Abstract
We have previously described novel cardioprotection in response to sustained morphine exposure, efficacious in young to aged myocardium and mechanistically distinct from conventional opioid or preconditioning (PC) responses. We further investigate opioid-dependent sustained ligand-activated preconditioning (SLP), assessing duration of protection, opioid receptor involvement, additivity with conventional responses, and signaling underlying preischemic induction of the phenotype. Male C57BL/6 mice were treated with morphine (75-mg subcutaneous pellet) for 5 days followed by morphine-free periods (0, 3, 5, or 7 days) before ex vivo assessment of myocardial tolerance to 25-min ischemia/45-min reperfusion. SLP substantially reduced infarction (by ∼50%) and postischemic contractile dysfunction (eliminating contracture, doubling force development). Cardioprotection persisted for 5 to 7 days after treatment. SLP was induced specifically by δ-receptor and not κ- or μ-opioid receptor agonism, was eliminated by δ-receptor and nonselective antagonism, and was additive with adenosinergic but not acute morphine- or PC-triggered protection. Cotreatment during preischemic morphine exposure with the phosphoinositide-3 kinase (PI3K) inhibitor wortmannin, but not the protein kinase A (PKA) inhibitor myristoylated PKI-(14-22)-amide, prevented induction of SLP. This was consistent with shifts in total and phospho-Akt during the induction period. In summary, data reveal that SLP triggers sustained protection from ischemia for up to 7 days after stimulus, is δ-opioid receptor mediated, is induced in a PI3K-dependent/PKA-independent manner, and augments adenosinergic protection. Mechanisms underlying SLP may be useful targets for manipulation of ischemic tolerance in young or aged myocardium.
Introduction
Amelioration of reversible and irreversible myocardial injuries with ischemia-reperfusion is a highly desirable goal, and substantial research effort has been directed at achieving clinical cardioprotection. Discovery of ischemic preconditioning (IPC) by Murry et al., (1986) invigorated research into cardioprotection, as have other discoveries regarding benefit with postconditioning (Zhao et al., 2003). However, despite potent protection experimentally, the therapeutic potential of conditioning-based approaches has yet to be realized clinically. A number of factors may limit translation of conventional conditioning responses, including negative influences of age, disease, and common pharmacological agents (Miura and Miki, 2008; Peart and Headrick, 2009). It is thus important to investigate alternate or unconventional modes of cardioprotection, such as opioid-mediated sustained ligand-activated preconditioning (SLP) (Peart and Gross, 2004a).
Opioids mediate acute or delayed PC-like effects (Gross, 2003; Peart et al., 2006, 2008), contribute to postconditioning (Zatta et al., 2008), and engage conventional kinase signaling in mediating these responses (i.e., the reperfusion injury salvage kinase or RISK path; Hausenloy and Yellon, 2007). It is noteworthy, however, that other work suggests that RISK components may actually be nonessential to cardioprotection (Skyschally et al., 2009), and alternate signal paradigms such as the survivor activating factor enhancement path have been identified (Lacerda et al., 2009). We have reported previously that 3 to 5 days of opioid agonism with morphine generates profound protection persisting for 48 h after stimulus removal (Peart and Gross, 2004a). The response, initially termed chronic morphine preconditioning but more accurately dubbed sustained ligand-activated preconditioning, is efficacious in young and aged hearts (Peart and Gross, 2004b), whereas conventional conditioning responses are impaired or eliminated with aging (Schulman et al., 2001; Peart et al., 2007; Przyklenk et al., 2008). Preservation of SLP during aging may relate to novel signaling involvement, distinct from conventional RISK paths (Peart and Gross, 2006). Mediation of protection during ischemia-reperfusion seems independent of mitochondrial KATP channels, only partially relies on PI3K/Akt, and involves Gs protein, PKA, and β2-adrenergic receptors (Peart and Gross, 2006). However, the precise mechanistic basis and protective features of SLP remain incompletely defined.
We sought to more fully characterize this novel response. Because induction of sustained protection is clinically attractive (Peart and Headrick, 2008), we first assessed persistence of protection after removal of the trigger stimulus. The identity of opioid receptor subtypes involved in inducing protection was also determined, together with assessing the ability of SLP to augment conventional protection. Finally, because we have examined only signaling involvement in protection during ischemia-reperfusion (Peart and Gross, 2006), we test here for kinase involvement in generating the SLP phenotype during the preischemic induction period. We specifically test for roles for PI3K (implicated in other forms of delayed cardioprotection; Kis et al., 2003) and PKA (contributing to SLP protection during ischemia-reperfusion; Peart and Gross, 2006).
Materials and Methods
Animals.
Investigations conformed to the guidelines of the Animal Care Committee of the Medical College of Wisconsin, which is accredited by the American Association of Laboratory Animal Care, and the Animal Ethics Committee of Griffith University. Hearts were isolated from fed 7- to 12-week-old, male, wild-type C57/BL6 mice.
The SLP Model and Experimental Design.
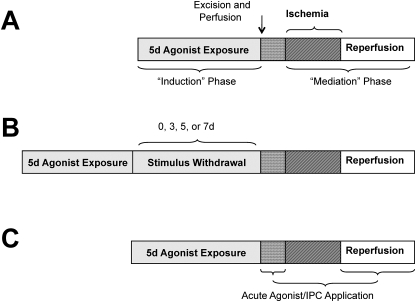
The general design (presented in Fig. 1A) involves induction of SLP via continuous opioid agonism in vivo for up to 5 days, followed by ex vivo perfusion of hearts to assess functional ischemic tolerance and infarct development. For morphine-induced SLP, mice were briefly anesthetized with halothane, a small incision was made at the base of the neck, and placebo or a 75-mg morphine pellet (National Institute of Drug Abuse, Bethesda, MD) was inserted into the dorsal subcutaneous space before it was closed with 9-mm wound clips. Pellets were in place for 5 days before analysis of cardiac ischemic tolerance ex vivo (Fig. 1A). To assess persistence of cardioprotection poststimulus, a subset of mice was reanesthetized with halothane at 5 days, pellets were removed, and mice were administered the nonselective antagonist naloxone (7 mg/kg i.p.) to limit further receptor agonism. These mice were allowed to recover for 3, 5, or 7 days before ex vivo assessment of ischemic tolerance (Fig. 1B).
Fig. 1.
Timeline for experimental procedures, outlining induction of SLP, and timing of drug treatments. Shown are generalized schemes for experiments to induce and mediate SLP (A), assess effects of stimulus withdrawal (B), and assess additivity of SLP with acute protective responses (C) (d, days).
