Abstract
Glutamatergic synaptic plasticity in the nucleus accumbens (NAc) is implicated in response to sensitization to psychomotor-stimulating agents, yet ethanol effects here are undefined. We studied the acute in vitro and in vivo effects of ethanol in medium spiny neurons from the shell NAc subregion of slices of C57BL/6 mice by using whole-cell voltage-clamp recordings of α-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid (AMPA) excitatory postsynaptic current (EPSCs). Synaptic conditioning (low-frequency stimulation with concurrent postsynaptic depolarization) reliably depressed AMPA EPSCs by nearly 30%; this accumbal long-term depression (LTD) was blocked by a nonselective N-methyl-d-aspartate (NMDA) receptor antagonist (dl-2-amino-5-phosphonovaleric acid) and a selective NMDA receptor 2B antagonist [R-(R*,S*)-α-(4-hydroxyphenyl)-β-methyl-4-(phenylmethyl)-1-piperidine propanol]. Acute ethanol exposure inhibited the depression of AMPA EPSCs differentially with increasing concentrations, but this inhibitory action of ethanol was occluded by a D1-selective dopamine receptor agonist. Ethanol dependence was elicited in C57BL/6 mice by two separate 4-day bouts of chronic intermittent ethanol (CIE) vapor exposure. When assessed 24 h after a single bout of in vivo CIE vapor exposure, NAc LTD was absent, and instead NMDA receptor-dependent synaptic potentiation [long-term potentiation (LTP)] was reliably observed. It is noteworthy that both LTP and LTD were completely absent after an extended withdrawal (72 h) after a single 3-day CIE vapor bout. These observations demonstrate that 1) accumbal synaptic depression is mediated by NR2B receptors, 2) accumbal synaptic depression is highly sensitive to both acute and chronic ethanol exposure, and 3) alterations in this synaptic process may constitute a neural adaptation that contributes to the induction and/or expression of ethanol dependence.
Introduction
GABAergic medium spiny neurons (MSNs) of the nucleus accumbens (NAc) in the ventral striatum are principal neurons in the mesocorticolimbic system that process information concerning reward behavior (Nestler, 2001). These neurons receive a dopaminergic projection from the ventral tegmental area (VTA) and glutamatergic projections from prefrontal cortex and other limbic structures. It is generally thought that neuroadaptations in response to chronic drug abuse underlie development of craving and other drug-seeking behaviors associated with dependence. Much evidence indicates that NAc MSNs are very likely involved in such aberrant neuroadaptive responses. Although neuroadaptations underlying chronic ethanol abuse remain undefined, interactions between dopaminergic, glutamatergic, and GABAergic systems probably are crucial in this regard (for reviews see Gonzales et al., 2004; Zhang et al., 2006).
Indeed, a large amount of literature indicates that ethanol reinforcement involves the activation of the VTA-accumbal dopamine system (Gonzales et al., 2004). Ethanol has unique pharmacological actions to excite VTA dopamine neurons, and withdrawal from chronic ethanol exposure reduces their firing (Brodie et al., 1990, 1999; Shen and Chiodo, 1993; Shen, 2003). Dopamine release increases in the NAc during operant self-administration of ethanol (Weiss et al., 1993; Gonzales and Weiss, 1998; Yim et al., 1998), and D1-dopamine receptor antagonists reduce operant ethanol responding (Rassnick et al., 1992; Hodge et al., 1993; Samson et al., 1993). In addition, ethanol self-administration is reduced in animals lacking D1 receptors (El-Ghundi et al., 1998) or one of its intracellular partners, dopamine and cAMP regulated phosphoprotein of 32 kDa (DARPP-32) (Risinger et al., 2001).
Neuroadaptations that contribute to ethanol abuse probably share common mechanisms with those seen in other abused reinforcers, especially the psychomotor-stimulating agents such as cocaine and amphetamine. Evidence indicates that adaptations in accumbal glutamatergic plasticity constitute a mechanism encoding repetitive drug experience to psychomotor stimulants. In control NAc MSNs, low-frequency conditioning stimulation paired with postsynaptic depolarization, which mimics the upstate of approximately −50 mV common in bistable MSNs, produces long-term depression (LTD) of AMPA EPSCs (Thomas et al., 2000, 2001). Like hippocampal NMDA LTD (Dudek and Bear, 1992; Man et al., 2000), NAc LTD is induced by a moderate increase in intracellular Ca2+ through NMDA receptor activation (Thomas et al., 2000).
Thomas et al. (2001) first reported marked differences in basal AMPA EPSCs in NAc MSNs from cocaine-sensitized animals. Furthermore, NAc LTD expression was completely occluded in these sensitized animals, suggesting that repetitive cocaine experience directly induced LTD. In a very elegant series of experiments, Brebner et al. (2005) also reported LTD occlusion in NAc MSNs after amphetamine sensitization and dissected the mechanisms underlying neuroadaptation to psychomotor stimulants. Because expression of hippocampal LTD had previously been demonstrated to depend on GluR2 subunit internalization (Luscher et al., 1999), those investigators also proposed that endocytotic process as a prime mechanism whereby amphetamine experience modulated glutamatergic plasticity. The role of GluR2 internalization in the expression of NAc LTD and sensitization to amphetamine was directly tested by using a peptide that disrupted internalization of GluR2-containing AMPA receptors. Active, but not inactive, peptides completely occluded NAc LTD, and the most critical observations further came from in vivo studies. Intravenous or intra-accumbal, but not intra-VTA, injection of active, but not inactive, peptides completely occluded expression of amphetamine sensitization in previously sensitized rats (Brebner et al., 2005).
No studies investigating neuroadaptive changes in glutamatergic synaptic plasticity in the nucleus accumbens after ethanol exposure exist to our knowledge. This is particularly significant because, in contrast with other drugs, ethanol has a unique action to inhibit NMDA receptors and disrupt NMDA receptor-dependent plasticity in hippocampal and other structures (Sinclair and Lo, 1986; Lovinger et al., 1989, 1990; Morrisett and Swartzwelder, 1993; Nie et al., 1993, 1994; Maldve et al., 2002; Zhang et al., 2005). Reports of plasticity changes in MSNs of the dorsal striatum in response to ethanol exposure do exist (Yamamoto et al., 1999; Xia et al., 2006; Wang et al., 2007); however, those studies involve different forms of plasticity from that involved herein. In addition, the MSNs of the dorsal striatum are involved in habit formation and are not thought to be involved in ethanol reinforcement and reward such as processed in the NAc (Everitt and Robbins, 2005). Taken together, these findings prompted us to investigate neuroadaptive changes in glutamatergic transmission in NAc medium spiny neurons after in vivo ethanol exposure. Because induction of NAc LTD is NMDA receptor-dependent, we analyzed the direct effects of in vitro ethanol exposure on LTD in the NAc as well.
Materials and Methods
Brain Slice Preparation.
Parasagittal slices (210–250 μm thick) containing the NAc were prepared from the brains of 4- to 8-week-old male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME). Mice were lightly anesthetized by inhalation of halothane, and the brains were rapidly removed and placed in ice-cold (4°C) oxygenated artificial cerebrospinal fluid (ACSF) containing 110 mM choline, 25 mM NaHCO3, 1.25 mM NaH2PO4, 2.5 mM KCl, 7 mM MgCl2, 0.5 mM CaCl2, 25 mM dextrose, 11.6 mM Na+-ascorbate, and 3.1 mM Na+-pyruvate, bubbled with 95% O2/5% CO2. Slices were then transferred to an incubation ACSF for a minimum of 45 to 60 min before recording that contained 120 mM NaCl, 25 mM NaHCO3, 1.23 mM NaH2PO4, 3.3 mM KCl, 2.4 mM MgCl2, 1.8 mM CaCl2, and 10 mM dextrose, bubbled with 95% O2/5% CO2, pH 7.4, 32°C. Unless otherwise noted, all drugs and chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Patch-Clamp Electrophysiology.
