Abstract
A distinct structural change in the trabecular meshwork (TM) of patients with primary open-angle glaucoma (POAH) is the increase in fibrillar extracellular matrix (ECM) in the juxtacanalicular region of the TM. Transforming growth factor (TGF)-β2 signaling may be involved, as TGF-β2 is significantly increased in the aqueous humor of patients with POAG. In cultured human TM cells, TGF-β2 causes an increase in ECM deposition, an effect that is blunted or prevented, if BMP7 is added in combination with TGF-β2. In order to know more about the signaling network that is induced in HTM cells treated with BMP7, TGF-β2 or the combination of both factors, we identified differentially regulated genes by microarray analysis, and confirmed selected genes by quantitative RT-PCR, Western blotting, or immunohistochemistry. We observed multiple effects of both TGF-β2 and BMP7 on the expression of a considerable number of genes involved in growth factor signaling, ECM structure and turnover, and modification of the cytoskeleton. Among the genes that were found to be regulated were CAPZA1, CDC42BPB, EFEMP1, FGF5, FSTL3, HBEGF, LTBP1, LTBP2, MATN2, NRP1, SERPINE1, SH3MD1, SMTN, SMAD7, TFPI2, TNFAIP6, and VEGF. Since SMAD7 encodes for Smad7, an inhibitory Smad that acts in a negative feedback loop to inhibit TGF-β activity, we silenced Smad7 mRNA in cultured human TM cells by a specific small interfering RNA. Silencing of its mRNA caused a substantial knock down of Smad7 in TM cells. Following combined BMP7/TGF-β2 treatment, the antagonizing effect of BMP7 on TGF-β2 induced CTGF expression was abolished. We conclude that Smad7 is the key molecular switch that inhibits TGF-β2 signaling, and mediates the blunting effects of BMP7 on TGF-β2 in TM cells. A therapeutic modulation of Smad7 might be a promising approach to influence ECM turnover in the TM and to treat POAG.
Keywords: Smad7, TGF-β2, BMP7, trabecular meshwork, extracellular matrix
1. Introduction
An intraocular pressure (IOP) that is too high for the health of the optic nerve head is the critical risk factor for primary open angle glaucoma (POAG) (Gordon et al., 2002; Leske et al., 2003; The AGIS Investigators, 2000), a major cause of blindness worldwide (Quigley, 1996). In most cases of POAG, IOP is increased because of an abnormally high aqueous humor outflow resistance in the juxtacanalicular region (JCT) of the trabecular meshwork (TM) (Johnson, 2006; Tamm et al., 2007). The characteristic structural change in the JCT of eyes with POAG is a significant increase in the amounts of extracellular banded fibrillar elements which have been termed “plaque material” (Lütjen-Drecoll et al., 1986; Rohen et al., 1993). The molecular nature of plaque material has not been identified, but there is evidence that collagen type VI (Lütjen-Drecoll, 1999; Lütjen-Drecoll et al., 1989) and fibronectin (Hann et al., 2001) are associated with it. Extracellular matrix synthesis of cultured human trabecular meshwork (HTM) cells is induced upon treatment with TGF-β2 (Fuchshofer et al., 2007; Li et al., 2000; Zhao et al., 2004; Zhao and Russell, 2005), and comparable effects have been observed in the TM of human anterior eye segment perfused organ cultures, in which perfusion with TGF-β2 causes an accumulation of fine fibrillar extracellular material, and an increase in fibronectin synthesis (Fleenor et al., 2006; Gottanka et al., 2004). The increase in ECM deposition in TGF-β2-treated anterior eye segment perfused organ cultures correlates with a reduction in outflow facility. Since higher than normal levels of TGF-β2 have been found in the aqueous humor of patients with POAG (Inatani et al., 2001; Ochiai and Ochiai, 2002; Picht et al., 2001; Tripathi et al., 1994), TGF-β2 signaling appears to be causatively involved in the pathogenetic mechanisms that lead to the structural and functional alterations of the trabecular meshwork outflow pathways in POAG (Tamm and Fuchshofer, 2007).
In a recent study, we identified bone morphogenetic protein-7 (BMP7) as a potent antagonist of the fibrogenic effects of TGF-β2 on HTM cells (Fuchshofer et al., 2007). BMP7, a growth factor of the bone morphogenetic protein family, plays a critical role during development of the kidney and the eye (Dudley et al., 1995; Luo et al., 1995). In the adult organism, the expression of BMP7 is retained in the eye, in which its expression and that of its receptors has been shown in cornea, trabecular meshwork, and optic nerve (Wordinger et al., 2002; You et al., 1999). Treatment of HTM cells with BMP7 prevents the TGF-β2-induced increase in the expression of fibronectin, collagen types IV and VI, plasminogen activator inhibitor, thrombospondin-1, and connective tissue growth factor (Fuchshofer et al., 2007). Comparable results have been reported by Wordinger and colleagues, who could show that BMP4, another member of the bone morphogenetic protein family, blocks the TGF-β2 induced expression of fibronectin in HTM cells (Wordinger et al., 2007).
Since BMP-signaling might be useful for the pharmacologic modulation of TGF-β2 signaling in the eyes of patients with POAG, we wanted to know more about the signaling network that is induced in HTM cells treated with BMP7, TGF-β2 or the combination of both factors. To this end, we performed a microarray study in order to identify differentially regulated genes. Selected genes were confirmed by quantitative RT-PCR or Western blotting. While we observed multiple antagonistic and synergistic effects of both TGF-β2 and BMP7 on the expression of a considerable number of genes with different functional properties, we also noted that both growth factors caused a substantial induction of Smad7. Smad7 belongs to the group of inhibitory Smads (I-Smads) in the TGF-β superfamily signaling pathway, and functions as intracellular antagonist of TGF-β signaling (Park, 2005). By performing subsequent experiments with small interfering (si) RNA specific for Smad7, we were able to show that the knock down of Smad7 mRNA expression in HTM cells completely inhibits the antagonizing effects of BMP7 on TGF-β2 signaling. Our results strongly indicate that Smad7 is a key molecule to prevent TGF-β2-induced gene expression in the TM.
2. Material and Methods
Cell Cultures
Cultures of human trabecular meshwork (HTM) cells were established from the eyes of nine human donors according to protocols published previously (Fuchshofer et al., 2003; Tamm et al., 1996). HTM cells of the third passage were seeded in 35 mm culture wells (4.0 × 105 cells per well) and grown to a confluent monolayer. After seven days of confluence, wells were incubated in serum-free medium F-10 (Invitrogen, Karlsruhe, Germany) for 24 h followed by an incubation in fresh serum-free medium supplemented with 300 pM BMP7, 300 pM TGF-β2 (both R&D Systems GmbH, Wiesbaden, Germany), or a combination of both for 72 hours. Treated cells were compared with those from control dishes that were incubated under identical conditions for 72 h, but without supplemented TGF-β2 or BMP7. Each of the described experiments was done with a minimum of three primary cell lines. Methods for securing human tissue were humane, included proper consent and approval, and complied with the Declaration of Helsinki.
cDNA Microarray Analysis
HTM cells from four different donors were treated with growth factors as described above and total RNA was isolated by using TRIzol (Invitrogen, Karlsruhe, Germany), according to the manufacturer’s instructions. The RNA concentration was determined by absorbance at 260 nm (Eppendorf BioPhotometer; Eppendorf, Hamburg, Germany). Doubled-stranded cDNA was synthesized from 5 μg purified total RNA with a kit (Superscript Double-Stranded cDNA Synthesis Kit, Invitrogen) and a T7-(dT)24 primer (Affymetrix, Santa Clara, CA). After the double-stranded cDNA was purified by phenol-chloroform extraction, in vitro transcription reactions were performed (Bioassay High Yield RNA Transcript Labeling Kit; Enzo Diagnostics, Farmingdale, NY), according to the manufacturer’s protocol. Biotin-labeled cRNA was purified (Qiagen, Valencia, CA) and quantified using a ND-1000 Nano-drop spectrophotometer (Nano-Drop Technologies, Wilmington, DE), before being fragmented to 35 to 200 base fragments in an alkaline buffer. For each of the four cell lines, four arrays were hybridized. Each of these four arrays was subjected to one of the following treatments: BMP7, TGF-β2, BMP7/TGF-β2, or untreated control. Thus, 16 samples were separately hybridized to Human Genome U133A Arrays (Affymetrix), which probe for 22,215 genes. Washing, staining, and scanning were performed on the GeneChip Fluidics Stations and Scanner (Affymetrix) as recommended in the manufacturer’s technical manual, and data were extracted and analyzed using GeneChip® Operating Software (GCOS; Affymetrix). The absolute intensity values of each chip were globally scaled a target intensity value of 150 in order to normalize the data for inter-array comparisons. The target intensity levels and detection calls of each gene were generated using the Statistical Expression Algorithm in GCOS (Affymetrix). Genes that passed the following filters were included in the analysis: Genes detected to be present in all eight arrays as determined by the Statistical Expression Algorithm, genes with a p-value less than 0.05 as determined using the Welch one-way Anova t-test, and genes with a fold-change greater than or equal to 1.5.
Generation and transfection of siRNA
Target sequences for human Smad7 siRNA were designed according to the Ambion web-based criteria and generated with a Silencer siRNA construction kit (Ambion, Austin, TX, USA). Different Smad7 siRNAs were tested in initial transfection experiments and subsequent RT-PCR analysis. Best results were obtained by transfecting 15 nM of the Smad7-519 siRNA (named after the nucleotide start site in the Smad7 sequence) using lipofectamine for transfection according to manufacturer’s instructions (Invitrogen, Karlsruhe, Germany). The primers used to generate Smad7-519 siRNA were Smad7-519 5′-AGGUCACCACCAUCCCCACUU-3′ (sense) and 5′-GUGGGGAUGGUGGUGACCUUU -3′ (antisense). To assess the effects of Smad7-519 siRNA on BMP7-, TGF-β2-, and BMP7/TGF-β2-induced changes in gene expression, cells were seeded as previously described, transfected with 15 nM Smad7-519 siRNA and supplemented after 4 h with media containing BMP7-, TGF-β2-, or BMP7/TGF-β2 at a final concentration of 300 pM. Cells were incubated for 48 h before harvesting for RNA isolation.
Western Blot Analysis
Culture medium of treated HTM cells was collected and aliquots (500 μl) were concentrated 50-fold by centrifugation through a Vivaspin500 tube (10 kD cutoff; Vivascience, Hannover, Germany), according to the manufacturer’s instructions. Protein contents were determined by a Bradford protein assay (Biorad, Munich, Germany). To obtain protein extracts of cells grown on tissue culture dishes, cells were directly lysed in RIPA (RadioImmuno Precipitation Assay) buffer (50 mM Tris–HCl pH 8, 150 mMNaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) and protein content was measured using the BCA protein assay reagent (Pierce, Rockford, IL, USA). All probes were supplemented with SDS-loading buffer and denaturated by boiling for 5 min. 2 μg of each sample were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred onto a polyvinyl difluoride membrane (PVDF; Roche) by semi-dry blotting. Membranes were blocked in TBST/BSA (Tris-buffered saline, 0.1% tween-20, 5% bovine serum albumin, pH 7.2) for 1 h. Primary antibodies were added in TBST (Table 1) and allowed to react overnight at 4°C. After washing with TBST, secondary antibodies were added in TBST at the appropriate dilution (Table 1) for 30 min at room temperature. For detection, CDP-star, a chemiluminescent substrate for alkaline phosphatase, was diluted 1:100 in detection buffer, and the membranes were incubated for 5 min at room temperature. Chemiluminescence signals were analyzed on a LAS3000 imaging workstation (Fujifilm Europe GmbH, Düsseldorf, Germany). Exposure times ranged between 1 and 5 min. Quantification was done using the AIDA Biopackage software (Raytest, Straubenhardt, Germany).
Table 1.