To identify opioid receptor involvement, a mini-pump containing either the nonselective antagonist naloxone (5 mg/kg/day), the δ-opioid selective antagonist 7-benzylidenenaltrexone (BNTX) (3.3 mg/kg/day), or the κ-opioid receptor antagonist nor-binaltorphimine (nor-BNI) (0.5 mg/kg/day) was simultaneously implanted with morphine pellets. The ability of selective opioid agonists or morphine metabolites to induce SLP was also tested: mice were implanted with mini-pumps containing either the δ-opioid receptor selective agonist (+)-4-[α(R)-α-[(2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl]-3-hydroxybenzyl]-N,N-diethylbenzamide (BW373U86) (0.5 mg/kg/day), the κ-opioid selective receptor agonist trans-(±)-3,4-dichloro-N-methyl-N-(2-(1-pyrrolidin)cyclohexyl)-benzeneacetamide methane sulfonate hydrate (U50,488H) (1.7 mg/kg/day), the morphine metabolite (and potent μ-opioid receptor agonist) morphine-6-glucuronide (0.3 mg/kg/day), or the metabolite morphine-3-glucuronide (5 mg/kg/day). In these studies, pellets and/or mini-pumps were left in place for 5 days before removal of hearts and analysis of ischemic tolerance ex vivo (Fig. 1).
To test for involvement of PI3K or PKA signals in the preischemic induction of SLP, osmotic mini-pumps (model 1007D; Alzet, Cupertino, CA) containing either wortmannin (30 μg/kg/day) or PKI-(14-22)-amide (200 μg/kg/day) were implanted together with morphine pellets to inhibit these signaling elements throughout opioid exposure. Ischemic tolerance was then assessed ex vivo. We also tested for shifts in expression and phosphorylation of Akt in membrane and cytosolic fractions of hearts removed after 12 or 72 h of induction (see Results).
In a final series of experiments we tested the ability of SLP to confer additional protection in hearts subjected to conventional stimuli (Fig. 1C). Specifically, protective effects of acute 10 μM morphine, 50 μM adenosine, or 10 μM of the poorly metabolized analog 2-chloroadenosine (all applied 5 min before ischemia) were assessed in hearts from placebo or SLP mice. The effects of morphine-induced SLP were also tested in mice overexpressing protective A1 adenosine receptors (A1ARs) in cardiomyocytes (Morrison et al., 2000). Finally, we tested the ability of IPC (three cycles of 1.5-min ischemia and 2-min reperfusion before the index ischemia) to provide additive protection in SLP hearts.
Langendorff Perfused Ex Vivo Heart Model.
Mice were anesthetized with 60 mg/kg sodium pentobarbital administered intraperitoneally, and hearts were isolated and perfused via the aorta in a Langendorff mode as described in detail previously (Reichelt et al., 2009). Contractile function was monitored via a water-filled balloon located in the left ventricle and inflated to yield an end-diastolic pressure of ∼5 mm Hg. Coronary flow was monitored via an ultrasonic flow-probe proximal to the aortic cannula, connected to a T206 flow meter (Transonic Systems Inc., Ithaca, NY). All functional data were recorded at 1 KHz on an 8/s MacLab system (ADInstruments Pty Ltd., Castle Hill, Australia) connected to an Apple 7300/180 computer (Apple Computer, Cupertino, CA). The ventricular pressure signal was digitally processed to yield peak systolic pressure, diastolic pressure, +differential of ventricular pressure development with time, −differential of ventricular pressure development with time, and heart rate.
After 20-min stabilization, hearts were switched to ventricular pacing at 420 beats per minute (SD9 stimulator; Grass Instruments, Quincy, MA). After an additional 10 min, baseline measurements were made and 25 min of global normothermic ischemia was initiated followed by 45 min of reperfusion. Pacing was terminated on initiation of ischemia and resumed after 1.5 min of reperfusion. Hearts were excluded from study after the initial stabilization period if they met one of the following criteria: 1) coronary flow >5 ml/min (near maximal dilation, or aortic tear), 2) unstable (fluctuating) contractile function, 3) left ventricular systolic pressure below 100 mm Hg, or 4) significant arrhythmias.
Infarct Size Determination via 2,3,5-Triphenyltetrazolium Chloride Staining.
Infarct size was assessed as described previously (Peart et al., 2008) in select groups. In brief, on termination of reperfusion, 1 ml of 0.1% (w/v) 2,3,5-triphenyltetrazolium chloride in phosphate-buffered saline was slowly infused retrogradely into the aorta, and hearts were incubated at 37°C for 3 min before setting in 5% agarose. Tissue was then sectioned and fixed overnight in 10% formalin. Images of slices were obtained and analyzed by independent observers using ImageJ (National Institutes of Health, Bethesda, MD).
Western Immunoblotting.
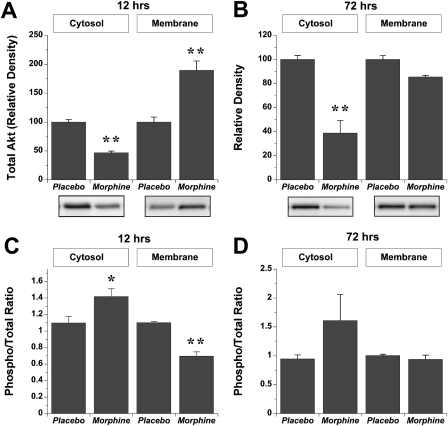
Changes in cytosolic or membrane expression of Akt after 12 or 72 h of morphine exposure (versus placebo) were assessed via immunoblot analysis. Hearts were excised, frozen in liquid N2, and homogenized with a glass dounce in lysis buffer containing protease and phosphatase inhibitors. Samples containing 30 μg of protein from cytosolic or detergent-soluble membrane fractions were loaded onto precast 10% acrylamide gels and separated at 150 V for ∼1.5 h. All placebo and treatment samples at each time point were run on a single gel to facilitate comparison. Equal loading was confirmed by Ponceau staining. Proteins were subsequently transferred to polyvinylidene difluoride membranes and blocked in 5% skim milk powder in Tris-buffered saline with Tween 20 for 60 min. Blots were incubated with primary antibody (total and phospho-Akt, 1:1000; Cell Signaling Technology, Danvers, MA) overnight at 4°C. After three washes in Tris-buffered saline with Tween 20 the blots were incubated with secondary antibody and visualized on a ChemiDoc XRS system (Bio-Rad Laboratories, Hercules, CA). Blot densities were normalized to baseline placebo densities on each gel, with mean expression in placebo groups set to an arbitrary 100 value. Variance in protein expression is low, as evidenced by modest S.E.M. values (relative to expression) in Fig. 6.
