltamethrin-Induced Ca2+ Influx.
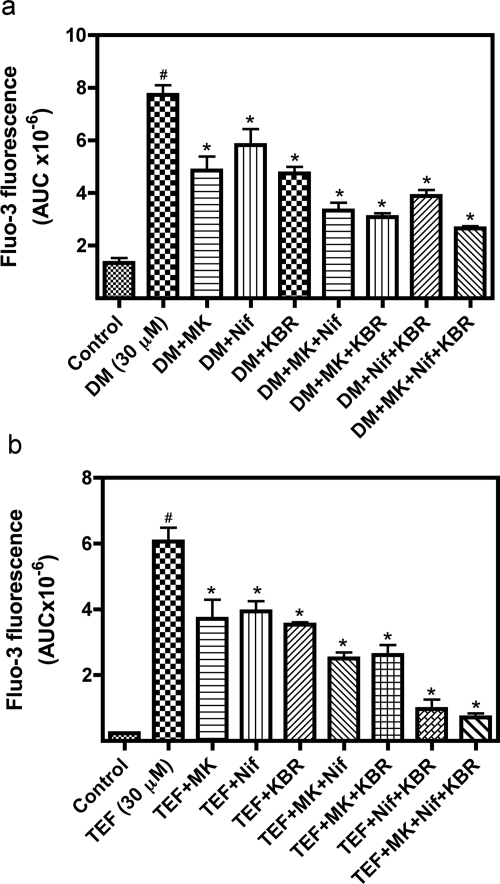
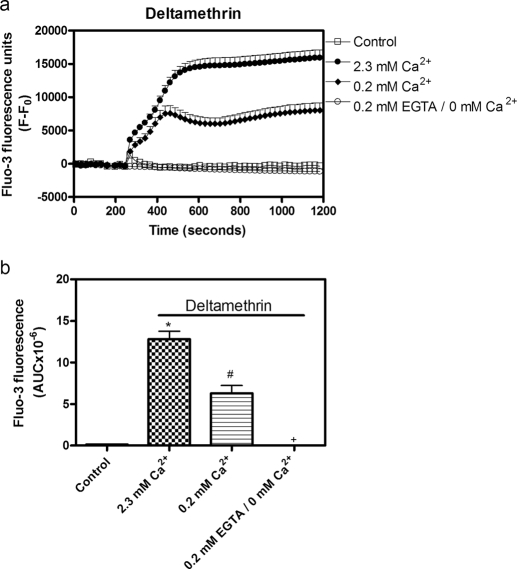
In cultured cerebellar granule cells the sodium channel neurotoxin site 5 activator, brevetoxin, stimulates Ca2+ influx through NMDA receptors, L-type Ca2+ channels, and reversed Na+/Ca2+ exchange (Berman and Murray, 2000). It was therefore of interest to determine the Ca2+ influx routes in neocortical neurons after pyrethroid exposure. We used both a type I pyrethroid, tefluthrin, and a type II compound, deltamethrin, as representative probes to explore the calcium influx pathways because of their high relative efficacies. As shown in Supplemental Fig. 2, the NMDA receptor antagonist MK-801 and the L-type Ca2+ channel antagonist nifedipine produced concentration-dependent partial inhibition of deltamethrin-induced Ca2+ influx. A 1 μM concentration of MK-801 or nifedipine was sufficient to produce maximal response. KBR-7943 has been shown to selectively inhibit the reverse mode of operation of the plasma membrane Na+/Ca2+ exchanger (Iwamoto et al., 1996). A concentration of 3 μM KBR-7943 has been demonstrated to attenuate brevetoxin-induced Ca2+ influx in cerebellar granule cells (Berman and Murray, 2000). We therefore used a single concentration of MK-801 (1 μM), nifedipine (1 μM), or KBR-7943 (3 μM) to evaluate the calcium pathways for deltamethrin- and tefluthrin-induced Ca2+ influx. MK-801 (1 μM) produced a significant reduction in the area under the fluo-3 fluorescence curve after deltamethrin (30 μM) exposure. Nifedipine (1 μM) and KBR-7943 (3 μM) also significantly reduced deltamethrin-induced Ca2+ influx. Nifedipine together with MK-801 or KBR-7943 produced additive reductions in deltamethrin-induced Ca2+ influx. Likewise, MK-801 produced a significant additional reduction in [Ca2+]i when combined with KBR-7943. Pretreatment with MK-801, nifedipine, and KBR-7943 together produced a nearly complete inhibition in deltamethrin-induced Ca2+ influx (Fig. 7a). Similar results were observed for pharmacological antagonism of tefluthrin-induced Ca2+ influx (Fig. 7b). Thus, the major routes of Ca2+ entry after deltamethrin or tefluthrin exposure in neocortical neurons were similar and involve NMDA receptors, L-type Ca2+ channels, and reversed Na+/Ca2+ exchange and therefore were similar to that previously observed for brevetoxin-induced Ca2+ influx in cerebellar granule cells (Berman and Murray, 2000). To further explore the Ca2+ source for pyrethroid-induced elevation of [Ca2+]i, we evaluated the role of extracellular Ca2+ and intracellular Ca2+ stores in the response to deltamethrin. As depicted in Fig. 