Antibodies used for Western blot and immunohistochemistry in the present study (WB, Western blot; IHC, immunohistochemistry)
| Antibodies | Abbreviation | Dilution | Companies |
|---|---|---|---|
| fluorescein swine anti goat IgG | Sw anti G-Cy3 | 1:100 | Dako, Glostrup, Denmark |
| horseradish peroxidase-conjugated chicken anti goat IgG | Ch anti G-HRP | 1:2000 | Santa Cruz Biotechnology, Santa Cruz, USA |
| horseradish peroxidase-conjugated chicken anti rabbit IgG | Ch anti R-HRP | 1:2000 | Santa Cruz Biotechnology, Santa Cruz, USA |
| polyclonal goat anti human CTGF | G anti hCTGF | 1:500 (WB) | Santa Cruz Biotechnology, Santa Cruz, USA |
| polyclonal rabbit anti human SMAD7 | rb anti hSMAD7 | 1:700 (WB) | Santa Cruz Biotechnology, USA |
| Polyclonal rabbit anti human VEGF | Rb anti hVEGF | 1:500 (WB) | Santa Cruz Biotechnology, USA |
| Polyclonal goat anti human TFPI | G anti hTFPI | 1:1000 (WB) 1:200 (IHC) | Santa Cruz Biotechnology, USA |
| Polyclonal goat anti human MRCKβ | G anti hMRCKβ | 1:300 (WB) | Santa Cruz Biotechnology, USA |
Immunohistochemistry
HTM cells were grown on microscopic slides to preconfluency and treated as described above. After incubation, cells were fixed with 4% paraformaldehyde for 15 min and subsequently washed twice with PBS containing 0.1% Triton X-100. Primary antibodies were added in appropriate dilutions (Table 1) in PBS/BSA (5%) and allowed to bind for 4 h at room temperature. After washing three times with PBS, fluorescein-conjugated secondary antibodies were added (Table 1) for 1 h at room temperature. 4′,6-diamidino-2-phenylindole (DAPI) was used to counterstain nuclear DNA. Slides were mounted using Vectashield mounting medium with DAPI (Vector Laboratories, Burlington, CA, USA), and analyzed under a Zeiss Axio Imager fluorescence microscope (Carl Zeiss AG, Oberkochen, Germany). To control for unspecific binding of secondary antibodies, negative controls were performed, which were handled similarly, but incubated in PBS/BSA without primary antibody.
Real-time RT-PCR Analysis
Quantification by real-time PCR was performed on a Biorad IQ5 real-time thermal analyzer (Bio-Rad Laboratories, Munich, Germany). The S9, GAPDH and GNB2L genes served as an endogenous control to normalize the differences in the amount of cDNA in each sample. Hot Star Taq polymerase (Qiagen) was used for PCR reaction according to the manufacturer’s protocol. PCR reaction was performed in a volume of 25 μl, consisting of 2.5 μl of 10x PCR buffer, 2.0 to 2.5 μl of MgCl2 (25 mM), 0.5 μl of dNTPs (10 mM each; Promega), 0.5 μl of Hot Star Taq (5 U/μl), 0.5 μl of primer mix (20 μM each) and 2.5 μl of 1x SYBR Green I solution (Sigma). All samples that had to be compared for expression differences were run in the same assay as duplicates. After completion of PCR amplification, data were analyzed with Biorad iQ5 Optical System Software (Version 2.0). Data were initially expressed as a threshold cycle and are expressed as fold increases in gene expression in untreated HTM cells compared with the expression of the different treatments investigated. For each experiment, the mean value of untreated cells was set at 100%. Each individual experiment was repeated four times and mean values and standard deviations were calculated. After amplification was complete, the PCR products were analyzed by agarose gel electrophoresis. Sequences of primers and PCR product sizes of primer pairs are given in Table 2.
Table 2.
Primers used for PCR amplification in the present study.
| Target | Sequence | Position | Product Size |
|---|---|---|---|
| VEGF | 5′-tgcccgctgctgtctaat–3′ 5′-tctccgctctgagcaagg-3 |
1540–1609 | 70 |
| FGF5 | 5′-ttctgccaagattcaagcag–3′ 5′-aggtggctttttcttttcagg-3 |
702–778 | 77 |
| FSTL3 | 5′-tacatctcctcgtgccacat–3′ 5′-ttctgcagactcaccacctg-3 |
687–806 | 120 |
| TNFAIP6 | 5′-ggccatctcgcaacttaca–3′ 5′-cagcacagacatgaaatccaa-3 |
263–326 | 64 |
| HBEGF | 5′-tggggcttctcatgtttagg–3′ 5′-tgcccaacttcactttctcttc-3 |
797–871 | 75 |
| NRP1 | 5′-cacatttcacaagaagattgtgc–3′ 5′-catcaattttaatttctgggttcttt-3 |
2930–2999 | 70 |
| SMTN | 5′-caaaaagtcctaacccctgct–3′ 5′-tcatgtcgtccacctccac-3 |
2954–3030 | 77 |
| Cdc42bpb | 5′-gacgacgtgctgagaaacac–3′ 5′-aatccagaaaagcctgtgtga-3 |
1352–1413 | 62 |
| Capza1 | 5′-ggaagttcaccatcacacca–3′ 5′-aaccaactgaacattgccatc-3 |
922–1013 | 92 |
| Matn2 | 5′-gctgaggatgggaagaggt–3′ 5′-tcacatccgtggttttctga-3 |
1165–1226 | 62 |
| EFEMP1 | 5′-aacccttcccaccgtatcc–3′ 5′-tgcagtgcactcgtctatgtc-3 |
589–672 | 84 |
| TFPI2 | 5′-cgccaacaatttctacacctg–3′ 5′-ggcaaactttgggaacttttt-3 |
291–364 | 74 |
| LTBP1 | 5′-tgctgtcatggctggagtaa–3′ 5′-acatggcggaacacagcta-3 |
517–585 | 69 |
| LTBP2 | 5′-ctgaacactgtgaacggacag–3′ 5′-cagcagtcctcctgggtagt-3 |
2053–2117 | 65 |
| SMAD7 | 5′-cgatggattttctcaaaccaa–3′ 5′-attcgttccccctgtttca-3 |
931–1004 | 74 |
Number of Experiments and Statistical Analysis
Immunohistochemistry or Western blot experiments were repeated at least three times with cells or protein extract from primary HTM cell lines of at least three different donors. Each real-time RT-PCR analysis was performed in duplicate and repeated at least three times with RNA from HTM cell lines of at least three different donors. Student’s t-test was used for statistical analysis.
3. Results
In order to identify TM genes that are modified in their expression pattern by the presence of TGF-β2, BMP7 or the combination of both factors, hybridization of DNA microarrays was performed with RNA from treated HTM cells, and untreated controls. For each of these conditions, four microarrays were hybridized. Table 3 lists those genes that fulfilled the following criteria: An intensity level and detection call that qualified “presence” in all eight arrays (experimental and control), a p-value less than 0.05 using the Welch one-way Anova t-test, and a fold-change of at least 1.5 (experiment relative to control, or control relative to experiment). 16 genes were found to be up- or downregulated following treatment with TGF-β2, 5 genes after treatment with BMP7, and 18 genes after combined TGF-β2/BMP7 treatment (Table 3). Following similar criteria, gene expression of cells treated with TGF-β2 was compared with that of cells treated with BMP7, and the effects of combined TGF-β2/BMP7 were compared with those after treatment with TGF-β2 or BMP7 alone (Table 3). The highest number of genes that were differentially regulated (57) was observed between BMP7-treated cells and those treated with the combination of TGF-β2 and BMP7. In contrast, only six genes were different in their expression profile when comparing TGF-β2-treated cells with those receiving combined TGF-β2/BMP7 treatment. 21 genes were differentially regulated when comparing TGF-β2 treatment with BMP7 treatment. Since the major purpose of our study was to shed light on the signaling network that is involved in the action of BMP7 and TGF-β2 on HTM cells, we sorted the genes that were identified by this approach and focused on those genes that encode for proteins belonging to the following groups: Cytokines and their receptors, proteins involved in TGF-β- or BMP-signaling, cytoskeletal proteins, ECM proteins, and proteins involved in the extracellular proteolytic system. Genes were selected from each of these groups to confirm the data obtained by microarray analysis with quantitative real-time RT-PCR, western blotting or immunohistochemistry.
Table 3.
Genes that are differentially in HTM cells treated with BMP7.
| Symbol | GenBank | CvsT | CvB | CvsBT | TvBT | BvBT | BvT | Gene Name | confirmed |
|---|---|---|---|---|---|---|---|---|---|
| ARFGAP1 | NM_018209 | 1.7* | ADP-ribosylation factor GTPase activating protein 1 | ||||||
| ARSB | NM_000046 | 1.6** | arylsulfatase B | ||||||
| ATAD3A | NM_018188 | 1.7* | ATPase family. AAA domain containing 3A | ||||||
| BCAT1 | NM_005504 | 2.2* | 1.9* | branched chain aminotransferase 1. cytosolic | |||||
| C1RL | NM_016546 | 0.5* | 0.4* | complement component 1. r subcomponent-like | |||||
| CAMK2D | NM_172115 | 0.5* | 0.5* | calcium/calmodulin-dependent protein kinase (CaM kinase) II delta | |||||
| CAPZA1 | NM_006135 | 0.3* | Capping protein (actin filament) muscle Z-line. alpha 1 | + | |||||
| CASP1 | NM_001223 | 0.5* | 0.5* | caspase 1. apoptosis-related cysteine peptidase (interleukin 1. beta. convertase) | |||||
| CDC2L6 | NM_015076 | 1.6* | cell division cycle 2-like 6 (CDK8-like) | ||||||
| CDC42BPB | NM_006035 | 0.5* | CDC42 binding protein kinase beta (DMPK-like) | + | |||||
| CDH2 | NM_001792 | 3.1* | cadherin 2. type 1. N-cadherin (neuronal) | ||||||
| CHST11 | NM_018413 | 2.1* | Carbohydrate (chondroitin 4) sulfotransferase 11 | ||||||
| CLCF1 | NM_013246 | 2.0* | 1.9* | cardiotrophin-like cytokine factor 1 | |||||
| COL5A1 | NM_000093 | 1.7** | 1.6* | Collagen. type V. alpha 1 | |||||
| CTHRC1 | NM_138455 | 2.9* | 3.6** | 3.2* | collagen triple helix repeat containing 1 | ||||
| CTPS | NM_001905 | 2.9* | 3.7* | 2.9* | CTP synthase | ||||
| CYP4V2 | NM_207352 | 1.6* | cytochrome P450. family 4. subfamily V. polypeptide 2 | ||||||
| DUSP1 | NM_004417 | 2.0* | dual specificity phosphatase 1 | ||||||
| EDD | NM_015902 | 1.6* | 0.6* | E3 ubiquitin protein ligase. HECT domain containing. 1 | |||||
| EFEMP1 | NM_004105 | 0.5* | EGF-containing fibulin-like extracellular matrix protein 1 | + | |||||