Fig. 6.
Effects of morphine-dependent induction of SLP on Akt expression in cytosolic and membrane fractions. Data are shown for total Akt at 12 h (A) or 72 h (B) and for the ratio of phosphorylated/total Akt at 12 h (C) or 72 h (D). Expression of total Akt (A and B) is normalized to levels in placebo hearts. Data are means ± S.E.M. *, P < 0.05 versus placebo; **, P < 0.01 versus placebo.
Chemicals.
PKI-(14-22)-amide was obtained from Calbiochem (La Jolla, CA), and BNTX, nor-BNI, BW373U86, and U50,488H were purchased from Tocris Bioscience (Ellisville, MO). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Drugs were infused through 0.22-μ filters at ≤1% of coronary flow to achieve the final concentrations indicated.
Statistical Analysis.
Data are expressed as mean ± S.E.M. For all groups the n value is ≥6. Final values for functional recovery and infarct size in Figs. 2, 3, 4, 5, and 7 were contrasted via one-way analysis of variance. For data in Fig. 6 a two-way analysis of variance was used to contrast effects of inhibitors on outcome and SLP protection. Appropriate planned comparisons were made for each group (placebo + inhibitor or SLP + inhibitor versus placebo or SLP; placebo + inhibitor versus SLP + inhibitor). Where significance was detected a post hoc Newman-Keuls test was used to identify individual differences. For all tests, evidence of significance was accepted at P < 0.05.
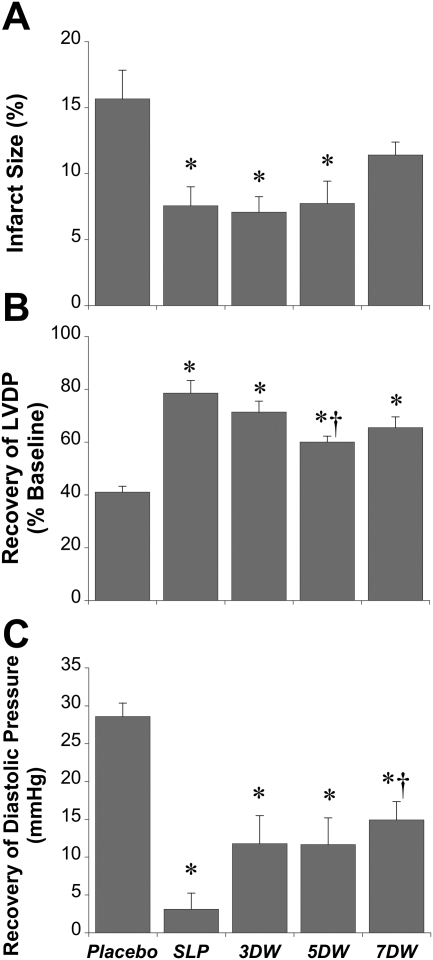
Fig. 2.
Cardioprotection with 5-day morphine-induced SLP and its long-term persistence. Data are shown for effects of SLP on myocardial infarct size (percentage of ventricular volume) (A) and recoveries for LVDP (percentage of baseline) (B) and end-diastolic pressure (mmHg) (C). Mice were subjected to 5-day morphine or placebo exposure followed by 0, 3, 5, or 7 days removal of morphine (or placebo). Hearts were then assessed for infarct development or functional recoveries after 25-min ischemia/45-min reperfusion. Data are mean ± S.E.M. *, P < 0.05 versus corresponding placebo group; †, P < 0.05 versus SLP alone. DW, days of stimulus withdrawal from SLP.
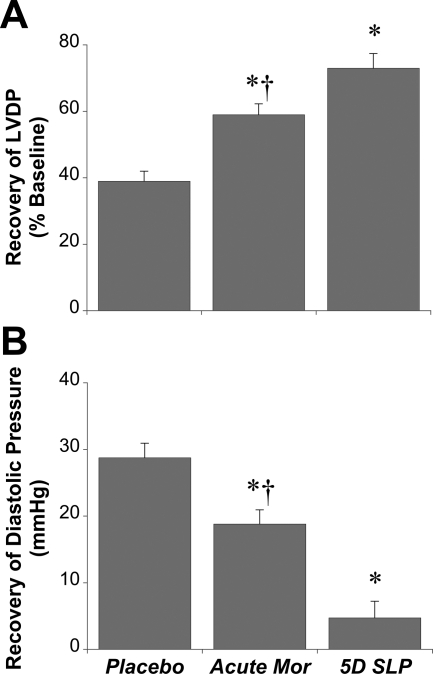
Fig. 3.
Efficacy of SLP versus acute morphine treatment. Data are shown for effects of continuous morphine exposure on recovery of LVDP (percentage of baseline) (A) and end-diastolic pressure (mmHg) (B). Mice were subjected to 5 days of morphine (SLP) or placebo (Plac) exposure before isolation of hearts and ex vivo assessment of functional sensitivity to 25-min ischemia/45-min reperfusion. Hearts were also isolated from untreated mice and subjected to acute 50 μM morphine treatment for the purposes of comparison. Data are means ± S.E.M. *, P < 0.05 versus placebo group; †, P < 0.05 versus 5-day (5D) SLP.
Fig. 4.
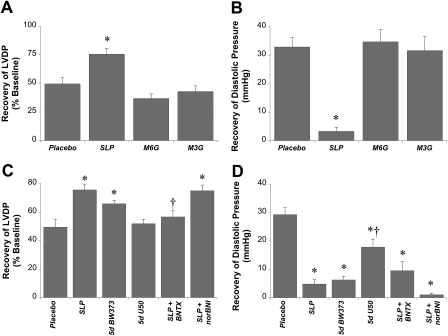
Relative roles of opioid receptor subtypes in triggering SLP. Effects of opioid receptor antagonists or agonists were assessed. A and B, data are shown for the effects on recoveries for LVDP (percentage of baseline) (A) and end-diastolic pressure (mmHg) (B) after 5-day exposure to placebo, morphine, morphine-6-glucuronide (M6G), or morphine-3-glucuronide (M3G). C and D, data are shown for recoveries for LVDP (percentage of baseline) (C) and end-diastolic pressure(mmHg) (D), where mice were subjected to 5-day (5d) morphine-induced SLP in the absence or presence of a δ-selective antagonist (BNTX) or κ-opioid antagonist (nor-BNI). We also assessed effects of 5-day exposure to a δ-opioid-selective agonist (BW373U86) or the κ-selective agonist U50,488H (U50). Hearts were isolated for ex vivo assessment of functional sensitivity to 25-min ischemia/45-min reperfusion. Data are means ± S.E.M. *, P < 0.05 versus placebo; †, P < 0.05 versus SLP alone.