8, decreasing the extracellular Ca2+ concentration from 2.3 to 0.2 mM produced a significant reduction on deltamethrin-induced elevation of [Ca2+]i. Complete removal of extracellular Ca2+ eliminated the deltamethrin-induced elevation of [Ca2+]i (Fig. 8). In contrast, depletion of intracellular Ca2+ stores by preincubation with thapsigargin (1 μM) for 1 h was without effect on the response to deltamethrin (Fig. 9). One of the main routes for the release of Ca2+ from intracellular Ca2+ store is the activation of inositol(1,4,5)triphosphate (IP3) receptors located on the membrane of the endoplasmic reticulum. As depicted in Fig. 9, 2-APB (10 μM), an IP3 receptor antagonist, was without effect on the response to deltamethrin. The level of IP3 is regulated by phospholipase C (PLC) activity. PLC catalyzes the cleavage of membrane-bound phosphatidylinositol 4,5-biphosphate into the second messengers IP3 and diacylglycerol. We therefore evaluated the influence of a PLC inhibitor U73122 on the response to deltamethrin. Pretreatment with U73122 (3 μM) had no significant effect on deltamethrin-induced elevation of [Ca2+]i in neocortical neurons (Fig. 9). A similar pharmacological profile was observed for tefluthrin-induced elevation of [Ca2+]i in neocortical neurons (data not shown). Considered together, these data suggest that, rather than the release of Ca2+ from intracellular stores, deltamethrin and tefluthrin stimulation of [Ca2+]i is derived primarily from extracellular Ca2+ influx through NMDA receptors, L-type Ca2+ channels, and reversed Na+/Ca2+ exchange.
Fig. 7.
Pharmacological evaluation of the Ca2+ influx routes involved in deltamethrin-induced (a) or tefluthrin-induced (b) elevation of [Ca2+]i in neocortical neurons. Each data point represents the mean ± S.E.M. from two experiments performed in triplicate. #, p < 0.01, control versus pyrethroid; *, p < 0.01, pyrethroid + inhibitors versus pyrethroid by ANOVA. DM, deltamethrin; TEF, tefluthrin; MK, MK-801; Nif, nifedipine; KBR, KBR-7943.
Fig. 8.
Role of extracellular Ca2+ in deltamethrin-induced Ca2+ influx. a, time-response relationships for the influence of 0.2 or 0 mM extracellular Ca2+ on deltamethrin-induced Ca2+ influx. b, quantification of the influence of 0.2 or 0 mM extracellular Ca2+ on deltamethrin-induced elevation of neuronal [Ca2+]i. Each data point represents mean ± S.E.M. from two experiments performed in duplicate. *, p < 0.01, control versus deltamethrin; #, p < 0.01, 0.2 mM extracellular Ca2+ versus 2.3 mM extracellular Ca2+; +, 0 mM extracellular Ca2+ versus 2.3 mM extracellular Ca2+ by ANOVA.
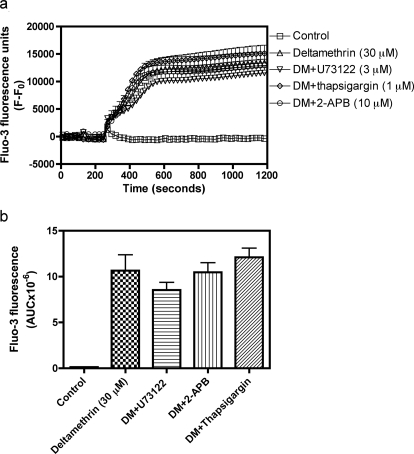
Fig. 9.