| ENOSF1 | NM_017512 | 0.6* | 0.5* | enolase superfamily member 1 | |||||
| FGF5 | NM_004464 | 0.5* | fibroblast growth factor 5 | + | |||||
| FOXO1A | NM_002015 | 1.8* | 0.7* | 2.1* | forkhead box O1A (rhabdomyosarcoma) | ||||
| FOXP1 | NM_032682 | 2.0* | 1.9* | Forkhead box P1 | |||||
| FSTL3 | NM_005860 | 4.4* | follistatin-like 3 (secreted glycoprotein) | + | |||||
| GNPTAB | NM_024312 | 1.6* | N-acetylglucosamine-1-phosphate transferase. alpha and beta subunits | ||||||
| HBEGF | NM_001945 | 2.4* | heparin-binding EGF-like growth factor | + | |||||
| IER3 | NM_003897 | 2.4* | immediate early response 3 | ||||||
| IFNAR2 | NM_207585 | 1.8* | interferon (alpha. beta and omega) receptor 2 | ||||||
| IRF2BP2 | NM_182972 | 2.1* | 1.7* | interferon regulatory factor 2 binding protein 2 | |||||
| KCTD16 | NM_020768 | 3.0* | 4.2* | potassium channel tetramerisation domain containing 16 | |||||
| KLF5 | NM_001730 | 1.9* | 1.6* | Kruppel-like factor 5 (intestinal) | |||||
| KRT7 | NM_005556 | 4.0* | 3.2* | keratin 7 | |||||
| KRT8 | NM_002273 | 1.6* | keratin 8 | ||||||
| LIMD1 | NM_014240 | 1.5* | 1.7* | LIM domains containing 1 | |||||
| LTBP1 | NM_206943 | 2.0* | 1.5* | latent transforming growth factor beta binding protein 1 | + | ||||
| LTBP2 | NM_000428 | 3.6** | latent transforming growth factor beta binding protein 2 | + | |||||
| LXN | NM_020169 | 0.3* | latexin | ||||||
| MAF | NM_005360 | 7.7* | v-maf musculoaponeurotic fibrosarcoma oncogene homolog (avian) | ||||||
| MAFK | NM_002360 | 1.6* | 1.6* | v-maf musculoaponeurotic fibrosarcoma oncogene homolog K (avian) | |||||
| MATN2 | NM_002380 | 0.4* | matrilin 2 | + | |||||
| MFAP5 | NM_003480 | 2.5* | microfibrillar associated protein 5 | ||||||
| MICAL2 | NM_014632 | 1.8* | microtubule associated monoxygenase. calponin and LIM domain containing 2 | ||||||
| MT1E | NM_175617 | 2.4** | metallothionein 1E (functional) | ||||||
| MT1F | NM_005949 | 1.7* | 2.2* | metallothionein 1F (functional) | |||||
| MT1G | NM_005950 | 1.6* | metallothionein 1G | ||||||
| MT1H | NM_005951 | 2.0* | metallothionein 1H | ||||||
| MT1K | NM_176870 | 2.2* | Metallothionein 1M | ||||||
| NAGA | NM_000262 | 0.6* | N-acetylgalactosaminidase. alpha- | ||||||
| NAV3 | NM_014903 | 0.6* | 1.8* | neuron navigator 3 | |||||
| NEDD9 | NM_006403 | 3.4* | 4.7* | neural precursor cell expressed. developmentally down-regulated 9 | |||||
| NPM3 | NM_006993 | 0.5** | 0.7* | 0.5* | nucleophosmin/nucleoplasmin. 3 | ||||
| NQO1 | NM_000903 | 0.4** | NAD(P)H dehydrogenase. quinone 1 | ||||||
| NRP1 | NM_003873 | 0.5* | neuropilin 1 | + | |||||
| NUAK1 | NM_014840 | 3.5* | NUAK family. SNF1-like kinase. 1 | ||||||
| NUBPL | NM_025152 | 2.2* | 0.5* | nucleotide binding protein-like | |||||
| OACT2 | NM_138799 | 2.6* | O-acyltransferase (membrane bound) domain containing 2 | ||||||
| ORMDL2 | NM_014182 | 1.6* | ORM1-like 2 (S. cerevisiae) | ||||||
| P4HA2 | NM_004199 | 2.3 | 2.2* | Procollagen-proline. 2-oxoglutarate 4-dioxygenase (proline 4-hydroxylase). alpha polypeptide II | |||||
| PDXK | NM_003681 | 1.8* | pyridoxal (pyridoxine. vitamin B6) kinase | ||||||
| PINK1 | NM_032409 | 0.8* | 1.6* | PTEN induced putative kinase 1 | |||||
| PLP2 | NM_002668 | 1.7* | proteolipid protein 2 (colonic epithelium-enriched) | ||||||
| PPP1R13L | NM_006663 | 2.5* | protein phosphatase 1. regulatory (inhibitor) subunit 13 like | ||||||
| PSD3 | NM_015310 | 0.5** | pleckstrin and Sec7 domain containing 3 | ||||||
| PTCH | NM_000264 | 2.0* | patched homolog (Drosophila) | ||||||
| PTHLH | NM_198965 | 3.7* | parathyroid hormone-like hormone | ||||||
| RARRES3 | NM_004585 | 0.5* | 0.4* | retinoic acid receptor responder (tazarotene induced) 3 | |||||
| SERPINE1 | NM_000602 | 3.9* | serpin peptidase inhibitor (plasminogen activator inhibitor type 1) | + | |||||
| SGCD | NM_000337 | 3.2* | 2.8* | 2.1* | sarcoglycan. delta (35kDa dystrophin-associated glycoprotein) | ||||
| SGPL1 | NM_003901 | 1.9* | sphingosine-1-phosphate lyase 1 | ||||||
| SH3MD1 | NM_014631 | 2.7* | 2.5* | SH3 multiple domains 1 | |||||
| SMAD7 | NM_005904 | 2.4* | 3.1** | 3.1** | 2.4* | SMAD. mothers against DPP homolog 7 (Drosophila) | + | ||
| SMTN | NM_134269 | 2.1 | smoothelin | + | |||||
| STAT1 | NM_007315 | 0.5* | signal transducer and activator of transcription 1. 91kDa | ||||||
| TCF3 | NM_003200 | 2.2* | transcription factor 3 (E2A immunoglobulin enhancer binding factors E12/E47) | ||||||
| TFPI2 | NM_006528 | 5.6* | Tissue factor pathway inhibitor 2 | + | |||||
| TGFBR1 | NM_004612 | 1.8* | 1.5* | transforming growth factor. beta receptor I (activin A receptor type II-like kinase. 53kDa) | |||||
| TMEPAI | NM_199171 | 9.1* | 9.2* | transmembrane. prostate androgen induced RNA | |||||
| TMPO | NM_003276 | 2.6* | 2.6* | thymopoietin | |||||
| TNFAIP6 | NM_007115 | 4.1* | 3.6* | 3.2* | 3.6 | tumor necrosis factor. alpha-induced protein 6 | + | ||
| VEGF | NM_003376 | 1.6* | vascular endothelial growth factor | + | |||||
| ZNF486 | NM_052852 | 2.1* | zinc finger protein 486 | ||||||
| ZNF673 | NM_017776 | 0.5* | zinc finger protein 673 |
TGF-β2 or the combination treatment of both factors (C = control; T = TGF-β2; B = BMP7; BT = BMP7/TGF-β2;
p < 0.05;
p < 0.01)
Cytokines and cytokine receptors
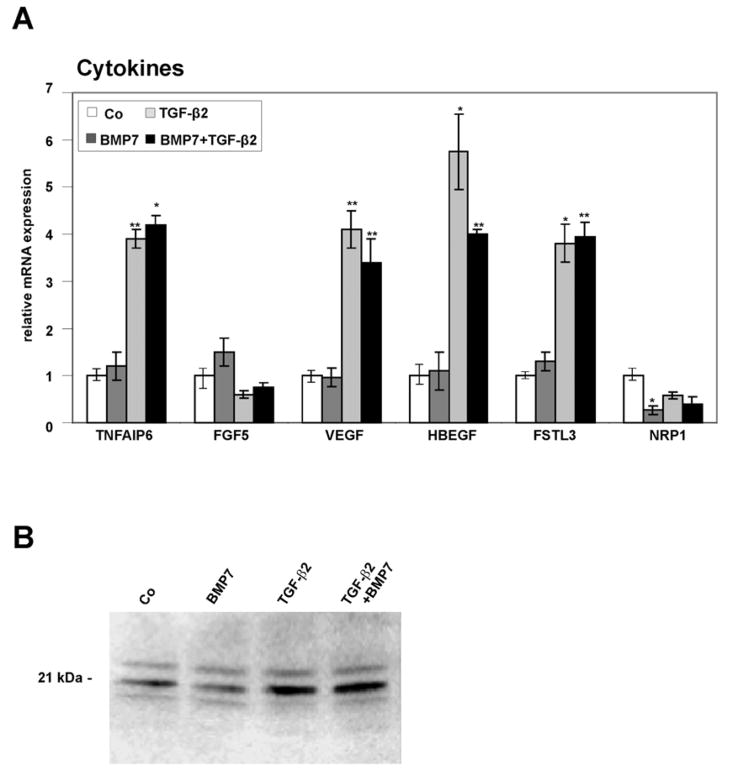
By quantitative real time RT-PCR, tumor necrosis factor α-induced protein 6 (TNFAIP6 or TSG6), a secretory protein containing a hyaluronan-binding domain and member of the hyaluronan-binding protein family (Milner et al., 2006), was found to be significantly upregulated following combined TGF-β2/BMP7 treatment (4.2 ± 0.2-fold, p < 0.01) or treatment with TGF-β2 alone (3.9 ± 0.2-fold, p < 0.05) corroborating the results obtained by microarray analysis (Fig. 1A). The expression of fibroblast growth factor 5 (FGF5), a secreted member of the FGF gene family (Allerstorfer et al., 2008), which was downregulated 0.5-fold by TGF-β2 in the microarray analysis, was found to be decreased using real time RT-PCR (Fig. 1A) albeit with marginal statistical significance (0.6 ± 0.08-fold, p > 0.06). Microarray data identified a higher expression of vascular endothelial cell growth factor (VEGF), heparin-binding EGF-like growth factor (HBEGF), and follistatin-like 3 (FSTL3) in HTM cells treated with the combination of BMP7/TGF-β2 as compared to HTM cells treated with BMP7 alone (Table 3). VEGF has important roles in mammalian vascular development and in diseases involving abnormal growth of blood vessels (Breen, 2007). HBEGF possesses a heparin binding domain that allows its interaction with cell surface and matrix heparin sulfate proteoglycans (Higashiyama et al., 1992; Thompson et al., 1994), while follistatin-like 3 is a glycoprotein that binds to various growth factors of the TGF-β superfamily and modulates their activity (Sidis et al., 2006). In contrast to VEGF, HBEGF, and follistatin-3, the expression of neuropilin 1 (NRP1), a membrane-bound co-receptor involved in VEGF and semaphoring signaling (Staton et al., 2007), was substantially lower in BMP7/TGF-β2-treated HTM cells than in BMP7 treated cells (Table 3). The influence of combined BMP7/TGF-β2-treatment on the expression of VEGF, HBEGF, follistatin-like 3, and neuropilin 1 could be confirmed by real time RT-PCR analysis. For VEGF, real time RT-PCR experiments showed a 4.11 ± 0.4-fold increase (p < 0.01) as compared to controls after treatment with TGF-β2. A smaller 3.4 ± 0.5-fold (p < 0.01) increase in mRNA for VEGF was observed after combined TGF-β/BMP7 treatment. Comparable findings were observed for HBEGF (5.8 ± 0.8-fold increase after TGFβ2, p < 0.05; 4.0 ± 0.1-fold increase after TGF-β2/BMP7, p < 0.01), and FSTL3 (3.81 ± 0.4-fold increase after TGFβ2, p < 0.05; 3.95 ± 0.3-fold increase after TGF-β2/BMP7, p < 0.01). Treatment with BMP7 had no substantial effects on the expression of VEGF, HBEGF, and follistatin-like 3. Real time RT-PCR showed a significant decrease in the expression of neuropilin 1 following BMP7 treatment (0.3 ± 0.1- fold, p < 0.01). To analyze, if the changes in VEGF mRNA expression correlated with the amounts of secreted VEGF, and to identify which VEGF isoform is predominantly expressed and regulated in HTM cells, western blot analysis was performed. The antibodies that were used bind to the 121 aa, 165 aa, and 189 aa VEGF splice variants, which migrate at around 14–18 kDa, 18–23 kDa, and 27 kDa, respectively (Ferrara et al., 1992; Ferrara et al., 1991). All three bands were detected in HTM cell culture medium (Fig. 1B). Similar to the results obtained by RT-PCR, BMP7-treatment had no effect on the amounts of VEGF, while treatment with TGF-β2 or TGF-β2/BMP7 substantially increased the amounts of detected VEGF165 that migrated at around 21 kDa. By densitometry, the increase in VEGF165 was 3.8 ± 0.4- fold after treatment with TGF-β2 and 3.0 ± 0.5-fold after combined TGF-β2/BMP7.
Fig. 1.
Differentially expressed growth factor-related genes in untreated HTM cells (Co), or cells treated with BMP-7, TGF-β2 or combined BMP-7/TGF-β2. A. Real-time RT-PCR analysis, means ± standard deviations of four independent experiments run in duplicate are shown. The mean value obtained with RNA from control cultures was set at 1 (* p < 0.05, ** p < 0.01). B. Western blot analysis for the presence of VEGF in cell culture medium of HTM cells.