Fig. 5.
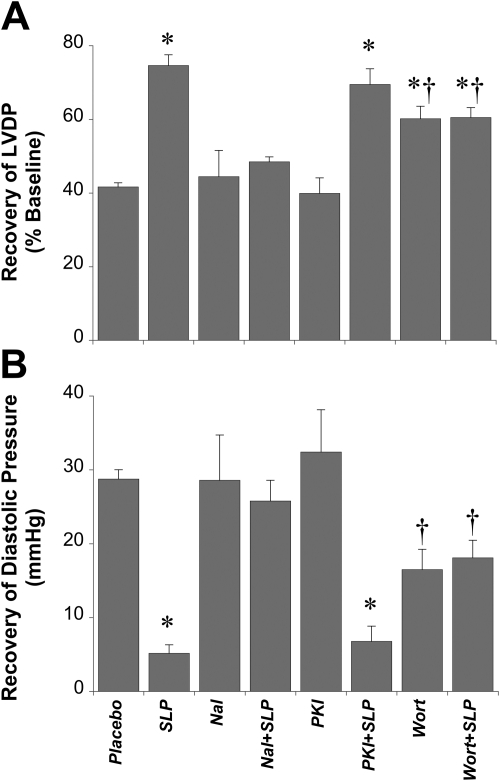
Effects of nonselective opioid receptor antagonism and PI3K or PKA inhibition on induction of the SLP phenotype. Data are shown for effects on recovery of LVDP (percentage of baseline) (A) and end-diastolic pressure (mmHg) (B). Mice were subjected to 5-day morphine-induced SLP in the absence or presence of the nonselective opioid antagonist naloxone (Nal), the PI3K inhibitor wortmannin (Wort), or the PKA inhibitor myristoylated PKI-(14-22)-amide (PKI) before isolation of hearts and ex vivo assessment of functional sensitivity to 25-min ischemia/45-min reperfusion. Data are means ± S.E.M. *, P < 0.05 versus placebo.; †, P < 0.05 versus SLP alone.
Fig. 7.
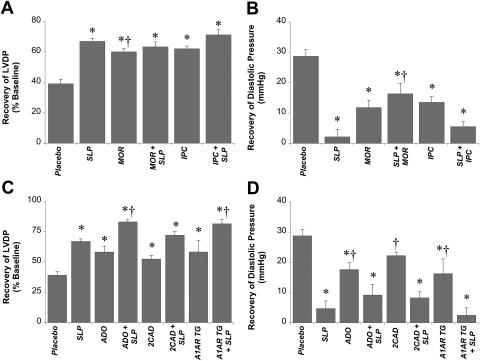
Additivity of SLP with conventional protection. Data are shown for recoveries of LVDP (A and C) and end-diastolic pressure (B and D). Mice were subjected to 5 days of morphine-induced SLP (or placebo), then isolated for ex vivo assessment of functional sensitivity to 25-min ischemia/45-min reperfusion. Isolated hearts were untreated or subjected to either acute 10 μM morphine (MOR) or IPC (three cycles × 1.5-min ischemia/2-min reperfusion) (A and B) or 50 μM adenosine (ADO), 10 μM 2-chloroadenosine (2CAD), or cardiac overexpression of A1 adenosine receptors (A1AR TG) (C and D). Data are means ± S.E.M. *, P < 0.05 versus corresponding placebo group; †, P < 0.05 versus SLP alone.
Results
Baseline.
None of the treatments studied modified normoxic cardiac function (Table 1).
TABLE 1.
Baseline contractile function and coronary flow in hearts isolated from placebo-treated or morphine-treated mice
Data were acquired after 30-min aerobic perfusion (and at a fixed pacing rate of 420 beats per minute). Data are means ± S.E.M.
| Group | LVEDP | LVDP | Heart Rate | Coronary Flow |
|---|---|---|---|---|
| mm Hg | beats/min | ml/min/g | ||
| SLP | ||||
| 5-Day placebo (n = 13) | 5 ± 1 | 153 ± 9 | 420 ± 3 | 2.7 ± 0.2 |
| 5-Day SLP (n = 11) | 6 ± 2 | 150 ± 3 | 422 ± 1 | 2.8 ± 0.4 |
| Effect of stimulus withdrawal (5-day SLP) | ||||
| 3-Day withdrawal (n = 8) | 5 ± 1 | 148 ± 4 | 427 ± 2 | 2.7 ± 0.2 |
| 5-Day withdrawal (n = 6) | 4 ± 2 | 153 ± 3 | 424 ± 1 | 2.7 ± 0.2 |
| 7-Day withdrawal (n = 8) | 3 ± 2 | 143 ± 10 | 430 ± 32 | 3.2 ± 0.2 |
| Inhibitors in the induction phase (5-day SLP) | ||||
| Naloxone (n = 6) | 5 ± 1 | 146 ± 4 | 425 ± 5 | 2.7 ± 0.1 |
| SLP + naloxone (n = 8) | 8 ± 1 | 152 ± 9 | 445 ± 19 | 3.6 ± 0.2 |
| PKI (n = 7) | 3 ± 1 | 148 ± 3 | 424 ± 5 | 2.5 ± 0.2 |
| SLP + PKI (n = 7) | 7 ± 1 | 142 ± 9 | 406 ± 11 | 2.8 ± 0.1 |
| Wortmannin (n = 6) | 3 ± 1 | 153 ± 6 | 419 ± 1 | 3.1 ± 0.1 |
| SLP + wortmannin (n = 10) | 3 ± 1 | 128 ± 5 | 435 ± 6 | 2.9 ± 0.5 |
| Opioid receptor specificity (5-day SLP) | ||||
| SLP + nor-BNI (n = 8) | 6 ± 1 | 149 ± 4 | 435 ± 39 | 2.5 ± 0.2 |
| SLP + BNTX (n = 6) | 4 ± 1 | 154 ± 3 | 416 ± 2 | 2.6 ± 0.1 |
| SLP (BW373U86) (n = 10) | 5 ± 1 | 137 ± 6 | 428 ± 1 | 2.5 ± 0.1 |
| SLP (U50,488) (n = 9) | 3 ± 2 | 148 ± 5 | 422 ± 5 | 3.2 ± 0.2 |
| Additivity of protection | ||||
| A1AR TG (n = 7) | 4 ± 1 | 138 ± 8 | 418 ± 2 | 3.2 ± 0.2 |
| SLP + A1AR TG (n = 8) | 5 ± 1 | 127 ± 8 | 424 ± 9 | 3.6 ± 0.3 |
LVEDP, left ventricular end-diastolic pressure.