Lack of effect of U73122, thapsigargin, and 2-APB on deltamethrin (DM)-induced Ca2+ influx. a, time-response relationships for U73122 (3 μM), thapsigargin (1 μM), and 2-APB (10 μM) on deltamethrin-induced Ca2+ influx. b, quantification of the influence of U73122, thapsigargin, and 2-APB on deltamethrin-induced elevation of neuronal [Ca2+]i. This experiment was repeated twice in quadruplicate with similar results.
Discussion
The present experiments demonstrate that in mouse neocortical neurons pyrethroid insecticides activate a TTX-sensitive Ca2+ influx with differing potencies and efficacies. These results indicate that the pyrethroid-induced activation of Ca2+ channels in neocortical neurons is a secondary, not primary, event.
We found that 9 of 11 pyrethroids increased [Ca2+]i in a concentration-dependent manner. These same pyrethroids also had distinct maximum efficacies to increase Ca2+ influx, whereas their potencies were within one order of magnitude. The rank order of potency for the nine compounds was esfenvalerate > cypermethrin > λ-cyhalothrin = β-cyfluthrin > fenpropathrin > deltamethrin > S-bioallethrin > bifenthrin > tefluthrin. These relative potency rankings agree well with the relative potencies for inhibition of motor activity by the same 11 compounds, in which the cyano-containing compounds were generally more potent than the noncyano-containing compounds (Wolansky et al., 2006). The greater potency of the cyano-containing compounds in both assays is also consistent with the relatively more potent LD50 values of these compounds. Resmethrin and permethrin were without effect on Ca2+ influx in the present experiments, and S-bioallethrin produced only a modest, transient Ca2+ influx. These compounds were also the least potent in a motor activity assay (Wolansky et al., 2006), stimulation of sodium influx (Cao et al., 2009), and inhibition of spontaneous network activity (Johnstone et al., 2009). The small and transient Ca2+ influx produced by bioallethrin could be caused by its inhibition of voltage-gated calcium channels, inasmuch as Hildebrand et al. (2004) demonstrated that allethrin, an isomeric mixture, blocked mammalian voltage-gated calcium channels expressed in HEK293 cells. However, Neal et al. (2010) have demonstrated that allethrin has both stimulatory and inhibitory actions, depending on the VGCC type investigated.
The action of pyrethroids on sodium channels is temperature-dependent, and the sensitivity to pyrethroid exposure can be augmented by lowering temperature (Motomura and Narahashi, 2000). We confirmed a modest temperature dependence for deltamethrin-induced Ca2+ influx in neocortical neurons. Lowering the temperature from 37 to 22°C produced a 2.6-fold increase in the potency for deltamethrin-induced Ca2+ influx (Supplemental Fig. 3).
The nine pyrethroids that stimulated Ca2+ influx displayed distinct efficacies. The rank order of efficacy for Ca2+ influx was similar to that for sodium influx (Cao et al., 2009) with the exception of S-bioallethrin, which is the least efficacious compound on Ca2+ influx. In rat primary cortical cultures, pyrethroids, including fenvalarate, cypermethrin, cyfluthrin, and deltamethrin, differentially increase BDNF mRNA expression levels by 15- to 150-fold over control (Imamura et al., 2006). This effect was thought to be triggered primarily through Ca2+ influx associated with the activation of TTX-sensitive VGSCs (Imamura et al., 2006). The present results are consistent with this mode of action for activation of BDNF expression. In contrast, permethrin was reported to be inactive in its ability to stimulate BDNF mRNA expression in rat cortical neurons (Imamura et al., 2006). Therefore, the distinct abilities of pyrethroids to elevate BDNF mRNA expression are consistent with the demonstration of a range of pyrethroid efficacies in the stimulation of Ca2+ influx. In vivo, deltamethrin has been reported to increase BDNF in the cortex and hippocampus (Imamura et al., 2006), and both deltamethrin and permethrin alter transcription profiles of activity-dependent genes in the cortex including c-fos, Egr1, and Camk1g (Harrill et al., 2008). Thus, activity-dependent changes in gene transcription after pyrethroid exposure can occur both in vitro and in vivo.