Cytoskeletal proteins
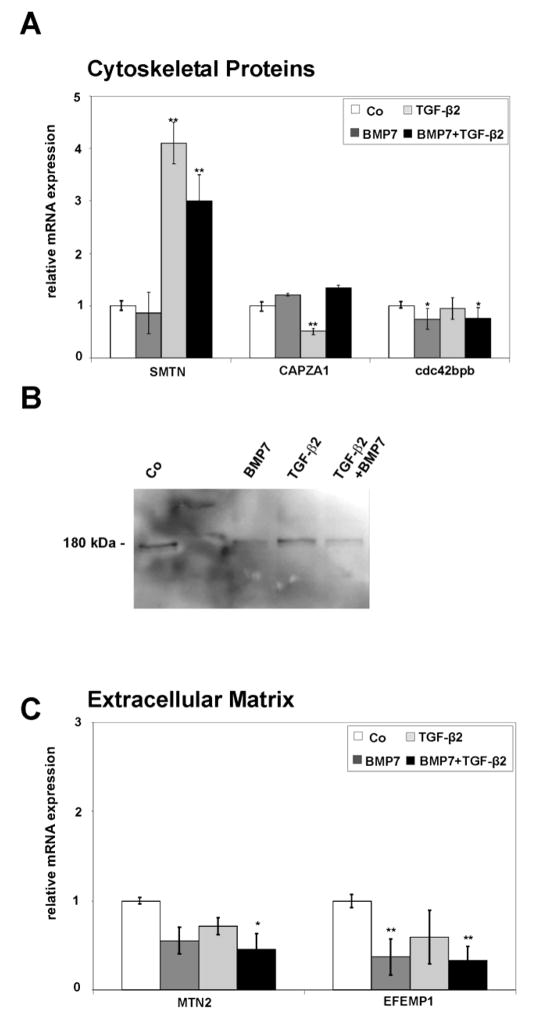
When gene expression profiles of BMP7/TGF-β2-treated HTM cells were compared with those of BMP7 treated cells, the expression of mRNA for smoothelin (SMTN), a structural protein that associates with actin stress fibers (Niessen et al., 2004; Niessen et al., 2005; van Eys et al., 2007) was found to be upregulated, while that of CAPZA1, another actin-binding protein (Wear and Cooper, 2004) was downregulated (Table 3). Real-time RT-PCR analysis confirmed the upregulation of SMTN mRNA following BMP7/TGF-β2 treatment as compared to untreated cells (3.0 ± 0.5-fold, p < 0.01) or BMP7-treated cells (Fig. 2A). The difference to control cultures was substantially higher after treatment with TGF-β2 alone (4.1 ± 0.4–fold, p < 0.01). In contrast, the expression of mRNA for CAPZA1 was not obviously different when comparing BMP7/TGF-β2-treated HTM cells with BMP7-treated ones. Still, treatment with TGF-β2 alone caused a substantial decrease of mRNA for CAPZA1 when compared with untreated HTM cells (0.5 ± 0.1-fold, p < 0.01), or those treated with BMP7 alone. By microarray analysis, mRNA for CDC42 binding protein kinase beta (cdc42bpb), a member of the serine/threonine protein kinase family and involved in cytoskeletal reorganization (Wilkinson et al., 2005) was downregulated in BMP7 treated cells when compared to controls (Table 3). Comparable results were observed by real time RT-PCR analysis in which less mRNA for cdc42bpb was found in BMP7-treated (0.78 ± 0.2-fold, p < 0.05) and BMP7/TGF-β2-treated cells (0.75 ± 0.14-fold, p < 0.05), when compared to untreated cells (Fig. 2A). A comparable decrease was observed after Western blot analysis with antibodies specific to cdc42bpb which detected the protein at its molecular weight of 180 kDa in all probes (Fig. 2B). Cells treated with BMP7 or BMP7/TGF-β2 contained less cdc42bpb than control cultures (0.7- fold) or those treated with TGF-β2 alone (Fig. 2B).
Fig. 2.
Differentially expressed cytoskeletal (A,B) or ECM (C) genes in untreated HTM cells (Co), or cells treated with BMP-7, TGF-β2 or combined BMP-7/TGF-β2. A,C. Real-time RT-PCR analysis, means ± standard deviations of four independent experiments run in duplicate are shown. The mean value obtained with RNA from control cultures was set at 1 (* p < 0.05, ** p < 0.01). B. Western blot analysis for the presence of cdc42bpb in HTM cells.
Extracellular matrix
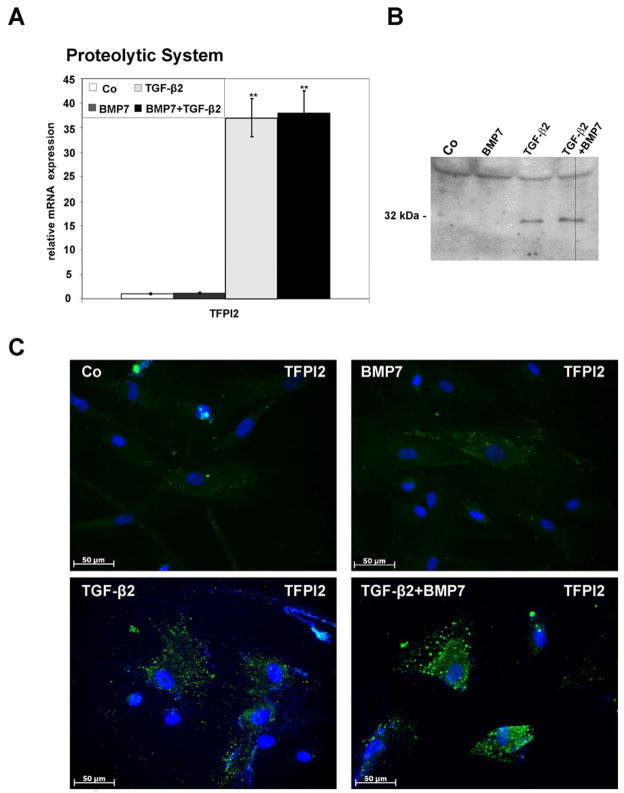
Two ECM proteins that were found to be regulated in their expression patterns by microarray analysis were matrilin-2 (MATN2), a protein involved in the formation of filamentous ECM (Deak et al., 1999; Wagener et al., 2005), and EGF-containing fibulin-like extracellular matrix protein1 (EFEMP1, fibulin-3), a secreted protein with unknown functions (Kobayashi et al., 2007). Both MATN2 and EFEMP1 were downregulated in their expression when BMP7/TGF-β2-treated HTM cells were compared with those treated with BMP7 only (Table 3). The results obtained by real time RT-PCR were at variance with those obtained by microarray analysis, as the expression of both MATN2 and EFEMP1 did not substantially differ between BMP7/TGF-β2- and BMP7-treated cells. Still, the expression of MATN2 was substantially downregulated by combined BMP7/TGF-β2 treatment (0.4 ± 0.2-fold, p < 0.05) when compared with control cultures (Fig. 2C). Comparable results were obtained for EFEMP1 which was downregulated 0.4 ± 0.2-fold by BMP7 treatment (p < 0.01) and 0.3 ± 0.1-fold by combined BMP7/TGF-β2 treatment (p < 0.01). In addition to structural ECM components, also genes involved in the extracellular proteolytic system were found to be regulated. Plasminogen activator inhibitor-1 (PAI-1, SERPINE1) showed higher expression in microarray analysis of BMP7/TGF-β2-treated HTM cells compared to cells treated with BMP7 (Table 3). The same was true for tissue factor pathway inhibitor 2 (TFPI2), a 32 kDa serine proteinase inhibitor and ECM protein that inhibits plasmin (Chand et al., 2005). By real-time RT-PCR analysis, a dramatic increase of TFPI2 mRNA (37.99 ± 4.6-fold, p < 0.01) was observed when RNA from BMP7/TGF-β2-treated HTM cells or from TGF-β2-treated cells (37.1 ± 3.9-fold, p < 0.01) was compared with control cultures (Fig. 3A). Comparable results were obtained by Western blot analysis with antibodies against TFPI2, in which a specific band at 32 kDa was detected in TGFβ2- and BMP7/TGFβ2-treated HTM cells, whereas no or only a very faint signal was observed in untreated control or in BMP7 treated cells (Fig. 3B). The cellular localization and the amounts of TFPI2 in HTM cells were also investigated by immunohistochemistry. Faint immunoreactivity in vesicular structures was observed in untreated control cells and cells treated with BMP7 (Fig. 3C). In contrast, after TGF-β2 treatment or treatment with BMP7/TGF-β2 more and brighter labeled vesicles were observed in the cytoplasm of HTM cells (Fig. 3C). Control cultures that were not incubated with the primary antibody showed no staining (data not shown). The induction of mRNA for PAI-1 following treatment with BMP7/TGFβ2 which was seen by microarray analysis was not confirmed by an independent assay, as the influence of TGFβ2 on the HTM expression of PAI-1 had been shown in several other studies (Fleenor et al., 2006; Fuchshofer et al., 2003; Fuchshofer et al., 2007; Zhao et al., 2004).
Fig. 3.
Expression of TFPI2 in untreated HTM cells (Co), or cells treated with BMP-7, TGF-β2 or combined BMP-7/TGF-β2. A. Real-time RT-PCR analysis, means ± standard deviations of four independent experiments run in duplicate are shown. The mean value obtained with RNA from control cultures was set at 1 (* p < 0.05, ** p < 0.01). B,C. Western blot analysis (B) and immunohistochemistry (C) for the presence of TFPI2 in HTM cells.
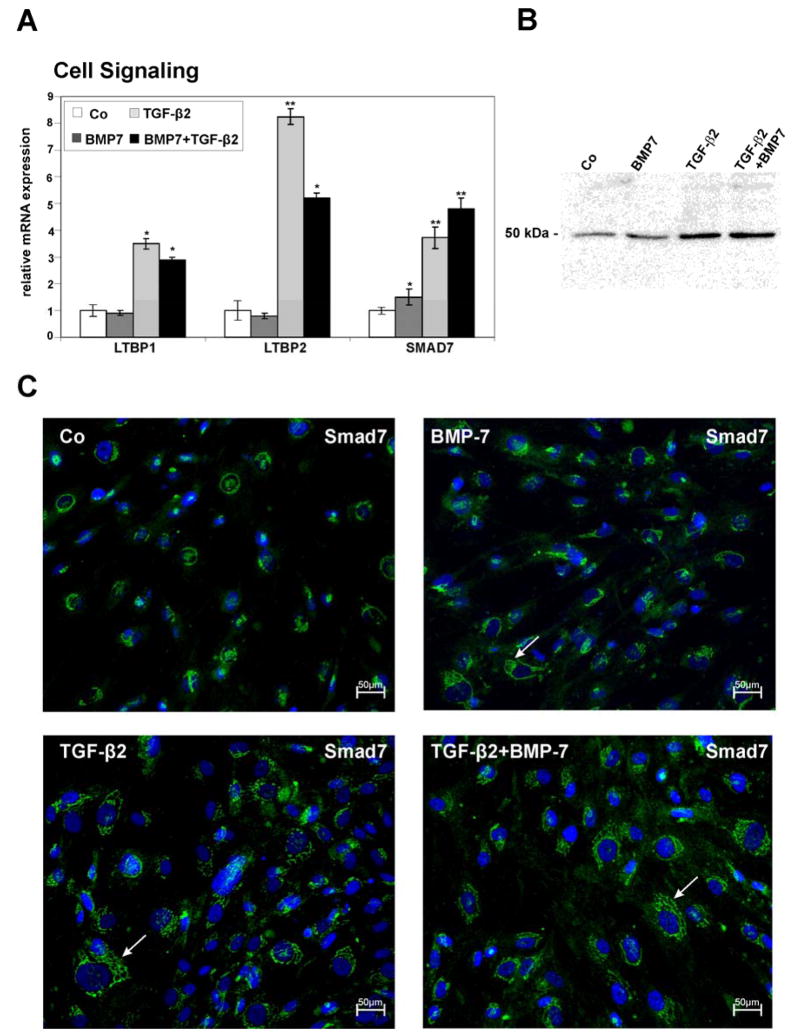
Proteins involved in TGF-β- and BMP-signaling
With regard to proteins involved in TGF-β-signaling, microarray data showed an upregulation of latent TGF-β binding protein-1 (LTBP-1) when comparing BMP7/TGF-β2-treated or TGF-β2-treated cells with those treated with BMP7 (Table 3). LTBP-1 targets latent complexes of TGF-β to the extracellular matrix, and is involved in the activation of TGF-β (Annes et al., 2004; Saharinen et al., 1999). Comparable results were observed for the closely related LTBP-2 which was also upregulated in BMP7/TGF-β2-treated cells as compared to BMP7 treated cells (Table 3). The upregulation was also seen by real time RT-PCR analysis which showed an increase in the expression of LTBP-1 and LTBP-2 both after treatment with TGF-β2 alone (3.5 ± 0.2-fold for LTBP-1, p < 0.05, and 8.25 ± 0.3 for LTBP-2, p < 0.01), and, at lower levels, after combined BMP7/TGF-β2-treatment (2.9 ± 0.1-fold for LTBP-1 p < 0.05, and 5.2 ± 0.2-fold for LTBP-2, p < 0.01) when compared with untreated cells (Fig. 4A). Another protein involved in TGF-β-signaling that microarray analysis identified as regulated in HTM cells both after TGF-β2- or BMP7/TGF-β2-treatment was Smad7, an inhibitory Smad in the TGF-β superfamily signaling pathway (Park, 2005). The upregulation in Smad7 expression was also seen by real time RT-PCR, in which treatment with TGF-β2 caused a 3.73 ± 0.4–fold (p < 0.01) increase as compared to controls, and combined BMP7/TGF-β2-treatment an increase of 4.8 ± 0.4 –fold (p < 0.01, Fig. 4A). Interestingly, real time RT-PCR experiments showed also an increase in the expression of Smad7 after treatment with BMP7 (1.5 ± 0.3-fold, p < 0.05), which was smaller than that observed after TGF-β2- or BMP7/TGF-β2-treatment. In order to analyze, if the differences in mRNA expression for Smad7 did also result in different amounts of proteins in HTM cells, Western blot analysis was performed. A specific band at 51 kDa was detected in HTM cells, which was faint in control cells, somewhat more intense in BMP7-treated cells, and considerably stronger in cells treated with TGF-β2 or BMP7/TGF-β2 (Fig. 4B). Densitometry showed a 2.9 ± 0.2-fold increase of Smad7 in HTM proteins after TGF-β2, and a 4.3 ± 0.5-fold increase after combined BMP7/TGF-β2-treatment. Data obtained by immunohistochemical analysis of HTM cells treated with BMP7, TGF-β2, or BMP7/TGF-β2 correlated markedly with those obtained by Western blotting. In untreated control cells, immunoreactivity for Smad7 was found to be restricted to a distinct perinuclear area, while the rest of the cytoplasm was largely unstained (Fig. 4C). In contrast, after treatment with BMP7, labeling for Smad7 were was seen to branch away from the perinuclear area into the periphery of the cytoplasm. This was even more prominent in cells treated with TGF-β2 or combined BMP7/TGF-β2, in which immunoreactivity for Smad7 was seen as a reticular network throughout the entire cytoplasm of HTM cells (Fig. 4C).