Longevity Of SLP Protection.
Prior data support persistence of protection with SLP for up to 48 h after complete removal of morphine (Peart and Gross, 2004b). Here, we show that a 5-day exposure to morphine markedly reduced infarct size (by ∼50%) in addition to improving postischemic contractile performance (Fig. 2). The effects of SLP on infarct size persisted for at least 5 days after removal of the stimulus (Fig. 2A). At 7 days after cessation of opioid stimulation the reduction in infarct size was intermediate between placebo and SLP without stimulus withdrawal (i.e., infarct size after 7 days of stimulus withdrawal did not differ from either placebo or SLP alone).
As a comparison, the protective efficacy of acute morphine (50 μM before and after ischemia) was assessed in a subset of hearts from placebo-treated mice. Functional recovery in these acutely treated hearts was intermediate: significantly greater than placebo but less than in hearts from the 5-day SLP group (Fig. 3).
Opioid Receptor Involvement in SLP Induction.
Morphine is a broad-spectrum opioid agonist, exhibiting a selectivity profile of μ>κ>δ. In contrast to morphine treatment, 5 days of treatment with the primary morphine metabolites morphine-6-glucuronide, also a potent μ-selective opioid agonist, or morphine-3-glucuronide failed to induce any protection against ischemia (Fig. 4). The κ-opioid-selective agonist U50488H was also ineffective in producing SLP, whereas 5 days of treatment with the δ-opioid receptor agonist BW373U86 mimicked the benefit with morphine alone (Fig. 4). Thus, induction of SLP seems to be specifically δ-opioid receptor-mediated. Further confirming δ-receptor versus κ-opioid receptor involvement, morphine-dependent induction of SLP was abolished by the δ-opioid receptor antagonist BNTX, but was insensitive to the κ-opioid receptor antagonist nor-BNI (Fig. 4).
Signaling Involvement in SLP Induction.
We showed previously that mediation of protection during ischemia-reperfusion seems to be PKA-dependent and partially PI3K/PKB-independent (Peart and Gross, 2006). Here, we tested signaling underlying preischemic induction of the SLP phenotype. Opioid antagonism with naloxone during this period completely abolished expression of protection (Fig. 5), confirming a requirement for opioid receptor activation. Opioid exposure was unable to augment resistance to ischemia-reperfusion in mice cotreated with the PI3K inhibitor wortmannin (Fig. 5). Unexpectedly, prolonged wortmannin treatment itself modestly improved ischemic tolerance. Nonetheless, recovery with SLP alone significantly exceeded that in the wortmannin group, yet SLP failed to improve outcome in hearts from wortmannin-treated animals. These hearts are also shown to be submaximally protected, because SLP can confer significantly greater protection under other conditions (see Fig. 7). In contrast to wortmannin, induction of SLP was insensitive to cotreatment with PKI (which failed to alter outcome when applied alone), supporting PKA-independent induction of the protected phenotype.
Given the evidence of PI3K involvement, we tested for changes in expression and phosphorylation of the key downstream target Akt, implicated in delayed forms of cardioprotection (Kis et al., 2003). After 12 h of morphine exposure total Akt was significantly modified, declining in the cytosolic and increasing in the membrane fraction (Fig. 6A). This may reflect translocation of Akt from cytosol to membrane. After 3 days of morphine exposure, when the protected phenotype was clearly apparent (Peart and Gross, 2004a), cytosolic Akt was again depressed, whereas there was no change in membrane levels (Fig. 6B). Relative phosphorylation of Akt (Fig. 6, C and D) was modestly altered at 12 h (increased phosphorylation in cytosol, reduced phosphorylation in membrane) with no significant changes at 72 h.
Additivity of SLP with Conventional Stimuli.
Because mechanisms of SLP may be distinct from those involved in conventional protection, it may be feasible to augment conventional responses with SLP. However, neither acute morphine nor a typical IPC protocol provided additional protection in hearts from SLP mice (Fig. 7). On the other hand, preischemic and postischemic adenosine or 2-chloroadenosine improved ischemic outcomes in both placebo and SLP hearts (Fig. 7). Likewise, cardiac specific expression of A1 adenosine receptors augmented ischemic tolerance in both groups (Fig. 7).
Discussion
Protection with prolonged opioid stimulation offers features relevant to clinical cardioprotection, including long-term benefit and efficacy in aged myocardium. However, the properties and mechanisms of SLP are incompletely defined. This study documents potent protection with SLP, which persists 7 days after stimulus removal. Protection arises specifically through δ-opioid receptor modulation of PI3K signaling before ischemia and is additive with adenosinergic responses but not acute opioids or IPC.
Persistent Protection with SLP.
For SLP (or related targets) to have clinical potential, it would ideally induce a broad protective window. We show that protection against infarction and dysfunction persists for 5 to 7 days poststimulus (Fig. 2), which is much broader than the 2- to 3-day window with delayed PC (Baxter and Ferdinandy, 2001). Proteins involved in SLP also differ from those in delayed PC, because the latter is NO- and potentially KATP channel-dependent (Baxter and Ferdinandy, 2001), whereas SLP is not (Peart and Gross, 2006). The longevity of protection with SLP is uncommon outside of genetic manipulation, although irreversible δ-opioid agonism affords protection for up to 5 days (Gross et al., 2005). Paralleling SLP, this response is abolished by coadministration of wortmannin, whereas PI3K inhibition during ischemia is only partially inhibitory (Gross et al., 2005).
Temporal profiles for protection against infarction versus dysfunction differed slightly (Fig. 2): protection against dysfunction remained significant at 7 days (Fig. 2, B and C), whereas anti-infarct effects were no longer significant (Fig. 2A). Although this might evidence a more sustained effect of SLP on the mechanisms of stunning versus infarction, infarct size at 7 days is still ∼30% lower than in placebo and intermediate between SLP and placebo groups. This suggests residual protection against infarction (as well as contractile dysfunction) after 7 days. One should not overinterpret this pattern, which may reflect issues of statistical power rather than biological significance.