Our data demonstrate that pyrethroid-induced Ca2+ influx depends on the activation of TTX-sensitive VGSCs inasmuch as the response was abrogated by TTX, a sodium channel blocker. Ca2+ influx induced by both type I and type II compounds occurred secondarily to activation of VGSCs. In addition, the parallel rank orders of efficacy between pryethroid-induced Na+ (Cao et al., 2009) and Ca2+ influx, with the exception of S-bioallethrin, indicates that these pyrethroids have no direct effect on VGCCs in neocortical neurons. These data in intact neurons are in contrast to previous reports of direct effects of pyrethroids on VGCCs. These include demonstrations that type I compounds inhibit VGCC function (Hagiwara et al., 1988; Someya and Yoshino 1990; Hildebrand et al., 2004) as well as reports that type II compounds increase Ca2+ flux into synaptosomes in a TTX-insensitive manner (Symington et al., 1999) or increase currents through VGCCs (Duce et al., 1999; Symington et al., 2007). Using rat brain synaptosomes, Symington et al. (2008) examined a group of 11 pyrethroids for their ability to stimulate high potassium-induced Ca2+ influx and glutamate release. For the 10 compounds common to these studies, five were reported in both studies to increase Ca2+ entry (esfenvalerate, cypermethrin, λ-cyhalothrin, β-cyfluthrin, and deltamethrin). However, there were divergent responses for the remaining five compounds; permethrin was reported to stimulate Ca2+ influx in synaptosomes, whereas bioallethrin, tefluthrin, and fenpropathrin were without effect, and bifenthrin was effective only at high concentrations (Symington et al., 2008). The TTX sensitivity of Ca2+ influx induced by the compounds tested by Symington et al. (2008) was not examined. A number of factors may contribute to these discordant results, including the use of neuronal subcompartments (synaptosomes) (Symington et al., 1999, 2008), insect neurons (Duce et al., 1999), or the method of VGCC stimulation (KCl depolarization: Symington et al., 1999; depolarizing voltage steps: Duce et al., 1999; Hagiwara et al., 1988; Someya and Yoshino 1990, Symington et al., 2008). Neal et al. (2010) have demonstrated that allethrin increased the amplitude of ω-conotoxin GVIA-insensitive (predominantly L-type), but decreased the amplitude of nimodipine-insensitive (predominantly N-type) calcium currents in PC12 cells. These results suggest that the subunit composition and/or post-translational modifications that may take place in mammalian neurons, but not other expression systems, also influence the nature of interactions between pyrethroids and VGCCs.
The present study used mammalian neocortical neurons that display spontaneous activity with no “external” stimulus (such as high potassium or voltage steps) required to activate VGCCs. Furthermore, the VGCC response was not isolated from the effects of pyrethroids on other channel types as is the case in patch-clamp recordings. Therefore, the present results demonstrate that VGCC responses of a neuronal network to pyrethroids are secondary to activation of VGSCs. Numerous studies that have focused specifically on VGCC function have reported pyrethroid effects in synaptosomes, oocytes, HEK cells, and other systems. However, a limited number of studies have attempted to examine potential roles of VGCC in pyrethroid effects using intact mammalian neuronal systems and in all cases failed to demonstrate a significant primary role for VGCC in pyrethroid effects (Meyer and Shafer, 2006; Meyer et al., 2008). Increased neurotransmitter release has been demonstrated after pyrethroid exposure in vivo (Hossain et al., 2004, 2005, 2008; Mubarak Hossain, 2006). However, it is unclear whether this is caused by direct effects of these compounds on presynaptic VGCCs, or whether it is secondary to depolarization caused by pyrethroid actions on VGSCs. Data from the current study are consistent with the latter possibility.