Fig. 4.
Differentially expressed genes involved in TGF-β-signaling in untreated HTM cells (Co), or cells treated with BMP-7, TGF-β2 or combined BMP-7/TGF-β2. A. Real-time RT-PCR analysis, means ± standard deviations of four independent experiments run in duplicate are shown. The mean value obtained with RNA from control cultures was set at 1. (* p < 0.05, ** p < 0.01). B. Western blot analysis for the presence of Smad7 in HTM cells. C. Immunohistochemical staining for Smad7 shows a perinuclear staining in untreated HTM cells, whereas in the BMP7, TGF-β2 and BMP7/TGF-β2 treated cells, an additional reticular staining is seen throughout the cytoplasm of cells (arrows).
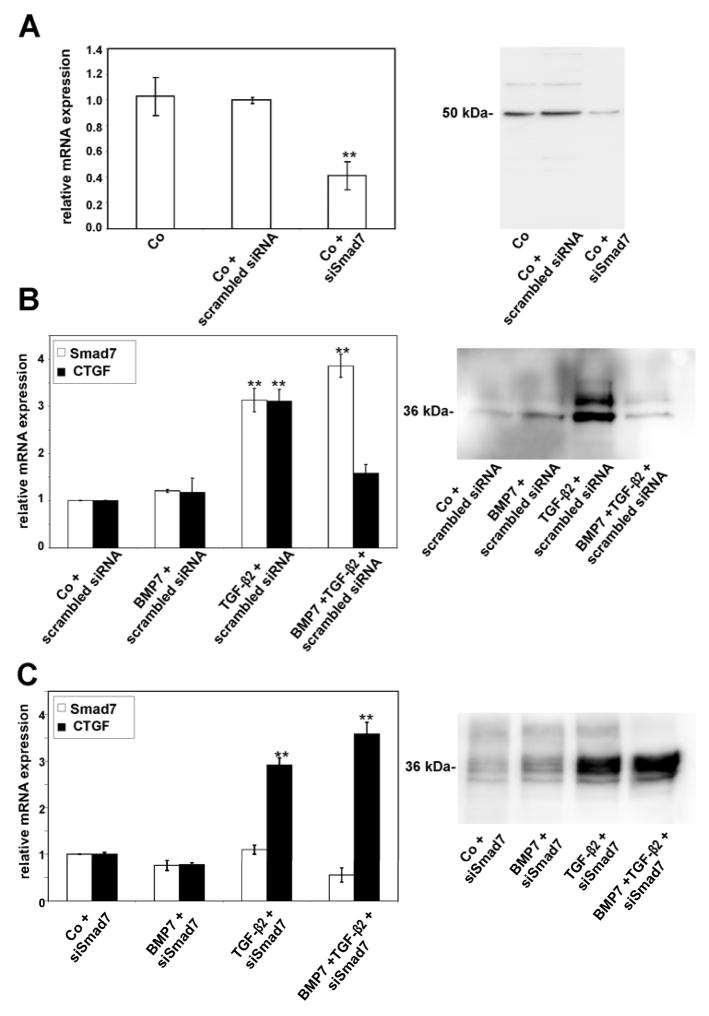
SMAD7 silencing
Since Smad7 functions as intracellular antagonist of TGF-β signaling (Park, 2005), we hypothesized that the substantial induction of Smad7 in HTM cells after combined BMP7/TGF-β2-treatment might be involved in the antagonizing effects of BMP7 on TGF-β2-signaling in HTM cells (Fuchshofer et al., 2007). To clarify this hypothesis, we used siRNA specific for Smad7 to knock down the expression of Smad7 mRNA in HTM cells. Quantitative real time RT-PCR analysis confirmed that following transfection with specific siRNA, the expression of mRNA for Smad7 was considerably downregulated (0.4 ± 0.1-fold, p < 0.01), an effect that was not seen when unspecific scrambled siRNA was used (Fig. 5A). Western blot analysis confirmed that the decrease in mRNA expression resulted in substantially lower amounts of Smad7 in transfected HTM cells (Fig. 5A). Again, transfection with scrambled siRNA had no effects. As a next step, we analyzed the expression of connective tissue growth factor (CTGF) in HTM cells after treatment with BMP7, TGF-β2 or the combination of both factors. We choose CTGF, as we had shown in a previous study that the expression of CTGF and its mRNA is substantially upregulated following treatment with TGF-β2, an effect that is completely prevented by adding BMP7 to TGF-β2 and treating HTM cells with combined BMP7/TGF-β2 (Fuchshofer et al., 2007). We started to transfect HTM cells with scrambled siRNA as described above in order to test, if transfection with scrambled siRNA would change the effects of BMP7, TGF-β2 or BMP7/TGF-β2 on the expression of mRNA for Smad7 or CTGF. Similar to experiments shown above, a substantial induction of Smad7 mRNA (p < 0.01) was observed after treatment with TGF-β2 or BMP7/TGF-β2 (Fig. 5B). In addition, treatment with TGF-β2 caused a substantial increase in mRNA for CTGF (p < 0.01), an effect that was completely prevented when BMP7 was added to TGF-β2 (Fig. 5B), corroborating the findings of our previous study (Fuchshofer et al., 2007). Western blot analysis confirmed that the increase in mRNA for CTGF after treatment TGF-β2, but not after combined BMP7/TGF-β2, resulted in an increase of secreted CTGF in culture medium of HTM cells (Fig. 5B). We concluded that transfection with scrambled siRNA has no effects on TGF-β2 and BMP7 signaling in HTM cells. Subsequently, we transfected HTM cells with Smad7 siRNA as described above and analyzed the amounts of CTGF and Smad7 mRNA by real time RT-PCR. In contrast to the results observed with untransfected cells or cells transfected with scrambled siRNA, the amounts of Smad7 mRNA remained unchanged following treatment with BMP7, TGF-β2 or BMP7/TGF-β2 (Fig. 5C). The expression of mRNA for CTGF was upregulated significantly following treatment with TGF-β2 (4.1 ± 0.6-fold, p < 0.01), an effect that was similar to that observed after treatment with combined BMP7/TGF-β2 (4.3 ± 0.2-fold, p < 0.01). Western blot analysis confirmed that the increase in mRNA for CTGF after treatment with Smad7 siRNA resulted in a considerable increase of secreted CTGF in culture medium of HTM cells following treatment with TGF-β2 or combined BMP7/TGF-β2 (Fig. 5C). Overall, the data obtained by siRNA experiments confirmed our hypothesis that the inhibitory action of Smad7 is required to mediate the antagonizing effects of BMP7 on TGF-β2-signaling.
Fig. 5.
Silencing of Smad7 mRNA following transfection with specific Smad7 siRNA (siSmad7) or scrambled siRNA, and its effects on Smad7 and CTGF expression in untreated HTM cells (Co), or cells treated with BMP-7, TGF-β2 or combined BMP-7/TGF-β2. A. Real-time RT-PCR for Smad7 mRNA and Western blot analysis for Smad7. B. Real-time RT-PCR for Smad7 and CTGF mRNA in HTM cells, and Western blot analysis for CTGF in culture medium of HTM cells transfected with scrambled siRNA. C. Real-time RT-PCR for Smad7 and CTGF mRNA in HTM cells, and Western blot analysis for CTGF in culture medium of HTM cells transfected with siRNA against Smad7 (for real time RT-PCR experiments, means ± standard deviations of four independent experiments run in duplicate are shown, * p < 0.05, ** p < 0.01. The amounts of Smad7 mRNA were normalized to that of GAPDH).
4. Discussion
Using microarray analysis and real time RT-PCR, we have identified a substantial number of HTM genes that are differentially regulated in their expression by treatment with TGF-β2, BMP7 or combined BMP7/TGF-β2. While the effects of BMP7 or BMP7/TGF-β2 on overall gene expression in HTM cells have not been investigated before, some of us have previously studied the genomic and proteomic expression changes in HTM cells following treatment with TGF-β2 (Zhao et al., 2004). Using comparable protocols for HTM cell culture, treatment with TGF-β2, and microarray analysis, 21 genes were identified as differentially regulated in the study by Zhao and colleagues, while 16 genes were found in the present study. It is certainly of interest to note, that out of these 37 genes, MAF was the only one to be found regulated in both of the studies. MAF, which was upregulated in its expression upon treatment with TGF-β2, encodes for a basic region leucine zipper transcription factor that plays an important role during development of the anterior eye (Kim et al., 1999; Ring et al., 2000). Mutations in MAF cause cataract, anterior segment dysgenesis and coloboma (Jamieson et al., 2002). In another microarray study, 10 genes were shown to be regulated in HTM cells after treatment with TGF-β2 (Fleenor et al., 2006). Again, only two of those (SERPINE1 and LTBP1) where also identified as regulated in the present study. In addition, several genes did not show up in our arrays, which in other studies that were using a candidate approach had been found to be regulated by TGF-β2 in HTM cells. Among these genes were those encoding for thrombospondin-1 (Flügel-Koch et al., 2004), fibronectin (Fuchshofer et al., 2007; Li et al., 2000; Welge-Lüssen et al., 2000), collagen types IV and VI (Fuchshofer et al., 2007), tissue transglutaminase (Welge-Lüssen et al., 2000), αB-crystallin (Welge-Lüssen et al., 1999), myocilin (Tamm et al., 1999), CTGF (Fuchshofer et al., 2007), and TGF-β1 (Li et al., 1996). We conclude that microarray analysis, at least as performed in our hands, is prone to false negative results, and is not an experimental approach that allows identification of a complete list of genes that are regulated upon treatment with a specific cytokine or growth factor. Nevertheless, microarray analysis is certainly a very powerful tool to identify new genes as being regulated, an advantage that allowed us to obtain substantial new insights on the action of BMP7 and/or TGF-β2 in HTM cells.