We conducted all recordings at 31 to 33°C in ACSF containing 120 mM NaCl, 25 mM NaHCO3, 1.23 mM NaH2PO4, 3.3 mM KCl, 0.9 mM MgCl2, 2 mM CaCl2, and 10 mM dextrose, bubbled with 95% O2/5% CO2. The GABAA receptor antagonist picrotoxin (50 μM) was added to the external recording solution throughout all recordings to inhibit GABAA receptor-mediated synaptic currents; this improves the reliability of synaptic plasticity in the dorsal and ventral striatum by favoring postsynaptic depolarization during conditioning stimuli (Berretta et al., 2008). Whole-cell voltage-clamp recordings were obtained from NAc shell MSNs visually identified by using the MRK200 Modular Imaging system (Siskiyou Corporation, Grants Pass, OR) mounted on a vibration isolation table under IR-Dodt optics (Siskiyou Corporation, San Diego, CA). MSNs represent ∼95% of the neurons in the NAc and have distinctly smaller cell bodies (approximately 10 μm in diameter). MSNs were also identified by their highly negative resting membrane potential (less than −75 mV). MSNs from the most rostral and ventral areas of the NAc were chosen to make sure all recordings arose from the NAc shell subregion. Only one neuron per slice was used for recording. ACSF continuously perfused the recording chamber at 2.0 to 2.5 ml/min. Recording electrodes (thin-wall glass; WPI Instruments, Sarasota, FL) were made by using a Brown-Flaming model P-88 electrode puller (Sutter Instruments, San Rafael, CA) to yield resistances between 3 and 5 MΩ and contained 135 mM KMeSO4, 12 mM NaCl, 0.5 mM EGTA, 10 mM HEPES, 2 mM Mg-ATP, 0.3 mM Tris-GTP, (pH 7.3 with KOH). Input and access resistances were monitored throughout all experiments, and the recording was terminated if either resistance varied by more than 20%. These parameters were measured by application of a −10-mV, 100-ms voltage step at 5- to 10-min intervals. Synaptic currents were monitored at a holding potential of −80 mV. Changes in the holding current were observed to detect any resealing or other instability of the patch.
Data Acquisition and Analysis.
Excitatory afferents, the majority of which arise from the prefrontal cortex, were stimulated with a stainless-steel bipolar stimulating electrode (FHC, Inc., Bowdoin, ME) placed between the recorded MSN and prefrontal cortex, typically 150 to 300 μm from the MSN cell body. EPSCs were acquired with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA), filtered at 1 kHz, and digitized at 10 to 20 kHz via a Digidata 1440A interface board using pClamp 10.2 (Molecular Devices). Standard evoked EPSCs elicited by local stimulation were established in NAc shell MSNs for at least 10 min (at 0.1 Hz) to ensure stable recordings. LTD induction was assessed by delivering conditioning stimuli (500 pulses at 1 Hz at baseline stimulation intensity) while continuously and simultaneously depolarizing the postsynaptic cell to −50 mV (referred to below as conditioning stimulation). EPSCs were then monitored for 30 to 45 min after pairing (at 0.1 Hz).
Peak EPSC values were determined by using Clampfit 10.2 software (Molecular Devices). For each recording, peak EPSC amplitude values were normalized to the average EPSC amplitude of the final 10 min of baseline (60 sweeps) for that single recording. The mean normalized EPSC amplitudes for 12 consecutive sweeps were condensed into 2-min bins and represented as a single data point in scatter plots for each treatment group. Each data point represents the average of 12 consecutive EPSC amplitudes at that time point from each neuron within its respective treatment group. We used two parameters to determine whether plasticity of EPSC amplitudes (either depression or potentiation) occurred. An unpaired Student's t test (p value < 0.05) was used to compare the five normalized EPSC values from 20 to 30 min (minutes 40–50 on figures) after the pairing protocol to the five normalized EPSC values during the last 10 min of baseline. In addition, the change in average EPSC amplitude after conditioning needed to be more than two standard deviations from baseline. If both of these criteria were met, that treatment group was determined to exhibit plasticity.
For each experiment, the 40- to 50-min time period was used to compare the magnitude of plasticity after different drug exposures. The five normalized EPSC values between min 40 and 50 were compared between groups by using a single-factor ANOVA with Bonferroni post hoc analyses. Statistical significance for between-treatment group comparisons was defined as p < 0.05. Thus, LTD was considered the control outcome to which all drug exposures (either in vitro or in vivo) were compared. LTD was determined to be reduced and not completely blocked in situations where the postpairing average EPSC amplitude (min 40–50) was significantly increased from control LTD (ANOVA) and significantly decreased from its respective baseline (Student's t test). Experiments testing different antagonists were interleaved with control experiments by using slices prepared from the same animals where possible.
Chronic Intermittent Ethanol Exposure.
Ethanol dependence was induced by exposing mice to chronic intermittent ethanol (CIE) vapor (Becker and Hale, 1993; Becker and Lopez, 2004; Lopez and Becker, 2005). Ethanol was volatilized by bubbling air through a flask containing 95% ethanol at a rate of 0.2 to 0.3 liter/min. The resulting ethanol vapor then combined with a separate air stream to give a total flow rate of approximately 4 liters/min, which was delivered to mice in special mouse chamber units (Allentown Inc., Allentown, NJ). These chambers resembled normal acrylic cages but contain an additional air-tight seal top, a vapor inlet, and an exhaust outlet. Food and water was available ad libitum on the wire cage tops. The ethanol flow rate was determined empirically to yield target blood ethanol concentrations (35–45 mM, or 150–200 mg/dl) measured from a 10-μl tail blood sample by using an Analox AM1 alcohol analyzer (Analox, Lunenberg, MA). Two identical cages of mice were always run simultaneously, one cage for exposure to ethanol vapor and the second cage for an air-only control. The ethanol group received a single daily intraperitoneal injection containing both ethanol (20% v/v, 1.5 g/kg) and pyrazole (68 mg/kg) in sterile phosphate-buffered saline. Mice were then immediately chambered and exposed to ethanol vapor or air (from 5:00 PM to 9:00 AM daily under a reverse light/dark cycle, lights off at noon) for 3 consecutive days. Air control mice received only the pyrazole injection but were otherwise handled exactly as the ethanol group. On the fourth day, animals were returned to their home cages for 24 or 72 h (depending on the experiment). On the fifth or seventh day, electrophysiological experiments were performed as described above.
Two-Bottle Choice Drinking.
Eight-week old C57BL/6J mice (The Jackson Laboratory) were acclimated to a 12:12-h reverse light environment (lights off at noon) for 2 weeks. The animals had free access to food and water throughout the experiment. Subjects were tested for baseline ethanol consumption (g/kg) by using a 2-h two-bottle choice limited-access drinking paradigm (15% ethanol/water or water choice) for 21 days, administered 30 min before the beginning of the dark cycle. Ethanol consumption was recorded for the last 5 days of the baseline period and used to divide subjects equally into control and experimental groups. Mice were subjected to ethanol vapor or control air chamber exposure as described above for 4 consecutive days followed by 74 h of rest. Post chamber ethanol consumption was recorded for 5 days as before. A second round of chamber exposure was administered for 4 consecutive days followed by 74 h of rest. Post chamber ethanol consumption was again recorded for 5 days. Statistical significance for average ethanol consumption over each 5-day period was defined as p < 0.05 using Student's t test with Bonferroni post hoc analyses.