Experiments to examine the routes of pyrethroid-induced Ca2+ entry using deltamethrin and tefluthrin as representative compounds demonstrated that extracellular Ca2+, rather than Ca2+ release from the intracellular Ca2+ stores, served as the source for pyrethroid-induced elevation of [Ca2+]i in neocortical neurons. We also demonstrated that L-type VGCCs, NMDA receptors, and the Na+/Ca2+ exchanger accounted for the majority of pyrethroid-induced Ca2+ entry. TTX completely abolished pyrethroid-induced Ca2+ entry, indicating that these pathways were activated as a result of pyrethroid actions on VGSCs. In the case of L-type VGCCs, activation by deltamethrin or tefluthrin is likely to have been secondary to depolarization of the cell membrane as a result of VGSC activation. Although it was not measured, it is likely that the depolarization and Ca2+ entry resulted in glutamate release, which then activated NMDA receptors, resulting in additional Ca2+ entry. Finally, Na+ entry through VGSC may have caused sodium loading of the neurons, which can result in a reversal of Na+/Ca2+ exchange, which accounts for the contribution of this component to pyrethroid-induced Ca2+ entry. The time-response profile for pyrethroid-induced Ca2+ influx occasionally appeared to be biphasic, and this may be caused by the distinct kinetics of the multiple calcium influx pathways. Deltamethrin- or tefluthrin-stimulated Ca2+ influx was similar to that previously reported for brevetoxins in that it was triggered by activation of TTX-sensitive VGSCs and the main routes for Ca2+ influx were through L-type Ca2+ channels, NMDA receptors, and the reversed mode of operation of the Na+-Ca2+ exchanger (Berman and Murray, 2000).
In summary, by monitoring [Ca2+]i in intact neurons we have compared the potencies and efficacies for an array of pyrethroids to stimulate Ca2+ influx. We demonstrated that, in addition to modest differences in potency, pyrethroids produced distinct maximum responses in the elevation of neuronal [Ca2+]i. This [Ca2+]i response was secondary to the effect of pyrethroids on VGSCs. More importantly, these data provide information regarding the mode of action of pyrethroids in intact mammalian neurons and indicate that the effects on VGCCs are secondary to the activation of VGSCs.
Supplementary Material
Acknowledgments
We thank Dr. April Neal of Michigan State University (East Lansing, MI) and Drs. Kevin M. Crofton, William R. Mundy, and Edward Scollon of the U.S. Environmental Protection Agency for useful comments on a draft version of this manuscript.
This work was supported by the U.S. Environmental Protection Agency [Grant PR-RT-08-00545] (to T.F.M.), and the National Institutes of Health National Center for Research Resources [Grant G20RR024001].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.171850.
 The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- VGSC
- voltage-gated sodium channel
- BDNF
- brain-derived neurotropic factor
- NMDA
- N-methyl-d-aspartic acid
- TTX
- tetrodotoxin
- VGCC
- voltage-gated calcium channel
- [Ca2+]i
- intracellular calcium concentration
- DIV
- days in vitro
- ANOVA
- analysis of variance
- HEK
- human embryonic kidney
- DMSO
- dimethyl sulfoxide
- IP3
- inositol(1,4,5)triphosphate
- PLC
- phospholipase C
- CI
- confidence interval
- AUC
- area under the curve
- MK-801
- (5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate
- 2-APB
- 2-aminoethoxydiphenylborane
- KBR-7943
- 2-[2-[4-(4-nitrobenzyloxy)phenyl]ethyl]isothiourea mesylate
- U73122
- 1-[6-[[(17b)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione.
Authorship Contributions
Participated in research design: Cao, Shafer, and Murray.
Conducted experiments: Cao.
Performed data analysis: Cao and Murray.
Wrote or contributed to the writing of the manuscript: Cao, Shafer, and Murray.
