Abstract
A brand three-step acne treatment system containing a solubilized 5% benzoyl lotion and a designated cleanser and moisturizer was compared with a brand benzoyl peroxide 5%/clindamycin 1% gel in subjects with acne vulgaris. The single-center, four-week study was investigator-blinded and randomized. The three-step acne treatment system proved to be comparable in efficacy and tolerability.
Benzoyl peroxide (BPO) is poorly soluble and many commercially available formulations often contain insoluble BPO macrocrystals, which trap much of the BPO in the interior of the clusters, thus reducing bioavailability.1–4 The larger BPO macrocrystals exhibit greater difficulty passing into the follicles as their size exceeds the diameter of the follicular orifice. As a result, intrafollicular access of BPO is reduced, likely limiting efficacy. Using patented technology to reduce the particulate size of BPO and to make it more uniform, a solubilized, 5% BPO lotion has been formulated to enhance follicular penetration of these smaller BPO particles.5 The 5% lotion formulation of solubilized BPO was designed for treating acne vulgaris in normal-to-dry skin, and is available as part of a three-step system (together with a proprietary gentle cream cleanser and a moisturizer containing glycerin and dimethicone).
Methods
Study design. The trial was performed as an investigator-blinded, randomized, single-center study.
Key inclusion criteria. The study included subjects age 12 to 45 years with acne vulgaris of mild-to-moderate severity who also had normal-to-dry skin. The lesion types and numbers allowed according to the inclusion criteria of the study protocol were as follows:
10–100 non-inflammatory lesions (open comedones + closed comedones)
17–40 inflammatory lesions (papules + pustules + nodules)
Up to two nodulocystic lesions.
Inclusion in the study mandated that subjects were willing to refrain from using other non-study acne medications, moisturizers, sunscreens, fragrances, aftershaves, or make-up on the face. Exceptions included oil-free, non-comedogenic make-up, mascara, eyeshadow, and lipstick. Included subjects also had to be willing to avoid excessive sun exposure and tanning booths.
Key exclusion criteria. Subjects allergic to benzoyl peroxide, clindamycin, lincomycin, salicylic acid, sunscreen, or any ingredient in study products were excluded from the study. Other exclusion criteria are listed as follows:
Papulopustular rosacea and other skin diseases on the face other than acne, which could interfere with study evaluation
Facial sunburn at baseline
Beard or sideburns that could interfere with study evaluation
Uncontrolled systemic disease or known infection with human immunodeficiency virus
History of regional enteritis, ulcerative colitis, or antibiotic-associated colitis
Concurrent use of other products on the face
Pregnancy, planning a pregnancy, or breastfeeding
Participation in another investigational study in the preceding 30 days.
Washout periods. The washout periods required prior to randomization were as follows:
One week for medicated facial cleansers
Two weeks for topical alpha-hydroxy acids, anti-acne medications, topical retinoids, topical and systemic antibiotics, and topical and systemic steroids
Three months for estrogens/birth control pills unless their use had already been stable for three months
Six months for systemic retinoids and facial cosmetic procedures.
Demographics. Of the 22 subjects enrolled, 21 (95%) completed the study. One subject was discontinued from the study due to poor compliance. Subjects were predominantly female (73%). The ethnic distribution was 68 percent Caucasian, 18 percent African American, 5 percent Asian, and 9 percent other. The Fitzpatrick skin type distribution was 55 percent type I, 14 percent type II, 18 percent type III, 9 percent type IV, and 4 percent type V. There were no significant between-group differences in demographic details.
Treatment regimen. Patients were randomly assigned to receive treatment with the three-step acne system or 5% BPO/1% clindamycin combination gel (containing dimethicone and glycerin) for four weeks. In the three-step acne system group, patients washed their face twice daily with the proprietary cream cleanser and then applied the solubilized 5% BPO lotion to the entire face each morning and the proprietary therapeutic moisturizer each evening. The moisturizer could also be used on an as needed basis. In the BPO/clindamycin combination gel group, patients washed their face twice daily with a specific over-the-counter cleansing wash and then applied the 5% BPO/1% clindamycin combination gel product to the entire face each morning. A specific over-the-counter moisturizer could be used as needed.
Outcome measures. The following parameters were used to evaluate treatment outcomes:
Non-inflammatory lesion count reduction
Inflammatory lesion count reduction
Erythema, dryness, peeling, burning/stinging, itching (Table 1)
Patient rating of effectiveness of treatment (Table 2)
Patient rating of acne improvement (Table 2)
Patient satisfaction with acne improvement (Table 2).
Table 1.