Results
NMDA Receptor-Mediated LTD in the NAc Shell of Ethanol-Naive Mice.
All data in this study were gathered by using whole-cell voltage clamp of MSNs solely in the mouse NAc shell region in the presence of the GABAA receptor antagonist picrotoxin (50 μM). Low-frequency stimulation (500 pulses at 1Hz) paired with postsynaptic depolarization to −50 mV has been reported to induce NMDA receptor-dependent LTD in the NAc core and shell (Thomas et al., 2000). Following a similar protocol involving coincident depolarization and low-frequency afferent stimulation (referred to below as conditioning stimulation), we consistently observed reliable LTD of EPSCs from all ethanol-naive MSNs examined (71.4 ± 0.7% of baseline, 21 neurons from 15 animals, p < 0.001, postconditioning versus baseline) (Fig. 1). Baseline and postconditioning EPSCs recorded at −80 mV were solely AMPA receptor-mediated, because they were completely abolished by the AMPA/kainate receptor antagonist, 6,7-dinitroquinoxaline-2,3-dione (10 μM; Supplemental Data).
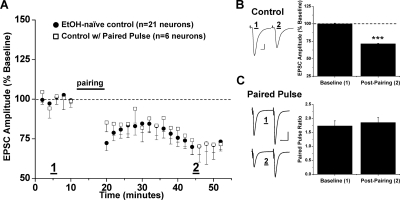
Fig. 1.
Low-frequency stimulation induces LTD in the NAc shell. A, conditioning stimulation (500 pulses at 1 Hz with concurrent depolarization to −50 mV, denoted as “pairing”) induces long-term depression of evoked AMPA receptor-mediated EPSCs in NAc shell MSNs of ethanol-naive mice (71.4 ± 0.7% of baseline, 21 neurons from 15 animals). Each data point represents an average of 12 consecutive normalized EPSC amplitudes condensed into 2-min bins (% baseline ± S.E.M.) from each neuron studied at that time point. Pairing stimulation also induced LTD when paired EPSCs (50 ms apart) were evoked during baseline and postpairing (72.9 ± 1.4% of baseline, six neurons from six animals, p < 0.001, versus baseline; p > 0.05, versus control LTD). B, left, sample EPSC traces from a single neuron. Scale bars represent 5 ms (horizontal) and 50 pA (vertical). B, right, bar graph representing the percentage change ± S.E.M. for average EPSC amplitude between baseline (min 0–10) and postpairing (min 40–50). ***, p < 0.001, postpairing versus baseline. C, left, sample traces of averaged baseline and postpairing EPSCs (60 sweeps, 10 min) of a single representative neuronal recording from single and paired-pulse groups. Scale bars represent 20 ms (horizontal) and 50 pA (vertical). C, right, bar graph represents the PPR ± S.E.M. between baseline and postpairing. PPR determined by dividing the amplitude of EPSC 2 by EPSC1 for each sweep. Average PPRs during baseline and postpairing were not significantly different (1.73 ± 0.18 and 1.85 ± 0.18, respectively, p > 0.05).
We measured the paired-pulse ratio (PPR) of EPSCs (two pulses, 50 ms apart) before and after conditioning stimulation to determine whether LTD expression was related to presynaptic and/or postsynaptic changes in glutamatergic transmission. In six neurons from different mice, we observed LTD by using paired test stimuli identical to that of LTD by using single stimuli (72.9 ± 1.4% of baseline, six neurons from six animals, p > 0.05, paired stimuli LTD versus single-stimuli LTD) (Fig. 1). In addition, we observed no significant difference (p > 0.05, Student's t test) in paired-pulse facilitation between baseline EPSCs (1.73 ± 0.18) and after induction of LTD (1.85 ± 0.18), indicating that changes in neurotransmitter release do not contribute to 1-Hz LTD in the NAc (Fig. 1C).
Nonspecific or Subunit-Specific NMDA Receptor Inhibition Blocks NAc LTD.
Thomas et al. (2000) first described low-frequency NAc LTD and showed its induction depended on NMDA receptor activation; therefore, we determined whether the plasticity we observed in response to conditioning likewise depended on NMDA receptors. In the presence of the nonselective NMDA receptor antagonist dl-APV (100 μM), LTD was completely blocked (96.6 ± 1.0% of baseline, four neurons from four animals, p > 0.05 versus baseline; p < 0.05 versus control LTD) (Fig. 2).
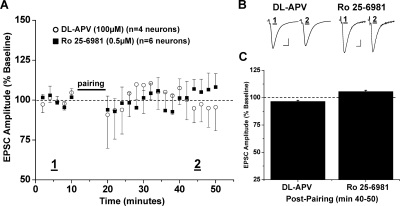
Fig. 2.
NR2B-containing NMDA receptors required for NAc LTD expression. A, pairing stimulation does not induce LTD expression in the presence of the nonselective NMDA receptor antagonist dl-APV (100 μM), (96.6 ± 1.0% of baseline, four neurons from four animals, p > 0.05 versus baseline) or the NR2B subunit-selective NMDA receptor antagonist Ro 25-6981 (0.5 μM), (105.6 ± 1.2% of baseline, five neurons from four animals, p < 0.05 versus baseline, versus dl-APV). B, sample traces of averaged baseline and postpairing EPSCs (60 sweeps, 10 min) of a single representative neuronal recording from each drug exposure group. Scale bars represent 5 ms (horizontal) and 50 pA (vertical). C, bar graph representing the percentage of baseline ± S.E.M. for average EPSC amplitude between baseline (min 0–10) and postpairing (min 40–50) for each drug exposure group.
We also tested whether NMDA receptors containing the NR2B subunit are required for induction of NAc LTD. In the presence of the specific NR2B antagonist R-(R*,S*)-α-(4-hydroxyphenyl)-β-methyl-4-(phenylmethyl)-1-piperidine propanol (Ro 25-6981) (0.5 μM), LTD in ethanol-naive mice was completely abolished (105.6 ± 1.2% of baseline, five neurons from four animals, p < 0.05 versus baseline; p < 0.05 versus control LTD, versus dl-APV) (Fig. 2). The small potentiation observed in the presence of Ro 25-6981 was significant from baseline; however, the magnitude of potentiation was not more than two standard deviations from baseline, which does not meet our criteria for plasticity expression.
Acute In Vitro Ethanol Exposure of Increasing Concentrations Differentially Inhibits NAc LTD.
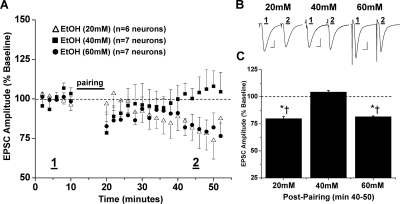
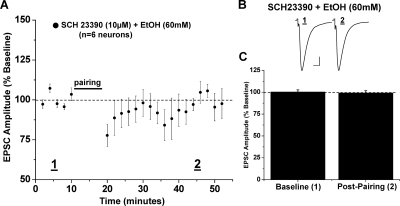
Ethanol is well known to inhibit NMDA receptors; therefore, we next tested whether ethanol inhibits NAc LTD expression. Bath application of a low intoxicating concentration of ethanol (20 mM) partially, but significantly, reduced NAc LTD (79.7 ± 2.0% of baseline, six neurons from five animals, p < 0.05 versus baseline; p < 0.05 versus control LTD) (Fig. 3). LTD expression was completely inhibited by a moderately to strongly intoxicating concentration of ethanol (40 mM) equivalent to the target concentration used in the in vivo vapor model described below (104.3 ± 1.3% of baseline, seven neurons from six animals, p > 0.05 versus baseline; p < 0.05 versus 20 mM EtOH, versus control LTD) (Fig. 3). It is noteworthy that when conditioning was performed in a highly intoxicating concentration of ethanol (60 mM) NAc LTD seemed similar in magnitude to that observed in the presence of the lowest concentration of ethanol (20 mM) tested (81.5 ± 0.7% of baseline, seven neurons from six animals, p < 0.05 versus baseline, versus 40 mM EtOH, versus control LTD; p > 0.05 versus 20 mM EtOH) (Fig. 3).