Among the genes that were newly identified as regulated by TGF-β2 and BMP7/TGF-β2 was TNFAIP6 which encodes for tumor necrosis factor-α-induced protein 6 (TSG6). TSG6 which already has been shown to be regulated by TGF-β1 (Zhao et al., 2004), tumor necrosis factor-α, and interleukin-1a (Acott and Kelley, 2008) in HTM cells, is a multifunctional secretory protein that interacts with a wide rage of glycosaminoglycans such as hyaluronan, chondroitin-4-sulphate, dermatan-sulfate, heparin, and heparan-sulfate, and with proteoglycans such as aggrecan and versican (Milner et al., 2006). In addition, it interacts with thrombospondin-1 and with fibronectin (Kuznetsova et al., 2005; Kuznetsova et al., 2008). In vitro, the interaction of TSG6 with other molecules of the extracellular matrix leads to significant functional changes, such as to an increase in hyaluronan cross-linking, or to an increase in fibronectin fibril assembly. Since all of the known binding partners of TSG6 have been identified in fresh HTM (Acott and Kelley, 2008), it is tempting to speculate that the functional properties of TSP6 are also important for TM cell biology in the living eye.
Regulation by TGF-β2, BMP7 and/or BMP7/TGF-β2 was also shown for several growth factors and related genes. FGF-5 and HB-EGF play important roles during embryonic development where they are involved in hair follicle formation (Hebert et al., 1994), or blastocyst implantation (Xie et al., 2007). In the adult organism, FGF-5 has been found to be expressed mainly in neurons of brain and spinal cord, on which it acts as neurotrophic factor (Hughes et al., 1993; Lindholm et al., 1994). HB-EGF appears to be involved in wound healing, renal injury, smooth muscle hyperplasia, and atherosclerosis (Raab and Klagsbrun, 1997). In the eye, HB-EGF is expressed in retinal pigment epithelial (RPE) cells and Müller glia, and has been identified in epiretinal membranes of patients with proliferative vitreoretinopathy (Hollborn et al., 2006; Hollborn et al., 2005). The role of both FGF-5 and HB-EGF for TM biology remains unclear at the moment. The expression of VEGF in HTM cells is surprising as VEGF plays important roles in vascular development and angiogenesis (Breen, 2007), while the normal TM is a tissue that needs to remain avascular for its proper function. Indeed, very high amounts of VEGF (40 to 113-fold more than in the AH of patients with cataract or POAG) have been found in the aqueous humor of patients with neovascular glaucoma caused by vascularization of the chamber angle (Tripathi et al., 1998). On the other hand, there is considerable evidence that in the adult organism, certain vessels depend on VEGF for the maintenance of a highly permeable and fenestrated endothelium (Eremina et al., 2007; Eremina et al., 2003; Kamba et al., 2006). VEGF165, the isoform that was found to be secreted by HTM cells in the present study, increases the permeability of venules and capillaries, in which it induces fenestration (Roberts and Palade, 1995). The endothelium of Schlemm’s canal (SC), the vessel that is directly adjacent to the TM, is highly porous providing one of the highest vascular permeabilities in the body (Johnson, 2006). It is tempting to speculate that the high permeability of SC endothelium depends on paracrine VEGF-signaling from the TM. Finally, follistatin-like 3 is an extracellular regulatory protein for several members of the TGFβ-superfamily including BMPs that acts via high-affinity binding to prevent interaction with the respective receptor (Sidis et al., 2006; Tsuchida et al., 2000). The upregulation of follistatin-like 3 expression following treatment with TGF-β2 might amplify the action of TGF-β2 by binding to BMP7 and/or BMP4 to modify the antagonizing effects of both factors on TGF-β2-signaling (Fuchshofer et al., 2007; Wordinger et al., 2007).
TGF-β1 is known to induce the synthesis of α-smooth muscle actin and to generate a myofibroblast-like contractile phenotype in fibroblasts (Desmoulière et al., 1993; Ronnov-Jessen and Petersen, 1993) and HTM cells (Tamm et al., 1996). Similar properties have been shown for TGF-β2 in lens epithelial (Hales et al., 2000; Wormstone et al., 2002) and RPE cells (Gamulescu et al., 2006). In correlation with these observations is the finding that two actin binding proteins, smoothelin and CAPZA1 were found do be differentially regulated by treatment with TGF-β2 in our study. Smoothelin, which was upregulated, is characteristically found in fully differentiated contractile smooth muscle cells and is regarded as marker for such cells (van Eys et al., 2007). Mice that are deficient in smoothelin die because the smooth muscle cells in the gastrointestinal tract do not contract properly (Niessen et al., 2005). CAPZA1 encodes for the α1-subunit of capping protein, a heterodimer composed of α and β subunits, which binds to the barbed ends of actin filaments (Wear and Cooper, 2004). The downregulation of CAPZA1 expression following treatment with TGF-β2 as observed in our study might be important in a scenario where actin fibrillogenesis, and elongation and reorganization of the actin cytoskeleton are happening.
Although TGF-β2 is known to induce the synthesis of a wide variety of structural ECM molecules in HTM cells (Tamm and Fuchshofer, 2007), the only such molecules that were identified as regulated in the present study were matrilin-2 and fibulin-3. Matrilins are adaptor proteins in the ECM that mediate interactions between collagen-containing fibrils and other matrix constituents such as proteoglycans. Fibulin-3 has been shown to bind to tropoelastin (Kobayashi et al., 2007), and is mutated in some forms of age-related macula degeneration where it is found in sub-RPE deposits (Marmorstein et al., 2007; Marmorstein et al., 2002). Matrilin-2 and fibulin-3 were found to be downregulated following all three kinds of treatment, a finding that is difficult to analyze in the absence of more detailed information on the function of both proteins. In previous work, we could show that TGF-β2 does not only act on the expression of structural ECM molecules, but does also modify the expression of various molecules involved in the extracellular proteolytic system (Fuchshofer et al., 2003). A critical molecule in this system is PAI-1, an inhibitor of the plasminogen/plasmin system that reduces the enzymatic activation of matrix-metalloproteinases (MMPs). Our finding that the expression of PAI-1 was increased upon treatment with TGF-β2 corroborates data of previous studies (Fleenor et al., 2006; Fuchshofer et al., 2003; Fuchshofer et al., 2007), and supports the hypothesis that inhibition of MMP activation may be a critical factor that leads to an increase in ECM in the TM of patients with POAG. In this context, the finding of the present study appears to be important that tissue factor pathway inhibitor-2 (TFPI2) is expressed in the TM and is highly induced following treatment with TGF-β2. TFPI2 is a matrix associated Kunitz-type serine proteinase inhibitor that inhibits plasmin gelatinolytic activity of MMP-2 and MMP-9 (Chand et al., 2005). It is tempting to speculate that PAI-1 and TFPI2 have synergistic roles on the extracellular proteolytic system in the HTM.
Two genes, LTBP-1 and SMAD7, which are directly involved in TGF-β-signaling were found to be regulated by TGF-β2. Together with the TGF-β propeptide, also called the latency-associated protein, LTBP-1 is part of the secreted inactive TGF-β complex (Saharinen et al., 1999). Extracellular activation of this complex is a critical but incompletely understood step in TGF-signaling which appears to involve binding of LTBP-1 to extracellular matrix compounds and/or cell-membrane associated integrins (Annes et al., 2004; Saharinen et al., 1999). The major fraction (>90%) of secreted LTBP-1 does not contain TGF-β (Saharinen et al., 1999) indicating that LTBP-1 also serves a separate role as structural ECM protein. LTBP-1 and the closely related LTBP-2, which was also induced in HTM cells following TGF-β2, share a high degree of homology with fibrillin and have been found to associate with microfibrils of elastic fibers (Hirai et al., 2007; Hirani et al., 2007; Isogai et al., 2003). In the normal human eye, LTBP-1 has been localized to ECM fibrils in the anterior eye segment including TM and scleral spur (Schlötzer-Schrehardt et al., 2001). The induction of LTBP-1 and -2 following treatment with TGF-β might be part of the overall fibrogenic effects of TGF-β2.
Our observation that both BMP7 and TGF-β2 induce the expression of Smad7 and its mRNA in HTM cells, an effect that appears to be additive when HTM cells are treated with combined BMP7/TGF-β2, lead to the hypothesis that Smad7 is responsible for the antagonizing effects of BMP7 on TGF-β2-signaling. This hypothesis could be supported by subsequent experiments using siRNA for Smad7, which showed that a knockdown of Smad7 completely prevents the antagonizing effects of BMP7 on the TGF-β2-induced expression of CTGF (Fuchshofer et al., 2007). Smad7 is an inhibitory Smad which functions as intracellular antagonist that inhibits TGF-β signaling, a situation that is in marked contrast to that of the group of receptor-associated Smads (Smad2/3) which mediate TGF-β signaling (Park, 2005). Several mechanisms have been suggested how Smad7 exerts its negative effects on TGF-β-signaling (ten Dijke and Hill, 2004). Smad7 inhibits signaling through stable binding to activated type I receptors and competition with receptor-associated Smads for receptor activation. In addition, Smad7 can recruit the E3 ubiquitin ligases Smurf1 and Smurf2 to the type I receptors, resulting in receptor ubiquitination, degradation, and termination of signaling. Finally, Smad7 appears also to act in the nucleus to disrupt the formation of the TGF-β-induced functional Smad-DNA complex (Zhang et al., 2007). Since TGF-β1 and BMP7 induce the expression of Smad7 in several cell types (Afrakhte et al., 1998), the concept has been proposed that Smad7 plays an essential role in an autoinhibitory negative-feedback regulation of TGF-β signaling (Itoh and ten Dijke, 2007; Park, 2005; ten Dijke and Hill, 2004). In HTM cells, the model would imply that TGF-β2 induces Smad7, an effect that shortens the time interval during which TGF-β2-signaling is promoting the expression of its specific target genes. If BMP7 is added to TGF-β2, the expression of target genes is blunted or completely prevented, as the available amounts of Smad7 are induced to much higher levels than when TGF-β2 would act alone. While this model is attractive and supported by the data of our study, it does not explain why some of the genes that were identified in the present study as regulated by TGF-β2 (TFPI2, TNFAIP6, FSTL3) were expressed to almost similar levels after treatment with BMP7/TGF-β2. The possibility that different pathways of TGF-β2-signaling exist in the TM, some of which cannot be inhibited by Smad7, is currently under investigation in our laboratory. Nevertheless, the data of the present study strongly indicate that Smad7 is a key molecular switch to inhibit TGF-β2-signaling in the TM. In other systems, the induction of Smad7 expression has already been successfully used to inhibit the pathogenic effects of TGF-β-signaling in experimental models of renal (Hou et al., 2005; Ka et al., 2007; Li et al., 2002) and liver fibrosis (Dooley et al., 2008), and proliferative vitreoretinopathy (Saika et al., 2007). Similar strategies appear to have promise to prevent the adverse effects of TGF-β2-signaling on the aqueous humor outflow pathways in eyes with POAG.