A final note regarding temporal properties of SLP relates to the possible impact of “withdrawal” itself. Opiate withdrawal involves complex biochemical changes that could conceivably modify ischemic tolerance. However, this is an unlikely explanation for SLP: we have documented previously SLP protection in the absence of any withdrawal per se (Peart and Gross, 2004a,b, 2006), and the efficacy of SLP is comparable with or without stimulus withdrawal plus naloxone (Fig. 2). Furthermore, SLP is induced specifically by δ-opioid agonism, whereas withdrawal effects are conventionally linked to μ- and κ- receptors but not δ-receptors.
Opioid Receptor Involvement.
We used the broad-spectrum agonist morphine as SLP stimulus in prior studies. Whether one or more receptor subtypes are involved, and whether morphine metabolites contribute, was not clear. Protection with morphine is commonly attributed to δ-receptors (Gross, 2003), despite low affinity for morphine (Gross, 2003). Both δ- and κ-receptors can generate protection (Chen et al., 2008; Peart et al., 2008), whereas there is little support for protection via μ-receptors.
The metabolite morphine-6-glucuronide is a selective μ-opioid receptor agonist (generally more potent than morphine in vivo) that may be responsible for morphine analgesia (Kilpatrick and Smith, 2005). Absence of protection with morphine-6-glucuronide, or the other primary metabolite morphine-3-glucuronide (Fig. 4), argues against roles for μ-receptors or metabolites in the induction of SLP. Lack of effect of κ-receptor agonism or antagonism discounts a major role for this subtype. In contrast, abrogation of SLP with δ-selective antagonism and induction of protection by δ-selective agonism strongly implicates this receptor (Fig. 4). This is important, because δ-agonism is less likely to invoke untoward effects of opioid exposure (primarily κ- or μ-dependent) and permits targeting of ischemic tolerance independently of analgesia.
Kinase Signals Underlying SLP Induction.
Prior data support unique signaling in the mediation phase of SLP, involving PKA and Gs-coupled paths (Peart and Gross, 2006). Here, we show that induction (preischemic opioid agonism) harnesses PI3K-dependent but PKA-independent signals (Fig. 5), rendering induction and mediation mechanistically distinct. Distinct signaling may contribute to SLP's efficacy in aged myocardium (Peart and Gross, 2004b), where conventional responses fail (Schulman et al., 2001; Peart et al., 2007). Pharmacological data support involvement of wortmannin-sensitive elements in SLP induction, implicating PI3K/Akt (Fig. 5). It was not possible to induce further protection in animals cotreated with wortmannin, although this is complicated by modest protection with wortmannin itself (Fig. 5). The basis of this unexpected observation is unclear, and we are unaware of prior studies of sustained PI3K inhibition. However, there are key parallels with signaling modulation in protective hibernation (discussed below). It is noteworthy that the benefit with prolonged wortmannin was significantly less than with SLP, and SLP was unable to enhance recovery in these hearts despite generating greater protection under other conditions (Fig. 7).
Consistent with evidence of PI3K involvement, we detected shifts in expression/phosphorylation of downstream Akt at 12 and 72 h (Fig. 6). Because PI3K/Akt activity during ischemia-reperfusion is not critical to SLP (Peart and Gross, 2006), PI3K-dependent induction must reflect PI3K-triggered changes in expression/function of proteins subsequently dictating ischemic tolerance. Reduced cytosolic or membrane levels of Akt (Fig. 6) may reflect reduced expression and/or translocation to other sites, including mitochondria or nuclei (Miyamoto et al., 2009), whereas reductions in phosphorylation reflect reduced activation. Although Akt translocation to mitochondria could acutely mediate protection (Miyamoto et al., 2009), this is not relevant to SLP (Peart and Gross, 2006). Alterations in nuclear Akt translocation may be involved through regulation of protein expression. Indeed, reductions in Akt activity may orchestrate global transcriptional responses in hibernation (Abnouset al., 2008).
Many parallels exist between SLP and hibernation: hibernation is triggered by δ-opioid agonism, which can also induce hibernation in nonhibernating primates (Oeltgen et al., 1982, 1988); δ-opioid triggered hibernation increases stress resistance (Bolling et al., 1997); hibernating and anoxia-tolerant species harness PKA signaling (Storey, 1996; Holden and Storey, 1998); and repression of Akt suppresses energy-costly anabolic/growth processes to maintain cell viability over extended periods (Cai et al., 2004; Abnous et al., 2008). The SLP phenotype is induced by δ-opioid agonism, involves PI3K-dependent signals and repression of Akt expression (Fig. 6), and leads to sustained PKA-dependent cytoprotection. Prolonged PI3K/Akt inhibition also induces some protection. Though speculative, these data support protection via sustained PI3K/Akt repression, consistent with the role of Akt in δ-opioid-mediated hibernation.
Additivity with Protective Stimuli.
If differing stimuli activate divergent signaling, it may be feasible to augment cardioprotection additively. However, research reveals commonality of signaling in PC, postconditioning, and with varied G protein-coupled receptors, converging on mitochondrial targets (Hausenloy and Yellon, 2007; Murphy and Steenbergen, 2008). As a result, conventional stimuli may be nonadditive (Halkos et al., 2004). In contrast, coactivation of differing upstream paths can augment activity of downstream effectors, as with erythropoietin and IPC (Nishihara et al., 2006). Because SLP seems to engage distinct signaling it offers potential for additive protection.
Protection via different adenosinergic stimuli was additive with SLP (Fig. 7). This further distinguishes SLP from IPC, because IPC is unable to augment protection via cardiac adenosine receptor overexpression (Morrison et al., 2000). It is noteworthy that neither acute morphine nor IPC elicited protection in SLP hearts (Fig. 7). Because opioid receptors and PI3K are involved in acute opioid and IPC responses (Gross et al., 2004; Peart and Gross, 2006; Chen et al., 2008; Peart et al., 2008) and in SLP induction, lack of additivity may reflect down-regulation of opioid receptors (Gintzler and Chakrabarti, 2006) and PI3K/Akt (Figs. 5 and 6) during SLP induction. On the other hand, adenosinergic stimuli may remain additive with SLP because chronic opiates can sensitize adenosine receptors (Brundege and Williams, 2002), and there is evidence protection with adenosine is uniquely PI3K/Akt-independent (Qin et al., 2003).