Fig. 3.
In vitro ethanol exposure, at increasing concentrations, differentially alters NAc LTD expression. A, escalating concentrations of ethanol applied to the recording bath have varying effects on the magnitude of LTD. Maximum inhibition of LTD expression is achieved through the application of ethanol (40 mM) (104.3 ± 1.3% of baseline, seven neurons from six animals, p > 0.05 versus baseline). In the presence of ethanol (20 and 60 mM), pairing stimulation induces an LTD magnitude that is decreased from control LTD but not completely occluded (79.7 ± 2.0% of baseline, six neurons from five animals; and 81.5 ± 0.7% of baseline, seven neurons from six animals). B, sample traces of averaged baseline and postpairing EPSCs (60 sweeps, 10 min) of a single representative neuronal recording from each ethanol exposure group. Scale bars represent 5 ms (horizontal) and 50 pA (vertical). C, bar graph representing the percentage of baseline ± S.E.M. for average EPSC amplitude between baseline (min 0–10) and postpairing (min 40–50) for each ethanol exposure group (*, p < 0.05 versus baseline; †, p < 0.05 versus 40 mM).
Activation of Dopamine D1 Receptors Restores LTD Expression in the Presence of Ethanol.
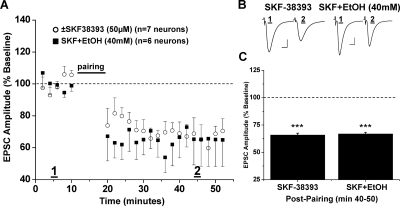
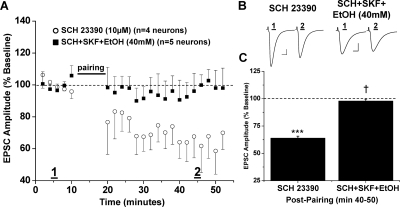
Previous work in our lab demonstrated that activation of D1-dopamine receptors decreased the ethanol sensitivity of NMDA receptors on MSNs in the NAc (Maldve et al., 2002; Zhang et al., 2005). Therefore, we next tested whether D1-dopamine receptor activation might antagonize the inhibitory effects of ethanol on NAc LTD. Bath application of the selective D1 receptor agonist (±)-1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzaazepine-7,8-diol hydrochloride [(±)SKF38393; 50 μM] did not affect the magnitude of LTD expression in control slices (65.9 ± 1.7% of baseline, seven neurons from six animals, p < 0.001 versus baseline) (Fig. 4). However, pretreatment of slices with SKF38393 for 30 min before ethanol (40 mM) application rescued LTD from acute ethanol inhibition (66.8 ± 1.5% of baseline, six neurons from five animals, p < 0.001 versus baseline) (Fig. 4). When LTD magnitude was compared between the (±)SKF38393 only, (±)SKF38393+ EtOH, and control NAc LTD groups, there were no significant differences apparent (p > 0.05, one-way ANOVA, Bonferroni post hoc). Likewise, a specific D1 receptor antagonist, R-(+)-8-chloro-2,3,4,5-tetrahydro-3-methyl-5-phenyl-1H-3-benzazepine-7-ol (SCH23390) (10 μM), had no effect on LTD expression when applied alone (63.9 ± 1.8% of baseline, four neurons from three animals, p < 0.001 versus baseline) (Fig. 5). Coapplication of both the D1 receptor agonist and antagonist 30 min before ethanol reversed the ability of the D1 agonist to occlude ethanol inhibition of NAc LTD. Thus, in the presence of both D1 agonist and antagonist and ethanol (40 mM), we observed occlusion of NAc LTD similar to that from the ethanol-alone treatment group (97.8 ± 1.7% of baseline, five neurons from four animals, p > 0.05 versus baseline) (Fig. 5).
Fig. 4.
D1 receptor activation occludes ethanol (40 mM) inhibition of NAc LTD. A, pretreatment (30 min) of NAc slices with the D1 receptor agonist (±)SKF38393 (50 μM) reverses ethanol inhibition of LTD expression (66.8 ± 1.5% of baseline, six neurons from five animals, p < 0.001, versus baseline; p < 0.05, versus 40 mM EtOH alone). Application of (±)SKF38393 alone did not have any effect on LTD expression (65.9 ± 1.7% of baseline, seven neurons from six animals; p < 0.001 versus baseline; p > 0.05 versus SKF+EtOH). B, sample traces of averaged baseline and postpairing EPSCs (60 sweeps, 10 min) of a single representative neuronal recording from each drug exposure group. Scale bars represent 5 ms (horizontal) and 50 pA (vertical). C, bar graph representing the percentage of baseline ± S.E.M. for average EPSC amplitude between baseline (min 0–10) and postpairing (min 40–50) for each drug exposure group. ***, p < 0.001 versus baseline.
Fig. 5.
Ethanol recovers inhibition of NAc LTD when D1 activation is occluded. A, bath application of the D1 receptor antagonist SCH23390 (10 μM), before (±)SKF38393 pretreatment, permits inhibition of LTD expression by ethanol (40 mM) similar to that observed with ethanol alone (97.8 ± 1.7% of baseline, five neurons from four animals, p > 0.05, versus baseline; p < 0.05 versus SCH23390 alone). SCH23390 alone does not alter the magnitude of LTD compared with control (63.9 ± 1.8% of baseline, four neurons from three animals, p < 0.001 versus baseline). B, sample traces of averaged baseline and postpairing EPSCs (60 sweeps, 10 min) of a single representative neuronal recording from each drug exposure group. Scale bars represent 5 ms (horizontal) and 50 pA (vertical). C, bar graph representing the percentage of baseline, mean ± S.E.M., for average normalized EPSC amplitude between baseline (min 0–10) and postpairing (min 40–50) for each drug exposure group. †. p < 0.05 versus SCH23390 alone. ***p < 0.001 versus baseline.
NAc LTD Is Completely Occluded in the Presence of Ethanol (60 mM) When D1 Receptors Are Inhibited.
We next investigated the possibility that the highest concentration of ethanol (60 mM) did not fully inhibit NAc LTD because of a subsequent release of dopamine upon ethanol application. When pretreated for 20 min with the specific D1 receptor antagonist SCH23390 (10 μM), the application of ethanol (60 mM) completely blocked NAc LTD (99.1 ± 2.6% of baseline, six from four animals, p > 0.05 versus baseline; p < 0.05 versus control LTD, versus 60 mM EtOH alone) (Fig. 6).
Fig. 6.
NAc LTD is completely inhibited by the highest ethanol concentration (60 mM) when D1 receptor activation is blocked. A, bath application of the D1 receptor antagonist SCH23390 (10 μM), 20 min before ethanol (60 mM), completely occludes NAc LTD (99.1 ± 2.6% of baseline, six neurons from four animals, p > 0.05, versus baseline; p < 0.05 versus 60 mM EtOH alone). B, sample traces of averaged baseline and postpairing EPSCs (60 sweeps, 10 min) of a single representative recording from all neurons recorded. Scale bars represent 5 ms (horizontal) and 50 pA (vertical). C, bar graph representing the percentage of baseline ± S.E.M. for average EPSC amplitude between baseline (min 0–10) and postpairing (min 40–50) for this drug exposure group.