Acknowledgments
The authors thank Angelika Pach and Tina Schiereis for excellent technical help. The study was supported by grants from the Deutsche Forschungsgemeinschaft (Research Unit (Forschergruppe) 1075, TP 3 and TP 5).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acott TS, Kelley MJ. Extracellular matrix in the trabecular meshwork. Exp Eye Res. 2008;86:543–61. doi: 10.1016/j.exer.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afrakhte M, Moren A, Jossan S, Itoh S, Sampath K, Westermark B, Heldin CH, Heldin NE, ten Dijke P. Induction of inhibitory Smad6 and Smad7 mRNA by TGF-beta family members. Biochem Biophys Res Commun. 1998;249:505–11. doi: 10.1006/bbrc.1998.9170. [DOI] [PubMed] [Google Scholar]
- Allerstorfer S, Sonvilla G, Fischer H, Spiegl-Kreinecker S, Gauglhofer C, Setinek U, Czech T, Marosi C, Buchroithner J, Pichler J, Silye R, Mohr T, Holzmann K, Grasl-Kraupp B, Marian B, Grusch M, Fischer J, Micksche M, Berger W. FGF5 as an oncogenic factor in human glioblastoma multiforme: autocrine and paracrine activities. Oncogene. 2008;27:4180–90. doi: 10.1038/onc.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annes JP, Chen Y, Munger JS, Rifkin DB. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol. 2004;165:723–34. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen EC. VEGF in biological control. J Cell Biochem. 2007;102:1358–67. doi: 10.1002/jcb.21579. [DOI] [PubMed] [Google Scholar]
- Chand HS, Foster DC, Kisiel W. Structure, function and biology of tissue factor pathway inhibitor-2. Thromb Haemost. 2005;94:1122–30. doi: 10.1160/TH05-07-0509. [DOI] [PubMed] [Google Scholar]
- Deak F, Wagener R, Kiss I, Paulsson M. The matrilins: a novel family of oligomeric extracellular matrix proteins. Matrix Biol. 1999;18:55–64. doi: 10.1016/s0945-053x(98)00006-7. [DOI] [PubMed] [Google Scholar]
- Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-β1 induces α-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley S, Hamzavi J, Ciuclan L, Godoy P, Ilkavets I, Ehnert S, Ueberham E, Gebhardt R, Kanzler S, Geier A, Breitkopf K, Weng H, Mertens PR. Hepatocyte-specific Smad7 expression attenuates TGF-beta-mediated fibrogenesis and protects against liver damage. Gastroenterology. 2008;135:642–59. doi: 10.1053/j.gastro.2008.04.038. [DOI] [PubMed] [Google Scholar]
- Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795–807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- Eremina V, Baelde HJ, Quaggin SE. Role of the VEGF - a signaling pathway in the glomerulus: evidence for crosstalk between components of the glomerular filtration barrier. Nephron Physiol. 2007;106:32–7. doi: 10.1159/000101798. [DOI] [PubMed] [Google Scholar]
- Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–16. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Houck K, Jakeman L, Leung DW. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992;13:18–32. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Houck KA, Jakeman LB, Winer J, Leung DW. The vascular endothelial growth factor family of polypeptides. J Cell Biochem. 1991;47:211–8. doi: 10.1002/jcb.240470305. [DOI] [PubMed] [Google Scholar]
- Fleenor DL, Shepard AR, Hellberg PE, Jacobson N, Pang IH, Clark AF. TGFbeta2-induced changes in human trabecular meshwork: implications for intraocular pressure. Invest Ophthalmol Vis Sci. 2006;47:226–34. doi: 10.1167/iovs.05-1060. [DOI] [PubMed] [Google Scholar]
- Flügel-Koch C, Ohlmann A, Fuchshofer R, Welge-Lüssen U, Tamm ER. Thrombospondin-1 in the trabecular meshwork: localization in normal and glaucomatous eyes, and induction by TGF-beta1 and dexamethasone in vitro. Exp Eye Res. 2004;79:649–63. doi: 10.1016/j.exer.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R, Welge-Lüssen U, Lütjen-Drecoll E. The effect of TGF-beta2 on human trabecular meshwork extracellular proteolytic system. Exp Eye Res. 2003;77:757–65. doi: 10.1016/s0014-4835(03)00220-3. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R, Yu AH, Welge-Lüssen U, Tamm ER. Bone morphogenetic protein-7 is an antagonist of transforming growth factor-beta2 in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2007;48:715–26. doi: 10.1167/iovs.06-0226. [DOI] [PubMed] [Google Scholar]
- Gamulescu MA, Chen Y, He S, Spee C, Jin M, Ryan SJ, Hinton DR. Transforming growth factor beta2-induced myofibroblastic differentiation of human retinal pigment epithelial cells: regulation by extracellular matrix proteins and hepatocyte growth factor. Exp Eye Res. 2006;83:212–22. doi: 10.1016/j.exer.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, 2nd, Wilson MR, Kass MA. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–20. doi: 10.1001/archopht.120.6.714. discussion 829–30. [DOI] [PubMed] [Google Scholar]
- Gottanka J, Chan D, Eichhorn M, Lütjen-Drecoll E, Ethier CR. Effects of TGF-β2 in perfused human eyes. Invest Ophthalmol Vis Sci. 2004;45:153–8. doi: 10.1167/iovs.03-0796. [DOI] [PubMed] [Google Scholar]
- Hales AM, Chamberlain CG, McAvoy JW. Susceptibility to TGFbeta2-induced cataract increases with aging in the rat. Invest Ophthalmol Vis Sci. 2000;41:3544–51. [PubMed] [Google Scholar]
- Hann CR, Springett MJ, Wang X, Johnson DH. Ultrastructural localization of collagen IV, fibronectin, and laminin in the trabecular meshwork of normal and glaucomatous eyes. Ophthalmic Res. 2001;33:314–24. doi: 10.1159/000055687. [DOI] [PubMed] [Google Scholar]
- Hebert JM, Rosenquist T, Gotz J, Martin GR. FGF5 as a regulator of the hair growth cycle: evidence from targeted and spontaneous mutations. Cell. 1994;78:1017–25. doi: 10.1016/0092-8674(94)90276-3. [DOI] [PubMed] [Google Scholar]
- Higashiyama S, Lau K, Besner GE, Abraham JA, Klagsbrun M. Structure of heparin-binding EGF-like growth factor. Multiple forms, primary structure, and glycosylation of the mature protein. J Biol Chem. 1992;267:6205–12. [PubMed] [Google Scholar]
- Hirai M, Horiguchi M, Ohbayashi T, Kita T, Chien KR, Nakamura T. Latent TGF-beta-binding protein 2 binds to DANCE/fibulin-5 and regulates elastic fiber assembly. Embo J. 2007;26:3283–95. doi: 10.1038/sj.emboj.7601768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirani R, Hanssen E, Gibson MA. LTBP-2 specifically interacts with the amino-terminal region of fibrillin-1 and competes with LTBP-1 for binding to this microfibrillar protein. Matrix Biol. 2007;26:213–23. doi: 10.1016/j.matbio.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Hollborn M, Iandiev I, Seifert M, Schnurrbusch UE, Wolf S, Wiedemann P, Bringmann A, Kohen L. Expression of HB-EGF by retinal pigment epithelial cells in vitreoretinal proliferative disease. Curr Eye Res. 2006;31:863–74. doi: 10.1080/02713680600888807. [DOI] [PubMed] [Google Scholar]
- Hollborn M, Tenckhoff S, Jahn K, Iandiev I, Biedermann B, Schnurrbusch UE, Limb GA, Reichenbach A, Wolf S, Wiedemann P, Kohen L, Bringmann A. Changes in retinal gene expression in proliferative vitreoretinopathy: glial cell expression of HB-EGF. Mol Vis. 2005;11:397–413. [PubMed] [Google Scholar]
- Hou CC, Wang W, Huang XR, Fu P, Chen TH, Sheikh-Hamad D, Lan HY. Ultrasound-microbubble-mediated gene transfer of inducible Smad7 blocks transforming growth factor-beta signaling and fibrosis in rat remnant kidney. Am J Pathol. 2005;166:761–71. doi: 10.1016/s0002-9440(10)62297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RA, Sendtner M, Goldfarb M, Lindholm D, Thoenen H. Evidence that fibroblast growth factor 5 is a major muscle-derived survival factor for cultured spinal motoneurons. Neuron. 1993;10:369–77. doi: 10.1016/0896-6273(93)90327-n. [DOI] [PubMed] [Google Scholar]
- Inatani M, Tanihara H, Katsuta H, Honjo M, Kido N, Honda Y. Transforming growth factor-β2 levels in aqueous humor of glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol. 2001;239:109–13. doi: 10.1007/s004170000241. [DOI] [PubMed] [Google Scholar]
- Isogai Z, Ono RN, Ushiro S, Keene DR, Chen Y, Mazzieri R, Charbonneau NL, Reinhardt DP, Rifkin DB, Sakai LY. Latent transforming growth factor beta-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J Biol Chem. 2003;278:2750–7. doi: 10.1074/jbc.M209256200. [DOI] [PubMed] [Google Scholar]
- Itoh S, ten Dijke P. Negative regulation of TGF-beta receptor/Smad signal transduction. Curr Opin Cell Biol. 2007;19:176–84. doi: 10.1016/j.ceb.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Jamieson RV, Perveen R, Kerr B, Carette M, Yardley J, Heon E, Wirth MG, van Heyningen V, Donnai D, Munier F, Black GC. Domain disruption and mutation of the bZIP transcription factor, MAF, associated with cataract, ocular anterior segment dysgenesis and coloboma. Hum Mol Genet. 2002;11:33–42. doi: 10.1093/hmg/11.1.33. [DOI] [PubMed] [Google Scholar]
- Johnson M. What controls aqueous humour outflow resistance? Exp Eye Res. 2006;82:545–57. doi: 10.1016/j.exer.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ka SM, Huang XR, Lan HY, Tsai PY, Yang SM, Shui HA, Chen A. Smad7 gene therapy ameliorates an autoimmune crescentic glomerulonephritis in mice. J Am Soc Nephrol. 2007;18:1777–88. doi: 10.1681/ASN.2006080901. [DOI] [PubMed] [Google Scholar]
- Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O’Brien SM, Davis RB, Gowen LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo CJ, McDonald DM. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–76. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- Kim JI, Li T, Ho IC, Grusby MJ, Glimcher LH. Requirement for the c-Maf transcription factor in crystallin gene regulation and lens development. Proc Natl Acad Sci U S A. 1999;96:3781–5. doi: 10.1073/pnas.96.7.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N, Kostka G, Garbe JH, Keene DR, Bachinger HP, Hanisch FG, Markova D, Tsuda T, Timpl R, Chu ML, Sasaki T. A comparative analysis of the fibulin protein family. Biochemical characterization, binding interactions, and tissue localization. J Biol Chem. 2007;282:11805–16. doi: 10.1074/jbc.M611029200. [DOI] [PubMed] [Google Scholar]
- Kuznetsova SA, Day AJ, Mahoney DJ, Rugg MS, Mosher DF, Roberts DD. The N-terminal module of thrombospondin-1 interacts with the link domain of TSG-6 and enhances its covalent association with the heavy chains of inter-alpha-trypsin inhibitor. J Biol Chem. 2005;280:30899–908. doi: 10.1074/jbc.M500701200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova SA, Mahoney DJ, Martin-Manso G, Ali T, Nentwich HA, Sipes JM, Zeng B, Vogel T, Day AJ, Roberts DD. TSG-6 binds via its CUB_C domain to the cell-binding domain of fibronectin and increases fibronectin matrix assembly. Matrix Biol. 2008;27:201–10. doi: 10.1016/j.matbio.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- Li J, Tripathi BJ, Chalam KV, Tripathi RC. Transforming growth factor-beta 1 and -beta 2 positively regulate TGF-beta 1 mRNA expression in trabecular cells. Invest Ophthalmol Vis Sci. 1996;37:2778–82. [PubMed] [Google Scholar]
- Li J, Tripathi BJ, Tripathi RC. Modulation of pre-mRNA splicing and protein production of fibronectin by TGF-β2 in porcine trabecular cells. Invest Ophthalmol Vis Sci. 2000;41:3437–3443. [PubMed] [Google Scholar]
- Li JH, Zhu HJ, Huang XR, Lai KN, Johnson RJ, Lan HY. Smad7 inhibits fibrotic effect of TGF-Beta on renal tubular epithelial cells by blocking Smad2 activation. J Am Soc Nephrol. 2002;13:1464–72. doi: 10.1097/01.asn.0000014252.37680.e4. [DOI] [PubMed] [Google Scholar]