References
- Amweg EL, Weston DP, Ureda NM. (2005) Use and toxicity of pyrethroid pesticides in the Central Valley, California, USA. Environ Toxicol Chem 24:966–972 [DOI] [PubMed] [Google Scholar]
- Barnes JM, Verschoyle RD. (1974) Toxicity of new pyrethroid insecticide. Nature 248:711. [DOI] [PubMed] [Google Scholar]
- Berman FW, Murray TF. (2000) Brevetoxin-induced autocrine excitotoxicity is associated with manifold routes of Ca2+ influx. J Neurochem 74:1443–1451 [DOI] [PubMed] [Google Scholar]
- Breckenridge CB, Holden L, Sturgess N, Weiner M, Sheets L, Sargent D, Soderlund DM, Choi JS, Symington S, Clark JM, et al. (2009) Evidence for a separate mechanism of toxicity for the type I and the type II pyrethroid insecticides. Neurotoxicology 30:S17–S31 [DOI] [PubMed] [Google Scholar]
- Cao Z, George J, Baden DG, Murray TF. (2007) Brevetoxin-induced phosphorylation of Pyk2 and Src in murine neocortical neurons involves distinct signaling pathways. Brain Res 1184:17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Shafer TJ, Murray TF. (2009) Influence of pyrethroid insecticides on sodium and calcium influx in neocortical neurons. Toxicologist 108:443 [Google Scholar]
- Casida JE, Quistad GB. (1998) Golden age of insecticide research: past, present, or future? Annu Rev Entomol 43:1–16 [DOI] [PubMed] [Google Scholar]
- Catterall WA, Cestèle S, Yarov-Yarovoy V, Yu FH, Konoki K, Scheuer T. (2007) Voltage-gated ion channels and gating modifier toxins. Toxicon 49:124–141 [DOI] [PubMed] [Google Scholar]
- Choi JS, Soderlund DM. (2006) Structure-activity relationships for the action of 11 pyrethroid insecticides on rat Na v 1.8 sodium channels expressed in Xenopus oocytes. Toxicol Appl Pharmacol 211:233–244 [DOI] [PubMed] [Google Scholar]
- Clark JM, Symington SB. (2007) Pyrethroid action on calcium channels: neurotoxicological implications. Invert Neurosci 7:3–16 [DOI] [PubMed] [Google Scholar]
- Duce IR, Khan TR, Green AC, Thompson AJ, Warburton SPM, Wong J. (1999) Calcium channels in insects, in Progress in Neuropharmacology and Neurotoxiology of Pesticides and Drugs (Beadle DJ. ed) pp 56–66, SCI Royal Society of Chemistry, Cambridge, UK [Google Scholar]
- Hagiwara N, Irisawa H, Kameyama M. (1988) Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J Physiol 395:233–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrill JA, Li Z, Wright FA, Radio NM, Mundy WR, Tornero-Velez R, Crofton KM. (2008) Transcriptional response of rat frontal cortex following acute in vivo exposure to the pyrethroid insecticides permethrin and deltamethrin. BMC Genomics 9:546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand ME, McRory JE, Snutch TP, Stea A. (2004) Mammalian voltage-gated calcium channels are potently blocked by the pyrethroid insecticide allethrin. J Pharmacol Exp Ther 308:805–813 [DOI] [PubMed] [Google Scholar]
- Hossain MM, Suzuki T, Sato I, Takewaki T, Suzuki K, Kobayashi H. (2004) The modulatory effect of pyrethroids on acetylcholine release in the hippocampus of freely moving rats. Neurotoxicology 25:825–833 [DOI] [PubMed] [Google Scholar]
- Hossain MM, Suzuki T, Sato I, Takewaki T, Suzuki K, Kobayashi H. (2005) Neuromechanical effects of pyrethroids, allethrin, cyhalothrin, and deltamethrin on the cholinergic processes in rat brain. Life Sci 77:795–807 [DOI] [PubMed] [Google Scholar]
- Hossain MM, Suzuki T, Unno T, Komori S, Kobayashi H. (2008) Differential presynaptic actions of pyrethroid insecticides on glutamatergic and GABAergic neurons in the hippocampus. Toxicology 243:155–163 [DOI] [PubMed] [Google Scholar]
- Imamura L, Yasuda M, Kuramitsu K, Hara D, Tabuchi A, Tsuda M. (2006) Deltamethrin, a pyrethroid insecticide, is a potent inducer for the activity-dependent gene expression of brain-derived neurotrophic factor in neurons. J Pharmacol Exp Ther 316:136–143 [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Watano T, Shigekawa M. (1996) A novel isothiourea derivative selectively inhibits the reverse mode of Na+/Ca2+ exchange in cells expressing NCX1. J Biol Chem 271:22391–22397 [DOI] [PubMed] [Google Scholar]
- Johnstone AM, Losa S, Shafer TJ. (2009) Type I and type II pyrethroid alterations in spontaneous bursting parameters in rat cortical networks measured using multielectrode array recordings. The Toxicologist 108:442 [Google Scholar]