Chronic Intermittent Ethanol Exposure Increases Voluntary Ethanol Consumption in C57BL/6J Mice.
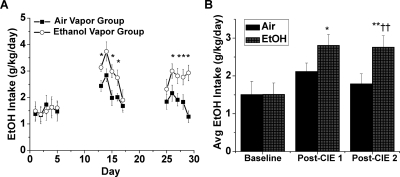
Repeated cycles of CIE exposure to C57BL/6 mice will significantly increase voluntary ethanol consumption (Becker and Lopez, 2004; Lopez and Becker, 2005; Griffin et al., 2009). To determine whether NAc LTD may represent an important synaptic process that may be altered in ethanol dependence, we first developed this mouse model in our lab. We examined ethanol intake in 2-h drinking bouts initiated 30 min before the dark cycle before and after two successive periods of 4 days of intermittent 16-h ethanol exposure. The average baseline ethanol consumption for air vapor control and ethanol vapor groups before CIE exposure was not significantly different (1.51 ± 0.08 versus 1.51 ± 0.05 g/kg/day, eight animals per group, p > 0.05, Student's t test, Bonferroni post hoc) (Fig. 7). After the first bout of CIE exposure (4 days, 16-h vapor exposure), the average ethanol intake of the ethanol vapor group was not significantly increased compared with the air vapor control group (2.90 ± 0.33 versus 2.19 ± 0.23 g/kg/day, eight animals per group, p > 0.05, Student's t test, Bonferroni post hoc) (Fig. 7). However, only the ethanol vapor group showed a significantly increased ethanol intake compared with baseline (p > 0.05 for air baseline versus air post-CIE 1; p < 0.05 for ethanol baseline versus ethanol post-CIE 1). After the second bout of CIE exposure, the average ethanol intake of the ethanol vapor group was significantly increased compared with the air vapor control group (2.77 ± 0.13 versus 1.75 ± 0.23 g/kg/day, eight animals per group, p < 0.01, Student's t test, Bonferroni post hoc) (Fig. 7), and neither group differed significantly between their respective post-CIE 1 average ethanol consumptions (p > 0.05).
Fig. 7.
C57BL/6J mice increase voluntary ethanol consumption after two CIE exposures. A, average daily EtOH intake (g/kg/day) in a 2-h drinking bout begun 30 min before the start of the dark cycle over 5 days in a baseline (prevapor exposure) and after two successive periods of 4 days of 16-h exposure to EtOH or air vapor. Baseline EtOH intake was measured during the last 5 days of the 21-day baseline period. The CIE vapor exposure periods were delivered over days 6 to 9 and 18 to 21. Animals rested 74 h after the final vapor exposure before resuming drinking. *, p < 0.05 ethanol versus air group. B, bar graph representing average EtOH intake (g/kg/day), shown as mean ± S.E.M., for air and EtOH vapor treatment groups measured over each 5-day drinking period. Baseline ethanol intake did not differ between the air (1.51 ± 0.08) and EtOH vapor (1.51 ± 0.05) groups (p > 0.05, Student's t test, Bonferroni post hoc). Only the ethanol vapor group significantly increased ethanol consumption after the first CIE vapor treatment compared with baseline (air, 2.19 ± 0.23, EtOH, 2.90 ± 0.33; *, p < 0.05). After the second CIE treatment, the air group (1.75 ± 0.23) showed significantly lower ethanol intake than the EtOH group (2.77 ± 0.13) (††, p < 0.01). **, p < 0.01, ethanol post-CIE 2 versus baseline. n = eight animals per group.
Synaptic Conditioning Elicits Long-Term Potentiation after In Vivo Chronic Intermittent Ethanol Vapor Exposure.
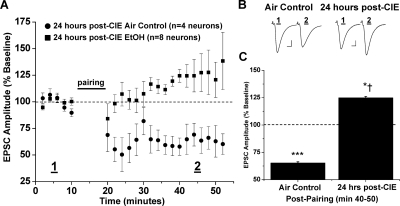
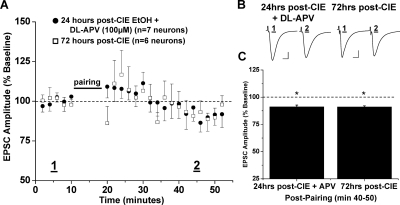
Repetitive exposure to psychomotor-stimulating agents occludes NAc LTD (Thomas et al., 2001; Brebner et al., 2005); therefore, we next investigated whether a similar neuroadaptation occurs after intermittent ethanol exposure. The standard CIE protocol involved two periods of ethanol exposure; however, because ethanol intake was significantly increased after the first ethanol exposure period, we chose to investigate the immediate effects after the first few 16-h bouts of intermittent ethanol exposure for 3 consecutive days. NAc MSNs prepared from mice 24 h after brief CIE exposure displayed marked differences in excitatory transmission in response to the standard conditioning stimulation (Fig. 8). Normal synaptic depression was present in NAc MSNs from the air control mouse group (65.3 ± 1.1% of baseline, four neurons from three animals, p < 0.001 versus baseline; p > 0.05 versus control LTD). Instead, synaptic conditioning elicited a striking, and highly significant, synaptic potentiation of EPSC amplitudes in NAc MSNs prepared 24 h after CIE treatment (124.9 ± 1.3% of baseline, eight neurons from eight animals, p < 0.05 versus baseline; p < 0.001, versus air control). Furthermore, dl-APV (100 μM) completely abolished this synaptic potentiation observed in slices from CIE-exposed mice (91.2 ± 1.6% of baseline, seven neurons from four animals, p < 0.05 versus baseline; p < 0.05, versus air control, versus 24 h post-CIE) (Fig. 9). This small depression satisfied our criteria for plasticity, yet it was still significantly different from air control LTD. Finally, we assessed NAc plasticity in slices from mice that had been allowed 72 h of recovery after the conclusion of their CIE exposure to investigate the endurance of these synaptic changes (Fig. 9). In these mice, conditioning elicited a slight, yet significantly less, depression of synaptic transmission than air control LTD (91.1 ± 0.9% of baseline, five neurons from three animals, p < 0.05 versus baseline, versus 24 h post-CIE, versus air control; p > 0.05 versus CIE-APV).
Fig. 8.
CIE vapor exposure converts NAc LTD to synaptic potentiation. A, 24 h after 3 consecutive days in vivo CIE vapor exposure, pairing stimulation induces synaptic potentiation rather than LTD of EPSCs (124.9 ± 1.3% of baseline, eight neurons from eight animals, p < 0.05 versus baseline; p < 0.05 versus air vapor control). In the air vapor control group, pairing stimulation induces a similar LTD to that observed in the ethanol-naive control group (65.3 ± 1.1% of baseline, four neurons from three animals, p < 0.001 versus baseline). B, sample traces of averaged baseline and postpairing EPSCs (60 sweeps, 10 min) of a single representative neuronal recording from each vapor (air or ethanol) exposure group. Scale bars represent 5 ms (horizontal) and 50 pA (vertical). C, bar graph representing the percentage of baseline ± S.E.M. for average EPSC amplitude between baseline (min 0–10) and postpairing (min 40–50) for each vapor (air or ethanol) exposure group. *, p < 0.05 versus baseline; †, p < 0.05 versus air vapor control; ***, p < 0.001 versus baseline.