Scales for outcome measures reported by the investigator
| SCORE | ERYTHEMA | DRYNESS | PEELING | BURNING/STINGING | ITCHING |
|---|---|---|---|---|---|
| 0 |
None May be minor discoloration |
None | None | None | None |
| 1 |
Mild Light pink, noticeable |
Mild Slight but definite roughness |
Mild Slight peeling |
Mild Light warm, tingling sensation, not really bothersome |
Mild Occasional, slight itching |
| 2 |
Moderate Pink-red, easily noticeable |
Moderate Moderate roughness |
Moderate Definitely noticeable peeling |
Moderate Definite warmth, tingling/stinging sensation that is somewhat bothersome |
Moderate Constant or intermittent itching that is somewhat bothersome |
| 3 |
Severe Deep or bright red, may be warm to the touch |
Severe Marked roughness |
Severe Extensive peeling |
Severe Hot tingling/stinging sensation which is disturbing normal activity |
Severe Bothersome itching which is disturbing normal activity |
Table 2.
Scales for outcome measures reported by the patients
| SCALE | PATIENT RATING OF EFFECTIVENESS OF TREATMENT | PATIENT RATING OF ACNE IMPROVEMENT | SATISFACTION WITH ACNE IMPROVEMENT |
|---|---|---|---|
| 0 | — |
Poor/no change No real improvement or noticeable difference in acne |
— |
| 1 | Ineffective |
Fair Some change or minor improvement |
Dissatisfied |
| 2 | Somewhat effective |
Good Visible difference in acne, but acne still present |
Indifferent |
| 3 | Effective |
Very good Very few acne lesions remaining |
Somewhat satisfied |
| 4 | Very effective |
Excellent Almost clear or clear of acne |
Satisfied |
| 5 | — | — | Very satisfied |
Statistical analyses. Statistical tests used to evaluate between-group differences were a two-sided Chi-square or Fisher's exact test for gender and race; a two-sided t test for age; a two-sided t test or ANCOVA for percent reduction in lesion counts; and a Wilcoxon rank-sum test for Fitzpatrick skin types, patient ratings, and tolerability assessments. A P value of ≤.05 was considered statistically significant.
Results
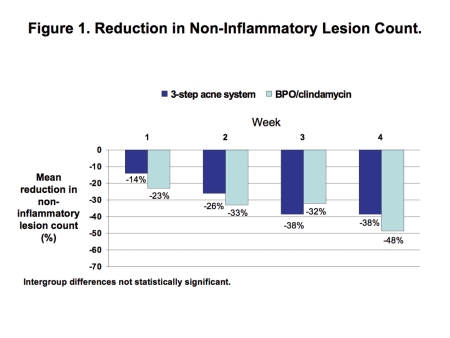
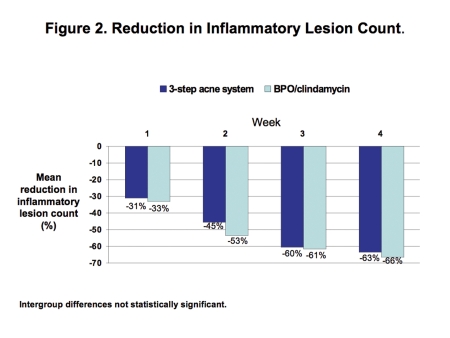
Efficacy. Investigator ratings showed that both groups achieved comparable improvements in efficacy. At Week 4, both therapies reduced the comedo count by a mean of 38 to 48% (Figure 1) and reduced the inflammatory lesion count by a mean of 63 to 66% (Figure 2).
Figure 1.
Reduction in non-inflammatory lesion count
Figure 2.
Reduction in inflammatory lesion count
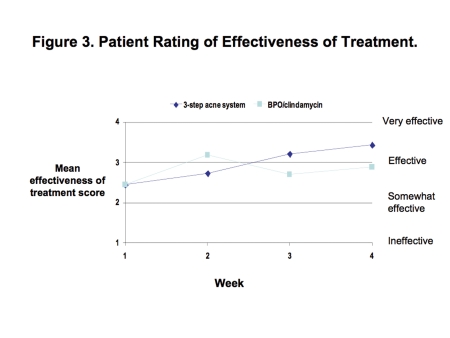
Patient ratings showed that the three-step, solubilized, 5%-BPO-lotion-based acne system was at least as effective as the BPO/clindamycin gel combination. Although not statistically significant, the three-step acne system achieved greater improvements from baseline through Week 4 in the following mean scores. The effectiveness of acne treatment score changed from 2.5 to 3.4 with the acne system versus 2.5 to 2.9 with BPO/clindamycin (Figure 3), and the improvement in acne score changed from 1.6 to 3.1 with the three-step acne system versus 1.8 to 2.6 with BPO/clindamycin (Figure 4).
Figure 3.
Patient rating of effectiveness of treatment
Figure 4.