Study Limitations.
The present study focuses on the recovery of contractile function (Table 1) as a primary endpoint. Although infarct size analysis was important in confirming the ability of SLP to limit cell death, this raises certain limitations. First, we assessed infarction after 45-min reperfusion, whereas infarct expansion may occur over days. Thus, early infarct size does not reflect the final extent of infarction. Nonetheless, we (Peart et al., 2008) and others commonly assess 45- to 60-min reperfusion periods in ex vivo studies, and although final size is not measured, there is clear differentiation in infarction between groups at this stage. Infarct analysis in every experimental group would also be informative; however, this precludes sampling of tissue for other purposes. We opted for infarct analysis when characterizing temporal properties of SLP.
Although we have identified SLP in rodent species (Peart and Gross, 2004a,b, 2006), we have yet to establish its existence in other mammals. Other responses, including PC and postconditioning, are well conserved across species, and opioid-mediated protection is documented in species from mouse to human (Gross, 2003). Indirect epidemiological evidence (Marmor et al., 2004) indicates that long-term opiate exposure in drug users attenuates coronary artery disease severity/mortality, implicating cardioprotection from prolonged opioid exposure in humans. A key future goal will be to test for occurrence of SLP in other species and ultimately humans.
Conclusions
We have further characterized novel protection with sustained exposure to opioids. Induction of SLP occurs via δ-opioid receptor-triggered PI3K-dependent signaling that is independent of PKA (contrasting signaling during ischemia-reperfusion). Protection persists for 5 to 7 days after stimulus removal and is additive with adenosinergic responses. The SLP phenotype shares several features with hibernation (δ-receptor triggered, repressed PI3K/Akt activity, protection involving PKA signaling). Molecular mechanisms underlying these responses may present useful targets for the sustained enhancement of myocardial stress resistance.
Acknowledgments
We thank Katherine Crane for technical assistance with the blinded analysis of infarct size and Dr. G. Paul Matherne for provision of mice overexpressing cardiac A1 adenosine receptors.
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grant R01HL008311-43], the National Health and Medical Research Council of Australia [Grant 481922], and the National Heart Foundation of Australia [Grants G 05B 2029, G O8B 3971, G 09B 4446]. J.N.P. is a recipient of a Career Development Award fellowship from the National Health and Medical Research Council of Australia.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.172593.
- PC
- preconditioning
- IPC
- ischemic PC
- RISK
- reperfusion injury salvage kinase
- A1AR
- A1 adenosine receptor
- BNTX
- 7-benzylidenenaltrexone
- BW373U86
- (+)-4-[α(R)-α-[(2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl]-3-hydroxybenzyl]-N,N-diethylbenzamide
- LVDP
- left ventricular developed pressure
- nor-BNI
- nor-binaltorphimine
- PI3K
- phosphoinositide-3 kinase
- PKA
- protein kinase A
- PKI
- myristoylated PKI-[14-22]-amide
- SLP
- sustained ligand-activated preconditioning
- U50,488H
- trans-(±)-3,4-dichloro-N-methyl-N-(2-(1-pyrrolidin)cyclohexyl)-benzeneacetamide methane sulfonate hydrate.
Authorship Contributions
Participated in research design: Peart, Gross, and Headrick.
Conducted experiments: Peart and See Hoe.
Performed data analysis: Peart and Headrick.
Wrote or contributed to the writing of the manuscript: Peart, Gross, and Headrick.
Other: Peart, Gross, and Headrick acquired funding for the research.
References
- Abnous K, Dieni CA, Storey KB. (2008) Regulation of Akt during hibernation in Richardson's ground squirrels. Biochim Biophys Acta 1780:185–193 [DOI] [PubMed] [Google Scholar]
- Baxter GF, Ferdinandy P. (2001) Delayed preconditioning of myocardium: current perspectives. Basic Res Cardiol 96:329–344 [DOI] [PubMed] [Google Scholar]
- Bolling SF, Tramontini NL, Kilgore KS, Su TP, Oeltgen PR, Harlow HH. (1997) Use of “natural” hibernation induction triggers for myocardial protection. Ann Thorac Surg 64:623–627 [DOI] [PubMed] [Google Scholar]
- Brundege JM, Williams JT. (2002) Increase in adenosine sensitivity in the nucleus accumbens following chronic morphine treatment. J Neurophysiol 87:1369–1375 [DOI] [PubMed] [Google Scholar]
- Cai D, McCarron RM, Yu EZ, Li Y, Hallenbeck J. (2004) Akt phosphorylation and kinase activity are down-regulated during hibernation in the 13-lined ground squirrel. Brain Res 1014:14–21 [DOI] [PubMed] [Google Scholar]
- Chen Z, Li T, Zhang B. (2008) Morphine postconditioning protects against reperfusion injury in the isolated rat hearts. J Surg Res 145:287–294 [DOI] [PubMed] [Google Scholar]
- Gintzler AR, Chakrabarti S. (2006) Post-opioid receptor adaptations to chronic morphine; altered functionality and associations of signaling molecules. Life Sci 79:717–722 [DOI] [PubMed] [Google Scholar]
- Gross ER, Hsu AK, Gross GJ. (2004) Opioid-induced cardioprotection occurs via glycogen synthase kinase β inhibition during reperfusion in intact rat hearts. Circ Res 94:960–966 [DOI] [PubMed] [Google Scholar]
- Gross ER, Peart JN, Hsu AK, Auchampach JA, Gross GJ. (2005) Extending the cardioprotective window using a novel delta-opioid agonist fentanyl isothiocyanate via the PI3-kinase pathway. Am J Physiol Heart Circ Physiol 288:H2744–H2749 [DOI] [PubMed] [Google Scholar]
- Gross GJ. (2003) Role of opioids in acute and delayed preconditioning. J Mol Cell Cardiol 35:709–718 [DOI] [PubMed] [Google Scholar]