Fig. 9.
Synaptic potentiation after CIE exposure is NMDA receptor-dependent and dissipates after 72-h withdrawal. A, 24 h after 3 consecutive days in vivo CIE vapor exposure, synaptic potentiation is blocked by the NMDA receptor antagonist dl-APV (100 μM), (91.2 ± 1.6% of baseline, seven neurons from four animals, p < 0.05, versus baseline, versus air vapor control, versus CIE EtOH 24 h). After 72 h of withdrawal from the identical CIE vapor exposure, pairing stimulation does not induce either synaptic potentiation or LTD (91.1 ± 0.9% of baseline, five neurons from three animals, p < 0.05, versus baseline, versus air vapor control, versus CIE EtOH 24 h; p > 0.05, versus CIE+APV). B, sample traces of averaged baseline and postpairing EPSCs (60 sweeps, 10 min) of a single representative neuronal recording from each drug/vapor exposure group. Scale bars represent 5 ms (horizontal) and 50 pA (vertical). C, bar graph representing the percentage of baseline ± S.E.M. for average EPSC amplitude between baseline (min 0–10) and postpairing (min 40–50) for each drug/vapor exposure group. *, p < 0.05.
Discussion
This article includes the following novel observations. First, synaptic depression of glutamatergic excitatory transmission onto medium spiny neurons of the shell of the nucleus accumbens requires activation of NR2B-containing NMDA receptors. Second, acute ethanol exposure blocks NAc LTD in a differential manner depending on the concentration. Third, activation of D1-dopamine receptors completely occludes the ability of ethanol to inhibit NAc LTD. Fourth, a repeated regimen of intermittent ethanol exposure enhances voluntary ethanol intake in C57BL/6J mice, and such intermittent exposure induces NAc metaplasticity from LTD to LTP. Occlusion of both NAc LTD and LTP is retained for at least 72 h after intermittent ethanol exposure.
NAc LTD, In Vitro Ethanol Exposure, and the Role of NMDA and Dopamine D1 Receptors.
Medium spiny neurons rest at more hyperpolarized membrane potentials (a “down-state” approximately −80 mV) and display transitions to a depolarized potential (the “up-state” approximately −50 mV) coupled with an ensemble of action potentials. When differing patterns of conditioning stimulation are delivered to NAc MSNs at least three forms of synaptic depression may result (Berretta et al., 2008). The focus of this article is the only form expressed via NMDA receptor activation and decreased postsynaptic AMPA receptor function (Brebner et al., 2005). Two forms of presynaptic LTD, independent of NMDA receptor activation, have been demonstrated; these are induced by endocannabinoid or metabotropic glutamate receptor activation, are caused by decreased glutamate release, and occur after delivery of higher-frequency synaptic stimuli than that used during NMDA LTD (Robbe et al., 2002a,b).
In the present study, we also observed that the low-frequency form of postsynaptic NAc LTD is NMDA receptor-dependent and that this LTD is mediated by NR2B subunit-containing NMDA receptors. This is a novel result not previously documented concerning NAc LTD, yet it is not surprising because NR2B-containing receptors are highly expressed and targeted on NAc MSNs (Chapman et al., 2003; Dunah and Standaert, 2003). We did not investigate the involvement of NR2A-containing NMDA receptors in LTD expression given the lack of a specific pharmacological tool (Neyton and Paoletti, 2006). There is likely limited involvement of NR2C or NR2D-containing NMDA receptors because their NAc expression is nearly nonexistent at this age (Standaert et al., 1994; Landwehrmeyer et al., 1995; Ghasemzadeh et al., 1996). The NR2A and NR2B subunits have been implicated to differentially contribute to LTP and LTD, respectively, in several brain regions (Liu et al., 2004; Massey et al., 2004; Zhao et al., 2005); however, these conclusions are controversial and specific contribution of one particular NMDA receptor subunit to exclusively LTD or LTP may not hold true in every instance (Berberich et al., 2005; Weitlauf et al., 2005; Morishita et al., 2007). Thus, we cannot derive any conclusions about the contributions of other subunits besides NR2B to LTD expression in the NAc. Future studies will have to address the possibility.
Our observations demonstrate that pharmacologically relevant concentrations (40 mM) of ethanol completely inhibit the expression of LTD when applied in vitro to a slice preparation from ethanol-naive mice. Evidence exists for both promoting and inhibiting effects of ethanol on LTD expression in other brain regions, including the hippocampus, dorsal striatum, and cerebellum (Hendricson et al., 2002; Izumi et al., 2005; Yin et al., 2007; Belmeguenai et al., 2008). Those reports certainly support the likelihood that ethanol modulates NAc LTD as we observed, but the different underlying mechanisms of plasticity expression interject a degree of complexity that tempers direct comparisons between those different reports.
Given the extensive literature that demonstrates ethanol inhibition of NMDA receptors, it is likely that a similar inhibition of NMDA receptors is the primary mechanism through which ethanol occludes the expression of NAc LTD. Excitatory transmission onto NMDA receptors is reduced by acute ethanol application in slice preparations of the hippocampus (Lovinger et al., 1989, 1990) and the nucleus accumbens (Nie et al., 1993, 1994; Maldve et al., 2002; Zhang et al., 2005; Wang et al., 2007). Our results indicate that both NR2B inhibition and application of ethanol prevent the expression of LTD, and it is tempting to speculate that ethanol blocks LTD expression by predominantly interacting with NR2B-containing NMDA receptors.
Our lab and others previously observed that activation of D1 receptors strongly suppressed the inhibitory effects of ethanol on NMDA receptor-mediated synaptic transmission dependent on activation of DARPP-32 and phosphorylation of Ser897 of the NR1 subunit (Maldve et al., 2002; Zhang et al., 2005; Lin et al., 2006; but also see Xu and Woodward, 2006). In the present article, we again observed that prior activation of D1-dopamine receptors rescues the expression of LTD in the NAc in the presence of ethanol. Thus, NAc LTD is ethanol-sensitive and displays characteristics consistent with NMDA receptor-mediated processes documented previously. One major goal is to determine how pharmacological manipulation of these processes can mitigate the long-term effects of ethanol exposure and therefore may be therapeutic in ethanol dependence; hence, modulation of D1-dopamine signaling may give insights to novel therapeutic targets. Furthermore, the observation that a high concentration of ethanol (60 mM) did not occlude NAc LTD is consistent with the idea that such concentrations of ethanol can induce dopamine release (Brodie et al., 1990, 1999) and DARPP-32 phosphorylation directly (Maldve et al., 2002). Both of these effects therefore increase Ser897-NR1 phosphorylation and thereby decrease ethanol sensitivity of NMDA receptors (Maldve et al., 2002; Zhang et al., 2005; Lin et al., 2006). Therefore, the observation of NAc LTD in the presence of a high concentration of ethanol, which is occluded by D1 receptor inhibition, is consistent with prior observations.
In Vivo Chronic Ethanol Exposure Increases Intake and Significantly Alters NAc LTD.
One major hindrance for basic research into the neurobiological mechanisms of alcoholism has been the difficulties associated with development of a convenient animal model. A model based on two 3- to 4-day regimens of passive ethanol administration via peripheral injection and subsequent 16-h vapor inhalation (termed chronic intermittent exposure) to C57BL/6 mice has become widely adopted (Becker and Lopez, 2004; Lopez and Becker, 2005; Griffin et al., 2009). In this study, we observed significant increases in voluntary ethanol consumption in our own cohort of animals after two bouts of CIE exposure. To test our hypothesis, as seen with chronic exposure to psychomotor stimulants, that occlusion of NAc LTD will result after CIE, we assessed the response of NAc MSNs to conditioning after a single bout of CIE as a baseline to determine the minimal level of ethanol exposure that might occlude LTD. Indeed, we were surprised that such a profound metaplasticity, a switch from LTD to LTP, occurred after such a brief ethanol exposure bout. We interpret such robust changes in NAc plasticity as an indicator of the potential importance of this process in ethanol neuroadaptation and therefore chose to first characterize this initial change in response to short-term CIE. Indeed, after a single 3-day cycle of this CIE model, we contend that significant alterations in glutamatergic synaptic plasticity in the NAc have already begun.