Patient rating of improvement in acne
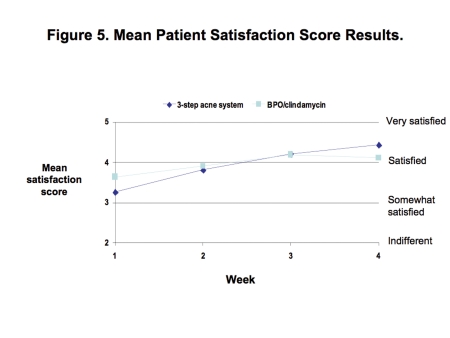
Patient satisfaction. Both regimens enhanced the mean patient ratings for satisfaction with acne improvement from “somewhat satisfied to satisfied” to “satisfied to very satisfied” (Figure 5). The three-step acne system group achieved a relatively greater mean improvement in satisfaction score (from 3.3 at baseline to 4.4 at Week 4 vs. from 3.6 to 4.1 in the BPO/clindamycin combination gel group) (Figure 5).
Figure 5.
Mean patient satisfaction score
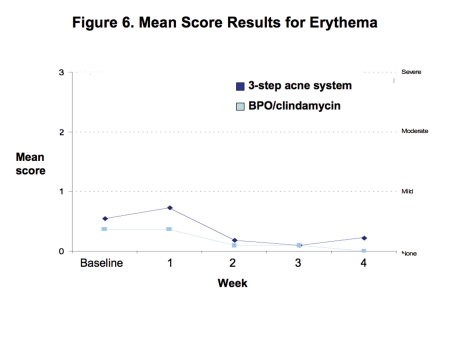
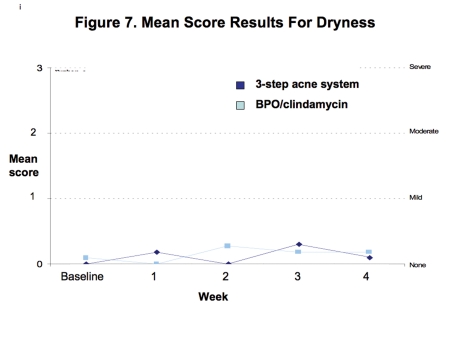
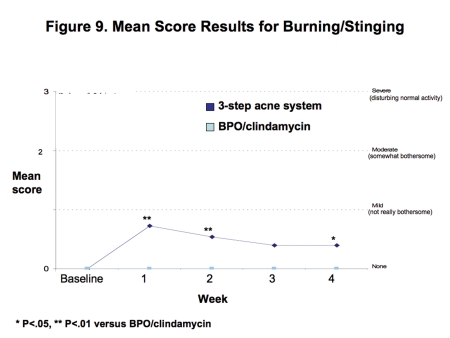
Tolerability. Both regimens were generally well tolerated with mean levels of erythema, dryness, peeling, and burning/stinging consistently less than mild at all time points (Figures 6–9).
Figure 6.
Mean score for erythema
Figure 7.
Mean score for dryness
Figure 8.
Mean score for peeling
Figure 9.
Mean score for burning/stinging
Although burning/stinging was reported with the three-step acne system, it was typically transient and resolved within a few minutes after application. In addition, facial skin burning/stinging occurred primarily in the first two weeks of therapy, lessened with continued treatment, and mean levels were less than “not really bothersome” (i.e., less than mild) at every time point. With both regimens, mean levels of itching were “none” at all time points. No serious adverse events were reported.
Conclusion
After four weeks, once-daily use of the three-step acne system (solubilized 5% BPO lotion plus proprietary cleanser and moisturizer) demonstrated comparable efficacy, patient satisfaction, and tolerability as compared to an established, commercially available, brand BPO/clindamycin combination gel product.
References
- 1.Wilson DC, Meadows KP, Ramirez J. A comparison of a novel benzoyl peroxide system with a combination benzoyl peroxide and clindamycin product: a 2-week split-face study of effectiveness and tolerability. Poster #146 presented at: The 65th Annual Meeting of the American Academy of Dermatology. Washington, DC; February 2-6, 2007.
- 2. Wilson DC. Evaluation of a novel acne treatment system designed to enhance the efficacy of benzoyl peroxide treatment: an investigator-blind, randomized study. Poster #103 presented at: The Summer Academy Meeting 2007 of the American Academy of Dermatology. New York, NY; August 1-5, 2007. [Google Scholar]
- 3. Tanghetti E, Kircik L, Wilson D, Shawan S. Solubilized benzoyl peroxide versus benzoyl peroxide/clindamycin in the treatment of moderate acne. J Drugs Dermatol. 2008;7:534–538. [PubMed] [Google Scholar]
- 4. Del Rosso JQ. Evaluation of a solubilized benzoyl peroxide gel: a pooled analysis from three randomized investigator-blinded clinical trials. Cosm Dermatol. 2008;21:201–206. [Google Scholar]
- 5. CLENZIderm MD.TM. Introducing a breakthrough acne solution from Obagi. [August 23, 2007]; http://www.obagi.com/article/forpatients/obagiclenzidermmd/clenziderm.html. [Google Scholar]