- Halkos ME, Kerendi F, Corvera JS, Wang NP, Kin H, Payne CS, Sun HY, Guyton RA, Vinten-Johansen J, Zhao ZQ. (2004) Myocardial protection with postconditioning is not enhanced by ischemic preconditioning. Ann Thorac Surg 78:961–969 [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. (2007) Reperfusion injury salvage kinase signalling: taking a RISK for cardioprotection. Heart Fail Rev 12:217–234 [DOI] [PubMed] [Google Scholar]
- Holden CP, Storey KB. (1998) Protein kinase A from bat skeletal muscle: a kinetic study of the enzyme from a hibernating mammal. Arch Biochem Biophys 358:243–250 [DOI] [PubMed] [Google Scholar]
- Kilpatrick GJ, Smith TW. (2005) Morphine-6-glucuronide: actions and mechanisms. Med Res Rev 25:521–544 [DOI] [PubMed] [Google Scholar]
- Kis A, Yellon DM, Baxter GF. (2003) Second window of protection following myocardial preconditioning: an essential role for PI3 kinase and p70S6 kinase. J Mol Cell Cardiol 35:1063–1071 [DOI] [PubMed] [Google Scholar]
- Lacerda L, Somers S, Opie LH, Lecour S. (2009) Ischemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res 84:201–208 [DOI] [PubMed] [Google Scholar]
- Marmor M, Penn A, Widmer K, Levin RI, Maslansky R. (2004) Coronary artery disease and opioid use. Am J Cardiol 93:1295–1297 [DOI] [PubMed] [Google Scholar]
- Miura T, Miki T. (2008) Limitation of myocardial infarct size in the clinical setting: current status and challenges in translating animal experiments into clinical therapy. Basic Res Cardiol 103:501–513 [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Rubio M, Sussman MA. (2009) Nuclear and mitochondrial signalling Akts in cardiomyocytes. Cardiovasc Res 82:272–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison RR, Jones R, Byford AM, Stell AR, Peart J, Headrick JP, Matherne GP. (2000) Transgenic overexpression of cardiac A1 adenosine receptors mimics ischemic preconditioning. Am J Physiol Heart Circ Physiol 279:H1071–H1078 [DOI] [PubMed] [Google Scholar]
- Murphy E, Steenbergen C. (2008) Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev 88:581–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. (1986) Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74:1124–1136 [DOI] [PubMed] [Google Scholar]
- Nishihara M, Miura T, Miki T, Sakamoto J, Tanno M, Kobayashi H, Ikeda Y, Ohori K, Takahashi A, Shimamoto K. (2006) Erythropoietin affords additional cardioprotection to preconditioned hearts by enhanced phosphorylation of glycogen synthase kinase-3β. Am J Physiol Heart Circ Physiol 291:H748–H755 [DOI] [PubMed] [Google Scholar]
- Oeltgen PR, Nilekani SP, Nuchols PA, Spurrier WA, Su TP. (1988) Further studies on opioids and hibernation: delta opioid receptor ligand selectively induced hibernation in summer-active ground squirrels. Life Sci 43:1565–1574 [DOI] [PubMed] [Google Scholar]
- Oeltgen PR, Walsh JW, Hamann SR, Randall DC, Spurrier WA, Myers RD. (1982) Hibernation “trigger”: opioid-like inhibitory action on brain function of the monkey. Pharmacol Biochem Behav 17:1271–1274 [DOI] [PubMed] [Google Scholar]
- Peart JN, Gross GJ. (2004a) Morphine-tolerant mice exhibit a profound and persistent cardioprotective phenotype. Circulation 109:1219–1222 [DOI] [PubMed] [Google Scholar]
- Peart JN, Gross GJ. (2004b) Chronic exposure to morphine produces a marked cardioprotective phenotype in aged mouse hearts. Exp Gerontol 39:1021–1026 [DOI] [PubMed] [Google Scholar]
- Peart JN, Gross GJ. (2006) Cardioprotective effects of acute and chronic opioid treatment are mediated via different signaling pathways. Am J Physiol Heart Circ Physiol 291:H1746–H1753 [DOI] [PubMed] [Google Scholar]
- Peart JN, Headrick JP. (2008) Sustained cardioprotection: exploring unconventional modalities. Vascul Pharmacol 49:63–70 [DOI] [PubMed] [Google Scholar]
- Peart JN, Headrick JP. (2009) Clinical cardioprotection and the value of conditioning responses. Am J Physiol Heart Circ Physiol 296:H1705–H1720 [DOI] [PubMed] [Google Scholar]
- Peart JN, Gross ER, Headrick JP, Gross GJ. (2007) Impaired p38 MAPK/HSP27 signaling underlies aging-related failure in opioid-mediated cardioprotection. J Mol Cell Cardiol 42:972–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peart JN, Gross ER, Reichelt ME, Hsu A, Headrick JP, Gross GJ. (2008) Activation of κ-opioid receptors at reperfusion affords cardioprotection in both rat and mouse hearts. Basic Res Cardiol 103:454–463 [DOI] [PubMed] [Google Scholar]
- Przyklenk K, Maynard M, Darling CE, Whittaker P. (2008) Aging mouse hearts are refractory to infarct size reduction with post-conditioning. J Am Coll Cardiol 51:1393–1398 [DOI] [PubMed] [Google Scholar]
- Qin Q, Downey JM, Cohen MV. (2003) Acetylcholine but not adenosine triggers preconditioning through PI3-kinase and a tyrosine kinase. Am J Physiol Heart Circ Physiol 284:H727–H734 [DOI] [PubMed] [Google Scholar]
- Reichelt ME, Willems L, Hack BA, Peart JN, Headrick JP. (2009) Cardiac and coronary function in the Langendorff-perfused mouse heart model. Exp Physiol 94:54–70 [DOI] [PubMed] [Google Scholar]
- Schulman D, Latchman DS, Yellon DM. (2001) Effect of aging on the ability of preconditioning to protect rat hearts from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 281:H1630–H1636 [DOI] [PubMed] [Google Scholar]
- Skyschally A, van Caster P, Boengler K, Gres P, Musiolik J, Schilawa D, Schulz R, Heusch G. (2009) Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res 104:15–18 [DOI] [PubMed] [Google Scholar]
- Storey KB. (1996) Metabolic adaptations supporting anoxia tolerance in reptiles: recent advances. Comp Biochem Physiol B Biochem Mol Biol 113:23–35 [DOI] [PubMed] [Google Scholar]
- Zatta AJ, Kin H, Yoshishige D, Jiang R, Wang N, Reeves JG, Mykytenko J, Guyton RA, Zhao ZQ, Caffrey JL, et al. (2008) Evidence that cardioprotection by postconditioning involves preservation of myocardial opioid content and selective opioid receptor activation. Am J Physiol Heart Circ Physiol 294:H1444–H1451 [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. (2003) Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 285:H579–H588 [DOI] [PubMed] [Google Scholar]