Although we observed increases in drinking at these short exposure durations, Becker and colleagues demonstrated that the maximum increases in voluntary ethanol consumption in mice are not observed until 3 days after withdrawal from CIE exposure (Becker and Lopez, 2004; Lopez and Becker, 2005). Thus, considerable study will be required to determine the temporal characteristics of the consolidation of this neuroadaptive response. Our results indicate that synaptic potentiation has subsided, whereas LTD remains occluded in the NAc shell at this same time point. Just as multiple CIE exposures are necessary to observe a sustained increase in ethanol consumption, we believe synaptic potentiation also persists after multiple CIE exposures. The current study indicates that synaptic activity is disrupted in the NAc shell at an early time point in a model of ethanol dependence. Future studies will address whether alterations in synaptic plasticity in the NAc shell directly contribute to the behaviors that lead toward increased voluntary ethanol consumption. The matter is made more complex because psychostimulants and ethanol have fundamentally distinct effects on NAc LTD. Chronic exposure to either drug seems to artificially induce an LTD-like state via occlusion of the response to in vitro conditioning stimulation. However, the unique NMDA receptor blocking actions of ethanol result in an additional potentiating action; thus, we contend that alterations in NAc shell glutamatergic synaptic function caused by CIE exposure are fundamentally distinct from those of psychostimulants. Nonetheless, disruption of GluR2 internalization suppressed the sensitized response to psychostimulants (Brebner et al., 2005), and therefore, the likelihood remains that LTD occlusion is critical to ethanol-seeking behavior. However, the ethanol-dependent state may conceivably be further and uniquely driven by the resultant LTP and subsequent enhancement of glutamatergic transmission. Could blockade of synaptic potentiation in the NAc after CIE exposure prevent an increase in voluntary ethanol consumption? Such possibilities deserve investigation. It is hoped that these results provide novel insights into the relevant contributions of NAc synaptic plasticity in the complex array of neuroadaptations that rewire the motivational circuitry in the ethanol-dependent animal.
Supplementary Material
This work was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grant U01AA16651-01] (to R.A.M.).
Parts of this work have been previously presented as research abstracts and posters: Jeanes ZM and Morrisett RA (2009) Dopamine receptor subtype expression determines plasticity direction in accumbal medium spiny neurons, at the Society for Neuroscience Annual Meeting, 2009 Oct 17–21; Chicago, IL. Society for Neuroscience, Washington, DC. Jeanes ZM and Morrisett RA (2009) D(1)-like dopamine receptor modulation of NMDA receptor-dependent long-term depression in the nucleus accumbens: interactions with ethanol, at the Research Society on Alcoholism Annual Meeting, 2009 June 20–24; San Diego, CA. Research Society on Alcoholism, Austin, TX.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.171009.
 The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- NAc
- nucleus accumbens
- MSN
- medium spiny neuron
- VTA
- ventral tegmental area
- AMPA
- α-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid
- NMDA
- N-methyl-d-aspartate
- ACSF
- artificial cerebrospinal fluid
- EPSC
- excitatory postsynaptic current
- LTD
- long-term depression
- LTP
- long-term potentiation
- EtOH
- ethanol
- CIE
- chronic intermittent ethanol
- DARPP-32
- dopamine and cAMP regulated phosphoprotein of 32 kDa
- dl-APV
- dl-2-amino-5-phosphonovaleric acid
- Ro 25-6981
- R-(R*,S*)-α-(4-hydroxyphenyl)-β-methyl-4-(phenylmethyl)-1-piperidine propanol
- (±)SKF38393
- (±)-1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzaazepine-7,8-diol hydrochloride
- SCH23390
- R-(+)-8-chloro-2,3,4,5-tetrahydro-3-methyl-5-phenyl-1H-3-benzazepine-7-ol
- ANOVA
- analysis of variance
- GluR2
- glutamate receptor 2
- PPR
- paired-pulse ratio.
Authorship Contributions
Participated in research design: Jeanes and Morrisett.
Conducted experiments: Jeanes and Buske.
Performed data analysis: Jeanes and Buske.
Wrote or contributed to the writing of the manuscript: Jeanes and Morrisett.
Other: Morrisett acquired funding for the research.
References
- Becker HC, Hale RL. (1993) Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal “kindling.” Alcohol Clin Exp Res 17:94–98 [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. (2004) Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res 28:1829–1838 [DOI] [PubMed] [Google Scholar]
- Belmeguenai A, Botta P, Weber JT, Carta M, De Ruiter M, De Zeeuw CI, Valenzuela CF, Hansel C. (2008) Alcohol impairs long-term depression at the cerebellar parallel fiber-Purkinje cell synapse. J Neurophysiol 100:3167–3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberich S, Punnakkal P, Jensen V, Pawlak V, Seeburg PH, Hvalby Ø, Köhr G. (2005) Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neurosci 25:6907–6910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta N, Nisticò R, Bernardi G, Mercuri NB. (2008) Synaptic plasticity in the basal ganglia: a similar code for physiological and pathological conditions. Prog Neurobiol 84:343–362 [DOI] [PubMed] [Google Scholar]
- Brebner K, Wong TP, Liu L, Liu Y, Campsall P, Gray S, Phelps L, Phillips AG, Wang YT. (2005) Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science 310:1340–1343 [DOI] [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB. (1999) Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res 23:1848–1852 [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV. (1990) Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res 508: 65–69 [DOI] [PubMed] [Google Scholar]
- Chapman DE, Keefe KA, Wilcox KS. (2003) Evidence for functionally distinct synaptic NMDA receptors in ventromedial versus dorsolateral striatum. J Neurophysiol 89:69–80 [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. (1992) Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-d-aspartate receptor blockade. Proc Natl Acad Sci USA 89:4363–4367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Standaert DG. (2003) Subcellular segregation of distinct heteromeric NMDA glutamate receptors in the striatum. J Neurochem 85:935–943 [DOI] [PubMed] [Google Scholar]
- El-Ghundi M, George SR, Drago J, Fletcher PJ, Fan T, Nguyen T, Liu C, Sibley DR, Westphal H, O'Dowd BF. (1998) Disruption of dopamine D1 receptor gene expression attenuates alcohol-seeking behavior. Eur J Pharmacol 353:149–158 [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. (2005) Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8:1481–1489 [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Sharma S, Surmeier DJ, Eberwine JH, Chesselet MF. (1996) Multiplicity of glutamate receptor subunits in single striatal neurons: an RNA amplification study. Mol Pharmacol 49:852–859 [PubMed] [Google Scholar]
- Gonzales RA, Weiss F. (1998) Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci 18:10663–10671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. (2004) The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther 103:121–146 [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Yanke AB, Middaugh LD, Becker HC. (2009) Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology (Berl) 201:569–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricson AW, Miao CL, Lippmann MJ, Morrisett RA. (2002) Ifenprodil and ethanol enhance NMDA receptor-dependent long-term depression. J Pharmacol Exp Ther 301:938–944 [DOI] [PubMed] [Google Scholar]
- Hodge CW, Haraguchi M, Erickson H, Samson HH. (1993) Ventral tegmental microinjections of quinpirole decrease ethanol and sucrose-reinforced responding. Alcohol Clin Exp Res 17:370–375 [DOI] [PubMed] [Google Scholar]