- Meyer DA, Shafer TJ. (2006) Permethrin, but not deltamethrin, increases spontaneous glutamate release from hippocampal neurons in culture. Neurotoxicology 27:594–603 [DOI] [PubMed] [Google Scholar]
- Meyer DA, Carter JM, Johnstone AF, Shafer TJ. (2008) Pyrethroid modulation of spontaneous neuronal excitability and neurotransmission in hippocampal neurons in culture. Neurotoxicology 29:213–225 [DOI] [PubMed] [Google Scholar]
- Morgan MK, Sheldon LS, Croghan CW, Jones PA, Chuang JC, Wilson NK. (2007) An observational study of 127 preschool children at their homes and daycare centers in Ohio: environmental pathways to cis- and trans-permethrin exposure. Environ Res 104:266–274 [DOI] [PubMed] [Google Scholar]
- Motomura H, Narahashi T. (2000) Temperature dependence of pyrethroid modification of single sodium channels in rat hippocampal neurons. J Membr Biol 177:23–39 [DOI] [PubMed] [Google Scholar]
- Mubarak Hossain M, Suzuki T, Sato N, Sato I, Takewaki T, Suzuki K, Tachikawa E, Kobayashi H. (2006) Differential effects of pyrethroid insecticides on extracellular dopamine in the striatum of freely moving rats. Toxicol Appl Pharmacol 217:25–34 [DOI] [PubMed] [Google Scholar]
- Narahashi T. (1996) Neuronal ion channels as the target sites of insecticides. Pharmacol Toxicol 79:1–14 [DOI] [PubMed] [Google Scholar]
- Neal AP, Yuan Y, Atchison WD. (2010) Allethrin differentially modulates voltage-gated calcium channel subtypes in rat PC12 cells. Toxicol Sci 116:604–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer TJ, Meyer DA. (2004) Effects of pyrethroids on voltage-sensitive calcium channels: a critical evaluation of strengths, weaknesses, data needs, and relationship to assessment of cumulative neurotoxicity. Toxicol Appl Pharmacol 196:303–318 [DOI] [PubMed] [Google Scholar]
- Smith TJ, Soderlund DM. (1998) Action of the pyrethroid insecticide cypermethrin on rat brain IIa sodium channels expressed in Xenopus oocytes. Neurotoxicology 19:823–832 [PubMed] [Google Scholar]
- Soderlund DM, Clark JM, Sheets LP, Mullin LS, Piccirillo VJ, Sargent D, Stevens JT, Weiner ML. (2002) Mechanisms of pyrethroid neurotoxicity: implications for cumulative risk assessment. Toxicology 171:3–59 [DOI] [PubMed] [Google Scholar]
- Someya T, Yoshino Y. (1990) Characterization of two types of calcium channel in smooth muscle cell membrane of guinea-pig taenia coli. Sapporo Med J 59:177–188 [Google Scholar]
- Spurlock F, Lee M. (2008) Synthetic Pyrethroid Use Patterns, Properties and Environmental Effects. American Chemical Society, Washington DC [Google Scholar]
- Symington SB, Clark JM. (2005) Action of deltamethrin on N-type (Cav2.2) voltage-sensitive calcium channels in rat brain. Pesticide Biochem Physiol 82:1–15 [Google Scholar]
- Symington SB, Frisbie RK, Clark JM. (2008) Characterization of eleven commercial pyrethroids on functional attributes of rat brain synaptosomes. Pesticide Biochem Physiol 92:61–69 [Google Scholar]
- Symington SB, Frisbie RK, Kim HJ, Clark JM. (2007) Mutation of threonine 422 to glutamic acid mimics the phosphorylation state and alters the action of deltamethrin on Cav2.2. Pesticide Biochem Physiol 88:312–320 [Google Scholar]
- Symington SB, Zhang A, Karstens W, Houten JV, Clark JM. (1999) Characterization of pyrethroid action on ciliary calcium channels in Paramecium tetraurelia. Pestic Biochem Physiol 65:181–193 [Google Scholar]
- Verschoyle RD, Aldridge WN. (1980) Structure-activity relationships of some pyrethroids in rats. Arch Toxicol 45:325–329 [DOI] [PubMed] [Google Scholar]
- Verschoyle RD, Barnes JM. (1972) Toxicity of natural and synthetic pyrethrins to rats. Pestic Biomech Physiol 2:308–311 [Google Scholar]
- Whyatt RM, Camann DE, Kinney PL, Reyes A, Ramirez J, Dietrich J, Diaz D, Holmes D, Perera FP. (2002) Residential pesticide use during pregnancy among a cohort of urban minority women. Environ Health Perspect 110:507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolansky MJ, Gennings C, Crofton KM. (2006) Relative potencies for acute effects of pyrethroids on motor function in rats. Toxicol Sci 89:271–277 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.