- Lindholm D, Harikka J, da Penha Berzaghi M, Castren E, Tzimagiorgis G, Hughes RA, Thoenen H. Fibroblast growth factor-5 promotes differentiation of cultured rat septal cholinergic and raphe serotonergic neurons: comparison with the effects of neurotrophins. Eur J Neurosci. 1994;6:244–52. doi: 10.1111/j.1460-9568.1994.tb00267.x. [DOI] [PubMed] [Google Scholar]
- Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995;9:2808–20. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- Lütjen-Drecoll E. Functional morphology of the trabecular meshwork in primate eyes. Prog Retin Eye Res. 1999;18:91–119. doi: 10.1016/s1350-9462(98)00011-1. [DOI] [PubMed] [Google Scholar]
- Lütjen-Drecoll E, Rittig M, Rauterberg J, Jander R, Mollenhauer J. Immunomicroscopical study of type VI collagen in the trabecular meshwork of normal and glaucomatous eyes. Exp Eye Res. 1989;48:139–147. doi: 10.1016/0014-4835(89)90027-4. [DOI] [PubMed] [Google Scholar]
- Lütjen-Drecoll E, Shimizu T, Rohrbach M, Rohen JW. Quantitative analysis of “plaque material” in the inner and outer wall of Schlemm’s canal in normal and glaucomatous eyes. Exp Eye Res. 1986;42:443–455. doi: 10.1016/0014-4835(86)90004-7. [DOI] [PubMed] [Google Scholar]
- Marmorstein LY, McLaughlin PJ, Peachey NS, Sasaki T, Marmorstein AD. Formation and progression of sub-retinal pigment epithelium deposits in Efemp1 mutation knock-in mice: a model for the early pathogenic course of macular degeneration. Hum Mol Genet. 2007;16:2423–32. doi: 10.1093/hmg/ddm199. [DOI] [PubMed] [Google Scholar]
- Marmorstein LY, Munier FL, Arsenijevic Y, Schorderet DF, McLaughlin PJ, Chung D, Traboulsi E, Marmorstein AD. Aberrant accumulation of EFEMP1 underlies drusen formation in Malattia Leventinese and age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:13067–72. doi: 10.1073/pnas.202491599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner CM, Higman VA, Day AJ. TSG-6: a pluripotent inflammatory mediator? Biochem Soc Trans. 2006;34:446–50. doi: 10.1042/BST0340446. [DOI] [PubMed] [Google Scholar]
- Niessen P, Clement S, Fontao L, Chaponnier C, Teunissen B, Rensen S, van Eys G, Gabbiani G. Biochemical evidence for interaction between smoothelin and filamentous actin. Exp Cell Res. 2004;292:170–8. doi: 10.1016/j.yexcr.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Niessen P, Rensen S, van Deursen J, De Man J, De Laet A, Vanderwinden JM, Wedel T, Baker D, Doevendans P, Hofker M, Gijbels M, van Eys G. Smoothelin-a is essential for functional intestinal smooth muscle contractility in mice. Gastroenterology. 2005;129:1592–601. doi: 10.1053/j.gastro.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Ochiai Y, Ochiai H. Higher concentration of transforming growth factor-β in aqueous humor of glaucomatous eyes and diabetic eyes. Jpn J Ophthalmol. 2002;46:249–53. doi: 10.1016/s0021-5155(01)00523-8. [DOI] [PubMed] [Google Scholar]
- Park SH. Fine tuning and cross-talking of TGF-beta signal by inhibitory Smads. J Biochem Mol Biol. 2005;38:9–16. doi: 10.5483/bmbrep.2005.38.1.009. [DOI] [PubMed] [Google Scholar]
- Picht G, Welge-Luessen U, Grehn F, Lütjen-Drecoll E. Transforming growth factor-β2 levels in the aqueous humor in different types of glaucoma and the relation to filtering bleb development. Graefes Arch Clin Exp Ophthalmol. 2001;239:199–207. doi: 10.1007/s004170000252. [DOI] [PubMed] [Google Scholar]
- Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–93. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab G, Klagsbrun M. Heparin-binding EGF-like growth factor. Biochim Biophys Acta. 1997;1333:F179–99. doi: 10.1016/s0304-419x(97)00024-3. [DOI] [PubMed] [Google Scholar]
- Ring BZ, Cordes SP, Overbeek PA, Barsh GS. Regulation of mouse lens fiber cell development and differentiation by the Maf gene. Development. 2000;127:307–17. doi: 10.1242/dev.127.2.307. [DOI] [PubMed] [Google Scholar]
- Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108 (Pt 6):2369–79. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- Rohen JW, Lütjen-Drecoll E, Flügel C, Meyer M, Grierson I. Ultrastructure of the trabecular meshwork in untreated cases of primary open-angle glaucoma (POAG) Exp Eye Res. 1993;56:683–692. doi: 10.1006/exer.1993.1085. [DOI] [PubMed] [Google Scholar]
- Ronnov-Jessen L, Petersen OW. Induction of alpha-smooth muscle actin by transforming growth factor- beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest. 1993;68:696–707. [PubMed] [Google Scholar]
- Saharinen J, Hyytiainen M, Taipale J, Keski-Oja J. Latent transforming growth factor-β binding proteins (LTBPs) - structural extracellular matrix proteins for targeting TGF-β action. Cytokine Growth Factor Rev. 1999;10:99–117. doi: 10.1016/s1359-6101(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Saika S, Yamanaka O, Nishikawa-Ishida I, Kitano A, Flanders KC, Okada Y, Ohnishi Y, Nakajima Y, Ikeda K. Effect of Smad7 gene overexpression on transforming growth factor beta-induced retinal pigment fibrosis in a proliferative vitreoretinopathy mouse model. Arch Ophthalmol. 2007;125:647–54. doi: 10.1001/archopht.125.5.647. [DOI] [PubMed] [Google Scholar]
- Schlötzer-Schrehardt U, Zenkel M, Küchle M, Sakai LY, Naumann GO. Role of transforming growth factor-beta1 and its latent form binding protein in pseudoexfoliation syndrome. Exp Eye Res. 2001;73:765–80. doi: 10.1006/exer.2001.1084. [DOI] [PubMed] [Google Scholar]
- Sidis Y, Mukherjee A, Keutmann H, Delbaere A, Sadatsuki M, Schneyer A. Biological activity of follistatin isoforms and follistatin-like-3 is dependent on differential cell surface binding and specificity for activin, myostatin, and bone morphogenetic proteins. Endocrinology. 2006;147:3586–97. doi: 10.1210/en.2006-0089. [DOI] [PubMed] [Google Scholar]
- Staton CA, Kumar I, Reed MW, Brown NJ. Neuropilins in physiological and pathological angiogenesis. J Pathol. 2007;212:237–48. doi: 10.1002/path.2182. [DOI] [PubMed] [Google Scholar]
- Tamm E, Russell P, Epstein DL, Johnson DH, Piatigorsky J. Modulation of Myocilin/TIGR expression in human trabecular meshwork. Invest Ophthalmol Vis Sci. 1999;40:2577–2582. [PubMed] [Google Scholar]
- Tamm ER, Fuchshofer R. What increases outflow resistance in primary open-angle glaucoma? Surv Ophthalmol. 2007;52(Suppl 2):S101–4. doi: 10.1016/j.survophthal.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Tamm ER, Siegner A, Baur A, Lütjen-Drecoll E. Transforming growth factor-β1 induces α-smooth muscle-actin expression in cultured human and monkey trabecular meshwork. Exp Eye Res. 1996;62:389–397. doi: 10.1006/exer.1996.0044. [DOI] [PubMed] [Google Scholar]
- Tamm ER, Toris CB, Crowston JG, Sit A, Lim S, Lambrou G, Alm A. Basic science of intraocular pressure. In: Weinreb RN, Brandt JD, Garway-Heath D, Medeiros F, editors. Intraocular pressure. Reports and consensus statements of the 4th global AIGS consensus meeting on intraocular pressure. Kugler Publications; Amsterdam: 2007. pp. 1–14. [Google Scholar]
- ten Dijke P, Hill CS. New insights into TGF-beta-Smad signalling. Trends Biochem Sci. 2004;29:265–73. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- The AGIS Investigators. The advanced glaucoma intervention study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- Thompson SA, Higashiyama S, Wood K, Pollitt NS, Damm D, McEnroe G, Garrick B, Ashton N, Lau K, Hancock N, et al. Characterization of sequences within heparin-binding EGF-like growth factor that mediate interaction with heparin. J Biol Chem. 1994;269:2541–9. [PubMed] [Google Scholar]
- Tripathi RC, Li J, Chan WFA, Tripathi BJ. Aqueous humor in glaucomatous eyes contains an increased level of TGF-β2. Exp Eye Res. 1994;58:723–727. doi: 10.1006/exer.1994.1158. [DOI] [PubMed] [Google Scholar]
- Tripathi RC, Li J, Tripathi BJ, Chalam KV, Adamis AP. Increased level of vascular endothelial growth factor in aqueous humor of patients with neovascular glaucoma. Ophthalmology. 1998;105:232–7. doi: 10.1016/s0161-6420(98)92782-8. [DOI] [PubMed] [Google Scholar]
- Tsuchida K, Arai KY, Kuramoto Y, Yamakawa N, Hasegawa Y, Sugino H. Identification and characterization of a novel follistatin-like protein as a binding protein for the TGF-beta family. J Biol Chem. 2000;275:40788–96. doi: 10.1074/jbc.M006114200. [DOI] [PubMed] [Google Scholar]
- van Eys GJ, Niessen PM, Rensen SS. Smoothelin in vascular smooth muscle cells. Trends Cardiovasc Med. 2007;17:26–30. doi: 10.1016/j.tcm.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Wagener R, Ehlen HW, Ko YP, Kobbe B, Mann HH, Sengle G, Paulsson M. The matrilins--adaptor proteins in the extracellular matrix. FEBS Lett. 2005;579:3323–9. doi: 10.1016/j.febslet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Wear MA, Cooper JA. Capping protein: new insights into mechanism and regulation. Trends Biochem Sci. 2004;29:418–28. doi: 10.1016/j.tibs.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Welge-Lüssen U, May CA, Eichhorn M, Bloemendal H, Lütjen-Drecoll E. AlphaB-crystallin in the trabecular meshwork is inducible by transforming growth factor-beta. Invest Ophthalmol Vis Sci. 1999;40:2235–2241. [PubMed] [Google Scholar]
- Welge-Lüssen U, May CA, Lütjen-Drecoll E. Induction of tissue transglutaminase in the trabecular meshwork by TGF- β1 and TGF-β2. Invest Ophthalmol Vis Sci. 2000;41:2229–2238. [PubMed] [Google Scholar]
- Wilkinson S, Paterson HF, Marshall CJ. Cdc42-MRCK and Rho-ROCK signalling cooperate in myosin phosphorylation and cell invasion. Nat Cell Biol. 2005;7:255–61. doi: 10.1038/ncb1230. [DOI] [PubMed] [Google Scholar]
- Wordinger RJ, Agarwal R, Talati M, Fuller J, Lambert W, Clark AF. Expression of bone morphogenetic proteins (BMP), BMP receptors, and BMP associated proteins in human trabecular meshwork and optic nerve head cells and tissues. Mol Vis. 2002;8:241–50. [PubMed] [Google Scholar]
- Wordinger RJ, Fleenor DL, Hellberg PE, Pang IH, Tovar TO, Zode GS, Fuller JA, Clark AF. Effects of TGF-beta2, BMP-4, and gremlin in the trabecular meshwork: implications for glaucoma. Invest Ophthalmol Vis Sci. 2007;48:1191–200. doi: 10.1167/iovs.06-0296. [DOI] [PubMed] [Google Scholar]
- Wormstone IM, Tamiya S, Anderson I, Duncan G. TGF-beta2-induced matrix modification and cell transdifferentiation in the human lens capsular bag. Invest Ophthalmol Vis Sci. 2002;43:2301–8. [PubMed] [Google Scholar]
- Xie H, Wang H, Tranguch S, Iwamoto R, Mekada E, Demayo FJ, Lydon JP, Das SK, Dey SK. Maternal heparin-binding-EGF deficiency limits pregnancy success in mice. Proc Natl Acad Sci U S A. 2007;104:18315–20. doi: 10.1073/pnas.0707909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You L, Kruse FE, Pohl J, Volcker HE. Bone morphogenetic proteins and growth and differentiation factors in the human cornea. Invest Ophthalmol Vis Sci. 1999;40:296–311. [PubMed] [Google Scholar]
- Zhang S, Fei T, Zhang L, Zhang R, Chen F, Ning Y, Han Y, Feng XH, Meng A, Chen YG. Smad7 antagonizes transforming growth factor beta signaling in the nucleus by interfering with functional Smad-DNA complex formation. Mol Cell Biol. 2007;27:4488–99. doi: 10.1128/MCB.01636-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Ramsey KE, Stephan DA, Russell P. Gene and protein expression changes in human trabecular meshwork cells treated with transforming growth factor-beta. Invest Ophthalmol Vis Sci. 2004;45:4023–34. doi: 10.1167/iovs.04-0535. [DOI] [PubMed] [Google Scholar]
- Zhao X, Russell P. Versican splice variants in human trabecular meshwork and ciliary muscle. Mol Vis. 2005;11:603–8. [PubMed] [Google Scholar]