- Izumi Y, Nagashima K, Murayama K, Zorumski CF. (2005) Acute effects of ethanol on hippocampal long-term potentiation and long-term depression are mediated by different mechanisms. Neuroscience 136:509–517 [DOI] [PubMed] [Google Scholar]
- Landwehrmeyer GB, Standaert DG, Testa CM, Penney JB, Jr, Young AB. (1995) NMDA receptor subunit mRNA expression by projection neurons and interneurons in rat striatum. J Neurosci 15:5297–5307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HH, Chang SJ, Shie HJ, Lai CC. (2006) Ethanol inhibition of NMDA-induced responses and acute tolerance to the inhibition in rat rostral ventrolateral medulla in vivo: involvement of cAMP-dependent protein kinases. Neuropharmacology 51:747–755 [DOI] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. (2004) Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 304:1021–1024 [DOI] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. (2005) Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 181:688–696 [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. (1989) Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science 243:1721–1724 [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. (1990) NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat. J Neurosci 10:1372–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, Nicoll RA. (1999) Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron 24:649–658 [DOI] [PubMed] [Google Scholar]
- Maldve RE, Zhang TA, Ferrani-Kile K, Schreiber SS, Lippmann MJ, Snyder GL, Fienberg AA, Leslie SW, Gonzales RA, Morrisett RA. (2002) DARPP-32 and regulation of the ethanol sensitivity of NMDA receptors in the nucleus accumbens. Nat Neurosci 5:641–648 [DOI] [PubMed] [Google Scholar]
- Man HY, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, Sheng M, Wang YT. (2000) Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron 25:649–662 [DOI] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. (2004) Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci 24:7821–7828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita W, Lu W, Smith GB, Nicoll RA, Bear MF, Malenka RC. (2007) Activation of NR2B-containing NMDA receptors is not required for NMDA receptor-dependent long-term depression. Neuropharmacology 52:71–76 [DOI] [PubMed] [Google Scholar]
- Morrisett RA, Swartzwelder HS. (1993) Attenuation of hippocampal long-term potentiation by ethanol: a patch-clamp analysis of glutamatergic and GABAergic mechanisms. J Neurosci 13:2264–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. (2001) Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci 2:119–128 [DOI] [PubMed] [Google Scholar]
- Neyton J, Paoletti P. (2006) Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci 26:1331–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Madamba SG, Siggins GR. (1994) Ethanol inhibits glutamatergic neurotransmission in nucleus accumbens neurons by multiple mechanisms. J Pharmacol Exp Ther 271:1566–1573 [PubMed] [Google Scholar]
- Nie Z, Yuan X, Madamba SG, Siggins GR. (1993) Ethanol decreases glutamatergic synaptic transmission in rat nucleus accumbens in vitro: naloxone reversal. J Pharmacol Exp Ther 266:1705–1712 [PubMed] [Google Scholar]
- Rassnick S, Pulvirenti L, Koob GF. (1992) Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate receptor antagonists into the nucleus accumbens. Psychopharmacology 109:92–98 [DOI] [PubMed] [Google Scholar]
- Risinger FO, Freeman PA, Greengard P, Fienberg AA. (2001) Motivational effects of ethanol in DARPP-32 knock-out mice. J Neurosci 21:340–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Chaumont S, Bockaert J, Manzoni OJ. (2002a) Role of p/q-Ca2+ channels in metabotropic glutamate receptor 2/3-dependent presynaptic long-term depression at nucleus accumbens synapses. J Neurosci 22:4346–4356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. (2002b) Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci USA 99:8384–8388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH, Hodge CW, Tolliver GA, Haraguchi M. (1993) Effect of dopamine agonists and antagonists on ethanol-reinforced behavior: the involvement of the nucleus accumbens. Brain Res Bull 30:133–141 [DOI] [PubMed] [Google Scholar]
- Shen RY. (2003) Ethanol withdrawal reduces the number of spontaneously active ventral tegmental area dopamine neurons in conscious animals. J Pharmacol Exp Ther 307:566–572 [DOI] [PubMed] [Google Scholar]
- Shen RY, Chiodo LA. (1993) Acute withdrawal after repeated ethanol treatment reduces the number of spontaneously active dopaminergic neurons in the ventral tegmental area. Brain Res 622:289–293 [DOI] [PubMed] [Google Scholar]
- Sinclair JG, Lo GF. (1986) Ethanol blocks tetanic and calcium-induced long-term potentiation in the hippocampal slice. Gen Pharmacol 17:231–233 [DOI] [PubMed] [Google Scholar]
- Standaert DG, Testa CM, Young AB, Penney JB., Jr (1994) Organization of N-methyl-d-aspartate glutamate receptor gene expression in the basal ganglia of the rat. J Comp Neurol 343:1–16 [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. (2001) Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci 4:1217–1223 [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Malenka RC, Bonci A. (2000) Modulation of long-term depression by dopamine in the mesolimbic system. J Neurosci 20:5581–5586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Carnicella S, Phamluong K, Jeanblanc J, Ronesi JA, Chaudhri N, Janak PH, Lovinger DM, Ron D. (2007) Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: implications for alcohol drinking behavior. J Neurosci 27:3593–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. (1993) Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther 267:250–258 [PubMed] [Google Scholar]
- Weitlauf C, Honse Y, Auberson YP, Mishina M, Lovinger DM, Winder DG. (2005) Activation of NR2A-containing NMDA receptors is not obligatory for NMDA receptor-dependent long-term potentiation. J Neurosci 25:8386–8390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia JX, Li J, Zhou R, Zhang XH, Ge YB, Ru Yuan X. (2006) Alterations of rat corticostriatal synaptic plasticity after chronic ethanol exposure and withdrawal. Alcohol Clin Exp Res 30:819–824 [DOI] [PubMed] [Google Scholar]
- Xu M, Woodward JJ. (2006) Ethanol inhibition of NMDA receptors under conditions of altered protein kinase A activity. J Neurochem 96:1760–1767 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Nakanishi H, Takai N, Shimazoe T, Watanabe S, Kita H. (1999) Expression of N-methyl-d-aspartate receptor-dependent long-term potentiation in the neostriatal neurons in an in vitro slice after ethanol withdrawal of the rat. Neuroscience 91:59–68 [DOI] [PubMed] [Google Scholar]
- Yim HJ, Schallert T, Randall PK, Gonzales RA. (1998) Comparison of local and systemic ethanol effects on extracellular dopamine concentration in rat nucleus accumbens by microdialysis. Alcohol Clin Exp Res 22:367–374 [PubMed] [Google Scholar]
- Yin HH, Park BS, Adermark L, Lovinger DM. (2007) Ethanol reverses the direction of long-term synaptic plasticity in the dorsomedial striatum. Eur J Neurosci 25:3226–3232 [DOI] [PubMed] [Google Scholar]
- Zhang TA, Hendricson AW, Morrisett RA. (2005) Dual synaptic sites of D(1)-dopaminergic regulation of ethanol sensitivity of NMDA receptors in nucleus accumbens. Synapse 58:30–44 [DOI] [PubMed] [Google Scholar]
- Zhang TA, Maldve RE, Morrisett RA. (2006) Coincident signaling in mesolimbic structures underlying alcohol reinforcement. Biochem Pharmacol 72:919–927 [DOI] [PubMed] [Google Scholar]
- Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, et al. (2005) Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron 47:859–872 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.